Abstract
Objectives:
Early detection of neurodevelopmental delay is crucial for intervention and treatment strategies. We analyzed associations between newborn DNA methylation (DNAm), neonatal magnetic resonance imaging (MRI) neuroimaging data, and neurodevelopment.
Methods:
Neurodevelopment was assessed in 161 children from the South African Drakenstein Child Health Study at two years of age using the Bayley Scales of Infant and Toddler Development III. We performed an epigenome-wide association study of neurodevelopmental delay using DNAm from cord blood. Subsequently, we analyzed if associations between DNAm and neurodevelopmental delay were mediated by altered neonatal brain volumes (subset of 51 children).
Results:
Differential DNAm at SPTBN4 (cg26971411, Δbeta=−0.024, p-value=3.28×10−08), and two intergenic regions (chromosome 11: cg00490349, Δbeta=−0.036, p-value=3.02×10−08; chromosome 17: cg15660740, Δbeta=−0.078, p-value = 6.49 × 10−08) were significantly associated with severe neurodevelopmental delay. While these associations were not mediated by neonatal brain volume, neonatal caudate volumes were independently associated with neurodevelopmental delay, particularly in language (Δcaudate volume=165.30mm3, p=0.0443) and motor (Δcaudate volume=365.36mm3, p-value=0.0082) domains.
Conclusions:
Differential DNAm from cord blood and increased neonatal caudate volumes were independently associated with severe neurodevelopmental delay at two years of age. These findings suggest that neurobiological signals for severe developmental delay may be detectable in very early life.
Keywords: Early child development, methylome-wide association study, MRI imaging data, early biomarkers, brain development
Introduction
The first two years of life are a critical period of rapid growth and brain development. Child cognitive development is influenced by genetic and environmental factors which interact to determine how the brain develops and functions (Tucker-Drob and Briley 2014). Multiple factors have been shown to affect neurodevelopment; these include poverty, maternal education, maternal physical and psychological health, substance use, and nutrition (Black et al. 2017; Britto et al. 2017; Chan et al. 2017; Daelmans et al. 2017; Donald et al. 2018). As a result, low- and middle-income countries in general, and in particular sub-Saharan Africa, have a very high proportion of young children at risk of developmental delay (Black et al. 2017). New research in early human development shows that epigenetic, immunological, physiological, and psychological adaptations to the environment occur from conception, and that these adaptations affect development throughout the life course (Britto et al. 2017). This highlights the importance of early identification of developmental risk and timely implementation of targeted interventions (Daelmans et al. 2017). However, screening for neurodevelopmental delay is inconsistent across different environments, and in low-resource contexts where children are at highest risk, often does not occur in a rigorous manner (Vitrikas et al. 2017). Therefore, exploring neonatal neurobiological signals that can potentially be used to detect early signs of severe neurodevelopmental delay could have implications for identification and intervention design.
As such, DNA methylation (DNAm) levels from cord blood could potentially be used as neurobiological signals to understand future disease risk. DNAm can be altered by psychological, environmental and genetic factors, and is one of the most studied modifications of the genome. Multiple studies have demonstrated that epigenetic modifications are associated with the risk of cognitive disability or impairment in adults (Roubroeks et al. 2017). However, the role of epigenetic variation on early cognitive development of infants remains largely unknown. Neuropsychiatric outcomes that are known to be associated with altered DNAm in newborns include attention-deficit/hyperactivity disorder (ADHD) (Walton, Pingault, et al. 2017), autism spectrum disorder (Mordaunt et al. 2020), and amygdala:hippocampal volume ratio, which is a marker for anxiety and aggression in later years (Walton, Cecil, et al. 2017). Associations between newborn DNAm and neurodevelopment are less well understood. A study of 80 children from India found associations between cord blood DNAm and mental development score but not with motor development at 18 months of age (Gurugubelli et al. 2021). However, their study was limited to the analysis of global DNAm. A study of 238 Mexican-American children (Huen et al. 2018) found some evidence that altered cord blood DNAm levels in the paraoxonase 1 (PON1) gene are associated with neurodevelopmental delay, but none of their associations remained significant after accounting for multiple comparisons (Huen et al. 2018).
Over the last decades, structural magnetic resonance imaging (MRI) studies in children have increasingly been used to ascertain relations between alteration in regional brain volumes and various functional measures of behavioral development (Paterson et al. 2006; Spann et al. 2014). Changes in brain volume have been associated with developmental disorders such as autism spectrum disorders (Schumann et al. 2004; Mosconi et al. 2009), ADHD (Castellanos et al. 2002), and specific language impairment (Gauger et al. 1997). However, most of these studies have been conducted in school-aged children and information regarding structural and functional brain development in the earliest years of life remains limited and is mainly focused on premature infants (Knickmeyer et al. 2008; Hinojosa-Rodríguez et al. 2017). A few studies have used MRI brain scans from neonates and infants at 6 or 7 months of age to predict cognitive development, suggesting involvement of the maturation of subcortical brain regions (Deniz Can et al. 2013; Spann et al. 2014). Given their association with prenatal environmental, psychological risk factors and genetics (Britto et al. 2017), we hypothesize that early changes in brain volume can potentially mediate the association between DNAm and neurodevelopmental delay.
In this study we will investigate associations between DNAm levels from cord blood, subcortical volumes from neonatal MRI imaging data and neurodevelopmental delay at two years of age in the South African Drakenstein Child Health Study (DCHS).
Methods
Study design and study population
The DCHS, a population-based birth cohort, has been described previously (Zar et al. 2015). Mothers were enrolled prenatally in their second trimester and followed through pregnancy at two primary care clinics serving two distinct populations (predominantly black African ancestry or predominantly mixed ancestry). Mother-child pairs were followed from birth and infants enrolled in the DCHS were followed until at least five years of age (Zar et al. 2015). All births occurred at a single, central facility, Paarl Hospital. The sample included in the present study were 161 children who had all measurements available, including cognitive Bayley Scales of Infant and Toddler Development, third edition (BSID III) scores at 2 years of age, methylation data from cord blood, genotyping data and all relevant covariates (Table 1). A sub-set of 51 children also had neonatal MRI data available.
Table 1.
