Abstract
Cardiac MRI has become a widely accepted standard for anatomic and functional assessment of complex Fontan physiology, because it is noninvasive and suitable for comprehensive follow-up evaluation after Fontan completion. The use of cardiac MRI in pediatric and adult patients after completion of the Fontan procedure are described, and a practical and experience-based cardiac MRI protocol for evaluating these patients is provided. The current approach and study protocol in use at the authors’ institution are presented, which address technical considerations concerning sequences, planning, and optimal image acquisition in patients with Fontan circulation. Additionally, for each sequence, the information that can be obtained and guidance on how to integrate it into clinical decision-making is discussed.
Keywords: Pediatrics, MRI, MRI Functional Imaging, Heart, Congenital
© RSNA, 2022
Keywords: Pediatrics, MRI, MRI Functional Imaging, Heart, Congenital
Summary
This review, which is intended for both radiologists and clinical cardiologists, highlights the use of cardiac MRI in pediatric and adult patients after completion of the Fontan procedure and provides a practical imaging protocol for patient evaluation.
Essentials
■ Although echocardiography remains a mainstay of diagnosis of single-ventricle physiology, additional imaging is required throughout staged palliation.
■ Cardiac MRI is a noninvasive, nonionizing modality that provides a unique combination of anatomic and functional data.
■ Cardiac MRI has become a widely accepted standard for monitoring and follow-up of patients before and after Fontan completion.
Introduction
Although echocardiography remains a mainstay of diagnosis for patients with single-ventricle physiology from fetal period into adulthood, additional imaging is required throughout all stages of palliation (1–4). Cardiac MRI is a noninvasive, nonionizing modality that provides a unique combination of anatomic and functional data about the complex intracardiac anatomy, Fontan pathways, systemic-to-pulmonary collaterals, and abdominal organs. Cardiac MRI also allows tissue characterization and stress perfusion imaging of the myocardium.
The role of cardiac MRI both before and after Fontan completion is well established. Cardiac MRI has been shown to be an excellent alternative to diagnostic cardiac catheterization in a substantial proportion of patients (5–7). It is also of crucial importance during follow-up, because it has been shown to substantially change patient management (ie, medical or interventional treatment strategy, ordering further diagnostic testing) after either clinically indicated studies or routine surveillance (8).
Although the role of cardiac MRI is recognized, no general agreement exists about the optimal frequency of examinations (9). Recent recommendations propose regular cardiac MRI follow-up be performed every 2 to 3 years in children and every 3 years in adolescents and adults (10).
Standard Cardiac MRI Protocol in Patients with Fontan Circulation
The cardiac MRI protocol at our institution is presented in the Table. Detailed discussion regarding the performance of each sequence and the clinical implications when scanning patients with Fontan circulation is provided in the text. More detailed guidelines on the performance of cardiac MRI in patients with congenital heart disease, presentation of planning instructions, and acquisition parameters for each sequence and with regard to pediatric and adult patients can be found elsewhere (3).
Cardiac MRI Protocol for Patients with Fontan Circulation at Our Institution
Assessment of Anatomy and Cardiac Function
Compared with echocardiography, cardiac MRI has a major advantage in the ability to assess all cardiovascular anatomy and function in any plane, independent of user and poor acoustic window, with high spatial and good temporal resolution.
Anatomic survey.— An initial anatomic survey is typically performed in one breath hold in the axial, coronal, and sagittal planes using dark-blood or bright-blood single-shot imaging. At our institution, bright-blood images in the axial, sagittal, and coronal planes are acquired, and dark-blood image sequences are performed only in the axial plane. These series are used for planning cardiac imaging planes (Fig 1). This survey also allows identification of extracardiac findings (eg, pleural effusions, liver disease) that may require further assessment.
Figure 1:
Cardiac imaging planes are essential for the evaluation of cardiac sizes and function. In this figure, each imaging plane is represented by a distinct color (frame colors and line colors correspond, ie, orange frame is sagittal view and orange line represents the same sagittal plane crossing through coronal image). Typically, evaluation begins with an initial anatomic survey (ie, scout imaging) in the coronal, axial, and sagittal planes. These series are used for planning the cardiac vertical long-axis (VLA) view (red frame), horizontal long-axis or four-chamber (4CH) view (blue frame), left ventricular outflow tract (LVOT) and LVOT cross-cut (XC) views (green frames), and the short-axis stack (SAX) at basal level (black frame) and SAX at mid ventricular level (light blue frame) views.
Cine imaging of the heart.— For anatomic and functional evaluation, balanced steady-state free precession (SSFP) cine images of the heart and great vessels are acquired. The SSFP sequence is fundamental to the visualization of intracardiac structures and Fontan pathways, because it provides high signal-to-noise ratio and excellent blood-tissue contrast (Figs 2–4). Additionally, turbulence resulting from valvular or conduit stenosis, valvular regurgitation, or intercompartment communication can be easily identified as dark signal within the bright-blood pool, because turbulent flow causes loss of signal from dephasing. When possible, breath-hold acquisitions are recommended, preferably at end expiration, because diaphragmatic position tends to be more consistent from breath hold to breath hold. However, small children may be more compliant with acquisition at end inspiration. Parallel imaging or partial Fourier techniques can be used to decrease breath-hold time, but at the expense of image quality. Alternatively, free-breathing imaging with the patient instructed to breathe regularly and quietly can be performed with a multiple signal averaging (two to four).
Figure 2:
Examples of different total cavopulmonary connections. (Left) MR images of the so-called old atriopulmonary Fontan procedure versus the (right) so-called modern extracardiac conduit Fontan procedure. ECC = extracardiac conduit, IVC = inferior vena cava, LPA = left pulmonary artery, RAA = right atrial appendage, RA = right atrium, RPA = right pulmonary artery, SVC = superior vena cava.
Figure 4:
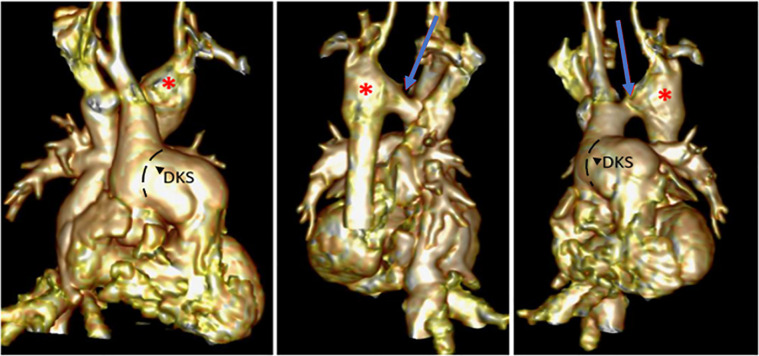
Cardiac MRI study in a 13-year-old child with hypoplastic left heart syndrome, double-outlet right ventricle (RV), and aortic coarctation after Damus-Kaye-Stansel (DKS) procedure, coarctation repair, and Fontan completion. Note the (1, 4) double-outlet RV, (1, 3, 11, 12) DKS anastomosis, (2, 7) hypoplastic left ventricle (LV), (3–5) Fontan pathways, and (5–12) persistent left superior vena cava (LSVC) connected to the left upper pulmonary vein (LUPV) draining into the left atrium (LA). AO = aorta, IVC = inferior vena cava, LLPV = left lower pulmonary vein, LPA = left pulmonary artery, RA = right atrium, RPA = right pulmonary artery, RPVS = right pulmonary veins, RSVC = right superior vena cava.
Figure 3:
Cardiac MRI study in a 3-year-old child with double-inlet, double-outlet right ventricle (RV) after right Blalock-Taussig (BT) shunt. Note the double-inlet aortic valve (AV) connection to the RV with (2, 5, 6) straddling left AV valve, (5–7) hypoplastic left ventricle (LV), (1–4, 9) double-outlet RV, and (1, 2, 5–7) large noncommitted ventricular septal defect. AO = aorta, IVC = inferior vena cava, LA = left atrium, LPA = left pulmonary artery, LPVS = left pulmonary veins, MV = mitral valve, PA = pulmonary artery, PV = pulmonary valve, RA = right atrium, RPA = right pulmonary artery, RPVS = right pulmonary veins, SVC = superior vena cava, TV = tricuspid valve.
