Abstract
Primers for PCR amplification of partial (1,102 of 1,680 bp) formyltetrahydrofolate synthetase (FTHFS) gene sequences were developed and tested. Partial FTHFS sequences were successfully amplified from DNA from pure cultures of known acetogens, from other FTHFS-producing organisms, from the roots of the smooth cordgrass, Spartina alterniflora, and from fresh horse manure. The amplimers recovered were cloned, their nucleotide sequences were determined, and their translated amino acid sequences were used to construct phylogenetic trees. We found that FTHFS sequences from homoacetogens formed a monophyletic cluster that did not contain sequences from nonhomoacetogens and that FTHFS sequences appear to be informative regarding major physiological features of FTHFS-producing organisms.
The homoacetogenic bacteria (acetogens) are a group of obligately anaerobic bacteria that utilize the acetyl coenzyme A (CoA) pathway to synthesize acetate from C1 precursors. Acetogens grow autotrophically on H2 and CO2 and/or heterotrophically on a variety of organic compounds, with mixotrophic growth on H2 and a suitable organic substrate being observed in some species (7, 46). Acetogenesis is of great importance to the global carbon cycle, producing an estimated 10% of the approximately 1013 kg of acetate formed annually in anaerobic habitats (46). As a group, acetogens are among the most metabolically versatile anaerobes (11, 36) and are also phylogenetically quite diverse. This diversity has greatly hindered studies of natural acetogen populations, and as a result, surprisingly little is known about their ecology in most anaerobic habitats.
Media that are selective for organisms capable of acid production under H2-CO2 incubation conditions have been developed (6), but pure-culture isolation from natural environments tends to result in collections of organisms that are not representative of the natural assemblages (9, 38). Molecular biological methods are widely used for studies of natural bacteria (15, 40, 43), but many of these methods are based on recovery and analysis of 16S rRNA gene sequences and therefore are not amenable to the study of a functional group of organisms that are phylogenetically diverse. A practical solution to this problem for bacterial functional groups is to exploit a unique and unifying function encoded by a conservative, non-16S rRNA gene. This approach has been employed with great success for several bacterial functional groups, including the nitrogen-fixing bacteria (nifH) (17, 26), the methylotrophic bacteria (pmoA) (16), the ammonia-oxidizing bacteria (amoA) (34), various groups of autotrophic organisms (rbcL) (29), the denitrifying bacteria (nirK, nirS) (4), and the sulfate-reducing bacteria (aprA) (10), among others. PCR amplification, which provides substantially greater sensitivity than direct DNA-DNA hybridization analyses (2), is particularly useful for ecological studies, but suitable primers have not been developed for the acetogens to date.
Previous studies have shown that some enzymes used in the acetyl-CoA pathway are both structurally and functionally conserved (20, 23, 32). In particular, the gene sequence encoding formyltetrahydrofolate synthetase (FTHFS), which catalyzes the ATP-dependent activation of formate, is highly conservative (20, 23) and has been used successfully as a functional group probe for acetogens in natural samples (24). This gene has been cloned and sequenced from three acetate-producing anaerobes, Moorella thermoacetica (formerly Clostridium thermoaceticum) (22, 23), Clostridium acidurici (44), and Clostridium cylindrosporum (33). The last two species are purinolytic organisms, which ferment purines and amino acids to acetate via the glycine synthase-glycine reductase pathway (14, 41), but are unable to produce acetate autotrophically. In this study we designed PCR primers for amplification of partial FTHFS and FTHFS-like gene sequences.
