Abstract
Background
Gastric or gastroesophageal junction (GEJ) adenocarcinoma is the most common form of gastric cancer diagnosed in the United States (US) each year. Diagnosis typically is in later stages of disease when it has advanced. Patients have been treated with a variety of regimens.
Methods
The goal of this retrospective study was to understand if treatment patterns were becoming more homogeneous or remaining heterogeneous using the Herfindahl-Hirschman index (HHI) and if treatments were becoming more concordant to treatment guidelines published by the National Comprehensive Cancer Network (NCCN). HHI scores were calculated for each site by 2-year increments. Trend analyses were conducted for HHI scores over time using a linear regression model. Concordance to Category 1 and any category NCCN guidelines was determined based on the date treatment was initiated with the version of the NCCN guidelines at that time. Time trend analyses were conducted using linear regression models. This study utilized data from the Flatiron Advanced Gastric/Esophageal cohort. This study also examined overall survival (OS) rates estimated by the Kaplan-Meier method by line of therapy.
Results
There were no statistically significant differences in HHI scores in the first-line setting over time, suggesting heterogeneity has not improved. Concordance to NCCN treatment guidelines for any category significantly increased over time, however Category 1 regimen concordance remained low in the first-line setting. Concordance over time improved in second-line treatment. Median OS from the start of first-line therapy was 13.57 months. There was no relationship between OS time from initiation of first-line therapy and HHI score, concordance with NCCN guidelines, or concordance with NCCN Category 1 guidelines in the first-line setting.
Conclusions
Treatment heterogeneity persists in gastric cancer care, though there is a significant association between heterogeneity and concordance with both Category 1 and any category in the NCCN treatment guidelines, and that concordance has increased over time.
Keywords: Treatment patterns, National Comprehensive Cancer Network (NCCN) guidelines, treatment heterogeneity, retrospective, overall survival (OS)
Introduction
Approximately 26,380 new cases of gastric or gastroesophageal junction (GEJ) adenocarcinoma, the most common form of gastric cancer (hereafter, simply gastric cancer) are diagnosed in the United States (US) each year (1). The majority of patients are diagnosed with advanced or metastatic disease when surgery and local therapies are no longer effective. Metastatic gastric cancer typically has poor survival; the 5-year overall survival (OS) rate is 5.5% (2). First-line treatment for patients diagnosed with advanced or metastatic disease currently consists of platinum and/or fluoropyrimidine-based regimens (3). Many of the agents used in the first-line setting are also used in subsequent lines of therapy in a wide variety of combinations (4). Although there have been recent US Food and Drug Administration approvals of novel targeted agents in later lines of therapy such as, ramucirumab (5,6) and pembrolizumab (7), there remains considerable variability in the treatment patterns of patients with gastric cancer (4,8-11).
The National Comprehensive Cancer Network (NCCN) has established and continuously updates oncology treatment guidelines (12). These guidelines provide a summary of the best evidence for the care for patients diagnosed with cancer. Because these guidelines are considered the best practices for treating cancer in the US, it was hypothesized that greater concordance with these guidelines should reduce treatment heterogeneity and is expected to lead to the optimal patient outcomes.
This study was designed to examine heterogeneity and NCCN guideline concordance over time among patients with advanced/metastatic gastric cancer in the US. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-890/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board approval (WCG IRB, protocol approval number 420180044) of the study protocol for data collection to build the electronic health record (EHR-derived database was obtained by Flatiron Health prior to study conduct. Informed consent for this retrospective analysis was waived.
Data source
This retrospective observational study utilized the Flatiron Health database, a nationwide longitudinal, demographically, and geographically diverse database derived from de-identified EHR data. The Flatiron Health EHR-derived database includes de-identified data from over 280 cancer clinics (~800 sites of care) representing more than 2.2 million US cancer patients. The Flatiron Advanced Gastric/Esophageal cohort is selected from the overall Flatiron database and contains additional enhanced data that are abstracted from patient records. The cohort for this retrospective study includes gastric, GEJ, and esophageal cancer diagnoses.