Careful, patient-specific planning of cardiac imaging planes is important in patients with congenital heart disease, and it is even more necessary in patients with Fontan circulation because of their peculiar anatomic features that make accuracy and reproducibility of functional assessment more challenging (eg, in double-inlet or double-outlet ventricle, entities involving hypoplastic left or right heart structures, and hearts that cannot undergo biventricular repair).
For a comprehensive evaluation of the heart in patients with Fontan circulation, cine imaging should always include the following views: a horizontal long-axis view that includes both atria and the dominant ventricle from base to apex (and the secondary ventricle when present); a vertical long-axis view that includes the dominant ventricle from base to apex and its respective atrium; a ventricular stack of contiguous sections encompassing the ventricular mass from base to apex; an inflow-to-outflow long-axis view that includes the dominant ventricle from base to apex and its respective atrioventricular valve inflow and outflow tract(s); and appropriate views of the outflow tract(s) and of the aortic arch in case of previous coarctation or other arch repair.
To calculate ventricular volumes and mass using the Simpson disk summation method (Fig 5), we usually collect a short-axis stack of contiguous sections parallel to the atrioventricular valve plane, acquiring 12 to 15 sections every 8 mm to 10 mm with a thickness of 6 mm to 8 mm according to the patient’s size.
Figure 5:
Cardiac MRI volumetric analysis in a patient with hypoplastic left heart syndrome. Ventricular volumes are calculated using the Simpson disk summation method, starting from endocardial contouring of the ventricular cavities in a short-axis stack of the functionally univentricular heart. The dominant single right ventricle is outlined in yellow, and the hypoplastic left ventricle is outlined in red.
Contrast-enhanced MR angiography.— Gadolinium-based contrast agents (GBCAs) strongly increase signal from the blood in T1-weighted spoiled gradient-echo sequences.
Single-phase contrast-enhanced MR angiography (CE-MRA) is an excellent method for visualizing Fontan pathways, great vessels, and venovenous and aortopulmonary collaterals (Fig 6). Ideally, the sequence should be performed when the contrast agent arrives in the vessel of interest at its maximum concentration (first pass). Scanning too early or too late may result in missing the passage of contrast material bolus, or inadequate vascular visualization owing to contrast material dilution or overlapping of venous and arterial phases. Consistently good results depend on sufficient expertise and ideally optimized planning that is individualized to the patient. Two data sets are routinely acquired for all patients undergoing cardiac MRI at our institution: The early data set is acquired to assess the venovenous collaterals and pulmonary arteries before the contrast material enters the heart, and the second data set is acquired 15 seconds after the first. This allows visualization of the aorta, aortopulmonary collaterals, and Fontan pathway. The acquired non–electrocardiographically (ECG) gated three-dimensional (3D) isotropic data sets can be displayed as maximum intensity projection, multiplanar reconstruction, or volume rendering (Fig 7).
Figure 6:
Three-dimensional volume-rendered contrast-enhanced MR angiographic images in a patient with double-outlet right ventricle and aortic stenosis and coarctation after Damus-Kaye-Stansel (DKS) anastomosis, coarctation repair, and Fontan completion. The images show discrete narrowing of the transverse arch (arrow) with poststenotic dilatation (*). Cavopulmonary anastomoses and branch pulmonary arteries appear unobstructed.
Figure 7:
The acquired three-dimensional (3D) isotropic data sets from contrast-enhanced MR angiography or 3D steady-state free precession can be displayed as maximum intensity projection (MIP), multiplanar reconstruction (MPR), or 3D volume rendering. (A) The MIP postprocessing algorithm selects the voxel with the maximum intensity along a ray through the 3D volume of data and displays these data as a two-dimensional projection. Shortcomings include decreased visibility of vessels passing among highly attenuating structures, poor depiction of 3D relationships, and volume averaging. (B) The MPR postprocessing algorithm generates coronal, sagittal, and axial reconstructions from source images and is useful in demonstrating and evaluating lesions after they have been detected or suspected using other means. Isolated examination of MPR images to evaluate sites of disease or abnormality, although providing the most detailed views, may be extremely time-consuming because of the large number of images generated. (C) 3D volume rendering is an interactive postprocessing algorithm that produces 3D volumetric images. The algorithm computes a weighted sum of the contributions of individual voxels along rays through the 3D volume of data. The interactive nature of volume rendering allows real-time manipulation of the 3D images and editing with clip planes. As a result, this reconstruction may provide clearer differentiation of overlapping vessels and better depiction of 3D spatial relationships.
Time-resolved CE-MRA has lower spatial resolution but provides dynamic information that helps clarify flow patterns, thereby highlighting potential stenoses or blockage of the Fontan pathway and collateral circulations (11). The advantage of time-resolved CE-MRA is the ability to follow blood flow in real time, including direction of flow through collaterals, which may be more difficult in a single data set. For example, time-resolved CE-MRA may detect complete obstruction of brachiocephalic vein and filling of the pulmonary veins via collateral vessels instead of the left lung, as shown in Figure 8. Although we routinely use conventional single-phase CE-MRA at our institution, time-resolved CE-MRA has a role in certain circumstances, such as when using a 3-T scanner or if particular collaterals are suspected and patient size allows sufficient spatial resolution when using a 1.5-T scanner.
Figure 8:
Time-resolved contrast-enhanced MR angiographic images in a 5-year-old boy with tricuspid atresia after a bidirectional Glenn procedure. Contrast material injection through the left arm shows contrast material filling the left subclavian vein (LSCV) which is obstructed at the costoclavicular space (2, 3). Contrast material proceeds through a major collateral to both lungs (arrow), rapidly filling the left upper pulmonary veins and the single ventricle (SV) without opacification of the pulmonary arteries (PAs), consistent with a systemic-to-pulmonary venovenous collateral (3, 4). The superior vena cava (SVC) and PAs are visualized later after contrast material returns from the head and neck (6). * = pulmonary arteriovenous malformation in the right lower lobe. Ao = aorta, RPA = right PA.
Neither the standard technique nor the time-resolved CE-MRA technique uses ECG gating. Thus, motion over the cardiac cycle causes blurring, particularly of the aortic root and intracardiac structures.
3D whole-heart SSFP.— 3D whole-heart SSFP is an ECG-triggered and respiratory-navigated sequence that allows collection of a single-phase 3D isotropic data set within a few minutes. It can be performed with or without contrast enhancement, but at our institution it is usually performed in every patient after CE-MRA. The data acquisition window in the cardiac cycle should be carefully adjusted during mid-diastole in adolescents and adults when the heart is still, but it may be necessary to center it on the end-systolic pause in smaller children with rapid heart rates owing to short diastolic intervals.
3D whole-heart SSFP has proved to be of substantial clinical value for diagnostic imaging in complex congenital heart disease (12). Whereas CE-MRA is intended primarily for assessment of extracardiac vascular anatomy, 3D SSFP allows comprehensive and detailed evaluation of intracardiac morphology and cardiovascular anatomy. This is possible because ECG triggering minimizes blurring resulting from cardiac motion, and multiplanar reconstruction permits arbitrary reformatting in any desired imaging plane during review without the loss of high spatial resolution (≤1.2 mm3). Thus, accurate and reproducible through-plane measurements are provided that are of particular importance when evaluating patients with Fontan circulation (Fig 7). The main disadvantages of 3D SSFP are its relatively long acquisition time (usually 7–10 minutes), during which the patient must be absolutely still, and its susceptibility to artifacts caused by turbulent flow and magnetic field inhomogeneity, which may cause misinterpretation when assessing structures around stenoses, regurgitant lesions, or stents.
ECG-gated and respiratory-navigated modified Dixon 3D MRA has been introduced in clinical practice. It has demonstrated superiority over the 3D SSFP sequence in terms of delineation of head and neck vasculature and pulmonary veins, as well as fewer artifacts from valve stenosis, vascular stents, and metallic implants (13).
Transaxial cine stack.— To evaluate the function of the Fontan circuit and clarify complex cardiovascular anatomy, an option is to acquire a continuous transaxial cine series through the thorax from the diaphragm to the neck. These acquisitions are slow, because they occur through many breath holds, and thus, they may be omitted in a less cooperative patient or if sufficient data are acquired using other sequences.
