Abstract
Toxocariasis, a neglected parasitic zoonosis with worldwide distribution, has been reportedly associated to different risk factors in several epidemiological and meta-analysis studies. However, dog and cat contact (environmental and animal exposure) as isolated associated risk factor for children and adults remains to be fully established. Accordingly, the present meta-analysis has aimed to directly assess dog and cat contact for toxocariasis seropositivity in under-18 and adult persons, using a survey strategy of PubMed/Medline, Embase, Scopus and Scielo Databases, from January 2009 to December 2021. A meta-analysis model of random effects was applied to estimate odds ratio (OR) with 95% Confidence Interval (CI). The statistical heterogeneity was evaluated by the Cochran Q-Test and I2 values. A total of 41 transversal studies (n = 20.515 individuals) from different geographic regions (classified by the World Health Organization) were included herein. In overall, 1,882/13,496 (13.95%; 95% IC = 13.4–14.5) youngers and 513/7.019 (7.3%; 95% CI = 6.7–7.9) adults in contact with dogs or cats were serologically reagent for anti-Toxocara antibodies. Association of dog and cat contact was observed only in youngers, with both dogs (OR = 1.53; p < 0.0001) and cats (OR = 1.64; p = 0.0001). In addition, association of dog and contact and serology was statistically significant in populations of Americas (OR = 1.37; 95% CI = 1.1–1.7), Middle East (OR = 2.9; 95% CI = 1.6–5.1) and West Pacific (OR = 1.6; 95% IC = 1.3–1.9). In conclusion, contact with dogs and cats, particularly by younger individuals and in regions such as Americas, Middle East, and West Pacific, should be always a public health concern for toxocariasis. Moreover, dogs and cats should be periodically dewormed, washed and hair cleaned prior to contact with youngers. Finally, robust statistical results herein may serve as basis for future strategies and preventive measures for safer dog and cat contact.
Keywords: companion animals, epidemiology, larva migrans, zoonoses, Toxocara spp.
Introduction
Toxocariasis is a worldwide parasitic zoonosis caused by nematodes Toxocara canis and Toxocara cati from dogs and cats as definitive hosts, respectively (1, 2). Classified amongst the top six parasitic infections of priority to public health by the World Health Organization and Centers of Disease Control (3), toxocariasis global seropositivity has been estimated in 19% by a recent meta-analysis study (CI 95% = 16, 6–21,4%) (4).
Primarily considered as geo-zoonosis, the main toxocariasis transmission occurs by accidental ingestion of larvae in eggs from the soil (5). In addition, fecal-oral transmission may occur after intake of contaminated soil with embryonated eggs of Toxocara spp., particularly in gardens, sandboxes, public squares, and parks (6, 7). The global prevalence of environmental contamination by eggs of Toxocara spp. has been estimated of 21% (CI = 16–27%) by another recent meta-analysis study (8).
Pet access to outdoors areas of human gathering and closer human: pet contact may predispose higher soil contamination and lead to higher human exposure to Toxocara spp. (9, 10). Although uncommon, transmission may also occur by ingestion of Toxocara spp. eggs from contact with non-dewormed dogs and cats (11–13).
Systematic reviews and meta-analysis studies have recently established clinical onset of toxocariasis, including disease association to respiratory (14, 15), neurological (16, 17), and dermatological disorders (18). Furthermore, prevalence has been established worldwide, including different regions such as Europe (19) and Western Pacific (20).
The concept of One Health highlights the interconnection among human, animal and environmental health, and the importance of multidisciplinary collaborations to address challenges in global health (21). Despite evidence of human recurrent exposure to Toxocara spp., comprehensive studies should be conducted to fully establish the impact on public health. In such scenario, One Health approach has been reportedly indicated as a vital tool to confront the complexity of human toxocariasis worldwide (22, 23).
Although contact with companion animals (dogs and cats) has already been established as risk factor for toxocariasis (4), age groups have shown different exposure characteristics, mostly related to immunological system, hygiene and food habits (24). Even so, no study to date has focused on risk association of disease to youth and adulthood as independent approaches. Accordingly, the present study aimed to individually assess younger (under-18) and adult age groups and contact with dogs and cats as associated risk factors for toxocariasis.
Methods
Search Strategy and Selection Criteria
The study herein has used components of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Supplementary Material), besides applying the guidelines for conception, execution, and interpretation of results (8, 25). The search strategy was based on screening of scientific articles that evaluated seroprevalence of toxocariasis in persons with dog or cat contact, from January 2009 to June 2019.
The search was performed in different databases including PubMed / Medline, Embase, Scopus e Scielo. A combination of terms was used, resulted in the following Mesh of (toxoc*) AND (prevalence OR seroepidem* OR serol* OR seroprevalence) AND (risk factor*), and was presented (Table 1). Literature surveyed in the present study included English, Spanish and Portuguese. Mostly to avoid overlapping information, theses and dissertations data were not assessed.
Table 1.
Search strategy in different databases utilized for meta-analysis for assessment of contact with dogs and cats as associated risk factor for toxocariasis from 2009 to 2021.
| Database | Search strategy |
|---|---|
| Embase | ((toxoc*) AND (prevalence OR seroepidem* OR serol* OR seroprevalence) AND (risk factor*)) |
| PubMed | ((toxoc*) AND (prevalence OR seroepidem* OR serol* OR seroprevalence) AND (risk factor*)) AND (“2009/01/01”: “2021/12/31”)) |
| Scielo | (toxoc*) AND (prevalence OR seroepidem* OR serol* OR seroprevalence) AND (risk factor*) AND year_cluster (“2021” OR “2020” OR “2019” OR “2018” OR “2017” OR “2016” OR “2015” OR “2014” OR “2013” OR “2012” OR “2011” OR “2010” OR “2009”) |
| Scopus | ((toxoc*) AND (prevalence OR seroepidem* OR serol* OR seroprevalence) AND (risk AND factor*)) AND (limit to pubyear 2021 to 2009)) |
Following removal of duplicates and screening of titles and abstracts, assessment of full texts was thoroughly performed for inclusion or exclusion in the meta-analysis study, with a final screening for solving conflicts, as previously described (8). Inclusion criteria included studies assessing contact, with adults or under-18 individuals, involving dogs and cats and that used serological methods for detection of anti-Toxocara (IgG) antibodies; geographical and idiom restrictions were not applied.
Articles failing to make the inclusion criteria were discharged, such as studies with comorbidities and/or without control groups, evaluating only dogs and cats, with no identification of animal species as risk factor, with no age stratification, case report or series of reports, clinical cases of diagnosed infection by Toxocara spp., and reviews, systematic reviews, or meta-analyses.
Data Extraction
Data extraction was independently performed following examination of eligibility criteria, with information gathered in a commercially available software (Excel, version 2016, Microsoft Co., Redmond, WA, USA). Data registered for analysis included author name, year of publication, country where the study was performed, age of population (children, under-18, adults, above-18), sample size, pet species assessed as associated risk factor (dog and/or cat), number of seropositive/seronegative individuals with and without contact with companion animals.