Patients are included in this cohort of advanced/metastatic if they were diagnosed with stage IV disease or with distant recurrence, had a second locoregional recurrence after any initial stage at diagnosis, a first locoregional recurrence that was not completely resected (or any locoregional recurrence), or did not have surgical resection of the primary tumor. The diagnosis of advanced or metastatic disease must have occurred on or after January 1, 2011–June 30, 2018 for patients to be included in this analysis.
Eligibility criteria
Patients were eligible for inclusion from the Flatiron Advanced Gastric/Esophageal cohort if they were diagnosed with advanced or metastatic gastric or GEJ adenocarcinoma. Patients were excluded who had no evidence of systemic therapy recorded in the database or if they had tumors with squamous histology. Patients were also ineligible if they were less than 18 years of age at the time of advanced diagnosis. Follow-up data were available through June 2018 at the time of this analysis.
Statistical analysis
Treatment heterogeneity
Treatment heterogeneity across the study cohort was evaluated using the Herfindahl-Hirschman index (HHI) (13,14). The HHI score is calculated using the following formula:
| [1] |
where N is the number of regimens in the line of therapy and si is the proportion of the ith regimen.
HHI scores range from 0.0000 (complete heterogeneity) to 1.0000 (complete homogeneity). A difference of 0.1000 in HHI scores was considered to be practically meaningful for this study. HHI scores were calculated for each practice site with ≥10 patients. HHI scores were calculated by 2-year increments (2011–2012, 2013–2014, 2015–2016, 2017–2018). Trend analyses were conducted for HHI scores over time using a linear regression model. To avoid overestimating heterogeneity, 5-FU and capecitabine, doxorubicin and epirubicin, and paclitaxel and docetaxel were considered interchangeable and were collapsed into ‘fluoropyrimidine’, ‘anthracycline’, and ‘taxane’, respectively. Cisplatin and carboplatin were considered interchangeable and collapsed into ‘platinum’. No other platinum agents (i.e., oxaliplatin) were collapsed.
Concordance with NCCN guidelines
The date each patient initiated first- or second-line therapy was associated with the version of NCCN guidelines at the time of treatment initiation, using versions 1.2011-1.2018. Each patient’s regimen as recorded by the oncologist in the EHR was categorized into either “NCCN concordant” or “NCCN non-concordant” groups for each line of therapy. Regimens were considered concordant for all levels of evidence. Analyses evaluated concordance to Category 1 guidelines only and then concordance to any category (e.g., 1 through 2B). Time trend analyses using linear regression models were conducted to evaluate concordance with NCCN guidelines by year of initiation of first- and second-line therapy, respectively.
A generalized linear regression model with binomial distribution and logit link function were used to explore the relationship between HHI score and NCCN concordance, controlling for baseline demographics and clinical characteristics [age, gender, prior surgery (yes, no), disease site (gastric vs. GEJ), HER2 (+, −, missing)].
OS
OS measured from the start of first-line of therapy to death was estimated using Kaplan-Meier method by line of therapy. Death date (recorded as month and year to meet de-identification standards) is populated from structured fields in the EHR as well as from information captured in unstructured documents such as clinician notes and condolence letters. The association of the HHI score in first-line therapy and OS from initiation of first-line therapy and the relationship between NCCN first-line treatment guideline concordance and survival outcomes were assessed using Cox proportional hazards regression model controlling for baseline demographic and clinical characteristics. Patients who were lost to follow-up or still alive at the end of the database were censored at the last activity date. No imputation was made for missing data.
