Abstract
Purpose: Elevated ferritin levels are associated with poor outcomes in Covid-19 patients. Optimal timing of ferritin assessment and the merit of longitudinal values remains unclear. Methods: Patients admitted to Henry Ford Hospital with confirmed SARS-CoV-2 were studied. Regression models were used to determine the relation between ferritin and mortality, need for mechanical ventilation, ICU admission, and days on the ventilator. Results: 2265 patients were evaluated. Patients with an initial ferritin of > 490 ng/mL had an increased risk of death (OR 3.4, P < .001), admission to the ICU (OR 2.78, P < .001) and need for mechanical ventilation (OR 3.9, P < .001). There was no difference between admission and Day 1 ICU ferritin levels (611.5 ng/mL vs. 649 ng/mL respectively; P = .07). The decline in ferritin over ICU days 1-4 was similar between survivors and non-survivors. A change in ferritin levels from admission to ICU Day 1 (P = .330), or from ICU Day 1 to 2 (P = .788), did not predict days on the ventilator. Conclusions: Initial Ferritin levels were highly predictive of ICU admission, the need for mechanical ventilation and in-hospital mortality. However, longitudinal measures of ferritin throughout the hospital stay did not provide additional predictive value.
Keywords: COVID-19, ferritin, biomarkers, intensive care unit
Introduction
The world witnessed the emergence of a novel virus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), in late December 2019. To date, there have been more than 360 million reported cases of Covid-19, the respiratory infection caused by SARS-CoV-2, with more than 5 million deaths worldwide.1
The spectrum of illness caused by SARS-CoV-2 ranges from asymptomatic infection to severe pneumonia with acute respiratory disease syndrome (ARDS) and multiple organ dysfunction syndrome (MODS).2 Factors contributing to the severity of illness include increased age, male gender, pre-existing comorbidities, obesity, pregnancy and immunosuppression.3
Several biomarkers have been associated with the severity of illness including elevation in C-reactive protein (CRP), procalcitonin, D-Dimer and ferritin.4 Ferritin elevation in patients with severe COVID-19 has been thought to represent uncontrolled inflammation and massive cytokine release, similar to that observed in hemophagocytic lymphohistiocytosis (HLH) or Macrophage Activation Syndrome (MAS).5,6 In one report, ferritin levels above 300 nanograms per milliliter (ng/mL) were associated with ARDS development with a hazard ratio (HR) of 3.53 (1.52-8.16).7 Meta-analyses have supported the association between high ferritin level and poor outcome from SARS-CoV-2 infection.3,4,8–10
While ferritin appears to be associated with in-hospital mortality in patients with Covid-19, knowledge gaps remain pertaining to the optimal timing of ferritin assessment and the value of longitudinal assessments of ferritin in the clinical course of SARS-CoV-2 infection evolves.7
Although clinical data lack a compelling rationale for the longitudinal evaluation of these biomarkers, they have been routinely followed in many centers (including ours) with the premise that changes over time may be predictive of clinical course and outcomes.8 However, there is no evidence to support a benefit to the longitudinal assessment of ferritin as a tool to predict respiratory decline and mortality in patients admitted to the hospital with COVID −19. The goal of this study was to evaluate the relationship between admission ferritin levels and outcomes in our large, urban medical center and to assess whether longitudinal assessment of ferritin levels would predict intensive care unit (ICU) admission, acute respiratory failure requiring intubation and mechanical ventilation, and mortality.
Methods
Study Population
We conducted a retrospective cohort study of patients admitted to Henry Ford Hospital- Detroit Campus for COVID-19 infection between March 7, 2020 and May 11, 2021. The study protocol was approved by the institutional review board. Patient consent was waived because of the retrospective design and inclusion of deceased patients. The study included adults over the age of 18 years who were admitted to the hospital with a laboratory-confirmed SARS-CoV-2 infection diagnosed using quantitative reverse transcriptase-polymerase chain reaction. The admission ferritin level was defined as the initial ferritin drawn within the first 24 h of admission.
Statistical Analysis
Continuous variables were compared using the student t test or the Kruskal-Wallis rank-sum test in the cases of nonnormally distributed variables and expressed, respectively, as means ± SD or median and interquartile range. Categorical variables were expressed as percentages and analyzed using a chi-squared test. A receiver operating characteristic (ROC) curve was used to test the ability of the admission ferritin level to predict in-hospital mortality. The optimal cut point (Liu, 2012) was used as a dichotomous variable to predict outcomes in this study.