Meta-Analysis
Estimative of toxocariasis seroprevalence in individuals with and without contact with companion animals were calculated using a model of random effects with Confidence Interval (CI) of 95%, providing an overall estimative. In addition, age population and region where the study was performed, along with correspondent geographical coordinates, were also calculated.
Heterogeneity among studies was calculated by the Q-test of Cochran, which has considered as significant the values of p < 0.05. Values of I2 ≥50% found were used to define the significant level of heterogeneity (8). Analysis of meta-regression were performed according to several parameters including country, animal species involved, human population age, presence of absence of contact to dog and/or cat, and seropositivity/seronegativity to anti-Toxocara (IgG) antibodies.
Contact with dogs and/or cats was considered in environmental and animal exposure, when individuals owned dogs or cats, had contact to dogs or cats, kept dogs or cats, played with dogs or cats, had intra-domiciliary or peri-domiciliary presence of dogs or cats, had dogs or cats at home, fed dogs or cats, raised dogs or cats, had contact with dogs or cats.
Assessment of potential associated risk factor related to seroprevalence of anti-Toxocara (IgG) antibodies and contact with dogs and cats was made by evaluation of odds ratio (OR) and Confidence Interval (CI) of 95%. Forest plots were constructed to present results of meta-analysis in a schematic fashion and with funnel graphics (each study was represented by a proportional effect size), and it was performed to assess publication bias (26, 27).
In order to evaluate the studies which overly contribute to the heterogeneity in meta-analysis, sensitivity analysis were conducted by Baujat plots (28).
Statistical analyses were conducted utilizing a commercially available package (29), implemented in the R Project (30). Results of statistical analyses were considered significant when p < 0.05.
Results
Study Characteristics
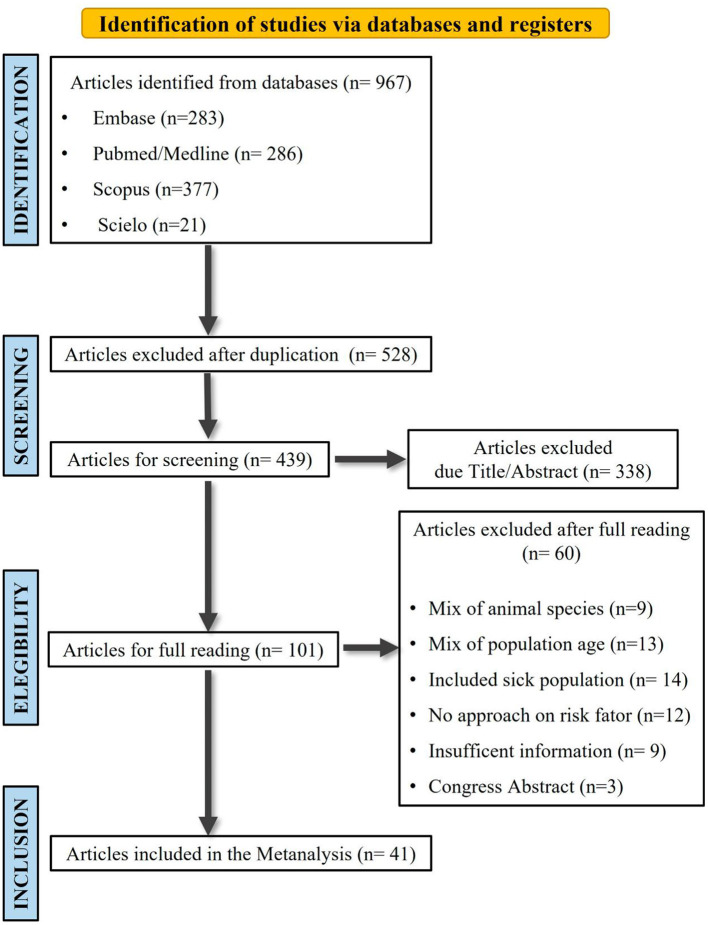
Initial bibliographical search comprised a total of 967 scientific articles, followed by application of eligibility criteria, and resulting in a final inclusion of 41 articles for the meta-analysis study (Figure 1).
Figure 1.
Flow-chart representing study search and selection strategy on seropositivity of (IgG) anti- Toxocara antibodies and contact with dogs and/or cats in articles of different databases included in the meta-analysis study, from 2009 to 2021.
Selected articles were from studies conducted in 13 different countries (Austria, Brazil, Colombia, China, Equator, Iran, Mexico, Norway, Nigeria, Serbia, Taiwan, Thailand, and Venezuela), representing different regions of the World Health Organization (OMS, 2021). Countries with most reports included Brazil with ten, Iran with six, Mexico with four, and Venezuela with three; followed by China and Taiwan with two each and the remaining countries with one study each. Regarding to geographic regions, 21/41 (51.2%) were taken in the Americas, 9/41 (22.0%) in Middle East, 5/41 (12.2%) in the Western Pacific, 3/41 (7.3%) in Europe, 2/41 (4.9%) in Africa, and one Southeast Asia 1/41 (2, 4%).
The 41 selected articles represented a total population of 20,515 individuals, with an overall prevalence of 24.1% (4,948/20,515; CI 95% = 23.5–24.7%) for anti-Toxocara antibodies. Considering the total population among studies a total of 6,826 (33.3%) participants were from the Americas, 6,113 (29.8%) from Western Pacific 4,859 (23.7%) from Middle East, 1,819 (8.9%) from Europe, 721 (3.5%) from Africa, and 177 (0.9%) from Southeast Asia.
Regarding to the presence of anti-Toxocara antibodies the highest prevalence was observed in the Africa with 497/721 (64.5%; CI 95% = 65.4–72.3%), followed by Americas with 2,774/6,826 (40.6%; CI 95% = 39.0–41.2%), Western Pacific with 865/6,113 (14.5%; CI 95% = 13.3–15.1%), Middle East with 548/4,859 (11.3%; CI 95% = 10.4–12.2%) and Europe with 161/1,819 (8.9%; CI 95% = 7.6–10.3%). Only one study was carried out in Southeast Asia resulting in a prevalence of 103/177 (58.2%/ CI 95% = 50.6–65.6%). The highest prevalence was observed in a serosurvey in Africa (92.5%) and the lowest in Iran (1.4%). Regarding geographic location, the highest prevalence was observed between latitudes of 0 and 20° (41.1%).
Assessment of having direct contact with dogs or cats showed the highest frequency in the population of Americas with 1,717/6,826 (25.2%; CI 95% = 24.1–26.2%), followed by Africa with 59/721 (8.2%; CI 95% = 6.3–10.4%), Western Pacific with 360/6,113 (5.89%; CI 95% = 5.3–6.5%), Middle East with 179/4,859 (3.7%; CI 95% = 3.2–4.3%), and Europe with 43/1,819 (2, 4%; CI 95% = 1.8–3.2%). In the solely Asian included study, 37 out of 177 (20.9%) individuals reported having contact with dog/cat.