Results
Demographics
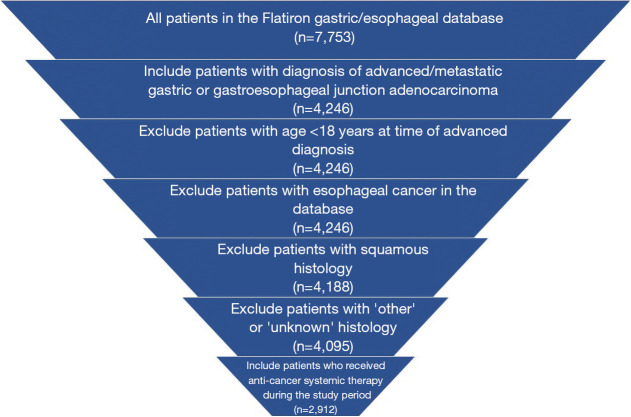
There were 2,912 patients who met eligibility criteria for inclusion in this study (Figure 1). The median age of the study cohort was 67 years, majority were male (70.9%) and white (61.1%). The majority of the cohort were diagnosed with gastric cancer (n=1,630, 56.0%) (Table 1). There were 1,230 patients who received second-line therapy in the cohort.
Figure 1.
Patient attrition.
Table 1. Demographics.
| Treated gastric/GEJ cohort (n=2,912) | Values |
|---|---|
| Age (median, range), years | 67 (23–85) |
| Gender, male | 70.9% |
| Race | |
| White | 61.1% |
| Black or African American | 8.5% |
| Asian | 4.5% |
| Other | 13.7% |
| Missing | 12.2% |
| Disease site | |
| Gastric | 56.0% |
| GEJ | 44.0% |
GEJ, gastroesophageal junction.
Treatment regimens
There were 102 and 96 regimens used in the first- and second-line settings, respectively. The most common first-line regimens were fluoropyrimidine (capecitabine or fluorouracil) + oxaliplatin (n=725, 24.9%), platinum (cisplatin or carboplatin) + taxane (n=511, 17.5%), and single-agent fluoropyrimidine (n=280, 9.6%). The most common second-line regimens were ramucirumab + taxane (n=203, 16.5%), fluoropyrimidine + oxaliplatin (n=155, 12.6%), and platinum + taxane (n=101, 8.2%) (Table 2).
Table 2. Most common treatment regimens across entire time period.
| Regimen | n (%) |
|---|---|
| Most common first-line regimens (n=2,912) | |
| Fluoropyrimidine†, oxaliplatin | 725 (24.9) |
| Platinum‡, taxane¶ | 511 (17.5) |
| Single-agent fluoropyrimidine† | 280 (9.6) |
| Fluoropyrimidine†, platinum‡, taxane¶ | 192 (6.6) |
| Anthracycline§, fluoropyrimidine†, oxaliplatin | 177 (6.1) |
| Doxorubicin or epirubicin, fluoropyrimidine†, platinum‡ | 117 (4.0) |
| Fluoropyrimidine†, oxaliplatin, trastuzumab | 109 (3.7) |
| Fluoropyrimidine†, platinum‡ | 90 (3.1) |
| Fluoropyrimidine†, oxaliplatin, taxane¶ | 70 (2.4) |
| Most common second-line regimens (n=1,230) | |
| Ramucirumab, taxane¶ | 203 (16.5) |
| Fluoropyrimidine†, oxaliplatin | 155 (12.6) |
| Platinum‡, taxane¶ | 101 (8.2) |
| Fluoropyrimidine† | 73 (5.9) |
| Fluoropyrimidine†, irinotecan | 73 (5.9) |
| Taxane¶ | 66 (5.4) |
| Ramucirumab | 53 (4.3) |
| Anthracycline§, fluoropyrimidine†, oxaliplatin | 31 (2.5) |
| Fluoropyrimidine†, oxaliplatin, trastuzumab | 31 (2.5) |
| Fluoropyrimidine†, platinum‡, taxane¶ | 30 (2.4) |
†, fluoropyrimidine = 5-FU or capecitabine; ‡, platinum = cisplatin or carboplatin; §, anthracycline = doxorubicin or epirubicin; ¶, taxane = paclitaxel or docetaxel.