Study characteristics of the whole study population as well as the subsample with MRI imaging data.
| Whole study sample | Children with MRI scan as neonates | |
|---|---|---|
| N | 161 | 51 |
| Female, n (%) | 71 (44.1%) | 24 (47.1%) |
| African ancestry, n (%) | 87 (54.0%) | 22 (43.1%) |
| Mixed ancestry, n (%) | 74 (46.0%) | 29 (56.9%) |
| Gestational age (weeks), mean ± sd | 38.7 ± 2.5 | 38.9 ± 2.2 |
| Socioeconomic status (SES)1 in quartiles | ||
| Low SES, n (%) | 37 (23.0%) | 5 (9.8%) |
| Low to medium SES, n (%) | 42 (26.1%) | 15 (29.4%) |
| Medium to high SES, n (%) | 42 (26.1%) | 13 (25.5%) |
| High SES, n (%) | 40 (24.8%) | 18 (35.3%) |
| SES z-score1, mean ± sd | 0.2 ± 2.3 | 0.8 ± 2.2 |
| Maternal smoking at enrolment, n (%) | 43 (26.7%) | 13 (25.5%) |
| Cognition severely delayed, n (%) | 11 (6.8%) | 4 (7.8%) |
| Language severely delayed, n (%) | 13 (8.1%) | 6 (11.8%) |
| Motor function severely delayed, n (%) | 6 (3.7%) | 2 (3.9%) |
| Age at MRI scan (in weeks), mean ± sd | n/a | 3.1 ± 0.9 |
| Intracranial volume (mm3), mean ± sd | n/a | 425906.8 ± 4235.7 |
| Total grey matter (mm3), mean ± sd | n/a | 235978.2 ± 12843.8 |
| Total white matter (mm3), mean ± sd | n/a | 122128.8 ± 11222.7 |
| Caudate volume (mm3), mean ± sd | n/a | 1877.1 ± 192.2 |
| Pallidum volume (mm3), mean ± sd | n/a | 715.6 ± 32.4 |
| Putamen volume (mm3), mean ± sd | n/a | 2847.0 ± 72.3 |
| Thalamus volume (mm3), mean ± sd | n/a | 3216.3 ± 107.7 |
| Amygdala volume (mm3), mean ± sd | n/a | 538.7 ± 25.1 |
| Hippocampus volume (mm3), mean ± sd | n/a | 1584.8 ± 144.4 |
socioeconomic composite score (see supplementary material for details)
Ethical approval for human subjects’ research was obtained from the Human Research Ethics Committee of the Faculty of Health Sciences of University of Cape Town (HREC UCT REF 401/2009; HREC UCT REF 525/2012). Written informed consent was signed by the mothers on behalf of herself and her infant for participation in this study.
DNA methylation
DNA was isolated from cord blood samples that were collected at time of delivery (Morin et al. 2017). DNA methylation was assessed with the Illumina Infinium HumanMethylation450 BeadChips (n=156) and the MethylationEPIC BeadChips (n=160). Pre-processing and statistics were done using R 3.5.1 (R Core Team 2018). Raw iDat files were imported to RStudio where intensity values were converted into beta values. The 450K and EPIC datasets were then combined using the minfi package (Aryee et al. 2014) resulting in 316 samples and 453,093 probes that were available on both arrays. Background subtraction, color correction and normalization were performed using the preprocessFunnorm function (Fortin et al. 2014). After sample and probe filtering, 273 samples and 409,033 probes remained for downstream analyses (see supplementary methods for details). Of these, 161 children had genotype data, BSID-III scores at two years and information on all relevant covariates and were therefore included in the analysis sample. Batch effects were removed using ComBat (Leek et al. 2012). Cord blood cell type composition was predicted using the most recent cord blood reference data set (Gervin et al. 2019)
Genotype data
Genome-wide genotyping was performed in 305 newborns using the Illumina Infinium PsychArray (n=150) and the Illumina Infinium Global Screening Array, GSA (n=155), of which 271 samples passed quality control (QC), including the 161 children from the final analysis sample. After QC SNPs were imputed on the 1000 Genomes reference panel (Phase III) using the Michigan Imputation Server (Das et al. 2016). Imputed genotypes reaching an R2≥0.3 in both arrays were used in analyses. Principal components were calculated using PLINK v1.90b4 64-bit.
Assessment of neurodevelopmental delay
At 24 months, children were assessed using the BSID-III (Bayley 2006). The BSID-III is a gold standard assessment of child development used internationally, and validated in South Africa (Rademeyer and Jacklin 2013). The assessment generates scores for cognitive, language and motor development. Trained assessors administered the BSID-III using direct observation to score children on their cognitive, language and motor development (Donald et al. 2019). BSID specialized software was used to produce normed and age-adjusted scores calculated using data from a US-reference group. Composite scores have previously been validated for use in a South African setting (Rademeyer and Jacklin 2013; Ballot et al. 2017). Composite scores for cognitive, motor and language scales were scaled to have a mean of 100 and standard deviation of 15. The scores were categorized into severe delay if they were two standard deviations from the BSID-III reference mean, and mild delay if they were one standard deviation from the mean (Bayley 2006). Due to its clinical relevance, we used severe neurodevelopmental delay as our main outcome. Mild delay and the Bayley score itself were used as secondary outcomes to validate our findings (see section “statistical analysis” for details).
MRI imaging data from neonates
Neonatal imaging was performed in a nested sub-study of 236 infants (20.6% of full cohort) from the DCHS at the Cape Universities Brain Imaging Centre, Tygerberg Hospital as has been described (Donald et al. 2018). Of these 236 infants, 80 were part of our main analysis sample of 161 children. These infants were selected in a convenience sample that fulfilled age requirements and was enriched for maternal depression exposure. The major exclusion criteria were: i) infant congenital abnormality, genetic syndrome, neurological disorder or HIV infection; ii) neonatal intensive care unit admission; iii) low Apgar score (<7 at 5 minutes); iv) premature birth (<36 weeks gestation); and v) MRI contra-indications, such as ferromagnetic implants.
