This systematic review and meta-analysis investigates the correlation of preoperative and intraoperative factors with physical function 1 year after total knee arthroplasty in patients with knee osteoarthritis.
Key Points
Question
What preoperative and intraoperative factors are correlated with physical function after total knee arthroplasty (TKA)?
Findings
In this systematic review and meta-analysis of 20 studies that included 11 317 patients with osteoarthritis, higher preoperative body mass index (BMI) was correlated with worse physical function, while better preoperative physical function and more severe osteoarthritis were correlated with better physical function 1 year after TKA.
Meaning
These findings suggest that presurgical BMI, physical function, and osteoarthritis severity may be important factors to include and test in models predicting TKA outcomes.
Abstract
Importance
More than 1 in 5 patients do not experience improved physical function after total knee arthroplasty (TKA). Identification of factors associated with physical function may be warranted to improve outcomes in these patients.
Objective
To identify preoperative and intraoperative factors associated with physical function at 12 months after TKA in a systematic review and meta-analysis.
Data Sources
Data from January 2000 to October 2021 were searched in Medline, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Library, and Physiotherapy Evidence Database (PEDro). No language restrictions were applied.
Study Selection
Prospective observational studies or randomized clinical trials on factors associated with physical function after TKA in adult patients with osteoarthritis were selected. A prespecified peer-reviewed protocol was followed.
Data Extraction and Synthesis
Following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline, 2 reviewers independently screened titles and abstracts and judged risk of bias using Quality in Prognosis Studies (QUIPS). Multivariate random-effects meta-analyses were performed to estimate mean correlations between factors and physical function with 95% CIs. Sensitivity analyses were conducted for each QUIPS domain. Certainty of evidence was evaluated using Grading of Recommendations, Assessment, Development and Evaluations (GRADE). This study was registered with the International Prospective Register of Systematic Reviews (PROSPERO).
Main Outcomes and Measures
The primary outcome was physical function 12 months after TKA. Secondary outcomes were physical function 3 and 6 months after TKA. All estimates are mean correlations between factors and postoperative function. Positive correlations correspond to better function.
Results
Among 12 052 articles, 20 studies (including 11 317 patients and 37 factors) were analyzed. Mean correlation with higher BMI was estimated to be −0.15 (95% CI, −0.24 to −0.05; P = .33; moderate-certainty evidence), while mean correlation with better physical function was estimated to be 0.14 (95% CI, 0.02 to 0.26; P = .03; low-certainty evidence) and mean correlation with more severe osteoarthritis was estimated to be 0.10 (95% CI, 0.01 to 0.19; P = .17; high-certainty evidence). In sensitivity analyses, mean correlation with better physical function was estimated to be 0.20 (95% CI, 0.04 to 0.36; P = .02), and so perhaps a larger coefficient than in the main analysis, while mean correlations were estimated to be similar for other factors (BMI: –0.17; 95% CI, –0.28 to –0.06; P < .001; osteoarthritis severity: 0.10; 95% CI, −0.01 to 0.20; P = .05).
Conclusions and Relevance
This study found that higher presurgical BMI was correlated with worse physical function (with moderate certainty) and that better physical function (low certainty) and osteoarthritis severity (high certainty) were correlated with better physical function after TKA. These findings suggest that these factors should be included when testing predictive models of TKA outcomes.
Introduction
Total knee arthroplasty (TKA) has become the third most common inpatient surgery in the United States, with 750 000 yearly procedures projected to double in the next decade.1,2 TKA is regarded as a cost-efficient and effective treatment for restoring physical function in patients with end-stage osteoarthritis.3 However, more than 1 in 5 patients do not regain physical function after TKA.4 Nonimprovement of physical function is a risk factor associated with more expensive revision surgery and an immense burden at individual, health care system, and socioeconomic levels.5,6
Factors identified in predictive models using high-quality evidence could improve patient outcomes, particularly for those who are unlikely to benefit from surgery or who have unrealistic expectations. Evidence on factors associated with physical function has been reviewed previously, but findings were contradictory, limited in scope, based on pooled data across short-term and longer-term outcomes, or did not address certainty of evidence.7,8,9,10,11,12,13 Thus, there is need for a new synthesis of evidence on short-term TKA outcomes that uses current systematic review methods and captures recently published studies. The aim of this systematic review and meta-analysis was to synthesize evidence on preoperative and intraoperative factors associated with physical function 12 months after TKA (primary outcome) and 3 and 6 months after TKA (secondary outcomes).
Methods
In this systematic review and meta-analysis, we followed a prespecified peer-reviewed protocol14 and a preprint15 registered in International Prospective Register of Systematic Reviews (PROSPERO; CRD42018079069), designed and conducted according to Cochrane Handbook guidelines.16 Results are reported according to the recently revised Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Search Strategy and Data Sources
The search strategy was collaboratively developed by researchers (U.O. and M.F.L.) and research librarians, with feedback from the research team.14 Published studies from January 1, 2000, to October 8, 2021, were systematically searched, with no language restrictions, in Medline (Ovid), Embase (Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL; EBSCO), Cochrane Library, and Physiotherapy Evidence Database. References were managed using Endnote X8 software version 20.2.1 (Clarivate Analytics). Subject headings and keywords for each database are described in eTable 5 in the Supplement, and full search strategies for each database are defined in the protocol.14
Eligibility Criteria
To be maximally inclusive, studies had to include estimates of association between preoperative or intraoperative factors and physical function at 3, 6, or 12 months after TKA. We considered studies eligible if participants were adults diagnosed with osteoarthritis scheduled for primary TKA. Prospective longitudinal observational studies and randomized clinical trials that provided sufficient estimates of association were eligible. We excluded retrospective and case-control studies, as well as conference abstracts. We also excluded studies with mixed patient populations (eg, rheumatoid arthritis, total hip arthroplasty, or unicompartmental arthroplasty) if separate outcome data were not reported for osteoarthritis and TKA.
Outcomes
The primary outcome was physical function at 12 months after TKA. Secondary outcomes were physical function 3 and 6 months after TKA.
Study Selection and Data Extraction
Data from included studies were extracted to a standardized extraction form, with details in the published protocol.14 Data included study design, sample size, country, age, sex, body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]), outcome measures used, data collection time points, statistical analyses, and estimates of association. One reviewer performed data extraction (U.O.), while another reviewer checked data accuracy against source material (M.F.L.). Two reviewers (U.O. and M.F.L.) evaluated titles and abstracts for applicability, then read and checked full-text publications against eligibility criteria. Another author (E.D.) was involved in resolving disagreements.
Methodological Quality
Risk of bias was assessed using the Quality in Prognosis Studies (QUIPS) tool,17 following the strategy described in the protocol,14 in which 2 reviewers (U.O. and M.F.L.) independently assessed risk of bias and had consensus discussions before arriving at consensus. In cases of disagreement, E.D. was involved in the final decision. QUIPS has 6 risk domains: study participation, attrition, prognostic factor measurement, statistical analysis and reporting, confounding, and outcome measurement.
Certainty of Evidence
Two researchers (U.O. and M.F.L.) rated certainty of evidence by consensus discussion using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework.18,19 In some cases, a third researcher (E.D.) was involved in discussions. Certainty of evidence was graded as high, moderate, low, or very low. We used GRADEpro GDT (McMaster University) to summarize evidence.
