Abstract
Neurodegenerative diseases are a diverse class of diseases attributed to chronic progressive neuronal degeneration and synaptic loss in the brain and/or spinal cord, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis. The pathogenesis of neurodegenerative diseases is complex and diverse, often involving mitochondrial dysfunction, neuroinflammation, and epigenetic changes. However, the pathogenesis of neurodegenerative diseases has not been fully elucidated. Recently, accumulating evidence revealed that ferroptosis, a newly discovered iron-dependent and lipid peroxidation-driven type of programmed cell death, provides another explanation for the occurrence and progression of neurodegenerative diseases. Here, we provide an overview of the process and regulation mechanisms of ferroptosis, and summarize current research progresses that support the contribution of ferroptosis to the pathogenesis of neurodegenerative diseases. A comprehensive understanding of the emerging roles of ferroptosis in neurodegenerative diseases will shed light on the development of novel therapeutic technologies and strategies for slowing down the progression of these diseases.
Keywords: ferroptosis, neurodegenerative diseases, iron metabolism, oxidative stress, redox regulation
Introduction
Dolma et al. (2003) discovered a new mechanism of cell death using a small molecule called erastin. In 2012, Scott Dixon discovered that erastin caused a non-apoptotic form of cell death with unique morphological, biochemical, and genetic properties, which was later termed ferroptosis (Dixon et al., 2012). As our knowledge of cell death continues to be updated, the concepts and mechanisms of ferroptosis are further elucidated. In 2018, the Nomenclature Committee on Cell Death defined ferroptosis as a Regulated Cell Death (RCD) caused by oxidative distress in the intracellular micro-environment, which can be inhibited by lipophilic antioxidants and iron chelators (Galluzzi et al., 2018). Recently, numerous studies have shown that ferroptosis is closely related to various diseases, including tumors (Mou et al., 2019), neurological disorders (Ren et al., 2020), metabolic diseases (Le et al., 2021), and cardiovascular diseases (Yu et al., 2021).
Neurodegenerative diseases (NDDs) are a group of diseases characterized by the progressive loss of a specific population of neurons, resulting in progressive cognitive decline, movement impairment, and other comorbidities, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and so on (Wells et al., 2019). Ferroptosis has been proven to be closely correlated with the occurrence and development of most NDDs. Here, we introduce the molecular mechanism of ferroptosis, the contribution of ferroptosis to the pathogenesis of NDDs, and the potential value of targeting ferroptosis in the treatment of NDDs.
The Process and Regulation of Ferroptosis
The Process of Ferroptosis
As an independent form of cell death, ferroptosis can be clearly distinguished from other forms of cell death by the accumulation of lethal reactive oxygen species (ROS) and lipid peroxidation products caused by iron-dependent reactions (Table 1; Dixon et al., 2012; Wu et al., 2012; Liu and Levine, 2015; Chen et al., 2016; Xie et al., 2016; Galluzzi et al., 2017; D’Arcy, 2019; Xu et al., 2019; Demarco et al., 2020; Mizushima and Levine, 2020; Nah et al., 2020; Khan et al., 2021; Li et al., 2021; Nadeem et al., 2021; Yang et al., 2021; Braicu et al., 2022; Lei et al., 2022). Ultrastructural changes in mitochondria are the most prominent morphological features of ferroptotic cells. Overall, the process of ferroptosis consists of multiple key steps including iron metabolism dysregulation and lipid peroxidation (Figure 1).
TABLE 1.
The main features of ferroptosis, apoptosis, autophagy, necroptosis, and pyroptosis.
| Ferroptosis | Apoptosis | Autophagy | Necroptosis | Pyroptosis | ||
| Morphological features | Cell morphology | Smaller and rounder; cell rounding up | Shrinkage; intercellular connections disappear |
Minor changes | Swelling | Swelling; formed bubble-like protrusions |
| Cell membrane | No rupture or blistering | Plasma membrane blebbing; membrane structure remains intact | Blebbing sometimes observed | Rupture of plasma membrane | Formation of plasma membrane pores; plasma membrane rupture |
|
| Cytoplasm | Small mitochondria with condensed mitochondrial membrane densities | Retraction of pseudopods; dense cellular contents |
Vacuolization of the cytoplasm; accumulation of double-membraned autophagic vacuoles |
Cytoplasmic swelling, swelling of cytoplasmic organelles | Osmotic swelling; cell contents leakage | |
| Nucleus | Normal nuclear size; lack of chromatin condensation |
Genetic materials fragmentation; marked chromatin condensation; nuclear fragmentation and condensation |
Lack of chromatin condensation | Mild-moderate chromatin condensation (Nuclear pyknosis) | Chromatin random breakage degradation | |
| Special features | Mitochondrial atrophy or fragmentation; mitochondrial membrane density condensed; mitochondrial cristae decreased |
Apoptotic bodies (or ApoBDs) | Numerous autophagosomes and autolysosomes | Necroptotic bodies | Pyroptotic bodies | |
| Biological features | Iron and ROS accumulation; lipid peroxides increased; system Xc– and GPX4 inhibition; GSH depletion; Δψm dissipation |
Caspase activation; DNA fragmentation; Δψm dissipation; intracellular calcium increased |
LC3-I to LC3-II conversion; ATG expression increased; increased lysosomal activity |
Activation of RIP1, RIP3, and MLKL; PARP1 hyperactivation; drop in ATP levels |
Dependent on caspase-1; GSDMD family activation |
|
| Immunological features | Release of DAMPs (e.g., inflammatory factor, arachidonic acid mediators, HMGB1) |
Release Ecto-CRT, Histone, HMGB1, and ATP under certain conditions | Regulation of immune cell differentiation and function | Release of DAMPs (e.g., DNA, IL-6 and HMGB1) | Release of proinflammatory cytokine | |
| Inflammation | Pro-inflammatory | Anti-inflammatory | Mostly anti-inflammatory | Mostly pro-inflammatory | Pro-inflammatory | |
| Major regulatory components | P53, HO-1, iron, systemXC-/GSH/GPX4 pathway, GCH1/DHFR/BH4 pathway, FSP1/CoQ10 pathway, DHODH/CoQ pathway, p62/Keap1/Nrf2 pathway |
P53, Bax and other Bcl-2 family proteins, Caspase family, endoplasmic reticulum pathway, death receptor |
ATG family proteins (e.g., Atg5 and Atg7), Beclin 1, PI3K-AKT-mTOR pathway, MAPK-ERK1/2-mTOR pathway |
Toll-like receptor family, RIP1, RIP3, MLKL, TNF-α, TRAIL, FasL, ROS |
Caspase-1, Caspase-4/5/11, GSDMD, IL-1β, IL-18, NLRP3-mediated signaling pathway |
|
| Main inducer and inhibitor |
Inducer | Erastin, sulfasalazine, sorafenib, altretamine, RSL3, ML162, ML210, SAS, lanperisone, DPI7, DPI10, FIN56, CIL56, artemisinin, FINO2 | Ca2+/Mg2+, TGF-β glucocorticoid, FASL |
Rapamycin, lithium, sodium, brefeldin A, thapsigargin, tunicamycin valproate, carbamazepine, xestospongin B/C, C2-ceramide |
Sorafenib, artesunate, shikonin, resibufogenin, 5-FU, SM-164 | Paclitaxel, VTPA, ZnO-NPs, ivermectin |
| Inhibitor | Deferoxamine, deferiprone, vitamin E, ferrostatin-1, Liproxstatin-1, DHO, SRS, CA-1, cycloheximide |
IAPs (XIAP, c-IAP1/2, ILP-2, NAIP, ML-IAP/livin, Z-VAD-FMK etc.), IL-2/3/4, GM/CSF |
SAR405, Bafilomycin A1, Wortmannin, LY294002, Spautin1, 3-Methyladenine, hydroxychloroquine |
Necrostatin-1, Necrostatin-2, NSA, Kongensin- A |
Necrosulfonamide, VX765, Z-VAD-FMK, Q-VD-Oph |
|
FIGURE 1.
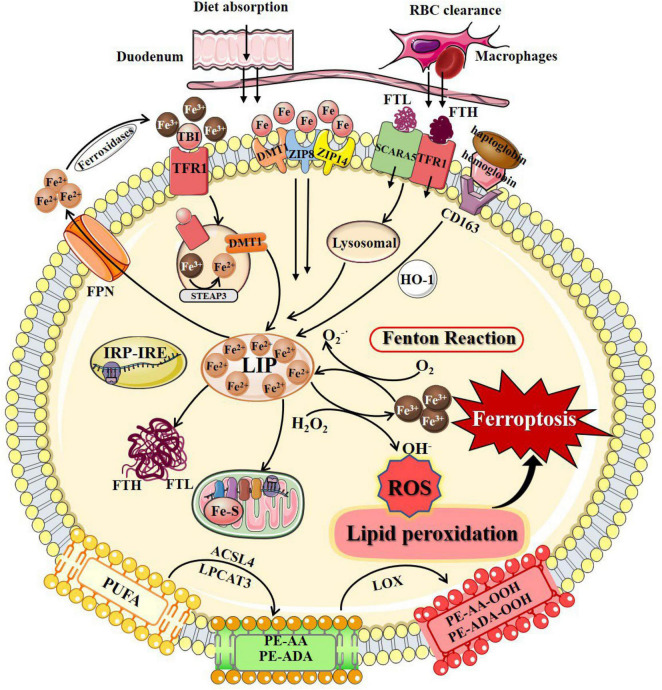
The process of ferroptosis. The occurrence of ferroptosis depends on the intracellular iron deposition caused by the disorder of iron metabolism. The body iron intake takes place by tissue macrophage-dependent aging red blood cells engulfing and duodenal enterocytes-mediated diet absorption into the bloodstream. Cellular iron absorption involves the following pathways: transferrin-bound iron pathway, non-transferrin bound iron pathway, SCARA5/TfR1-dependent endocytosis, and hemoglobin-dependent pathway. Intracellular Fe2+ formed LIP, stored in FTL and FTH in the cytosol, or as Fe–S in the mitochondrial respiratory chain. IRP/IRE regulates iron homeostasis by regulating the transcription of iron metabolism genes. When cellular iron metabolism is disordered, accumulated intracellular Fe2+ undergo Fenton’s reaction with H2O2 to generate OH⋅. OH⋅ and other ROS attack the PUFAs on the membrane surface to cause membrane peroxidation and release oxidative toxic substances such as 4-HNE and MDA. Consequently, membrane structure, proteins, and genetic materials are damaged, which affects organelles’ functions and cell homeostasis, causing ferroptosis at last. 4-HNE, 4-hydroxynonenal; ACSL4, long-chain-fatty-acid-CoA ligase 4; DMT1, divalent metal transporters 1; FPN, ferroportin; Fe–S, iron-sulfur clusters; FTH, ferritin heavy chain; FTL, ferritin light chain; HO-1, heme oxygenase 1; IRP/IRE, iron-regulatory protein/iron-responsive element; LIP, labile iron pool; LPCAT3, lysophosphatidylcholine acyltransferase 3; LOX, lipoxygenase; MDA, malondialdehyde; PUFAs, polyunsaturated fatty acids; PE-AA, phosphatidylethanolamines-arachidonoyl; PE-AA-OOH, hydroperoxides of phosphatidylethanolamines-arachidonoyl; PE-ADA, phosphatidylethanolamines-adrenoyl; PE-ADA-OOH, hydroperoxides of phosphatidylethanolamines- adrenoyl; ROS, reactive oxygen species; SCARA5, scavenger receptor class A member 5; TfR1, transferrin receptor 1; ZIP8, Zrt/Irt-related protein 8; ZIP14, Zrt/Irt-related protein 14.
Iron Metabolism
Iron overload caused by intracellular iron metabolism disorder is an important factor that induces ferroptosis. The cellular iron metabolism involves regulation of iron absorption, storage, utilization, excretion, and some special cell iron regulatory elements.
Cellular Iron Absorption, Storage, and Excretion
Iron is internalized through diet absorption by duodenal enterocytes into the bloodstream (Winter et al., 2014). The circulating iron levels can be further regulated by tissue macrophages that engulf senescent red blood cells to release iron via ferroportin (FPN) (Winter et al., 2014). Cellular iron absorption involves the following pathways: (1) Transferrin-bound iron (TBI) pathway refers to that circulating iron binds to transferrin (Tf) and is recognized by the transferrin receptors (TfRs) to form a Fe3+-containing Tf/TfR1 complex that entry into cells through receptor-mediated endocytosis. TfRs are divided into two types according to their expression patterns, TfR1 and TfR2. Iron enters cells primarily via binding to cell surface TfR1, while TfR2 is mainly expressed in hepatocytes and erythroid precursor cells (Kawabata, 2019). The Fe3+-containing Tf/TfR1 complex is phagocytosed, followed by the detachment of Fe3+ from Tf in the acidic environment of the endosome, reduced to Fe2+ by six-transmembrane epithelial antigen of prostrate 3 (STEAP3) or duodenal cytochrome b (DCYTB), two metalloreductases, and then crosses the endosomal membrane enters the cytoplasm via divalent metal transporter 1 (DMT1) (Kawabata, 2019; Kosman, 2020). (2) non-transferrin bound iron (NTBI) pathway refers to that free iron is available as NTBI when iron levels exceed the binding capacity of available Tf. NTBI uptake at the plasma membrane involves both Zrt/Irt-related protein 14 (ZIP14) and Zrt/Irt-related protein 8 (ZIP8) on the cell membrane surface (Ji and Kosman, 2015). Subsequently, iron is transported from the endosome to the cytoplasm via ZIP14 and DMT1 (van Raaij et al., 2019). (3) SCARA5/TfR1-dependent endocytosis refers to that ferritin can be endocytosed by scavenger receptor class A member 5 (SCARA5) (light chain) or TfR1 (heavy chain) on the cell membrane surface and then degraded by lysosomes (Kawabata, 2019). (4) Hemoglobin-dependent pathway refers to that iron binds to haptoglobin and is endocytosed into cells by the scavenger receptor CD163, which is cleaved in the cytoplasm by heme oxygenase 1 (HO-1) to generate Fe2+ (Li et al., 2009). Ferritin, the major form of iron storage in organisms, is widespread in the cytoplasm, nucleus, mitochondria, and serum (MacKenzie et al., 2008). Ferritin is a spherical polymer composed of ferritin heavy chain (FTH) and ferritin light chain (FTL) that stores iron in its shell as inactive Fe3+, thus preventing damage due to free iron overload. FTH has ferroxidase activity, which can convert Fe2+ into Fe3+ and store it in the shell, and Fe2+ can flow out through the channel formed by ferritin H or L subunits (Liu et al., 2022).