A total of 27/41 (65.9%) studies were conducted with children and under-18 participants, while 12 (27/41; 29.3%) studies were conducted with adults, and two (4.9%) with adults and children independently. Overall prevalence of 3,665/13,496 (27.2%; CI 95% = 26.4–27.9%) was observed in under-18 participants, while 1,283/7,019 (18.3%; CI 95% = 17.4–19.2%) in adults.
Most of the studies (24/41; 58.5%) used indirect ELISA for detection of anti-Toxocara antibodies followed by 4/41 (9.8%) studies with Western blot and 5/41 (12.2%) using both diagnostic tests; commercial ELISA kits were used in the remaining 8/41 (19.5%) articles.
Meta-Analysis Results
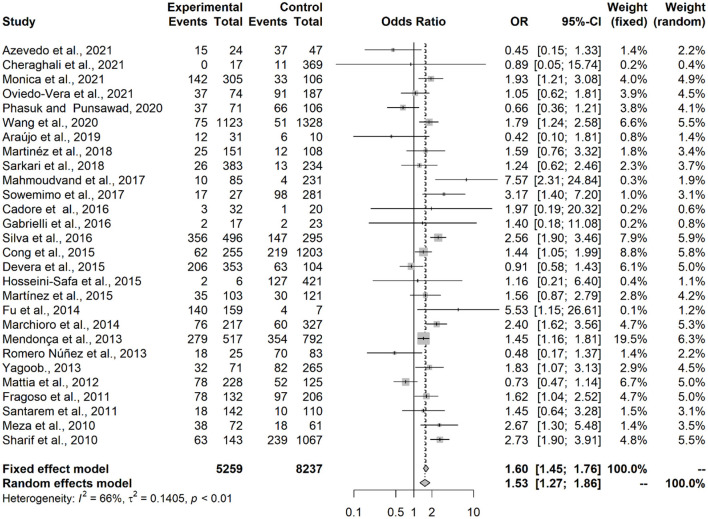
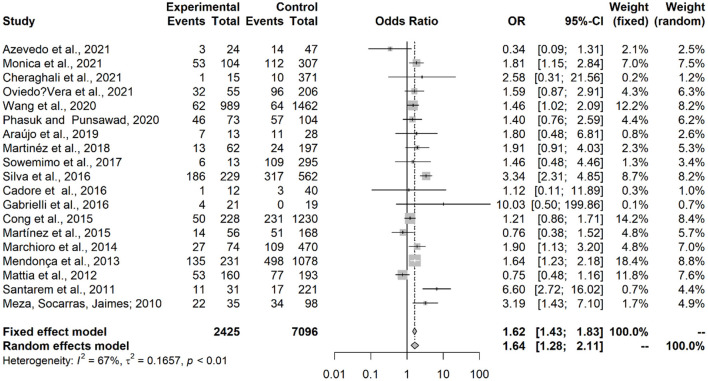
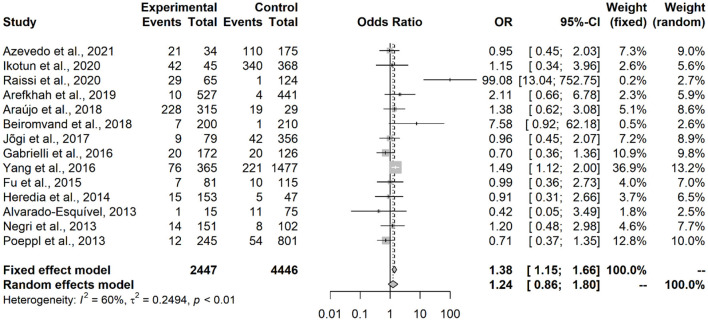
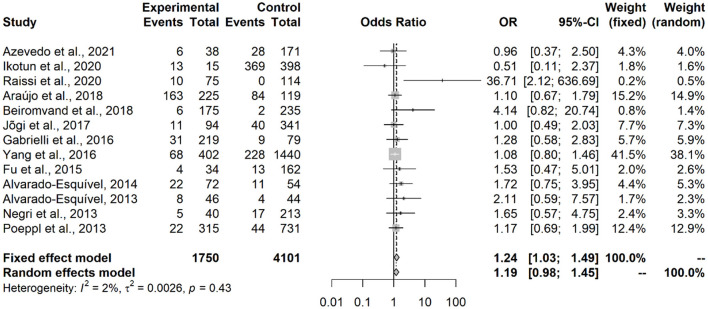
Results showed that contact with dogs (OR = 1.53; IC = 1.27–1.86; p < 0.0001) or with cats (OR = 1.64; IC = 1.28–2.11; p = 0.0001) represented an associated risk factor to seropositivity in under-18 participants (Figures 2, 3). On the other hand, no statistical difference of dogs (OR = 1.24; CI 95% = 0.86–1.80; p = 0.2494) and cats (OR = 1,20; CI 95% = 0.98–1.45; p = 0.0735) was observed in adult participants (Figures 4, 5).
Figure 2.
Forest plot for assessment of odds-ratio of influence of contact with dogs in the frequency of anti-Toxocara antibodies in under-18 participants, according to selected articles used in the meta-analysis from 2009 to 2021.
Figure 3.
Forest plot for assessment of odds-ratio of influence of contact with cats in the frequency of anti-Toxocara antibodies in under-18 participants, according to selected articles used in the meta-analysis from 2009 to 2021.
Figure 4.
Forest plot for assessment of odds-ratio of influence of contact with dogs in the frequency of anti-Toxocara antibodies in adult participants, according to selected articles used in the meta-analysis from 2009 to 2021.
Figure 5.
Forest plot for assessment of odds-ratio of influence of contact with cats in the frequency of anti-Toxocara antibodies in adult participants, according to selected articles used in the meta-analysis from 2009 to 2021.
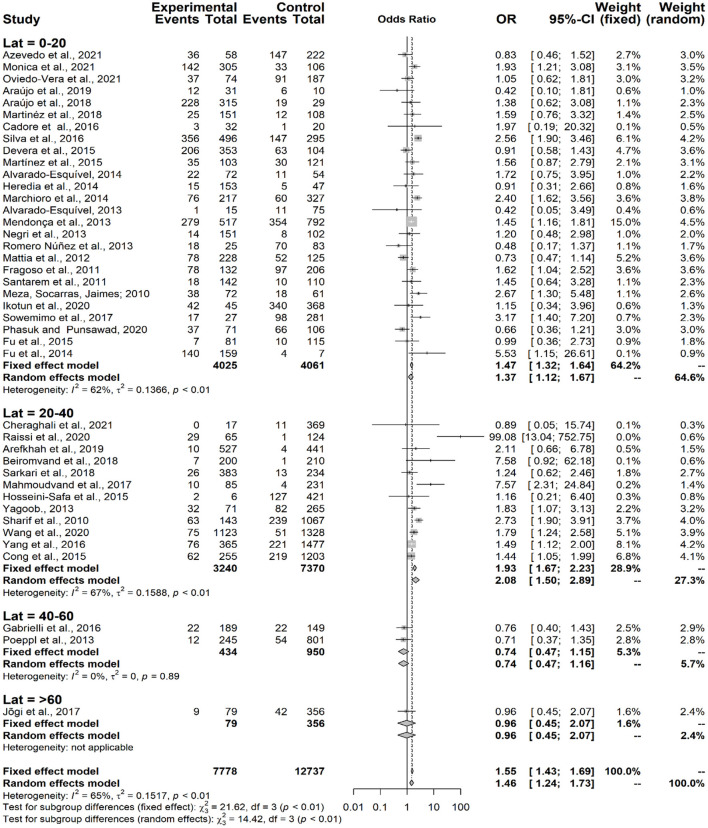
The assessment of seropositivity for anti-Toxocara antibodies relative to the geographic coordinates showed that the higher the latitude, the lower the seroprevalence (χ2 = 14.42; p = 0.0024) (Figure 6).