Statistical Analysis
Findings for all included studies were synthesized by outcomes at 3, 6, or 12 months after TKA as described in the protocol.14 We were unable to complete planned multivariate random-effects meta-analysis because extracted data were too sparse (with a large number of factors reported by relatively few studies). Accordingly, we used a frequentist version of the bayesian multivariate model.15 Additional protocol deviations are explained in eMethods in the Supplement.
To quantify associations between potential factors and the outcome, we extracted odds ratios (ORs), risk ratios (RRs), linear model coefficients (including differences), or correlations using discrete or continuous scales. We meta-analyzed hyperbolic arctangent–transformed correlation coefficients,20 which under reasonable assumptions can be imputed for these measures of association and are invariant under linear transformation. This approach allowed inclusion of studies using various measurement tools and analyses in the meta-analysis.
We anticipated that studies would use different instruments and statistical methods that could lead to between-study heterogeneity. Therefore, multivariate random-effects meta-analysis was conducted to estimate mean correlations (ie, not common correlations) between factors and postoperative physical function.
Heterogeneity was quantified using I2 statistics. We used P scores that measured the certainty that the mean correlation for a factor was larger than those for all other factors.21 We also performed exploratory univariate meta-analyses and multivariate meta-analyses (after removing factors supported by few studies to reduce the problem of sparsity of estimation). Estimates from 3 models were compared for consistency. Finally, sensitivity analyses on physical function at 12 months after TKA were conducted for each QUIPS domain by excluding studies judged as high risk of bias and rerunning multivariate meta-analysis.
Statistical analyses were performed using Stata statistical software version 16 (StataCorp). We report mean correlations with 95% CIs. We did not prespecify any hypothesis testing but report 2-sided P values for completeness.
Results
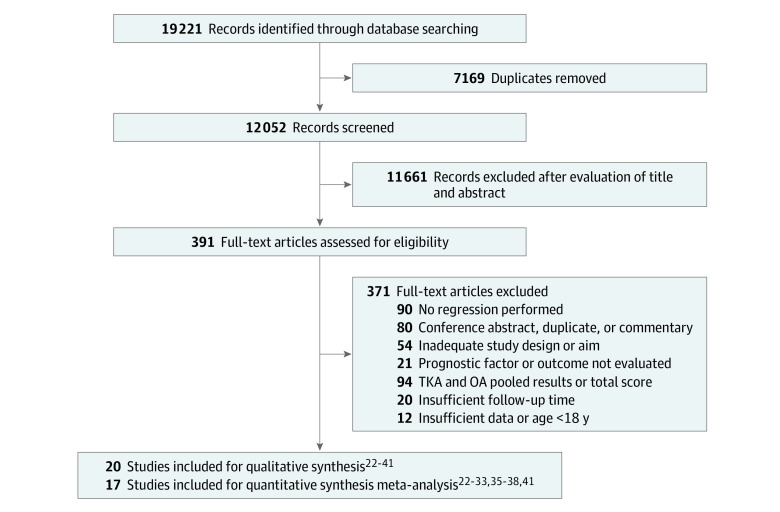
The Figure 1 study flow diagram outlines study selection and reasons for exclusion.22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 From 12 052 articles screened for title and abstracts, 391 articles were selected for full-text examination, with 20 studies22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 (total sample = 11 317 patients) for qualitative analysis at 3, 6, and 12 months and 17 studies22,23,24,25,26,27,28,29,30,31,32,33,35,36,37,38,41 for quantitative analysis at 6 and 12 months. Individual study characteristics are detailed in the Table.22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 All were prospective longitudinal observational designs; no randomized trial met inclusion criteria. We identified 37 factors across 20 studies. There were 8 studies26,27,28,29,30,34,37,38 conducted in Europe, 6 studies24,31,32,33,39,40 in Asia, 4 studies25,35,36,41 in North America, and 1 study22 in Australia, and 1 study23 was multicontinental (ie, Australia, Europe, and North America). Sample sizes ranged from 49 patients36 to 5309 patients.31 Mean age varied from 63 years35 to 75 years,32 and representation of women ranged from 49.3%36 to 90.0%.32 The most common physical function measure was the Western Ontario and McMaster Universities Arthritis Index (WOMAC). We excluded 6 studies from analysis.42,43,44,45,46,47 owing to unsuccessful attempts to obtain missing data. Sedentary behavior,40 lack of energy,38 drowsiness,38 sleeping difficulties,38 bloating,38 worrying,38 and problems with sexuality were reported once38 and were not included in the meta-analysis.
Figure 1. Flowchart of Included Studies.
OA indicates osteoarthritis; TKA, total knee arthroplasty.
Table. Characteristics of Reviewed Studies.
| Source | Country | Design | Patients analyzed, No. | Data collection | Follow-up, mo | Baseline age, y | Patients, No./Total No. (%) | Analysis | Factors measured | Outcome measured | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | ||||||||||
| Berghmans et al,37 2019a | Netherlands | PC | 146 | NA | 3 | Mean, 66.4 | 79/150 (53) | 71/150 (47) | Stepwise multiple linear regression | Mental health (SF-36), physical function (WOMAC), knee stiffness (WOMAC) | WOMAC |
| Lindner et al,34 2018 | Germany | PC | 61 | NA | 3 | Mean, 67 | 37/61 (61) | 24/61 (39) | Stepwise multiple linear regression | Pain (WOMAC) | WOMAC |
| Lingard et al,23 2007a | UK, US, Canada, Australia | PC | 676 | 1997-1999 | 3 | Distress: median, 70 |
574/676 (85) | 102/ 676 (15) | Repeated measures | Psychological distress (SF-36) | WOMAC |
| Nondistress: median, 71 | |||||||||||
| Luo et al,39 2019 | China | PC | 471 | 2017-2018 | 3 | Mean, 64.3 | 357/471 (76) | 114/471 (24) | Pearson correlation | Sleep dysfunction (PSQI), daytime sleepiness (ESS), sleep quality (self-developed scale [0-10]) | KSS |
| Bugada et al,29 2017 | Italy | PC | 563 | 2012-2015 | 6 | Median, 72 | 421/606 (69) | 185/606 (31) | Logistic regression | Comorbidity (ASA Physical Status Classification System) | NRS |
| Engel et al,36 2004 | US | PC | 54 | NA | 6 | Mean, 68 | 26/74 (49%) | 28 /74 (51) | Multiple hierarchical regression | AHI | WOMAC |
| Escobar et al,30 2007 | Spain | PC | 640 | 1999-2000 | 6 | Mean, 72 | 473/471 (74%) | 167/471 (26) | General linear model | Age (y), sex (men/women), social support (yes/no), comorbidity (CCI), physical function (WOMAC), low back pain (yes/no), mental health (SF-36) | WOMAC |
