Abstract
Background
There is lack of studies on sequential regorafenib after sorafenib and lenvatinib treatment failure in patients with unresectable hepatocellular carcinoma (HCC). This study was to explore the safety and prognosis of sequential regorafenib after sorafenib and lenvatinib failure in HCC patients.
Methods
This study was a retrospective, real-world study that included 50 HCC patients who received sequential regrafinib after sorafenib and lenvatinib failure. The safety and prognosis of two groups were compared.
Results
The incidence of all grade and III/IV adverse events were 68% and 24%. According to Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 and modified (m) RECIST standards, the objective response rates (ORRs) after receiving regorafenib were 14.0% and 22.0%, respectively. The disease control rates (DCRs) were 62.0% and 60.0%, respectively. Based on different first-line targeted drugs, 50 patients were divided into sorafenib (n=22) and lenvatinib group (n=28). There was no differences between two groups except age and bilirubin. And there was no differences in other treatments before or after regorafenib. The baseline between two groups was basically same and had good comparability. There was no difference in incidence of all grade and III/IV adverse events, ORR and DCR between two groups (P>0.05). On long-term prognosis, total overall survival (TOS) in sorafenib and lenvatinib group were 23.0 (95% CI: 15.1–30.9) vs. 29.7 (95% CI: 21.4–38.1) months. The difference was statistically significant (P=0.041). Besides, regorafenib overall survival (ROS) in sorafenib and lenvatinib group were 11.7 (95% CI: 7.1–16.3) vs. 15.9 (95% CI: 8.3–23.5) months. The difference was statistically significant ( P=0.045). The regorafenib progression-free survival (RPFS) was 5.6 (95% CI: 1.9–9.2) vs. 8.0 (95% CI: 5.1–10.9) months in sorafenib and lenvatinib group, respectively, and difference was not statistically significant (P=0.380).
Conclusions
Regorafenib is an effective drug for second-line treatment of HCC, with fewer severe adverse events, ORR and DCR was 14–22% and 62–60%, respectively. Both TOS and ROS in lenvatinib group were better than those in sorafenib group. For HCC patients whose first-line targeted drug is lenvatinib, it is safe and effective to accept regorafenib after disease progresses.
Keywords: Hepatocellular carcinoma (HCC), targeted drugs, regorafenib, efficacy
Introduction
Primary liver cancer is the seventh most commonly occurring malignant tumor worldwide, and ranks fourth in terms of cancer-related mortality worldwide. There are about 841,080 new cases and 781,631 deaths per year (1). China is a country with a high incidence rate of liver cancer, and the number of new cases and deaths accounts for about 50% of the worldwide cases (2). Hepatocellular carcinoma (HCC) is the most common type of liver cancer. HCC patients are mostly at the advanced stage when they are diagnosed (3), and the prognosis of these patients is poor.
Targeted drug therapy is an important method for the treatment of patients with advanced HCC, and can effectively improve the prognosis of HCC patients. A previous study reported that sorafenib and lenvatinib are first-line targeted drugs for HCC, and the median overall survival (OS) can reach 13.6 months (95% CI: 12.1–14.9) and 12.3 months (95% CI: 10.4–13.9), respectively (4). Regorafenib is a second-line targeted drug for HCC. As a multi-target kinase inhibitor, regorafenib can comprehensively inhibit angiogenesis targets. For example, vascular endothelial growth factor receptors (VEGFR) 1-3, platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and Tie-2 play an anti-angiogenesis role and comprehensively inhibit angiogenesis (5). Receiving regorafenib can significantly improve the prognosis of HCC patients after treatment with a first-line targeted drug. A previous study reported that the OS of regorafenib after first-line targeted drug treatment can reach 16.4 months, which is significantly better than 7.5 months without second-line treatment (6). As a second-line targeted drug for HCC, regorafenib has a median OS of 10.6 months (95% CI: 9.1–12.1) (7). It has been recommended as the choice of drug for disease progression after systemic therapy (8). However, there was no study reported regrafinib after different first-line targeted drugs failure. We know that targeted drugs act mainly through targets. However, sorafenib and lenvatinib as a first-line targeted drug, act on different targets. Whether sequential regorafenib after sorafenib and lenvatinib treatment failure could lead to different clinical efficacy, safety and long term prognosis has not yet been studied. It is necessary and clinically important to investigate whether sequential regrafinib after sorafenib and lenvatinib treatment failure leads to different adverse effects and prognosis. Based on real-world studies, this investigation systematically reported the total overall survival (TOS) of different first-line targeted drugs followed by regorafenib, the regorafenib overall survival (ROS), and regorafenib progression-free survival (RPFS), as well as the adverse events and efficacy evaluation of sequential regorafenib after sorafenib and lenvatinib treatment failure in HCC patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-404/rc).