The iron exportation pathway is relatively simple. Fe2+ is mainly transported out of cells by FPN and then oxidized to Fe3+ by ferroxidase. Following transferrin binding, Fe3+ is reabsorbed into the intracellular iron metabolism (Masaldan et al., 2019). Increased cellular iron absorption, weakened iron storage capacity, or blocked iron excretion, will finally lead to an abnormal accumulation of iron and possible ferroptosis.
Labile Iron Pool
Small amounts of iron exist in the free state to form the cellular labile iron pool (LIP), also considered as the cellular chelatable pool or the redox-active iron complex pool (Kakhlon and Cabantchik, 2002). Free iron in LIP is mainly found in free ferrous iron, which can bind to various ligand groups and transported by iron chaperones, such as poly(rC)-binding protein 1 (PCBP1), mediate binding to iron-requiring or iron-containing proteins and enzymes, enabling cells to meet their metabolic needs for iron (Lv and Shang, 2018; Philpott et al., 2020). LIP is homeostatic to other forms of intracellular iron regulation, while minimizing its involvement in the formation hydroxyl radicals (OH⋅) from hydrogen peroxide (H2O2), further reducing the occurrence of cytotoxic chemical reactions in intracellular oxygen-rich environments (Lv and Shang, 2018).
Mitochondrial Iron
Mitochondria are critical sites for iron utilization and accumulation. Cytoplasmic ferrous irons can be imported into the inner mitochondrial membrane (IMM) in a membrane potential-dependent manner or be transported by ferritin and traverse the IMM via mitoferrin 1 or mitoferrin 2 (Wang et al., 2020). Imported iron is mainly used for heme synthesis, biosynthesis of iron-sulfur clusters, and iron storage in mitochondria (Gao et al., 2021). Fe–S clusters act as cofactors in a variety of biological processes and are required for the function of enzymes related to energy metabolism, redox reactions, DNA synthesis, and other cellular physiological processes. Fe–S clusters are essential for the function of aconitine and succinate dehydrogenase in the TCA cycle and mediate functional electron transport in the respiratory complex of the electron transport chain (Paul et al., 2017). Furthermore, Fe–S clusters are important cofactors to ensure the normal functioning of key enzymes in DNA metabolism, such as DNA helicases and DNA polymerases (Puig et al., 2017). Mitochondrial ferritin (FtMt) is a specific protein that stores iron in mitochondria, and its main function is to participate in the formation of mitochondrial iron pools to maintain iron homeostasis (Campanella et al., 2009).
Iron Regulation
Iron regulatory proteins (IRPs), including IRP1 and IRP2, play important roles in maintaining cellular iron homeostasis. IRP regulates iron metabolizing genes transcripts by binding to the iron response element (IRE) in 3′-untranslated region (UTR) or 5′-UTR (Gao et al., 2019). When intracellular iron is low, Fe–S occupying the active site of the IRP is released, allowing DMT1 and TfR gene transcripts to bind to IRE and increase their translation, while IRPs bind to 5′-UTR-bound FPN gene transcripts inhibits its translation, thereby reducing cellular iron excretion and increasing absorption to promote intracellular free iron growth (Wei et al., 2020). The accumulated Fe2+ undergoes Fenton’s reaction with H2O2 to produce a large amount of OH⋅, one of the ROS with strong oxidative capability, which leads to the destruction of the cell membrane by oxidative damage. Regulation of iron homeostasis prevents intracellular iron accumulation by regulating iron absorption, utilization, storage, and excretion, which is one of the most important ways to prevent ferroptosis. Iron chelators, such as deferoxamine (DFO), deferiprone (DFP), and ciclopirox olamine, can suppress ferroptosis by profound depletion of intracellular iron (Eberhard et al., 2009; Nikseresht et al., 2019). Zinc protoporphyrin IX (ZnPPIX) is a specific HO-1 inhibitor that reduces Fe2+ produced by intracellular heme breakdown and inhibits erastin-induced ferroptosis (Gammella et al., 2015). Decreased Recombinant iron responsive element binding protein 2 (IREB2) expression can also inhibit ferroptosis by improving the intracellular storage capacity of ferritin (Kwon et al., 2015).
Lipid Peroxidation
Lipid metabolism is essential for ferroptosis, and lipid peroxidation induced by ROS represents the state of oxidative stress, which is a triggering factor of ferroptosis. Polyunsaturated fatty acids (PUFAs) with labile bis-allylic hydrogen atoms are especially susceptible to ROS damage (Yang et al., 2016). PUFAs and polyunsaturated acyl-tailed phospholipids (PUFA-PL) are necessary for the normal execution of ferroptosis, which can generate ROS and lipid peroxides such as malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and lipid hydroperoxides (LOOHs) through enzymatic catalysis or autooxidation (Dixon and Stockwell, 2014; Stockwell et al., 2017). In cells under ferroptotic stress, Fenton’s reaction-mediated OH⋅ production and lipid peroxidation generate toxic products that damage cellular proteins and nucleic acids, resulting in cellular dysfunction and even death.
The key phospholipids involved in ferroptosis are phosphatidylethanolamines (PE), including phosphatidylethanolamines-arachidonoyl (PE-AA) and phosphatidylethanolamines-adrenoyl (PE-ADA) (Kagan et al., 2017). PE-AA and PE-ADA are important substrates for lipid peroxidation. Long-chain-fatty-acid-CoA 4 (ACSL4) esterifies coenzyme A (CoA) and catalyzes AA to intermediate AA-CoA (Doll et al., 2017). In the endoplasmic reticulum, lysophosphatidylcholine acyltransferase 3 (LPCAT3) binds PUFAs to AA-COAs using PE as a substrate, forming a PE-AA-rich membrane microenvironment (Lee et al., 2021). Finally, PE-AA and PE-ADA undergo peroxidation by lipoxygenases (LOXs) to generate PUFAs, which leads to ferroptosis ultimately (Wenzel et al., 2017). LOXs, especially LOX15, are catalysts for highly selective and specific oxidation reactions of PE-AA and PE-ADA (Stoyanovsky et al., 2019). LOX15 forms a PEBP1/LOX15 complex with phosphatidylethanolamine-binding protein 1 (PEBP1), which promotes the peroxidation of PE to PE-AA-hydrogen peroxide (OOH) metabolites (Wenzel et al., 2017). High concentrations of PE-AA-OOH in cell and organelle membranes are also prone to oxidative cleavage of loosely bound iron, and electrophilic ions attack functional proteins, resulting in impaired cellular integrity and function (Anthonymuthu et al., 2021).
Lipid peroxidation inhibitors represented by the Vitamin E family can effectively inhibit the activities of LOX, ACSL4, and LPCAT3, and prevent ferroptosis by reducing the accumulation of executioners (Mabalirajan et al., 2009; Zhang et al., 2022). Furthermore, activated protein kinase (AMPK) may regulate a mitochondrial-independent mechanism of acetyl-CoA carboxylase under energy stress by reducing PUFA biosynthesis and inhibiting ferroptosis (Lee et al., 2020).
The Regulation of Ferroptosis
Ferroptosis is under the regulation of multiple pathways. Besides the aforementioned iron metabolism and oxidative distress, there are anti-ferroptosis pathways that inhibit ferroptosis by inhibiting lipid peroxidation, including systemXC-/GSH/GPX4 pathway, GCH1/DHFR/BH4 pathway, FSP1/CoQ10 pathway, DHODH/CoQ pathway, and p62/Keap1/Nrf2 pathway (Figure 2). When cellular anti-ferroptosis defense pathways are interrupted, free lipid radicals are generated in the presence of free iron. It makes polyunsaturated fatty acids susceptible to lipid peroxidation, leading to irreversible oxidative distress damage to biofilms or genetic material and resulting in cell death eventually (Guichardant et al., 2011; Hadian and Stockwell, 2020).
FIGURE 2.
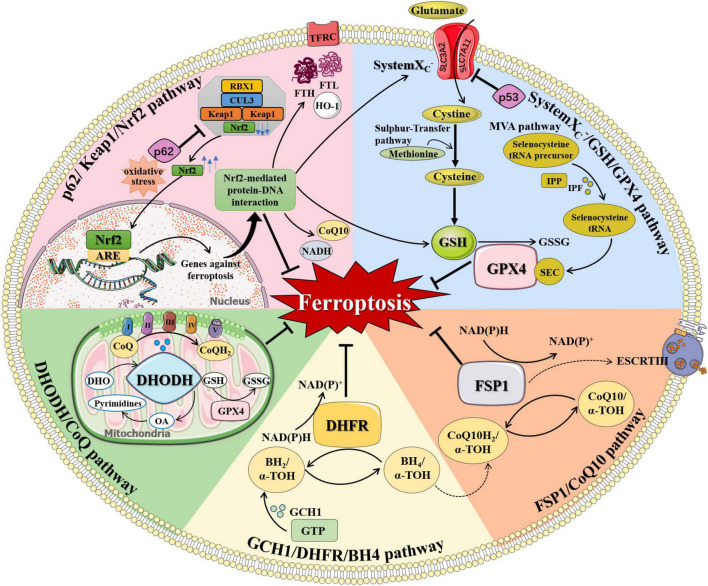
The regulation of ferroptosis. Multiple pathways inhibit ferroptosis by resisting oxidative distress and inhibiting lipid peroxidation, including systemXC–/GSH/GPX4 pathway, FSP1/CoQ10 pathway, GCH1/DHFR/BH4 pathway, DHODH/CoQ pathway, and p62/Keap1/Nrf2 pathway. (1) systemXC–/GSH/GPX4 pathway: systemXC– consists of SLC7A11 and SLC3A2 and is capable to exchange glutamate and cystine equally. Cystine is obtained from methionine through the Sulfur-transfer pathway and then converted to cysteine intracellularly to generate GSH. Selenoprotein GPX4 detects GSH, leading to the reduction of lipid hydroperoxides to lipid alcohols and the simultaneous oxidization of two GSH into oxidized GSSG. IPP, produced by the MVA pathway, transfers isopentyl groups to isopentyl transferases-catalyzed Sec-tRNA precursors which mediate the maturation of Sec-tRNAs responsible for Sec insertion into GPX4. (2) FSP1/CoQ10 pathway: FSP1 converts CoQ10 to CoQ10H2 using NAD(P)H, which quenches lipid free radicals generated by lipid peroxidation. Membrane-associated protein complex ESCRT-III regulates membrane regeneration through membrane germination and cleavage. FSP1 transported in the plasma membrane can resist ferroptosis by enrolling ESCRT-III to activate the membrane restore mechanism. (3) GCH1/DHFR/BH4 pathway: BH4 is a potent free radical scavenger that exerts antioxidant effects in cells. The interconversion between the oxidation and reduction forms of BH4 is controlled by two enzymes, GCH1 and DHFR. The synthesis of BH4 by GCH1 expression selectively prevents PUFA-PL depletion-induced membrane lipid remodeling. DHFR is an essential enzyme for BH4 regeneration, and its inhibition may synergize with GPX4 inhibitors to induce ferroptosis. BH4 can also quench ROS by promoting the synthesis of CoQ10. (4) DHODH/CoQ pathway: DHODH is an enzyme present on the inner surface of mitochondria that catalyzes substrates DHO to produce OA. DHODH cooperates with mitochondrial GPX4 to regulate mitochondrial ferroptosis by reducing CoQ to CoQH2 on the mitochondrial intima independently of the cytoplasmic GPX4 or FSP1 pathways. (5) p62/Keap1/Nrf2 pathway: Under oxidative distress conditions, p62 prevents the degradation of Nrf2 in the Keap1-CUL3-RBX1 E3 ubiquitin ligase complex. Nrf2 undergoes nuclear translocation and binds to ARE to initiate transcription of multiple cytoprotective genes against ferroptosis. Nrf2-mediated protein-DNA interaction regulates the expression of FTH, FTL, FPN, TfR, HO-1, and so on for controlling cellular iron metabolism, promoting SLC7A11 expression, and increasing the production of NADPH, GSH, and CoQ10 to enhance the antioxidant capacity of cells. α-TOH, α-tocopherol; ARE, antioxidant response element; BH2, dihydrobiopterin; BH4, tetrahydrobiopterin; CoQ, coenzyme Q; CoQ10, coenzyme Q10; CoQH2, reduced coenzyme Q; CUL3, cullin 3; DHFR, dihydrofolate reductase; DHO, dihydroorotate acid; DHODH, dihydroorotate dehydrogenase; ESCRT-III, endosome sorting complex; FTH, ferritin heavy chain; FTL, ferritin light chain; FSP1, ferroptosis suppressor protein 1; GCH1, GTP cyclohydrolase-1; GPX4, glutathione peroxidase 4; GSH, glutathione; GSSG, oxidized glutathione; GTP, guanosine triphosphate; HO-1, heme oxygenase 1; IPF, isopentenyl transferase; IPP, isopentenyl pyrophosphate; Keap1, KELCH-ECH-associated protein 1; MVA pathway, mevalonate Regulation pathway; NADH, reduced nicotinamide adenine dinucleotide; NAD(P)+, oxidized nicotinamide adenine dinucleotide (phosphate); NAD(P)H, reduced nicotinamide adenine dinucleotide (phosphate); Nrf2, nuclear factor 2-related erythroid factor 2; OA, orotate; RBX1, RING-box protein 1; Sec, selenocysteine; SLC3A2, solute carrier family 3 member 2; SLC7A11, solute carrier family 7 member 11; TFRC, transferrin receptor.