Figure 6.
Forest plot for assessment of odds-ratio of influence of geographical latitude (Lat = degrees) in the frequency of anti-Toxocara antibodies in participants, according to selected articles used in the meta-analysis from 2009 to 2021.
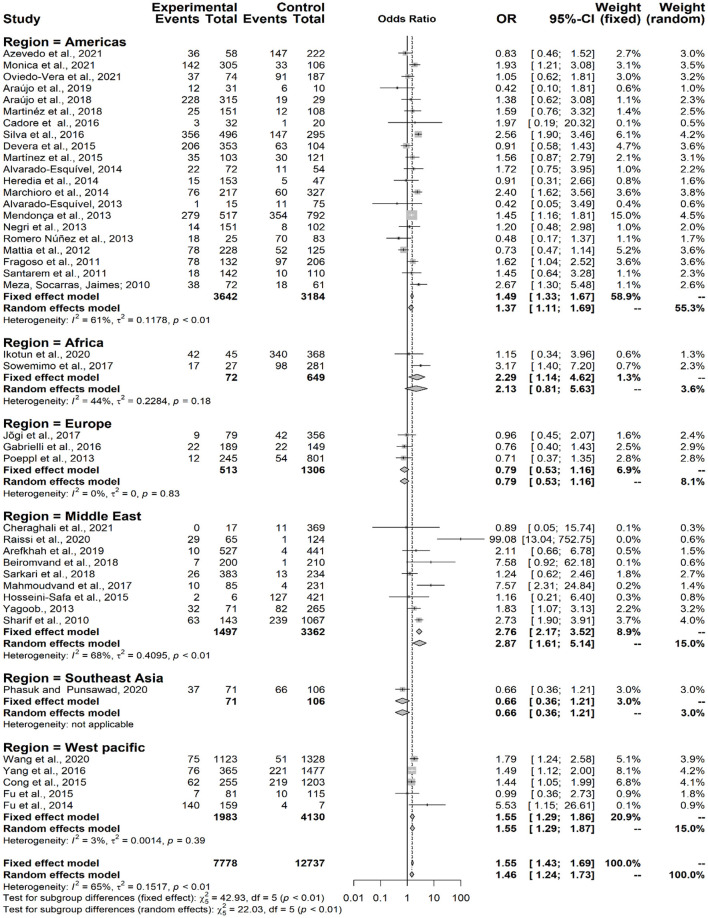
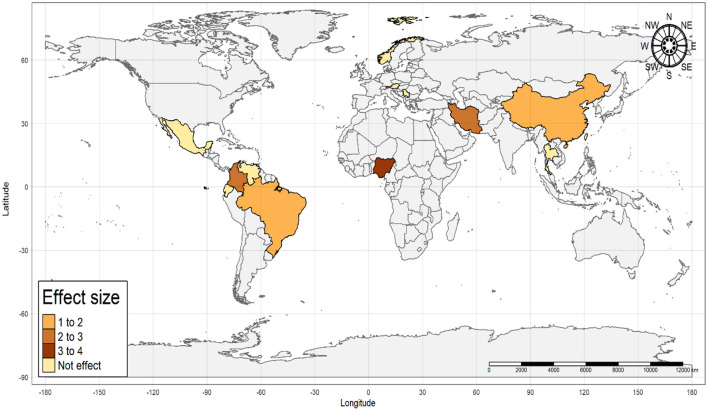
Considering the presence of anti-Toxocara spp. antibodies in populations from different geographical regions, according to the classification by the World Health Organization (WHO) (χ2 = 22.03; p < 0.0001), a statistically significant difference was observed in the Americas (OR = 1.37; CI 95% = 1.11–1.69), Middle East (OR = 2.87; CI 95% = 1.61–5.14) and Western Pacific (OR = 1.39; CI 95% = 1.03–1.88), but neither observed in Europe (OR = 0.79; CI 95% = 0.53–1.16) nor in Africa (OR = 2.13; CI 95% = 0.81–5.63), The single study from Southeast Asia has also no statistically significant difference (OR = 0.66; CI 95% = 0.36–1.21) concerning the presence of anti-Toxocara spp. antibodies (Figure 7). An illustrative map was created for a better pinpoint view of seroprevalence per country of study (Figure 8). The funnel graphic utilized for assessment of publication bias showed asymmetry among some studies, probably due to bigger size of effects and lower sample size (Figure 9).
Figure 7.
Forest plot for assessment of odds-ratio of influence of anti-Toxocara spp. antibodies in different world geographical regions (WHO- World Health Organization), according to selected articles used in the meta-analysis from 2009 to 2021.
Figure 8.
Illustrative map of effect size from studies on the frequency of anti-Toxocara spp. antibodies in under-18 and adults, according to selected articles used in the meta-analysis from 2009 to 2021.
Figure 9.
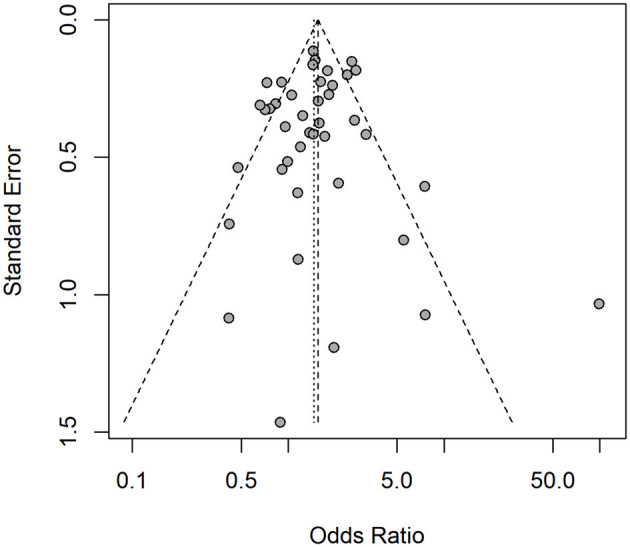
Funnel graphic utilized for assessment of publication bias, which has assessed the contact of dogs and cats and frequency of anti-Toxocara spp. antibodies in under-18 and adults, according to selected articles used in the meta-analysis from 2009 to 2021.