We used univariate logistic regression to identify independent risk factors for mortality, and need for mechanical ventilation and ICU admission. The variables used in these analyses included ferritin at the cut off determined by ROC curve analysis, race/ethnicity, sex, median income by zip code, body mass index (BMI), chronic obstructive pulmonary disease/asthma, diabetes mellitus, congestive heart failure (CHF), and chronic kidney disease (CKD). Variables with a p value of less than or equal to 0.1 were included in the multivariate regression models.
To examine the changes in ferritin levels over time among those who lived or died, a mixed model was used, which included the effects of time, mortality and the interaction between time and mortality.
For those patients in the ICU, simple linear regression was used to test if changes in ferritin levels over time significantly predicted the duration of mechanical ventilation.
Dichotomous variables were assigned to each biomarker: CRP (positive ≥10 mg/L) or negative, Procalcitonin positive (> 0.5 ng/mL) or negative, or d-dimer (positive ≥ 1.4 mcg/mL) or negative.
Results
2265 patients were admitted to the hospital with COVID-19 between March 2020 and May 2021. Of these, 700 patients (31%) required an ICU admission and 284 patients (12.5%) died. Of the total number of patients admitted, 1904 patients (84%) had a documented ferritin level within the first 24 h of admission. The median ferritin level for the 1904 patients who had a ferritin drawn within 24 h of hospital admission was 417 (95% CI 181-857). Patients with and without an admission ferritin level had similar baseline demographics and co-morbidities including age, gender, BMI, Charlson comorbidity index9 and a medical history of COPD/ Asthma, hypertension, CHF, and CKD (Table 1). Patients without an admission ferritin were less likely to have diabetes (38.2% vs. 45.2%, p 0.035), and more likely to have received glucocorticoids (78.5% vs. 46.8% P < .001) and Remdesivir (36% vs. 18.8%, P < .001). Both groups had similar mortality and ICU admission rates (13.9% vs. 12.3%, P = .412) and (34.9% vs. 30.2%, P = .073) respectively.
Table 1.
Patient Characteristics.
| No Admission Ferritin N = 361 |
Ferrin drawn N = 1904 |
P value | |
|---|---|---|---|
| Median Ferritin, ng/mL | N/A | 417 (181-857) | |
| Age, SD | 59.5 ± 17.2 | 60.5 ± 16.1 | .427 |
| Male | 180 (49.9) | 955 (50.1) | .911 |
| POC | 240 (66.5) | 1567 (82.3) | <.001 |
| Median BMI | 30.2 (25.5-36.1) | 31.0 (26.5-37.1) | .09 |
| Comorbid conditions | |||
| COPD/ Asthma | 75 (20.8) | 385 (20.2) | .810 |
| Hypertension | 246 (68.1) | 1340 (70.4) | .396 |
| Diabetes | 138 (38.2) | 842 (45.2) | .035 |
| CHF | 23 (6.4) | 124 (6.5) | .920 |
| Chronic kidney disease | 94 (26) | 412 (21.6) | .066 |
| Median Charlson Comorbidity index | 3 (2-6) | 3 (1-5) | <.001 |
| Inpatient medications | |||
| Glucocorticoids | 169 (46.8) | 1494 (78.5) | <.001 |
| Antibiotics | 201 (55.7) | 1038 (54.5) | .684 |
| Tociluzimab | 12 (3.32) | 69 (3.6) | .778 |
| Remdesivir | 68 (18.8) | 685 (36) | <.001 |
| ICU admission | 126 (34.9) | 574 (30.2) | .073 |
| In hospital mortality | 50 (13.9) | 234 (12.3) | .412 |
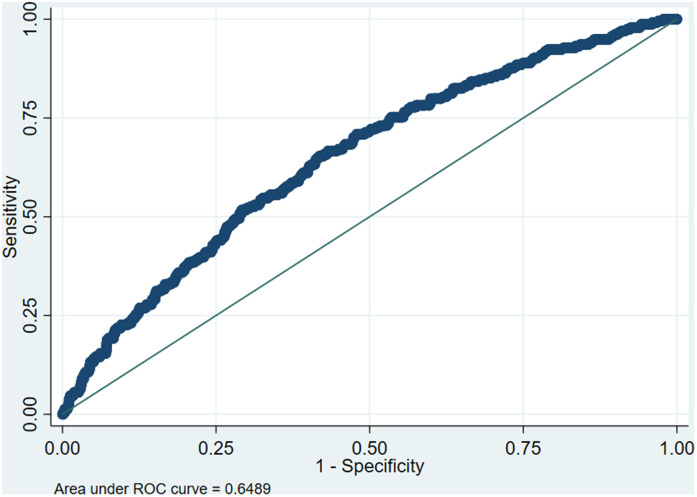
Generation of a ROC curve for predicting hospital mortality identified an optimal cutoff of admission ferritin level of 490 (AUC 0.65; sensitivity .65 and specificity 0.58). (Figure 1).
