Abstract
Background
Repeated transcatheter arterial chemoembolization (TACE) could cause ischemia of the tumor tissue and increases production of angiogenic factors in patients with hepatocellular carcinoma (HCC). Lenvatinib can inhibit the expression of angiogenic factors induced by ischemia after TACE and reduce angiogenesis and tumor recurrence. TACE-lenvatinib sequential therapy may improve clinical outcomes. There have been few investigations of TACE-lenvatinib sequential therapy for the treatment of unresectable HCC. We aimed to evaluate the efficacy and safety of TACE-lenvatinib sequential therapy for unresectable HCC.
Methods
From May 2018 to May 2021, 53 consecutive patients who underwent TACE-lenvatinib sequential therapy were retrospectively reviewed. Of these, 30 patients who met the inclusion criteria were selected. Lenvatinib treatment started within 1 or 2 weeks after TACE at a dose of 8 or 12 mg once daily. Treatment response was assessed using dynamic magnetic resonance imaging (MRI) according to the modified response evaluation criteria in solid tumor (mRECIST). Blood tests were also performed at every response evaluation. Patients with complete response (CR) or partial response (PR) and stable disease (SD) received continuous lenvatinib therapy, and patients with progressive disease (PD) received repeated TACE. The progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), and adverse events (AEs) were calculated. Statistical analysis was performed using the Kaplan-Meier method.
Results
The median age was 58.5±9.1 years, and 16.7% (5/30) of patients were female. A total of 12 patients were categorized as Barcelona Clinic Liver Cancer (BCLC) Stage B and 18 were BCLC Stage C. The mean follow-up time was 15.7 months. The ORR was 76.7% (23/30), and the DCR was 96.7% (29/30). The median PFS was 6.1 months, and the median OS was 20.7 months. The most common lenvatinib-related AE was rash, and the most common TACE-related AE was elevated aspartate aminotransferase (AST). No treatment-related mortality was observed.
Conclusions
From our findings, TACE-lenvatinib sequential therapy may prolong OS and PFS in patients with unresectable HCC, and the side effects are acceptable. The efficacy and safety of the sequential therapy should be confirmed in multiple center randomized controlled trials (RCTs) with a large sample and sufficient follow-up period.
Keywords: Transcatheter arterial chemoembolization (TACE), lenvatinib, hepatocellular carcinoma (HCC), unresectable, sequential therapy
Introduction
Hepatocellular carcinoma (HCC) is currently the fifth most common malignancy in the world and the third cause of cancer-related deaths (1). However, only about 25% of HCC patients have surgical opportunities, because 70–80% patients are diagnosed at an advanced stage and can only choose non-surgical treatments (2). Transcatheter arterial chemoembolization (TACE) is the standard treatment for HCC patients of Barcelona Clinic Liver Cancer (BCLC) Stage B. Systemic treatment is the standard treatment for HCC patients with BCLC Stage C (1,2). However, because of the limitations of TACE (easy recurrence and metastasis), systemic treatment based on TACE has become the main treatment for patients with unresectable HCC (3-6).
In the past decade, many breakthroughs have been made in the clinical exploration of molecular targeted drugs in the treatment of HCC. Although sorafenib is the most popular first-line treatment for HCC, its response rate is only 2% (7). Lenvatinib is another first-line drug which was approved by the US Food and Drug Administration (FDA) in August 2018 to treat HCC. A previous study showed that lenvatinib was not inferior to sorafenib in overall survival (OS) in untreated advanced HCC, and significantly improved median progression-free survival (PFS), median time to progression, and objective response rates (ORR) compared with sorafenib (8). In addition, lenvatinib has more advantages in terms of mechanism of action, showing stronger inhibitory activity against multiple targets related to tumor vascular regulation and good synergistic inhibitory effects, especially against vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), and other receptors (9). Before the TACTICS trial, many studies failed to demonstrate the efficacy of TACE combined with molecular targeted agents (sorafenib, brivanib, orantinib) (10). The TACTICS trial showed that TACE plus sorafenib significantly improved PFS compared with TACE alone in unresectable HCC patients (3). Research on TACE-lenvatinib sequential therapy for the treatment of HCC has been scarce. The aim of this study was to evaluate the efficacy and safety of TACE-lenvatinib sequential therapy for unresectable HCC. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-525/rc).