SystemXC–/GSH/GPX4 Pathway
Among anti-ferroptosis defense pathways, the systemXC–/GSH/GPX4 pathway related to amino acid metabolism is widely studied. SystemXC– consists of two subunits, solute carrier family 7 member 11 (SLC7A11) and solute carrier family 3 member 2 (SLC3A2). SystemXC– is widely distributed in the phospholipid bilayer and play an central role in the exchange of glutamate and cystine. After entering the cell, cystine converts to cysteine, which can also be obtained from methionine through the Sulfur-transfer pathway (Zheng and Conrad, 2020). Afterward, glutathione (GSH), an important free radical scavenger in organisms, is generated from cysteine, glutamate, and glycine under the action of glutathione synthase (Lin et al., 2020; Chen L. et al., 2021). Cysteine provides sulfhydryl, an active group with antioxidant effect for GSH. Glutathione peroxidase 4 (GPX4) is a selenoprotein whose main cofactor is GSH, which reduces lipid hydroperoxides to lipid alcohols and simultaneously oxidizes two GSH to oxidized glutathione (GSSG) (Seibt et al., 2019).
The systemXC–/GSH/GPX4 pathway plays an important role in the downregulation of ferroptosis. Increasing extracellular glutamate concentration or decreasing cystine uptake inhibits systemXC– to promote ferroptosis (Hambright et al., 2017). The classic ferroptosis inducers such as erastin and sulfadiazine block the absorption of GSH by inhibiting the systemXC– (Dixon and Stockwell, 2014). p53 is a tumor suppressor protein that inhibits cystine cellular uptake by decreasing the expression of SLC7A11, resulting in reduced GPX4 activity and elevated susceptibility to ferroptosis (Jiang et al., 2015). Glutaminase 2 (GLS2), an enzyme necessary for glutamine hydrolysis is considered as an important target protein of p53. The GLS2 inhibitor compound 968 and artificial oocyte activation (AOA) inhibit erastin-induced ferroptosis (Gao et al., 2015; Jennis et al., 2016).
GPX4 is the first major regulator of ferroptosis. The compounds including RSL3, DP17, and DP110 can inhibit GPX4 directly, resulting in the accumulation of lipid peroxides and ROS for ferroptosis (Yang et al., 2014; Yang and Stockwell, 2016). The activity of GPX4 is also associated with the Mevalonate Regulation pathway (MVA pathway) (Dixon et al., 2014). Selenocysteine (Sec) is the active central amino acid of GPX4, and the Sec-tRNA is responsible for embedding Sec into GPX4. Isopentenyl pyrophosphate (IPP) produced by the MVA pathway transfers the isopentyl group to the Sec-tRNA precursor catalyzed by isopentenyl transferase, mediating the maturation of that specific tRNA (Wirth et al., 2014). The use of statins downregulates the MVA pathway, reduces IPP production, and disrupts Sec-tRNA maturation, resulting in deficiency of selenoprotein and cellular antioxidant capacity, ultimately, promoting ferroptosis (Watanabe et al., 2021).
GCH1/DHFR/BH4 Pathway
The GCH1/DHFR/BH4 pathway, discovered by Kraft and Soula in 2018, is a unique anti-ferroptosis mechanism independent of GPX4. Tetrahydrobiopterin (BH4) is a potent free radical scavenger that exerts antioxidant effects in cells through two enzymes, GTP cyclohydrolase-1 (GCH1) and dihydrofolate reductase (DHFR). Two enzymes are responsible for the interconversion between the oxidation and reduction forms of BH4. The synthesis of BH4 by GCH1 expression selectively prevents PUFA-PL depletion-induced membrane lipid remodeling (Kraft et al., 2020). DHFR is an essential enzyme for BH4 regeneration, and its inhibition may synergize with GPX4 inhibitors to induce ferroptosis (Soula et al., 2020). In addition, BH4 can also quench ROS by promoting the synthesis of Coenzyme Q10 (CoQ10) (Kraft et al., 2020). CoQ10/CoQ10H2 is an antioxidant that scavenges free radicals on cell membranes and maintains the fluidity and integrity of cell membranes.
FSP1/CoQ10 Pathway
The FSP1/CoQ10 pathway was discovered by Bersuker et al. (2019) and Doll et al. (2019). Ferroptosis suppressor protein 1 (FSP1) converts CoQ10 to CoQ10H2 using NAD(P)H, which quenches lipid free radicals generated by lipid peroxidation. FSP1 inhibits lipid peroxidation and ferroptosis independently of systemXC–/GSH/GPX4 pathway and still has a protective effect after GPX4 knockout (Stockwell, 2019). Homologous murine double minute 2 homolog (MDM2) and murine double minute X (MDMX) proteins are the two negative regulators of p53. Inhibition of MDM2 or MDMX increases the levels of FSP1 and CoQ10, leading to inhibition of ferroptosis in a p53-independent manner (Venkatesh et al., 2020). In addition, the endosomal sorting complex required for transport III (ESCRT-III) is a membrane-associated protein complex that regulates membrane regeneration through membrane germination and cleavage. FSP1 transported in the plasma membrane can resist ferroptosis by enrolling ESCRT-III to activate the membrane restore mechanism (Dai et al., 2020b). Activation of ferroptosis inducers such as sorafenib by regulating FSP1-ESCRT-III has been emerged as a novel anti-tumor strategy (Dai et al., 2020a).
DHODH/CoQ Pathway
In 2021, Mao et al. discovered a mitochondrial anti-ferroptosis defense mechanism mediated by dihydroorotate dehydrogenase (DHODH), which inhibits ferroptosis by reducing CoQ to CoQH2 on the IMM (Mao et al., 2021). DHODH is an enzyme present on the inner surface of mitochondria, whose substrates are dihydroorotate acid (DHO) and its reaction product is orotate (OA). DHO and OA have opposite effects on GPX4 inhibitor-induced ferroptosis. DHO exerts a protective role, while OA just the reverse. DHODH loss-of-function leads to extensive lipid peroxidation in mitochondria and ferroptosis in cancer cells with low GPX4 levels. Taken together, DHODH regulates mitochondrial ferroptosis independently of the cytoplasmic GPX4 or FSP1 pathways, while cooperates with mitochondrial GPX4.
p62/Keap1/Nrf2 Pathway
Nuclear factor 2-related erythroid factor 2 (Nrf2) is a major antioxidant element against oxidative distress in cells and a key transcriptional regulator of ferroptosis. Nrf2 is normally degraded by specific proteasome ubiquitination mediated by the Keap1-CUL3-RBX1 E3 ubiquitin ligase complex. The double-glycine repeat (DGR) domains of the Keap1 homodimer bind with the DLG and ETGE domains in Nrf2 (Bellezza et al., 2018). p62 direct interacts with Keap1, which inhibits the activity of Keap1 to bind Nrf2 (Komatsu et al., 2010). The release of DLG motif in Nrf2 from Keap1 blocks Nrf2 ubiquitination and degradation, therefore increasing the antioxidant capacity of cells (Bellezza et al., 2018). Activation of the p62/Nrf2/Keap1 pathway increases the antioxidant capacity of cells and inhibits the occurrence of ferroptosis (Sun et al., 2016). When stimulated by oxidative distress, Nrf2 undergoes nuclear translocation and binds to antioxidant response element (ARE) to initiate transcription of a variety of cytoprotective genes (Matsumaru and Motohashi, 2021). For example, Nrf2 regulates the expression of FTH and FTL for iron storage, FPN and TFRC for iron transport, and HO-1 for intracellular iron production, therefore controlling cellular iron metabolism (Zhao et al., 2021). Nrf2 can promote the expression of SLC7A11, glutathione synthase (GSS), and glucose-6-phosphate dehydrogenase (G6PD), which increases the production of NADPH, GSH, and CoQ10 to enhance the antioxidant capacity of cells (Shin et al., 2017; Carpi-Santos and Calaza, 2018; Dodson et al., 2019; Zheng and Conrad, 2020). Besides, Nrf2 regulates oxidative distress-induced lipid accumulation by regulating autophagy proteins including ATG5 and lipogenesis proteins such as Sterol-regulatory element binding proteins (SREBPs) (Bai et al., 2019; Sun et al., 2020).
Emerging Links of Ferroptosis to Neurodegenerative Diseases
NDDs are a group of diseases characterized by protein intra- and extracellular deposition and gradual loss of a specific population of neurons, with progressive motor and cognitive decline (Baloni et al., 2021). There is growing evidence that ferroptosis and NDDs are inextricably linked. Currently, studies on ferroptosis in NDDs mainly focus on AD, PD, HD, and ALS. Although different NDDs have different mechanisms in disease evolution, ferroptosis is proven to be involved in all of them and characterized by altered brain iron homeostasis, dysregulation of antioxidant system and oxidative damage (Table 2).
TABLE 2.
Pathological changes of NDDs associated with pathological features of ferroptosis.
| Diseases | Ferroptosis-related Pathological Features | Potential pathological outcome | References |
| AD | Abnormal iron metabolism and iron deposition | Accumulation and aggregation of Aβ and Tau proteins | Ayton et al., 2020; Derry et al., 2020 |
| Abnormal increase in APP level and tau hyperphosphorylation and aggregation; | Altamura and Muckenthaler, 2009; McIntosh et al., 2019; Derry et al., 2020 | ||
| Microglial activation and neuroinflammation | van Duijn et al., 2017; McIntosh et al., 2019 | ||
| Neuronal death and NFT deterioration | Wan et al., 2019 | ||
| Redox imbalance and oxidative stress | Abnormal increase of Aβ oligomer and neuroinflammation | Saito et al., 2019 | |
| Decreased GPX4 protein levels and elevated lipid peroxidation products | Hippocampal neurodegeneration | Hambright et al., 2017 | |
| PD | Oxidative distress and lipid peroxidation | Degeneration and loss of dopaminergic neurons | Dias et al., 2013; Hare and Double, 2016; Dionísio et al., 2021 |
| Abnormal iron metabolism and lipid peroxidation | α-synuclein aggregation | Angelova et al., 2020; Riederer et al., 2021 | |
| Increased iron in SN | Aggregation and dopaminergic neuron death | Wang et al., 2009; Zhu et al., 2017; Zhang Y. et al., 2020 | |
| Decreased GPX4 in SN | Dopaminergic neuron axon damage | Bellinger et al., 2011 | |
| Decrease FPN expression | Microglia proinflammatory transformation | Zhang et al., 2014 | |
| Iron homeostasis and iron deposition | Microglia activation and dopaminergic cell death | Wang Z. L. et al., 2022 | |
| Down-regulation of the SLC7A11 gene and decreased SLC7A11 protein level | Neuroinflammation | Vallerga et al., 2020 | |
| Elevated lipid peroxidation products and decreased GSH protein level | Increased Lewy bodies | Jenner et al., 1992 | |
| DJ-1 gene mutation | Decreased GPX4 and GSH activity | Cao et al., 2020 | |
| HD | Iron overload and increased lipid peroxidation | Mitochondrial dysfunction and oxidative distress damage | Agrawal et al., 2018 |
| Decreased GPX4 activity | HTTP gene variants | Mason et al., 2013 | |
| Recurrent glutamate abnormalities | Mitochondrial dysfunction | Reddy and Shirendeb, 2012 | |
| ALS | GPX4 depletion | Loss of motor neurons | Evans et al., 2022 |
| Changes in iron metabolizing proteins and elevated iron levels | SOD1 gene mutations | Jeong et al., 2009 | |
| GSH/GPX4 depletion and increased lipid peroxidation | SOD1 gene mutations | Wang et al., 2021; Peng et al., 2022 | |
| MS | Decreased GSH protein level and systemXc– activity | Microglial activation and neuroinflammation | Hu et al., 2019 |
| Glutathione deficiency and lipid peroxidation | Oligodendrocyte loss and demyelination | Jhelum et al., 2020 |
Ferroptosis and Alzheimer’s Disease
Alzheimer’s disease is the most common NDD manifested as progressive decline in cognitive function, including impairment of multiple cognitive domains such as memory, executive function, and language (Tiwari et al., 2019). The typical pathologic features of AD are the presence of senile plaques (SP) formed by extracellular amyloid (Aβ) deposition and intracellular neurofibrillary tangles (NFTs) formed by hyperphosphorylated tau protein, ultimately leading to neuronal dysfunction and synapse loss (Lane et al., 2018). Multiple theories have been proposed regarding the mechanism of AD progression, which include neuroinflammation, Aβ overproduction/aggregation, tau hyperphosphorylation, neurotransmitter disorders, and mitochondrial dysfunction (Xia et al., 2021). Aging, genetic factors (e.g., altered APOE gene), environmental factors, and vascular diseases are considered as risk factors for AD (Tang et al., 2022). However, this does not appear to be fully sufficient to explain the pathogenesis of AD.
In recent years, the theory of the metal homeostasis mechanism represented by ferroptosis has been supported by increasing amounts of evidence (Hambright et al., 2017; Zhang et al., 2021). Human brain tissue from AD patients exhibits abnormal iron metabolism and glutamate levels, damaged systemXC–, and induced apparent lipid peroxidation (Hambright et al., 2017; Ayton et al., 2020). Iron deposition occurs in the hippocampus and the inferior temporal cortex of AD patients, and is strongly associated with loss of memory and cognitive decline (Ayton et al., 2020; You et al., 2021). Quantification of iron deposition using susceptibility-weighted imaging (SWI) indicates iron deposition in eight brain regions including prefrontal, parietal, temporal, amygdala, putamen, globus pallidus, cingulate cortex, and caudate nucleus in advanced AD patients (Ashraf et al., 2020). Being the only exit for iron excretion through cells, FPN1 has been found to be downregulated in the brains of AD patients and AD animal models to induce intracellular iron accumulation (Raha et al., 2013; Bao et al., 2021). Furthermore, they found that differentially expressed genes (DEGs) in ferroptosis were highly enriched in AD-related gene concentrations through Gene Set Enrichment Analysis (GSEA), which was consistent with the conclusion of Majerníková et al. (2021) after analysis of four datasets of AD DEGs. These literatures prompt a strong association of ferroptosis with the pathogenesis of AD.
