Abstract
Background
Locally advanced gastric cancer (LAGC) presents a therapeutic dilemma, particularly as it often involves adjacent organs through desmoplasia or true pathologic invasion. To obtain a margin-negative resection, these tumors require en bloc gastrectomy with multivisceral resection (G+MVR), and contention remains regarding its safety and oncologic benefit.
Methods
We used the National Cancer Database to retrospectively evaluate the short- and long-term outcomes of patients with LAGC treated in the USA between 2004 and 2016. Associations with margin status and perioperative outcomes were calculated using logistic regression. Survival was estimated using Cox proportional hazards regression and the Kaplan-Meier method.
Results
Overall, 785 pathologic stage T4b (pT4b) patients diagnosed with LAGC underwent gastrectomy (n = 438) or G+MVR (n = 347). There was no association between G+MVR and short- or long-term mortality. Positive resection margins (HR 1.68, 95% CI 1.40–2.03), the presence of nodal disease (HRs 1.46–1.50), treatment at a high-volume center (HR 0.76, 95% CI 0.68–0.85), and the receipt of adjuvant chemotherapy (HR 0.64, 95% CI 0.51–0.80) were independently associated with overall survival. Diffuse-type histology was associated with higher rates of an R1 resection (OR 3.60, 95% CI 2.20–5.87). Perioperative and long-term survival metrics were comparable between patients with pT4a and pT4b LAGC who underwent a margin-negative G+MVR. Undergoing a margin-negative G+MVR imparted a 6-month survival benefit over non-curative gastrectomy alone (p < 0.001).
Conclusions
Our study demonstrates the safety and long-term feasibility of G+MVR for disease clearance in well-selected patients with LAGC, and we advocate for their referral to high-volume centers for optimal care.
Keywords: Multivisceral resection, Locally advanced, Gastric cancer
Introduction
Gastric cancer (GC) is the world’s third most lethal malignancy, accounting for over 780,000 deaths annually.1 In the Western world, patients often present at advanced stages, leaving them with less opportunity for cure.1–5 A multimodal approach incorporating surgery and chemotherapy generally provides patients the best chance for a favorable outcome.6 Adequate locoregional control in GC includes either subtotal or total gastrectomy with regional lymphadenectomy.7 Resection of adjacent organs, such as the distal pancreas, spleen, or colon, may be necessary to maximize local control of disease. Advances in perioperative systemic chemotherapy have led to improved oncologic outcomes of resection.6 However, despite improvements in both treatment modalities, overall GC prognosis remains poor.6,8–10
A subset of GC patients present with stage T4b locally advanced gastric cancer (LAGC) that extends through the stomach wall and into adjacent organs in the absence of remote spread.11 This locally aggressive behavior is believed to represent a more favorable disease biology than GC that develops regional or distant dissemination early in the disease course.12,13 For such patients, a potentially curative resection remains possible but may require en bloc multivisceral resection (MVR) of adjacent organs to achieve negative margins.7,14,15 Routine staging studies do not reliably identify patients who require MVR for disease clearance, as preoperative clinical T stage accuracy ranges from 43 to 88%.16–22 Many LAGCs are densely adherent to adjacent organs due to an intense desmoplastic inflammatory response, which may be interpreted as T4b disease while tumor itself does not traverse the gastric serosa. As such, rates of pathologic T4 (pT4) disease in the setting of desmoplasia are as low as 46%.7,11,14,15 Given this uncertainty, these patients are presumed to have pT4b disease until proven otherwise, and curative-intent resection in such cases should include MVR. The risk of additional perioperative complications and mortality associated with MVR in LAGC may create a nihilistic view of aggressive treatment, which in turn may impede referral to surgical oncologists and potentially dissuade their attempts at a curative-intent operation.7,23,24
Treatment of these complex patients requires a nuanced approach, and long-term outcomes in GC have improved through the development of increasingly effective systemic therapy regimens.6,8 However, resection of the primary tumor remains a critical component of GC management, and this task is further complicated in patients with locally aggressive tumors.7 In order to assess whether MVR is beneficial for patients with LAGC and adjacent organ involvement, we used a national database to evaluate short- and long-term outcomes for this population from across the USA.7,14,23 Specifically, we aimed to determine the safety and efficacy of MVR, the biological effect of pathologically verified tumor invasion into an adjacent organ in the setting of a margin-negative resection, and the survival implications of a radical operation to achieve negative margins for LAGC. Additionally, we sought to better characterize the centers at which these patients receive care and to assess the relationships between outcomes and hospital volume, database entry completion, and center type.
Methods
Data Source
The National Cancer Database participant user files (NCDB PUFs) are a repository of de-identified tumor- and outcome-related cancer patient data from across the USA. These data are collated from the submissions of over 1,500 Commission on Cancer (CoC)-accredited programs, accounting for over 70% of incident US cancer diagnoses per year.25 Of note, the CoC is a collaboration between the American College of Surgeons and the American Cancer Society that evaluates and accredits hospitals based on their cancer care metrics. The CoC does not verify the data supplied in the NCDB, the methodology used in its analysis, or the conclusions that are drawn from those results. NCDB was the source of all data in our study, and because only de-identified data was included, the study was exempt from institutional review board review.
Cohort Selection
We selected all patients with malignancy of the stomach diagnosed between 2004 and 2016, as delineated by the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography codes 16.1–16.9. Patients with cancer of the gastric cardia, indicated by topography code 16.0, were excluded from our analysis to eliminate the potential confounding factors associated with undergoing esophagogastrectomy. We selected only patients with gastric adenocarcinoma, as indicated by ICD-O-3 morphology codes 8140, 8141, 8142, 8143, 8144, 8145, and 8490, with the American Joint Committee on Cancer (AJCC) 7th edition pathologic stages T4a and T4b (pT4a and pT4b) disease. Patients with distant metastasis were excluded. Using the Facility Oncology Registry Data Standards, patients who underwent gastrectomy were identified using site-specific procedure codes 30–52 and 80, and those who underwent gastrectomy with MVR were indicated by procedure codes 60–63. Patients who did not undergo gastrectomy were excluded. Patients who underwent gastrectomy alone were designated to the “gastrectomy” cohort, while those who also underwent MVR were designated to the “G+MVR” cohort (Fig. 1). Patients who underwent a “margin-positive resection” consisted of those who underwent a microscopically margin-positive resection (R1), a macroscopically positive margin (R2), and a margin-positive resection not otherwise specified (R1+R2).
Fig. 1.