Baujat plots showed that three studies (31–33) add substantially to the heterogeneity in the meta-analysis. However, these studies do not have a large impact on the overall results and were kept in the meta-analysis. One study was considered a potential source of publication bias, standing out as a point to the right in the funnel chart (31). However, it was found that this study has a substantially high odds ratio estimate compared to the others, more due to the low proportion of seropositive individuals in the control group than to a small study size. Thus, it was assumed that the effect size associated with this study does not imply a significant risk of publication bias.
Discussion
The present meta-analysis study has found significant association between contact with dogs and cats and seropositivity for anti-Toxocara antibodies in under-18, but not for adult populations. Thus, contact with dogs and cats has been confirmed as an associated risk factor for Toxocara exposure in younger individuals. Based on the herein results, frequency of anti-Toxocara antibodies in under-18 with contact with dogs and cats (16.2%) was statistically higher than in adults (7.8%). Previous studies have shown that most seropositive youngers for anti-Toxocara antibodies were children from 2 to 8 years old with clinical history of onychophagia, geophagia, and exposure to animals (34–37).
A recent meta-analysis study has shown that frequency of anti-Toxocara antibodies in pediatric population worldwide was approximately 30% (IC = 22–37%: I2 = 99.11%; p < 0.05), similarly to the present study of 31.8% prevalence in under-18 individuals (26). Despite another up-to-date survey has also found youth as likely risk factor for toxocariasis (OR = 1.89; IC = 1.72–12.8), authors have not assessed presence of dog and cat contact as associated risk factor for youngers (4).
Children may be more exposed to toxocariasis agents due to habits of putting dirty hands into mouth, geophagia, and onychophagia (1, 38–40). Besides, children have a higher direct soil contact in recreation places such as parks and squares, which may be contaminated with Toxocara spp. eggs shed by dogs and cats (7, 41–43) Not surprisingly, one fifth of recreational public areas worldwide were contaminated with Toxocara spp. eggs (8). Another mechanism for Toxocara spp. transmission may rely on physical egg transfer from soil to owner shoes and animal paws, making even dewormed dogs and cats as potential helminth carriers (44).
Reportedly presence of Toxocara spp. eggs in dog/cat hair has been supported the hypothesis of transmission by ingestion of contaminated hair (13, 45, 46). Despite the contact with well-taken care dogs may represent low risk of infection, such potential transmission cannot be ruled out (11, 47). Children with contact with dogs or cats may present a higher tendency of acquiring infection by Toxocara spp. (48), with a higher risk of accidental intake of embryonated eggs of T. canis or de T. cati from contaminated pet hair (49). Daily contact with dogs and geophagia were the two most significant and influential factors for failure of toxocariasis treatment with albendazole in children from two to 16 years old in Poland, with prolonged treatment for toxocariasis more likely in children with daily contact with dogs (50).
The present study has found no association between contact with dogs or cats and presence of anti-Toxocara spp. antibodies in adults, which has been previously indicated as an occupational disease (51, 52). Moreover, toxocariasis in adults has been associated to consumption of non-treated water, raw and unwashed vegetables, raw or uncooked meat of paratenic hosts such as chickens, pigs, and rodents (53–55).
Regarding to the geographical regions, association of owning dogs or cats and be seropositive was observed in the Americas, Middle East and Western Pacific, corroborating to previous studies indicating areas of South America (27.8%) and Western Pacific (22.8%) with high frequency of anti-Toxocara antibodies (4). Besides climate conditions, socio-demographic factors such as low human development index (HDI), lack of veterinary assistance, pet outdoor access and risk of parasitic infection, and precarious self-hygiene may contribute altogether for a higher environmental exposure (1, 56, 57).
Although the frequency of anti-Toxocara antibodies in Middle East was relatively low, a statistical association was found with contact with companion animals. Besides the high temperature with low pluviometry in such region may be responsible for low environmental contamination and reduced seroprevalence (4), contact with dogs and cats may be limited due to religious reasons and legal restrictions for pet ownership (4, 42, 58, 59). On the other hand, the high feral, owned, and stray cat populations observed in Middle East countries such as Iran may favor infection risk due to contact with dogs and cats (20).
As a single study from Nigeria represented the entire Africa region, extrapolation of results and interpretations are limited and should be carefully taken. Toxocariasis studies in Africa have been mostly restricted and underreported (57, 60). In addition, seroprevalence studies such as in northern Africa (61) have not met inclusion criteria for the present meta-analysis, including case reports, lack of dog or cat assessment as associated risk factors, lack of population stratification, toxocariasis with comorbidities and potential bias in methodology, data extraction and/or interpretation. Despite report limitations, Africa may provide optimum toxocariasis transmission environment, including climatic settings, poor infrastructure, low socioeconomic conditions, and lack of veterinarian care (1, 56, 62). Such scenario strongly suggests that future studies should be conducted on prevalence and associated risk factors in throughout African countries as guidance for mitigation and prophylactic measures, as already proposed (57).
In Europe, no statistical association was found herein between owning a dog or cat and be seropositive for anti-Toxocara antibodies, which confirms previous studies showing that high income and HDI may present the lowest prevalence rates, consequence of fully access to information and prevention of infectious diseases, and to both human and animal health care (4, 63).
In Southeast Asia, only one study was included herein (64). This serosurvey evaluated children (5 to 15 years old), seroprevalence was 58.2% (103/177) and only the lack of handwashing before a meal was a significant risk factor (adjusted odds ratio (AOR) = 2.20; 95% CI 1.11–4.34; p = 0.023). Despite the high seroprevalence, interpretations of the results should be carefully taken since further investigation in Southeast Asia may provide robust data for a metanalytic investigation.
The observed trend of decreased frequency in anti-Toxocara antibodies related to increase of geographical latitude can be explained mostly due to colder climate of higher latitudes such as in Europe, unfavorable and limiting the life cycle of Toxocara spp. (4, 8, 65). On the other hand, countries located in low latitudes mostly present favorable climatic and environmental conditions to survival of Toxocara spp. eggs, associated to more non-dewormed stray dogs and cats in public areas (6, 8, 66).
As limitations, evaluated studies were incomplete and lacked information on sex of tested individuals, not allowing adequate assessment or comparisons of human gender involved on serologies. Thus, no assessment was made between contact with dogs or cats and gender of participants. In addition, few studies were included in the meta-analysis from certain regions, a single study from Africa and none from Asia, impairing an ideal analysis. Despite authors of studies in other languages, besides those included herein, were contacted to provide further information, no response was received back.
Studies included in the present metanalysis mainly used ELISA test for anti-Toxocara spp. antibody detection, mostly by Toxocara excretory-secretory (TES) antigens. As such specific IgG detection frequently persist for years, tests have not allowed differentiation between active and persistent infection (67). In addition, false-positive results in serological assays may occur in coinfections with other helminths due to cross-reactivity, as all Ascarid parasites may share a high homology of antigenicity with Toxocara spp. (68). Reduction of cross-reactivity in the Toxocara ELISA test has been obtained by preincubation with extract of adult Ascaris suum, removing antibodies elicited by exposure to Ascaris (10, 69). In addition, cross-reaction has been also reported between Toxocara spp. Toxocara spp. and Trichinella spp. (70), Angiostrongylus cantonensis (71), Echinococcus spp. (72). Consequently, seroprevalence assessed in metanalytic studies should be carefully interpreted to avoid under or overestimation due to differences in sensitivity and specificity among different serological methods, particularly in populations living in areas of endemic polyparasitism (4).