Methods
Patients
Primary HCC was diagnosed according to the American Academy of Hepatology Guidelines (2018 Edition) (1). From May 2018 to May 2021, 53 patients received TACE-lenvatinib sequential therapy at The First Medical Center of Chinese PLA General Hospital. We selected 30 patients according to the follo wing inclusion criteria: (I) diagnosis of unresectable HCC; (II) aged 18–75 years; (III) Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2; (IV) Child–Pugh Class A or B liver function; (V) categorized as BCLC Stage B–C; (VI) a treatment interval >28 days since previous tyrosine kinase inhibitor (TKI) therapy; (VII) lesions able to be measured by magnetic resonance imaging (MRI). The exclusion criteria were as follows: (I) Child–Pugh Class C: (II) ECOG PS 3–4; (III) abnormal and uncorrectable coagulation function; (IV) heart, lung, or kidney dysfunction; (V) a lenvatinib duration of less than 4 weeks; (VI) any contraindication for therapy with TACE or lenvatinib. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethical approval was waived by the ethics committee of The First Medical Center of Chinese PLA General Hospital. The informed consent was not required because of the retrospective nature of this study.
Therapeutic method
TACE
All interventional procedures were performed by an expert with over 10 years of experience in the interventional treatment of HCC and followed the standard operating methods in our hospital. After femoral artery catheterization using modified Seldinger techniques, hepatic artery and superior mesenteric artery angiography were routinely performed with a 4F catheter (RH, Terumo Corporation, Tokyo, Japan) to evaluate the blood supply of the tumor and the patency of the portal vein. Selective hepatic artery angiography was performed if necessary. The tumor supplying the artery was super-selectively intubated with the 2.7F microcatheter (Progreat, Terumo Corporation, Japan). An emulsion of 6–20 mL of lipiodol (Lipiodol Ultrafluide; Guerbet, Paris, France), 30–50 mg of epirubicin (Farmorubicin; Pharmacia, Tokyo, Japan), 100–150 mg of oxaliplatin, 500–750 mg of 5-fluorouracil, 200–300 mg of calcium folinate, and 10–14 mg of mitomycin C (Kyowa Hakko Kogyo, Tokyo, Japan) was injected into the supply vessel of the tumor through the microcatheter. Ployvinyl alcohol (PVA; Cook Inc., Bloomington, IN, USA) and gelatin sponge particles (Gelfoam; Hangzhou Alc Ltd., Zhejiang, China) were used for embolization after the infusion of emulsion. Collateral artery embolization was performed if there were collateral vessels involved in the blood supply (e.g., inferior phrenic artery, internal thoracic artery, adrenal artery).
Lenvatinib treatment and assessment of adverse events (AEs)
Within 1 or 2 weeks after TACE, if the Child–Pugh score of liver function was A or B, and there were no contraindications for lenvatinib, the lenvatinib treatment was initiated. Lenvatinib (Levima®, Eisai, Tokyo, Japan) was administered once daily at a dose of 8 mg for patients weighing <60 kg and at a dose of 12 mg for patients weighing ≥60 kg. If a patient developed grade ≥3 severe lenvatinib-related AEs, stepwise dose modification was recommended as follows: reduced to 8 mg and 4 mg/day, or 4 mg every other day, according to the REFLECT trial (8). If the symptoms did not resolve to either grade 1 or 2 after the dose reduction, drug withdrawal was permitted. The AE grading of AEs complied with the Common Terminology Criteria for Adverse Events v4.03 (CTCAE; https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm).