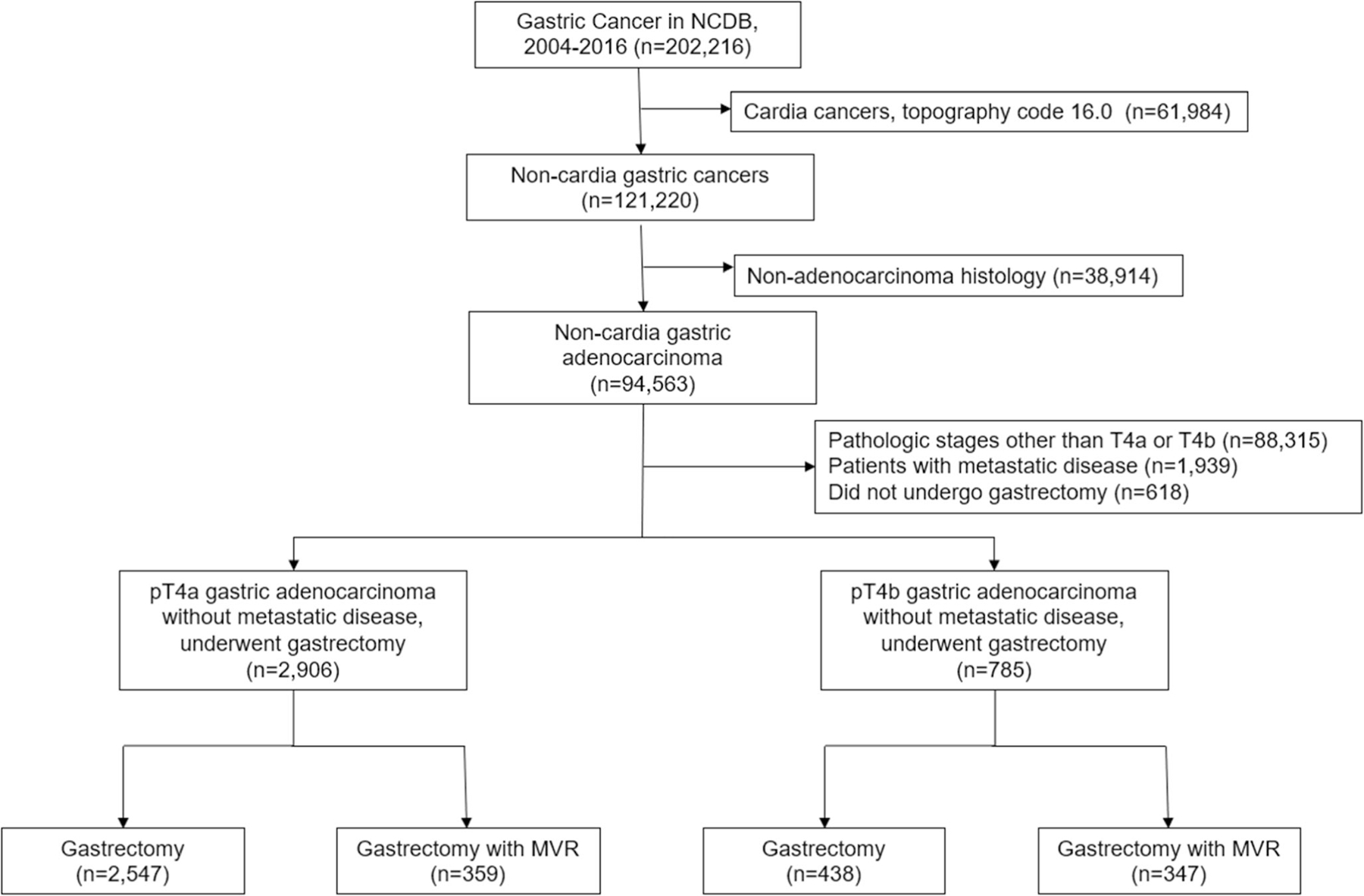
Consolidated standards of reporting trials (CONSORT) diagram for locally advanced gastric cancer
Data Analysis
We collected available demographic, clinicopathologic, and outcomes data on all patients and subsequently performed Wilcoxon rank-sum tests and Pearson’s chi-squared tests for continuous and categorical variables, respectively. We performed a subgroup analysis using logistic regression to compare diffuse versus intestinal histologic subtypes of LAGC and the odds of specifically obtaining an R1 (versus R0) resection margin. Logistic regression was also used to compare perioperative outcomes (30-day unplanned readmission, 30-day mortality, and 90-day mortality). Bivariable survival analyses were performed using Cox proportional hazards regression. Clinically significant variables and variables demonstrating statistically significant associations in bivariable analysis were subsequently included as predictors in a multivariable model.
We also performed a facility-based analysis based on case volume and database completion percentage. We compiled facility case totals based on G+MVR contributions to the entire gastric cancer NCDB PUFs and considered facilities who contributed a total number of G+MVR cases within the top 10% of all contributing centers to be “high-volume centers” (HVCs). We hypothesized that a hierarchical clustering association by treatment in HVCs may affect these data, so we performed multilevel modeling to measure that difference. While incorporating both random intercepts and random slopes within our model, Akaike information criteria did at times slightly decrease, but these iterative changes did not appreciably change the covariates’ fixed effects. Based on these findings, we believed that the use of a fixed effects model in this situation was simpler and more appropriate for these data.
Additionally, we evaluated the database entry completeness of each contributing center by calculating the percentage of missing or ambiguous data (with respect to the total number of data entry opportunities) from each center and subtracting that value from 1 to generate a “completion rate.” Of note, we applied further scrutiny to pertinent variables. Specifically, we considered entries of “not otherwise specified” (NOS) for margin status (positive margin NOS), primary tumor site (NOS, greater curvature, lesser curvature), and extent of gastrectomy (NOS) as incomplete entries. Similarly, we considered clinical and pathologic staging entries as incomplete if they had “Tx” or “Nx” values. We categorized centers whose completion rates were below the median as “low completion centers” (LCCs) and those whose completion rates were at or above the median as “high completion centers” (HCCs). We used Pearson’s chi-squared testing to determine associations between being an HCC and an HVC or academic center.
Statistical significance was defined as p < 0.05. Survival curves were estimated using the Kaplan-Meier method, and the curves were compared using the log-rank test. Of note, perioperative and long-term survival data were unavailable for patients who were diagnosed with LAGC in 2016 (n = 97), which constituted over 95% of patients with missing follow-up data. These data were not considered incomplete entries with respect to our database completion analysis. All statistical analyses were performed using SAS version 9.4 (SAS Inc, Cary, NC), and graphics were produced using GraphPad Prism 8.1.1 (GraphPad Software, San Diego, CA).
Stage pT4b: Gastrectomy vs. G+MVR
The first portion of our analysis evaluated treatment effect while controlling for disease biology by comparing patients with pT4b tumors who underwent gastrectomy alone versus those who underwent G+MVR. We analyzed baseline characteristics, margin status, perioperative outcomes, and long-term survival outcomes as above.
True vs. Suspected Tumor Invasion–Margin-negative G+MVR: pT4a vs. pT4b
The second portion of our analysis evaluated disease biology while controlling for treatment effect by comparing patients who underwent a margin-negative G+MVR with pT4a disease versus those with pT4b disease. We analyzed baseline characteristics, perioperative outcomes, and long-term survival outcomes as above.
Subgroup Survival Analysis
Finally, the third portion of our analysis approximated the survival advantage afforded patients with pT4b disease who underwent a margin-negative G+MVR when compared with those with pT4b disease who underwent a margin-positive gastrectomy (effectively, a non-curative gastrectomy). This analysis eliminated patients who underwent a margin-positive G+MVR (i.e., aborted after adjacent organ resection due to operative difficulty, positive margins on final pathology) and those who underwent a margin-negative gastrectomy alone (i.e., invasion into an adjacent structure such as the omentum, mesentery, or peritoneum but not into an adjacent major organ). We believed that comparing these two groups best highlighted the balance between the short-term morbidity of an extended operation and the potential long-term survival benefit of a complete resection.
Results
Stage pT4b: Gastrectomy vs. G+MVR
Patient Demographics and Clinicopathologic Variables
Overall, 785 patients with pT4b disease met inclusion criteria, and a slightly higher proportion (n = 438, 55.8%) underwent gastrectomy alone compared with G+MVR (n = 347, 44.2%, Table 1). Factors associated with G+MVR were younger age, larger tumor size, receipt of care at an academic or high-volume center, receipt of neoadjuvant chemotherapy, total gastrectomy, higher lymph node yield, and longer length of stay (p < 0.05). Among patients who underwent a margin-positive resection, only 4.7% (n = 15) underwent an R2 resection, while the remainder underwent an R1 resection (n = 185, 58.4%) or a margin-positive resection that was not otherwise specified (n = 117, 36.9%). In a multivariable model, diffuse histology was independently associated with undergoing an R1 resection (OR 3.60, 95% CI 2.20–5.87), and treatment at an HVC was associated with undergoing an R0 resection (OR 1.67, 1.08–2.60). Treatment at an HCC was not associated with R1 status (OR 1.01, 95% CI 0.71–1.42). Of note, the receipt of neoadjuvant therapy was not associated with undergoing an R0 resection among patients who underwent gastrectomy alone (OR 1.59, 95% CI 0.89–2.85).
Table 1.