The acetogenic and nonacetogenic organisms used in this study included Acetobacterium woodii ATCC 29683, Azospirillum lipoferum Sp59b, Azotobacter vinelandii UW, Bacillus circulans ATCC 61, Bacillus cereus ATCC 14579, Clostridium aceticum DSM 1496, C. acidurici ATCC 7906, C. cylindrosporum ATCC 7905, Clostridium formicoaceticum ATCC 23439, Clostridium magnum WoBdP1, Clostridium perfringens 876, Desulfosporosinus orientis (formerly Desulfotomaculum orientis) ATCC 19365, Escherichia coli B, Klebsiella oxytoca ATCC 50231, M. thermoacetica DSM 521, Proteus vulgaris ATCC 13315, Pseudomonas aeruginosa ATCC 27853, Rhizobium leguminosarum bv. viceae USDA 2370, Rhizobium meliloti USDA 1025, Rhodospirillum rubrum Molisch s1, Ruminococcus productus (formerly Peptostreptococcus productus) U1, Sporomusa ovata H1, Sporomusa termitida DSM 4440, Staphylococcus aureus ATCC 12600, Thermoanaerobacter kivui (formerly Acetogenium kivui) DSM 1428, and Xanthomonas maltophilia ATCC 13637. The sources of these organisms, as well as the methods used for purification of DNA from bacterial cultures and from fresh horse manure, have been described previously (24). DNA was purified from fresh roots of the smooth cordgrass, Spartina alterniflora, by using a direct lysis procedure (25, 30). FTHFS amino acid and nucleotide sequences from M. thermoacetica, C. acidurici, and C. cylindrosporum were obtained from the National Center for Biotechnology Information and were aligned by using ClustalW (39). Stretches of six or more consecutive conserved residues were identified, and the degeneracy of these potential priming sequences was determined. Primers identified in initial studies were used to recover a 1,374-bp segment of the approximately 1,680-bp FTHFS gene from A. woodii, C. formicoaceticum, C. magnum, and T. kivui. However, these initial primers yielded some nonspecific products from other acetogens. Analysis of the additional FTHFS sequences revealed less degenerate PCR primer sequences that amplified a 1,102-bp stretch of the FTHFS gene specifically and with a high yield. The M. thermoacetica nucleotide sequence was substantially different from other acetogen sequences and was dropped from consideration in the design of these primers (see below). The final primer sequences were as follows: 5′-TTY ACW GGH GAY TTC CAT GC-3′ (forward, 24-fold degenerate) and 5′-GTA TTG DGT YTT RGC CAT ACA-3′ (reverse, 12-fold degenerate), where Y is C or T, W is A or T, H is A, C, or T, D is A, G, or T, and R is A or G. PCR amplification conditions were optimized by using DNA from known acetogen pure cultures with Dynazyme EXT Tbr polymerase (MJ Research, Waltham, Mass.). The reaction system consisted of 1.5 mM MgCl2, 0.4 mg of bovine serum albumin per ml, 0.2 mmol of each deoxynucleoside triphosphate per ml, and 25 ng of target DNA per ml. The touchdown thermal cycling protocol used included initial denaturation at 94°C for 2 min, followed by nine cycles of denaturation at 94°C for 30 s, annealing at 63°C for 30 s (decreased by 1°C per cycle to 55°C), and elongation at 72°C for 30 s. After the touchdown portion of the protocol was completed, 25 additional cycles in which an annealing temperature of 55°C was used were performed, and this was followed by a final elongation step consisting of 72°C for 2 min. Amplimers from a representative collection of acetogens, from known nonacetogens, and from Spartina root and horse manure DNA were cloned into pGEM-T (Promega, Madison, Wis.). Recombinant plasmids were purified from selected clones, and sequences were determined for both strands of each cloned amplimer with a DNA4000LS sequencer (Li-Cor, Lincoln, Nebr.). Cloned partial FTHFS sequences were translated by using SeqPup (version 0.6f; D. G. Gilbert, Indiana University, Bloomington). Alignments of translated sequences were constructed by using ClustalW (39). Distance matrix calculations and tree construction were performed by using MEGA (version 1.0; The Pennsylvania State University, University Park). The partial FTHFS sequence of P. vulgaris served as the outgroup. Bootstrapping (12) was used to estimate the reliability of phylogenetic reconstructions (500 replicates).