Abnormal iron metabolism can result in post-translational production of inappropriately modified Aβ, leading to an abnormal increase in Aβ oligomers (Derry et al., 2020). Iron deposition promotes the expression and abnormal degradation of amyloid precursor protein (APP) through the intracellular IRE/IRP regulatory system, a control element for cellular iron homeostasis (Altamura and Muckenthaler, 2009; McIntosh et al., 2019; Derry et al., 2020). Abnormal elevation of divalent iron in AD brain induces tau hyperphosphorylation and aggregation, leading to the formation of NFTs (Wan et al., 2019). Amyloid plaques and NFTs therefore mediate ferroptosis-mediated neuronal death and AD-related cytotoxicity. Microglial activation and neuroinflammation are also typical changes during the pathogenesis of AD. Hippocampal microglia and astrocytes of AD patients contain high levels of ferritin (Zeineh et al., 2015). Iron accumulation induces the phenotype transition of microglia to a pro-inflammatory one and the reprogramming of cellular metabolism that reduces the Aβ clearance capacity of microglia (van Duijn et al., 2017; McIntosh et al., 2019).
Studies of ferroptosis suggest potential biomarkers for early prediction of AD. As mentioned above, abnormal iron metabolism has emerged as an important pathological characteristic. Quantitative susceptibility mapping (QSM) was used to observe the spatial co-localization of brain iron deposition and Aβ plaques in pre-AD patients, which demonstrated Aβ accumulation along with iron deposition (van Bergen et al., 2016b). Therefore, the imaging evidence of iron deposition has been proposed as an important diagnostic index of AD (Tao et al., 2014; Ayton et al., 2020). APOEε4, a risk factor for the development of AD, increases carrier susceptibility by increasing ferritin levels (Ayton et al., 2015). Cerebrospinal fluid (CSF) ferritin levels are positively associated with the risk of cognitive decline in AD patients with APOEε4 genotype, hence researchers also recommend CSF ferritin levels as a biomarker for AD (Ayton et al., 2018; Diouf et al., 2019).
Inspiringly, several studies have shown favorable therapeutic effects of ferroptosis inhibitors on AD animals (Zhang et al., 2018; Komaki et al., 2019; Rao et al., 2020; Farr and Xiong, 2021). The iron chelators DFO and DFP are widely used as specific inhibitors of ferroptosis. Preclinical evidence suggests that iron chelators modulate iron homeostasis, mitigate oxidative distress, improve cognition and behavioral outcomes in AD mice (Rao et al., 2020; Farr and Xiong, 2021). CoQ10 has been shown to inhibit ferroptosis in AD mice by inhibiting lipid peroxidation. After pretreatment of AD mice with CoQ10, MDA was decreased and oxidative distress was alleviated significantly, suggesting that CoQ10 exerts neuroprotective effects on Aβ-induced nerve damage (Komaki et al., 2019). The antioxidant alpha-lipoic acid (ALA) significantly inhibited tau-induced iron overload and reduced NFTs formation by enhancing GPX4 expression (Zhang et al., 2018). Similarly, Forsythoside A, the main component of Forsythia suspensa (Thunb.) Vahl, and Ginkgolide B, a terpene lactone derivative of Ginkgo biloba, also exhibited anti-AD properties via inhibiting ferroptosis-mediated neuroinflammation by activating Nrf2/GPX4 axis (Shao et al., 2021; Wang C. et al., 2022). Vitamin E can neutralize peroxidative free radicals, terminate lipid peroxidation, and reduce the risk of cognitive decline following high vitamin E supplementation in AD patients (Basambombo et al., 2017). CMS121, a lipoxygenase inhibitor, reduces the relative levels of fatty acids and PUFAs in AD mice and improves cognitive dysfunction by inhibiting lipid peroxidation (Everett et al., 2020). However, although ferroptosis inhibitors have exhibited promising therapeutic effects in AD animal models, there are still in lack of clinical evidence to support these findings, which is urgently needed to confirm ferroptosis as an important therapeutic target for AD.
Ferroptosis and Parkinson’s Disease
Parkinson’s disease is the second most common NDD. Clinical symptoms of PD include motor symptoms (e.g., resting tremor, muscle rigidity, bradykinesia, and postural disorders) and non-motor symptoms (e.g., sleep disorders, depression, and cognitive dysfunction) (Parkinson, 2002). The most outstanding character of PD is the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), resulting in striatal dopamine depletion, neuromelanin loss, and the appearance of α-synuclein (α-syn)-rich Lewy bodies in neurons (Hayes, 2019).
Numerous studies have implicated the increase of ferroptosis-associated oxidative distress and lipid peroxidative damage as important features of PD pathophysiology (Dionísio et al., 2021; Mahoney-Sánchez et al., 2021; Riederer et al., 2021). The SNpc and brainstem of PD patients have obvious iron deposition, and the iron concentration in the substantia nigra (SN) is related to the severity of the disease (Wieler et al., 2015). Animal studies also identified SN as the most susceptible brain region for iron accumulation during aging (Jia et al., 2018). The magnitude of elevated iron levels significantly correlated with the magnitude of α-syn aggregation, iron deposition, and dopaminergic (DA) neuron death in early-stage PD patients and PD animal models (Wang et al., 2009; Zhu et al., 2017; Zhang Y. et al., 2020). The studies of possible mechanisms revealed that iron combines with DA to form a potent oxide, which simultaneously produces dopamine quinine and 6-hydroxydopamine (6-OHDA). 6-OHDA can release iron from ferritin while exerting a new round of oxidative distress and neurotoxicity damage (Dias et al., 2013; Hare and Double, 2016). Meanwhile, high levels of iron in dopaminergic neurons exacerbate oxidative distress due to Fenton’s reaction (Dias et al., 2013; Hare and Double, 2016). Furthermore, total GPX4 levels are significantly reduced in the substantia nigra of PD patients (Bellinger et al., 2011). SLC7A11 was also hypermethylated in PD, severely affecting systemXC– activity (Vallerga et al., 2020). Hence, the abnormal activities of systemXC–/GSH/GPX4 pathway in the SN have been proposed as an early event in PD pathogenesis (Jenner et al., 1992). In recent years, α-syn oligomers have been reported to induce ferroptosis by interacting with cell membranes and promoting lipid peroxidation (Angelova et al., 2020). In addition, an in vitro PD model further showed that FPN1 activity in SN microglia is inhibited and iron secretion is blocked, causing pro-inflammatory transformation (Zhang et al., 2014). Activation of glial cells and iron homeostasis-induced iron deposition form a “partners in crime” that alter cellular metabolic state, exacerbate oxidative distress, and induce ferroptosis on DA neurons (Wang Z. L. et al., 2022).
Due to the importance of ferroptosis in the pathogenesis of PD, iron metabolism and systemXC–/GSH/GPX4 pathway have been considered as promising therapeutic targets of PD. Iron chelators such as DFO have been shown to reduce oxidative distress damage and increase dopaminergic neuron activity, thereby improving motor symptoms (Do Van et al., 2016). Human brain imaging studies have shown that DFP decreases brain iron levels, alters cerebrospinal fluid ferritin, and mitigates dyskinesia (Martin-Bastida et al., 2017). Similarly, both the blockage of ferritin degradation by ferritinophagy inhibitors chloroquine and bafilomycin A1 and the application of an iron-free form of ferritin, apoferritin, have been reported to chelate excess iron to protect DA neurons against PD (Tian et al., 2020; Song et al., 2021). Moreover, NADPH oxidase inhibitor apocynin was also found to inhibit iron accumulation and lipid peroxidation, therefore ameliorating dopaminergic neurodegeneration and motor function abnormality in PD mice (Hou et al., 2019). Early-onset autosomal recessive PD is often accompanied by mutations in the DJ-1 gene. Using the antioxidant enzyme DJ-1 as a ferroptosis inhibitor can stabilize the sulfur transmission pathway, ensure the activity of GPX4 and GSH, and reduce susceptibility to ferroptosis (Cao et al., 2020). Elimination of oxidative distress is a novel therapeutic strategy. Diacetylbis (4-methyl-3-thiosemicarbazonato) copperII [CuII(atsm)], a hypoxia-sensitive positron emission tomography imaging agent, has the greatest potential in the treatment of various NDDs (Zilka et al., 2021). In vivo and in vitro PD model experiments confirmed that CuII(atsm)-mediated activation of Nrf2-related antioxidant enzymes improved motor and cognitive functions, protected SN cells against lipid peroxidation, and improved DA metabolism (Hung et al., 2012; Southon et al., 2020). Encouragingly, CuII(atsm) has achieved preliminary positive treatment outcomes in a phase I clinical trial in PD patients (NCT03204929).
Ferroptosis and Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis is a degenerative motor neuron disease featured by the progressive loss of motor neurons in the spinal cord, brainstem, and motor cortex, resulting in progressive muscle weakness and loss of respiratory function, leading to premature death (Vahsen et al., 2021). ALS is currently thought to be associated with gene mutation (e.g., SOD1 gene mutation), glutamate excitotoxicity, oxidative distress, immune disorders, mitochondrial dysfunction, and reduced axonal transport, but the exact etiology and pathogenesis remain unclear (Brown and Al-Chalabi, 2017; Hemerková and Vališ, 2021).
Ferroptosis has been implicated in the pathological process of ALS. Iron deposits were observed in the spinal cord, motor cortex, basal ganglia, and thalamus in ALS patients by MRI. Abnormally elevated iron levels were also found in the cerebrospinal fluid (Ignjatović et al., 2012; Kwan et al., 2012). The development of ALS is associated with increased oxidative damage caused by mutations in the free radical scavenging protein superoxide dismutase 1 (SOD1) gene. Deregulated ferritin and blocked intracellular iron efflux in neurons have been found in SOD1 transgenic mice, thereby increasing intracellular iron load (Jeong et al., 2009). SOD1 mutation also activates hypochlorous acid (HOCl)-myeloperoxidase (MPO) pathway, accelerating ROS accumulation and inhibiting GPX4 expression and thus leading to irreversible lipid peroxidation (Peng et al., 2022). Similar to SOD1 transgenic mice, other ALS animal models including TDP-43 and C9orf72 transgenic mice show GPX4 depletion and dysregulation of glutathione synthesis and iron-binding proteins as well (Wang et al., 2021). The in vivo studies have been corroborated by in vitro ALS model. hTBK1-c.978T > A mutation induces severe cell ferroptosis, which significantly inhibits the proliferation of NSC-34 cells (Zhang P. et al., 2020).
The involvement of ferroptosis in the progression of ALS has been widely examined. Gpx4 neuron inducible knockout (Gpx4NIKO) mice display ALS-like paralytic symptoms and spinal motor neuron death (Evans et al., 2022). The overexpression of GPX4 in ALS mouse models exhibits reduced systemic cytotoxicity of SOD1, late delayed disease-onset, improved motor function, and longer survival, which are associated with faster recovery from spinal motor neuron injury and repressed lipid peroxidation (Chen X. et al., 2021). Recent studies have further shown that human iPS cell-derived motor neuron (hiMN) death is associated with GPX4, which can be rescued by iron chelators and lipid peroxidase inhibitors, suggesting that iron toxicity has an important pathophysiological role in hiMN death (Matsuo et al., 2021). These evidences indicate a great contribution of ferroptosis to motor neuron degeneration in ALS.
The important role of ferroptosis in ALS also provides novel insights into the treatment and prognosis of ALS. The use of high-affinity, lipophilic iron chelator SIH results in improved spinal motor neuron survival and restored motor function (Jeong et al., 2009). Similarly, the treatment of conservative ferroptosis inhibitors such as Edaravone and DFP also exhibits promising neuroprotective effects on ALS (Devos et al., 2020). Edaravone is a clinically approved free radical scavenger for the treatment of ALS that largely inhibits ferroptosis in cystine deficiency and systemXC–/GPX4 inhibition, preventing devastating motor neuron damage (Spasić et al., 2020; Al-Chalabi et al., 2021). In a single-center pilot clinical study, patients treated with DFP (30 mg/kg/day) for 3 months showed improved ALS functional scores, mitigated oxidative distress, and reduced levels of cerebral iron and neurofilament light chain in the CSF. This study is the first to demonstrate the feasibility of iron chelators in the clinical management of ALS (Moreau et al., 2018). Furthermore, higher levels of baseline neurofilament light chain, 4-HNE, 8-oxo’2’-desoxyguanosine, and ferritin have been found to be independently associated with greater ALSFRS-r decline, suggesting the predictive value of these ferroptosis-related biomarkers for ALS diagnosis and prognosis (Devos et al., 2019).
Ferroptosis and Huntington’s Disease
Huntington’s disease is an autosomal dominant NDD, which is featured by involuntary random movements, cognitive decline and personality changes (Wyant et al., 2017). In the first exon of the HTTP gene, the CAG triplet expands and repeats, the HTTP variants predispose to abnormal conformation of the HD protein, severing high levels of toxic molecules in the brain, resulting in disruption of antioxidant gene transcription, and disruption of cellular protein damage processing systems leads to neuronal degeneration and cell death due to oxidative distress (Ross and Tabrizi, 2011).
Neuronal death in HD shows the typical symptoms of ferroptosis, namely recurrent glutamate abnormalities, increased lipid peroxidation, decreased GSH, and persistent iron accumulation (Klepac et al., 2007; Agrawal et al., 2018; Mi et al., 2019). QSM revealed elevated iron levels in the caudate nucleus and putamen of the forebrain in HD patients (van Bergen et al., 2016a). Typical phenotypes of ferroptosis with reduced GSH levels and GPX4 activity, massive iron accumulation in neurons and lipid peroxidation were found in HD animal models (Reddy and Shirendeb, 2012; Mason et al., 2013). In vitro models also demonstrated the importance of ferroptosis in HD pathogenesis as ferrostatin-1 effectively prevented ferroptosis in HTT exon-overexpressing cells (Skouta et al., 2014).