Clinicopathologic characteristics of patients with pT4b locally advanced gastric cancer
| Gastrectomy (n = 438)a | G+MVR (n = 347)a,b | p value | |
|---|---|---|---|
| Age (years) | |||
| 18–49 | 44 (10.1) | 51 (14.7) | < 0.001 |
| 50–64 | 121 (27.6) | 134 (38.6) | |
| 65–74 | 123 (28.1) | 85 (24.5) | |
| ≥ 75 | 150 (34.2) | 77 (22.2) | |
| Female sex | 195 (44.5) | 152 (43.8) | 0.84 |
| Race | 0.85 | ||
| White | 289 (66.0) | 237 (68.3) | |
| African-American | 96 (21.9) | 72 (20.8) | |
| Asian | 43 (9.8) | 29 (8.4) | |
| Hispanic | 0.33 | ||
| Yes | 67 (15.3) | 43 (12.4) | |
| No | 358 (81.7) | 297 (85.6) | |
| Charlson-Deyo score | 0.24 | ||
| 0 | 283 (64.6) | 244 (70.3) | |
| 1 | 113 (25.8) | 75 (21.6) | |
| ≥ 2 | 42 (9.6) | 28 (8.1) | |
| Tumor size (cm)b | 6.0 (4.0–8.5) | 7.0 (5.0–9.5) | 0.006 |
| Tumor location | 0.29 | ||
| Fundus | 25 (5.7) | 21 (6.1) | |
| Body | 40 (9.1) | 46 (13.3) | |
| Antrum/pylorus | 172 (39.3) | 124 (35.7) | |
| Unspecified | 201 (45.9) | 156 (45.0) | |
| Gastrectomy type | < 0.001 | ||
| Subtotal | 269 (61.4) | 149 (42.9) | |
| Total | 169 (38.6) | 151 (43.5) | |
| Gastrectomy unspecified | 0 (0) | 47 (13.5) | |
| Differentiation | 0.03 | ||
| Well- and moderately differentiated | 99 (22.6) | 53 (15.3) | |
| Poorly and undifferentiated | 328 (74.9) | 287 (82.7) | |
| Pathologic nodal stage | 0.57 | ||
| 0 | 89 (20.3) | 64 (18.4) | |
| 1 | 76 (17.4) | 59 (17.0) | |
| 2 | 89 (20.3) | 71 (20.5) | |
| 3 | 171 (39.0) | 148 (42.7) | |
| Number of lymph nodes harvestedc | 15 (9–23) | 19 (12–28) | < 0.001 |
| ≥ 15 lymph nodes harvested | < 0.001 | ||
| Yes | 230 (52.5) | 232 (66.9) | |
| No | 205 (46.8) | 109 (31.4) | |
| Resection margin status | 0.26 | ||
| Positive | 188 (42.9) | 129 (37.2) | |
| Negative | 243 (55.5) | 211 (60.8) | |
| Neoadjuvant chemotherapy | 0.003 | ||
| Yes | 73 (16.7) | 92 (26.5) | |
| No | 360 (82.2) | 252 (72.6) | |
| Neoadjuvant radiation | 0.77 | ||
| Yes | 9 (2.1) | 7 (2.0) | |
| No | 422 (96.4) | 332 (95.7) | |
| Adjuvant chemotherapy | 0.77 | ||
| Yes | 182 (41.6) | 152 (43.8) | |
| No | 251 (57.3) | 192 (55.3) | |
| Adjuvant radiation | 0.74 | ||
| Yes | 112 (25.6) | 85 (24.5) | |
| No | 319 (72.8) | 254 (73.2) | |
| Treatment at an academic center | < 0.001 | ||
| Yes | 148 (33.8) | 158 (45.5) | |
| No | 277 (63.2) | 168 (48.4) | |
| Unspecified | 13 (3.0) | 21 (6.1) | |
| Treatment at a high-volume center | 0.01 | ||
| No | 332 (75.8) | 236 (68.0) | |
| Yes | 106 (24.2) | 111 (32.0) | |
| Treatment at a high-completion center | 0.48 | ||
| No | 222 (50.7) | 167 (48.1) | |
| Yes | 216 (49.3) | 180 (51.9) | |
| Hospital length-of-stayb | 9 (7–14) | 11 (7–15) | 0.004 |
| Unplanned readmission within 30 days of surgery | |||
| Yes | 38 (8.7) | 39 (11.2) | 0.35 |
| No | 399 (91.1) | 306 (88.2) | |
| Alive at 30 days | 0.21 | ||
| Yes | 345 (78.8) | 269 (77.5) | |
| No | 43 (9.8) | 26 (7.5) | |
| Unspecified | 50 (11.4) | 52 (15.0) | |
| Alive at 90 days | 0.42 | ||
| Yes | 317 (72.3) | 245 (70.6) | |
| No | 69 (15.8) | 50 (14.4) | |
| Unspecified | 52 (11.9) | 52 (15.0) |
For some variables, a column percent will sum to less than 100% as the NCDB user agreement prohibits reporting of small cell sizes
Gastrectomy with multivisceral resection
Median (interquartile range)
The median data completion rate (interquartile range, IQR) for all centers was 0.90 (0.85–0.95). Overall, just 67.1% (n = 53) of HVCs were HCCs while 62.7% (n = 229) of HVCs were LCCs (OR 1.21, 95% CI 0.72–2.03; p = 0.47). However, there was an association between academic centers and being an HCC, as 75% (n = 105) were HCCs (OR 2.01, 95% CI 1.28–3.14; p < 0.001), as opposed to just 60% (n = 172) of non-academic centers. When considering variables that highly associated with gastric cancer outcomes, just 17.7% (n = 41) of pT4a tumors were staged as cT4a tumors and just 19.4% of pT4b tumors (n = 41) were staged as cT4b tumors. Importantly, a considerable fraction of both pT4a (46.1%) and pT4b (47.3%) patients either had cX or a “missing” value recorded as their clinical T stage. Additionally, a considerable fraction of patients had a missing or ambiguous code with respect to tumor location (45.5%) and clinical N stage (19.4%).
Perioperative Outcomes
Thirty-day readmission data were available for nearly all patients (n = 782, 99.6%). Younger age and a positive resection margin were associated with an unplanned 30-day readmission (Fig. 2a). The 30-day mortality rate was 8.8% (n = 69), and the 90-day mortality rate was 15.2% (n = 119). Older age and a margin-positive resection were independently associated with 30-and 90-day mortality, while pN1 nodal disease was associated with 90-day mortality (Figs. 2b–c). Specifically, patients aged 75 years and above, who constituted over one-quarter of the cohort, had a 90-day mortality rate of 22.9%. Of note, G+MVR was not associated with 30-day readmission (OR 1.34, 95% CI 0.84–2.14), 30-day mortality (OR 0.78, 95% CI 0.47–1.29), or 90-day mortality (OR 0.94, 95% CI 1.40) using bivariable logistic regression. Treatment at an HVC was inversely associated with 90-day mortality (OR 0.56, 0.33–0.95). Additionally, treatment at an HCC was not associated with 30-day readmission (OR 0.85, 95% CI 0.53–1.36), 30-day mortality (OR 0.87, 0.53–1.43), or 90-day mortality (OR 0.96, 95% CI 0.65–1.44).
Fig. 2.