Flow Assessment
Phase-contrast imaging (PCI) has a proven ability to accurately quantify velocities and volumes of blood flow in vascular structures, relying on the fundamental concept that nuclei flowing through a specifically designed magnetic field gradient accumulate a predictable phase shift that is proportional to flow velocity (14).
Although two-dimensional (2D) PCI remains the clinical standard for flow quantification, four-dimensional (4D) flow imaging has become more established in clinical practice. With 4D flow imaging, time-resolved, three-directional velocities of the whole heart are obtained within a single acquisition.
The ability to assess flow in all major vessels and in any orientation makes cardiac MRI ideal for assessment of the patency and efficiency of the Fontan circulation. In the absence of regurgitant lesions, patent fenestration, or significant systemic-to-collateral flow, aortic forward flow should be equal to total systemic venous return (ie, [superior vena cava (SVC) + inferior vena cava (IVC)] flows) and to total pulmonary venous return. Discrepancies in flows should indicate the presence of any of the aforementioned lesions, whereas segmental analysis of flows in the circuit may allow quantification of their entity at each level (Figs 9, 10).
Figure 9:
![Illustration of sites of the through-plane flows used for complete hemodynamics assessment of patients with Fontan circulation. In the absence of regurgitant lesions, patent fenestration, or significant systemic-to-collateral flow, aortic forward flow should be equal to total systemic venous return (superior vena cava [SVC] + inferior vena cava [IVC] flows) and also to total pulmonary venous return (right pulmonary veins [RPVs] + left pulmonary veins [LPVs] flows). Discrepancy in flows should indicate the presence of any of the aforementioned lesions, whereas segmental analysis of flows in the circuit may allow quantification of their entity at each level. Ao = aorta, ECC = extracardiac conduit, LPA = left pulmonary artery, RPA = right pulmonary artery.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/fd64/9274315/14e61cf52e70/ryct.210235.fig9.jpg)
Illustration of sites of the through-plane flows used for complete hemodynamics assessment of patients with Fontan circulation. In the absence of regurgitant lesions, patent fenestration, or significant systemic-to-collateral flow, aortic forward flow should be equal to total systemic venous return (superior vena cava [SVC] + inferior vena cava [IVC] flows) and also to total pulmonary venous return (right pulmonary veins [RPVs] + left pulmonary veins [LPVs] flows). Discrepancy in flows should indicate the presence of any of the aforementioned lesions, whereas segmental analysis of flows in the circuit may allow quantification of their entity at each level. Ao = aorta, ECC = extracardiac conduit, LPA = left pulmonary artery, RPA = right pulmonary artery.
Figure 10:
Flow assessment in a 15-year-old patient with double-outlet right ventricle and pulmonary atresia after extracardiac Fontan procedure. Flows are assessed by imaging the vessel perpendicular to its long axis using phase-contrast imaging. Through-plane velocity maps of the ascending aorta, superior vena cava (SVC), inferior vena cava (IVC), right pulmonary artery (RPA), left pulmonary artery (LPA), right pulmonary veins (RPVs), and left pulmonary veins (LPVs) are shown. The pulmonary arteries appear unobstructed (RPA:LPA net flow split ratio, approximately 60%:40%). Estimated systemic-to-pulmonary collateral flow is approximately 10% (systemic estimator: AO − [SVC + IVC]; pulmonary estimator: [RPV + LPV] − [RPA + LPA]).
2D flow imaging.— Retrospectively gated, free-breathing 2D PCI is the standard in most centers owing to the effects of breath holding on passive pulmonary filling. For a comprehensive hemodynamic evaluation, it is necessary to acquire through-plane flows at the level of the ascending aorta, IVC and SVC (or any conduit if present), right and left pulmonary arteries, and, possibly, total pulmonary venous return. Velocity encoding should be chosen to encompass the highest velocities likely to be encountered within the vessel of interest: 50–80 cm/sec for systemic and pulmonary venous flow and 150–200 cm/sec for aortic flow.
4D flow imaging.— 4D flow imaging has been validated for flow visualization and quantification in patients with congenital heart disease, and it also allows assessment of advanced hemodynamic markers such as kinetic energy, viscous energy loss, vorticity, and helicity (15–19). 4D flow imaging was found to be reliable, operator-independent, and even more time-efficient when compared with 2D flows; 4D imaging also provides greater insight into Fontan hemodynamics (20). Software providers now include specific tools that allow relatively fast analysis of the image data (Fig 11). Studies have shown the practicability of 4D flow for flow assessment in routine clinical practice (21). Beyond simple flow measurements, however, 4D flow imaging also allows the assessment of energetics of the Fontan circulation and ventricular efficiency. Rijnberg et al (18) used 4D flow imaging to analyze flow patterns at the IVC-conduit junction together with associated kinetic energy and energy loss and demonstrated that the IVC-conduit junction is a potential source of increased energy loss. Other authors found that ventricular kinetic energy appears higher in patients with Fontan circulation than in healthy controls and that this potentially leads to lower cardiac efficiency in these patients (22). Advanced hemodynamic markers derived from 4D flow may also be correlated with clinical markers such as exercise capacity, which suggests that in the future these hemodynamic markers may be able to serve as clinically relevant imaging biomarkers (23).
Figure 11:
Four-dimensional (4D) flow assessment in an 11-year-old patient with hypoplastic left heart syndrome after intra-atrial lateral tunnel Fontan procedure. (A) Streamline visualization with velocity color coding of 4D flow data. (B) Flow quantification in the aorta (Ao) (red), superior vena cava (SVC) and lateral tunnel (LT) (green), left pulmonary artery (LPA) (yellow), and right pulmonary veins (RPVs) (blue). IVC = inferior vena cava, SV = single ventricle.
Although long scanning time was historically a main disadvantage of 4D flow, most scans can now be performed in 5 to 8 minutes. This may be less than the time required to perform 2D PCI throughout all sites of flow assessment when including the time for planning and recovery between breath holds (24). Moreover, it was recently shown that 3D flow imaging, which acquires a cardiac cycle–averaged 3D velocity field within a volume of interest irrespective of the phase in the cardiac cycle, is capable of measuring flow rates in Fontan pathways with good to excellent agreement compared with both 2D and 4D flow, and with reduced scan times and higher image quality than 4D flow (25).
Additional Sequences (Selected Cases)
Late gadolinium enhancement (LGE) imaging should be performed in cases of unexplained heart failure symptoms, recent deterioration in cardiac function, or new onset of complex arrhythmias to detect the presence and extension of myocardial fibrosis. The presence of implanted metal devices may require additional sequences such as spoiled gradient-echo (SPGR) cine imaging, whereas suspicion of thrombus formation may dictate the performance of dedicated sequences (see below).
Assessment of Common Complications Associated with Fontan Circulation
Although creation of Fontan circulation is palliative by nature, many patients with ideal hemodynamics may lead almost normal lives. Some patients experience substantial morbidity and mortality, however, particularly those with unfavorable hemodynamics (eg, poor ventricular function, elevated pulmonary pressures, or Fontan pathway obstruction).
Cardiac MRI is a comprehensive tool for anatomic and functional assessment of most common complications associated with Fontan circulation.
Ventricular Failure
The Fontan circulation is characterized by chronic preload depletion, which may result in impaired ventricular filling and, eventually, low cardiac output. Moreover, the systemic ventricle can be a morphologic right ventricle that may fail after years of systemic loading.
Serial assessment of ventricular volumes and function using a standard and reproducible analysis technique is crucial to detect and track any deterioration in cardiovascular function during follow-up of these patients. In contrast to standard echocardiography, cardiac MRI volumetry does not rely on geometric assumptions and is widely accepted as the reference standard for ventricular volumes, particularly in regard to the right ventricle and univentricular hearts (1–4,26). Cardiac MRI–derived end-diastolic volume index is a strong predictor of death and transplant-free survival (27).
Moreover, atrioventricular valve regurgitation is common in patients with Fontan circulation and may lead to volume overload and back-pressure elevation in the Fontan circuit, potentially resulting in Fontan failure (28). Atrioventricular valve regurgitation volume and fraction can be accurately quantified by cardiac MRI as the difference between systemic forward flow and stroke volume of the single functional ventricle (29).