Treatment heterogeneity
The median HHI score for first-line treatment throughout the study period was 0.1850 (min-max: 0.0900–0.4400) (Table 3). From 2011–2012, median HHI score was 0.1771 and was 0.1901 for the 2017–2018 time period (Table 4). There were no statistically significant differences in HHI scores in the first-line setting over time (P=0.25) (Table 4).
Table 3. HHI statistics (limited to sites with ≥10 patients).
| Line of therapy | Number of patients in calculation | Number of sites in calculation | HHI score | ||
|---|---|---|---|---|---|
| Mean (SD) | Median | Min, max | |||
| 1L | 2,583 | 85 | 0.2042 (0.0830) | 0.1850 | (0.0900, 0.4400) |
| 2L | 818 | 34 | 0.1364 (0.0437) | 0.1260 | (0.0700, 0.2400) |
HHI, Herfindahl-Hirschman index; SD, standard deviation; 1L, first-line; 2L, second-line.
Table 4. HHI over time by line of therapy (limited to sites with ≥10 patients).
| Grouped by year | Patients | Sites | HHI score | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Median | Min | Max | ||||
| 1L | ||||||||
| 2011–2012 | 197 | 12 | 0.1946 | 0.0531 | 0.1771 | 0.1357 | 0.3156 | 0.25* |
| 2013–2014 | 571 | 25 | 0.1930 | 0.0881 | 0.1851 | 0.1116 | 0.5690 | |
| 2015–2016 | 739 | 34 | 0.2291 | 0.0953 | 0.1950 | 0.1074 | 0.5400 | |
| 2017–2018 | 536 | 27 | 0.2161 | 0.0807 | 0.1901 | 0.1296 | 0.4438 | |
| 2L | ||||||||
| 2011–2012 | 24 | 2 | 0.1561 | 0.0621 | 0.1561 | 0.1122 | 0.2000 | 0.17* |
| 2013–2014 | 246 | 16 | 0.1397 | 0.0402 | 0.1312 | 0.0892 | 0.2089 | |
| 2015–2016 | 324 | 19 | 0.1803 | 0.0773 | 0.1531 | 0.1029 | 0.4000 | |
| 2017–2018 | 123 | 7 | 0.1961 | 0.0791 | 0.1735 | 0.1332 | 0.3633 | |
*, linear regression was performed on the trend analysis. HHI, Herfindahl-Hirschman index; 1L, first-line; 2L, second-line.
The median HHI score for second-line therapy was 0.1260 (min-max: 0.0700–0.2400) (Table 3) during the study period. Due to limited number of clinical sites with ≥10 patients, time trend analyses could not be conducted for the second-line setting.
Concordance with NCCN guidelines
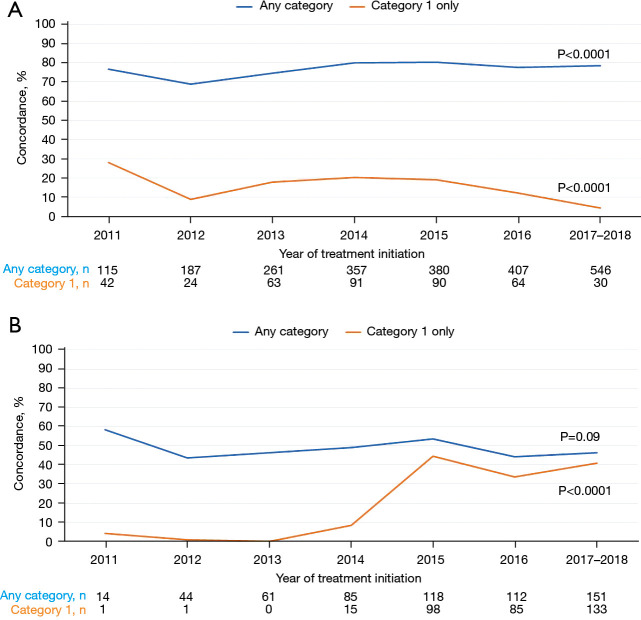
In the first-line setting, more than 70% of patients were treated in concordance with NCCN treatment guidelines for any category. There was a significant increase in concordance with NCCN treatment guidelines over time (P<0.0001, Figure 2A). However, Category 1 regimens were used with a low concordance with NCCN treatment guidelines (<30%) and a statistically significant decrease over time in the first-line setting (P<0.0001, Figure 2A).