Structural T2-weighted images were acquired during natural sleep. As described previously (Donald et al. 2016), images were acquired on a Siemens Magnetom 3T Allegra MRI system (Erlangen, Germany) with a RF transmit/receive head coil. To overcome limitations with scanning smaller volumes of tissue, voltage was reduced to optimize signal, and the head coil was loaded with a wet clay inlay (40×40 cm with a thickness of 2 cm, standard sculpting clay commercially bought – white stoneware clay with grog). A 3D T2 image was acquired on the 3T Siemens Allegra in the sagittal direction with the following parameters: FOV = 160×160 mm, TR = 3500 ms, TE = 354 ms, 128 slices, in-plane resolution = 1.3×1.3 mm and a slice thickness of 1.0 mm. The sequence scan time was 5 min 41 s.
Images were brain extracted with FSL 5.0 brain extraction tool (BET) (Smith et al. 2006) and exported to SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8) for further processing. The processing steps consisted of registration, normalization with modulation, and segmentation into grey matter, white matter, and cerebrospinal fluid according to neonate probabilistic maps (Shi et al. 2011). Image alignment to the template and grey matter segmentations were visually inspected for accuracy. Overall, 17 of the 80 infants had to be excluded because no eligible scan was obtained and 12 infants were excluded because the scan did not pass the quality control. Consequently, a subset of 51 of the 161 children had neonatal imaging data available for inclusion in this analysis. Due to the limited intensity contrast between different types of brain tissue observed in neonates (Prastawa et al. 2005), the analysis was restricted to grey matter volumes of subcortical regions. Volumetric data was extracted for the following subcortical regions: caudate, pallidum, putamen, thalamus, amygdala and hippocampus. The mean volume of the left and right hemispheres was used in all analyses. In addition, total grey matter, total white matter, and total cerebrospinal fluid (CSF) volumes were extracted and summed to obtain a measure of intracranial volume.
Statistical analysis
To identify DNA methylation patterns in cord blood that are associated with severe delay in neurodevelopment at two years of age, we conducted an epigenome-wide association study (EWAS) on single CpG sites, as well as an analysis of differentially methylated regions (DMRs). For the EWAS, we ran a multivariate robust linear regression model with empirical Bayes from the R package limma (version 3.40.6) (Ritchie et al. 2015) using severe neurodevelopmental delay as the independent variable and methylation at each CpG site as a dependent variable, adjusting for sex, gestational age (in weeks), maternal smoking, socioeconomic status (see supplementary methods for details), the first five genetic PCs to control for population stratification (see Figure S1 for details) and the first three PCs from the estimated cell type proportions (after centered log-ratio transformation), which explained >90% of the cell type heterogeneity (Pawlowsky-Glahn and Egozcue 2006) (see Table S1 for details). We applied a Bonferroni threshold to correct for multiple testing based on the number of tested CpG sites (threshold: 0.05/403933=1.24 × 10−07). Fine-mapping of our epigenome-wide associations was done with comet (Martin et al. 2015), which is a visualization tool of EWAS results. We conducted the following sensitivity analyses to validate our findings: 1) Robustness across cognitive domains – Do we find similar associations across all three cognitive domains (cognition, language, motor function)?, 2) Robustness across different scales - Do we find similar associations across different scales (severe neurodevelopmental delay, mild neurodevelopmental delay, continuous Bayley scores)?, 3) Robustness to unmeasured confounding – We used two models to validate the robustness of our findings to unmeasured confounding: i) We used the R package “cate” (Wang and Zhao 2019) to adjust for unmeasured confounding; ii) We included the raw estimated cell type proportions instead of their first three principal components. 4) Robustness to potential violation of model assumptions – We validated our findings from the multivariate robust linear regression analysis using a traditional linear regression analysis (R function lm()) with asymptotic (default, p-values obtained from normal theory) and exact p-values (permutation test).
To identify plausible pathways associated with severe delay in neurodevelopment, we performed an over-representation analysis based on the CpGs with p-values < 0.001 for the association with severe delay in neurodevelopment using the R Bioconductor package missMethyl (Phipson et al. 2016). Differentially methylated regions (DMRs) in severe neurodevelopmental delay were identified using DMRcate, that identifies DMRs from tunable kernel smoothing processes of association signals (Peters et al. 2015). Input files were our single-CpG EWAS results on severe neurodevelopmental delay including regression coefficients, standard deviations and uncorrected p-values. DMRs were defined based on the following criteria: a) a DMR should contain more than one probe; b) regional information can be combined from probes within 1,000 bp; c) the region showed FDR corrected p-value < 0.05.
Finally, we analyzed if associations between differential methylation and neurodevelopmental delay were mediated by neonatal brain volume as follows: (1) We analyzed associations between CpG sites, that were associated with neurodevelopmental delay, and MRI imaging data from neonates (total grey matter, total white matter and subcortical brain volumes) using linear regression models adjusted for the same covariates as the EWAS analyses, plus age at MRI scan, child sex and intracranial volume. (2) We analyzed associations between MRI imaging data and severe delay in neurodevelopment using linear regression models adjusted for age at MRI scan, child sex and intracranial volume. These associations were validated in two sensitivity analyses: First, associations were additionally adjusted for gestational age, maternal smoking, socioeconomic status and the first five genetic PCs to control for population stratification and second, the findings were validated by using mild neurodevelopmental delay and the continuous Bayley scores as independent variables. Lastly, if the associations in (1) and (2) were significant, a formal mediation analysis was conducted using the R package “mediation”, an approach that relies on the quasi-Bayesian Monte Carlo method based on normal approximation (Imai et al. 2010).
Results
Description of Study Participants
The study sample included 161 children with DNA methylation and genotype data, BSID-III scores at two years and information on all relevant covariates (Table 1). Of these, neonatal MRI imaging data were available in 51 children and the average age at MRI scan was 3.1 weeks (sd = 0.9). Almost half of our study sample was female, half of them were of African ancestry and the other half were of mixed ancestry, and a quarter of the population was exposed to maternal smoking during pregnancy. Cognitive development at two years of age was severely delayed in 4 to 12% of the study sample, depending on the tested domain. The most prevalent delay was in language (8% in the whole study sample and 12% in the subsample with imaging data had severe language delay), followed by severe cognitive delay (7–8%) and severe delay in motor function (4%).