Methods
Study design and participants
A total of 73 patients with unresectable HCC who accepted regorafenib in our hospital from April 2020 to August 2021 were selected. After screening by the inclusion and exclusion criteria, 50 patients were finally included in this study. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The retrospective study protocol was reviewed and approved by The Ethics Committee of Eastern Hepatobiliary Surgery Hospital. All patients provided informed consent before treatment.
The inclusion criteria of this study were as follows: (I) Eligible patients were adults with HCC confirmed by pathological assessment or non-invasive assessment according to the American Association for the Study of Liver Diseases criteria for patients with confirmed cirrhosis (9); (II) ≥18 years old, ≤75 years old; (III) Eastern Cooperative Oncology Group (ECOG) performance status score 0–2; (IV) not suitable for radical surgical resection; (V) at least 1 measurable lesion by the modified Response Evaluation Criteria in Solid Tumors for HCC (mRECIST) and RECIST version 1.1 (10); (VI) Child-Pugh grade A or B; (VII) no history of other tumors; (VIII) in the course of treatment, targeted therapy only used first-line drugs sorafenib and lenvatinib and the second-line drug regorafenib; (IX) the clinical data of the included patients were complete; (X) the patient signed an informed consent form.
The exclusion criteria were as follows: (I) Incomplete clinical data; (II) received targeted drugs other than sorafenib, lenvatinib, and regorafenib during treatment; (III) voluntarily discontinuing regorafenib for no reason for more than 1 week; (IV) sorafenib followed by regorafenib, and lenvatinib was received again after regorafenib failure or lenvatinib followed by regorafenib, and sorafenib was received again after regorafenib failure; (V) received traditional Chinese medicine (TCM) during the course of treatment.
Procedures
According to the use of first-line targeted drugs, 50 patients were divided into the sorafenib group (n=22) and lenvatinib group (n=28). If the patient’s disease progressed after receiving sorafenib or lenvatinib, sequential regorafenib was administered. Regorafenib was administered orally at 160 mg once daily during weeks 1–3 of each 4-week cycle. Regorafenib was received until the disease progressed according to the criteria in RECIST version 1.1 or mRECIST, the patient died, or had intolerable adverse events.
Efficacy and safety analyses
The records of adverse events included skin reactions, weight, sleep quality, fatigue, vital signs, blood biochemical indicators, urine and stool routine tests, and cardiopulmonary function. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (11). The evaluation of intrahepatic lesions was performed using liver magnetic resonance imaging (MRI), which was evaluated every 2 months until the patient stopped receiving regorafenib or died. According to the size of the lesion on the liver MRI and the extent of the lesion enhancement, the RECIST version 1.1 or mRECIST standard was used to evaluate the therapeutic effect (10,12). At the same time, the therapeutic effect was evaluated according to the changes in alpha fetoprotein (AFP) level (13,14).
Endpoints of study
The main endpoints of this study were ROS, RPFS, and TOS. The secondary endpoints were adverse events and the efficacy of regorafenib. ROS was calculated from the time of the beginning of regorafenib treatment to the patient’s death or the last follow-up. RPFS was calculated from the beginning of regorafenib treatment to the end of receiving regorafenib due to disease progression or complications, or the last follow-up. TOS was calculated from the time of the beginning of sorafenib or lenvatinib treatment to the patient’s death or the last follow-up.
Follow-up
Patients were followed up every 2 months after receiving regorafenib. The tests of blood biochemistry, tumor markers [AFP, carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA 199)], urine and stool routine tests, heart and lung function examination, abdominal B-ultrasound, and liver MRI were performed at each of the follow-up visits. At the same time, a detailed record was obtained of the patient’s adverse events after receiving regorafenib, such as hypertension, rash, fatigue, and weight loss, among others. According to the patient’s adverse events, the attending physician decided whether to continue the original regimen of regorafenib or reduce the dose or stop regorafenib.
Statistical analysis
Measurement data are described by median (range), and the independent sample t-test or Mann-Whitney U test was performed to compare differences between groups. Count data are described by number (percentage), and differences were compared by the chi-square test or Fisher’s exact test. The Kaplan-Meier method was used to draw survival and PFS curves. 95% CI represent relative risk. P<0.05 was considered to indicate statistical significance. All data were analyzed by IBM SPSS Statistics for Windows software, version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Figure 1 shows the flow chart of this study. Initially, 73 patients were included in the study. After screening by the inclusion and exclusion criteria, a total of 50 patients were finally included. Twenty-three patients were excluded from this study, of which 3 patients were lost to follow-up. Five patients voluntarily stopped regorafenib for no reason. Four patients received targeted drugs other than sorafenib and lenvatinib. Six patients received lenvatinib after sorafenib and sequential regorafenib failed. Two patients received sorafenib after regorafenib and sequential regorafenib failed. Three patients received TCM treatment.