Fontan Pathway Obstruction
Unobstructed blood flow throughout the venous systemic connections and pulmonary arteries is essential for optimal Fontan hemodynamics. Significant stenosis or obstruction of the pulmonary arteries and surgically constructed conduits (eg, extracardiac conduits, intra-atrial baffles) are not uncommon both early and late after the Fontan procedure (Fig 12) (30).
Figure 12:
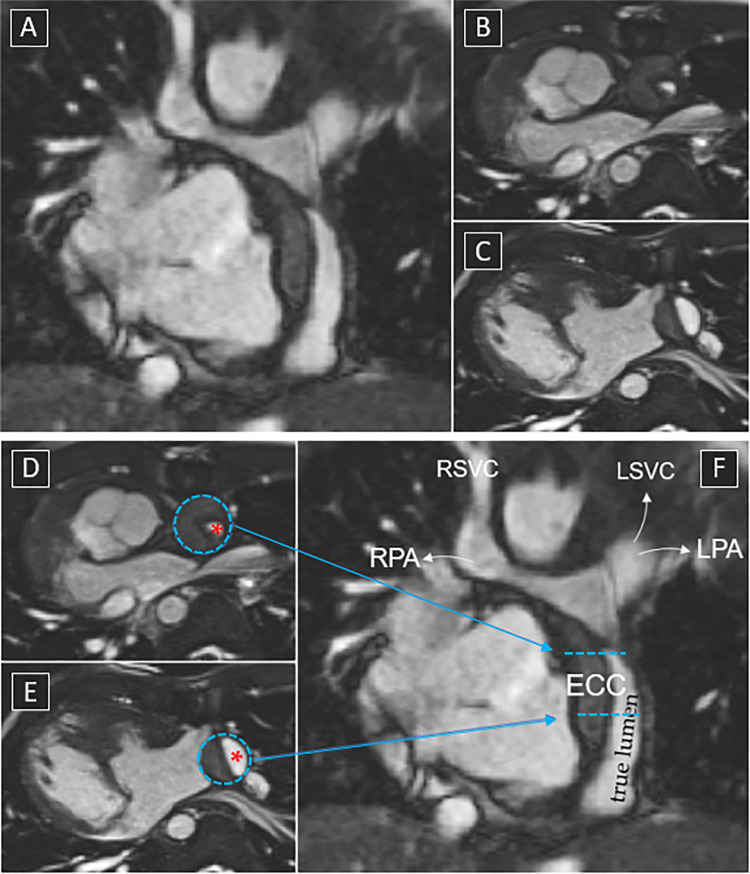
Cardiac MRI scan in a 15-year-old patient with situs inversus, dextrocardia, bilateral superior vena cavas (SVC), atrioventricular discordance with single outlet and pulmonary atresia, large perimembranous ventricular septal defect, and right aortic arch after extracardiac Fontan procedure. (The patient refused cannulation, so angiography could not be performed.) These still images from balanced steady-state free precession cines show incidental finding of dissection of the extracardiac conduit (ECC). (A) Long-axis view of the dissected conduit. (B, C) Short-axis view with very narrowed true lumen (red * in D and E) at the conduit insertion to the pulmonary artery. (D–F) The same images as B, C, and A, respectively, but with labeling showing the Fontan pathway and the respective acquisition planes. Arrows indicate the direction of flow. LPA = left pulmonary artery, LSVC = left SVC, RPA = right pulmonary artery, RSVC = right SVC.
CE-MRA performed during the venous phase and dedicated cines allow detection of any stenosis in the Fontan pathway. In contrast, flow imaging allows quantification of any obstruction based on distribution patterns of caval flows or pulmonary artery flows to the lungs, thus establishing indications for transcatheter or surgical reinterventions.
Thrombus Formation
The lifetime risk of thromboembolic events in patients with Fontan circulation is significant, involving up to 20% of these patients, and carries a substantial burden of morbidity and mortality; in patients who experience thromboembolic complications, the mortality rate is as high as 38%, even with aggressive intervention (31,32). Thus, detection of clot formation is essential (33).
On images generated from bright-blood cine sequences, the thrombus appears as a mass of low signal intensity within Fontan pathways or ventricular chambers. On T1-weighted spin-echo sequences, signal intensity of the thrombus is lower than that of myocardium and can be distinguished from slowly flowing blood, which has a higher signal intensity. More sensitive sequences have been developed, such as early gadolinium enhancement with typically high inversion time (approximately 450 msec) to detect the low signal intensity avascular thrombus surrounded by the higher signal intensity contrast material–filled blood pool and myocardium (Fig 13).
Figure 13:
MR images of thrombus formation in a 28-year-old man with tricuspid atresia after atriopulmonary Fontan procedure. Two crescent-shaped thrombi are noted along the anterior and posterior walls of the right atrium (RA) (black and white *). (1, 2, 5) Balanced steady-state free precession, (3, 6) early gadolinium enhancement, and (4) T1-weighted spin-echo sequences (please refer to the text for signal intensity in each sequence).
Myocardial Fibrosis
Areas of fibrosis are common in patients with Fontan circulation and result from developmental cardiomyopathic processes, such as endocardial fibroelastosis associated with hypoplastic left heart syndrome, from previous open-heart surgery, and from further myocardial damage secondary to cardiac stress of the single ventricle.
Myocardial fibrosis can be assessed by LGE imaging. This technique is based on differences in the uptake and washout kinetics of the contrast agent between healthy and infarcted or scarred myocardium; the larger interstitial space caused by cell death or increased collagen retains more gadolinium than normal myocardium and thus, appears bright on strongly T1-weighted images obtained 10 to 20 minutes after contrast material administration (Fig 14). In patients with Fontan circulation, LGE has been associated with a more dilated, hypertrophied, and poorly functioning single ventricle and increased incidence of ventricular arrhythmia (34,35).
Figure 14:
Late gadolinium enhancement images in a 3-year-old patient with hypoplastic left heart syndrome after a Norwood procedure and a bidirectional Glenn procedure. The diffuse subendocardial enhancement of the hypoplastic left ventricle (LV) is compatible with endocardial fibroelastosis. Enhancement of the septal papillary muscle of the tricuspid valve is also observed (white arrow). RV = right ventricle.
Additionally, although not routinely performed, it is possible to perform MR relaxometry with T1 mapping before and after contrast material administration for calculation of extracellular volume fraction and evaluation of diffuse interstitial myocardial fibrosis (36).
Myocardial Perfusion Defects
More than half of patients with Fontan circulation exhibit myocardial perfusion abnormalities. Interestingly, a significant number of these perfusion defects did not conform to the usual coronary territories (37).
By means of ultrafast sequences, myocardial perfusion imaging follows the effect of the first pass of a bolus of intravenous GBCA through multiple planes of the myocardium, showing the presence of perfusion defects with high spatial resolution. Additionally, stress perfusion imaging, using pharmacologic stress agents such as adenosine or dobutamine, has been shown to be an accurate technique for detecting inducible perfusion defects (38,39). At our institution, we generally use regadenoson as a pharmacologic stressor in children and adults, because it is better tolerated and easier to use than adenosine (40).
Systemic-to-Pulmonary Collateral Circulation
Systemic-to-pulmonary collateral flow frequently occurs in patients with Fontan circulation, and it may typically be responsible for volume overload or cyanosis (41). Flows in each individual pulmonary vein can also be measured, and the difference between cumulative pulmonary venous flow and pulmonary arterial flow allows the quantification of systemic-to-pulmonary collateral burden (21,42) (Fig 10). Alternatively, collateral flow can be estimated by subtracting the sum of SVC and IVC flows from aortic forward flow: AO − [SVC + IVC]. Good correlation between these two techniques has been shown (43). An association has been demonstrated between the amount of aortopulmonary collateral flow and postoperative outcomes, including length of hospital stay and chest tube duration (44,45).