In summary, the present study has shown a statistical influence of contact with dogs or cats and serological exposure to Toxocara spp. in under-18 individuals. Such robust finding on associated risk factor strongly indicates special attention on preventive measures for toxocariasis, particularly to youngers in contact with dogs or cats. In addition, other measures such as preventive anti-helminthic treatment for dogs and cats, adequate removal and disposal of pet feces from parks and other public areas, population management of stray dogs and cats, and preventive educational programs for toxocariasis, particularly to youngers (4, 43).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YM, RG, and VS: conception or design of the work and final approval of the version to be published. YM: data collection. YM, RG, RS, LK, AS, AB, and VS: data analysis, interpretation, and drafting the article. LK, AS, and AB: critical revision of the article. All authors contributed to the article and approved the submitted version.
Funding
This research was funded through the Araucária Foundation of Paraná State (Protocol # SUS2020111000010) to AB.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Authors kindly thank the Brazilian Higher Education Improvement Coordination (CAPES) for sponsoring the PhD fellowship (code 001) of YM.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.854468/full#supplementary-material
References
- 1.Ma G, Holland C V, Wang T, Hofmann A, Fan C-K, Maizels RM, et al. Human toxocariasis. Lancet Infect Dis. (2018) 18:e14–24. 10.1016/S1473-3099(17)30331-6 [DOI] [PubMed] [Google Scholar]
- 2.Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. (2001) 39:1–11. 10.3347/kjp.2001.39.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC - Parasites - Neglected Parasitic Infections (NPIs) in the United States . [Internet].. Available online at: https://www.cdc.gov/parasites/npi/ (accessed January 12, 2022).
- 4.Rostami A, Riahi SM, Holland C V, Taghipour A, Khalili-Fomeshiid M, Fakhri Y, et al. Seroprevalence estimates for toxocariasis in people worldwide: a systematic review and meta-analysis. PLoS Negl Trop Dis. (2019) 13:e0007809. 10.1371/journal.pntd.0007809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macpherson CNL. The epidemiology and public health importance of toxocariasis: a zoonosis of global importance. Int J Parasitol. (2013) 43:999–1008. 10.1016/j.ijpara.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 6.Bojanich M, Alonso J, Caraballo N, Schöller M, López M, García L, et al. Assessment of the presence of toxocara eggs in soils of an arid area in Central-Western Argentina. Rev Inst Med Trop São Paulo. (2015) 57:73–6. 10.1590/S0036-46652015000100010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otero D, Alho AM, Nijsse R, Roelfsema J, Overgaauw P, Madeira de Carvalho L. Environmental contamination with Toxocara spp. eggs in public parks and playground sandpits of Greater Lisbon, Portugal. J Infect Public Health. (2018) 11:94–8. 10.1016/j.jiph.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 8.Fakhri Y, Gasser RB, Rostami A, Fan CK, Ghasemi SM, Javanian M, et al. Toxocara eggs in public places worldwide - a systematic review and meta-analysis. Environ Pollut. (2018) 242(Pt B):1467–75. 10.1016/j.envpol.2018.07.087 [DOI] [PubMed] [Google Scholar]
- 9.Marques JP, Guimarães C de R, Vilas Boas A, Carnaúba PU, de Moraes J. Contamination of public parks and squares from Guarulhos (São Paulo State, Brazil) by Toxocara spp. and Ancylostoma spp. Rev Inst Med Trop São Paulo. (2012) 54:267–71. 10.1590/S0036-46652012000500006 [DOI] [PubMed] [Google Scholar]
- 10.Ramires LM. CPF:33500019803, http://lattes.cnpq.br/4744555689623781. Impacto do tratamento antihelmíntico de cães na contaminação de vias públicas por agentes de Larva Migrans. (2014). Available online at: http://bdtd.unoeste.br:8080/jspui/handle/tede/693 (accessed January 13, 2022).
- 11.Merigueti YFFB, Santarém VA, Ramires LM, da Silveira Batista A, da Costa Beserra LV, Nuci AL, et al. Protective and risk factors associated with the presence of Toxocara spp. eggs in dog hair Vet Parasitol. (2017) 244:39–43. 10.1016/j.vetpar.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 12.Roddie G, Holland C, Stafford P, Wolfe A. Contamination of fox hair with eggs of Toxocara canis. J Helminthol. (2008) 82:293–6. 10.1017/S0022149X08996954 [DOI] [PubMed] [Google Scholar]
- 13.Wolfe A, Wright IP. Human toxocariasis and direct contact with dogs. Vet Rec. (2003) 152:419–22. 10.1136/vr.152.14.419 [DOI] [PubMed] [Google Scholar]
- 14.Aghaei S, Riahi SM, Rostami A, Mohammadzadeh I, Javanian M, Tohidi E, et al. Toxocara spp. infection and risk of childhood asthma: a systematic review and meta-analysis. Acta Trop. (2018) 182:298–304. 10.1016/j.actatropica.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 15.Li L, Gao W, Yang X, Wu D, Bi H, Zhang S, et al. Asthma and toxocariasis. Ann Allergy, Asthma Immunol. (2014) 113:187–92. 10.1016/j.anai.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 16.Luna J, Cicero CE, Rateau G, Quattrocchi G, Marin B, Bruno E, et al. Updated evidence of the association between toxocariasis and epilepsy: systematic review and meta-analysis. PLoS Negl Trop Dis. (2018) 12:e0006665. 10.1371/journal.pntd.0006665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quattrocchi G, Nicoletti A, Marin B, Bruno E, Druet-Cabanac M, Preux PM. Toxocariasis and epilepsy: systematic review and meta-analysis. PLoS Negl Trop Dis. (2012) 6:e1775. 10.1371/journal.pntd.0001775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammadzadeh I, Riahi SM, Saber V, Darvish S, Amrovani M, Arefkhah N, et al. The relationship between Toxocara species seropositivity and allergic skin disorders: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. (2018) 112:529–37. 10.1093/trstmh/try094 [DOI] [PubMed] [Google Scholar]
- 19.Strube C, Raulf MK, Springer A, Waindok P, Auer H. Chapter Nineteen - Seroprevalence of human toxocarosis in Europe: a review and meta-analysis. In: Bowman DDBT-A in P, editor. Toxocara and Toxocariasis. Academic Press (2020). p. 375–418. 10.1016/bs.apar.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 20.Abbaszadeh Afshar MJ, Zahabiun F, Heydarian P, Mozafar Saadati H, Mohtasebi S, Khodamoradi F, et al. A systematic review and meta-analysis of toxocariasis in Iran: is it time to take it seriously? Acta Parasitol. (2020) 65:569–84. 10.2478/s11686-020-00195-1 [DOI] [PubMed] [Google Scholar]
- 21.One Health | CDC . Available online at: https://www.cdc.gov/onehealth/index.html (accessed January 13, 2022).