Follow-up and efficacy assessment
To assess the efficacy of the treatment, the patients were regularly followed up. The first response evaluation was performed using dynamic MRI 4 weeks after sequential therapy, and dynamic MRI was performed every 8 weeks after the first evaluation. Blood tests were also performed at every response evaluation, including blood routine, liver function, and serum hepatitis B virus deoxyribonucleic acid (HBV DNA) and alpha-fetoprotein (AFP). According to the modified response evaluation criteria in solid tumor (mRECIST) (9), complete response (CR): the disappearance of all tumor; partial response (PR): at least a 30% decrease of the tumor size; progressive disease (PD): at least a 20% increase of the tumor size; stable disease (SD): less than 20% increase or 30% decrease of the tumor size. The disease control rate (DCR) was the percentage of CR, PR, and SD. The ORR was the percentage of CR and PR. The OS was defined as the time from first treatment to death or last follow-up (1 May 2021). The PFS was the time from first treatment to disease progression, death, or last follow-up. Patients with CR or PR and stable SD received continuous lenvatinib therapy. If treatment response was evaluated as PD, TACE was repeated.
Statistical analyses
Statistical analysis was performed using IBM SPSS™ Statistics software 21.0 (IBM Corp., Armonk, NY, USA). Most statistical analyses are descriptive. Continuous variables are described as median ± standard deviation (SD). Categorical variables are described as frequency and proportion. The OS and PFS were estimated using the Kaplan-Meier method.
Results
Patient characteristics
From May 2018 to May 2021, a total of 53 unresectable HCC patients who were treated with TACE-lenvatinib were reviewed. Of these, 30 patients meet the inclusion criteria. Of the 23 patients excluded from our study, 4 patients missed the enrolment date, 7 patients were lost to follow-up, 10 patients had received other therapy before study, and 2 patients received other therapy during the study (Figure 1). Baseline patient characteristics are summarized in Table 1. The median age was 58.5±9.1 years, and 16.7% (5/30) of patients were female. A total of 12 patients were categorized as BCLC Stage B and 18 were BCLC Stage C. Hepatitis B virus was the most common etiology of HCC (n=24, 80%). Extrahepatic metastases were observed in the lungs (n=7, 23.3%), retroperitoneal lymph node (n=5, 16.7%), bone (n=2, 6.7%), paranephros (n=2, 6.7%), pleura (n=1, 3.3%), and brain (n=1, 3.3%). Initial doses of lenvatinib were 12 mg (n=14, 46.7%) or 8 mg (n=16, 53.3%) daily. The initial dose was reduced for 7 patients (23.3%) because of stomachache (n=2, 6.7%), hypertension (n=2, 6.7%), diarrhea (n=2, 6.7%), vomiting (n=1, 3.3%), and hand-foot skin reaction (n=1, 3.3%). The mean follow-up time was 15.7 months.
Figure 1.
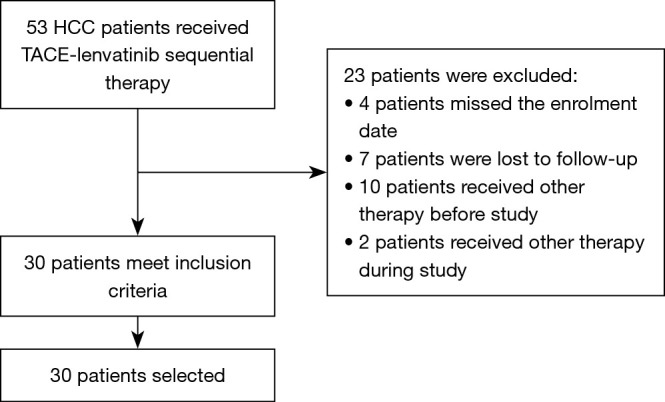
Flowchart of patient selection. HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization.