Inhibition of ferroptosis is beneficial in impeding the pathological progression of HD. Mounting studies have demonstrated that CoQ10 supplementation for HD patients can improve mitochondrial function and alleviate membrane lipid peroxidation caused by oxidative distress (Andrich et al., 2004). CoQ10 supplementation in R6/2HD mice had neuroprotective effects on motor performance and prolonged survival of HD mice, by alleviating the reduced glutathione production, lipid peroxidation and oxidative DNA damage (Yang et al., 2009). Besides, targeting iron and activating Nrf2-mediated pathway can be potential therapeutic strategies for HD (Mi et al., 2019). Both the activation of Nrf2 by the cyanoenone triterpenoids CDDO-ethyl amide/CDDO-trifluoroethyl amide and the disruption of Keap1-Nrf2 interaction by KEAP1-modifying small molecule MIND4-17 significantly enhance antioxidant functions of brain cells and ameliorate the behavioral phenotype in HD mouse (Stack et al., 2010; Quinti et al., 2017). Therefore, the above evidences demonstrated a key role of ferroptosis in the pathogenesis of HD, suggesting ferroptosis as an important target for treating and/or preventing HD.
Ferroptosis and Multiple Sclerosis
Multiple sclerosis is a chronic autoimmune disease of the human central nervous system (CNS), characterized by neuroinflammation, demyelination, oligodendrocyte loss, and neurodegeneration (Lucchinetti et al., 1996). Recent progress in understanding the pathogenesis of MS suggests major roles for microglia in the disease, in which microglia alter their transcriptional profile and activate into profuse inflammatory phenotypes (Voet et al., 2019).
Pioneer studies were carried out to unveil the association of ferroptosis with microglia-driven neuroinflammation and MS. Hu et al. (2019) reported reduced GPX4 expression levels in the gray matter of MS patients and in the spinal cord of experimental autoimmune encephalomyelitis (EAE) mice, a classic MS mouse model. Further studies demonstrated that ferroptosis mediated rapid loss of oligodendrocytes and demyelination induced by cuprizone, a widely used copper chelator to induce MS-like pathological phenotype (Jhelum et al., 2020). The inflammatory responses of microglia in vitro can be triggered by ferroptosis and lipopolysaccharides (LPS)-stimulated systemic inflammation in vivo can be partially blocked by inhibiting ferroptosis via Ferrostatin-1 treatment (Cui et al., 2021). Interestingly, multiple publications suggested ferroptosis resistance of microglia. Pro-inflammatory microglia display higher ferroptosis resistance than alternatively activated anti-inflammatory ones highly likely due to their abundant expression of NRF2 and enrichment of iNOS/NO⋅ (Jhelum et al., 2020; Kapralov et al., 2020). Pro-inflammatory microglia, but not anti-inflammatory ones, promote distant suppression of ferroptosis, implying anti-ferroptotic effects of neuroinflammation. Therefore, the exact roles of ferroptosis in MS remain unclear.
Besides, literatures have implied the involvement of ferroptosis in the pathogenesis of other NDDs. For example, ferroptosis has been found in light-induced retinal degeneration and Ferrostatin-1 has been reported to protect retina against degeneration via elevating the expression of SLC7A11 and GPX4 protein expression and suppress neuroinflammation (Tang et al., 2021). However, the pathological roles of ferroptosis in these unmentioned NDDs remain vague, which requires further investigations to clarify.
Hence, ferroptosis has been emerged as a key contributor to the pathogenesis of various NDDs. Given that, ferroptotic factors and ferroptosis-related signaling pathways have now been considered as potential diagnostic/prognostic biomarkers and therapeutic targets of NDDs. It is also worth-noting that ferroptosis has been reported to participate in neuroinflammation and neuronal death post-acute brain damage, which may play a role in the pathogenesis of NDDs as well (Mao et al., 2020; Cao et al., 2021; Cui et al., 2021; Ge et al., 2022; Qu et al., 2022).
Conclusion
In summary, NDDs are a group of pathologically and clinically heterogeneous diseases that create a heavy burden on healthcare systems, families, and individuals. However, the pathogenesis of NDDs has not been fully elucidated. To date, sufficient evidence has demonstrated that ferroptosis is closely related to the occurrence and progression of NDDs. The underlying mechanisms involve altered activities of Gpx4- and Nrf2-mediated signaling pathways, the deregulation of iron metabolism, oxidative distress, and glutamate-mediated excitotoxicity. In the future, more comprehensive investigations will greatly expand our understanding on the roles of ferroptosis in the pathogenesis of NDDs and shed light on the development of novel therapeutic technologies and strategies for treating incurable NDDs.
Author Contributions
JL, JH, and JZ conceived the manuscript. YS and JL collected the references. YS, XX, DB, JL, and JH wrote the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Qihui Wu for proofreading the manuscript.
Glossary
- 4-HNE
4-hydroxynonenal
- 5-FU
5-fluorouracil
- 6-OHDA
6-hydroxydopamine
- AA
arachidonoyl
- ACSL4
long-chain-fatty-acid-CoA 4
- AD
Alzheimer’s disease
- ADA
adrenoyl
- ALA
alpha-lipoic acid
- ALS
amyotrophic lateral sclerosis
- AKT
threonine protein kinase
- AMPK
activated protein kinase
- APP
amyloid precursor protein
- ARE
antioxidant response element
- ATG
autophagy-related
- ATP
adenosine triphosphate
- Aβ
amyloid β
- Bax
Bcl2-associated X.
- Bcl-2
B-cell lymphoma-2
- BH2
dihydrobiopterin
- BH4
tetrahydrobiopterin
- CDDO
2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid
- c-IAP 1/2
cellular inhibitor of apoptosis proteins ½
- CIL56
caspase-independent lethal 56
- CNS
central nervous system
- CoA
coenzyme A
- CoQ10
coenzyme Q10
- CoQH2
reduced coenzyme Q
- CSF
cerebrospinal fluid
- CuII(atsm)
diacetylbis (4-methyl-3-thiosemicarbazonato) copperII
- CUL3
cullin 3
- DA
dopaminergic
- DAMPs
damage-associated molecular patterns
- DCYTB
duodenal cytochrome b
- DFO
deferoxamine
- DEGs
differentially expressed genes
- DFP
deferiprone
- DGR
double-glycine repeat
- DHFR
dihydrofolate reductase
- DHO
dihydroorotate acid
- DHODH
dihydroorotate dehydrogenase
- DMT1
divalent metal transporters 1
- DNA
deoxyribonucleic acid
- Ecto-CRT
surface-exposed calreticulin
- EAE
experimental autoimmune encephalomyelitis
- ERK
extracellular signal-regulated kinase
- ESCRT-III
endosomal sorting complex required for transport III
- FASL
factor associated suicide ligend
- Fe2+
ferrous iron
- Fe3+
ferric iron
- Fe–S
iron–sulfur clusters
- FIN56
ferroptosis inducing 56
- FINO2
1,2-dioxolane
- FPN
ferroportin
- FSP1
ferroptosis suppressor protein 1
- FTH
ferritin heavy chain
- FTL
ferritin light chain
- FtMt
mitochondrial ferritin
- G6PD
glucose-6-phosphate dehydrogenase
- GCH1
GTP cyclohydrolase-1
- GLS2
glutaminase 2
- GM/CSF
granulocyte-macrophage colony-stimulating factor
- GPX4
glutathione peroxidase 4
- Gpx4NIKO
Gpx4 neuron inducible knockout
- GSDMD
gasdermin D
- GSEA
gene set enrichment analysis
- GSH
glutathione
- GSS
glutathione synthase
- GSSG
oxidized glutathione
- H2O2
hydrogen peroxide
- HD
Huntington’s disease
- HMGB1
high mobility group box 1 protein
- HO-1
heme oxygenase 1
- HOCl
hypochlorous acid
- IAPs
the inhibitor of apoptosis proteins
- IL-2/3/4
interleukin-2/3/4
- ILP-2
inhibitor of apoptosis protein-like protein-2
- IMM
inner mitochondrial membrane
- IPF
isopentenyl transferase
- IPP
isopentenyl pyrophosphate
- IRE
iron-responsive element
- IREB2
iron responsive element binding protein 2
- IRP
iron-regulatory protein
- Keap1
KELCH-ECH-associated protein 1
- LIP
labile iron pool
- LOOHs
lipid hydroperoxides
- LOX
lipoxygenase
- LPCAT3
lysophosphatidylcholine acyltransferase 3
- LPS
lipopolysaccharides
- MAPK
mitogen-activated protein kinase
- MDA
malondialdehyde
- MDM2
murine double minute 2 homolog
- MDMX
murine double minute X
- MLKL
mixed lineage kinase domain-like protein
- MPO
myeloperoxidase
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- mTOR
mammalian target of rapamycin
- MVA pathway
Mevalonate Regulation Pathway
- NAD(P)+
oxidized nicotinamide adenine dinucleotide (phosphate)
- NAD(P)H
reduced nicotinamide adenine dinucleotide (phosphate)
- NADH
reduced nicotinamide adenine dinucleotide
- NDDs
neurodegenerative diseases
- NFTs
neurofibrillary tangles
- NLRP3
NLR family pyrin domain-containing protein 3
- Nrf2
nuclear factor 2-related erythroid factor 2
- NSA
necrosulfonamide
- NTBI
non-transferrin bound iron
- OA
orotate
- OH⋅
hydroxyl radical
- PARP-1
Poly (ADP-ribose) polymerase 1
- PCBP1
poly(rC)-binding protein 1
- PD
Parkinson’s disease
- PE
phosphatidylethanolamines
- PE-AA
phosphatidylethanolamines-arachidonoyl
- PE-AA-OOH
hydroperoxides of phosphatidylethanolamines-arachidonoyl
- PE-ADA
phosphatidylethanolamines-adrenoyl
- PE-ADA
phosphatidylethanolamines-adrenoyl
- PE-ADA-OOH
hydroperoxides of phosphatidylethanolamines- adrenoyl
- PEBP1
phosphatidylethanolamine-binding protein 1
- PI3K
phosphoinositide 3-kinase
- PUFAs
polyunsaturated fatty acids
- PUFA-PL
polyunsaturated acyl-tailed phospholipids
- RCD
regulated cell death
- RIP-1
receptor interacting protein-1
- RIP-3
receptor interacting protein-1
- ROS
reactive oxygen species
- RSL3
RAS-selective lethal 3
- SAS
sulfasalazine
- SCARA5
scavenger receptor class A member 5
- Sec
selenocysteine
- SLC3A2
solute carrier family 3 member 2
- SLC7A11
solute carrier family 7 member 11
- SM-164
Smac mimetics-164
- SOD1
superoxide dismutase
- SN
substantia nigra
- SP
senile plaques
- SREBPs
sterol-regulatory element-binding proteins
- STEAP3
six-transmembrane epithelial antigen of prostrate 3
- SWI
susceptibility-weighted imaging
- TBI
transferrin-bound iron
- TCA cycle
tricarboxylic acid cycle
- Tf
transferrin
- TfR
transferrin receptor 1
- TGF-β
transforming growth factor beta
- TRAIL
tumor necrosis factor- related apoptosis-inducing ligand
- tRNA
transfer ribonucleic acid
- UTR
untranslated region
- VTPA
virus-spike tumor-activatable pyroptotic agent
- XIAP
X-linked inhibitor of apoptosis
- ZIP14
Zrt/Irt-related protein 14
- ZIP8
Zrt/Irt-related protein 8
- ZnO-NPs
zinc oxide nanoparticles
- ZnPPIX
zinc protoporphyrin IX
- α-Syn
α-synuclein
- α-TOH
α-tocopherol
- Δψm
mitochondrion membrane potential.
Funding
This work was supported in part by research grants from the National Natural Science Foundation of China (Nos. 82171194 and 81974155 to JL, Nos. 91949204 and 81830037 to JZ, Nos. 81971145 and 81901333 to XX) and by Biomedical Technology Support Project of Shanghai Science and Technology Commission (No. 22S31902600).