| Hylkema et al,35 2019 | US | PC | 131 | 2012-2014 | 6 | Mean, 61 | 114/ 183 (62) | 69/ 183 (38) | Univariate linear regression | Pain catastrophizing (PCS) | WPAI:SHP |
| Oka et al,40 2020 | Japan | PC | 82 | 2017-2019 | 6 | Mean, 72.1 | 67/82 (82) | 15/82 (18) | Multiple linear regression | Sedentary behavior (MET) | New KSS |
| Pua et al,31 2019 | Singapore | PC | 4026 | 2013-2017 | 6 | Mean, 68 | 3003/4026 (75) | 1026/4026 (25) | Proportional odds ordinal regression | Age (y), sex (men/women), BMI, education (primary, secondary, tertiary), ethnicity (Chinese, Indian, Malay, other), social support (yes/no), comorbidities (yes/no) contralateral knee pain (KSS), pain (OKQ), knee extension and flexion (goniometer), physical function (categories), depression (SF-36) | OKQ |
| Sugawara et al,32 2017 | Japan | PC | 59 | 2011-2012 | 6 | Mean, 75 | 53/59 (90) | 6/59 (10) | Stepwise multiple regression | TSLS | JKOM |
| Taniguchi et al,33 2016 | Japan | PC | 81 | 2013-2014 | 6 | Mean, 72 | 73/81 (90) | 8/81 (10) | Multiple linear regression | TUG | TUG |
| Yang et al,41 2019 | US | PC | 107 | 2010-2011 | 6 | Mean, 65 | 55/107 (51) | 42/107 (49) | Multivariate logistic regression | Mental health (SF-36), pain catastrophizing (PCS), comorbidity (CCI), use device (yes/no) | WOMAC |
| Berghmans et al,37 2019a | Netherlands | PC | 144 | NA | 12 | Mean, 66.4 | 79/150 (53) | 71/150 (47) | Stepwise multiple linear regression | Physical function (WOMAC), knee function (KSS) | WOMAC |
| Dowsey et al,222012 | Australia | PC | 473 | 2006-2007 | 12 | Mean, 71 | 331/478 (69) | 142/478 (31) | Multivariate linear regression | Age (y), sex (men/women), BMI, comorbidity (CCI), pain (IKSS), physical function (IKSS), mental health (SF-12), K-L grade, cruciate retaining, patella resurface, multicompartment OA | IKSS |
| Lindberg et al,38 2020 | Norway | PC | 182 | 2012-2014 | 12 | Mean, 67 | 124/ 182 (68) | 58/182 (32) | Multivariate logistic regression | Age (y), sex (men/women), pain (BPI), lack of energy, drowsiness, sleeping difficulties, bloating, worrying, sexuality problems (MSAS-SF) | BPI |
| Lingard et al,23 2007a | UK, US, Canada, Australia | PC | 676 | 1997-1999 | 12 | Distress: median, 70 |
574/676 (85) | 102/ 676 (15) | Logistic regression | Psychological distress (SF-36) | WOMAC |
| Nondistress: median, 71 | |||||||||||
| Nankaku et al,24 2018 | Japan | PC | 115 | 2013-2015 | 12 | Mean, 71 | 99/115 (86) | 16/115 (14) | Stepwise multiple regression | Age (y), physical function (KSS), TUG | KSS |
| Sullivan et al,252011 | Canada | PC | 120 | NA | 12 | Mean, 67 | 73/120 (61) | 47/120 (39) | Multiple regression | Age (y), sex (men/women), BMI, comorbidity (CCI), physical function and pain (WOMAC), pain catastrophizing (PCS), depression (PHQ-9), kinesiophobia (TSK), surgery duration (min) | WOMAC |
| Tilbury et al,26 2018 | Netherlands | PC | 146 | 2011-2012 | 12 | Mean, 67 | 101/146 (69) | 87/146 (31) | Multivariate linear regression | BMI, mental health (SF-36), physical function (KOOS), outcome expectancies (HSS hip replacement and knee replacement expectations surveys) | KOOS |
| van de Water et al,27 2019 | Netherlands | PC | 559 | 2012-2015 | 12 | Mean, 67 | 378/559 (68) | 181/559 (32) | Multivariate linear regression | Pain (KOOS), K-L grade | KOOS |
| Wylde et al,28 2012 | UK | PC | 220 | NA | 12 | Median, 70 | 136/220 (62) | 84/220 (38) | Ordinary least square regression | Age (y), sex (men/women), comorbidity (SCQ), physical function (WOMAC), depression and anxiety (HADS), pain self-efficacy (PSEQ) | WOMAC |
Abbreviations: AHI, Arthritis Helplessness Index; ASA, American Society of Anesthesiologists; BMI, body mass index; BPI, Brief Pain Inventory; CCI, Charlson Comorbidity Index; ESS, Epworth Sleepiness Scale; HADS, Hospital Anxiety and Depression Scale; HSS, Hospital for Special Surgery; IKSS, International Knee Society Score; JKOM, Japanese Knee Osteoarthritis Measure; K-L, Kellgren-Lawrence; KOOS, Knee Injury and Osteoarthritis Outcome Score; KSS, Knee Society Clinical Rating System; MET, Metabolic Equivalent of Tasks; MSAS-SF, Memorial Symptom Assessment Scale Short Form; NA, not applicable; NRS, numerical rating scale; OKQ, Oxford Knee Questionnaire; PC, prospective cohort; PCS, Pain Catastrophizing Scale; PHQ-9, Patient Health Questionnaire; PSEQ, Pain Self-Efficacy Questionnaire; PSQI, Pittsburgh Sleep Quality Index; SCQ, Self-Administered Comorbidity Questionnaire; SF-12, 12-Item Short-Form Health Survey 12; SF-36, 36-Item Short Form Health Survey; TSK, Tampa Scale of Kinesiophobia; TSLS, time single legged stand with eyes open; TUG, Timed Up and Go; WPAI:SHP, Work Productivity and Activity Impairment Questionnaire: Specific Health Problem; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Study with 2 follow-up times.
Estimates of correlations of factors with function are reported separately for 6-month and 12-month outcomes (Figure 2 and Figure 3). Results from 2 or more studies that could be statistically combined in multivariate meta-analysis are reported subsequently. Explorations of sensitivity analysis are in eFigure 1 and eTable 1 in the Supplement, while explorations of potential inconsistencies and results from exploratory univariate meta-analyses are in eFigures 2 and 3 in the Supplement. Labels for included factors are defined in eTable 3 and reason for exclusion of the individual studies are described in eTable 6 in the Supplement. Positive correlations correspond to better function postoperatively.
Figure 2. Forest Plot of Factors Associated With Physical Function at 12 mo.