Figure 2.
Concordance with NCCN guidelines over time. (A) First-line setting. (B) Second-line setting. NCCN, National Comprehensive Cancer Network.
In the second-line setting, approximately half of all patients were treated with NCCN concordant regimens with a numeric decrease over time in concordance with NCCN treatment guidelines (P=0.09). Although the concordance with NCCN treatment guidelines was very low in Category 1 regimens in early years (2011–2014), there was a statistically significant increase in the use of Category 1 NCCN concordant regimens in the second-line setting over time (P<0.0001, Figure 2B).
There was a statistically significant relationship between HHI score and concordance with NCCN treatment guidelines (P=0.001) and concordance with Category 1 guidelines (P=0.05) in the first-line setting (Table 5). The analysis could not be done in the second-line setting due to limited number of clinical sites with ≥10 patients.
Table 5. Association analyses.
| Analysis | N | P value |
|---|---|---|
| Association of 1L HHI and OS from initiation of 1L therapy | 2,390 | 0.71* |
| Concordance with NCCN 1L Category 1 guidelines and OS from initiation of 1L therapy | 2,912 | 0.45* |
| Concordance with any category NCCN 1L guideline and OS from initiation of 1L therapy | 2,912 | 0.76* |
| Association of HHI in 1L therapy and concordance with NCCN 1L Category 1 guidelines | 2,390 | 0.05** |
| Association of HHI in 1L therapy and concordance with any category NCCN 1L guidelines | 2,390 | 0.001** |
*, P values are from Cox proportional hazard regression model with covariates: age, gender, prior surgery (yes, no), disease site (gastric vs. GEJ), HER2 status (+, −, missing); **, P values are from generalized linear regression model with binomial distribution and logit link function with covariates: age, gender, prior surgery (yes, no), disease site (gastric vs. GEJ), HER2 status (+, −, missing). 1L, first-line; HHI, Herfindahl-Hirschman index; OS, overall survival; NCCN, National Comprehensive Cancer Network; GEJ, gastroesophageal junction.
OS
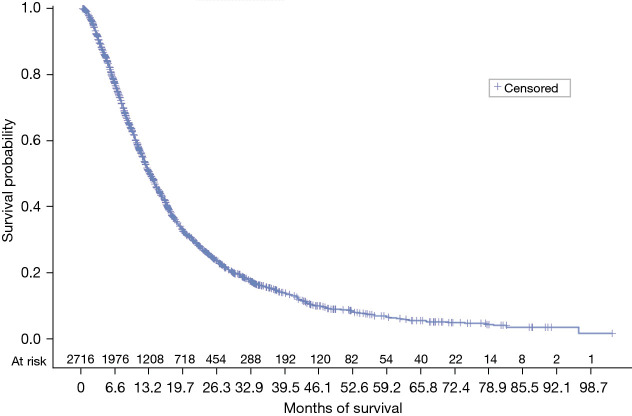
Median OS from the start of first-line therapy was 13.57 months (95% CI: 12.83–14.2) (Figure 3). Patients who were lost to follow-up or still alive at the end of the database were censored at the last activity date. There was no relationship between OS time from initiation of first-line therapy and HHI score (P=0.71), concordance with NCCN guidelines (P=0.76), or concordance with NCCN Category 1 guidelines (P=0.45) in the first-line setting (Table 5).
Figure 3.
OS from start of first-line therapy. OS, overall survival.