Differential Methylation as an Early Sign of Neurodevelopmental Delay
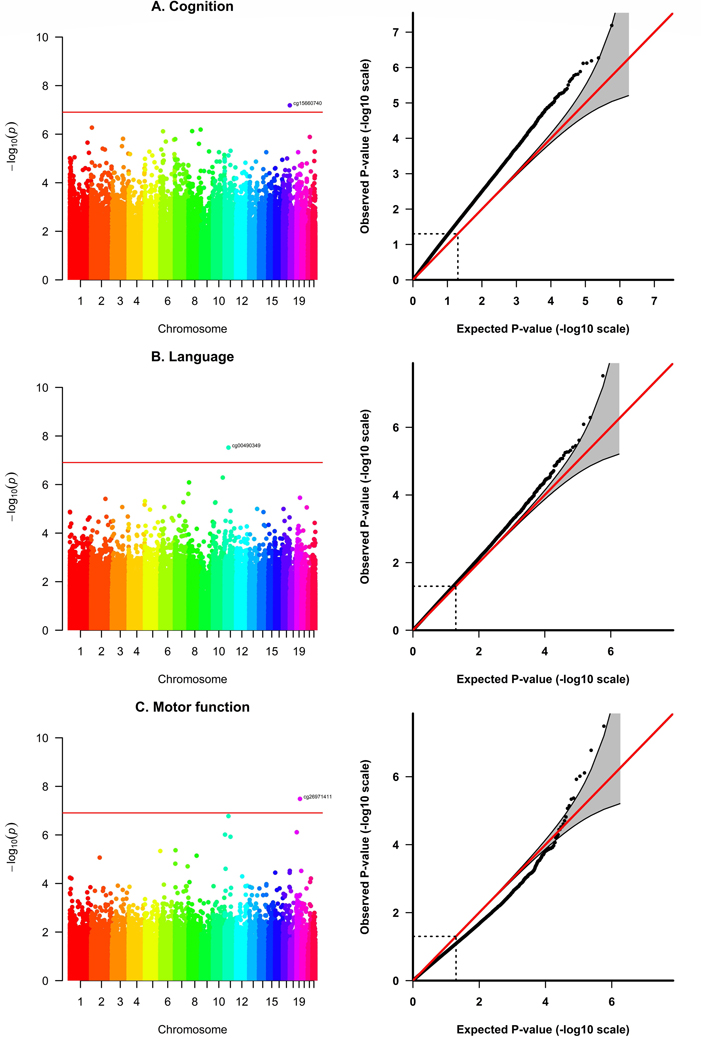
Differentially methylated CpG sites in the SPTBN4 (Spectrin Beta, Non-Erythrocytic 4) locus (cg26971411) and in intergenic regions on chromosome 11 (cg00490349) and chromosome 17 (cg15660740) were associated with severe neurodevelopmental delay at the epigenome-wide significance level (Bonferroni-adjustment) after adjusting for sex, gestational age, maternal smoking, socioeconomic status, the first five genetic PCs and cell type proportions (Table 2, Figure 1, Figures S2–S5). Differential methylation in cg26971411 was significantly associated with severe delay in motor function (Δ beta = −0.024, p-value = 3.28 × 10−08) and nominally significant for cognitive development (Δ beta = −0.013, p-value = 1.96 × 10−05) and language development (Δ beta = −0.014, p-value = 3.52 × 10−06). Differential methylation in cg00490349 was significantly associated with severe delay in language development (Δ beta = −0.036, p-value = 3.02 × 10−08) and nominally significant for cognitive development (Δ beta = −0.032, p-value = 7.52 × 10−06) and motor function (Δ beta = −0.051, p-value = 1.68 × 10−07). Differential methylation in cg15660740 was significantly associated with severe delay in cognitive function (Δ beta = −0.078, p-value = 6.49 × 10−08), but not associated with severe delay in language development or motor function. The significant associations were robust to unmeasured confounding and potential violations of model assumptions (Figure S6, Table S2). Associations with the continuous Bayley scores were weaker, but still nominally significant, for differential methylation in cg26971411 and cg00490349 (p-values < 0.05, Table 2C). Associations with mild neurodevelopmental delay were not significant (Table 2B).
Table 2.
Validation of the three significant CpG sites from the main analysis of severe neurodevelopmental delay for A. the two other cognitive domains, and for the secondary outcomes B. mild neurodevelopmental delay and C. Bayley scores.
| A. Severe neurodevelopmental delaya | Cognition | Language | Motor function | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| CpG | chr | position | Gene | Δ beta | p-value | Δ beta group | p-value | Δ beta group | p-value |
| cg00490349 | 11 | 45740105 | n/a | −0.0315 | 7.52E-06 | −0.0362 | 3.02E-08 | −0.0508 | 1.68E-07 |
| cg15660740 | 17 | 81023473 | n/a | −0.0779 | 6.49E-08 | −0.0108 | 0.4478 | −0.0263 | 0.2109 |
| cg26971411 | 19 | 41016501 | SPTBN4 | −0.0134 | 1.96E-05 | −0.0136 | 3.52E-06 | −0.0236 | 3.28E-08 |
|
| |||||||||
| B. Mild neurodevelopmental delayb | Cognition | Language | Motor function | ||||||
|
| |||||||||
| CpG | chr | position | Gene | Δ beta | p-value | Δ beta group | p-value | Δ beta group | p-value |
| cg00490349 | 11 | 45740105 | n/a | −0.0112 | 0.0025 | −0.0050 | 0.1768 | −0.0046 | 0.2742 |
| cg15660740 | 17 | 81023473 | n/a | −0.0152 | 0.0506 | −0.0024 | 0.7580 | −0.0073 | 0.4016 |
| cg26971411 | 19 | 41016501 | SPTBN4 | −0.0040 | 0.0152 | −0.0016 | 0.3467 | −0.0030 | 0.0987 |
|
| |||||||||
| C. Bayley score | Cognition | Language | Motor function | ||||||
|
| |||||||||
| CpG | chr | position | Gene | Δ beta per unit | p-value | Δ beta per unit | p-value | Δ beta per unit | p-value |
| cg00490349 | 11 | 45740105 | n/a | 0.0010 | 8.63E-07 | 0.0006 | 0.0004 | 0.0002 | 0.0899 |
| cg15660740 | 17 | 81023473 | n/a | 0.0012 | 0.0070 | 0.0003 | 0.4515 | 0.0003 | 0.3341 |
| cg26971411 | 19 | 41016501 | SPTBN4 | 0.0004 | 8.18E-06 | 0.0002 | 0.0035 | 0.0001 | 0.0163 |
Bayley score < −2 SD below the mean score
Bayley score < −1 SD below the mean score
Δ beta: This coefficient represents the mean difference of DNAm beta values between children with and without neurodevelopmental delay. Negative coefficients refer to smaller mean DNAm beta values in children with neurodevelopmental delay and positive coefficients refer to larger mean DNAm beta values in children with neurodevelopmental delay.