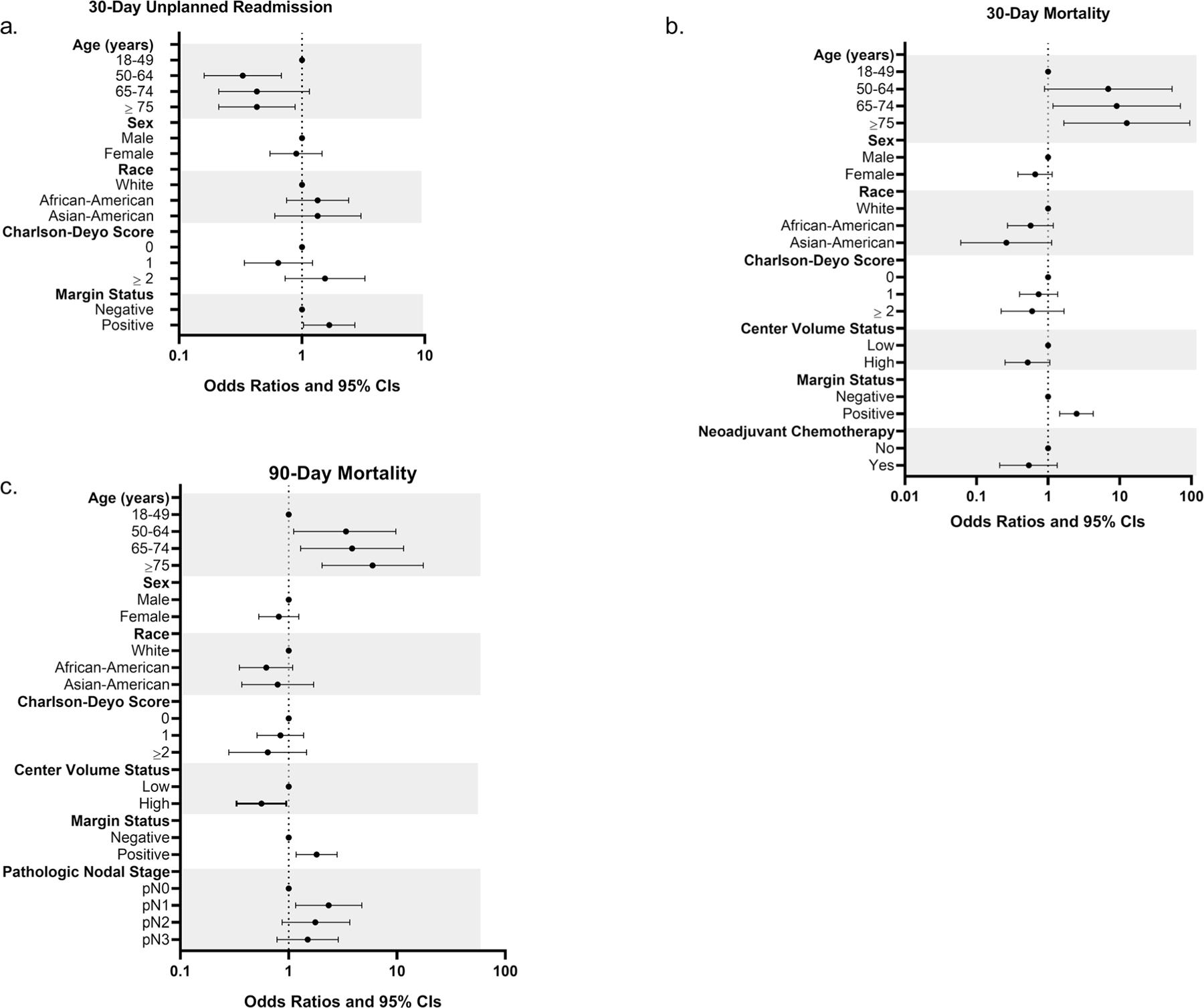
Perioperative outcomes for patients with pT4b gastric cancer. Odds ratios and 95% confidence intervals calculated using multivariable logistic regression. Horizontal axis uses a log-10 based scale. a 30-day unplanned readmission. b 30-day mortality. c 90-day mortality
Survival Analysis
Long-term follow-up information was available for 88% (n = 688) of pT4b patients. Unadjusted median (interquartile range, IQR) overall survival (OS) for all patients with pT4b tumors was 13.5 months (IQR 6.2–30.3). Unadjusted median OS for gastrectomy and G+MVR pT4b cohorts was 13.7 (IQR 6.1–31.5) months and 12.9 (IQR 6.2–26.6) months (p= 0.62), respectively. Among all patients, a positive resection margin and the presence of nodal disease (pN1 and pN3) were independently associated with shortened OS, while undergoing care at an HVC and the receipt of adjuvant radiation or chemotherapy were independently associated with prolonged survival (Table 2). Treatment at an HCC was not associated with survival (HR 1.07, 95% CI 0.90– 1.30). G+MVR subgroup analyses demonstrated that positive margin status (HR 1.49, 95% CI 1.11–2.00) and the presence of extensive nodal disease (pN3) were associated with shorter survival (HR 1.97, 95% CI 1.33–2.91), and the receipt of care at an HVC (HR 0.72, 95% CI 0.54–0.96) and adjuvant chemotherapy (HR 0.58, 95% CI 0.42–0.80) were independently associated with prolonged survival (Supplemental 1).
Table 2.
Bivariable and multivariable survival analysis for patients with pT4b locally advanced gastric cancer
| Bivariable HR (95% CI)* | Multivariable HR (95% CI)* | |
|---|---|---|
| Age (years) | ||
| 18–49 | Reference | Reference |
| 50–64 | 0.84 (0.63–1.13) | 0.87 (0.65–1.17) |
| 65–74 | 0.97 (0.72–1.31) | 1.02 (0.75–1.38) |
| ≥ 75 | 1.42 (1.07–1.90) | 1.20 (0.88–1.65) |
| Female sex | 1.21 (1.02–1.43) | 1.11 (0.93–1.33) |
| Race | ||
| White | Reference | |
| African-American | 0.84 (0.68–1.04) | |
| Asian-American | 0.77 (0.56–1.07) | |
| Hispanic Ethnicity | ||
| No | Reference | |
| Yes | 0.82 (0.62–1.08) | |
| Charlson-Deyo score | ||
| 0 | Reference | Reference |
| 1 | 1.03 (0.84–1.26) | 0.87 (0.71–1.08) |
| ≥ 2 | 0.97 (0.71–1.33) | 0.85 (0.61–1.19) |
| MVR | ||
| Yes | 1.04 (0.88–1.24) | |
| Gastrectomy type | ||
| Subtotal | Reference | |
| Total | 1.14 (0.95–1.36) | |
| Gastrectomy unspecified | 1.36 (0.96–1.94) | |
| Pathologic nodal stage | ||
| 0 | Reference | Reference |
| 1 | 1.54 (1.16–2.05) | 1.50 (1.12–2.00) |
| 2 | 1.30 (0.98–1.72) | 1.31 (0.98–1.75) |
| 3 | 1.68 (1.31–2.14) | 1.46 (1.13–1.88) |
| Resection margin status | ||
| Positive | 1.76 (1.48–2.10) | 1.63 (1.35–1.97) |
| Neoadjuvant chemotherapy | ||
| Yes | 0.79 (0.64–0.98) | 0.81 (0.64–1.03) |
| Neoadjuvant radiation | ||
| Yes | 0.79 (0.42–1.48) | |
| Adjuvant chemotherapy | ||
| Yes | 0.60 (0.51–0.72) | 0.64 (0.51–0.80) |
| Adjuvant radiation therapy | ||
| Yes | 0.63 (0.52–0.77) | 0.77 (0.61–0.98) |
| Treatment at a high-volume center | ||
| Yes | 0.73 (0.60–0.89) | 0.76 (0.68–0.85) |
| Treatment at a high-completion center | ||
| Yes |
Hazard ratios and 95% confidence intervals
True vs. Suspected Tumor Invasion–Margin-negative G + MVR: pT4a vs. pT4b
A total of 443 patients had a margin-negative (R0) gastrectomy with multivisceral resection. A slight majority had pT4a (n = 232, 52.4%) versus pT4b (n = 211, 47.6%) tumors. While patients with pT4a disease more commonly had poorly and undifferentiated tumors and slightly higher readmission rates, demographic and clinicopathologic variables were quite similar between groups (Supplemental 2). Pathologic T4b disease was not predictive of a 30-day unplanned readmission (OR 2.17, 95% CI 0.98–4.77), 30-day mortality (OR 1.22, 95% CI 0.48–3.07), or 90-day mortality (OR 1.23, 95% CI 0.63–2.40). There were few variables associated with perioperative outcomes, but older age was independently associated with a higher rate of 90-day mortality (Supplemental 3). Unadjusted median OS was 22.6 (IQR 10.4–46.3) and 17.7 (IQR 8.8–39.2) months for pT4a and pT4b groups, respectively (Fig. 3, log-rank p = 0.21). For both pT4a and pT4b groups, nodal disease and a higher comorbidity score were associated with shortened survival (Table 3).