- 22.Hartnack S, Alobo G, Kankya C. Toxocariasis in Africa: a one health perspective. Travel Med Inf Dis. (2017) 20:3–4. 10.1016/j.tmaid.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 23.Bovenkerk B, Herten J., van, Verweij M. The animal factor in human health. Am J Bioeth. (2017) 17:28–30. 10.1080/15265161.2017.1353171 [DOI] [PubMed] [Google Scholar]
- 24.Santos LM., Dos, Magalhães CG, Telmo P de L, Cerqueira MP, Donassolo RA, Leite FPL, et al. Sensitivity and specificity of recombinant proteins in Toxocara spp for serodiagnosis in humans: differences in adult and child populations. PLoS One. (2018) 13:e0208991. 10.1371/journal.pone.0208991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abedi B, Akbari M, KhodaShenas S, Tabibzadeh A, Abedi A, Ghasemikhah R, et al. The global prevalence of Toxocara spp. in pediatrics: a systematic review and meta-analysis. Clin Exp Pediatr. (2021) 64:575–81. 10.3345/cep.2020.01039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuijfzand S, Creswell C, Field AP, Pearcey S, Dodd H. Research review: is anxiety associated with negative interpretations of ambiguity in children and adolescents? A systematic review and meta-analysis. J Child Psychol Psychiatry. (2018) 59:1127–42. 10.1111/jcpp.12822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baujat B, Mahé C, Pignon JP, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. (2002) 21:2641–52. 10.1002/sim.1221 [DOI] [PubMed] [Google Scholar]
- 29.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R: The R Project for Statistical Computing . Available online at: https://www.r-project.org/ (accessed January 12, 2022).
- 31.Raissi V, Taghipour A, Navi Z, Etemadi S, Sohrabi Z, Sohrabi N, et al. Seroprevalence of Toxoplasma gondii and Toxocara spp. infections among pregnant women with and without previous abortions in the west of Iran. J Obstet Gynaecol Res. (2020) 46:382–8. 10.1111/jog.14184 [DOI] [PubMed] [Google Scholar]
- 32.Silva MB, Amor ALM, Santos LN, Galvão AA, Oviedo Vera A V, Silva ES, et al. Risk factors for Toxocara spp. seroprevalence and its association with atopy and asthma phenotypes in school-age children in a small town and semi-rural areas of Northeast. Brazil Acta Trop. (2017) 174:158–64. 10.1016/j.actatropica.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 33.Mattia S, Colli C, Adami CM, Guilherme GF, Nishi L, Rubinsky-Elefant G, et al. Seroprevalence of Toxocara infection in children and environmental contamination of urban areas in Parana State, Brazil. J Helminthol. (2011) 86:1–6. 10.1017/S0022149X11000666 [DOI] [PubMed] [Google Scholar]
- 34.Fragoso RP, Monteiro MBM, Lemos EM, Pereira FEL. Anti-Toxocara antibodies detected in children attending elementary school in Vitoria, State of Espírito Santo, Brazil: prevalence and associated factors. Rev Soc Bras Med Trop. (2011) 44:461–6. 10.1590/S0037-86822011000400012 [DOI] [PubMed] [Google Scholar]
- 35.Wiśniewska-Ligier M, Wozniakowska-Gesicka T, Sobolewska-Dryjańska J, Markiewicz-Józwiak A, Wieczorek M. Analysis of the course and treatment of toxocariasis in children—a long-term observation. Parasitol Res. (2012) 110:2363–71. 10.1007/s00436-011-2772-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prestes-Carneiro LE, Rubinsky-Elefant G, Ferreira AW, Araujo PR, Troiani C, Zago SC, et al. Seroprevalence of toxoplasmosis, toxocariasis and cysticercosis in a rural settlement, São Paulo State, Brazil. Pathog Glob Health. (2013) 107:88–95. 10.1179/2047773213Y.0000000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoenardie ER, Scaini CJ, Brod CS, Pepe MS, Villela MM, McBride AJA, et al. Seroprevalence of Toxocara infection in children from southern Brazil. J Parasitol. (2013) 99:537–9. 10.1645/GE-3182 [DOI] [PubMed] [Google Scholar]
- 38.Glickman LT, Schantz PM. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. (1981) 3:230–50. 10.1093/oxfordjournals.epirev.a036235 [DOI] [PubMed] [Google Scholar]
- 39.Maroufi Y, Faridi A, Khademerfan M, Bahrami F, Zamini G. Seroepidemiological study of toxocariasis in children aged 6–14 year old in Sanandaj, Western Iran. Iran J Parasitol. (2020) 15:435–9. 10.18502/ijpa.v15i3.4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoicescu RM, Mihai CM, Giannakopoulou AD. Marked hypereosinophilia in a toddler: a case report. J Med Life. (2011) 4:105–8. [PMC free article] [PubMed] [Google Scholar]
- 41.Mazur-Melewska K, Mania A, Figlerowicz M, Kemnitz P, Słuzewski W, Michalak M. The influence of age on a clinical presentation of Toxocara spp. infection in children. Ann Agric Environ Med. (2012) 19:233–6. [PubMed] [Google Scholar]
- 42.Nijsse R, Overgaauw P, Ploeger H, Mughini-Gras L. Sources of environmental contamination with Toxocara spp. An omnipresent parasite. Adv Parasitol. (2020) 109:585–614. 10.1016/bs.apar.2020.01.010 [DOI] [PubMed] [Google Scholar]
- 43.Ozlati M, Spotin A, Shahbazi A, Mahami-Oskouei M, Hazratian T, Adibpor M, et al. Genetic variability and discrimination of low doses of Toxocara spp. from public areas soil inferred by loop-mediated isothermal amplification assay as a field-friendly molecular tool. Vet world. (2016) 9:1471–7. 10.14202/vetworld.2016.1471-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panova OA, Khrustalev A V. Dog walking brings Toxocara eggs to people's homes. Vet Parasitol. (2018) 262:16–9. 10.1016/j.vetpar.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 45.Aydenizöz-Özkayhan M, Yagci BB, Erat S. The investigation of Toxocara canis eggs in coats of different dog breeds as a potential transmission route in human toxocariasis. Vet Parasitol. (2008) 152:94–100. 10.1016/j.vetpar.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 46.Öge H, Öge S, Özbakiş G, Gürcan S. Comparison of Toxocara eggs in hair and faecal samples from owned dogs and cats collected in Ankara, Turkey. Vet Parasitol. (2014) 206:227–31. 10.1016/j.vetpar.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 47.Keegan JD, Holland C V. Contamination of the hair of owned dogs with the eggs of Toxocara spp. Vet Parasitol. (2010) 173:161–4. 10.1016/j.vetpar.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Li H, Yao Z, Li P, Wang D, Zhang H, et al. Toxocara infection: seroprevalence and associated risk factors among primary school children in central China. Parasite. (2020) 27:30. 10.1051/parasite/2020028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodhall DM, Fiore AE. Toxocariasis: a review for pediatricians. J Pediatric Infect Dis Soc. (2014) 3:154–9. 10.1093/jpids/pit066 [DOI] [PubMed] [Google Scholar]