Δ beta per unit: This coefficient represents the increase of mean DNAm beta values per increase of 1 unit in the Bayley score. Negative coefficients refer to smaller mean DNAm beta values in children with higher Bayley scores and positive coefficients refer to larger mean DNAm beta values in children with higher Bayley scores. Since a low Bayley score refers to neurodevelopmental delay, effect signs in analysis C are different than in A and B.
Adjusted for sex, gestational age, maternal smoking, socioeconomic status, the first five genetic PCs to control for population stratification and the first three PCs from cell type proportions (after centered log-ratio transformation). Bonferroni-threshold (0.05/403933=1.24e-07) was used to correct for multiple testing in the single CpG analyses. Significant p-values in bold.
Figure 1.
Manhattan and QQ-Plots from the EWAS on severe neurodevelopmental delay in different cognitive domains (A. Cognition, B. Language, C. Motor function). Adjusted for sex, gestational age, maternal smoking, socioeconomic status, the first five genetic PCs to control for population stratification and the first three PCs from cell type proportions (after centered log-ratio transformation). Bonferroni-threshold: 0.05/403933 = 1.24 × 10−07
We did not find any significant DMR and no significantly enriched pathway among the most significant CpG sites (p-values < 0.001) from the EWAS of severe neurodevelopmental delay (Tables S3–S5).
Changes in Neonatal Subcortical Brain Volumes Associated with Neurodevelopmental delay
Increased caudate volumes were associated with severe neurodevelopmental delay at two years of age (Table 3, Figure 2), particularly for language development (Δ caudate volume = 165.30 mm3, p-value = 0.0443) and delay in motor function (Δ caudate volume = 365.36 mm3, p-value = 0.0082). The association between caudate volumes and delay in motor function was also significant for mild delay in motor function (Δ caudate volume = 156.62 mm3, p-value = 0.0148) and when using the continuous Bayley score (p-value = 0.0150) (Figure S5, Table S6). Furthermore, the associations were robust towards additional adjustment for gestational age, maternal smoking, socioeconomic status and the first five genetic PCs to control for population stratification (Δ caudate volume = 388.79 mm3, p-value = 0.0071, Table S7). Associations with language development were only significant for severe delay (Table S6) and not significant after including additional covariates (Table S8). There were no associations with other subcortical brain volumes or with total grey or white matter (Table 3).
Table 3.
Association between MRI imaging data from neonates and severe neurodevelopmental delay at two years of age.
| Cognition | Language | Motor function | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| MRI | Δ MRI (mm3) | p-value | Δ MRI (mm3) | p-value | Δ MRI (mm3) | p-value |
| Total grey matter | 8790.70 | 0.1788 | 4878.53 | 0.3611 | 4605.74 | 0.6151 |
| Total white matter | −5404.95 | 0.3679 | 527.50 | 0.9143 | 12192.75 | 0.1408 |
| Caudate volume | 117.61 | 0.2520 | 165.30 | 0.0443 | 365.36 | 0.0082 |
| Pallidum volume | 9.03 | 0.5850 | −15.72 | 0.2394 | 0.56 | 0.9806 |
| Putamen volume | 27.69 | 0.4555 | −18.37 | 0.5429 | 34.08 | 0.5089 |
| Thalamus volume | −2.12 | 0.9694 | −70.01 | 0.1149 | 26.07 | 0.7347 |
| Amygdala volume | 11.57 | 0.3855 | 3.77 | 0.7289 | 29.38 | 0.1092 |
| Hippocampus volume | 105.80 | 0.1510 | 64.80 | 0.2814 | 171.76 | 0.0919 |
Adjusted for age at scan, child sex and intracranial volume.
Δ MRI: This coefficient represents the mean difference of MRI imaging values between children with and without severe neurodevelopmental delay. Negative coefficients refer to smaller MRI imaging values in children with severe neurodevelopmental delay and positive coefficients refer to larger mean MRI imaging values in children with severe neurodevelopmental delay.
Figure 2.
Association between caudate volume in neonates and severe neurodevelopmental delay. Adjusted for age at scan, child sex and intracranial volume.
However, since we did not find associations between methylation in the significant CpG sites from the EWAS of neurodevelopmental delay (cg26971411, cg00490349 and cg15660740) and changes in caudate volumes (Table S9), our data did not support a mediating effect of altered brain volumes on the association between methylation and neurodevelopmental delay.
Discussion
In this study of infants from a peri-urban region in a low-resourced community in South Africa, we showed that differential methylation levels from cord blood were associated with severe neurodevelopmental delay at two years of age. While these associations were not mediated by neonatal brain volume, neonatal caudate volumes were independently associated with severe neurodevelopmental delay, particularly in language and motor domains. These results suggest that methylation levels and brain imaging data from newborns can potentially be useful as unique neurobiological signals for neurodevelopmental delay and highlight the need to understand the biological pathways of how prenatal environmental and psychological risk factors, and genetics affect neurodevelopment. Future studies are needed to determine whether these findings could have implications for early detection of neurodevelopmental delay, which could lead to timely implementation of interventions (Daelmans et al. 2017) which are essential for optimal cognitive functioning across the life course (McCormick et al. 2006).
Comparison with previous studies
The role of epigenetic variation on early cognitive development of infants remains largely unknown. Some evidence that altered methylation levels in neonates are associated with neurodevelopmental delay comes from a study of 238 Mexican-American children (Huen et al. 2018) which found associations between two methylation sites (cg15887283 and cg22798737 both located in PON1) and working memory and processing speed. The paraoxonase 1 (PON1) gene encodes a multifunctional enzyme that is involved both in detoxification of certain organophosphate (OP) pesticides and oxidative stress pathways (Huen et al. 2018). However, none of their associations remained significant after accounting for multiple comparisons (Huen et al. 2018). Of these two CpG sites, only cg15887283 was nominally associated with severe delay in cognition (Δ beta = −0.007, p-value = 0.0227, Table S10). This finding is in line with Huen et al. who showed an inverse relationship between cord blood methylation at cg15887283 with working memory. The other CpG site (cg22798737) was not associated with severe cognitive delay in the DCHS. However, as Huen et al. found an association with 7-year-old DNAm for this CpG site and DNAm is highly tissue and age-dependent, the lack of an association in our data is not in contradiction to Huen et al. In addition, our relatively small sample size might have further limited our chances for a successful replication of their findings. As Huen et al. conducted a targeted analysis, in which they focused on PON1 DNA methylation, we could not assess our associations in their data.