Figure 1.
Receiver operating characteristic curve (ROC) demonstrating the ability of ferritin level to predict in-hospital mortality. The optimal discriminatory cutoff point corresponded to a ferritin level of 490 ng/mL. Area under the ROC curve 0.65.
Patients with an initial ferritin of > 490 ng/mL had an increased risk of death (OR 3.4; 95%CI 2.45 to 4.76, P < .001) and increased risk of admission to the ICU (OR 2.78; 95% CI 2.26 to 3.41, P < .001). Moreover, in those patients admitted to the ICU, ferritin was a strong predictor of requiring mechanical ventilation (OR 3.9 [2.98 to 5.17], P < .001) (Table 2).
Table 2.
Predictors of in-Hospital Mortality.
| Factor | Adjusted OR | P value |
|---|---|---|
| Ferritin > 490 ng/mL | 3.41 (2.45-4.76) | <.001 |
| Male | 1.27 (0.95-1.70) | .111 |
| Age > 65 | 3.38 (2.45-4.68) | <.001 |
| BMI | 1.00 (0.985-1.02) | .782 |
| History HTN | 0.91 (0.624-1.33) | .624 |
| History DM | 1.20 (0.90-1.61) | .212 |
| History CHF | 1.66 (0.990-2.80) | .055 |
| History CKD | 1.15 (0.83-1.59) | .411 |
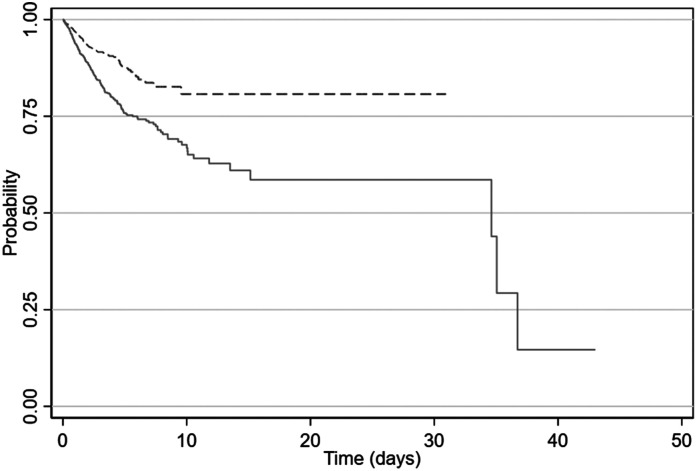
Of the 1859 patients initially admitted to an inpatient service, 294 patients required transfer to the ICU. These patients had a higher admission ferritin levels (594 ng/mL [95% CI 275 to 1126]) compared to the patients who did not require ICU transfer (360.5 ng/mL [95% CI 156 to 737]). Moreover, among the patients who were transferred to the ICU, a higher admission ferritin level (above 490 ng/ml) was associated with a more rapid need for transfer to the ICU setting (hazard ratio 2.0 [CI 1.5 to 2.6]; P < .001) (Figure 2) (Tables 3 and 4).
Figure 2.
Probability of requiring transfer from general ward to ICU was higher among those patients with an admission ferritin level > 490 (solid line) compared to patients with a level < 490 (dashed line) (hazard ratio 2.0 [CI 1.5 to 2.6]; P < .001).
Table 3.
Predictors of ICU Admission.
| Factor | Adjusted OR | P value |
|---|---|---|
| Ferritin > 490 ng/mL | 2.78 (2.26-3.41) | <.001 |
| Male | 1.06 (0.87-1.29) | .575 |
| Age > 65 | 1.28 (1.05-1.55) | .013 |
| History DM | 1.52 (1.25-1.84) | <.001 |
Table 4.