Figure 1.
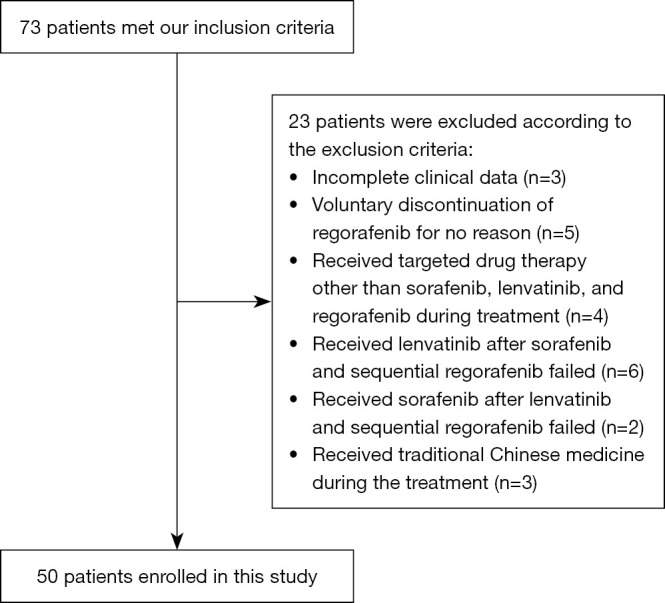
The flow chart of this study.
Table 1 shows the basic information of the 50 patients included in this study. There were no significant differences between the 2 groups of patients in age, sex, ECOG, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), prealbumin (pre-ALB), prothrombin time (PT), platelet (PLT), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), prognostic nutritional index (PNI), hepatitis B virus (HBV)-DNA level, HbsAg, liver cirrhosis, AFP, CEA, CA 199, protein induced by vitamin K absence or antagonist-II (PIVKA-II), Barcelona clinic liver cancer (BCLC) stage, tumor diameter, tumor number, vascular invasion, and extrahepatic metastasis (P>0.05). There were significant differences in age and total bilirubin (TBIL) between the 2 groups. The lenvatinib group was younger and had lower TBIL levels (P=0.033 and 0.018).
Table 1. Clinical characteristics of patients who accepted regorafenib.
| Variable | Sorafenib group | Lenvatinib group | Total (%) | P |
|---|---|---|---|---|
| Age, years | 58.5 (33.0–71.0) | 50.0 (33.0–75.0) | 54.5 (33.0–75.0) | 0.033 |
| Sex | ||||
| Male | 20 (90.9) | 25 (89.3) | 45 (90.0) | 0.849 |
| Female | 2 (9.1) | 3 (10.7) | 5 (10.0) | |
| ECOG performance status | ||||
| 0 | 8 (36.4) | 14 (50.0) | 22 (44.0) | 0.371 |
| 1 | 13 (59.1) | 14 (50.0) | 27 (54.0) | |
| 2 | 1 (4.5) | 0 (0) | 1 (2.0) | |
| TBIL, μmol/L | 17.3 (6.7–60.8) | 14.6 (4.0–34.4) | 15.9 (4.0–60.8) | 0.018 |
| ALT, U/L | 27.0 (12.0–189.0) | 34.0 (13.0–71.0) | 31.5 (12.0–189.0) | 0.646 |
| AST, U/L | 34.0 (16.0–200.0) | 35.5 (16.0–111.0) | 34.5 (16.0–200.0) | 0.914 |
| ALB, g/L | 38.9 (8.7–48.2) | 40.5 (9.5–50.6) | 40.4 (8.7–50.6) | 0.338 |
| Pre-ALB, mg/L | 157.0 (42.0–254.0) | 177.5 (64.0–334.0) | 165 (42.0–334.0) | 0.107 |
| PT, seconds | 11.9 (10.0–13.7) | 11.7 (10.2–13.3) | 11.9 (10.0–13.7) | 0.145 |
| PLT, 109/L | 125.0 (33.0–222.0) | 118.0 (61.0–220) | 118.0 (33.0–222.0) | 0.538 |
| NLR | 3.1 (0.5–14.8) | 3.2 (0.9–7.9) | 3.2 (0.5–14.8) | 0.369 |
| PLR | 8.15 (5.8–23.2) | 5.5 (2.1–16.9) | 5.5 (1.8–23.2) | 0.845 |
| PNI | 39.4 (9.4–49.2) | 41.0 (9.9–51.4) | 40.9 (9.4–51.4) | 0.348 |
| HBV-DNA level, IU/mL | ||||
| <2,000 | 16 (72.7) | 22 (78.6) | 38 (57.1) | 0.631 |
| ≥2,000 | 6 (27.3) | 6 (21.4) | 12 (42.9) | |
| HbsAg | ||||
| Negative | 1 (4.5) | 4 (14.3) | 5 (10.0) | 0.254 |
| Positive | 21 (95.5) | 24 (85.7) | 45 (90.0) | |
| Liver cirrhosis a | ||||
| N | 2 (9.1) | 7 (25.0) | 9 (18.0) | 0.146 |
| Y | 20 (90.9) | 21 (75.0) | 41 (82.0) | |
| AFP, μg/L | 75.7 (1.6–108571.0) | 176.5 (1.2–162793.0) | 83.8 (1.2–162791.8) | 0.883 |
| AFP, μg/L | ||||
| <400 | 13 (59.1) | 16 (57.1) | 29 (58.0) | 0.890 |