Table 1. Baseline characteristics.
| Characteristics | n (%)/mean ± SD |
|---|---|
| Gender | |
| Male | 25 (83.3) |
| Female | 5 (16.7) |
| Age (years) | 58.5±9.1 |
| Etiology | |
| Hepatitis B | 24 (80.0) |
| Hepatitis C | 4 (13.3) |
| Unknown | 2 (6.7) |
| BCLC stage | |
| A | 0 (0.0) |
| B | 12 (40.0) |
| C | 18 (60.0) |
| AST (U/L) | 37.9±18.3 |
| ALT (U/L) | 30.9±22.1 |
| PLT (×109) | 139.7±99.1 |
| TB (μmol/L) | 16.6±7.1 |
| ALB (g/L) | 38.0±5.6 |
| PT (s) | 20.1±18.5 |
| AFP (μg/L) | 4,810.0±12,421.5 |
| Macrovascular invasion | 12 (40.0) |
| Extrahepatic metastasis | 13 (13.3) |
| Lung | 7 (23.3) |
| Retroperitoneal lymph node | 5 (16.7) |
| Bone | 2 (6.7) |
| Paranephros | 2 (6.7) |
| Pleura | 1 (3.3) |
| Brain | 1 (3.3) |
| Initial dose of lenvatinib | |
| 12 mg | 14 (46.7) |
| 8 mg | 16 (53.3) |
| Reduced initial dose of lenvatinib | 7 (23.3) |
Measurement data is presented as mean ± SD; count date is presented as numbers and percentages. SD, standard deviation; BCLC stage, Barcelona Clinic Liver Cancer stage; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PLT, platelet; TB, total bilirubin; ALB albumin; PT, prothrombin time; AFP, alpha-fetoprotein.
Tumor response and survival outcomes
At the time of the best response evaluation based on the mRECIST, 7 patients achieved a CR, 16 patients achieved a PR, 6 patients exhibited an SD, and only 1 patient showed a PD after sequential therapy. The ORR was 76.7% (23/30) and the DCR was 96.7% (29/30). The details of tumor response are shown in Table 2. At the time of survival assessment, 14 of 30 (46.7%) patients had died. The median OS and PFS from the start of TACE-lenvatinib sequential therapy was 6.1 and 20.7 months, respectively. Kaplan-Meier survival curves are shown in Figures 2,3.
Table 2. The best tumor response of the patients.
| Tumor response | n (%) |
|---|---|
| CR | 7 (23.3) |
| PR | 16 (53.3) |
| SD | 6 (20.0) |
| PD | 1 (3.3) |
| ORR | 23 (76.7) |
| DCR | 29 (96.7) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Figure 2.
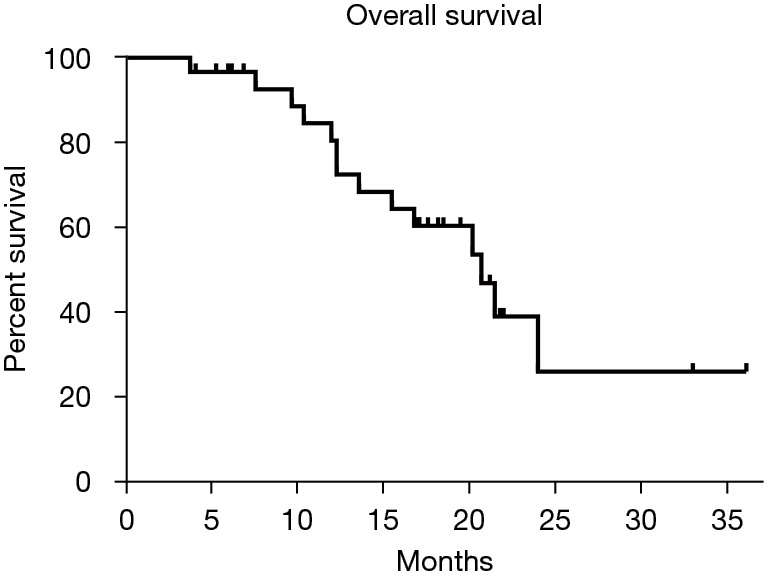
The median OS of the patients. OS, overall survival.