Based on the amino acid numbering system for the sequence of M. thermoacetica FTHFS (23), the only complete FTHFS sequence from a true acetogen, the amplimer begins with Val 136 and ends with Val 487 exclusive of primers and begins with Phe 129 and ends with Tyr 494 including primers. All sequences were examined for amino acid residues that are universally conserved in known FTHFS sequences and are thought to be important to FTHFS structure or catalysis (31). These include a hexapeptide, encompassing residues 197 to 202, thought to be involved in tetrahydrofolate binding (23) and the putative adenosine ring-stabilizing residue, Trp 412 (31). In addition, residues 271 to 284 are highly conserved in all known FTHFS sequences. These residues are useful markers for performing accurate sequence alignment and provide additional confidence in the identity of FTHFS sequences.
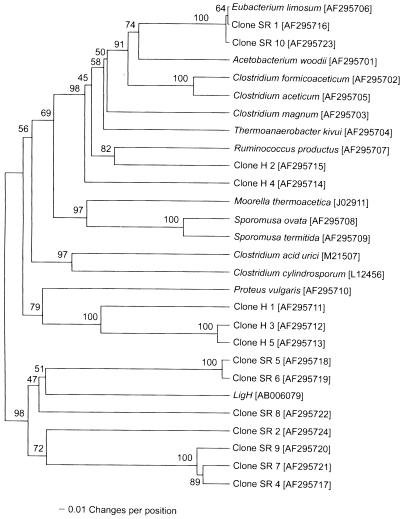
The known acetogens C. formicoaceticum and C. aceticum formed a monophyletic clade based on partial FTHFS sequences (93.3% sequence similarity) (Fig. 1), as did S. ovata and S. termitida (91.3% sequence similarity). Both of these results were supported in 100% of 500 bootstrap replicates. The M. thermoacetica sequence grouped with the Sporomusa clade in 99% of the bootstrap replicates. However, the levels of sequence similarity between the M. thermoacetica and Sporomusa sequences were not very high (73.7% between S. ovata and M. thermoacetica and 72.6% between S. termitida and M. thermoacetica). The Eubacterium limosum and A. woodii sequences formed a tight group as well, which exhibited 83% sequence similarity and was supported in 72% of the bootstrap replicates. FTHFS sequences from the two thermophilic acetogens, M. thermoacetica and T. kivui, were substantially separated, and the T. kivui sequence was more similar to the sequences from the clostridial species than to the Moorella sequence. The average level of similarity for the known acetogen FTHFS sequences was 73.3%.
FIG. 1.
Phylogenetic relationships of FTHFS and LigH-like sequences (partial sequences; 359 amino acids; accession numbers are given in brackets). The dendrogram was generated by using the gamma distance (2.0) analysis method with the unweighted pair group with mathematical average algorithm. P. vulgaris (accession no. AF295710) was used as the outgroup. The values at the nodes are the percentages of 500 bootstrap replicates supporting the branching order. Clones obtained from Spartina root and horse manure DNA have designations that begin with SR and H, respectively.
The FTHFS sequences from the purine fermenters C. acidurici and C. cylindrosporum formed a monophyletic clade which did not contain any of the sequences from genuine acetogens. The sequences from purinolytic clostridia were most similar to the Sporomusa clade sequences, but the average level of similarity to the Sporomusa sequences was only 65.2%. An additional FTHFS sequence from P. vulgaris fell outside the acetogen and purinolytic clostridium clusters and was designated the outgroup in subsequent analyses. The P. vulgaris sequence was 58.7% similar to C. cylindrosporum and C. acidurici sequences and only 63.4% similar to the most similar sequence from a true acetogen, the T. kivui sequence.