Fig. 3.
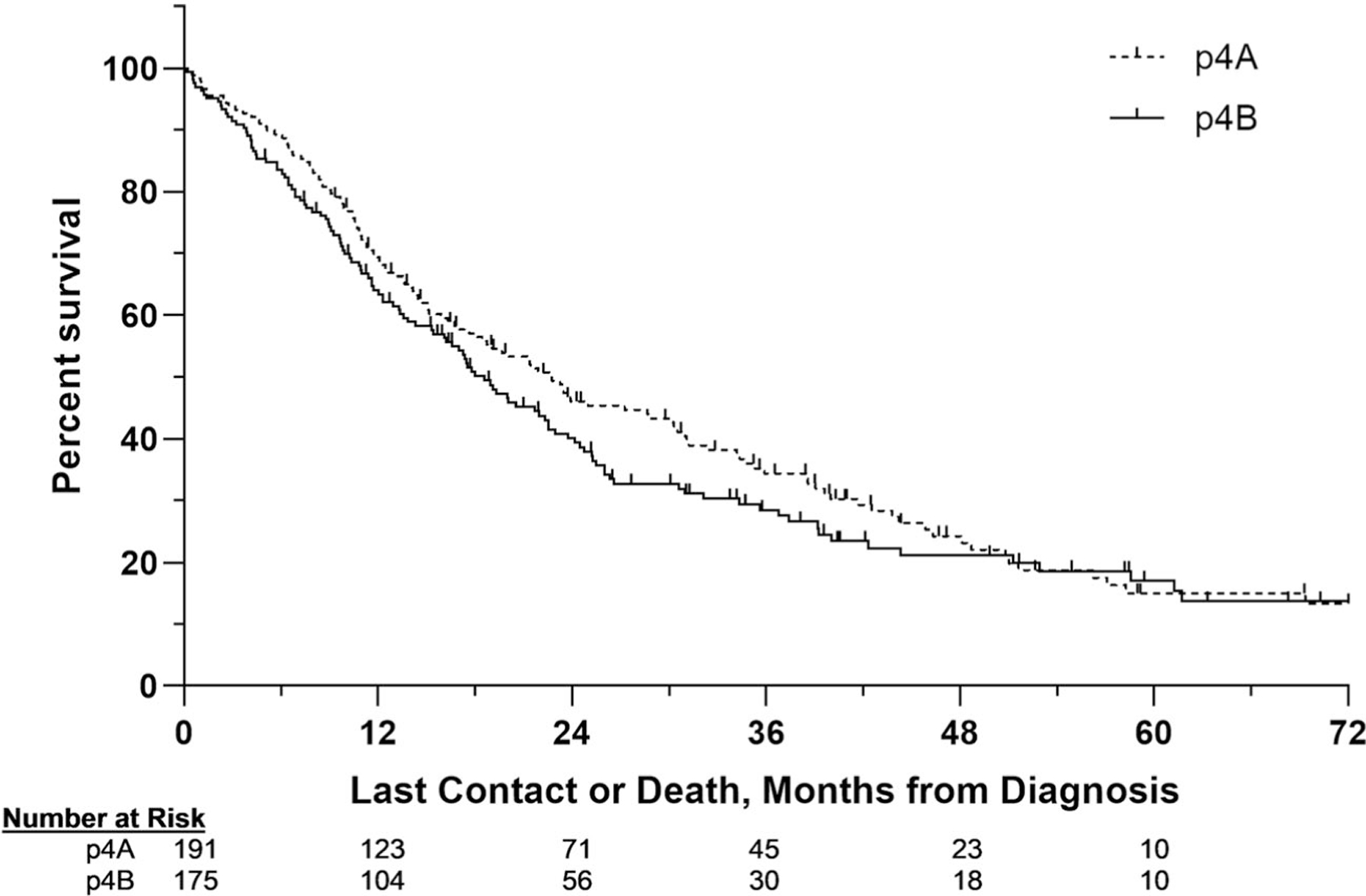
Kaplan-Meier estimates comparing patients with pT4a and pT4a gastric cancer. Patients who underwent margin-negative gastrectomy with multivisceral resection
Table 3.
Bivariable and multivariable survival analysis for patients with pT4a and pT4 locally advanced gastric cancer who underwent margin-negative gastrectomy with multivisceral resection
| Bivariable HR (95% CI)* | Multivariable HR (95% CI)* | |
|---|---|---|
| Age (years) | ||
| 18–49 | Reference | Reference |
| 50–64 | 0.98 (0.67–1.45) | 0.93 (0.62–1.40) |
| 65–74 | 0.78 (0.51–1.18) | 0.84 (0.54–1.30) |
| ≥ 75 | 1.54 (1.03–2.30) | 1.36 (0.89–2.07) |
| Female sex | 0.99 (0.62–1.59) | 1.32 (1.02–1.71) |
| Race | ||
| White | Reference | |
| African-American | 1.11 (0.82–1.50) | |
| Asian-American | 0.89 (0.55–1.42) | |
| Hispanic ethnicity | ||
| Yes | 1.07 (0.75–1.53) | |
| Charlson-Deyo score | ||
| 0 | Reference | Reference |
| 1 | 1.57 (1.17–2.09) | 1.41 (1.05–1.90) |
| ≥ 2 | 1.13 (0.72–1.75) | 1.29 (0.74–1.92) |
| Gastrectomy type | ||
| Subtotal | Reference | |
| Total | 1.12 (0.86–1.45) | |
| Gastrectomy unspecified | 1.05 (0.72–1.55) | |
| MVR | ||
| Yes | 0.75 (0.47–1.20) | |
| Primary tumor stage | ||
| T4b | 1.17 (0.92–1.49) | |
| Pathologic nodal stage | ||
| 0 | Reference | Reference |
| 1 | 1.36 (0.90–2.06) | 1.61 (1.06–2.47) |
| 2 | 1.18 (0.79–1.76) | 1.45 (0.96–2.19) |
| 3 | 2.35 (1.69–3.27) | 2.69 (1.91–3.78) |
| Neoadjuvant chemotherapy | ||
| Yes | 0.88 (0.66–1.16) | |
| Neoadjuvant radiation | ||
| Yes | 0.72 (0.27–1.94) | |
| Adjuvant chemotherapy | ||
| Yes | 0.59 (0.46–0.75) | 0.75 (0.55–1.03) |
| Adjuvant radiation | ||
| Yes | 0.64 (0.49–0.84) | 0.77 (0.55–1.08) |
Hazard ratios and 95% confidence intervals
Subgroup Survival Analysis
A slightly higher proportion of patients with pT4b disease underwent a margin-negative G+MVR (n = 211, 52.8%) than margin-positive gastrectomy alone (n = 188, 47.1%). Follow-up information was available for 86% of patients (n = 344). Median OS (IQR) for the entire cohort was 13.3 (IQR 5.9– 27.9) months. Median OS for patients who underwent a margin-negative G+MVR was 6.6 months longer than for those who underwent a margin-positive gastrectomy alone (17.8 [IQR 8.8–39.2] vs. 11.2 [IQR 4.1–20.1] months, respectively; Fig. 4).
Fig. 4.