| ≥400 | 9 (40.1) | 12 (42.9) | 21 (42.0) | |
| CEA, ng/mL | 2.8 (1.0–44.4) | 2.6 (0.8–6.3) | 2.7 (0.8–44.4) | 0.322 |
| CA 199, U/mL | 16.4 (2.0–138.0) | 15.3 (2.0–216.0) | 15.7 (2.0–216.0) | 0.272 |
| PIVKA-II, μg/L | 1868.5 (24.0–912229.0) | 2025.5 (20.0–125947.0) | 1929 (20.0–912229.0) | 0.777 |
| BCLC stage | ||||
| A | 2 (9.1) | 2 (7.1) | 4 (8.0) | 0.916 |
| B | 11 (50.0) | 8 (46.4) | 19 (38.0) | |
| C | 9 (40.9) | 13 (46.4) | 22 (44.0) | |
| Tumor diameter, cm a | ||||
| <5 | 8 (36.4) | 9 (32.1) | 17 (34.0) | 0.754 |
| ≥5 | 14 (63.6) | 19 (67.9) | 33 (66.0) | |
| Number of tumors a | ||||
| Single | 2 (9.1) | 5 (17.9) | 7 (14.0) | 0.375 |
| Multiple | 20 (90.9) | 23 (82.1) | 43 (86.0) | |
| Vascular invasion a | ||||
| N | 14 (63.6) | 18 (64.3) | 32 (64.0) | 0.962 |
| Y | 8 (36.4) | 10 (35.7) | 18 (36.0) | |
| Extrahepatic metastasis a | ||||
| N | 17 (77.3) | 23 (82.1) | 40 (80.0) | 0.669 |
| Y | 5 (22.7) | 5 (17.9) | 10 (20.0) | |
a, Based on pre-treatment imaging studies. Data were presented as number (%) or median (IQR). ECOG, Eastern Cooperative Oncology Group; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; pre-ALB, prealbumin; PT, prothrombin time; PLT, platelet count; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; HBV, hepatitis B virus; AFP, alpha fetoprotein; CEA, carcinoembryonic antigen; CA 199, carbohydrate antigen 199; PIVKA-II, protein induced by vitamin K absence or antagonist-II; BCLC, Barcelona clinic liver cancer; N, no; Y, yes.
Other treatments before and after receiving regorafenib
Before receiving regorafenib, 13 patients (59.1%) in the sorafenib group underwent surgery, 5 patients (22.7%) received ablation treatment, 19 patients (86.4%) received transcatheter arterial chemoembolization (TACE), 1 patient (4.5%) received radiotherapy, and 14 patients (63.6%) received immunotherapy. In the lenvatinib group, the numbers of patients who accepted surgery, ablation, TACE, radiotherapy, and immunotherapy were 14 (50.0%), 5 (17.9%), 23 (82.1%), 5 (17.9%), and 20 (71.4%), respectively. There was no significant difference in other treatments which were received before regorafenib (P>0.05). During the period of receiving regorafenib treatment, 2 patients (9.1%) in the sorafenib group received ablation, 11 patients (50.0%) received TACE, 1 patient (4.5%) received radiotherapy, and 16 patients (72.7%) received immunotherapy. In the lenvatinib group, the numbers of patients who received ablation, TACE, radiotherapy, and immunotherapy were 3 (10.7%), 13 (46.4%), 2 (7.1%), and 25 (89.3%), respectively. There was no significant difference in other treatments which were received during the period of receiving regorafenib (P>0.05; Table 2).