Figure 3.
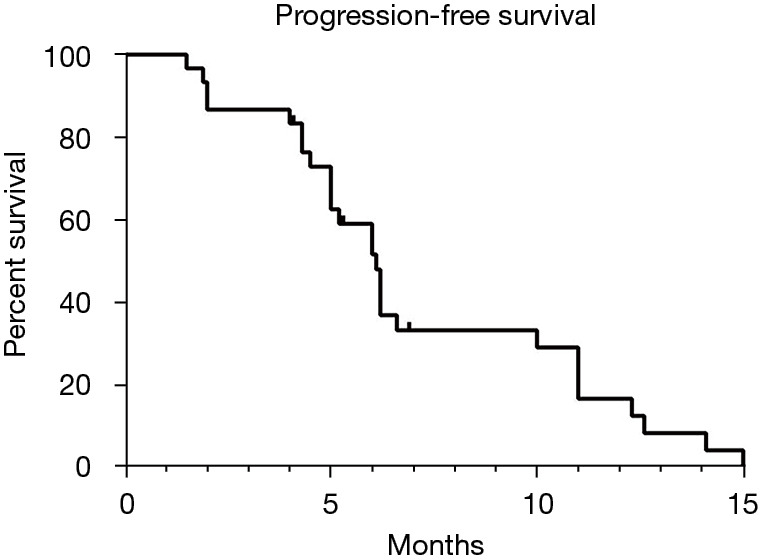
The median PFS of the patients. PFS, progression-free survival.
Adverse events and safety evaluation
The TACE-related AEs of any grade were elevated aspartate aminotransferase (AST; n=27, 90%), elevated alanine aminotransferase (ALT; n=27, 90%), elevated bilirubin (n=18, 60%), stomachache (n=22, 73.4%), fever (n=20, 66.7%), vomiting (n=7, 23.3%), constipation (n=7, 23.3%), abdominal distension (n=3, 10%), hiccups (n=3, 10%), and liver abscess (n=1, 3.3%).
The lenvatinib-related AEs of any grade were rash (n=6, 20%), hypertension (n=5, 16.7%), diarrhea (n=4, 13.3%), decreased appetite (n=3, 10%), fatigue (n=3, 10%), hand-foot skin reaction (n=2, 6.7%), stomachache (n=2, 6.7%), oral ulcer (n=2, 6.7%), myalgia (n=1, 3.3%), vomiting (n=1, 3.3%), obstructive jaundice (n=1, 3.3%), epistaxis (n=1, 3.3%), and gingival bleeding (n=1, 3.3%). There were no treatment-related deaths. The TACE-related AEs are shown in Table 3; lenvatinib-related AEs are shown in Table 4.
Table 3. TACE-related AEs.
| AEs | All grade n (%) | Grade 3 n (%) | Grade 4 n (%) |
|---|---|---|---|
| Elevated AST | 11 (90.0) | 8 (26.7) | 2 (6.7) |
| Elevated ALT | 11 (90.0) | 7 (23.3) | 1 (3.3) |
| Elevated bilirubin | 18 (60.0) | 3 (10.0) | 0 |
| Stomachache | 22 (73.4) | 0 | 0 |
| Fever | 20 (66.7) | 0 | 0 |
| Vomiting | 7 (23.3) | 0 | 0 |
| Constipation | 7 (23.3) | 0 | 0 |
| Abdominal distension | 3 (10.0) | 0 | 0 |
| Hiccups | 3 (10.0) | 0 | 0 |
| Liver abscess | 1 (3.3) | 0 | 1 (3.3) |
AEs, adverse events; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Table 4. Lenvatinib-related AEs.