Predictors of Mechanical Ventilation.
| Factor | Adjusted OR | P value |
|---|---|---|
| Ferritin > 490 ng/mL | 3.92 (2.98-5.17)) | <.001 |
| Male | 1.18 (0.93-1.50) | .179 |
| Age > 65 | 1.52 (1.19-1.94) | .001 |
| History HTN | 1.02 (0.76-1.37) | .907 |
| History DM | 1.50 (1.18-1.91) | .001 |
| History CHF | 1.63 (1.05-2.52) | .029 |
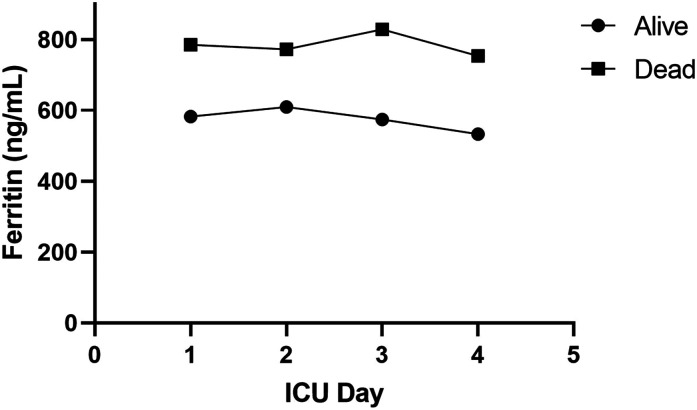
Among patients admitted directly to the ICU or transferred to the ICU from another inpatient unit, the ICU day 1 ferritin levels were not significantly different from the admission ferritin levels (649 ng/mL [95% CI 308 −1232] vs. 611.5 ng/mL [95% CI 287 to 1126] respectively; P = .07). Ferritin levels were significantly higher in those who died during ICU days 1-4.
While both survivors and non-survivors experienced a decrease in ferritin over days 1-4, the extent of decline in ferritin levels was similar between these 2 groups (Figure 3). This suggests that a change in ferritin over the first 96 h of ICU care did not provide additional prognostic information (interaction p-value = 0.4562).
Figure 3.
Overall, median ferritin levels were higher among non-survivors, but the decline in ferritin levels over the first 4 days in the ICU was similar between survivors (Circle) and non-survivors (Square) (interaction P-value = .4562).
Similarly, in patients requiring mechanical ventilation, a change in ferritin levels from admission to ICU day 1 (regression coefficient −0.0003 [95% CI −0.001 to −0.003], P = .330), or from ICU day 1 to ICU day 2 (regression coefficient −0.00066 [95% CI −0.0005 to −0.0004];, P = .788), did not predict number of days requiring mechanical ventilation.
In addition to ferritin, other biomarkers were found to be significant predictors of mortality when elevated (CRP: OR 2.1 ([1.57-2.82], P < .001), Procalcitonin: OR 3.76 ([2.7-5.1], P < .001) and D-Dimer: OR 3.2 ([2.3-4.4], P < .001)). (Table 5)
Table 5.
Biomarkers as Predictors of in-Hospital Mortality.
| Biomarker | Unadjusted OR | Adjusted OR | P |
|---|---|---|---|
| Ferritin > 490 | 3.88 (2.8-5.3) | 3.41 (2.45-4.76) | <.001 |
| CRP > 10 | 2.0 (1.5-2.63) | 2.1 (1.57-2.82) | <.001 |
| Procalcitonin positive | 4.2 (3.2-5.7) | 3.76 (2.7-5.1) | <.001 |
| D-Dimer > 1.4 | 4.1 (3.0-5.6) | 3.2 (2.3-4.4) | <.001 |
Finally, 977 patients had an elevated serum ferritin > 490 ng/mL at time of admission even when CRP, procalcitonin and D-Dimer levels were negative; this also predicted mortality (adjusted OR 3.19 [2.21-4.59]; P < .0001).
Discussion
The primary finding of this study was that, in hospitalized patients with COVID-19, ferritin levels obtained within 24 h of admission were highly predictive of ICU admission, the need for mechanical ventilation and in-hospital mortality. The predictive ability of admission ferritin levels was also not dependent on clinical status at the time of admission; ferritin predicted outcomes equally well in patients requiring direct ICU admission and admission to a general medical ward (non-ICU) at the time of presentation.