Restriction fragment length polymorphism analysis of 16 Spartina root DNA clones and five horse manure DNA clones showed that 11 of the Spartina root DNA clones were different, as were all five sequences cloned from horse manure. The clones from Spartina roots were designated SR 1 through SR 11, and those from horse manure were designated H 1 through H 5. Two of the Spartina root sequences (SR 1 and SR 10) fell in the A. woodii-E. limosum clade and were very similar to the E. limosum sequence (levels of similarity, 99.7 and 99.4%, respectively). Interestingly, a recent study employing a 16S ribosomal DNA probe specific for A. woodii and E. limosum detected these acetogens on the root surfaces and inside the roots of the seagrass Halodule wrightii (18). Two horse manure clones, H 2 and H 4, were 74.5% similar to each other and 78.5 and 73.5% similar, respectively, to the sequence from R. productus, an acetogen that was originally isolated from a sewage digester (21) and has also been detected in the intestinal tracts of a variety of mammals (42). H 2 grouped with the R. productus sequence in 81% of the bootstrap replicates. There was also substantial similarity between these clones and the FTHFS sequence from M. thermoacetica (H 4 more so than H 2), which was originally isolated from a horse manure pile (13). Three other clones from horse manure, H 1, H 3, and H 5, formed a cluster with the enteric, nonacetogenic P. vulgaris sequence. P. vulgaris is a known producer of FTHFS activity (45), and a presumed FTHFS gene was previously detected in this organism with an FTHFS-specific probe (24). This cluster apparently contained divergent sequences (average level of similarity, 74.0%) but was well supported by bootstrapping.
Seven other unique Spartina root clones, SR 2 and SR 4 through SR 9, were also found. These sequences clustered together but were quite different from the FTHFS sequences. The ortho-demethylating enzyme encoded by ligH in Sphingomonas paucimobilis has 60% sequence similarity to the FTHFS from M. thermoacetica (28). The LigH sequence clustered with these seven root clones with sequence similarities ranging from 61.9 to 65.9%. It should be noted that these levels of similarity are not sufficient to conclusively identify the unknown root sequences as ligH products.
We tested the PCR primers by using a broad range of organisms isolated from a wide variety of environments; these organisms included A. woodii from estuarine sediment (3), C. aceticum from soil (5), C. formicoaceticum from sewage (1), C. magnum from freshwater sediment (35), E. limosum from sheep rumen fluid (37), R. productus from sewage digester sludge (21), S. ovata from silage (27), S. termitida from termite hindgut (8), and T. kivui from freshwater sediment (19). All of the homoacetogens tested except M. thermoacetica yielded strong FTHFS-specific amplimers without nonspecific products. An amplimer of the correct size was obtained from M. thermoacetica; however, spurious products dominated the amplification mixture. This was also the case for amplification of D. orientis DNA. While the M. thermoacetica FTHFS was clearly quite congruent with other FTHFSs at the amino acid sequence level and clearly fell within the acetogen cluster, it was substantially different from the other acetogen FTHFSs at the nucleotide sequence level. The reasons underlying the low levels of similarity between the M. thermoacetica FTHFS nucleotide sequence and the sequences of other acetogen FTHFS genes are not clear at present, but the primers used in this study are probably not suitable for amplification of this sequence from natural samples. Successful amplification of the M. thermoacetica FTHFS sequence was accomplished by increasing the degeneracy of the primer pair in accordance with the M. thermoacetica nucleotide sequence (data not shown). The more degenerate primers produced spurious products from some other known acetogens and are not recommended for use with environmental samples. Analysis of additional FTHFS sequences may help resolve this issue.
The FTHFS primers developed here permit examination of natural samples to determine the presence and diversity of acetogenic bacteria. Sequences of purinolytic clostridia, as well as other FTHFS-producing organisms, may also be recovered, but they are sufficiently different from acetogen FTHFS sequences that they can be accurately discriminated. The phylogenetic inferences drawn from FTHFS sequence data are congruent with phylogenetic inferences drawn from 16S rRNA gene sequence data in most cases (data not shown), and where the two phylogenies differ, FTHFS sequence analysis is more informative concerning physiological distinctions among FTHFS-producing organisms. Use of the PCR primers described here should greatly facilitate discovery of unknown acetogens and determination of acetogen distributions and dynamics in complex natural environments.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study have been deposited in the GenBank database under accession no. AF295701 to AF295724.