Table 2. Summary of other treatments before or after regorafenib.
| Treatment | Sorafenib group (%) | Lenvatinib group (%) | Total (%) | P |
|---|---|---|---|---|
| Surgery a | ||||
| N | 9 (40.9) | 14 (50.0) | 23 (46.0) | 0.522 |
| Y | 13 (59.1) | 14 (50.0) | 27 (54.0) | |
| Ablation a | ||||
| N | 17 (77.3) | 23 (82.1) | 40 (80.0) | 0.669 |
| Y | 5 (22.7) | 5 (17.9) | 10 (20.0) | |
| TACE a | ||||
| N | 3 (13.6) | 5 (17.9) | 8 (16.0) | 0.686 |
| Y | 19 (86.4) | 23 (82.1) | 42 (84.0) | |
| Radiotherapy a | ||||
| N | 21 (95.5) | 23 (82.1) | 44 (88.0) | 0.131 |
| Y | 1 (4.5) | 5 (17.9) | 6 (12.0) | |
| Immunotherapy a | ||||
| N | 8 (13.6) | 8 (14.3) | 16 (32.0) | 0.558 |
| Y | 14 (63.6) | 20 (71.4) | 34 (68.0) | |
| Ablation b | ||||
| N | 20 (90.9) | 25 (89.3) | 45 (90.0) | 0.849 |
| Y | 2 (9.1) | 3 (10.7) | 5 (10.0) | |
| TACE b | ||||
| N | 11 (50.0) | 15 (53.6) | 26 (52.0) | 0.802 |
| Y | 11 (50.0) | 13 (46.4) | 24 (48.0) | |
| Radiotherapy b | ||||
| N | 21 (95.5) | 26 (92.9) | 47 (94.0) | 0.698 |
| Y | 1 (4.5) | 2 (7.1) | 3 (6.0) | |
| Immunotherapy b | ||||
| N | 2 (9.1) | 4 (14.3) | 6 (12.0) | 0.683 |
| Y | 20 (90.9) | 24 (85.7) | 44 (88.0) |
a, treatment before accepting regorafenib; b, treatment after accepting regorafenib; TACE, transcatheter arterial chemoembolization; N, no; Y, yes.
Adverse events of regorafenib
Table 3 shows the adverse events of the 50 patients after receiving regorafenib. The results showed that the incidence of adverse events in the 50 patients was 68.0%, the incidence of grade I/II adverse events was 60.0%, and the incidence of grade III/IV adverse events was 24.0%. The most common adverse events were hand-foot skin reactions (16.0%), fatigue (14.0%), and diarrhea (12.0%). The incidence rates of adverse events in the sorafenib and lenvatinib groups were 72.7% and 64.3%, respectively. The incidence rates of grade III/IV adverse events in the sorafenib and lenvatinib groups were 27.3% and 21.4%, respectively. The difference in the incidence of all adverse events and grade III/IV adverse events was not statistically significant (P=0.525 and 0.631).
Table 3. Complications of patients who accepted regorafenib.
| Complications | All grades (%) | III/IV grades (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Both groups | Sorafenib group | Lenvatinib group | P | Both groups | Sorafenib group | Lenvatinib group | P | ||
| Total, patients | 34 (68.0) | 16 (72.7) | 18 (64.3) | 0.525 | 12 (24) | 6 (27.3) | 6 (21.4) | 0.631 | |
| Hypertension | 3 (6.0) | 1 (4.5) | 2 (7.1) | 2 (4.0) | 1 (4.5) | 1 (3.6) | |||
| Hand-foot skin reaction | 8 (16.0) | 4 (18.2) | 4 (14.3) | 2 (4.0) | 1 (4.5) | 1 (3.6) | |||
| Diarrhea | 6 (12.0) | 2 (9.1) | 4 (14.3) | 2 (4.0) | 1 (4.5) | 1 (3.6) | |||
| Fatigue | 7 (14.0) | 4 (18.2) | 3 (10.7) | 1 (2.0) | 0 | 1 (3.6) | |||
| Increased ALT | 3 (6.0) | 2 (9.1) | 1 (3.6) | 3 (6.0) | 2 (9.1) | 1 (3.6) | |||
| Increased AST | 3 (6.0) | 3 (13.6) | 0 | 2 (4.0) | 2 (9.1) | 0 | |||
| Increased TBIL | 6 (12.0) | 3 (13.6) | 3 (10.7) | 2 (4.0) | 0 | 2 (7.1) | |||
| Weight loss | 5 (10.0) | 2 (9.1) | 3 (10.7) | 0 | 0 | 0 | |||
| Proteinuria | 2 (4.0) | 1 (4.5) | 1 (3.6) | 0 | 0 | 0 | |||
| Others | 3 (6.0) | 2 (9.1) | 1 (3.6) | 0 | 0 | 0 | |||
TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
The efficacy of regorafenib
Of the 50 patients included in this study, 24 patients died, including 15 deaths (68.2%) in the sorafenib group and 9 deaths (32.1%) in the lenvatinib group. According to the RECIST 1.1 standard, the numbers of patients with complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were 0, 7 (14.0%), 24 (48.0%), and 19 (38.0%), respectively. The corresponding objective response rate (ORR) was 14.0% and the disease control rate (DCR) was 62.0%. The ORRs of the sorafenib group and the lenvatinib group were 18.2% and 10.7%, respectively (P=0.684). The corresponding DCRs of the sorafenib group and the lenvatinib group were 63.7% and 60.7%, respectively (P=0.833).