Differential Methylation as an Early Sign of Neurodevelopmental Delay
Differential methylation in three loci (SPTBN4 (cg26971411) and intergenic regions on chromosome 11 (cg00490349) and chromosome 17 (cg15660740)) was significantly associated with severe neurodevelopmental delay at two years of age in this cohort. Differential methylation in the SPTBN4 locus (cg26971411) showed the strongest association with severe delay in motor function. SPTBN4 (Spectrin Beta, Non-Erythrocytic 4) is a protein coding gene, which is, according to the Human Protein Atlas, primarily expressed in brain tissue and particularly in the cerebellum, the brain region that functions as a co-processor of movement in concert with the cortex and basal ganglia (Koziol et al. 2014). In line, mutations and loss-of-function variants in SPTBN4 were reported in association with arthrogryposis, a neuromuscular condition (Pehlivan et al. 2019), and with a severe neurological syndrome that includes congenital hypotonia, intellectual disability, and motor axonal and auditory neuropathy (Wang et al. 2018). To the best of our knowledge, only one study has identified differential DNAm in SPTBN4 in association with neurocognitive outcomes (Alzheimer’s disease) and this study was conducted in mice (Sanchez-Mut et al. 2013). Therefore, our study extends the current literature by highlighting that not only genetic variants or methylation levels from brain tissue, but also methylation levels from cord blood are linked to cognitive outcomes by providing to our knowledge the first report of an association between neonatal differential methylation in SPTBN4 in cord blood and early neurodevelopmental outcomes.
Changes in Neonatal Caudate Volumes Associated with Severe Neurodevelopmental Delay
While we did not find that altered neonatal brain volumes mediate the association between DNAm and neurodevelopmental delay, we demonstrated that larger neonatal caudate volumes were associated with neurodevelopmental delay at two years of age, particularly in motor function. The caudate nucleus is one of the structures that make up the corpus striatum, which is a component of the basal ganglia. The caudate nucleus plays a prominent role in motor processes, and caudate nucleus dysfunction has been found in Parkinson’s disease, Huntington’s chorea, dyskinesias, obsessive–compulsive disorder and other movement and cognitive disorders (Schultz 2016). Similarly, studies of children have found that the caudate is involved in executive function processes and is associated with autism spectrum disorders (Voelbel et al. 2006).
The human brain undergoes rapid change in the first year of life, where the growth rates of subcortical grey-matter structures are similar to cortical grey-matter growth rates, where the amygdala, thalamus, caudate, putamen, and pallidum grow by about 105% in the first year and roughly 15% in the second (Gilmore et al. 2018). However, different subcortical structures are known to have varying developmental trajectories early in life (Gilmore et al. 2012). Our study finding that larger neonatal caudate volumes were associated with severe neurodevelopmental delay suggest that risk factors for neurodevelopmental delay may impact structural brain development in utero, resulting in deviations in maturation that may already be detected soon after birth. This finding suggests the potential use of neonatal brain imaging data as a neurobiological signal for neurodevelopmental delay in addition to DNAm. There is very limited literature around the association between subcortical volumes and neurodevelopmental delay in young children (Gilmore et al. 2018; Wedderburn et al. 2020). Given brain growth is dynamic in the first few years it is possible that either abnormally large or small volumes may signal deviation from the normal developmental trajectory and associate with adverse outcomes. For example, enlarged head sizes (proxy for larger brain volumes) in children have been associated with adverse neurodevelopment including autism spectrum disorder (Knickmeyer et al. 2008). One study of pre-adolescent children found that outward surface deformation (both expansion and contraction) of areas in the basal ganglia associated with cognitive performance (Sandman et al. 2014). Further work is needed to explore the significance of the association between neonatal caudate volume and neurodevelopmental delay.
Strengths & Limitations
Strengths of this study include the very well characterized group of infants as part of a population-based prospective study design with (epi)genetic data from newborns, early MRI scanning in the neonatal period and detailed assessment of neurodevelopment at two years of age. Furthermore, our study population is of African and mixed ancestry, populations which have been understudied in current genetic and epigenetic studies. Beside genome-wide DNAm levels from newborns, our study also included genome-wide genotype data, which we used to correct for population stratification. For this study, 2 to 4-week-old infants were chosen for imaging as the last weeks of gestation and the early postnatal period are a time of marked cerebral maturation. Imaging during the early postnatal period may more accurately reflect the effects of prenatal environmental, psychological risk factors and genetics on brain structure, given the limited exposure to postnatal factors.
Our study has a number of limitations. First, our findings were based on a relatively small sample size, particularly the analyses of neonatal brain imaging data, which may have impacted our results and limited the statistical power to detect mediation effects. Therefore, our study should be seen as preliminary investigation that requires replication in independent cohorts. As premature children were excluded from the MRI assessment, children with neonatal brain imaging data tended to have a greater gestational age and a higher socioeconomic status. These differences to whole study population may be another reason why we could not validate our associations between DNAm and severe neurodevelopmental delay in this subgroup. Another limitation of our analyses was the small number with severe neurodevelopmental delay in our sample. However, to reduce the risk of false positive findings due to the imbalanced study design, we validated our findings by using different modelling approaches in our EWAS analyses (limma, linear regression, permutation tests, DMR analysis) and different variables to measure neurodevelopmental delay in all our analyses (severe neurodevelopmental delay, mild neurodevelopmental delay, continuous Bayley scores). Another possible limitation is that we used estimated cell counts in our analyses because measured cell types or single-cell methylation data were not available. However, such estimated cell type adjustments have been shown to be appropriate in epidemiological settings (Kaushal et al. 2017). Methylation signatures are tissue and cell-type specific, and therefore, selection of relevant tissues and cell-type is of crucial importance for epigenetic analyses. While brain tissue would be the most relevant tissue in association with neurodevelopment, we analyzed methylation levels in cord blood, which has the advantage that it is easily assessible from living samples and could therefore be linked to future cognitive outcomes.