References
- Agrawal S., Fox J., Thyagarajan B., Fox J. H. (2018). Brain mitochondrial iron accumulates in Huntington’s disease, mediates mitochondrial dysfunction, and can be removed pharmacologically. Free Radic. Biol. Med. 120 317–329. 10.1016/j.freeradbiomed.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Chalabi A., Chiò A., Merrill C., Oster G., Bornheimer R., Agnese W., et al. (2021). Clinical staging in amyotrophic lateral sclerosis: analysis of Edaravone Study 19. J. Neurol. Neurosurg. Psychiatry 92 165–171. 10.1136/jnnp-2020-323271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura S., Muckenthaler M. U. (2009). Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis. J. Alzheimers Dis. 16 879–895. 10.3233/jad-2009-1010 [DOI] [PubMed] [Google Scholar]
- Andrich J., Saft C., Gerlach M., Schneider B., Arz A., Kuhn W., et al. (2004). Coenzyme Q10 serum levels in Huntington’s disease. J. Neural Transm. Suppl. 68 111–116. 10.1007/978-3-7091-0579-5_13 [DOI] [PubMed] [Google Scholar]
- Angelova P. R., Choi M. L., Berezhnov A. V., Horrocks M. H., Hughes C. D., De S., et al. (2020). Alpha synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 27 2781–2796. 10.1038/s41418-020-0542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonymuthu T. S., Tyurina Y. Y., Sun W. Y., Mikulska-Ruminska K., Shrivastava I. H., Tyurin V. A., et al. (2021). Resolving the paradox of ferroptotic cell death: Ferrostatin-1 binds to 15LOX/PEBP1 complex, suppresses generation of peroxidized ETE-PE, and protects against ferroptosis. Redox Biol. 38:101744. 10.1016/j.redox.2020.101744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf A., Jeandriens J., Parkes H. G., So P. W. (2020). Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer’s disease: evidence of ferroptosis. Redox Biol. 32:101494. 10.1016/j.redox.2020.101494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S., Diouf I., Bush A. I. (2018). Evidence that iron accelerates Alzheimer’s pathology: a CSF biomarker study. J. Neurol. Neurosurg. Psychiatry 89 456–460. 10.1136/jnnp-2017-316551 [DOI] [PubMed] [Google Scholar]
- Ayton S., Faux N. G., Bush A. I. (2015). Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat. Commun. 6:6760. 10.1038/ncomms7760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S., Wang Y., Diouf I., Schneider J. A., Brockman J., Morris M. C., et al. (2020). Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol. Psychiatry 25 2932–2941. 10.1038/s41380-019-0375-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Meng L., Han L., Jia Y., Zhao Y., Gao H., et al. (2019). Lipid storage and lipophagy regulates ferroptosis. Biochem. Biophys. Res. Commun. 508 997–1003. 10.1016/j.bbrc.2018.12.039 [DOI] [PubMed] [Google Scholar]
- Baloni P., Funk C. C., Readhead B., Price N. D. (2021). Systems modeling of metabolic dysregulation in neurodegenerative diseases. Curr. Opin. Pharmacol. 60 59–65. 10.1016/j.coph.2021.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W. D., Pang P., Zhou X. T., Hu F., Xiong W., Chen K., et al. (2021). Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 28 1548–1562. 10.1038/s41418-020-00685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basambombo L. L., Carmichael P. H., Cote S., Laurin D. (2017). Use of vitamin E and C supplements for the prevention of cognitive decline. Ann. Pharmacother. 51 118–124. 10.1177/1060028016673072 [DOI] [PubMed] [Google Scholar]
- Bellezza I., Giambanco I., Minelli A., Donato R. (2018). Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 1865 721–733. 10.1016/j.bbamcr.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Bellinger F. P., Bellinger M. T., Seale L. A., Takemoto A. S., Raman A. V., Miki T., et al. (2011). Glutathione peroxidase 4 is associated with neuromelanin in substantia nigra and dystrophic axons in putamen of Parkinson’s brain. Mol. Neurodegener. 6:8. 10.1186/1750-1326-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersuker K., Hendricks J. M., Li Z., Magtanong L., Ford B., Tang P. H., et al. (2019). The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575 688–692. 10.1038/s41586-019-1705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braicu C., Zanoaga O., Zimta A. A., Tigu A. B., Kilpatrick K. L., Bishayee A., et al. (2022). Natural compounds modulate the crosstalk between apoptosis- and autophagy-regulated signaling pathways: controlling the uncontrolled expansion of tumor cells. Semin. Cancer Biol. 80 218–236. 10.1016/j.semcancer.2020.05.015 [DOI] [PubMed] [Google Scholar]
- Brown R. H., Al-Chalabi A. (2017). Amyotrophic lateral sclerosis. N. Engl. J. Med. 377 162–172. 10.1056/NEJMra1603471 [DOI] [PubMed] [Google Scholar]
- Campanella A., Rovelli E., Santambrogio P., Cozzi A., Taroni F., Levi S. (2009). Mitochondrial ferritin limits oxidative damage regulating mitochondrial iron availability: hypothesis for a protective role in Friedreich ataxia. Hum. Mol. Genet. 18 1–11. 10.1093/hmg/ddn308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Chen X., Jiang L., Lu B., Yuan M., Zhu D., et al. (2020). DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat. Commun. 11:1251. 10.1038/s41467-020-15109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Li Y., He C., Yan F., Li J. R., Xu H. Z., et al. (2021). Selective ferroptosis inhibitor Liproxstatin-1 attenuates neurological deficits and neuroinflammation after subarachnoid hemorrhage. Neurosci. Bull. 37 535–549. 10.1007/s12264-020-00620-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpi-Santos R., Calaza K. C. (2018). Alterations in system x(c)(−) expression in the retina of type 1 diabetic rats and the role of Nrf2. Mol. Neurobiol. 55 7941–7948. 10.1007/s12035-018-0961-8 [DOI] [PubMed] [Google Scholar]
- Chen L., Na R., Danae McLane K., Thompson C. S., Gao J., Wang X., et al. (2021). Overexpression of ferroptosis defense enzyme Gpx4 retards motor neuron disease of SOD1G93A mice. Sci. Rep. 11:12890. 10.1038/s41598-021-92369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., He W. T., Hu L., Li J., Fang Y., Wang X., et al. (2016). Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 26 1007–1020. 10.1038/cr.2016.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Yu C., Kang R., Kroemer G., Tang D. (2021). Cellular degradation systems in ferroptosis. Cell Death Differ. 28 1135–1148. 10.1038/s41418-020-00728-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Zhang Z., Zhou X., Zhao Z., Zhao R., Xu X., et al. (2021). Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression. J. Neuroinflamm. 18:249. 10.1186/s12974-021-02231-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai E., Meng L., Kang R., Wang X., Tang D. (2020a). ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem. Biophys. Res. Commun. 522 415–421. 10.1016/j.bbrc.2019.11.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai E., Zhang W., Cong D., Kang R., Wang J., Tang D. (2020b). AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem. Biophys. Res. Commun. 523 966–971. 10.1016/j.bbrc.2020.01.066 [DOI] [PubMed] [Google Scholar]
- D’Arcy M. S. (2019). Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 43 582–592. 10.1002/cbin.11137 [DOI] [PubMed] [Google Scholar]
- Demarco B., Chen K. W., Broz P. (2020). Cross talk between intracellular pathogens and cell death. Immunol. Rev. 297 174–193. 10.1111/imr.12892 [DOI] [PubMed] [Google Scholar]
- Derry P. J., Hegde M. L., Jackson G. R., Kayed R., Tour J. M., Tsai A. L., et al. (2020). Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. Prog. Neurobiol. 184:101716. 10.1016/j.pneurobio.2019.101716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D., Cabantchik Z. I., Moreau C., Danel V., Mahoney-Sanchez L., Bouchaoui H., et al. (2020). Conservative iron chelation for neurodegenerative diseases such as Parkinson’s disease and amyotrophic lateral sclerosis. J. Neural Transm. 127 189–203. 10.1007/s00702-019-02138-1 [DOI] [PubMed] [Google Scholar]
- Devos D., Moreau C., Kyheng M., Garçon G., Rolland A. S., Blasco H., et al. (2019). A ferroptosis-based panel of prognostic biomarkers for amyotrophic lateral sclerosis. Sci. Rep. 9:2918. 10.1038/s41598-019-39739-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias V., Junn E., Mouradian M. M. (2013). The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 3 461–491. 10.3233/jpd-130230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionísio P. A., Amaral J. D., Rodrigues C. M. P. (2021). Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 67:101263. 10.1016/j.arr.2021.101263 [DOI] [PubMed] [Google Scholar]
- Diouf I., Fazlollahi A., Bush A. I., Ayton S. (2019). Cerebrospinal fluid ferritin levels predict brain hypometabolism in people with underlying β-amyloid pathology. Neurobiol. Dis. 124 335–339. 10.1016/j.nbd.2018.12.010 [DOI] [PubMed] [Google Scholar]
- Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149 1060–1072. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S. J., Patel D. N., Welsch M., Skouta R., Lee E. D., Hayano M., et al. (2014). Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3:e02523. 10.7554/eLife.02523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S. J., Stockwell B. R. (2014). The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10 9–17. 10.1038/nchembio.1416 [DOI] [PubMed] [Google Scholar]
- Do Van B., Gouel F., Jonneaux A., Timmerman K., Gelé P., Pétrault M., et al. (2016). Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis. 94 169–178. 10.1016/j.nbd.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Dodson M., Castro-Portuguez R., Zhang D. D. (2019). NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 23:101107. 10.1016/j.redox.2019.101107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S., Freitas F. P., Shah R., Aldrovandi M., da Silva M. C., Ingold I., et al. (2019). FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575 693–698. 10.1038/s41586-019-1707-0 [DOI] [PubMed] [Google Scholar]
- Doll S., Proneth B., Tyurina Y. Y., Panzilius E., Kobayashi S., Ingold I., et al. (2017). ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13 91–98. 10.1038/nchembio.2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolma S., Lessnick S. L., Hahn W. C., Stockwell B. R. (2003). Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3 285–296. 10.1016/s1535-6108(03)00050-3 [DOI] [PubMed] [Google Scholar]
- Eberhard Y., McDermott S. P., Wang X., Gronda M., Venugopal A., Wood T. E., et al. (2009). Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood 114 3064–3073. 10.1182/blood-2009-03-209965 [DOI] [PubMed] [Google Scholar]
- Evans R. C., Chen L., Na R., Yoo K., Ran Q. (2022). The Gpx4NIKO mouse is a versatile model for testing interventions targeting ferroptotic cell death of spinal motor neurons. Neurotox Res. 40 373–383. 10.1007/s12640-021-00469-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett J., Brooks J., Lermyte F., O’Connor P. B., Sadler P. J., Dobson J., et al. (2020). Iron stored in ferritin is chemically reduced in the presence of aggregating Aβ(1-42). Sci. Rep. 10:10332. 10.1038/s41598-020-67117-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. C., Xiong M. P. (2021). Challenges and opportunities of deferoxamine delivery for treatment of Alzheimer’s disease, Parkinson’s disease, and intracerebral hemorrhage. Mol. Pharm. 18 593–609. 10.1021/acs.molpharmaceut.0c00474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Kepp O., Chan F. K., Kroemer G. (2017). Necroptosis: mechanisms and relevance to disease. Annu. Rev. Pathol. 12 103–130. 10.1146/annurev-pathol-052016-100247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Vitale I., Aaronson S. A., Abrams J. M., Adam D., Agostinis P., et al. (2018). Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25 486–541. 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammella E., Recalcati S., Rybinska I., Buratti P., Cairo G. (2015). Iron-induced damage in cardiomyopathy: oxidative-dependent and independent mechanisms. Oxid. Med Cell Longev. 2015:230182. 10.1155/2015/230182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Li J., Zhang Y., Chang Y. Z. (2019). Cellular iron metabolism and regulation. Adv. Exp. Med. Biol. 1173 21–32. 10.1007/978-981-13-9589-5_2 [DOI] [PubMed] [Google Scholar]
- Gao J., Zhou Q., Wu D., Chen L. (2021). Mitochondrial iron metabolism and its role in diseases. Clin. Chim. Acta 513 6–12. 10.1016/j.cca.2020.12.005 [DOI] [PubMed] [Google Scholar]
- Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. (2015). Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59 298–308. 10.1016/j.molcel.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H., Xue X., Xian J., Yuan L., Wang L., Zou Y., et al. (2022). Ferrostatin-1 alleviates white matter injury via decreasing ferroptosis following spinal cord injury. Mol. Neurobiol. 59 161–176. 10.1007/s12035-021-02571-y [DOI] [PubMed] [Google Scholar]
- Guichardant M., Chen P., Liu M., Calzada C., Colas R., Véricel E., et al. (2011). Functional lipidomics of oxidized products from polyunsaturated fatty acids. Chem. Phys. Lipids 164 544–548. 10.1016/j.chemphyslip.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Hadian K., Stockwell B. R. (2020). SnapShot: ferroptosis. Cell 181:1188. 10.1016/j.cell.2020.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambright W. S., Fonseca R. S., Chen L., Na R., Ran Q. (2017). Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 12 8–17. 10.1016/j.redox.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare D. J., Double K. L. (2016). Iron and dopamine: a toxic couple. Brain 139(Pt 4), 1026–1035. 10.1093/brain/aww022 [DOI] [PubMed] [Google Scholar]
- Hayes M. T. (2019). Parkinson’s disease and parkinsonism. Am. J. Med. 132 802–807. 10.1016/j.amjmed.2019.03.001 [DOI] [PubMed] [Google Scholar]
- Hemerková P., Vališ M. (2021). Role of oxidative stress in the pathogenesis of amyotrophic lateral sclerosis: antioxidant metalloenzymes and therapeutic strategies. Biomolecules 11:437. 10.3390/biom11030437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Huang R., Sun F., Zhang L., Wang Q. (2019). NADPH oxidase regulates paraquat and maneb-induced dopaminergic neurodegeneration through ferroptosis. Toxicology 417 64–73. 10.1016/j.tox.2019.02.011 [DOI] [PubMed] [Google Scholar]
- Hu C. L., Nydes M., Shanley K. L., Morales Pantoja I. E., Howard T. A., Bizzozero O. A. (2019). Reduced expression of the ferroptosis inhibitor glutathione peroxidase-4 in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurochem. 148 426–439. 10.1111/jnc.14604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L. W., Villemagne V. L., Cheng L., Sherratt N. A., Ayton S., White A. R., et al. (2012). The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson’s disease. J. Exp. Med. 209 837–854. 10.1084/jem.20112285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatović A., Stević Z., Lavrnić D., Nikolić-Kokić A., Blagojević D., Spasić M., et al. (2012). Inappropriately chelated iron in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. 13 357–362. 10.3109/17482968.2012.665929 [DOI] [PubMed] [Google Scholar]