Discussion
The Flatiron Advanced Gastric cohort is a representative cohort of the gastric cancer population. While there is some degree of difference in diagnosis stage and race between Flatiron compared to SEER and NPCR data, the sex and geographic demographics are consistent (15). In a previous study conducted by Abrams and colleagues examining first-line treatment heterogeneity in gastric cancer using Truven Health MarketScan claims and Intercontinental Medical Statistics (IMS) Oncology EMR, the demographics of age and gender are consistent (8). The current study demographics show that the median age is 67 and the percentage of male patients is 70.9% (Table 1). Abrams and colleagues reported age at diagnosis between 60.4 (SD: 11.9) and 62.7 (SD: 12.1) and percentage male ranged from 64.7–74.2% across the three cohorts in their study (8).
The Flatiron Advanced Gastric cohort contains a subset of the overall Flatiron Health dataset with enhanced information. The records are randomly selected for inclusion by a randomized algorithm and then the data are abstracted electronically to enhance the data in the cohort.
Consistent with previous research using claims and IMS EMR data, which found HHI scores to be very heterogeneous (0.1400 for first-line and 0.1300 for second-line therapy) in claims and EMR databases (8), patients with gastric cancer are treated with a variety of regimens, and heterogeneity was also high in this study (median HHI score in the first-line setting =0.1850 and median HHI score in the second-line setting =0.1260) (Table 3). In the first-line setting, concordance with regimens considered Category 1 in the NCCN guidelines was low and decreased over time periods. This is likely in part due to the frequent use of FOLFOX, which remains Category 2A level evidence in the NCCN guidelines, though is a ‘preferred’ NCCN regimen. Overall, concordance with NCCN guidelines was high in the first-line setting throughout the study period. Over the study period, the number of treatments that met the NCCN Category 1 threshold increased in both first- and second-line.
In the second-line setting, concordance with Category 1 NCCN treatment guidelines increased over time, but concordance rates remain less than 50%. Heterogeneity in first-line treatment would impact what options are available in second-line. By the last period evaluated (2017–2018), 78.6% of first-line and 46.3% of second-line treatments were consistent with NCCN guidelines in general, demonstrating a higher level of NCCN guideline-supported care for these patients in recent years. As of the end of this study period, for first-line, there was one Category 1, nine Category 2A, and five Category 2B options (12). The few numbers of Category 1 treatment options may explain the low concordance observed in this study. In second-line, there were five Category 1, three Category 2A, and one Category 2B treatments.
Heterogeneity in the treatment of gastric cancer persists, despite the continued refinement of guidelines. This observation holds true across different data sources (8,16). Heterogeneity in the treatment of gastric cancer has been previously described in the literature and it was expected that this study would have shown improvement in heterogeneity, given there was a longer time period for treatments to be available and used and demonstrate improvements in patient outcomes (8,9). OS was measured from initiation of first-line therapy to death. OS has improved since 2004 where it was reported that OS was only 3–6 months (17). More recent figures from 2016 of 10–12 months are consistent with the current study (18).
There was no association observed between HHI or NCCN concordance and OS from initiation of first-line therapy. This may be in part due to the limitations of a de-identified data set, which does not allow for exact dates to be provided and limited follow-up information from patients in the most recent years, where concordance was higher.
A longer period of follow-up would be needed to reduce censoring of patients who remained alive at the cut-off of the study period.
As with all studies using retrospective data, there are limitations. Causality cannot be inferred from this study; therefore, it is not possible to determine if concordance with guidelines is the causal factor that leads to better or worse outcomes in a retrospective study design, this design can only demonstrate the significant associations between factors. Other factors (e.g., differences in therapeutic options used) could have led changes in observed survival over time. Over the study period, there were 21 versions of gastric cancer treatment guidelines, and the heterogeneity of these guidelines could have influenced the ability to identify associations with survival outcomes.