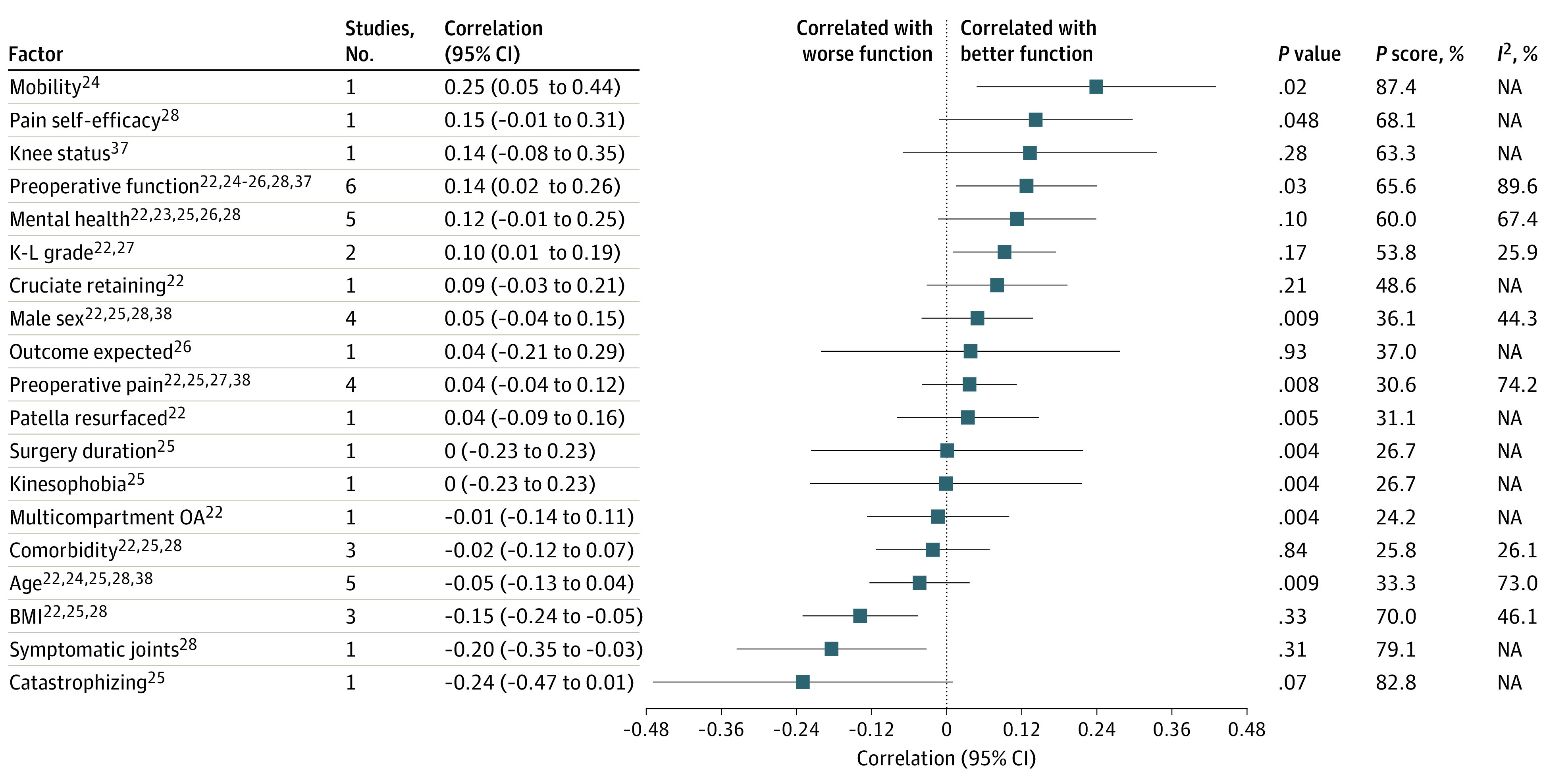
BMI indicates body mass index; K-L, Kellgren-Lawrence; NA, not applicable; OA, osteoarthritis. Direction of correlation: increased values of factors correlate with better postoperative function for all factors except dichotomous values (ie, cruciate retaining, male sex, patella resurfaced, and multicompartment OA), for which presence of factor correlates with better postoperative function.
Figure 3. Forest Plot of Factors Associated With Physical Function at 6 mo.
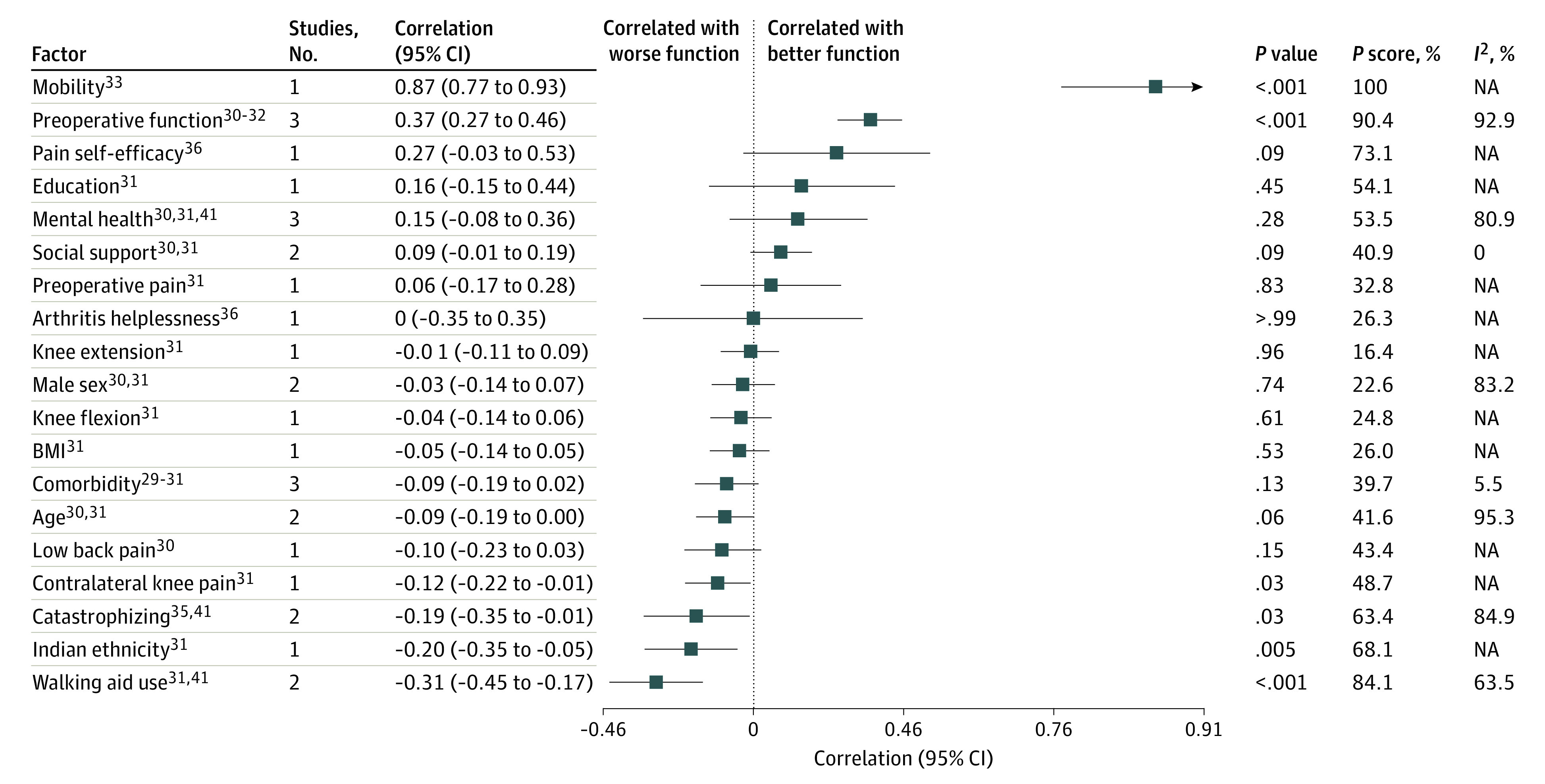
BMI indicates body mass index; NA, not applicable. Direction of correlation: increased values of factors correlate with better postoperative function for all factors except dichotomous values (ie, male sex, Indian ethnicity, and walking aid use), for which presence of factor correlates with better postoperative function.
