Huangkui capsule, as a traditional Chinese medicine, is made from the ethanol extract of flowers in Abelmoschus manihot and currently used for treatment of patients with kidney diseases in China (1). Irbesartan, as an angiotensin receptor blocker, is renoprotective independently of its blood pressure–lowering effect in patients with type 2 diabetes (T2D) and microalbuminuria (2,3). In this study, we have undertaken a multicenter randomized double-blind parallel controlled clinical trial to evaluate the efficacy and safety of huangkui capsule alone and in combination with irbesartan for reduction of albuminuria and proteinuria in T2D patients with diabetic kidney disease (DKD).
This clinical trial was conducted in nine hospitals in Jiangsu Province, China. The first patient was enrolled on 9 May 2017 and the last patient on 26 March 2021. All enrolled patients signed a written informed consent form before enrollment, and the project was approved by the ethics committees. All patients (men 61.5% and women 38.5%) included in this study were Han Chinese, and average age was 57.8 years old. The patients met the 1999 World Health Organization diagnostic criteria for T2D. The diagnostic criteria for DKD in this study followed the 2007 American Diabetes Association and 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) guidelines. Of 697 DKD patients screened, 413 met the criteria for inclusion and were randomly assigned to the groups of irbesartan (n = 138), A. manihot (n = 137), and combined treatment (n = 138) at a 1:1:1 ratio. Irbesartan [Sanofi Pharmaceutical Co., Ltd., Hangzhou, China] was prescribed at a dose of 150 mg/day. In the A. manihot group, a 2.5-g huangkui capsule (Suzhong Pharmaceutical Group Co., Ltd., Taizhou, China) was prescribed for three times per day. In the combined treatment group, both A. manihot and irbesartan were used. The duration of intervention was 24 weeks. Urine albumin-to-creatinine ratio (ACR) was assessed every 4 weeks. The 24-h urine proteinuria, urine protein-to-creatine ratio (PCR), estimated glomerular filtration rate, hs-CRP, glycosylated hemoglobin (HbA1c) and other clinical parameters were examined at 0, 12, and 24 weeks.
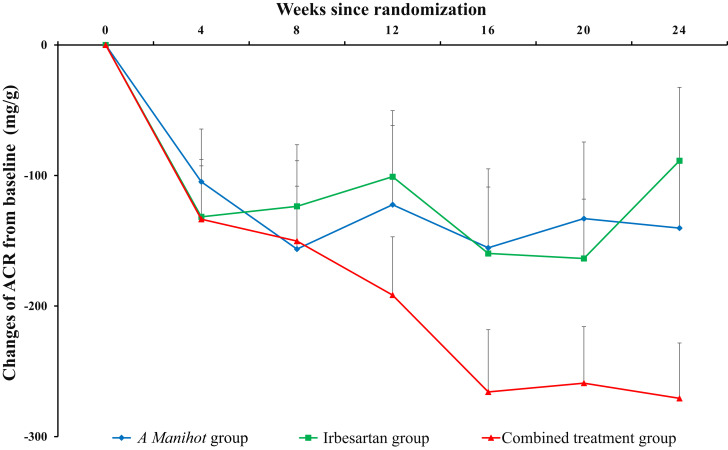
The primary outcomes were changed values of ACR from baseline after treatment. At baseline, mean ± SE ACR in the irbesartan, A. manihot, and combined groups was similar (847.04 ± 39.28, 761.69 ± 39.77, and 797.23 ± 37.59 mg/g, respectively). From baseline to after 24 weeks of treatment, ACR in these three groups was reduced by −89.07 ± 51.167, −146.06 ± 45.520, and −262.31 ± 39.081 mg/g (A. manihot vs. irbesartan, P = 0.087; combined treatment vs. irbesartan, P < 0.001). The dynamic changes in ACR in the irbesartan, A. manihot, and combined groups from baseline and after 24-week treatment are represented in Fig. 1. Similar results were founded for the intention-to-treat estimated with LSmeans difference. After 24 weeks of treatment, the least squares means changes in ACR were −56.02 (95% CI −138.96 to 26.91), −155.55 (−238.52 to −72.57), and −253.96 (−336.68 to −171.25) in the irbesartan, A. manihot, and combined groups. The adjusted mean difference in ACR for the A. manihot group compared with the irbesartan group was −99.52 (95% CI −213.64 to 14.60, P = 0.087) and for the combined treatment group compared with irbesartan group −197.94 (−311.63 to −84.25; P < 0.001).
Figure 1.
Relative changes of urine ACR since randomization. Data are means ± SE.