According to the mRECIST standard, the numbers of patients with CR, PR, SD, and PD were 2 (4.0%), 9 (18.0%), 19 (38.0%), and 20 (40.0%), respectively. The corresponding ORR was 22.0% and the DCR was 60.0%. The ORRs of the sorafenib group and lenvatinib group were 22.7% and 21.4%, respectively (P=0.912). The corresponding DCRs of the sorafenib group and lenvatinib group were 59.1% and 60.7%, respectively (P=0.907), and there was no significant difference (Table 4).
Table 4. Evaluation according to RECIST 1.1 and mRECIST standards.
| Efficacy evaluation | RECIST 1.1 (%) | mRECIST (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Sorafenib group | Lenvatinib group | P | Total | Sorafenib group | Lenvatinib group | P | ||
| CR | 0 | 0 | 0 | 0.684 | 2 (4.0) | 1 (4.5) | 1 (3.5) | 0.912 | |
| PR | 7 (14.0) | 4 (18.2) | 3 (10.7) | 9 (18.0) | 4 (18.2) | 5 (17.9) | |||
| SD | 24 (48.0) | 10 (45.5) | 14 (50.0) | 0.833 | 19 (38.0) | 8 (36.4) | 11 (39.3) | 0.907 | |
| PD | 19 (38.0) | 8 (36.3) | 11 (39.3) | 20 (40.0) | 9 (40.9) | 11 (39.3) | |||
RECIST, Response Evaluation Criteria in Solid Tumours; mRECIST, modified Response Evaluation Criteria In Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Long-term prognosis of regorafenib
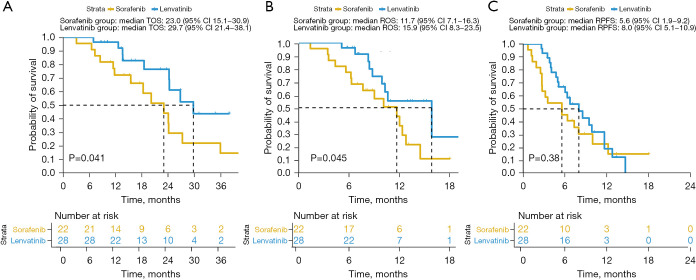
The median TOS of the 50 HCC patients was 24.2 months (95% CI: 21.1–27.3), the median ROS was 11.7 months (95% CI: 9.4–14.0), and the median RPFS was 6.7 months (95% CI: 4.5–9.0). The median TOS of the sorafenib group was 23.0 months (95% CI: 15.1–30.9) and the median TOS of the lenvatinib group was 29.7 months (95% CI: 21.4–38.1). There was a significant difference in TOS between the 2 groups (P=0.041; Figure 2A). The median ROS of the sorafenib group was 11.7 months (95% CI: 7.1–16.3). The median ROS of the lenvatinib group was 15.9 months (95% CI: 8.3–23.5), with a significant difference (P=0.045; Figure 2B). The RPFS of the sorafenib group was 5.6 months (95% CI: 1.9–9.2), the median RPFS of the lenvatinib group was 8.0 months (95% CI: 5.1–10.9), and there was no significant difference (P=0.380; Figure2C).
Figure 2.
Kaplan-Meier estimate of total overall survival (A), regorafenib overall survival (B), and regorafenib progression-free survival (C) for the sorafenib and lenvatinib groups of HCC patients. HCC, hepatocellular carcinoma.