- Jenner P., Dexter D. T., Sian J., Schapira A. H., Marsden C. D. (1992). Oxidative stress as a cause of nigral cell death in Parkinson’s disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann. Neurol. 32(Suppl.), S82–S87. 10.1002/ana.410320714 [DOI] [PubMed] [Google Scholar]
- Jennis M., Kung C. P., Basu S., Budina-Kolomets A., Leu J. I., Khaku S., et al. (2016). An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 30 918–930. 10.1101/gad.275891.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S. Y., Rathore K. I., Schulz K., Ponka P., Arosio P., David S. (2009). Dysregulation of iron homeostasis in the CNS contributes to disease progression in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 29 610–619. 10.1523/jneurosci.5443-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhelum P., Santos-Nogueira E., Teo W., Haumont A., Lenoël I., Stys P. K., et al. (2020). Ferroptosis mediates cuprizone-induced loss of oligodendrocytes and demyelination. J. Neurosci. 40 9327–9341. 10.1523/jneurosci.1749-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C., Kosman D. J. (2015). Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J. Neurochem. 133 668–683. 10.1111/jnc.13040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F., Song N., Wang W., Du X., Chi Y., Jiang H. (2018). High dietary iron supplement induces the nigrostriatal dopaminergic neurons lesion in transgenic mice expressing mutant A53T human alpha-synuclein. Front. Aging Neurosci. 10:97. 10.3389/fnagi.2018.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Kon N., Li T., Wang S. J., Su T., Hibshoosh H., et al. (2015). Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520 57–62. 10.1038/nature14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan V. E., Mao G., Qu F., Angeli J. P., Doll S., Croix C. S., et al. (2017). Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13 81–90. 10.1038/nchembio.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakhlon O., Cabantchik Z. I. (2002). The labile iron pool: characterization, measurement, and participation in cellular processes(1). Free Radic. Biol. Med. 33 1037–1046. 10.1016/s0891-5849(02)01006-7 [DOI] [PubMed] [Google Scholar]
- Kapralov A. A., Yang Q., Dar H. H., Tyurina Y. Y., Anthonymuthu T. S., Kim R., et al. (2020). Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 16 278–290. 10.1038/s41589-019-0462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata H. (2019). Transferrin and transferrin receptors update. Free Radic. Biol. Med. 133 46–54. 10.1016/j.freeradbiomed.2018.06.037 [DOI] [PubMed] [Google Scholar]
- Khan I., Yousif A., Chesnokov M., Hong L., Chefetz I. (2021). A decade of cell death studies: breathing new life into necroptosis. Pharmacol. Ther. 220:107717. 10.1016/j.pharmthera.2020.107717 [DOI] [PubMed] [Google Scholar]
- Klepac N., Relja M., Klepac R., Hećimović S., Babić T., Trkulja V. (2007). Oxidative stress parameters in plasma of Huntington’s disease patients, asymptomatic Huntington’s disease gene carriers and healthy subjects : a cross-sectional study. J. Neurol. 254 1676–1683. 10.1007/s00415-007-0611-y [DOI] [PubMed] [Google Scholar]
- Komaki H., Faraji N., Komaki A., Shahidi S., Etaee F., Raoufi S., et al. (2019). Investigation of protective effects of coenzyme Q10 on impaired synaptic plasticity in a male rat model of Alzheimer’s disease. Brain Res. Bull. 147 14–21. 10.1016/j.brainresbull.2019.01.025 [DOI] [PubMed] [Google Scholar]
- Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., et al. (2010). The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 12 213–223. 10.1038/ncb2021 [DOI] [PubMed] [Google Scholar]
- Kosman D. J. (2020). A holistic view of mammalian (vertebrate) cellular iron uptake. Metallomics 12 1323–1334. 10.1039/d0mt00065e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft V. A. N., Bezjian C. T., Pfeiffer S., Ringelstetter L., Müller C., Zandkarimi F., et al. (2020). GTP Cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 6 41–53. 10.1021/acscentsci.9b01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan J. Y., Jeong S. Y., Van Gelderen P., Deng H. X., Quezado M. M., Danielian L. E., et al. (2012). Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7:e35241. 10.1371/journal.pone.0035241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M. Y., Park E., Lee S. J., Chung S. W. (2015). Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 6 24393–24403. 10.18632/oncotarget.5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C. A., Hardy J., Schott J. M. (2018). Alzheimer’s disease. Eur. J. Neurol. 25 59–70. 10.1111/ene.13439 [DOI] [PubMed] [Google Scholar]
- Le Y., Zhang Z., Wang C., Lu D. (2021). Ferroptotic cell death: new regulatory mechanisms for metabolic diseases. Endocr. Metab. Immune Disord. Drug Targets 21 785–800. 10.2174/1871530320666200731175328 [DOI] [PubMed] [Google Scholar]
- Lee H., Zandkarimi F., Zhang Y., Meena J. K., Kim J., Zhuang L., et al. (2020). Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22 225–234. 10.1038/s41556-020-0461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Kim W. K., Bae K. H., Lee S. C., Lee E. W. (2021). Lipid metabolism and ferroptosis. Biology 10:e2100396. 10.3390/biology10030184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei G., Zhuang L., Gan B. (2022). Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer [Epub ahead of print]. 10.1038/s41568-022-00459-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Y., Paragas N., Ned R. M., Qiu A., Viltard M., Leete T., et al. (2009). Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev. Cell 16 35–46. 10.1016/j.devcel.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Jiang M., Qi L., Wu Y., Song D., Gan J., et al. (2021). Pyroptosis, a new bridge to tumor immunity. Cancer Sci. 112 3979–3994. 10.1111/cas.15059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Wang C., Liu G., Bi C., Wang X., Zhou Q., et al. (2020). SLC7A11/xCT in cancer: biological functions and therapeutic implications. Am. J. Cancer Res. 10 3106–3126. [PMC free article] [PubMed] [Google Scholar]
- Liu M., Kong X. Y., Yao Y., Wang X. A., Yang W., Wu H., et al. (2022). The critical role and molecular mechanisms of ferroptosis in antioxidant systems: a narrative review. Ann. Transl. Med. 10:368. 10.21037/atm-21-6942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Levine B. (2015). Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ. 22 367–376. 10.1038/cdd.2014.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti C. F., Brück W., Rodriguez M., Lassmann H. (1996). Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol. 6 259–274. 10.1111/j.1750-3639.1996.tb00854.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H., Shang P. (2018). The significance, trafficking and determination of labile iron in cytosol, mitochondria and lysosomes. Metallomics 10 899–916. 10.1039/c8mt00048d [DOI] [PubMed] [Google Scholar]
- Mabalirajan U., Aich J., Leishangthem G. D., Sharma S. K., Dinda A. K., Ghosh B. (2009). Effects of vitamin E on mitochondrial dysfunction and asthma features in an experimental allergic murine model. J. Appl. Physiol. 107 1285–1292. 10.1152/japplphysiol.00459.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie E. L., Iwasaki K., Tsuji Y. (2008). Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid. Redox Signal. 10 997–1030. 10.1089/ars.2007.1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney-Sánchez L., Bouchaoui H., Ayton S., Devos D., Duce J. A., Devedjian J. C. (2021). Ferroptosis and its potential role in the physiopathology of Parkinson’s disease. Prog. Neurobiol. 196:101890. 10.1016/j.pneurobio.2020.101890 [DOI] [PubMed] [Google Scholar]
- Majerníková N., den Dunnen W. F. A., Dolga A. M. (2021). The potential of ferroptosis-targeting therapies for Alzheimer’s disease: from mechanism to transcriptomic analysis. Front. Aging Neurosci. 13:745046. 10.3389/fnagi.2021.745046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C., Liu X., Zhang Y., Lei G., Yan Y., Lee H., et al. (2021). DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593 586–590. 10.1038/s41586-021-03539-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H., Zhao Y., Li H., Lei L. (2020). Ferroptosis as an emerging target in inflammatory diseases. Prog. Biophys. Mol. Biol. 155 20–28. 10.1016/j.pbiomolbio.2020.04.001 [DOI] [PubMed] [Google Scholar]
- Martin-Bastida A., Ward R. J., Newbould R., Piccini P., Sharp D., Kabba C., et al. (2017). Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci. Rep. 7:1398. 10.1038/s41598-017-01402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaldan S., Bush A. I., Devos D., Rolland A. S., Moreau C. (2019). Striking while the iron is hot: iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 133 221–233. 10.1016/j.freeradbiomed.2018.09.033 [DOI] [PubMed] [Google Scholar]
- Mason R. P., Casu M., Butler N., Breda C., Campesan S., Clapp J., et al. (2013). Glutathione peroxidase activity is neuroprotective in models of Huntington’s disease. Nat. Genet. 45 1249–1254. 10.1038/ng.2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumaru D., Motohashi H. (2021). The KEAP1-NRF2 system in healthy aging and longevity. Antioxidants 10:1929. 10.3390/antiox10121929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Adachi-Tominari K., Sano O., Kamei T., Nogami M., Ogi K., et al. (2021). Involvement of ferroptosis in human motor neuron cell death. Biochem. Biophys. Res. Commun. 566 24–29. 10.1016/j.bbrc.2021.05.095 [DOI] [PubMed] [Google Scholar]
- McIntosh A., Mela V., Harty C., Minogue A. M., Costello D. A., Kerskens C., et al. (2019). Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol. 29 606–621. 10.1111/bpa.12704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Y., Gao X., Xu H., Cui Y., Zhang Y., Gou X. (2019). The emerging roles of ferroptosis in Huntington’s disease. Neuromol. Med. 21 110–119. 10.1007/s12017-018-8518-6 [DOI] [PubMed] [Google Scholar]
- Mizushima N., Levine B. (2020). Autophagy in human diseases. N. Engl. J. Med. 383 1564–1576. 10.1056/NEJMra2022774 [DOI] [PubMed] [Google Scholar]
- Moreau C., Danel V., Devedjian J. C., Grolez G., Timmerman K., Laloux C., et al. (2018). Could conservative iron chelation lead to neuroprotection in amyotrophic lateral sclerosis? Antioxid. Redox Signal. 29 742–748. 10.1089/ars.2017.7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Y., Wang J., Wu J., He D., Zhang C., Duan C., et al. (2019). Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J. Hematol. Oncol. 12:34. 10.1186/s13045-019-0720-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem S., Yang C., Du Y., Li F., Chen Z., Zhou Y., et al. (2021). A virus-spike tumor-activatable pyroptotic agent. Small 17:e2006599. 10.1002/smll.202006599 [DOI] [PubMed] [Google Scholar]
- Nah J., Zablocki D., Sadoshima J. (2020). Autosis: a new target to prevent cell death. JACC Basic Transl. Sci. 5 857–869. 10.1016/j.jacbts.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikseresht S., Bush A. I., Ayton S. (2019). Treating Alzheimer’s disease by targeting iron. Br. J. Pharmacol. 176 3622–3635. 10.1111/bph.14567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. (2002). An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 14 223–236. 10.1176/jnp.14.2.223 [DOI] [PubMed] [Google Scholar]
- Paul B. T., Manz D. H., Torti F. M., Torti S. V. (2017). Mitochondria and Iron: current questions. Expert Rev. Hematol. 10 65–79. 10.1080/17474086.2016.1268047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Pan J., Mo J., Peng Y. (2022). MPO/HOCl facilitates apoptosis and ferroptosis in the SOD1(G93A) motor neuron of amyotrophic lateral sclerosis. Oxid. Med. Cell Longev. 2022:8217663. 10.1155/2022/8217663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott C. C., Patel S. J., Protchenko O. (2020). Management versus miscues in the cytosolic labile iron pool: the varied functions of iron chaperones. Biochim. Biophys. Acta Mol. Cell Res. 1867:118830. 10.1016/j.bbamcr.2020.118830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S., Ramos-Alonso L., Romero A. M., Martínez-Pastor M. T. (2017). The elemental role of iron in DNA synthesis and repair. Metallomics 9 1483–1500. 10.1039/c7mt00116a [DOI] [PubMed] [Google Scholar]
- Qu W., Cheng Y., Peng W., Wu Y., Rui T., Luo C., et al. (2022). Targeting iNOS alleviates early brain injury after experimental subarachnoid hemorrhage via promoting ferroptosis of M1 microglia and reducing neuroinflammation. Mol. Neurobiol. 59 3124–3139. 10.1007/s12035-022-02788-5 [DOI] [PubMed] [Google Scholar]
- Quinti L., Dayalan Naidu S., Träger U., Chen X., Kegel-Gleason K., Llères D., et al. (2017). KEAP1-modifying small molecule reveals muted NRF2 signaling responses in neural stem cells from Huntington’s disease patients. Proc. Natl. Acad. Sci. U.S.A. 114 E4676–E4685. 10.1073/pnas.1614943114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha A. A., Vaishnav R. A., Friedland R. P., Bomford A., Raha-Chowdhury R. (2013). The systemic iron-regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer’s disease. Acta Neuropathol. Commun. 1:55. 10.1186/2051-5960-1-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. S., Portbury S. D., Lago L., Bush A. I., Adlard P. A. (2020). The iron chelator deferiprone improves the phenotype in a mouse model of tauopathy. J. Alzheimers Dis. 78:1783. 10.3233/jad-209009 [DOI] [PubMed] [Google Scholar]
- Reddy P. H., Shirendeb U. P. (2012). Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington’s disease. Biochim. Biophys. Acta 1822 101–110. 10.1016/j.bbadis.2011.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J. X., Sun X., Yan X. L., Guo Z. N., Yang Y. (2020). Ferroptosis in neurological diseases. Front. Cell Neurosci. 14:218. 10.3389/fncel.2020.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P., Monoranu C., Strobel S., Iordache T., Sian-Hülsmann J. (2021). Iron as the concert master in the pathogenic orchestra playing in sporadic Parkinson’s disease. J. Neural Transm. 128 1577–1598. 10.1007/s00702-021-02414-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. A., Tabrizi S. J. (2011). Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 10 83–98. 10.1016/s1474-4422(10)70245-3 [DOI] [PubMed] [Google Scholar]
- Saito T., Hisahara S., Iwahara N., Emoto M. C., Yokokawa K., Suzuki H., et al. (2019). Early administration of galantamine from preplaque phase suppresses oxidative stress and improves cognitive behavior in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Free Radic. Biol. Med. 145, 20–32. 10.1016/j.freeradbiomed.2019.09.014 [DOI] [PubMed] [Google Scholar]
- Seibt T. M., Proneth B., Conrad M. (2019). Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 133 144–152. 10.1016/j.freeradbiomed.2018.09.014 [DOI] [PubMed] [Google Scholar]