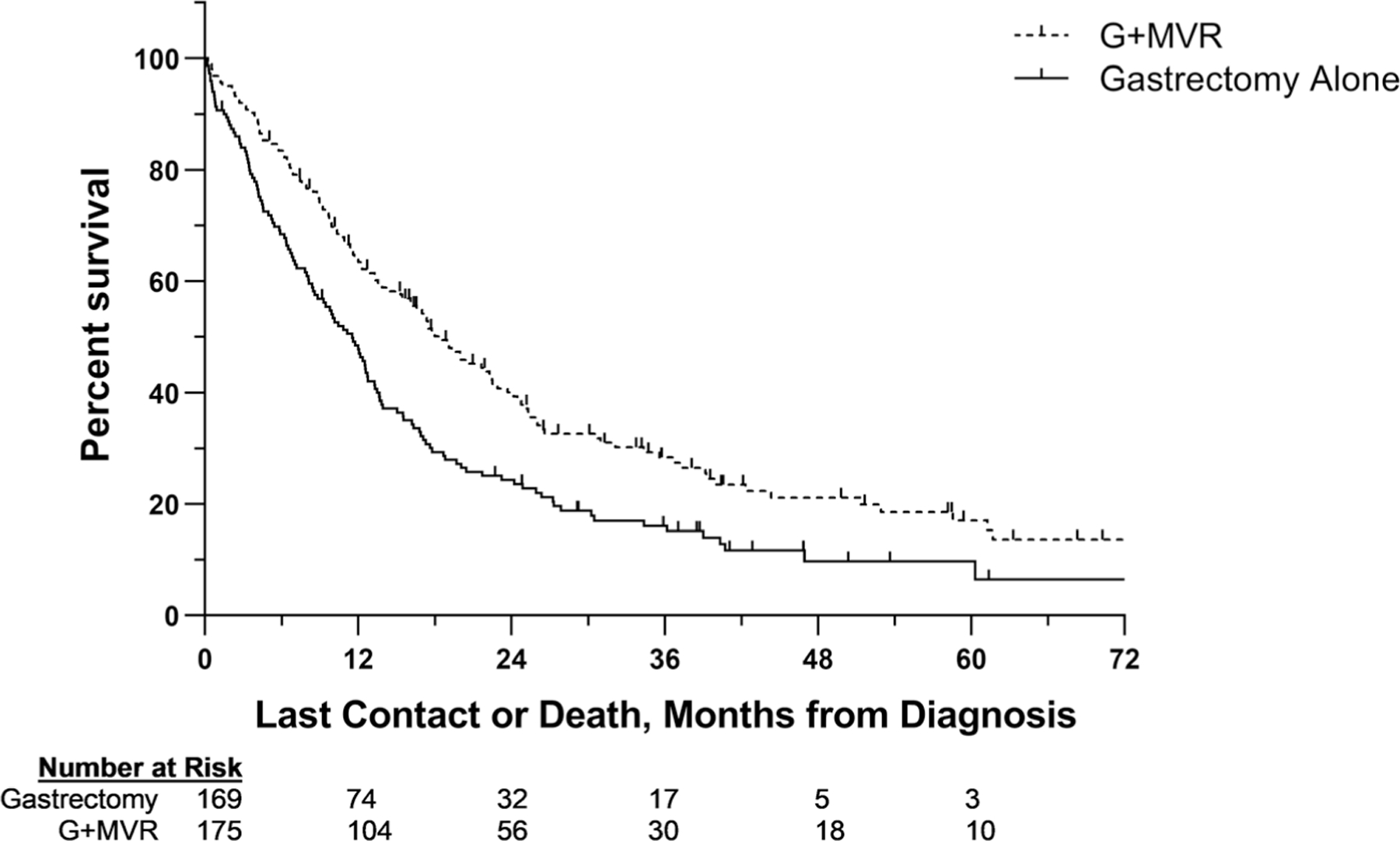
Kaplan-Meier estimates comparing patients with locally advanced gastric cancer. Patients who underwent margin-negative gastrectomy with multivisceral resection versus those who underwent margin-positive gastrectomy alone
Discussion
We evaluated the short- and long-term outcomes of patients with LAGC treated with gastrectomy and multivisceral resection using a national database. First, we selected the most locally advanced (T4b) tumors treated with G+MVR compared with gastrectomy alone and demonstrated that the addition of MVR was not associated with worse short- or long-term outcomes. Undergoing a margin-positive resection was associated with decreased long-term survival. Furthermore, while older patient age and a positive resection margin were associated with increased perioperative mortality, treatment at an HVC was associated with a lower 90-day mortality rate and better long-term survival, highlighting the importance of patient selection and referral to experienced centers. Additionally, diffuse-type histology was associated with a higher rate of undergoing an R1 resection. Next, we compared patients with pT4a and pT4b tumors who underwent a margin-negative G+MVR to determine differences in disease biology between true pathologic invasion versus those with organ-adherent desmoplasia. In doing so, we showed that short- and long-term outcomes were similar between the two groups. Finally, we approximated the survival advantage afforded to a well-selected patient with pT4b LAGC by comparing survival between those who underwent a margin-negative G+MVR versus a margin-positive gastrectomy. Our findings suggest that when necessary for disease clearance of LAGC, G+MVR is a practical and potentially beneficial treatment option for highly selected patients.
Our results are consistent with the well-documented advantages conferred by margin-negative resections for long-term oncologic outcomes.26,27 We selected patients with the most locally invasive biology, finding little overall survival difference compared with patients who underwent an extended resection for desmoplasia.16,21,28 When considering that up to half of patients with apparent pathologic invasion may only have organ-adherent desmoplasia, surgeons are potentially subjecting these patients to “over-resection,” but the practice does not appear to inflict additional harm compared with gastrectomy alone. However, our data also emphasize the risks of undergoing a margin-positive resection, and this suboptimal outcome seems to be associated with the more insidious diffuse histologic subtype of GC. This risk of a margin-positive resection is potentially related to subepithelial spread, and is independently associated both with diffuse-type cancers and locally-advanced tumors.29,30 An overly aggressive approach in an unfit candidate may increase the risk of perioperative morbidity and mortality, which further underscores the importance of patient selection in these complex cases. This increase in perioperative mortality seems to be most profound in older patients. For those with presumed T4b LAGC, G+MVR represents the only potentially curative treatment option and seems to impart a marginal increase in morbidity in order to achieve negative margins and potentially prolong survival in a well-selected patient.
While the addition of MVR to gastrectomy is oncologically sound, indiscriminate application of this approach can be perilous even in patients with excellent functional status who undergo treatment in experienced centers.31,32 Any patient being considered for MVR must be able to sustain the procedure’s associated morbidity, and a patient’s physiologic reserve is often related to the mechanical and functional effects of the locally aggressive primary tumor on per oral nutrition.33,34 This biology often manifests clinically as profound anorexia, sarcopenia, and malnutrition antecedent to operative intervention and can result in an increased rate of perioperative complications.33,34 Patients who underwent MVR in our pT4b cohort did have a slightly longer median hospital length-of-stay, suggesting that their courses may have been more complicated than their gastrectomy-only counterparts. However, despite this longer initial hospital stay, the addition of MVR was not associated with increased rates of hospital readmission or perioperative mortality. In addition to proper patient selection for an extended resection, the referral of G+MVR candidates to high-volume surgeons and centers ensures the highest probability of a safe and margin-negative resection.35,36 Admittedly, the perioperative mortality rate of our cohort is higher than previously reported in patients with T4b disease.7,14,15 We believe that much of this can be attributed to the substantial mortality rates among our group’s older patients. More advanced chronologic age may reflect increased frailty in this subgroup, and it often serves as a surrogate for diminished physiologic reserve. Additionally, the diffuse histologic subtype of GC seems to carry higher risk of achieving a margin-positive resection, which is a major predictor of poorer short- and long-term outcomes. Our findings underscore the importance of careful patient selection for this radical operation.
Historical and contemporary cohorts evaluating the feasibility and utility of MVR for LAGC have yielded variable results, and more recent studies have reflected improvements in the execution of a complex operation and the efficacy of systemic therapy regimens.6–8,23,37,38 Most studies questioning the role of MVR come from an era when systemic options for LAGC were limited; however, despite that, the authors felt that these patients may benefit from margin-negative locoregional tumor control.12,13,39–41 Results from single- and multi-institutional series have demonstrated the feasibility and utility of MVR for LAGC patients and have identified positive margin status, extensive nodal disease, and the inclusion of pancreatectomy in MVR as main predictors of shortened survival.7,14,23 Our findings support the results of prior studies but are more generalizable, as they are derived from a wider spectrum of care centers.