Longitudinal measures of ferritin throughout the hospital stay did not provide additional predictive value for respiratory deterioration or mortality in our population. While ferritin levels were higher in non survivors across all 4 days in the ICU, changes in ferritin levels over this time period was not predictive of in hospital mortality. Indeed, the absolute admission value of ferritin was more closely associated with clinical deterioration than the rate of change in ferritin. Thus, the admission ferritin level might have clinical utility in the initial risk stratification and triage of patients presenting with COVID-19, but repeated measurements are of limited value. These results are consistent with the results of a prior study of 235 patients showing that daily ferritin levels did not predict length of ICU stay.10 To our knowledge, this is the largest study evaluating the association between serial measurements of ferritin levels and mortality in hospitalized patients with COVID-19.
Ferritin has previously been shown to be a predictor of poor outcomes11,12 in patients with COVID-19. One meta-analysis of 25 studies and 5350 patients found that a higher serum ferritin level was independently associated with ARDS, mortality, and severe COVID-19.4 Another meta-analysis included 189 studies with data from 57 563 COVID-19 patients and showed significant differences in mean ferritin levels between survivors and non-survivors.13
Not all studies have found correlations between ferritin and mortality. One early report showed that elevated ferritin levels were associated with development of ARDS, but not with death.14 Another study demonstrated that ferritin levels over the 25th percentile were associated with more severe pulmonary involvement but not with outcomes.15 Finally, a retrospective study evaluated 942 COVID-19 patients and showed that neither presentation ferritin levels nor the maximum value over the duration of hospitalization was associated with all-cause mortality, new mechanical ventilation, or a new renal replacement therapy requirement.16
Ferritin is known to be elevated in inflammatory conditions, both as an acute-phase reactant and as a marker for developing a cytokine storm.17 The pathogenesis of COVID-19 has been shown to have similarities with macrophage activating syndrome and catastrophic antiphospholipid syndrome.18 Similar to other viral infections, iron metabolism helps support the innate immune system during the early and acute phases of SARS-CoV-2 infection by decreasing the iron bioavailability and replication of the virus.19 While other inflammatory diseases have shown ferritin levels in excess of 1000 ng/mL to be associated with mortality, others have shown that a level > 300 ng/mL leads to worsening outcomes.20–24 This is consistent with our study in which ROC analysis indicated a cutoff of 490 ng/mL to predict in-hospital mortality.
We found that CRP, D-Dimer and procalcitonin were also predictive of in-hospital mortality. These findings are in agreement with other studies that found an association between inflammatory markers and poor outcomes in patients with COVID-19.25–28 However, in contrast to ferritin, CRP, procalcitonin and D-Dimer levels are commonly incorporated into clinical decision making. For example, serum procalcitonin has value in decisions to deescalate antibiotics in patients with bacterial infections and to initiate antibacterial antibiotics in specific patients.29,30 A D-Dimer is frequently obtained as part of the clinical assessment for presence of venous thromboembolism31 and more recently has been used to guide anticoagulation treatment in patients with COVID-19.32 Finally, serum CRP has been used to stratify patients for tocilizumab in severe COVID-19.33 In contrast to these biomarkers, ferritin is not used to guide specific management or treatment strategies in patients with COVID-19. In addition, the utility of ferritin as a predictor of poor outcome was independent of the values of the other inflammatory makers; a substantial number of patients had ferritin elevations > 490 in the absence of an elevated CRP, D-dimer or procalcitonin. This finding suggests that ferritin serves as an independent predictor of deterioration and death in patients hospitalized with severe COVID-19. Notably, these other biomarkers might predict poor outcomes because they identify cohorts of patients enriched for secondary clinical processes such as venous thromboembolism or bacterial superinfection.
There are a few limitations in our study. First, this is a single-center retrospective study performed in a large, urban, tertiary-care hospital. The results may not be broadly applicable in other clinical settings. Since ferritin levels were known to the primary team, it is feasible that patient care was approached differently for patients with different ferritin levels; however, as discussed, absolute ferritin levels in patients with Covid-19 infection have not led to specific medical interventions. Finally, there was no validation cohort for the ferritin cutoff of 490. Future studies could be aimed at validating this cutoff value in a separate group of patients.