Acknowledgments
We acknowledge Christopher Bagwell, who provided the S. alterniflora root DNA used in this study, as well as helpful comments on the manuscript.
This research was supported by NSF grants MCB-9873606 and DEB-9903623 to C.R.L.
REFERENCES
- 1.Andreesen J R, Gottschalk G, Schlegel H G. Clostridium formicoaceticum nov. spec. isolation, description, and distinction from C. aceticum and C. thermoaceticum. Arch Microbiol. 1970;72:154–174. doi: 10.1007/BF00409521. [DOI] [PubMed] [Google Scholar]
- 2.Bagwell C E, Lovell C R. Persistence of selected Spartina alterniflora rhizoplane diazotrophs exposed to natural and manipulated environmental variability. Appl Environ Microbiol. 2000;66:4625–4633. doi: 10.1128/aem.66.11.4625-4633.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch W E, Schoberth S, Tanner R S, Wolfe R S. Acetobacterium, a new genus of hydrogen-oxidizing, carbon dioxide-reducing, anaerobic bacteria. Int J Syst Bacteriol. 1977;27:355–361. [Google Scholar]
- 4.Braker G, Zhou J, Lu L, Devol A H, Tiedje J M. Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in Pacific Northwest marine sediment communities. Appl Environ Microbiol. 2000;66:2096–2104. doi: 10.1128/aem.66.5.2096-2104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun M, Mayer F, Gottschalk G. Clostridium aceticum (Wieringa), a microorganism producing acetic acid from molecular hydrogen and carbon dioxide. Arch Microbiol. 1981;128:288–293. doi: 10.1007/BF00422532. [DOI] [PubMed] [Google Scholar]
- 6.Braun M, Schoberth S, Gottschalk G. Enumeration of bacteria forming acetate from H2 and CO2 in anaerobic habitats. Arch Microbiol. 1979;120:201–204. doi: 10.1007/BF00423066. [DOI] [PubMed] [Google Scholar]
- 7.Breznak J A, Kane M D. Microbial H2/CO2 acetogenesis in animal guts: nature and nutritional significance. FEMS Microbiol Rev. 1990;87:309–313. doi: 10.1111/j.1574-6968.1990.tb04929.x. [DOI] [PubMed] [Google Scholar]
- 8.Breznak J A, Switzer J M, Seitz H-J. Sporomusa termitida sp. nov., an H2/CO2-utilizing acetogen isolated from termites. Arch Microbiol. 1988;150:282–288. [Google Scholar]
- 9.Brock T D. The study of microorganisms in situ: progress and problems. Symp Soc Gen Microbiol. 1987;41:1–17. [Google Scholar]
- 10.Deplancke B, Hristova K R, Oakley H A, McCracken V J, Aminov R, Mackie R I, Gaskins H R. Molecular ecological analysis of the succession and diversity of sulfate-reducing bacteria in the mouse gastrointestinal tract. Appl Environ Microbiol. 2000;66:2166–2174. doi: 10.1128/aem.66.5.2166-2174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake H L. Acetogenesis, acetogenic bacteria, and the acetyl CoA “Wood/Ljungdahl” pathway: past and current perspectives. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 3–60. [Google Scholar]
- 12.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine F E, Peterson W H, McCoy E, Johnson M J, Ritter G J. A new type of glucose fermentation by Clostridium thermoaceticum n. sp. J Bacteriol. 1942;43:701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs G. CO2 fixation in acetogenic bacteria: variations on a theme. FEMS Microbiol Rev. 1986;39:181–213. [Google Scholar]
- 15.Head I M, Saunders J R, Pickup R W. Microbial evolution, diversity, and ecology: a decade of ribosomal RNA analysis of uncultivated organisms. Microb Ecol. 1998;35:1–21. doi: 10.1007/s002489900056. [DOI] [PubMed] [Google Scholar]