This analysis encountered variable levels of data reporting within a national database for a rare tumor subset. Most centers reported approximately 90% of requested variables. While there was no evidence of a difference in outcomes between HCCs and LCCs, reporting in academic centers was more complete than in non-academic centers. This short-coming represents an opportunity for participating centers and their specialists who treat advanced gastric cancer to redouble efforts to provide the most accurate, detailed, and complete reporting of patient data to national registries. More reliable patient-level data likely provides more realistic insights into the care of these complex patients as a whole. A universal commitment by surgeons, oncologists, radiologists, gastroenterologists, pathologists, and data registrars should center around providing the most complete and granular data to the designated databases for subsequent outcomes research. Specific to this study, more accurate and better reported tumor characterization and clinical staging could have potentially provided more information about advanced gastric cancers, for which clinical and pathologic data are frequently disparate yet treatment decisions are made based on clinical staging.
Our study highlights a cohort of GC patients who may benefit from a nuanced approach, calling attention to the heterogeneity of GC’s natural history. For example, while some GCs metastasize to distant organs prior to their invasion of the gastric serosa, other GCs invade adjacent organs without even seeding nearby lymph nodes. The basis of our investigation hinges on the biological differences between small primary GCs with multiple early metastases versus more indolent, large, locally aggressive GCs with or without oligometastatic nodal foci. We examined a cohort that has been pathologically confirmed to represent the most locally aggressive subset of tumors. Given the implications of this tumor biology, we believe that while there may be some added perioperative morbidity with MVR, it is reasonable to pursue an extended resection in well-selected patients to achieve disease clearance.
While our use of a national database for this study allows for an evaluation of LAGC treatment across a variety of care centers, it also has some inherent limitations. Coding errors and incomplete or ambiguous data are a peril of the use of large databases. The non-randomized, retrospective nature of the study limits our insight into patient selection for particular treatments and detailed knowledge of clinical decision-making. The lack of GC genomic data within the NCDB also precludes a nuanced analysis pertaining to prognostication or targeted systemic therapies.42–45 Further, we were unable to analyze LAGC patients with the granularity of single- and multi-institutional studies. Specifically, we were unable to know the sites of margin positivity, and degree of margin positivity (R1 vs. R2) for much of our cohort, which organs were included in MVR, the extent of organ resection in each MVR, or why an extended resection was needed at all (vascular involvement, direct organ involvement, suspected tumor invasion, iatrogenic injury). Finally, follow-up information on these patients is limited, not collected at regular intervals, and lacks details of disease recurrence.
Conclusion
Some patients will present with locally advanced gastric cancer, leading to either desmoplastic adherence or infiltration of adjacent organs. Such patients may require multivisceral resection to achieve disease clearance and negative resection margins. Patients with good functional status, the absence or low number of clinically positive regional lymph nodes, and ability to tolerate perioperative systemic chemotherapy are most likely to tolerate a more radical resection. Despite potential nihilism surrounding multivisceral resection for gastric cancer, short-term complication rates are no worse. Importantly, achieving a margin-negative resection is a primary driver of short- and long-term survival among this patient population, and patients with the intestinal subtype of GC may be more likely to benefit from an aggressive approach. We advocate for the referral of LAGC cases to high-volume centers for consideration of G+MVR in well-selected patients to minimize complications and to maximize the likelihood of a successful perioperative and survival-prolonging outcome.
Supplementary Material
Funding Information
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11605-020-04719-y) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards
Disclosure of Potential Conflicts of Interest Dr. Dominguez reports personal fees from Verb Surgical, outside the submitted work.
Drs. Aversa, Diggs, Hagerty, Ituarte, Hernandez, Davis, and Blakely: none declared.
Ethical Approval Due to this study’s inclusion of only de-identified data, it was exempt from institutional board review.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF, Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. Jama 1991;265(10):1287–9. [PubMed] [Google Scholar]
- 3.Steevens J, Botterweck AA, Dirx MJ, van den Brandt PA, Schouten LJ. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. European journal of gastroenterology & hepatology 2010;22(6):669–78. doi: 10.1097/MEG.0b013e32832ca091. [DOI] [PubMed] [Google Scholar]
- 4.Jim MA, Pinheiro PS, Carreira H, Espey DK, Wiggins CL, Weir HK. Stomach cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24:4994–5013. doi: 10.1002/cncr.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crew KD, Neugut AI. Epidemiology of gastric cancer. World journal of gastroenterology 2006;12(3):354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Batran S-E, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp H-G, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastrooesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. The Lancet 2019;393(10184):1948–57. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 7.Molina JC, Al-Hinai A, Gosseling-Tardif A, Bouchard P, Spicer J, Mulder D, Mueller CL, Ferri LE. Multivisceral Resection for Locally Advanced Gastric and Gastroesophageal Junction Cancers-11-Year Experience at a High-Volume North American Center. J Gastrointest Surg 2019;23(1):43–50. doi: 10.1007/s11605-018-3746-5. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Participants MT. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. The New England journal of medicine 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 9.Wanebo HJ, Kennedy BJ, Chmiel J, Steele G, Jr., Winchester D, Osteen R. Cancer of the stomach. A patient care study by the American College of Surgeons. Annals of surgery 1993;218(5): 583–92. doi: 10.1097/00000658-199321850-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin AN, Das D, Turrentine FE, Bauer TW, Adams RB, Zaydfudim VM. Morbidity and Mortality After Gastrectomy: Identification of Modifiable Risk Factors. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2016;20(9):1554–64. doi: 10.1007/s11605-016-3195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Power DG, Schattner MA, Gerdes H, Brenner B, Markowitz AJ, Capanu M, Coit DG, Brennan M, Kelsen DP, Shah MA. Endoscopic Ultrasound Can Improve the Selection for Laparoscopy in Patients with Localized Gastric Cancer. Journal of the American College of Surgeons 2009;208(2):173–8. doi: 10.1016/j.jamcollsurg.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Martin RC 2nd, Jaques DP, Brennan MF, Karpeh M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Annals of Surgery 2002;236(2):159–65. doi: 10.1097/00000658-200208000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carboni F, Lepiane P, Santoro R, Lorusso R, Mancini P, Sperduti I, Carlini M, Santoro E. Extended multiorgan resection for T4 gastric carcinoma: 25-year experience. Journal of surgical oncology 2005;90(2):95–100. doi: 10.1002/jso.20244. [DOI] [PubMed] [Google Scholar]
- 14.Pacelli F, Cusumano G, Rosa F, Marrelli D, Dicosmo M, Cipollari C, Marchet A, Scaringi S, Rausei S, di Leo A, Roviello F, de Manzoni G, Nitti D, Tonelli F, Doglietto GB, Italian Research Group for Gastric Cancer ft. Multivisceral Resection for Locally Advanced Gastric Cancer: An Italian Multicenter Observational Study. JAMA Surgery 2013;148(4):353–60. doi: 10.1001/2013.jamasurg.309 [DOI] [PubMed] [Google Scholar]