| AEs | All grade n (%) | Grade 3 n (%) | Grade 4 n (%) |
|---|---|---|---|
| Rash | 6 (20.0) | 1 (3.3) | 0 |
| Hypertension | 5 (16.7) | 2 (6.7) | 0 |
| Diarrhea | 4 (13.3) | 0 | 0 |
| Decreased appetite | 3 (10.0) | 0 | 0 |
| Fatigue | 3 (10.0) | 0 | 0 |
| Hand-foot skin reaction | 2 (6.7) | 0 | 0 |
| Stomachache | 2 (6.7) | 0 | 0 |
| Oral ulcer | 2 (6.7) | 0 | 0 |
| Myalgia | 1 (3.3) | 0 | 0 |
| Vomiting | 1 (3.3) | 0 | 0 |
| Obstructive jaundice | 1 (3.3) | 0 | 1 (3.3) |
| Epistaxis | 1 (3.3) | 0 | 0 |
| Gingival bleeding | 1 (3.3) | 0 | 0 |
AEs, adverse events.
Typical cases
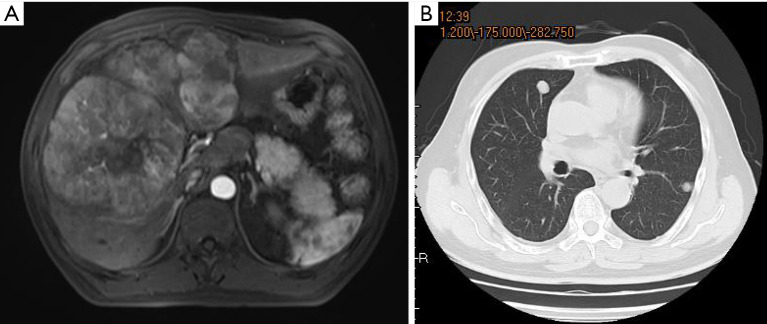
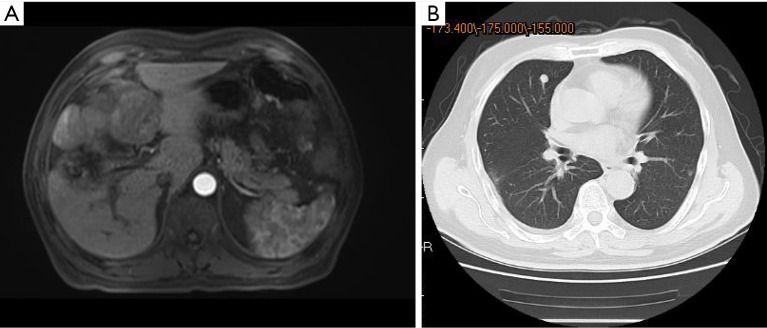
Examples of follow-up MRIs in patients who achieved a PR after TACE-lenvatinib sequential therapy are shown in Figures 4,5.
Figure 4.
A 58-year-old male patient diagnosed with liver cancer (A) and multiple pulmonary metastases (B).
Figure 5.
The intrahepatic tumor (A) and pulmonary metastases (B) significantly shrunk after TACE-lenvatinib sequential therapy. TACE, transcatheter arterial chemoembolization.
Discussion
This study provided important clinical results. We retrospectively analyzed 30 HCC patients to evaluate the efficacy and safety of TACE-lenvatinib sequential therapy. The results showed that the ORR was 76.7%, the DCR was 96.7%, and the median PFS was 6.1 months. In contrast, the ORR, DCR, and PFS of a Korean study on the treatment of HCC with lenvatinib were 16.2%, 83.8%, and 4.6 months respectively (11). Through comparison with the Korean study, we can find that sequential therapy performed significantly better than lenvatinib monotherapy.
Although TACE is the standard treatment for intermediate-stage HCC (12), repeated TACE could cause ischemia of the tumor tissue and increases the expression of hypoxia-inducible factor 1-α (13,14), which leads to increased production of angiogenic factors such as VEGF and FGF in tumor tissues, thereby promoting tumor angiogenesis (15). Lenvatinib was approved to treat unresectable HCC in 2018. It has been shown to be more effective than sorafenib in the treatment of unresectable HCC after TACE failure in the real world (16). In addition, because of disease progression and side effects in most patients at the early stage of lenvatinib treatment (17), the treatment effect is not completely satisfactory. Therefore, it is important to find other combination therapies to treat HCC.