Discussion
Most HCC patients are in the middle and advanced stages at the time of diagnosis, and the prognosis of these patients is poor (3). With the advent of targeted drugs, the prognosis of patients with advanced HCC has been greatly improved. Sorafenib and lenvatinib are the first-line drugs for targeted therapy of HCC. With the treatment of targeted drugs, resistance to targeted drugs will inevitably appear after a period of PFS (4,15). Regorafenib is a second-line drug for targeted therapy of HCC (7), which can be used to overcome ineffectiveness and resistance to first-line targeted drugs (8).
However, research on second-line targeted drugs for HCC is not clear, and there is a lack of research on sequential second-line targeted treatments after different first-line targeted drugs. Especially, there is a lack of studies on sequential regorafenib after sorafenib and lenvatinib treatment failure in HCC patients. This study is a real-world study of regorafenib used for unresectable HCC patients. It is also the first study reporting the adverse reactions, clinical efficacy, and long-term prognosis of sequential regorafenib after sorafenib and lenvatinib treatment failure in HCC patients in detail. Besides, our study first reported that there is a difference in the long-term prognosis of patients on sequential regorafenib after sorafenib and lenvatinib treatment failure. There are great significance for the treatment of HCC, especially for the treatment of targeted drugs for HCC. Our results show that regorafenib is safe for HCC patients. The overall incidence of adverse events was 68%, most of which were grade I/II adverse events. The incidence of grade III/IV adverse events was 24%, which is basically consistent with the reports of previous study (7). A previous study reported that the ORR of regorafenib was 7.7% and the DCR was 53.4% (16). Another study also reported that the ORR of regorafenib combined with TACE was 35.3% and the DCR was 76.5% (17). In this study, as a second-line targeted drug, regorafenib had an ORR of 14.0–22.0% and a DCR of 62.0–60.0%. The median TOS of the 50 HCC patients was 24.2 months (95% CI: 21.1–27.3), which was basically consistent with the results of the phase III RESORCE trial (18). A real-world study in South Korea showed that the median ROS was 12.1 months and the median RPFS was 3.2 months (16). In our study, the median ROS was 11.7 months (95% CI: 9.4–14.0) and the median RPFS was 6.7 months (95% CI: 4.5–9.0). The median RPFS reported in our study is better than that reported in previous studies. This may be due to the fact that 50% of the patients included in our study were treated with TACE while receiving regorafenib. A study reported that the combination of regorafenib and TACE can improve the RPFS of HCC patients. In patients treated with regorafenib combined with TACE, the median RPFS can reach 9.1 months, and the median ROS can reach 14.3 months (19). In addition, a previous study has reported that regorafenib results in an anti-tumor immune response for other cancers (20). In this study, 82.0% of patients received programmed cell death protein 1 (PD-1) treatment while receiving regorafenib. Whether receiving regorafenib combined with PD-1 treatment can improve the efficacy for HCC patients is still unclear, making it worthy of further thought and attention. Further analysis found that the median TOS and ROS of the lenvatinib group were longer than those of the sorafenib group. However, the results showed that the RPFS of the lenvatinib group was not significantly different from that of the sorafenib group. The curve trend showed that the RPFS of the lenvatinib group was better than that of the sorafenib group. The lack of significant differences may be due to the small sample size of our study. Regarding the different prognoses of different first-line targeted drugs followed by regorafenib, we suspect that the reason may be related to the different targets of the first-line targeted drugs. Although sorafenib and lenvatinib are both multikinase inhibitors (21,22), the specific targets and pathways of the 2 targeted drugs are different. Sorafenib mainly acts on c-CRAF, BRAF, mutant BRAF, KIT, FLT-3, RET, and VEGFR 1-3, while lenvatinib mainly acts on VEGFR 1-3, FGFR 1-4, PDGFα, KIT, and RET (23,24). Compared with sorafenib, lenvatinib can act on FGFR. One study reported that after inhibiting FGFR in HCC cells, epidermal growth factor receptor (EGFR) can feedback activation of its downstream PAK2-ERK5 signaling pathway and its common downstream MEK1/2-ERK1/2 signaling pathway with FGFR, resulting in HCC cells having a strong ability to survive and proliferate under the condition of continuous use of targeted drugs (25). Therefore, the 2 targets of EGFR and FGFR play an important role in the efficacy of targeted drugs. Lenvatinib can act on FGFR 1-4. However, EGFR would be upregulated when lenvatinib inhibits FGFR, and results in the resistance of HCC cells to lenvatinib (25). Regorafenib can act on EGFR. However, sorafenib cannot have an effect on these 2 targets. This may be the reason why the efficacy of lenvatinib followed by regorafenib is better than that of sorafenib followed by regorafenib. Regarding the specific reasons for the different prognoses of different first-line targeted drugs followed by regorafenib, we are conducting further exploration and verification using cytology.