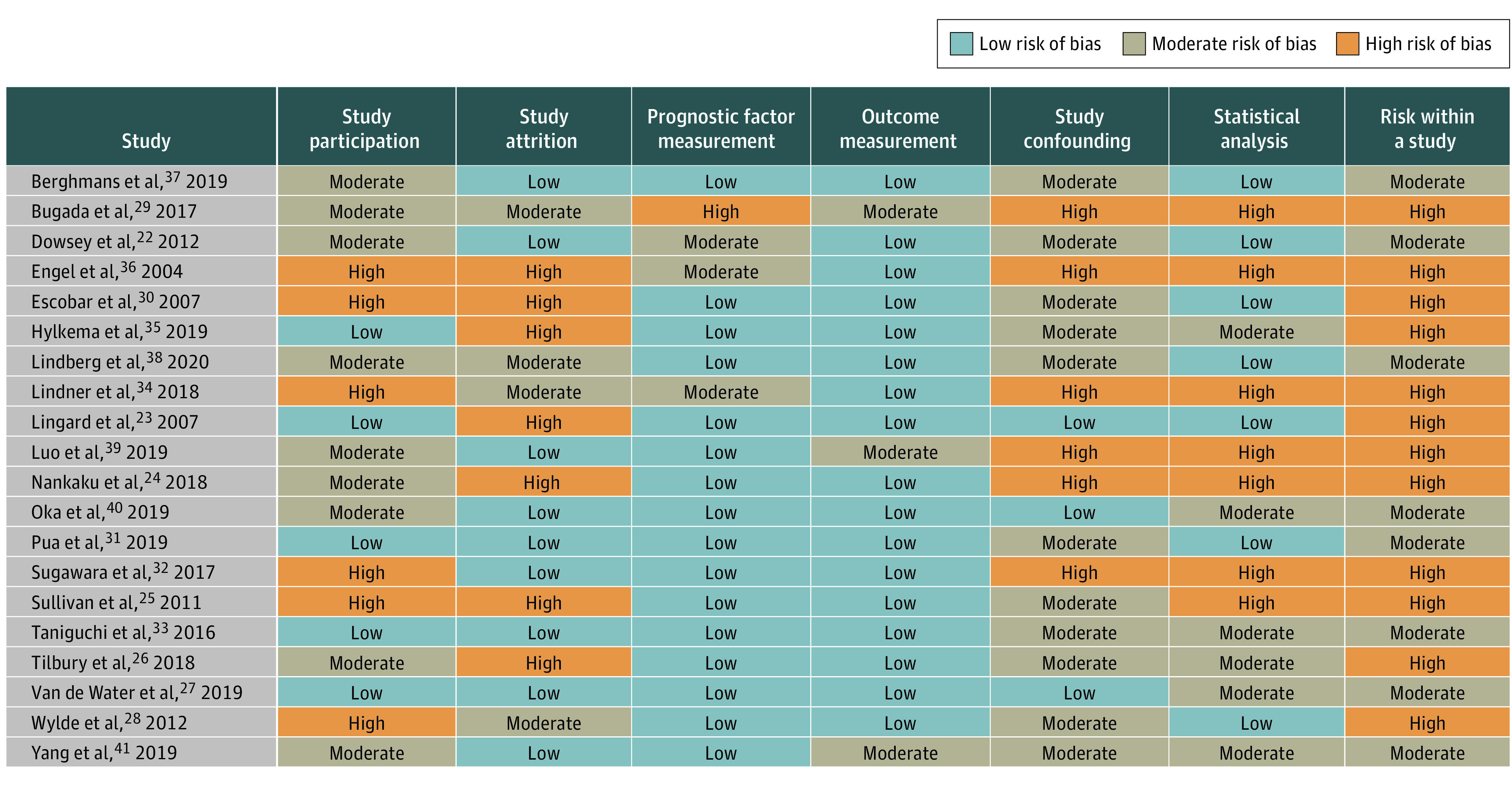
There were 9 studies with 2637 patients that reported estimates for 25 potential factors for our primary outcome, physical function at 12 months after TKA.22,23,24,25,26,27,28,37,38 Preoperative function (6 studies),22,24,25,26,28,37 mental health (including anxiety, depression, and psychological distress [5 studies]),22,23,25,26,28 and age (5 studies)22,24,25,28,38 were the most frequently reported factors. Several studies were judged as at high risk of bias on 1 or more domains (Figure 4).23,24,25,26,28,29,30,32,34,35,36,39 Multivariate meta-analytical correlation coefficient estimates are in Figure 2.22,23,24,25,26,27,28,37,38
Figure 4. Risk of Bias.
Mean correlation with higher BMI was estimated to be −0.15 (95% CI −0.24 to −0.05; P = .33; P score = 70.0%; 3 studies22,25,26; moderate-certainty evidence and moderate heterogeneity among reported estimates of association [I2 = 46%]). Mean correlation with better physical function was estimated to be 0.14 (95% CI, 0.02 to 0.26; P = .03; P score = 65.6%; 6 studies22,24,25,26,28,37; low-certainty evidence and substantial heterogeneity among estimates of association [I2 = 90%]), while mean correlation with better mental health was estimated to be 0.12 (95% CI, –0.01 to 0.25; P = .10; P score = 60.0%; 5 studies22,23,25,26,28; moderate-certainty evidence and substantial heterogeneity among reported estimates of association [I2 = 67%]) and mean correlation with more severe osteoarthritis was estimated to be 0.10 (95% CI, 0.01 to 0.19; P = .17; P score = 53.8%; 2 studies22,27; high-certainty evidence and heterogeneity between reported estimates [I2 = 26%]). High-certainty evidence and heterogeneity for osteoarthritis may not be important. We were unable to conclude that clinically meaningful correlations did not exist for the other 15 factors owing to limited evidence (ie, wide CIs).
In the prespecified sensitivity analysis (eTable 1 in the Supplement), mean correlation with better physical function was estimated to be 0.20 (95% CI, 0.04 to 0.36; P = .02 vs coefficient = 0.14; 95% CI, 0.02 to 0.26 when including all studies). Mean correlation with BMI was estimated to be –0.17; 95% CI, –0.28 to –0.06; P < .001 vs coefficient = –0.15; 95% CI, –0.24 to –0.05 when including all studies), while mean correlation with mental health was estimated to be 0.13 (95% CI, –0.04 to 0.29; P = .02 vs coefficient = 0.12; 95% CI, –0.01 to 0.25 when including all studies), and mean correlation with osteoarthritis severity was estimated to be 0.10 (95% CI, –0.01 to 0.20; P = .05 vs coefficient = 0.10; 95% CI, 0.01 to 0.19 when including all studies).
For the secondary outcome, physical function 6 months after TKA, 9 studies with 5743 participants reported estimates on 20 potential factors.29,30,31,32,33,35,36,40,41 Estimated correlation coefficients from multivariate meta-analysis are in Figure 3.29,30,31,32,33,35,36,41 Mean correlation with more catastrophizing was estimated to be –0.19 (95% CI, –0.35 to –0.01; P = .03; P score = 63.4%; 2 studies35,41; very low–certainty evidence and substantial heterogeneity between reported estimates of association [I2 = 85%]), while mean correlation with walking use was estimated to be –0.31 (95% CI, –0.45 to –0.17; P < .001, P score = 84.1%; 2 studies31,41; high-certainty evidence and substantial heterogeneity between reported estimates of association [I2 = 63%]). Mean correlation with better physical function was estimated to be 0.37 (95% CI, 0.27 to 0.46; P < .001; P score = 90.4; 3 studies30,31,32; moderate-certainty evidence and substantial heterogeneity among reported estimates of association [I2 = 93%]), while mean correlation with better mental health was estimated to be 0.15 (95% CI, –0.08 to 0.36; P = .28; P score = 53.5; 3 studies30,31,41; high-certainty evidence and substantial heterogeneity among reported estimates of association [I2 = 81%]). We were unable to conclude that clinically meaningful correlations did not exist for the other 15 factors owing to limited evidence (ie, wide CIs). For the 3-month outcome, we were unable to perform multivariate meta-analysis, as shown in eTable 2 in the Supplement.