Previous study has attempted to explore the TACE plus molecular targeted drugs combination therapy for unresectable HCC to reduce the frequency of TACE that tends to impair liver functional reserve during tumor progression (10). The TACTICS studies show that TACE plus sorafenib combination therapy significantly improves PFS compared with TACE alone, and that it prevents the progression from intermediate stage HCC to advanced stage HCC (3). An OPTIMIS study showed that the prognosis of patients who received sorafenib after TACE failure was better than patients who received repeated TACE (18). When TACE was repeated until the TACE failure, the liver function of 25% of the patients deteriorated to Child–Pugh Class B or C (19). At this time, the physical condition of the patients was not suitable for the targeted drugs treatment. Therefore, TACE combined with targeted therapy at an earlier time could maximize the benefit for patients with HCC. Earlier study has suggested that the efficacy of TACE after the targeted drug therapy failure is limited (20).
Compared with receiving TACE treatment after targeted therapy failure, TACE-lenvatinib sequential therapy has the following advantages: TACE can effectively reduce tumor volume by directly killing tumor cells with chemotherapeutic drugs and blocking the supply vessel of the tumor with embolization agents. Lenvatinib can inhibit the expression of angiogenic factors induced by ischemia after TACE and reduce angiogenesis and tumor recurrence. However, after targeted drug induction, because of ischemia and “normalization” of tumor vessels, blood flow of the liver and the diameter of hepatic arteries significantly decreased compared with that before targeted drug induction (20). In clinical practice, we also observed the phenomenon of hepatic artery thinning and even occlusion after targeted drug therapy. If TACE is performed at this time, the therapeutic effect is limited, because the hepatic artery thinning results in a relative decrease in the chemotherapeutic drugs dose that can reach the tumor. In addition, because of the hepatic artery thinning, it is difficult for the microcatheter to super-selectively reach the distal branch of the hepatic artery, and chemoembolization can be performed only in the large branch or trunk of the hepatic artery, which leads to an increase of the area of chemoembolization and results in liver function deterioration.
The median PFS in this study was lower than that in the TACTICS study (6.1 vs. 25.2 months), which may be because BCLC Stage C patients in our study accounted for 60% and BCLC Stage B patients accounted for 40%, while 33.8% patients in the TACTICS study were BCLC Stage A. Patients of early stage HCC may have a better response to targeted drugs and TACE than advanced-stage patients, so the PFS in the TACTICS study was higher than in the present study. Notably, the ORR and DCR of this study were significantly higher than the TACTICS study. The ORR and DCR of the TACTICS study were 57% and 67%, respectively. The median PFS was 6 months in another retrospective study of combination treatment with TACE and sorafenib, which was the same as in our study, while the median OS was lower than in our study (13 vs. 20.7 months), indicating that TACE-lenvatinib sequential therapy could significantly prolong the OS of patients (21).
Elevated AST and ALT, the most frequent TACE-related AEs, occurred in 90% of patients. Rashes (20%) were the most frequent lenvatinib-related AEs. Elevated AST (33.4%) and hypertension (6.7%) were the most frequent grade 3–4 TACE-related and lenvatinib-related AEs, respectively. The rates of grades 3 and 4 AEs were relatively low. Most AEs were mild and recoverable. Experience in the management of lenvatinib-related AEs may decrease discontinuation rate for AEs, and lead to a longer survival.
There were several limitations to our study. First, it was a single-center, retrospective study, which limits the generalizability of the outcomes and makes results vulnerable to potential biases. Second, the sample size was relatively small. Third, the present study had a lack of pathological data, and follow-up in our study should be continued.
In conclusion, TACE-lenvatinib sequential therapy may prolong OS and PFS in patients with unresectable HCC, and the side effects are acceptable. The efficacy and safety of the sequential therapy should be confirmed in multiple center randomized controlled trials (RCTs) with a large sample and sufficient follow-up period.