- 15.Ozer I, Bostanci EB, Orug T, Ozogul YB, Ulas M, Ercan M, Kece C, Atalay F, Akoglu M. Surgical outcomes and survival after multiorgan resection for locally advanced gastric cancer. American journal of surgery 2009;198(1):25–30. doi: 10.1016/j.amjsurg.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, Schmidt C, Weber SM, Votanopoulos K, Maithel SK, Pawlik TM. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. Journal of the American College of Surgeons 2015;220(1):48–56. doi: 10.1016/j.jamcollsurg.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Willis S, Truong S, Gribnitz S, Fass J, Schumpelick V. Endoscopic ultrasonography in the preoperative staging of gastric cancer: accuracy and impact on surgical therapy. Surgical endoscopy 2000;14(10):951–4. doi: 10.1007/s004640010040. [DOI] [PubMed] [Google Scholar]
- 18.Meyer L, Meyer F, Schmidt U, Gastinger I, Lippert H. Endoscopic ultrasonography (EUS) in preoperative staging of gastric cancer–demand and reality. Polski przeglad chirurgiczny 2012;84(3):152–7. doi: 10.2478/v10035-012-0024-1. [DOI] [PubMed] [Google Scholar]
- 19.Ganpathi IS, So JB, Ho KY. Endoscopic ultrasonography for gastric cancer: does it influence treatment? Surgical endoscopy 2006;20(4):559–62. doi: 10.1007/s00464-005-0309-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Yang CC, Yeh YH. Preoperative staging of gastric cancer by endoscopic ultrasound: the prognostic usefulness of ascites detected by endoscopic ultrasound. Journal of clinical gastroenterology 2002;35(4):321–7. doi: 10.1097/00004836-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Serrano OK, Huang K, Ng N, Yang J, Friedmann P, Libutti SK, Kennedy TJ. Correlation between preoperative endoscopic ultrasound and surgical pathology staging of gastric adenocarcinoma: A single institution retrospective review. Journal of surgical oncology 2016;113(1):42–5. doi: 10.1002/jso.24098. [DOI] [PubMed] [Google Scholar]
- 22.Seevaratnam R, Cardoso R, McGregor C, Lourenco L, Mahar A, Sutradhar R, Law C, Paszat L, Coburn N. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 2012;15 Suppl 1:S3–18. doi: 10.1007/s10120-011-0069-6. [DOI] [PubMed] [Google Scholar]
- 23.Tran TB, Worhunsky DJ, Norton JA, Squires MH 3rd, Jin LX, Spolverato G, Votanopoulos KI, Schmidt C, Weber S, Bloomston M, Cho CS, Levine EA, Fields RC, Pawlik TM, Maithel SK, Poultsides GA. Multivisceral Resection for Gastric Cancer: Results from the US Gastric Cancer Collaborative. Ann Surg Oncol 2015;22 Suppl 3:S840–7. doi: 10.1245/s10434-015-4694-x. [DOI] [PubMed] [Google Scholar]
- 24.Kasakura Y, Fujii M, Mochizuki F, Kochi M, Kaiga T. Is there a benefit of pancreaticosplenectomy with gastrectomy for advanced gastric cancer? American journal of surgery 2000;179(3):237–42. doi: 10.1016/s0002-9610(00)00293-2. [DOI] [PubMed] [Google Scholar]
- 25.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Annals of surgical oncology 2008;15(3):683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S-Y, Yeh C-N, Lee H-L, Liu Y-Y, Chao T-C, Hwang T-L, Jan Y-Y, Chen M-FJAoSO. Clinical Impact of Positive Surgical Margin Status on Gastric Cancer Patients Undergoing Gastrectomy 2009;16(10):2738–43. doi: 10.1245/s10434-009-0616-0. [DOI] [PubMed] [Google Scholar]
- 27.Nagata T, Ichikawa D, Komatsu S, Inoue K, Shiozaki A, Fujiwara H, Okamoto K, Sakakura C, Otsuji E. Prognostic impact of microscopic positive margin in gastric cancer patients. Journal of surgical oncology 2011;104(6):592–7. doi: 10.1002/jso.22022. [DOI] [PubMed] [Google Scholar]
- 28.Colen KL, Marcus SG, Newman E, Berman RS, Yee H, Hiotis SP. Multiorgan resection for gastric cancer: intraoperative and computed tomography assessment of locally advanced disease is inaccurate. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2004;8(7):899–902. doi: 10.1016/j.gassur.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Raziee HR, Cardoso R, Seevaratnam R, Mahar A, Helyer L, Law C, Coburn N. Systematic review of the predictors of positive margins in gastric cancer surgery and the effect on survival. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 2012;15 Suppl 1:S116–24. doi: 10.1007/s10120-011-0112-7. [DOI] [PubMed] [Google Scholar]
- 30.Lee YM, Kang SH, Kim JS, Eun HS, Joo JS, Rou WS, Park JH, Moon HS, Lee ES, Kim SH, Sung JK, Lee BS, Jeong HY, Yeo MK, Song KS, Yoo HM. Subepithelial spread of early gastric signet ring cell carcinoma: How far they can reach? Digestive diseases (Basel, Switzerland) 2020. doi: 10.1159/000507322. [DOI] [PubMed]
- 31.Iwatsuki M, Yamamoto H, Miyata H, Kakeji Y, Yoshida K, Konno H, Seto Y, Baba H. Effect of hospital and surgeon volume on postoperative outcomes after distal gastrectomy for gastric cancer based on data from 145,523 Japanese patients collected from a nationwide web-based data entry system. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 2019;22(1):190–201. doi: 10.1007/s10120-018-0883-1. [DOI] [PubMed] [Google Scholar]
- 32.Lee JA, Park JH, Lee EJ, Kim SY, Kim Y, Lee SI. High-Quality, Low-Cost Gastrectomy Care at High-Volume Hospitals: Results From a Population-Based Study in South Korea. Archives of Surgery 2011;146(8):930–6. doi: 10.1001/archsurg.2011.81. [DOI] [PubMed] [Google Scholar]
- 33.Zheng ZF, Lu J, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Lin M, Huang CM. A Novel Prognostic Scoring System Based on Preoperative Sarcopenia Predicts the Long-Term Outcome for Patients After R0 Resection for Gastric Cancer: Experiences of a High-Volume Center. Annals of surgical oncology 2017;24(7): 1795–803. doi: 10.1245/s10434-017-5813-7. [DOI] [PubMed] [Google Scholar]
- 34.Wang SL, Zhuang CL, Huang DD, Pang WY, Lou N, Chen FF, Zhou CJ, Shen X, Yu Z. Sarcopenia Adversely Impacts Postoperative Clinical Outcomes Following Gastrectomy in Patients with Gastric Cancer: A Prospective Study. Annals of surgical oncology 2016;23(2):556–64. doi: 10.1245/s10434-015-4887-3. [DOI] [PubMed] [Google Scholar]
- 35.Claassen YHM, van Sandick JW, Hartgrink HH, Dikken JL, De Steur WO, van Grieken NCT, Boot H, Cats A, Trip AK, Jansen EPM, Meershoek-Klein Kranenbarg WM, Braak J, Putter H, van Berge Henegouwen MI, Verheij M, van de Velde CJH. Association between hospital volume and quality of gastric cancer surgery in the CRITICS trial. The British journal of surgery 2018;105(6):728–35. doi: 10.1002/bjs.10773. [DOI] [PubMed] [Google Scholar]
- 36.Jensen LS, Nielsen H, Mortensen PB, Pilegaard HK, Johnsen SP. Enforcing centralization for gastric cancer in Denmark. European Journal of Surgical Oncology (EJSO) 2010;36:S50–S4. doi: 10.1016/j.ejso.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet (London, England) 1996;347(9007):995–9. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 38.Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet (London, England) 1995;345(8952):745–8. doi: 10.1016/s0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 39.Nanthakumaran S, Fernandes E, Thompson AM, Rapson T, Gilbert FJ, Park KG. Morbidity and mortality rates following gastric cancer surgery and contiguous organ removal, a population based study. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2005;31(10):1141–4. doi: 10.1016/j.ejso.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Kodama I, Takamiya H, Mizutani K, Ohta J, Aoyagi K, Kofuji K, Takeda J, Shirouzu K. Gastrectomy with combined resection of other organs for carcinoma of the stomach with invasion to adjacent organs: clinical efficacy in a retrospective study. Journal of the American College of Surgeons 1997;184(1):16–22. [PubMed] [Google Scholar]
- 41.Kim DY, Joo JK, Seo KW, Park YK, Ryu SY, Kim HR, Kim YJ, Kim SK. T4 gastric carcinoma: the benefit of non-curative resection. ANZ journal of surgery 2006;76(6):453–7. doi: 10.1111/j.1445-2197.2006.03751.x. [DOI] [PubMed] [Google Scholar]
- 42.Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513(7517):202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nature medicine 2015;21(5):449–56. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 44.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London, England) 2010;376(9742):687–97. doi: 10.1016/s0140-6736(10)61121-x. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs CS, Doi T, Jang RW-J, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges J-P, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DVT, Bang Y-J, Wang J, Koshiji M, Dalal RP, Yoon HH. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer 2017;35(15_suppl):4003-. doi: 10.1200/JCO.2017.35.15_suppl.4003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