Fontan-associated Liver Disease
Fontan-associated liver disease is a growing concern for this young population, affecting nearly all patients with Fontan circulation (Fig 15). Elevated central venous pressure, lymphatic dysfunction, and hypoxia-induced sinusoidal stress result in chronic hepatocyte injury and fibrosis. Fibrosis can be observed early after Fontan completion but may develop into end-stage cirrhosis during different phases of adolescence and adulthood, when most complications occur (eg, portal hypertension with ascites and/or variceal bleeding, hepatocellular carcinoma).
Figure 15:
Extracardiac findings in a 26-year-old patient affected by hypoplastic left heart syndrome after Fontan operation. Bright-blood single-shot images generated using T2-weighted MRI, true fast imaging with steady-state free precession in the (left) axial, (middle) coronal, and (right) sagittal planes. Distortion of the gross architecture of the liver with irregular nodular margins and hypertrophy of the caudate lobe as well as severe ascites in the abdominal cavity are seen. These findings are in line with end-stage liver cirrhosis, and dedicated liver imaging and testing are required.
Recent techniques, including MR liver elastography and MR relaxometry with T1, T2, and T1ρ mapping, have been reported to be useful in the diagnosis of Fontan-associated liver disease and in the assessment of central venous pressure, thus demonstrating promising clinical value in the longitudinal follow-up of patients with Fontan circulation (46,47).
Lymphatic Dysfunction
Fontan circulation operates at or sometimes beyond the functional limits of the lymphatic system. Elevated venous pressures may impair thoracic duct drainage, resulting in pleural effusions, plastic bronchitis, or protein-losing enteropathy. Congenital lymphatic malformations may also predispose to lymphatic dysfunction.
T2-weighted noncontrast MR lymphangiography of the thorax has been shown to allow detection of lymphatic abnormalities associated with postoperative and long-term outcomes, which shows promise for guiding treatment in the near future (48,49).
Challenging Scenarios: Tips and Tricks
Cardiac MRI in patients with Fontan circulation is gaining in importance and appreciation for its ability to provide a comprehensive anatomic and functional evaluation of the Fontan circulation. It provides 3D imaging of nearly any structure (independent of user and anatomic problems), represents the reference standard for ventricular volumetry and functional assessment, and allows quantification of valvular regurgitation, shunt estimation, and tissue characterization. All such assessment is possible with this noninvasive technique with no ionizing radiation.
However, similar to other imaging techniques, cardiac MRI also has some challenges and limitations.
Challenges Caused by Lack of Cooperation
It is beneficial to dedicate time for preparation of patients before cardiac MRI. Careful guidance of pediatric patients before and during the scan by play therapists may help the patients overcome fear and allow performance of imaging without any sedation.
Short protocols may help overcome problems caused by the requirement for breath holding and lack of cooperation. The complexity of the patient with Fontan circulation requires good teamwork between the radiographer and the cardiologist and/or the radiologist to adjust the scanning protocol and sequences according to the patient’s needs and address clinical questions.
Free breathing complicates the process of assembling multiple cardiac planes acquired at differing respiratory phases. A somewhat overlooked simple, established strategy for free breathing is to collect multiple so-called outer-level averages of segmented raw-data acquisition; this process is not necessarily time-consuming, because each average can be quickly generated by using parallel imaging.
Standard real-time imaging during free breathing cannot obtain the resolution of a breath-hold segmented collection of more detailed raw data. However, more recent real-time techniques have shown impressive clinical applications and are available at specialized centers (50,51).
Sedation in infants younger than 6 months can be achieved by allowing them to fall into a natural sleep after a feeding. Children younger than 6 years usually require either deep sedation or general anesthesia. The type of sedation depends on the available facilities and hospital protocols and requires a well-trained multidisciplinary team that strictly follows safety measures and uses appropriate facilities (52,53). Although most patients do not experience adverse events, it is important to be aware that the complication rate is higher in patients with complex congenital heart disease than in the general population (54).
Arrhythmias
Arrhythmias are prevalent in patients with Fontan circulation, with increasing prevalence as this population ages (55). Bradyarrhythmias are present in approximately 7% of patients 10 years after the Fontan procedure and in 15% of patients 20 years after the procedure, with tachyarrhythmias present in 9% and 31% of patients at those time points, respectively (56).
Irregular cardiac cycles can degrade image quality, and technologists should be aware of the options available for improved consistency in clinical service.
When arrhythmia affects a small fraction of the cardiac cycles, it is possible to set a so-called acceptance range of R-R intervals so that data acquired during an R-R interval that turns out to be abnormally short or long can be rejected and reacquired in the next cycle. If arrhythmia affects more than approximately 20% of cycles on average, it is common to use prospectively triggered rather than retrospectively gated sequences for cine imaging. Prospective triggering often does not image late diastole; it may be acceptable for assessing ventricular function, but it may not capture the entire volume flow of a cardiac cycle in situations in which diastolic flow is continuing. As an alternative, free-breathing retrospectively gated segmented cine imaging with multiple averages may be successful in situations in which predominantly normal cycles are averaged with a few abnormal ones.
For the most severely chaotic or variable arrhythmia, real-time cine cardiac MRI based on single-shot acquisitions within each cycle must be used, because it is inherently insensitive to arrhythmia. Scanning and analysis become laborious if reliability is to be achieved, because it is necessary to image multiple cycles of each section and painfully identify the normal cycles to be analyzed. Historically, real-time imaging necessitated a compromise in spatial and temporal resolution with substantial reduction in image quality compared with standard cine cardiac MRI. More recently, however, new, highly accelerated techniques have allowed acceptable diagnostic image quality with good agreement in ventricular volumetry between standard and real-time cine cardiac MRI. In a 2016 study by Kido et al (57), the high spatial (1.7 mm × 1.7 mm) and temporal (41 msec) resolutions of compressed sensing real-time cine cardiac MRI were identical to those of standard cine imaging and translated into good image quality and high agreement for all left ventricular measurements.
Implanted Metal Devices
Patients with Fontan circulation regularly undergo transcatheter or surgical procedures that may involve implantation of devices containing ferromagnetic material such as stents, occluder devices, embolization coils, prosthetic valves, and pacing wires with or without a permanent pacemaker or implantable cardioverter defibrillator. These devices may severely degrade image quality, precluding complete assessment of the single ventricle in up to 20% of patients (58). Frequently, owing to the susceptibility of cine SSFP imaging to magnetic field inhomogeneity and its tendency to produce more image artifacts, cine SPGR imaging may be preferred (Fig 16). Occasionally, even with SPGR imaging it may not be possible to assess in-stent stenoses. CT may be a suitable alternative in such cases. However, cardiac MRI can show indirect signs of such stenoses, such as a diastolic tail in the flow pattern of the descending aorta when assessing a stented coarctation or a discrepancy in right-to-left pulmonary artery split flow ratio when assessing stented pulmonary arteries.
Figure 16:
(A) Steady-state free precession (SSFP) image in a patient with mesocardia, double-inlet indeterminate ventricle with pulmonary atresia, bilateral superior vena cava after a bilateral bidirectional Glenn procedure and Fontan completion with left-sided extracardiac conduit (ECC). There is metal artifact from a stent implanted at the site of inferior vena cava-ECC anastomosis. (B) Spoiled gradient-recalled echo (SPGR) image in a patient after Fontan procedure with dual-chamber epicardial pacemaker (dark artifact) implanted for sick sinus syndrome. Cardiac MRI was performed as part of the transplant assessment workup, specifically, for assessment of pulmonary flow and estimation of pulmonary vascular resistance. Imaging was performed in line with protocol for non–MRI-conditional devices and was limited to half-Fourier single-shot turbo spin-echo images and flow maps. (C, D) SPGR images in a patient with double-outlet right ventricle with pulmonary atresia after extracardiac Fontan procedure. The patient underwent left pulmonary artery stent placement for branch stenosis. Unlike SSFP imaging (panel A), SPGR imaging minimized metal artifacts from the stent.
Notably, CE-MRA reportedly has comparable sensitivity and specificity to CT in assessing stent stenoses (59,60). Additionally, the inversion-recovery sequences applied for LGE imaging have been modified for increased tolerance to device distortion of the main field (61).