QUIPS domains most frequently assessed as at low risk of bias were prognostic factor measurement (16 studies23,24,25,26,27,28,30,31,32,33,37,38,39,40,41) and outcome measurement (17 studies22,23,24,25,26,27,28,30,31,32,33,34,35,36,37,38,40). For high risk of bias, QUIPS domains most often assessed were attrition (7 studies23,24,25,26,30,35,37) and statistical analysis (7 studies24,25,29,32,34,36,39), as shown in Figure 4.
Our GRADE certainty of evidence judgements are included in previously listed data and in eTable 4 in the Supplement. The most common reasons for downgrading certainty of evidence were risk of bias and imprecision.
Discussion
To our knowledge, this study is the first prespecified systematic review and meta-analysis using wide eligibility criteria and evaluating certainty of evidence to identify preoperative and intraoperative factors correlated with physical function at 12 months after TKA. Evidence from 7 observational studies22,24,25,26,27,28,37 suggested that higher BMI was correlated with poorer physical function 12 months after TKA and that better preoperative physical function and more severe osteoarthritis were correlated with better physical function 12 months after TKA. Importantly, our findings did not suggest that individual patients with a poor risk factor profile will not experience functional improvement if they undergo TKA. Our findings merely suggest that identified factors were correlated with poorer or better physical function in an absolute sense and may therefore be useful for guiding expectations about TKA outcomes.
We found moderate-certainty evidence for a correlation between higher preoperative BMI and worse function at 12 months, with equal correlation in the sensitivity analysis, in which studies judged to be at high risk of bias were removed. This finding is similar to that of another meta-analysis,13 in which participants without obesity reported lower rates of disability than participants with obesity. The evidence was not graded, however, and the study included retrospective studies with follow-up at 6 months to 10 years. Although we found a correlation between obesity and poorer physical function after TKA, patients with obesity still experience improved function from baseline48 and should thus be considered for surgery. However, the surgeon needs to consider the functional benefit against the risk for complications (eg, septic revisions are more prevalent in patients with severe obesity and super obesity49) for each patient and discuss these issues with the patient to encourage realistic expectations before proceeding with TKA.49
We found a correlation between better preoperative and better postoperative function at 12 months (low-certainty evidence) and 6 months (moderate-certainty evidence). The correlation remained, with increased coefficients, in the sensitivity analysis. It is not surprising that patients who were healthier before surgery may also have been healthier after surgery. However, our results conflict with those of a systematic review8 concluding that lower preoperative function was associated with better function 12 months after TKA. To resolve these conflicting findings, evidence is needed from well-conducted studies using standardized methods to measure factors and outcomes. We also estimated a correlation between more severe osteoarthritis (Kellgren-Lawrence grade) and better physical function at 12 months (high-certainty evidence) in multivariate meta-analysis and sensitivity analysis. These findings are consistent with those of a systematic review8 that included retrospective studies with follow-up extending to 1 year. Uncertainty remains regarding evidence for osteoarthritis severity as a factor associated with the outcome at 12 months.50,51
Major strengths of our study include following the recently revised Cochrane Handbook16 and guidelines for peer-reviewed protocols,14 including longitudinal prospective studies reporting associations at predefined times after TKA, and using multivariate meta-analysis when the number of factors was large compared with the number of studies.15 Several previous systematic reviews were unable to perform meta-analysis owing to heterogeneity associated with measurement issues, and others used vote counting, a method discouraged in current guidelines.16 We used recommended tools to assess risk of bias (QUIPS) and certainty of evidence (GRADE). Additionally, we prioritized transparency with the systematic use of prespecified methods documented in the protocol,14 preprint,15 and this article’s supplemental materials.
Limitations
This study has several limitations. To obtain trustworthy estimates without prejudging which factors may have been associated with the outcome, we included a wide range of factors but only from prospective studies reporting associations at specific postoperative times. This necessarily included estimates from studies measuring factors using a range of methods, and so we accounted for heterogeneity in our random-effects meta-analyses. Less heterogeneity was observed across studies using a common measure, particularly 9 studies that used WOMAC to measure physical function. Narrower inclusion criteria could increase the potential for excluding important evidence.16 Some studies had large sample sizes and therefore provided precise estimates (ie, narrow CIs). I2 may be misleading when study estimates are very precise because it is statistically easier to distinguish (ie, detect heterogeneity) between study estimates. In this situation, it is important to consider the degree to which study estimates vary from one another and whether this is clinically important, rather than relying solely on I2. In particular, I2 from prognostic studies may be misleading so I2 statistics should be interpreted cautiously.18 Because studies with high risk of bias can lead to biased main results and heterogeneity, we performed prespecified sensitivity analyses and excluded studies assessed as high risk for each QUIPS domain.14 We planned to perform analyses of nonreporting bias, small study effects, and subgroup analyses,14 but the number of included studies did not meet our prespecified threshold.
We also downgraded certainty of evidence if we judged studies to be at risk of bias. Several studies11,52,53,54 had insufficient reporting of important QUIPS domains (such as attrition and statistical analysis), thus lowering the certainty that study estimates were unbiased. We suggest that researchers use tools like QUIPS at the study design stage to encourage low risk of bias in their findings regarding prognostic factors. This review identified some key areas for future research. Uncertainty remains regarding which patients may benefit most from TKA. Because patient preoperative status (ie, BMI, physical function, and osteoarthritis severity) may be correlated with overall outcomes, evidence from high-quality studies is fundamental for developing a prediction model to better identify patients at increased risk of poor outcomes after TKA. Prehabilitation interventions to improve modifiable factors (eg, better mental health) are not well-established.55,56 We could not synthesize data for a number of factors given that they were studied only once. For these and other factors and outcomes, such as associations between physical function during the first year after TKA and biomechanical aspects of surgery (eg, implant) or pain management, evidence is lacking, highlighting the need for research from these perspectives with appropriate design and power. Additionally, our study provided evidence at the population level not at the level of individual patients. Our results are important for investigating factors to include in predictive models but should be used with caution at the individual level.
Conclusions
This study found that there is evidence (with moderate certainty) that higher BMI was correlated with worse physical function and that better physical function (low-certainty evidence) and more severe osteoarthritis (high-certainty evidence) were correlated with better physical function 12 months after TKA. Our findings suggest that these factors should be included in development of predictive models aimed at identifying patients at increased risk of poor function after TKA.