Finally, we were unable to assess the cerebellum and white matter structures due to limited contrast between tissue types in the neonatal group and further work is needed to understand the association between these structures, methylation and neurodevelopment.
Conclusions
We have presented evidence that differential neonatal methylation in SPTBN4, a regulatory region on chromosome 1, and an intergenic region on chromosome 11, as well as increased neonatal caudate volumes, were independently associated with severe neurodevelopmental delay at two years of age. These findings suggest that neurobiological signals for severe developmental delay may be detectable in very early life with implications for identification and intervention design. However, as our study is based on a relatively small sample size, it should be seen as a preliminary investigation. More studies are needed to replicate our findings in larger study populations and to evaluate their generalizability across different countries and ancestries.
Supplementary Material
Acknowledgments:
The authors thank the study and clinical staff at Paarl Hospital, Mbekweni and TC Newman clinics, as well as the CEO of Paarl Hospital, and the Western Cape Health Department for their support of the study. The authors thank the families and children who participated in this study.
Funding:
The Drakenstein Child Health Study was funded by the Bill & Melinda Gates Foundation (OPP 1017641), Discovery Foundation, Medical Research Council South Africa, National Research Foundation South Africa, CIDRI Clinical Fellowship and Wellcome Trust (204755/2/16/z). Additional support for the DNA methylation work was by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NICHD) under Award Number R21HD085849, and the Fogarty International Center (FIC). AH was supported by a research fellowship from the Deutsche Forschungsgemeinschaft (DFG; HU 2731/1–1) and by the HERCULES Center (NIEHS P30ES019776). MPE was supported by NIH grant R01 GM117946. NAG was supported by a Claude Leon Fellowship. DJS, HJZ and KAD are supported by the South African Medical Research Council (SAMRC). CJW was supported by the Wellcome Trust through a Research Training Fellowship [203525/Z/16/Z]. Support for the neuroimaging was also received by KAD from an ABMRF young investigator grant, the Brain & Behavior Research Foundation Independent Investigator grant (24467) and NIH-R21AA023887 and the Harry Crossley Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of manuscript.
Footnotes
Competing financial interests declaration:
All authors declare they have no actual or potential competing financial interest.
Ethics approval:
Ethical approval for human subjects’ research was obtained from the Human Research Ethics Committee of the Faculty of Health Sciences of University of Cape Town (HREC UCT REF 401/2009; HREC UCT REF 525/2012). Written informed consent was signed by the mothers on behalf of herself and her infant for participation in this study.
References
- Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. 2014. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballot DE, Ramdin T, Rakotsoane D, Agaba F, Davies VA, Chirwa T, et al. 2017. Use of the Bayley Scales of Infant and Toddler Development, Third Edition, to Assess Developmental Outcome in Infants and Young Children in an Urban Setting in South Africa. Int. Sch. Res. Not. 2017:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. 2006. Bayley scales of infant and toddler development, Technical manual. 3rd ed. Bloomington [Google Scholar]
- Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C, et al. 2017. Early childhood development coming of age: science through the life course. Lancet 389:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto PR, Lye SJ, Proulx K, Yousafzai AK, Matthews SG, Vaivada T, et al. 2017. Nurturing care: promoting early childhood development. Lancet 389:91–102. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. 2002. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 288:1740–1748. [DOI] [PubMed] [Google Scholar]
- Chan M, Lake A, Hansen K. 2017. The early years: silent emergency or unique opportunity? Lancet 389:11–13. [DOI] [PubMed] [Google Scholar]
- Daelmans B, Darmstadt GL, Lombardi J, Black MM, Britto PR, Lye S, et al. 2017. Early childhood development: the foundation of sustainable development. Lancet 389:9–11. [DOI] [PubMed] [Google Scholar]
- Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, et al. 2016. Next-generation genotype imputation service and methods. Nat. Genet. 48:1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniz Can D, Richards T, Kuhl PK. 2013. Early gray-matter and white-matter concentration in infancy predict later language skills: A whole brain voxel-based morphometry study. Brain Lang. 124:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald KA, Fouche JP, Roos A, Koen N, Howells FM, Riley EP, et al. 2016. Alcohol exposure in utero is associated with decreased gray matter volume in neonates. Metab. Brain Dis. 31:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald KA, Hoogenhout M, du Plooy CP, Wedderburn CJ, Nhapi RT, Barnett W, et al. 2018. Drakenstein Child Health Study (DCHS): investigating determinants of early child development and cognition. BMJ Paediatr Open [Internet] 2:e000282. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29942867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald KA, Wedderburn CJ, Barnett W, Nhapi RT, Rehman AM, Stadler JAM, et al. 2019. Risk and protective factors for child development: An observational South African birth cohort. PLoS Med. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin J-P, Labbe A, Lemire M, Zanke BW, Hudson TJ, Fertig EJ, et al. 2014. Functional normalization of 450k methylation array data improves replication in large cancer studies. Genome Biol. 15:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. 1997. Brain morphology in children with specific language impairment. J. Speech, Lang. Hear. Res. 40:1272–1284. [DOI] [PubMed] [Google Scholar]
- Gervin K, Salas LA, Bakulski KM, Van Zelm MC, Koestler DC, Wiencke JK, et al. 2019. Systematic evaluation and validation of reference and library selection methods for deconvolution of cord blood DNA methylation data. Clin. Epigenetics 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, Gao W. 2018. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 19:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, et al. 2012. Longitudinal Development of Cortical and Subcortical Gray Matter from Birth to 2 Years. Cereb. Cortex 22:2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurugubelli KR, Ballambattu VB, Bobby Z. 2021. Global DNA Methylation in Cord Blood and Neurodevelopmental Outcome at 18 Months of Age among Intrauterine Growth Restricted and Appropriate for Gestational Age Infants. J. Trop. Pediatr. 67:1–8. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Rodríguez M, Harmony T, Carrillo-Prado C, Van Horn JD, Irimia A, Torgerson C, et al. 2017. Clinical neuroimaging in the preterm infant: Diagnosis and prognosis. NeuroImage Clin. 16:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Solomon O, Kogut K, Eskenazi B, Holland N. 2018. PON1 DNA methylation and neurobehavior in Mexican-American children with prenatal organophosphate exposure. Environ. Int. 121:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Keele L, Tingley D. 2010. A general approach to causal mediation analysis. Psychol. Methods 15:309–334. [DOI] [PubMed] [Google Scholar]