Implanted metal devices also present some safety issues. Guidelines indicate that MRI can be performed safely in patients with a permanent pacemaker or implantable cardioverter defibrillator if strict safety conditions are met (62). Patients with MRI-conditional devices and a proper exemption period after implantation can undergo MRI following the appropriate conditions of use (including programming), in the context of appropriate clinical expertise and a rigorously applied standardized institutional workflow. In patients with non–MRI-conditional devices, MRI may be considered if there is no imaging alternative and the result of the test is crucial, provided that there are no epicardial leads, connected fractured leads, or abandoned leads; such cases require careful judgment of risks versus benefits by the clinician and informed consent from the patient (62).
MRI safety websites have been created to gather data on all devices checked for safety in 1.5-T and 3-T scanners. However, it remains mandatory to consult data published by the manufacturer rather than rely on data offered by any third party.
Contrast Agent Toxicity
GBCAs are widely used in MRI and are considered safe and well-tolerated when used in recommended doses (3). Residual gadolinium has been found within the brain tissue of patients who were administered multiple doses of GBCAs over their lifetimes, even in the absence of clinically evident disease and in the setting of an intact blood-brain barrier (63,64). Although the clinical significance of residual gadolinium remains unclear, caution is required, and the risks versus benefits of GBCA use must be weighed carefully, particularly in patients with Fontan circulation who are likely to undergo repeated administrations over time. A recent study on a cohort of pediatric and adult patients with congenital heart disease undergoing contrast-enhanced cardiac MRI demonstrated a low incidence of qualitative and no significant quantitative imaging-based evidence of gadolinium brain deposition (65).
Ferumoxytol is an ultrasmall superparamagnetic iron oxide (USPIO) nanoparticle that is approved for intravenous iron replacement therapy. This agent is also recognized for its remarkable MRI properties, acting as a potent T1 and T2 relaxation agent (66). However, the manufacturers of ferumoxytol elected to pursue a therapy rather than a diagnostic label; therefore, although recently published imaging studies have reported outstanding results with no safety concerns, they were all limited by small patient sample sizes. Multicenter pooled analysis of safety data related to diagnostic use of this contrast agent is currently underway (67).
Many researchers have explored the use of USPIO agents in diagnostic MRI and have shown that ferumoxytol has the potential for use in clinical applications well beyond the scope of extracellular gadolinium agents and may open up new vistas in clinical practice and workflow (66). The stable blood pool signal of this agent has been leveraged to generate high-resolution, steady-state 4D images of dynamic anatomy and blood flow in children with congenital heart disease, thereby simplifying and shortening acquisition time in this complex population, particularly in neonates and small infants (68,69).
Conclusions and Future Perspectives
Although cardiac MRI is sometimes challenging, it has become a widely accepted standard for anatomic and functional assessment of complex Fontan physiology because it is noninvasive and is suitable for comprehensive evaluation of patient follow-up evaluation.
The development of faster cardiac MRI techniques that are robust to breathing motion and arrhythmia will markedly shorten scanning time and enable the use of cardiac MRI even in challenging situations. Furthermore, 3D and 4D whole-heart cine and flow imaging techniques are in development that would allow acquisition of dynamic data with full anatomic coverage in a single scan and reformatting in any plane during postprocessing, thus reducing dependency on patient cooperation and operator skills (70,71). These approaches have the potential to be the solution to performing MRI in patients with complex congenital issues.
Finally, MRI may allow for comprehensive assessment of the most common extracardiac complications associated with Fontan circulation (eg, liver disease, lymphatic dysfunction) in a single examination.
Authors declared no funding for this work.
Disclosures of conflicts of interest: F.P. No relevant relationships. I.V. No relevant relationships. P.G. No relevant relationships. M.R. No relevant relationships. C.I. No relevant relationships. D.J.P. Siemens research grant. S.K. No relevant relationships.
Abbreviations:
- CE-MRA
- contrast-enhanced MR angiography
- ECG
- electrocardiography
- 4D
- four-dimensional
- GBCA
- gadolinium-based contrast agent
- IVC
- inferior vena cava
- LGE
- late gadolinium enhancement
- PCI
- phase-contrast imaging
- SPGR
- spoiled gradient echo
- SSFP
- steady-state free precession
- SVC
- superior vena cava
- 3D
- three-dimensional
- 2D
- two-dimensional
- USPIO
- ultrasmall superparamagnetic iron oxide
References
- 1. Geva T , Powell AJ . Magnetic resonance imaging – Single ventricle and Fontan . In: Allen HD , Driscoll DJ , Shaddy RE , Feltes TF , eds. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult . 8th ed. Philadelphia, Pa: : Lippincott Williams & Wilkins, 2013. ; 207 – 246 . [Google Scholar]
- 2. Valsangiacomo Buechel ER , Grosse-Wortmann L , Fratz S , et al . Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: an expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI . Eur Heart J Cardiovasc Imaging 2015. ; 16 ( 3 ): 281 – 297 . [DOI] [PubMed] [Google Scholar]
- 3. Fratz S , Chung T , Greil GF , et al . Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease . J Cardiovasc Magn Reson 2013. ; 15 ( 1 ): 51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeong M , Loughborough W , Hamilton M , Manghat N . Role of cardiac MRI and CT in Fontan circulation . J Congenit Heart Dis 2017. ; 1 : 8 . [Google Scholar]
- 5. Prakash A , Khan MA , Hardy R , Torres AJ , Chen JM , Gersony WM . A new diagnostic algorithm for assessment of patients with single ventricle before a Fontan operation . J Thorac Cardiovasc Surg 2009. ; 138 ( 4 ): 917 – 923 . [DOI] [PubMed] [Google Scholar]
- 6. Fogel MA , Pawlowski TW , Whitehead KK , et al . Cardiac magnetic resonance and the need for routine cardiac catheterization in single ventricle patients prior to Fontan: a comparison of 3 groups: pre-Fontan CMR versus cath evaluation . J Am Coll Cardiol 2012. ; 60 ( 12 ): 1094 – 1102 . [DOI] [PubMed] [Google Scholar]
- 7. Hughes ML , Broadhead M , McEwan A , Tann O , Krupickova S , Muthurangu V . Pre-Operative Grade of Decompressing Systemic Venous Collaterals, But Not Jugular Venous Pressure, Predicts Short- and Medium-Term Outcome After Completion of the Total Cavopulmonary Connection . JACC Cardiovasc Imaging 2019. ; 12 ( 6 ): 1109 – 1111 . [DOI] [PubMed] [Google Scholar]
- 8. Zaki NC , Kelleman MS , James Parks W , Slesnick TC , McConnell ME , Oster ME . The utility of cardiac magnetic resonance imaging in post-Fontan surveillance . Congenit Heart Dis 2019. ; 14 ( 2 ): 140 – 146 . [DOI] [PubMed] [Google Scholar]
- 9. Baumgartner H , De Backer J , Babu-Narayan SV , et al . 2020 ESC Guidelines for the management of adult congenital heart disease . Eur Heart J 2021. ; 42 ( 6 ): 563 – 645 . [DOI] [PubMed] [Google Scholar]
- 10. Rychik J , Atz AM , Celermajer DS , et al . Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association . Circulation 2019. ; 140 ( 6 ): e234 – e284 . [DOI] [PubMed] [Google Scholar]
- 11. Wagner M , Nguyen KL , Khan S , et al . Contrast-enhanced MR angiography of cavopulmonary connections in adult patients with congenital heart disease . AJR Am J Roentgenol 2012. ; 199 ( 5 ): W565 – W574 . [DOI] [PubMed] [Google Scholar]