This study was nevertheless able to identify a significant association between heterogeneity and concordance with both Category 1 and any category in the NCCN treatment guidelines. This, in part, is to be expected as the overall heterogeneity of treatment would be expected to decline as regimens narrow to those with NCCN-level evidence supporting their use. It also was able to demonstrate that concordance with guidelines has increased over time. Additional research is needed with longer follow-up to evaluate the impact of evidence-based care on patient outcomes.
Acknowledgments
This study was supported by employee time and materials provided by Eli Lilly and Company.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board approval (WCG IRB, protocol approval number 420180044) of the study protocol for data collection to build the EHR-derived database was obtained by Flatiron Health prior to study conduct. Informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-890/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-890/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-890/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-890/coif). All authors are employees of Eli Lilly and Company. JAD, ZLC, XL, CW are also shareholders of Eli Lilly and Company.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.SEER. Cancer Stat Facts: Stomach Cancer. National Institutes of Health 2022. Available online: https://seer.cancer.gov/statfacts/html/stomach.html
- 3.Barzi A, Hess LM, Zhu YE, et al. Real-World Outcomes and Factors Associated With the Second-Line Treatment of Patients With Gastric, Gastroesophageal Junction, or Esophageal Adenocarcinoma. Cancer Control 2019;26:1073274819847642. 10.1177/1073274819847642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess LM, Michael D, Mytelka DS, et al. Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective analysis of electronic medical record (EMR) and administrative claims data. Gastric Cancer 2016;19:607-15. 10.1007/s10120-015-0486-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, andomized, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 6.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, andomized phase 3 trial. Lancet Oncol 2014;15:1224-35. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 7.Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams T, Hess LM, Zhu YE, et al. Predictors of heterogeneity in the first-line treatment of patients with advanced/metastatic gastric cancer in the U.S. Gastric Cancer 2018;21:738-44. 10.1007/s10120-018-0802-5 [DOI] [PubMed] [Google Scholar]
- 9.Hess LM, Zhu YE, Fang Y, et al. Health care resource utilization and treatment variability in the care of patients with advanced or metastatic colorectal or gastric cancer. J Med Econ 2021;24:930-8. 10.1080/13696998.2021.1958607 [DOI] [PubMed] [Google Scholar]
- 10.Karve S, Lorenzo M, Liepa AM, et al. Treatment Patterns, Costs, and Survival among Medicare-Enrolled Elderly Patients Diagnosed with Advanced Stage Gastric Cancer: Analysis of a Linked Population-Based Cancer Registry and Administrative Claims Database. J Gastric Cancer 2015;15:87-104. 10.5230/jgc.2015.15.2.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liepa A, Mitchell S, Batson S, et al. 2314 Systematic review and meta-analysis of recommended second-line therapies for advanced gastric cancer (GC). ScienceDirect 2015;51:S437. abstract 2314. 10.1016/S0959-8049(16)31230-8 [DOI] [Google Scholar]
- 12.NCCN. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Guideline Gastric V.1.2013-V.2.2018. To view the most recent and complete version of the guideline, available online: https://www.nccn.org
- 13.Cutler DM, Scott Morton F. Hospitals, market share, and consolidation. JAMA 2013;310:1964-70. 10.1001/jama.2013.281675 [DOI] [PubMed] [Google Scholar]
- 14.Rhoades SA. The Herfindahl-Hirschman index. Fed Res Bull 1993;79:188-9. [Google Scholar]
- 15.Ma X, Long L, Moon S, et al. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv 2020. doi: . 10.1101/2020.03.16.20037143 [DOI]
- 16.Paulson S, Hess LM, Chatterjee A, et al. The impact of a clinical decision support system for advanced gastric or gastroesophageal junction cancer. J Clin Pathways 2020;6:55-64. [Google Scholar]
- 17.Yang D, Hendifar A, Lenz C, et al. Survival of metastatic gastric cancer: Significance of age, sex and race/ethnicity. J Gastrointest Oncol 2011;2:77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol 2016;22:2403-14. 10.3748/wjg.v22.i8.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]