- 50.Kroten A, Toczylowski K, Oldak E, Sulik A. Toxocarosis in children: poor hygiene habits and contact with dogs is related to longer treatment. Parasitol Res. (2018) 117:1513–9. 10.1007/s00436-018-5833-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alvarado-Esquivel C, Hernández-Tinoco J, Sánchez-Anguiano LF. Toxocara infection in gardeners: a case control seroprevalence study. Asian Pac J Trop Med. (2014) 7:S79–81. 10.1016/S1995-7645(14)60207-8 [DOI] [PubMed] [Google Scholar]
- 52.Haagsma JA, Tariq L, Heederik DJ, Havelaar AH. Infectious disease risks associated with occupational exposure: a systematic review of the literature. Occup Environ Med. (2012) 69:140–6. 10.1136/oemed-2011-100068 [DOI] [PubMed] [Google Scholar]
- 53.Choi D, Lim JH, Choi DC, Paik SW, Kim SH, Huh S. Toxocariasis and ingestion of raw cow liver in patients with Eosinophilia. Korean J Parasitol. (200)8 46:139–43. 10.3347/kjp.2008.46.3.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seo M, Yoon SC. A seroepidemiological survey of toxocariasis among eosinophilia patients in Chungcheongnam-do. Korean J Parasitol. (2012) 50:249–51. 10.3347/kjp.2012.50.3.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strube C, Heuer L, Janecek E. Toxocara spp. infections in paratenic hosts. Vet Parasitol. (2013) 193:375–89. 10.1016/j.vetpar.2012.12.033 [DOI] [PubMed] [Google Scholar]
- 56.Gawor J, Borecka A, Marczyńska M, Dobosz S, Zarnowska-Prymek H. Risk of human toxocarosis in Poland due to Toxocara infection of dogs and cats. Acta Parasitol. (2015) 1:2015. 10.1515/ap-2015-0012 [DOI] [PubMed] [Google Scholar]
- 57.Omonijo AO, Kalinda C, Mukaratirwa S. A systematic review and meta-analysis of canine, feline and human Toxocara infections in sub-Saharan Africa. J Helminthol. (2020) 94:e96. 10.1017/S0022149X19000889 [DOI] [PubMed] [Google Scholar]
- 58.Animals in Islamic Tradition and Muslim Cultures . Available online at: https://oneworld-publications.com/animals-in-islamic-tradition-and-muslim-cultures.html (accessed January 12, 2022).
- 59.Zibaei M, Sadjjadi SM. Trend of toxocariasis in Iran: a review on human and animal dimensions. Iran J Vet Res. (2017) 18:233–42. [PMC free article] [PubMed] [Google Scholar]
- 60.Gyang PV, Akinwale OP, Lee YL, Chuang TW, Orok AB, Ajibaye O, et al. Seroprevalence, disease awareness, and risk factors for Toxocara canis infection among primary schoolchildren in Makoko, an urban slum community in Nigeria. Acta Trop. (2015) 146:135–40. 10.1016/j.actatropica.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 61.Adeel AA. Chapter Twenty-Five - Seroepidemiology of human toxocariasis in North Africa. In: Bowman DDBT-A in P, editor. Toxocara and Toxocariasis: Academic Press (2020). p. 501–34. Available online at: https://www.sciencedirect.com/science/article/pii/S0065308X20300233 [DOI] [PubMed]
- 62.Liao CW, Sukati H, D'lamini P, Chou CM, Liu YH, Huang YC, et al. Seroprevalence of Toxocara canis infection among children in Swaziland, southern Africa. Ann Trop Med Parasitol. (2013) 104:73–80. 10.1179/136485910X12607012373795 [DOI] [PubMed] [Google Scholar]
- 63.Water and sanitation in developing countries: including health in the equation . Environ Sci Technol. (2007) 41:17–24. 10.1021/es072435t [DOI] [PubMed] [Google Scholar]
- 64.Phasuk N, Punsawad C. Seroprevalence of Toxocara canis infection and associated risk factors among primary schoolchildren in rural Southern Thailand. Trop Med Health. (2020) 48:23. 10.1186/s41182-020-00211-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Azam D, Ukpai OM, Said A, Abd-Allah GA, Morgan ER. Temperature and the development and survival of infective Toxocara canis larvae. Parasitol Res. (2012) 110:649–56. 10.1007/s00436-011-2536-8 [DOI] [PubMed] [Google Scholar]
- 66.Dantas-Torres F, Otranto D. Dogs, cats, parasites, and humans in Brazil: opening the black box. Parasit Vectors. (2014) 7:22. 10.1186/1756-3305-7-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rudzińska M, Kowalewska B, Sikorska K. Clinical usefulness of Western blotting and ELISA avidity for the diagnosis of human toxocariasis. Parasite Immunol. (2017) 39:12400. 10.1111/pim.12400 [DOI] [PubMed] [Google Scholar]
- 68.Nguyen YTH, Hayata Y, Sonoda S, Nonaka N, Maruyama H, Yoshida A. Establishment of a serodiagnosis system for the detection of Toxocara spp. and Ascaris suum infection in chickens. Parasitol Int. (2020) 75:102022. 10.1016/j.parint.2019.102022 [DOI] [PubMed] [Google Scholar]
- 69.de Oliveira Azevedo P, Lescano SZ, Giuffrida R, Kmetiuk LB, Dos Santos AP, Dangoudoubiyam S, et al. Serosurvey of anti-Toxocara antibodies and risk factors in adolescent and adult pregnant women of southeastern Brazil. PLoS Negl Trop Dis. (2021) 15:e0009571. 10.1371/journal.pntd.0009571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raulf MK, Jordan D, Auer H, Warnecke JM, Lepenies B, Strube C, et al. new ELISA and western blot technique based on recombinant TES antigen and/or larval antigen for the detection of toxocariasis in humans. Parasitology. (2021) 148:333–40. 10.1017/S0031182020002085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morassutti AL, Rascoe LN, Handali S, DA Silva AJ, Wilkins PP, Graeff-Teixeira C. Cross-reactivity of the 31 kDa antigen of Angiostrongylus cantonensis - Dealing with the immunodiagnosis of meningoencephalitis. Parasitology. (2017) 144:459–63. 10.1017/S0031182016001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fathi S, Jalousian F, Hosseini SH, Parsa H, Kordafshari S. A Study of cross-reactivity between recombinant EPC1 antigen of echinococcus granulosus in serum from patients with confirmed cystic echinococcosis infection and other parasitic infections. Am J Trop Med Hyg. (2016) 94:1313–7. 10.4269/ajtmh.15-0680 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.