- 12. Sørensen TS , Körperich H , Greil GF , et al . Operator-independent isotropic three-dimensional magnetic resonance imaging for morphology in congenital heart disease: a validation study . Circulation 2004. ; 110 ( 2 ): 163 – 169 . [DOI] [PubMed] [Google Scholar]
- 13. Kourtidou S , Jones MR , Moore RA , et al . mDixon ECG-gated 3-dimensional cardiovascular magnetic resonance angiography in patients with congenital cardiovascular disease . J Cardiovasc Magn Reson 2019. ; 21 ( 1 ): 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Powell AJ , Maier SE , Chung T , Geva T . Phase-velocity cine magnetic resonance imaging measurement of pulsatile blood flow in children and young adults: in vitro and in vivo validation . Pediatr Cardiol 2000. ; 21 ( 2 ): 104 – 110 . [DOI] [PubMed] [Google Scholar]
- 15. Dyverfeldt P , Bissell M , Barker AJ , et al . 4D flow cardiovascular magnetic resonance consensus statement . J Cardiovasc Magn Reson 2015. ; 17 ( 1 ): 72 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sundareswaran KS , Haggerty CM , de Zélicourt D , et al . Visualization of flow structures in Fontan patients using 3-dimensional phase contrast magnetic resonance imaging . J Thorac Cardiovasc Surg 2012. ; 143 ( 5 ): 1108 – 1116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McLennan D , Schäfer M , Mitchell MB , et al . Usefulness of 4D-flow MRI in mapping flow distribution through failing Fontan circulation prior to cardiac intervention . Pediatr Cardiol 2019. ; 40 ( 5 ): 1093 – 1096 . [DOI] [PubMed] [Google Scholar]
- 18. Rijnberg FM , Elbaz MSM , Westenberg JJM , et al . Four-dimensional flow magnetic resonance imaging-derived blood flow energetics of the inferior vena cava-to-extracardiac conduit junction in Fontan patients . Eur J Cardiothorac Surg 2019. ; 55 ( 6 ): 1202 – 1210 . [DOI] [PubMed] [Google Scholar]
- 19. Sjöberg P , Heiberg E , Wingren P , et al . Decreased diastolic ventricular kinetic energy in young patients with Fontan circulation demonstrated by four-dimensional cardiac magnetic resonance imaging . Pediatr Cardiol 2017. ; 38 ( 4 ): 669 – 680 . [Published correction appears in Pediatr Cardiol 2017;38(5):1087.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Isorni MA , Moisson L , Moussa NB , et al . 4D flow cardiac magnetic resonance in children and adults with congenital heart disease: Clinical experience in a high volume center . Int J Cardiol 2020. ; 320 ( 168 ): 177 . [DOI] [PubMed] [Google Scholar]
- 21. Valverde I , Nordmeyer S , Uribe S , et al . Systemic-to-pulmonary collateral flow in patients with palliated univentricular heart physiology: measurement using cardiovascular magnetic resonance 4D velocity acquisition . J Cardiovasc Magn Reson 2012. ; 14 ( 1 ): 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rutkowski DR , Barton G , François CJ , Bartlett HL , Anagnostopoulos PV , Roldán-Alzate A . Analysis of cavopulmonary and cardiac flow characteristics in fontan Patients: Comparison with healthy volunteers . J Magn Reson Imaging 2019. ; 49 ( 6 ): 1786 – 1799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamphuis VP , Elbaz MSM , van den Boogaard PJ , et al . Stress increases intracardiac 4D flow cardiovascular magnetic resonance -derived energetics and vorticity and relates to VO2max in Fontan patients . J Cardiovasc Magn Reson 2019. ; 21 ( 1 ): 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demirkiran A , van Ooij P , Westenberg JJM , et al . Clinical intra-cardiac 4D flow CMR: acquisition, analysis, and clinical applications . Eur Heart J Cardiovasc Imaging 2022. ; 23 ( 2 ): 154 – 165 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rijnberg FM , van Assen HC , Juffermans JF , et al . Reduced scan time and superior image quality with 3D flow MRI compared to 4D flow MRI for hemodynamic evaluation of the Fontan pathway . Sci Rep 2021. ; 11 ( 1 ): 6507 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Margossian R , Schwartz ML , Prakash A , et al . Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study) . Am J Cardiol 2009. ; 104 ( 3 ): 419 – 428 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rathod RH , Prakash A , Kim YY , et al . Cardiac magnetic resonance parameters predict transplantation-free survival in patients with fontan circulation . Circ Cardiovasc Imaging 2014. ; 7 ( 3 ): 502 – 509 . [Published correction appears in Circ Cardiovasc Imaging 2018;11(11):e000021.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Honjo O , Mertens L , Van Arsdell GS . Atrioventricular valve repair in patients with single-ventricle physiology: mechanisms, techniques of repair, and clinical outcomes . Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2011. ; 14 ( 1 ): 75 – 84 . [DOI] [PubMed] [Google Scholar]
- 29. Mathew RC , Löffler AI , Salerno M . Role of Cardiac Magnetic Resonance Imaging in Valvular Heart Disease: Diagnosis, Assessment, and Management . Curr Cardiol Rep 2018. ; 20 ( 11 ): 119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sreeram N , Emmel M , Bennink G . Stent therapy for acute and chronic obstructions in extracardiac Fontan conduits . Cardiol Young 2013. ; 23 ( 5 ): 766 – 768 . [DOI] [PubMed] [Google Scholar]
- 31. Firdouse M , Agarwal A , Chan AK , Mondal T . Thrombosis and thromboembolic complications in fontan patients: a literature review . Clin Appl Thromb Hemost 2014. ; 20 ( 5 ): 484 – 492 . [DOI] [PubMed] [Google Scholar]
- 32. Gewillig M . The Fontan circulation . Heart 2005. ; 91 ( 6 ): 839 – 846 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takawira F , Ayer JG , Onikul E , et al . Evaluation of the extracardiac conduit modification of the Fontan operation for thrombus formation using magnetic resonance imaging . Heart Lung Circ 2008. ; 17 ( 5 ): 407 – 410 . [DOI] [PubMed] [Google Scholar]
- 34. Rathod RH , Prakash A , Powell AJ , Geva T . Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after Fontan operation . J Am Coll Cardiol 2010. ; 55 ( 16 ): 1721 – 1728 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kato A , Riesenkampff E , Yim D , Yoo SJ , Seed M , Grosse-Wortmann L . Pediatric Fontan patients are at risk for myocardial fibrotic remodeling and dysfunction . Int J Cardiol 2017. ; 240 ( 172 ): 177 . [DOI] [PubMed] [Google Scholar]
- 36. Kato A , Riesenkampff E , Yim D , Yoo SJ , Seed M , Gross-Wortmann L . Diffuse myocardial fibrosis in patients after Fontan operation: a T1 relaxometry magnetic resonance pilot study . J Cardiovasc Magn Reson 2016. ; 18 ( Suppl 1 ): O28 . [Google Scholar]
- 37. Priyadarshini A , Saxena A , Patel C , Paul VK , Lodha R , Airan B . Myocardial perfusion abnormalities in patients occurring more than 1 year after successful univentricular (Fontan surgery) and biventricular repair (complete repair of tetralogy of Fallot) . Pediatr Cardiol 2013. ; 34 ( 4 ): 786 – 794 . [DOI] [PubMed] [Google Scholar]
- 38. Prakash A , Powell AJ , Krishnamurthy R , Geva T . Magnetic resonance imaging evaluation of myocardial perfusion and viability in congenital and acquired pediatric heart disease . Am J Cardiol 2004. ; 93 ( 5 ): 657 – 661 . [DOI] [PubMed] [Google Scholar]
- 39. Schwitter J , Wacker CM , Wilke N , et al . MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial . Eur Heart J 2013. ; 34 ( 10 ): 775 – 781 . [DOI] [PubMed] [Google Scholar]
- 40. Noel CV , Krishnamurthy R , Masand P , et al . Myocardial Stress Perfusion MRI: Experience in Pediatric and Young-Adult Patients Following Arterial Switch Operation Utilizing Regadenoson . Pediatr Cardiol 2018. ; 39 ( 6 ): 1249 – 1257 . [DOI] [PubMed] [Google Scholar]
- 41. Whitehead KK , Harris MA , Glatz AC , et al . Status of systemic to pulmonary arterial collateral flow after the fontan procedure . Am J Cardiol 2015. ; 115 ( 12 ): 1739 – 1745 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grosse-Wortmann L , Al-Otay A , Yoo SJ . Aortopulmonary collaterals after bidirectional cavopulmonary connection or Fontan completion: quantification with MRI . Circ Cardiovasc Imaging 2009. ; 2 ( 3 ): 219 – 225 . [DOI] [PubMed] [Google Scholar]