Acknowledgments
Funding: This study was supported by National Key R&D Program of China (No. 2020YFC2008900) and National Natural Science Foundation of China (Nos. 81671790, 82104404).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the declaration of Helsinki (as revised in 2013). The ethical approval was waived by the ethics committee of The First Medical Center of Chinese PLA General Hospital. The informed consent was not required because of the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-525/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-525/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-525/coif). The authors have no conflicts of interest to declare.
References
- 1.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver . Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-501. 10.1136/gutjnl-2019-318934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M, Ueshima K, Chan S, et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers (Basel) 2019;11:1084. 10.3390/cancers11081084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudo M. A New Treatment Option for Intermediate-Stage Hepatocellular Carcinoma with High Tumor Burden: Initial Lenvatinib Therapy with Subsequent Selective TACE. Liver Cancer 2019;8:299-311. 10.1159/000502905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu HH, Kim JH, Yoon HK, et al. Chemoembolization Combined with Radiofrequency Ablation for Medium-Sized Hepatocellular Carcinoma: A Propensity-Score Analysis. J Vasc Interv Radiol 2019;30:1533-43. 10.1016/j.jvir.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 9.Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014;2014:638747. 10.1155/2014/638747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M, Arizumi T. Transarterial Chemoembolization in Combination with a Molecular Targeted Agent: Lessons Learned from Negative Trials (Post-TACE, BRISK-TA, SPACE, ORIENTAL, and TACE-2). Oncology 2017;93 Suppl 1:127-34. 10.1159/000481243 [DOI] [PubMed] [Google Scholar]
- 11.Cheon J, Chon HJ, Bang Y, et al. Real-World Efficacy and Safety of Lenvatinib in Korean Patients with Advanced Hepatocellular Carcinoma: A Multicenter Retrospective Analysis. Liver Cancer 2020;9:613-24. 10.1159/000508901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 13.Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 2004;10:2878-82. 10.3748/wjg.v10.i19.2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Xu H, Gao ZQ, et al. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol 2008;49:523-9. 10.1080/02841850801958890 [DOI] [PubMed] [Google Scholar]
- 15.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 2008;103:914-21. 10.1111/j.1572-0241.2007.01712.x [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Sung PS, Yang H, et al. A Real-World Comparative Analysis of Lenvatinib and Sorafenib as a Salvage Therapy for Transarterial Treatments in Unresectable HCC. J Clin Med 2020;9:4121. 10.3390/jcm9124121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamura Y, Kobayashi M, Shindoh J, et al. Lenvatinib-Transarterial Chemoembolization Sequential Therapy as an Effective Treatment at Progression during Lenvatinib Therapy for Advanced Hepatocellular Carcinoma. Liver Cancer 2020;9:756-70. 10.1159/000510299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peck-Radosavljevic M, Kudo M, Raoul J, et al. Outcomes of patients (pts) with hepatocellular carcinoma (HCC) treated with transarterial chemoembolization (TACE): Global OPTIMIS final analysis. J Clin Oncol 2018;36:4018. 10.1200/JCO.2018.36.15_suppl.4018 [DOI] [Google Scholar]
- 19.Ogasawara S, Chiba T, Ooka Y, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology 2014;87:330-41. 10.1159/000365993 [DOI] [PubMed] [Google Scholar]
- 20.Matsuda N, Imai N, Kuzuya T, et al. Progression After Molecular Targeted Agents: Hepatic Arterial Changes and Transarterial Chemoembolization in Hepatocellular Carcinoma. In Vivo 2021;35:1185-9. 10.21873/invivo.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu Z, Shen L, Jiang Y, et al. Transarterial chemoembolization (TACE) combined with apatinib versus TACE combined with sorafenib in advanced hepatocellular carcinoma patients: a multicenter retrospective study. Ann Transl Med 2021;9:283. 10.21037/atm-20-5360 [DOI] [PMC free article] [PubMed] [Google Scholar]