In conclusion, our retrospective study demonstrates a strong association between the admission ferritin level, ICU admission, need for mechanical ventilation and ultimate mortality in patients with COVID-19 infection. Importantly, this predictive value was independent of other commonly used biomarkers and was not enhanced by longitudinal assessments. Together, these data support the clinical value of a single measurement of ferritin at the time of hospital admission for the purposes of risk-stratification while also demonstrating the lack of value in serial assessments.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jeffrey H. Jennings https://orcid.org/0000-0001-6617-399X
References
- 1.Johns Hopkins. COVID-19 dashboard by the center for science and engineering. 2021. https://coronavirus.jhu.edu/map.html.
- 2.COVID-19 Treatment Guidelines Panel. (2022). Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. [PubMed]
- 3.Gao Y€, Ding M, Dong Xet al. 19 Patients: a review. Allergy. 2021;76(2):428-455. [DOI] [PubMed] [Google Scholar]
- 4.Huang I, et al. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li Xet al. 2019 Novel coronavirus in wuhan, China. The Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P, Mcauley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. 19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng L, et al. Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Lab Anal. 2020;34(10):e23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samprathi M, Jayashree M. Biomarkers in COVID-19: an up-to-date review. Front Pediatr. 2021;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 10.Nadeem R, Elhoufi AM, Iqbal NEet al. 19 Admitted to ICU: do markers tell the story? Dubai Med J. 2021;4(2):142-150. [Google Scholar]
- 11.Karaboyas A, Morgenstern H, Pisoni RLet al. Association between serum ferritin and mortality: findings from the USA, Japan and European dialysis outcomes and practice patterns study. Nephrol Dial Transplant. 2018;33(12):2234-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tornai D, Antal-Szalmas P, Tornai Tet al. et al. Abnormal ferritin levels predict development of poor outcomes in cirrhotic outpatients: a cohort study. BMC Gastroenterol. 2021;21(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taneri PE, GóMez-Ochoa SA, Llanaj Eet al. 19: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35(8):763-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C, Chen X, Cai Yet al. 2019 Pneumonia in wuhan, China. JAMA Intern Med. 2020;180(7):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carubbi F, Salvati L, Alunno Aet al. 19: data from two Italian COVID-19 units. Sci Rep. 2021;11(1):4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feld J, Tremblay D, Thibaud S, Kessler A, Naymagon L. 19: a poor predictor of mortality and hemophagocytic lymphohistiocytosis. Int J Lab Hematol. 2020;42(6):773-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;19(6):102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drakesmith H, Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6(7):541-552. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed S, Ansar Ahmed Z, Siddiqui I, Haroon Rashid N, Mansoor M, Jafri L. 19- A cross sectional study. Ann Med Surg (Lond). 2021;63:102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alroomi M, Rajan R, Omar AAet al. 19 Patients. Immun Inflamm Dis. 2021;9(4):1648-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen T, Wu D, Chen Het al. 113 Deceased patients with coronavirus disease 2019: retrospective study. Br Med J. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng F, et al. Increased levels of ferritin on admission predicts intensive care unit mortality in patients with COVID-19. Med Clin (Engl Ed). 2021;156(7):324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruan Q, Yang K, Wang W, Jiang L, Song J. 19 Based on an analysis of data of 150 patients from wuhan, China. Intensive Care Med. 2020;46(5):846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poudel A, Poudel Y, Adhikari Aet al. et al. 19 Prognosis: d-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLOS ONE. 2021;16(8):e0256744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhomwegen C, Veliziotis I, Malinverni Set al. 19 Patients admitted to intensive care unit. Ir J Med Sci. 2021;190(4):1649-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J-, Xu C, Zhang Ret al. et al. 19 Patients in China. Sci Rep. 2020;10(1):15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Yan X, Fan Qet al. et al. 19. J Thromb Haemost. 2020;18(6):1324-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Self WH, Balk RA, Grijalva CGet al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia. Clin Infect Dis. 2017;65(2):183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans L, Rhodes A, Alhazzani Wet al. 2021. Crit Care Med. 2021;49(11):e1063-e1143. [DOI] [PubMed] [Google Scholar]
- 31.Pulivarthi S, Gurram MK. Effectiveness of d-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci. 2014;6(10):491-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spyropoulos AC, Goldin M, Giannis Det al. 19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariette X, Hermine O, Tharaux P-Let al. et al. 19: a follow-up of the CORIMUNO-TOCI-1 randomized clinical trial. JAMA Intern Med. 2021;181(9):1241-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]