- 43. Odenwald T , Quail MA , Giardini A , et al . Systemic to pulmonary collateral blood flow influences early outcomes following the total cavopulmonary connection . Heart 2012. ; 98 ( 12 ): 934 – 940 . [DOI] [PubMed] [Google Scholar]
- 44. Glatz AC , Rome JJ , Small AJ , et al . Systemic-to-pulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-Fontan clinical outcomes . Circ Cardiovasc Imaging 2012. ; 5 ( 2 ): 218 – 225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grosse-Wortmann L , Drolet C , Dragulescu A , et al . Aortopulmonary collateral flow volume affects early postoperative outcome after Fontan completion: a multimodality study . J Thorac Cardiovasc Surg 2012. ; 144 ( 6 ): 1329 – 1336 . [DOI] [PubMed] [Google Scholar]
- 46. Emamaullee J , Zaidi AN , Schiano T , et al . Fontan-Associated Liver Disease: Screening, Management, and Transplant Considerations . Circulation 2020. ; 142 ( 6 ): 591 – 604 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Lange C , Reichert MJE , Pagano JJ , et al . Increased extracellular volume in the liver of pediatric Fontan patients . J Cardiovasc Magn Reson 2019. ; 21 ( 1 ): 39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dori Y , Keller MS , Fogel MA , et al . MRI of lymphatic abnormalities after functional single-ventricle palliation surgery . AJR Am J Roentgenol 2014. ; 203 ( 2 ): 426 – 431 . [DOI] [PubMed] [Google Scholar]
- 49. Biko DM , DeWitt AG , Pinto EM , et al . MRI Evaluation of Lymphatic Abnormalities in the Neck and Thorax after Fontan Surgery: Relationship with Outcome . Radiology 2019. ; 291 ( 3 ): 774 – 780 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Steeden JA , Kowalik GT , Tann O , Hughes M , Mortensen KH , Muthurangu V . Real-time assessment of right and left ventricular volumes and function in children using high spatiotemporal resolution spiral bSSFP with compressed sensing . J Cardiovasc Magn Reson 2018. ; 20 ( 1 ): 79 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haji-Valizadeh H , Rahsepar AA , Collins JD , et al . Validation of highly accelerated real-time cardiac cine MRI with radial k-space sampling and compressed sensing in patients at 1.5T and 3T . Magn Reson Med 2018. ; 79 ( 5 ): 2745 – 2751 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilson SR , Shinde S , Appleby I , et al . Guidelines for the safe provision of anaesthesia in magnetic resonance units 2019: Guidelines from the Association of Anaesthetists and the Neuro Anaesthesia and Critical Care Society of Great Britain and Ireland . Anaesthesia 2019. ; 74 ( 5 ): 638 – 650 . [DOI] [PubMed] [Google Scholar]
- 53. Fogel MA , Weinberg PM , Parave E , et al . Deep sedation for cardiac magnetic resonance imaging: a comparison with cardiac anesthesia . J Pediatr 2008. ; 152 ( 4 ): 534 – 539 . 539 . e1 . [DOI] [PubMed] [Google Scholar]
- 54. Stockton E , Hughes M , Broadhead M , Taylor A , McEwan A . A prospective audit of safety issues associated with general anesthesia for pediatric cardiac magnetic resonance imaging . Paediatr Anaesth 2012. ; 22 ( 11 ): 1087 – 1093 . [DOI] [PubMed] [Google Scholar]
- 55. Stephenson EA , Lu M , Berul CI , et al . Arrhythmias in a contemporary fontan cohort: prevalence and clinical associations in a multicenter cross-sectional study . J Am Coll Cardiol 2010. ; 56 ( 11 ): 890 – 896 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carins TA , Shi WY , Iyengar AJ , et al . Long-term outcomes after first-onset arrhythmia in Fontan physiology . J Thorac Cardiovasc Surg 2016. ; 152 ( 5 ): 1355 – 1363 . e1 . [DOI] [PubMed] [Google Scholar]
- 57. Kido T , Kido T , Nakamura M , et al . Compressed sensing real-time cine cardiovascular magnetic resonance: accurate assessment of left ventricular function in a single-breath-hold . J Cardiovasc Magn Reson 2016. ; 18 ( 1 ): 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garg R , Powell AJ , Sena L , Marshall AC , Geva T . Effects of metallic implants on magnetic resonance imaging evaluation of Fontan palliation . Am J Cardiol 2005. ; 95 ( 5 ): 688 – 691 . [DOI] [PubMed] [Google Scholar]
- 59. Nordmeyer J , Gaudin R , Tann OR , et al . MRI may be sufficient for noninvasive assessment of great vessel stents: an in vitro comparison of MRI, CT, and conventional angiography . AJR Am J Roentgenol 2010. ; 195 ( 4 ): 865 – 871 . [DOI] [PubMed] [Google Scholar]
- 60. den Harder AM , Suchá D , van Hamersvelt RW , et al . Imaging of pediatric great vessel stents: Computed tomography or magnetic resonance imaging? PLoS One 2017. ; 12 ( 1 ): e0171138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rashid S , Rapacchi S , Vaseghi M , et al . Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices . Radiology 2014. ; 270 ( 1 ): 269 – 274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brignole M , Auricchio A , Baron-Esquivias G , et al . 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) . Eur Heart J 2013. ; 34 ( 29 ): 2281 – 2329 . [DOI] [PubMed] [Google Scholar]
- 63. Kanda T , Fukusato T , Matsuda M , et al . Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy . Radiology 2015. ; 276 ( 1 ): 228 – 232 . [DOI] [PubMed] [Google Scholar]
- 64. Harvey HB , Gowda V , Cheng G . Gadolinium Deposition Disease: A New Risk Management Threat . J Am Coll Radiol 2020. ; 17 ( 4 ): 546 – 550 . [DOI] [PubMed] [Google Scholar]
- 65. Zaki N , Parra D , Wells Q , et al . Assessment of gadolinium deposition in the brain tissue of pediatric and adult congenital heart disease patients after contrast enhanced cardiovascular magnetic resonance . J Cardiovasc Magn Reson 2020. ; 22 ( 1 ): 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Finn JP , Nguyen KL , Hu P . Ferumoxytol vs. Gadolinium agents for contrast-enhanced MRI: Thoughts on evolving indications, risks, and benefits . J Magn Reson Imaging 2017. ; 46 ( 3 ): 919 – 923 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nguyen KL , Yoshida T , Kathuria-Prakash N , et al . Multicenter Safety and Practice for Off-Label Diagnostic Use of Ferumoxytol in MRI . Radiology 2019. ; 293 ( 3 ): 554 – 564 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Han F , Zhou Z , Han E , et al . Self-gated 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) using rotating cartesian K-space (ROCK): Validation in children with congenital heart disease . Magn Reson Med 2017. ; 78 ( 2 ): 472 – 483 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cheng JY , Hanneman K , Zhang T , et al . Comprehensive motion-compensated highly accelerated 4D flow MRI with ferumoxytol enhancement for pediatric congenital heart disease . J Magn Reson Imaging 2016. ; 43 ( 6 ): 1355 – 1368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xu J , Kim D , Otazo R , et al . Towards a five-minute comprehensive cardiac MR examination using highly accelerated parallel imaging with a 32-element coil array: feasibility and initial comparative evaluation . J Magn Reson Imaging 2013. ; 38 ( 1 ): 180 – 188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Markl M , Kilner PJ , Ebbers T . Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance . J Cardiovasc Magn Reson 2011. ; 13 ( 1 ): 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]











![Flow assessment in a 15-year-old patient with double-outlet right ventricle and pulmonary atresia after extracardiac Fontan procedure. Flows are assessed by imaging the vessel perpendicular to its long axis using phase-contrast imaging. Through-plane velocity maps of the ascending aorta, superior vena cava (SVC), inferior vena cava (IVC), right pulmonary artery (RPA), left pulmonary artery (LPA), right pulmonary veins (RPVs), and left pulmonary veins (LPVs) are shown. The pulmonary arteries appear unobstructed (RPA:LPA net flow split ratio, approximately 60%:40%). Estimated systemic-to-pulmonary collateral flow is approximately 10% (systemic estimator: AO − [SVC + IVC]; pulmonary estimator: [RPV + LPV] − [RPA + LPA]).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/fd64/9274315/ae7b050bfa10/ryct.210235.fig10.jpg)