eMethods. Multivariate Meta-analysis
eFigure 1. Sensitivity Analysis
eFigure 2. Exploring Potential Inconsistency at 6 and 12 mo
eFigure 3. Univariate Meta-analysis
eTable 1. Sensitivity Analysis
eTable 2. Reported Associations at 3 mo After TKA
eTable 3. Definition and Labels of Factors
eTable 4. Grading of Recommendation Assessment, Development and Evaluation
eTable 5. Search Strategy
eTable 6. Reason for Exclusion of Individual Studies
References
- 1.Agency for Healthcare Research and Quality . Healthcare cost and utilization project (HCUP). Accessed June 21, 2022. http://www.hcup-us.ahrq.gov [PubMed]
- 2.Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100(17):1455-1460. doi: 10.2106/JBJS.17.01617 [DOI] [PubMed] [Google Scholar]
- 3.Price AJ, Alvand A, Troelsen A, et al. Knee replacement. Lancet. 2018;392(10158):1672-1682. doi: 10.1016/S0140-6736(18)32344-4 [DOI] [PubMed] [Google Scholar]
- 4.Wieczorek M, Rotonda C, Guillemin F, Rat AC. What have we learned about the course of clinical outcomes after total knee or hip arthroplasty? Arthritis Care Res (Hoboken). 2020;72(11):1519-1529. doi: 10.1002/acr.24045 [DOI] [PubMed] [Google Scholar]
- 5.Maradit Kremers H, Kremers WK, Berry DJ, Lewallen DG. Patient-reported outcomes can be used to identify patients at risk for total knee arthroplasty revision and potentially individualize postsurgery follow-up. J Arthroplasty. 2017;32(11):3304-3307. doi: 10.1016/j.arth.2017.05.043 [DOI] [PubMed] [Google Scholar]
- 6.Weber M, Renkawitz T, Voellner F, et al. Revision surgery in total joint replacement is cost-intensive. Biomed Res Int. 2018;2018:8987104. doi: 10.1155/2018/8987104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorel JC, Veltman ES, Honig A, Poolman RW. The influence of preoperative psychological distress on pain and function after total knee arthroplasty: a systematic review and meta-analysis. Bone Joint J. 2019;101-B(1):7-14. doi: 10.1302/0301-620X.101B1.BJJ-2018-0672.R1 [DOI] [PubMed] [Google Scholar]
- 8.Harmelink KEM, Zeegers AVCM, Hullegie W, Hoogeboom TJ, Nijhuis-van der Sanden MWG, Staal JB. Are there prognostic factors for one-year outcome after total knee arthroplasty: a systematic review. J Arthroplasty. 2017;32(12):3840-3853.e1. doi: 10.1016/j.arth.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 9.Vissers MM, Bussmann JB, Verhaar JA, Busschbach JJ, Bierma-Zeinstra SM, Reijman M. Psychological factors affecting the outcome of total hip and knee arthroplasty: a systematic review. Semin Arthritis Rheum. 2012;41(4):576-588. doi: 10.1016/j.semarthrit.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Dai X, Li L, Chen Z, Cui H, Lv S. Patellar resurfacing versus nonresurfacing in total knee arthroplasty: an updated meta-analysis of randomized controlled trials. J Orthop Surg Res. 2021;16(1):83. doi: 10.1186/s13018-020-02185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santaguida PL, Hawker GA, Hudak PL, et al. Patient characteristics affecting the prognosis of total hip and knee joint arthroplasty: a systematic review. Can J Surg. 2008;51(6):428-436. [PMC free article] [PubMed] [Google Scholar]
- 12.Shohat N, Heller S, Sudya D, et al. Mild radiographic osteoarthritis is associated with increased pain and dissatisfaction following total knee arthroplasty when compared with severe osteoarthritis: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2022;30(3):965-981. doi: 10.1007/s00167-021-06487-x [DOI] [PubMed] [Google Scholar]
- 13.Pozzobon D, Ferreira PH, Blyth FM, Machado GC, Ferreira ML. Can obesity and physical activity predict outcomes of elective knee or hip surgery due to osteoarthritis: a meta-analysis of cohort studies. BMJ Open. 2018;8(2):e017689. doi: 10.1136/bmjopen-2017-017689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen U, Lindberg MF, Denison EM, et al. Predictors of chronic pain and level of physical function in total knee arthroplasty: a protocol for a systematic review and meta-analysis. BMJ Open. 2020;10(9):e037674. doi: 10.1136/bmjopen-2020-037674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose CJ, Olsen U, Lindberg MF, Denison EML, Aamodt A, Lerdal A. A new multivariate meta-analysis model for many variates and few studies. arXiv. Published online September 24, 2020. Updated February 12, 2021. doi: 10.48550/arXiv.2009.11808 [DOI]
- 16.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Cochrane. Accessed May 14, 2021. https://training.cochrane.org/handbook/archive/v6.2 [Google Scholar]
- 17.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280-286. doi: 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 18.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870 [DOI] [PubMed] [Google Scholar]
- 19.Schünemann HBJ, Brozek J, Guyatt G, Oxman A, eds. GRADE Handbook. The GRADE Working Group; 2013. Accessed May 4, 2020. https://gdt.gradepro.org/app/handbook/handbook.html [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. John Wiley & Sons; 2009. doi: 10.1002/9780470743386 [DOI] [Google Scholar]
- 21.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowsey MM, Nikpour M, Dieppe P, Choong PF. Associations between pre-operative radiographic changes and outcomes after total knee joint replacement for osteoarthritis. Osteoarthritis Cartilage. 2012;20(10):1095-1102. doi: 10.1016/j.joca.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 23.Lingard EA, Riddle DL. Impact of psychological distress on pain and function following knee arthroplasty. J Bone Joint Surg Am. 2007;89(6):1161-1169. doi: 10.2106/00004623-200706000-00002 [DOI] [PubMed] [Google Scholar]
- 24.Nankaku M, Ito H, Furu M, et al. Preoperative factors related to the ambulatory status at 1 year after total knee arthroplasty. Disabil Rehabil. 2018;40(16):1929-1932. doi: 10.1080/09638288.2017.1323025 [DOI] [PubMed] [Google Scholar]
- 25.Sullivan M, Tanzer M, Reardon G, Amirault D, Dunbar M, Stanish W. The role of presurgical expectancies in predicting pain and function one year following total knee arthroplasty. Pain. 2011;152(10):2287-2293. doi: 10.1016/j.pain.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 26.Tilbury C, Haanstra TM, Verdegaal SHM, et al. Patients’ pre-operative general and specific outcome expectations predict postoperative pain and function after total knee and total hip arthroplasties. Scand J Pain. 2018;18(3):457-466. doi: 10.1515/sjpain-2018-0022 [DOI] [PubMed] [Google Scholar]
- 27.van de Water RB, Leichtenberg CS, Nelissen RGHH, et al. Preoperative radiographic osteoarthritis severity modifies the effect of preoperative pain on pain/function after total knee arthroplasty: results at 1 and 2 years postoperatively. J Bone Joint Surg Am. 2019;101(10):879-887. doi: 10.2106/JBJS.18.00642 [DOI] [PubMed] [Google Scholar]
- 28.Wylde V, Dixon S, Blom AW. The role of preoperative self-efficacy in predicting outcome after total knee replacement. Musculoskeletal Care. 2012;10(2):110-118. doi: 10.1002/msc.1008 [DOI] [PubMed] [Google Scholar]
- 29.Bugada D, Allegri M, Gemma M, et al. Effects of anaesthesia and analgesia on long-term outcome after total knee replacement: a prospective, observational, multicentre study. Eur J Anaesthesiol. 2017;34(10):665-672. doi: 10.1097/EJA.0000000000000656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escobar A, Quintana JM, Bilbao A, et al. Effect of patient characteristics on reported outcomes after total knee replacement. Rheumatology (Oxford). 2007;46(1):112-119. doi: 10.1093/rheumatology/kel184 [DOI] [PubMed] [Google Scholar]