- Shao L., Dong C., Geng D., He Q., Shi Y. (2021). Ginkgolide B protects against cognitive impairment in senescence-accelerated P8 mice by mitigating oxidative stress, inflammation and ferroptosis. Biochem. Biophys. Res. Commun. 572 7–14. 10.1016/j.bbrc.2021.07.081 [DOI] [PubMed] [Google Scholar]
- Shin C. S., Mishra P., Watrous J. D., Carelli V., D’Aurelio M., Jain M., et al. (2017). The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat. Commun. 8:15074. 10.1038/ncomms15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouta R., Dixon S. J., Wang J., Dunn D. E., Orman M., Shimada K., et al. (2014). Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 136 4551–4556. 10.1021/ja411006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L. M., Xiao Z. X., Zhang N., Yu X. Q., Cui W., Xie J. X., et al. (2021). Apoferritin improves motor deficits in MPTP-treated mice by regulating brain iron metabolism and ferroptosis. iScience 24:102431. 10.1016/j.isci.2021.102431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soula M., Weber R. A., Zilka O., Alwaseem H., La K., Yen F., et al. (2020). Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 16 1351–1360. 10.1038/s41589-020-0613-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southon A., Szostak K., Acevedo K. M., Dent K. A., Volitakis I., Belaidi A. A., et al. (2020). Cu(II) (atsm) inhibits ferroptosis: implications for treatment of neurodegenerative disease. Br. J. Pharmacol. 177 656–667. 10.1111/bph.14881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasić S., Nikolić-Kokić A., Miletić S., Oreščanin-Dušić Z., Spasić M. B., Blagojević D., et al. (2020). Edaravone may prevent ferroptosis in ALS. Curr. Drug Targets 21 776–780. 10.2174/1389450121666200220123305 [DOI] [PubMed] [Google Scholar]
- Stack C., Ho D., Wille E., Calingasan N. Y., Williams C., Liby K., et al. (2010). Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington’s disease. Free Radic. Biol. Med. 49 147–158. 10.1016/j.freeradbiomed.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell B. R. (2019). A powerful cell-protection system prevents cell death by ferroptosis. Nature 575 597–598. 10.1038/d41586-019-03145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell B. R., Friedmann Angeli J. P., Bayir H., Bush A. I., Conrad M., Dixon S. J., et al. (2017). Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171 273–285. 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanovsky D. A., Tyurina Y. Y., Shrivastava I., Bahar I., Tyurin V. A., Protchenko O., et al. (2019). Iron catalysis of lipid peroxidation in ferroptosis: regulated enzymatic or random free radical reaction? Free Radic. Biol. Med. 133 153–161. 10.1016/j.freeradbiomed.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Li X., Jia H., Wang H., Shui G., Qin Y., et al. (2020). Nuclear factor E2-related factor 2 mediates oxidative stress-induced lipid accumulation in adipocytes by increasing adipogenesis and decreasing lipolysis. Antioxid. Redox Signal. 32 173–192. 10.1089/ars.2019.7769 [DOI] [PubMed] [Google Scholar]
- Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., et al. (2016). Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63 173–184. 10.1002/hep.28251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Guo J., Liu W., Ma J., Xu G. (2021). Ferrostatin-1 attenuates ferroptosis and protects the retina against light-induced retinal degeneration. Biochem. Biophys. Res. Commun. 548 27–34. 10.1016/j.bbrc.2021.02.055 [DOI] [PubMed] [Google Scholar]
- Tang Y., Zhang D., Gong X., Zheng J. (2022). A mechanistic survey of Alzheimer’s disease. Biophys. Chem. 281:106735. 10.1016/j.bpc.2021.106735 [DOI] [PubMed] [Google Scholar]
- Tao Y., Wang Y., Rogers J. T., Wang F. (2014). Perturbed iron distribution in Alzheimer’s disease serum, cerebrospinal fluid, and selected brain regions: a systematic review and meta-analysis. J. Alzheimers Dis. 42 679–690. 10.3233/jad-140396 [DOI] [PubMed] [Google Scholar]
- Tian Y., Lu J., Hao X., Li H., Zhang G., Liu X., et al. (2020). FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson’s disease. Neurotherapeutics 17 1796–1812. 10.1007/s13311-020-00929-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S., Atluri V., Kaushik A., Yndart A., Nair M. (2019). Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int. J. Nanomedicine 14 5541–5554. 10.2147/ijn.S200490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahsen B. F., Gray E., Thompson A. G., Ansorge O., Anthony D. C., Cowley S. A., et al. (2021). Non-neuronal cells in amyotrophic lateral sclerosis - from pathogenesis to biomarkers. Nat. Rev. Neurol. 17 333–348. 10.1038/s41582-021-00487-8 [DOI] [PubMed] [Google Scholar]
- Vallerga C. L., Zhang F., Fowdar J., McRae A. F., Qi T., Nabais M. F., et al. (2020). Analysis of DNA methylation associates the cystine-glutamate antiporter SLC7A11 with risk of Parkinson’s disease. Nat. Commun. 11:1238. 10.1038/s41467-020-15065-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen J. M., Hua J., Unschuld P. G., Lim I. A., Jones C. K., Margolis R. L., et al. (2016a). Quantitative susceptibility mapping suggests altered brain iron in premanifest huntington disease. AJNR Am. J. Neuroradiol. 37 789–796. 10.3174/ajnr.A4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen J. M., Li X., Hua J., Schreiner S. J., Steininger S. C., Quevenco F. C., et al. (2016b). Colocalization of cerebral iron with amyloid beta in mild cognitive impairment. Sci. Rep. 6:35514. 10.1038/srep35514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijn S., Bulk M., van Duinen S. G., Nabuurs R. J. A., van Buchem M. A., van der Weerd L., et al. (2017). Cortical iron reflects severity of Alzheimer’s disease. J. Alzheimers Dis. 60 1533–1545. 10.3233/jad-161143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Raaij S. E. G., Srai S. K. S., Swinkels D. W., van Swelm R. P. L. (2019). Iron uptake by ZIP8 and ZIP14 in human proximal tubular epithelial cells. Biometals 32 211–226. 10.1007/s10534-019-00183-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh D., O’Brien N. A., Zandkarimi F., Tong D. R., Stokes M. E., Dunn D. E., et al. (2020). MDM2 and MDMX promote ferroptosis by PPARα-mediated lipid remodeling. Genes Dev. 34 526–543. 10.1101/gad.334219.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voet S., Prinz M., van Loo G. (2019). Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol. Med. 25 112–123. 10.1016/j.molmed.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Wan W., Cao L., Kalionis B., Murthi P., Xia S., Guan Y. (2019). Iron deposition leads to hyperphosphorylation of tau and disruption of insulin signaling. Front. Neurol. 10:607. 10.3389/fneur.2019.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Chen S., Guo H., Jiang H., Liu H., Fu H., et al. (2022). Forsythoside a mitigates Alzheimer’s-like pathology by inhibiting ferroptosis-mediated neuroinflammation via Nrf2/GPX4 axis activation. Int. J. Biol. Sci. 18 2075–2090. 10.7150/ijbs.69714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu C., Zhao Y., Gao G. (2020). Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 99:151058. 10.1016/j.ejcb.2019.151058 [DOI] [PubMed] [Google Scholar]
- Wang J., Xu H. M., Yang H. D., Du X. X., Jiang H., Xie J. X. (2009). Rg1 reduces nigral iron levels of MPTP-treated C57BL6 mice by regulating certain iron transport proteins. Neurochem. Int. 54 43–48. 10.1016/j.neuint.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Wang T., Tomas D., Perera N. D., Cuic B., Luikinga S., Viden A., et al. (2021). Ferroptosis mediates selective motor neuron death in amyotrophic lateral sclerosis. Cell Death Differ. [Epub ahead of print]. 10.1038/s41418-021-00910-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. L., Yuan L., Li W., Li J. Y. (2022). Ferroptosis in Parkinson’s disease: glia-neuron crosstalk. Trends Mol. Med. 28 258–269. 10.1016/j.molmed.2022.02.003 [DOI] [PubMed] [Google Scholar]
- Watanabe L. M., Hashimoto A. C., Torres D. J., Alfulaij N., Peres R., Sultana R., et al. (2021). Effect of statin treatment in obese selenium-supplemented mice lacking selenocysteine lyase. Mol. Cell Endocrinol. 533:111335. 10.1016/j.mce.2021.111335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Yi X., Zhu X. H., Jiang D. S. (2020). Posttranslational modifications in ferroptosis. Oxid. Med. Cell Longev. 2020:8832043. 10.1155/2020/8832043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C., Brennan S. E., Keon M., Saksena N. K. (2019). Prionoid proteins in the pathogenesis of neurodegenerative diseases. Front. Mol. Neurosci. 12:271. 10.3389/fnmol.2019.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel S. E., Tyurina Y. Y., Zhao J., St Croix C. M., Dar H. H., Mao G., et al. (2017). PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171 628.e6–641.e6. 10.1016/j.cell.2017.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieler M., Gee M., Martin W. R. (2015). Longitudinal midbrain changes in early Parkinson’s disease: iron content estimated from R2*/MRI. Parkinsonism Relat. Disord. 21 179–183. 10.1016/j.parkreldis.2014.11.017 [DOI] [PubMed] [Google Scholar]
- Winter W. E., Bazydlo L. A., Harris N. S. (2014). The molecular biology of human iron metabolism. Lab. Med. 45 92–102. 10.1309/lmf28s2gimxnwhmm [DOI] [PubMed] [Google Scholar]
- Wirth E. K., Bharathi B. S., Hatfield D., Conrad M., Brielmeier M., Schweizer U. (2014). Cerebellar hypoplasia in mice lacking selenoprotein biosynthesis in neurons. Biol. Trace Elem. Res. 158 203–210. 10.1007/s12011-014-9920-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Liu P., Li J. (2012). Necroptosis: an emerging form of programmed cell death. Crit. Rev. Oncol. Hematol. 82 249–258. 10.1016/j.critrevonc.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Wyant K. J., Ridder A. J., Dayalu P. (2017). Huntington’s disease-update on treatments. Curr. Neurol. Neurosci. Rep. 17:33. 10.1007/s11910-017-0739-9 [DOI] [PubMed] [Google Scholar]
- Xia X., Wang Y., Zheng J. (2021). COVID-19 and Alzheimer’s disease: how one crisis worsens the other. Transl. Neurodegener. 10:15. 10.1186/s40035-021-00237-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., et al. (2016). Ferroptosis: process and function. Cell Death Differ. 23 369–379. 10.1038/cdd.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Lai Y., Hua Z. C. (2019). Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci. Rep. 39:BSR20180992. 10.1042/bsr20180992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Hu S., Bian Y., Yao J., Wang D., Liu X., et al. (2021). Targeting cell death: pyroptosis, ferroptosis, apoptosis and necroptosis in osteoarthritis. Front. Cell Dev. Biol. 9:789948. 10.3389/fcell.2021.789948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Calingasan N. Y., Wille E. J., Cormier K., Smith K., Ferrante R. J., et al. (2009). Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J. Neurochem. 109 1427–1439. 10.1111/j.1471-4159.2009.06074.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S., Kim K. J., Gaschler M. M., Patel M., Shchepinov M. S., Stockwell B. R. (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U.S.A. 113 E4966–E4975. 10.1073/pnas.1603244113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S., SriRamaratnam R., Welsch M. E., Shimada K., Skouta R., Viswanathan V. S., et al. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell 156 317–331. 10.1016/j.cell.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S., Stockwell B. R. (2016). Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 26 165–176. 10.1016/j.tcb.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You P., Li X., Wang Z., Wang H., Dong B., Li Q. (2021). Characterization of brain iron deposition pattern and its association with genetic risk factor in alzheimer’s disease using susceptibility-weighted imaging. Front. Hum. Neurosci. 15:654381. 10.3389/fnhum.2021.654381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Yan Y., Niu F., Wang Y., Chen X., Su G., et al. (2021). Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 7:193. 10.1038/s41420-021-00579-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh M. M., Chen Y., Kitzler H. H., Hammond R., Vogel H., Rutt B. K. (2015). Activated iron-containing microglia in the human hippocampus identified by magnetic resonance imaging in Alzheimer disease. Neurobiol. Aging 36 2483–2500. 10.1016/j.neurobiolaging.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Zhang Y., Shen Y., Wang Y., Zhao M., Sun L. (2021). The potential role of ferroptosis in Alzheimer’s disease. J. Alzheimers Dis. 80 907–925. 10.3233/jad-201369 [DOI] [PubMed] [Google Scholar]
- Zhang P., Chen L., Zhao Q., Du X., Bi M., Li Y., et al. (2020). Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson’s disease. Free Radic. Biol. Med. 152 227–234. 10.1016/j.freeradbiomed.2020.03.015 [DOI] [PubMed] [Google Scholar]
- Zhang X., Wu S., Guo C., Guo K., Hu Z., Peng J., et al. (2022). Vitamin E exerts neuroprotective effects in pentylenetetrazole kindling epilepsy via suppression of ferroptosis. Neurochem. Res. 47 739–747. 10.1007/s11064-021-03483-y [DOI] [PubMed] [Google Scholar]
- Zhang Y., Fan D., Liu X., Liu X., He J., Zhang N., et al. (2020). hTBK1-c.978T>A mutation promotes the ferroptosis in NSC-34 cells via mediation of KEAP1/NRF2/p62 signaling. Am. J. Transl. Res. 12 7386–7394. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. H., Wang D. W., Xu S. F., Zhang S., Fan Y. G., Yang Y. Y., et al. (2018). α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 14 535–548. 10.1016/j.redox.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Hou L., Song J. L., Song N., Sun Y. J., Lin X., et al. (2014). Pro-inflammatory cytokine-mediated ferroportin down-regulation contributes to the nigral iron accumulation in lipopolysaccharide-induced Parkinsonian models. Neuroscience 257 20–30. 10.1016/j.neuroscience.2013.09.037 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Lu J., Mao A., Zhang R., Guan S. (2021). Autophagy inhibition plays a protective role in ferroptosis induced by alcohol via the p62-Keap1-Nrf2 pathway. J. Agric. Food Chem. 69 9671–9683. 10.1021/acs.jafc.1c03751 [DOI] [PubMed] [Google Scholar]
- Zheng J., Conrad M. (2020). The metabolic underpinnings of ferroptosis. Cell Metab. 32 920–937. 10.1016/j.cmet.2020.10.011 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Wang B., Tao K., Yang H., Wang Y., Zhou T., et al. (2017). Iron accumulation and microglia activation contribute to substantia nigra hyperechogenicity in the 6-OHDA-induced rat model of Parkinson’s disease. Parkinsonism Relat. Disord. 36 76–82. 10.1016/j.parkreldis.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Zilka O., Poon J. F., Pratt D. A. (2021). Radical-trapping antioxidant activity of copper and nickel Bis(thiosemicarbazone) complexes underlies their potency as inhibitors of ferroptotic cell death. J. Am. Chem. Soc. 143 19043–19057. 10.1021/jacs.1c08254 [DOI] [PubMed] [Google Scholar]