The secondary efficacy changes from baseline to after treatment included the changed value of 24-h proteinuria, urine protein-to-creatinine ratio, estimated glomerular filtration rate, and hs-CRP. Mean ± SE 24-h proteinuria in the irbesartan, A. manihot, and combined treatment groups decreased by −102.55 ± 129.15, −387.04 ± 127.99, and −526.99 ± 100.24 mg, respectively (P = 0.005 and 0.001). All these indicators were tested from baseline to 12 and 24 weeks. After 24 weeks of treatment, the changed PCR values from baseline in the irbesartan, A. manihot, and combined groups decreased, by −46.77 ± 122.50, −232.50 ± 152.45, and −428.54 ± 143.152 mg/g, and the difference was significant (P = 0.001). However, the changed values of other clinical parameters from baseline to the end of the 24-week follow-up period, such as estimated glomerular filtration rate, hs-CRP, and HbA1c in the three groups were not statistically significant.
Safety evaluation was performed from baseline and every 4 weeks after treatment. The overall incidence of adverse events was low and generally similar among treatment groups. There were no severe adverse events regarding liver function or decreases in kidney function in the A. manihot or combined treatment groups. Huangkui capsule received approval from the China Food and Drug Administration in 1999.
Several hypoglycemic drugs, including sodium–glucose cotransporter 2 inhibitors, PLP-1R agonists, and dipeptidyl peptidase 4 inhibitors, have been used to treat patients with DKD for intrinsic renal protection (4). Herein, we report that A. manihot, in the form of huangkui capsule, combined with irbesartan is an effective therapy for T2D patients with DKD in reduction of albuminuria and proteinuria.
Article Information
Acknowledgments. This is a Sino-French cooperative research project, in which Chinese and French scientists and clinicians have jointly participated in the study design, experiment implementation, and quality control of the study. The authors thank all subjects in this study for their participation, Dr. Qingshan Zheng (Shanghai University of Traditional Chinese Medicine) for his support in data analysis, Jiangsu Famesheng Pharmaceutical Co., Ltd., for quality control during the implementation of the project, and Drs. Jiangyi Yu, Xu Wang, Liji Huang, Qiong Liu, Jin Yan, Yuanyuan Zhu, and Jianjiang Shen (First Affiliated Hospital of Nanjing University of Chinese Medicine); Drs. Hui Jin and Chenchen Wang (Zhongda Hospital, Southeast University); Dr. Meiyue Lv (The First People’s Hospital of Xuzhou); Drs. Liang Wang and Yue Zhang (Wuxi People’s Hospital); Drs. Qianru Tao and Jia Di (The First People’s Hospital of Changzhou); Dr. Ling Zhang (Changzhou Hospital of Traditional Chinese Medicine); Dr. Zhiwei Zhou (Taizhou Hospital of Traditional Chinese Medicine); Dr. Yan Yu (Affiliated Hospital of Nantong University); and Dr. Kun Hu (The First Affiliated Hospital of Soochow University) for contributions during the project implementation.
Funding. This project is supported by a project grant from Paris Public Hospital Group of France (COMPL126, P131001), International Cooperation Project Grant of China-France Paris Traditional Chinese Medicine Center (1601500000027-6, GJZX2016005, GZYYGJ2018018, GZYYGJ2020025, 0610-2040NF020931, 0610-2140NF020629) from China State Administration of Traditional Chinese Medicine, and International Joint Research and Development Project of Nanjing Science and Technology Commission (201402068). In this study, Suzhong Pharmaceutical Group Co., Ltd., provided experimental drugs and related placebos.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. I.T. and W.S. coordinated and designed the study. J.Z., L.X., L.G., J.-J.B., M.Y., L.W., Z.S., X.C., A.-L.S., A.B., T.M., G.L., L.L., D.C., L.P., B.L., X.Q., W.H., and Y.W. carried out the study. J.H. conducted the statistical analyses. I.T., H.F.G., and W.S. interpreted data and reviewed and edited the manuscript. All authors contributed to the discussion and agreed to the final version. W.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
J.Z., I.T., and L.X. contributed equally to this work.
Clinical trial reg. no. NCT03016832, clinicaltrials.gov
References
- 1. Li N, Tang H, Wu L, et al. Chemical constituents, clinical efficacy and molecular mechanisms of the ethanol extract of Abelmoschus manihot flowers in treatment of kidney diseases. Phytother Res 2021;35:198–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870–878 [DOI] [PubMed] [Google Scholar]
- 3. Lewis EJ, Hunsicker LG, Clarke WR, et al.; Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 4. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020;98(Suppl.): S1–S115 [DOI] [PubMed] [Google Scholar]