- 16.Henckel T, Jäckel U, Schnell S, Conrad R. Molecular analyses of novel methanotrophic communities in forest soil that oxidize atmospheric methane. Appl Environ Microbiol. 2000;66:1801–1808. doi: 10.1128/aem.66.5.1801-1808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirshtein J D, Paerl H W, Zehr J. Amplification, cloning, and sequencing of a nifH segment from aquatic microorganisms and natural communities. Appl Environ Microbiol. 1991;57:2645–2650. doi: 10.1128/aem.57.9.2645-2650.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Küsel K, Pinkart H C, Drake H L, Devereux R. Acetogenic and sulfate-reducing bacteria inhabiting the rhizoplane and deep cortex cells of the sea grass Halodule wrightii. Appl Environ Microbiol. 1999;65:5117–5123. doi: 10.1128/aem.65.11.5117-5123.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leigh J A, Mayer F, Wolfe R S. Acetogenium kivui, a new thermophilic, hydrogen-oxidizing, acetogenic bacterium. Arch Microbiol. 1981;129:275–280. [Google Scholar]
- 20.Ljungdahl L G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- 21.Lorowitz W H, Bryant M P. Peptostreptococcus productus strain that grows rapidly with CO2 as the energy source. Appl Environ Microbiol. 1984;47:961–964. doi: 10.1128/aem.47.5.961-964.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovell C R, Przbyla A, Ljungdahl L G. Cloning and expression in Escherichia coli of the Clostridium thermoaceticum gene encoding thermostable formyltetrahydrofolate synthetase. Arch Microbiol. 1988;149:280–285. doi: 10.1007/BF00411642. [DOI] [PubMed] [Google Scholar]
- 23.Lovell C R, Przbyla A, Ljungdahl L G. Primary structure of the thermostable formyltetrahydrofolate synthetase from Clostridium thermoaceticum. Biochemistry. 1990;29:5687–5694. doi: 10.1021/bi00476a007. [DOI] [PubMed] [Google Scholar]
- 24.Lovell C R, Hui Y. Design and testing of a functional group-specific DNA probe for the study of natural populations of acetogenic bacteria. Appl Environ Microbiol. 1991;57:2602–2609. doi: 10.1128/aem.57.9.2602-2609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovell C R, Piceno Y M. Purification of DNA from estuarine sediments. J Microbiol Methods. 1994;20:161–174. [Google Scholar]
- 26.Lovell C R, Piceno Y M, Quattro J M, Bagwell C E. Molecular analysis of diazotroph diversity in the rhizosphere of the smooth cordgrass, Spartina alterniflora. Appl Environ Microbiol. 2000;66:3814–3822. doi: 10.1128/aem.66.9.3814-3822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Möller B, Oßmer R, Howard B H, Gottschalk G, Hippe H. Sporomusa, a new genus of gram-negative anaerobic bacteria including Sporomusa sphaeroides spec. nov. and Sporomusa ovata spec. nov. Arch Microbiol. 1984;139:388–396. [Google Scholar]
- 28.Nishikawa S, Sonoki T, Kasahara T, Obi T, Kubota S, Kawai S, Morohoshi N, Katayama Y. Cloning and sequencing of the Sphingomonas (Pseudomonas) paucimobilis gene essential for the O-demethylation of vanillate and syringate. Appl Environ Microbiol. 1998;64:836–842. doi: 10.1128/aem.64.3.836-842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul J H, Alfreider A, Wawrik B. Micro- and macrodiversity in rbcL sequences in ambient phytoplankton populations from the southeastern Gulf of Mexico. Mar Ecol Prog Ser. 2000;198:9–18. [Google Scholar]
- 30.Piceno Y M, Noble P A, Lovell C R. Spatial and temporal assessment of diazotroph assemblage composition in vegetated salt marsh sediments using denaturing gradient gel electrophoresis analysis. Microb Ecol. 1999;35:157–167. doi: 10.1007/s002489900164. [DOI] [PubMed] [Google Scholar]