It should be noted that this study is a single-center, small-sample, real-world study. Thus, the results need to be further validated in large-sample prospective studies.
Conclusions
This is the first study to report the adverse reactions, clinical efficacy, and long-term prognosis of regorafenib in HCC patients in detail. Regorafenib is an effective drug for the second-line treatment of HCC, with an ORR of 14.0–22.0% and a DCR of 62.0–60.0%. Besides, this study first reported that there is a difference in the long-term prognosis of patients receiving sequential regorafenib after different first-line targeted drug treatment. The TOS of the first-line targeted drug followed by regorafenib is better, especially for patients on lenvatinib followed by regorafenib. In addition, regorafenib has fewer severe adverse events. Therefore, for patients with unresectable HCC, it is safe and effective to accept regorafenib in time when the disease progresses after first-line targeted drugs, especially for patients whose first-line targeted drug is lenvatinib. Timely acceptance of regorafenib may result in patients achieving a better prognosis.
Acknowledgments
Funding: This study was funded in full by the WuMengchao talent plan fund.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The retrospective study protocol was reviewed and approved by The Ethics Committee of Eastern Hepatobiliary Surgery Hospital. All patients provided informed consent before treatment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-404/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-404/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-404/coif). The authors have no conflicts of interest to declare.
(English Language Editor: C. Betlazar-Maseh)
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Feng RM, Zong YN, Cao SM, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. 10.1186/s40880-019-0368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-66. 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Adjei AA. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015;20:660-73. 10.1634/theoncologist.2014-0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogasawara S, Chiba T, Ooka Y, et al. Characteristics of patients with sorafenib-treated advanced hepatocellular carcinoma eligible for second-line treatment. Invest New Drugs 2018;36:332-9. 10.1007/s10637-017-0507-3 [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 8.Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv238-55. 10.1093/annonc/mdy308 [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. Protocol development. Cancer therapy evaluation program. (accessed March 21, 2017). Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- 12.Sager S, Akgün E, Uslu-Beşli L, et al. Comparison of PERCIST and RECIST criteria for evaluation of therapy response after yttrium-90 microsphere therapy in patients with hepatocellular carcinoma and those with metastatic colorectal carcinoma. Nucl Med Commun 2019;40:461-8. 10.1097/MNM.0000000000001014 [DOI] [PubMed] [Google Scholar]
- 13.Nakazawa T, Hidaka H, Takada J, et al. Early increase in α-fetoprotein for predicting unfavorable clinical outcomes in patients with advanced hepatocellular carcinoma treated with sorafenib. Eur J Gastroenterol Hepatol 2013;25:683-9. 10.1097/MEG.0b013e32835d913b [DOI] [PubMed] [Google Scholar]
- 14.Patel T, Harnois D. Assessment of response to therapy in hepatocellular carcinoma. Ann Med 2014;46:130-7. 10.3109/07853890.2014.891355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin S, Bi F, Gu S, et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol 2021;39:3002-11. 10.1200/JCO.21.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo C, Byeon S, Bang Y, et al. Regorafenib in previously treated advanced hepatocellular carcinoma: Impact of prior immunotherapy and adverse events. Liver Int 2020;40:2263-71. 10.1111/liv.14496 [DOI] [PubMed] [Google Scholar]
- 17.Cao F, Zheng J, Luo J, et al. Treatment efficacy and safety of regorafenib plus drug-eluting beads-transarterial chemoembolization versus regorafenib monotherapy in colorectal cancer liver metastasis patients who fail standard treatment regimens. J Cancer Res Clin Oncol 2021;147:2993-3002. 10.1007/s00432-021-03708-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol 2018;69:353-8. 10.1016/j.jhep.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Cao G, Sun B, et al. Regorafenib combined with transarterial chemoembolization for unresectable hepatocellular carcinoma: a real-world study. BMC Gastroenterol 2021;21:393. 10.1186/s12876-021-01967-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu RY, Kong PF, Xia LP, et al. Regorafenib Promotes Antitumor Immunity via Inhibiting PD-L1 and IDO1 Expression in Melanoma. Clin Cancer Res 2019;25:4530-41. 10.1158/1078-0432.CCR-18-2840 [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 22.Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014;2014:638747. 10.1155/2014/638747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008;7:3129-40. 10.1158/1535-7163.MCT-08-0013 [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 2014;6:18. 10.1186/2045-824X-6-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin H, Shi Y, Lv Y, et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature 2021;595:730-4. 10.1038/s41586-021-03741-7 [DOI] [PubMed] [Google Scholar]