- 31.Pua YH, Poon CL, Seah FJ, et al. Predicting individual knee range of motion, knee pain, and walking limitation outcomes following total knee arthroplasty. Acta Orthop. 2019;90(2):179-186. doi: 10.1080/17453674.2018.1560647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugawara Y, Ishijima M, Kurosawa H, et al. Preoperative timed single leg standing time is associated with the postoperative activity of daily living in aged disabled patients with end-stage knee osteoarthritis at six-months after undergoing total knee arthroplasty. Mod Rheumatol. 2017;27(2):326-331. doi: 10.1080/14397595.2016.1192759 [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi M, Sawano S, Kugo M, Maegawa S, Kawasaki T, Ichihashi N. Physical activity promotes gait improvement in patients with total knee arthroplasty. J Arthroplasty. 2016;31(5):984-988. doi: 10.1016/j.arth.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 34.Lindner M, Nosseir O, Keller-Pliessnig A, Teigelack P, Teufel M, Tagay S. Psychosocial predictors for outcome after total joint arthroplasty: a prospective comparison of hip and knee arthroplasty. BMC Musculoskelet Disord. 2018;19(1):159. doi: 10.1186/s12891-018-2058-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hylkema TH, Stevens M, Selzer F, Amick BA, Katz JN, Brouwer S. Activity impairment and work productivity loss after total knee arthroplasty: a prospective study. J Arthroplasty. 2019;34(11):2637-2645. doi: 10.1016/j.arth.2019.06.015 [DOI] [PubMed] [Google Scholar]
- 36.Engel C, Hamilton NA, Potter PT, Zautra AJ. Impact of two types of expectancy on recovery from total knee replacement surgery (TKR) in adults with osteoarthritis. Behav Med. 2004;30(3):113-123. doi: 10.3200/BMED.30.3.113-123 [DOI] [PubMed] [Google Scholar]
- 37.Berghmans DDP, Lenssen AF, Emans PJ, van Rhijn LW, de Bie RA. Limited predictive value of pre-surgical level of functioning for functioning at 3 and 12 months after TKA. Knee Surg Sports Traumatol Arthrosc. 2019;27(5):1651-1657. doi: 10.1007/s00167-018-5288-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindberg MF, Schweitz TU, Aamodt A, Gay C, Lerdal A. High pre- and postoperative symptom burden in non-responders to total knee arthroplasty. PLoS One. 2020;15(5):e0233347. doi: 10.1371/journal.pone.0233347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo ZY, Li LL, Wang D, Wang HY, Pei FX, Zhou ZK. Preoperative sleep quality affects postoperative pain and function after total joint arthroplasty: a prospective cohort study. J Orthop Surg Res. 2019;14(1):378. doi: 10.1186/s13018-019-1446-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oka T, Ono R, Tsuboi Y, et al. Effect of preoperative sedentary behavior on clinical recovery after total knee arthroplasty: a prospective cohort study. Clin Rheumatol. 2020;39(3):891-898. doi: 10.1007/s10067-019-04849-y [DOI] [PubMed] [Google Scholar]
- 41.Yang HY, Losina E, Lange JK, Katz JN, Collins JE. Longitudinal trajectories of pain and function improvement following total knee replacement. ACR Open Rheumatol. 2019;1(5):308-317. doi: 10.1002/acr2.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lingard EA, Katz JN, Wright EA, Sledge CB; Kinemax Outcomes Group . Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am. 2004;86(10):2179-2186. doi: 10.2106/00004623-200410000-00008 [DOI] [PubMed] [Google Scholar]
- 43.Amusat N, Beaupre L, Jhangri GS, et al. Diabetes that impacts on routine activities predicts slower recovery after total knee arthroplasty: an observational study. J Physiother. 2014;60(4):217-223. doi: 10.1016/j.jphys.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 44.Jain D, Nguyen LL, Bendich I, et al. Higher patient expectations predict higher patient-reported outcomes, but not satisfaction, in total knee arthroplasty patients: a prospective multicenter study. J Arthroplasty. 2017;32(9S):S166-S170. doi: 10.1016/j.arth.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 45.Papakostidou I, Dailiana ZH, Papapolychroniou T, et al. Factors affecting the quality of life after total knee arthroplasties: a prospective study. BMC Musculoskelet Disord. 2012;13:116. doi: 10.1186/1471-2474-13-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belford K, Gallagher N, Dempster M, et al. Psychosocial predictors of outcomes up to one year following total knee arthroplasty. Knee. 2020;27(3):1028-1034. doi: 10.1016/j.knee.2020.03.006 [DOI] [PubMed] [Google Scholar]
- 47.Sharma S, Kumar V, Sood M, Malhotra R. Effect of preoperative modifiable psychological and behavioural factors on early outcome following total knee arthroplasty in an Indian population. Indian J Orthop. 2021;55(4):939-947. doi: 10.1007/s43465-020-00325-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overgaard A, Lidgren L, Sundberg M, Robertsson O, W-Dahl A. Patient-reported 1-year outcome not affected by body mass index in 3,327 total knee arthroplasty patients. Acta Orthop. 2019;90(4):360-365. doi: 10.1080/17453674.2019.1604940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhry H, Ponnusamy K, Somerville L, McCalden RW, Marsh J, Vasarhelyi EM. Revision rates and functional outcomes among severely, morbidly, and super-obese patients following primary total knee arthroplasty: a systematic review and meta-analysis. JBJS Rev. 2019;7(7):e9. doi: 10.2106/JBJS.RVW.18.00184 [DOI] [PubMed] [Google Scholar]
- 50.Chen H, Li S, Ruan T, Liu L, Fang L. Is it necessary to perform prehabilitation exercise for patients undergoing total knee arthroplasty: meta-analysis of randomized controlled trials. Phys Sportsmed. 2018;46(1):36-43. doi: 10.1080/00913847.2018.1403274 [DOI] [PubMed] [Google Scholar]
- 51.Almeida GJ, Khoja SS, Zelle BA. Effect of prehabilitation in older adults undergoing total joint replacement: an overview of systematic reviews. Curr Geriatr Rep. 2020;9(4):280-287. doi: 10.1007/s13670-020-00342-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agrawal M, Jain V, Yadav VP, Bhardwaj V. Patellar resurfacing in total knee arthroplasty. J Clin Orthop Trauma. 2011;2(2):77-81. doi: 10.1016/S0976-5662(11)60048-9 [DOI] [Google Scholar]
- 53.Tibbo ME, Limberg AK, Salib CG, et al. Acquired idiopathic stiffness after total knee arthroplasty: a systematic review and meta-analysis. J Bone Joint Surg Am. 2019;101(14):1320-1330. doi: 10.2106/JBJS.18.01217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Jonbergen HP, Reuver JM, Mutsaerts EL, Poolman RW. Determinants of anterior knee pain following total knee replacement: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):478-499. doi: 10.1007/s00167-012-2294-x [DOI] [PubMed] [Google Scholar]
- 55.Bay S, Kuster L, McLean N, Byrnes M, Kuster MS. A systematic review of psychological interventions in total hip and knee arthroplasty. BMC Musculoskelet Disord. 2018;19(1):201. doi: 10.1186/s12891-018-2121-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whale K, Wylde V, Beswick A, Rathbone J, Vedhara K, Gooberman-Hill R. Effectiveness and reporting standards of psychological interventions for improving short-term and long-term pain outcomes after total knee replacement: a systematic review. BMJ Open. 2019;9(12):e029742. doi: 10.1136/bmjopen-2019-029742 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Multivariate Meta-analysis
eFigure 1. Sensitivity Analysis
eFigure 2. Exploring Potential Inconsistency at 6 and 12 mo
eFigure 3. Univariate Meta-analysis
eTable 1. Sensitivity Analysis
eTable 2. Reported Associations at 3 mo After TKA
eTable 3. Definition and Labels of Factors
eTable 4. Grading of Recommendation Assessment, Development and Evaluation
eTable 5. Search Strategy
eTable 6. Reason for Exclusion of Individual Studies