- 31.Radfar R, Shin R, Sheldrick G M, Minor W, Lovell C R, Odom J D, Dunlap R B, Lebioda L. The crystal structure of N10-formyltetrahydrofolate synthetase from Moorella thermoacetica. Biochemistry. 2000;39:3920–3926. doi: 10.1021/bi992790z. [DOI] [PubMed] [Google Scholar]
- 32.Ragsdale S W. Enzymology of the acetyl-CoA pathway of CO2 fixation. Crit Rev Biochem Mol Biol. 1991;26:261–300. doi: 10.3109/10409239109114070. [DOI] [PubMed] [Google Scholar]
- 33.Rankin C A, Haslam G C, Himes R H. Sequence and expression of the gene for N-10-formyltetrahydrofolate synthetase from Clostridium cylindrosporum. Protein Sci. 1993;2:197–205. doi: 10.1002/pro.5560020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakano Y, Kerkhof L. Assessment of changes in microbial community structure during operation of an ammonia biofilter with molecular tools. Appl Environ Microbiol. 1998;64:4877–4882. doi: 10.1128/aem.64.12.4877-4882.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schink B. Clostridium magnum sp. nov., a non-autotrophic homoacetogenic bacterium. Arch Microbiol. 1984;137:250–255. [Google Scholar]
- 36.Schink B. Diversity, ecology, and isolation of acetogenic bacteria. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 197–235. [Google Scholar]
- 37.Sharak-Genthner B R, Davies C L, Bryant M P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981;42:12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staley J T, Konopka A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol. 1985;39:321–346. doi: 10.1146/annurev.mi.39.100185.001541. [DOI] [PubMed] [Google Scholar]
- 39.Thompson J D, Higgins D G, Gibson T J. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tunlid A. Molecular biology: a linkage between microbial ecology, general ecology and organismal biology. Oikos. 1999;85:177–189. [Google Scholar]
- 41.Vogels G D, Van der Drift C. Degradation of purines and pyrimidines by microorganisms. Bacteriol Rev. 1976;40:403–468. doi: 10.1128/br.40.2.403-468.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang R F, Cao W W, Cerniglia C E. PCR detection and quantification of predominant anaerobic bacteria in human and animal fecal samples. Appl Environ Microbiol. 1996;62:1242–1247. doi: 10.1128/aem.62.4.1242-1247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward D M, Ferris M J, Nold S C, Bateson M M. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol Mol Biol Rev. 1998;62:1353–1370. doi: 10.1128/mmbr.62.4.1353-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitehead T R, Rabinowitz J C. Nucleotide sequence of the Clostridium acidiurici (“Clostridium acidi-urici”) gene for 10-formyltetrahydrofolate synthetase shows extensive amino acid homology with the trifunctional enzyme C1-tetrahydrofolate synthase from Saccharomyces cerevisiae. J Bacteriol. 1988;170:3255–3261. doi: 10.1128/jb.170.7.3255-3261.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitehead T R, Park M, Rabinowitz J C. Distribution of 10-formyltetrahydrofolate synthetase in eubacteria. J Bacteriol. 1988;170:995–997. doi: 10.1128/jb.170.2.995-997.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood H G, Ljungdahl L G. Autotrophic character of the acetogenic bacteria. In: Barton L L, Shively J, editors. Variations in autotrophic life. San Diego, Calif: Academic Press; 1991. pp. 201–250. [Google Scholar]