- Kaushal A, Zhang H, Karmaus WJJ, Ray M, Torres MA, Smith AK, et al. 2017. Comparison of different cell type correction methods for genome-scale epigenetics studies. BMC Bioinformatics [Internet] 18:216. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28410574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, et al. 2008. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 28:12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. 2014. Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum 13:151–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. 2012. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28:882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TC, Yet I, Tsai PC, Bell JT. 2015. coMET: Visualisation of regional epigenome-wide association scan results and DNA co-methylation patterns. BMC Bioinformatics 16:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MC, Brooks-Gunn J, Buka SL, Goldman J, Yu J, Salganik M, et al. 2006. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics 117:771–780. [DOI] [PubMed] [Google Scholar]
- Mordaunt CE, Jianu JM, Laufer BI, Zhu Y, Hwang H, Dunaway KW, et al. 2020. Cord blood DNA methylome in newborns later diagnosed with autism spectrum disorder reflects early dysregulation of neurodevelopmental and X-linked genes. Genome Med. 12:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin AM, Gatev E, McEwen LM, Macisaac JL, Lin DTS, Koen N, et al. 2017. Maternal blood contamination of collected cord blood can be identified using DNA methylation at three CpGs. Clin. Epigenetics 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. 2009. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch. Gen. Psychiatry 66:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson SJ, Heim S, Friedman JT, Choudhury N, Benasich AA. 2006. Development of structure and function in the infant brain: implications for cognition, language and social behaviour. Neurosci. Biobehav. Rev. 30:1087–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowsky-Glahn V, Egozcue JJ. 2006. Compositional data and their analysis: An introduction. Geol. Soc. Spec. Publ. 264:1–10. [Google Scholar]
- Pehlivan D, Bayram Y, Gunes N, Coban Akdemir Z, Shukla A, Bierhals T, et al. 2019. The Genomics of Arthrogryposis, a Complex Trait: Candidate Genes and Further Evidence for Oligogenic Inheritance. Am. J. Hum. Genet. 105:132–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters TJ, Buckley MJ, Statham AL, Pidsley R, Samaras K, V Lord R, et al. 2015. De novo identification of differentially methylated regions in the human genome. Epigenetics Chromatin 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipson B, Maksimovic J, Oshlack A. 2016. missMethyl: an R package for analyzing data from Illumina’s HumanMethylation450 platform. Bioinformatics 32:286–288. [DOI] [PubMed] [Google Scholar]
- Prastawa M, Gilmore JH, Lin W, Gerig G. 2005. Automatic segmentation of MR images of the developing newborn brain. Med. Image Anal. 9:457–466. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2018. R: A language and environment for statistical computing. Available from: https://www.r-project.org/
- Rademeyer V, Jacklin L. 2013. A study to evaluate the performance of black South African urban infants on the Bayley Scales of Infant Development III. South African J. Child Heal. 7:54–59. [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubroeks JAY, Smith RG, van den Hove DLA, Lunnon K. 2017. Epigenetics and DNA methylomic profiling in Alzheimer’s disease and other neurodegenerative diseases. J. Neurochem. 143:158–170. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mut JV, Aso E, Panayotis N, Lott I, Dierssen M, Rabano A, et al. 2013. DNA methylation map of mouse and human brain identifies target genes in Alzheimer’s disease. Brain 136:3018–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Head K, Muftuler LT, Su L, Buss C, Davis EP. 2014. Shape of the basal ganglia in preadolescent children is associated with cognitive performance. Neuroimage 99:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. 2016. Reward functions of the basal ganglia. J. Neural Transm. 123:679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. 2004. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J. Neurosci. 24:6392–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, et al. 2011. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–505. [DOI] [PubMed] [Google Scholar]
- Spann MN, Bansal R, Rosen TS, Peterson BS. 2014. Morphological features of the neonatal brain support development of subsequent cognitive, language, and motor abilities. Hum. Brain Mapp. 35:4459–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Briley DA. 2014. Continuity of genetic and environmental influences on cognition across the life span: a meta-analysis of longitudinal twin and adoption studies. Psychol. Bull. 140:949–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitrikas K, Savard D, Bucaj M. 2017. Developmental Delay: When and How to Screen. Am. Fam. Physician 96:36–43. [PubMed] [Google Scholar]
- Voelbel GT, Bates ME, Buckman JF, Pandina G, Hendren RL. 2006. Caudate nucleus volume and cognitive performance: Are they related in childhood psychopathology? Biol. Psychiatry 60:942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton E, Cecil CAM, Suderman M, Liu J, Turner JA, Calhoun V, et al. 2017. Longitudinal epigenetic predictors of amygdala:hippocampus volume ratio. J. Child Psychol. Psychiatry 58:1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton E, Pingault JB, Cecil CAM, Gaunt TR, Relton CL, Mill J, et al. 2017. Epigenetic profiling of ADHD symptoms trajectories: A prospective, methylome-wide study. Mol. Psychiatry 22:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Ortiz-González XR, Yum SW, Gill SM, White A, Kelter E, et al. 2018. βIV Spectrinopathies Cause Profound Intellectual Disability, Congenital Hypotonia, and Motor Axonal Neuropathy. Am. J. Hum. Genet. 102:1158–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhao Q. 2019. cate: High Dimensional Factor Analysis and Confounder Adjusted Testing. Available from: https://cran.r-project.org/package=cate
- Wedderburn CJ, Subramoney S, Yeung S, Fouche J-P, Joshi SH, Narr KL, et al. 2020. Neuroimaging young children and associations with neurocognitive development in a South African birth cohort study. Neuroimage 219:116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar HJ, Barnett W, Myer L, Stein DJ, Nicol MP. 2015. Investigating the early-life determinants of illness in Africa: The Drakenstein Child Health Study. Thorax 70:592–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.