Abstract
Social behaviour is an essential component of human life and deficits in social function are seen across multiple psychiatric conditions with high morbidity. However, there are currently no FDA-approved treatments for social dysfunction. Since social cognition and behaviour rely on multiple signalling processes acting in concert across various neural networks, treatments aimed at social function may inherently require a combinatorial approach. Here, we describe the social neurobiology of the oxytocin and endocannabinoid signalling systems as well as translational evidence for their use in treating symptoms in the social domain. We leverage this systems neurobiology to propose a network-based framework that involves pharmacology, psychotherapy, non-invasive brain stimulation and social skills training to combinatorially target trans-diagnostic social impairment. Lastly, we discuss the combined use of oxytocin and endocannabinoids within our proposed framework as an illustrative strategy to treat specific aspects of social function. Using this framework provides a roadmap for actionable treatment strategies for neuropsychiatric social impairment.
This article is part of the theme issue ‘Interplays between oxytocin and other neuromodulators in shaping complex social behaviours’.
Keywords: endocannabinoids, social behaviour, social cognition, oxytocin, combinatorial
1. Understanding neural mechanisms of social behaviour
Social function is crucial to survival, and is evolutionarily conserved in many animals, from nematodes to humans [1–7]. Animals with simpler nervous systems engage in social behaviours such as courtship, mating, parenting and aggression, and animals with greater neural complexity exhibit more complex social behaviours such as alliance formation, cooperative hunting, empathy and altruism [8–10]. In humans, social behaviour relies on multiple processes that can be conceptualized into overlapping domains such as social anxiety, reward and attachment.
The importance of social behaviour is underscored by the significant impact of social isolation and social stress on human health [11,12]. Social isolation leads to pro-inflammatory gene expression [13], increases overall mortality [14–16], is linked to functional decline [17], and increases cardiovascular risk [18]. Aversive social environments have serious negative impacts on health [19,20] and can decrease the efficacy of mental health treatment [21,22]. Many psychiatric conditions such as depression, anxiety and schizophrenia show early social impairment and isolation, which further contribute to functional decline [23,24]. Other conditions such as autism spectrum disorder (ASD) and borderline personality disorder exhibit symptoms that are cardinal in the social domain [25–27].
There has been a concerted effort to understand the mechanisms that enable social cognition—the processing and integration of multisensory inputs to represent social information and control social behaviour. Many lines of research have established that social cognition requires the integration of multiple signalling networks such as those involving dopamine, serotonin, oxytocin and endocannabinoids [3,10,12,28,29]. Here, we focus on describing the social neurobiology of oxytocinergic and endocannabinoid signalling, which provides important principles to design combinatorial treatment approaches. We then expand on these principles to encompass various treatment modalities (such as psychotherapy and brain stimulation) and propose a network-based, mechanistic framework to design combinatorial treatment approaches with improved specificity and efficacy.
2. Oxytocin in the control of social behaviour
An extensive body of research indicates that oxytocin signalling plays a central role in mediating social behaviours across multiple species [2,30–33]. Oxytocin is a neuropeptide that is produced in the hypothalamus and acts both centrally and peripherally, via a combination of local and hormonal transmission, to facilitate critical social functions such as mother–child bonding and nurturing, pair-bonding, social recognition and social reward [30,34–39]. Central oxytocinergic signalling is thought to be composed of oxytocin neurons in the hypothalamus [40–42] and their synaptic and/or diffuse oxytocin-receptor-mediated actions in specific neural circuits spanning many cortical and subcortical regions [36,40,43–49]. Oxytocin is hypothesized to amplify the salience of social stimuli via sensory circuits, temper social anxiety via hypothalamic and limbic circuitry, and enhance social reward via signalling in ventral tegmental area and nucleus accumbens [36,50,51].
More recent cognitive and translational neuroscience studies have suggested that oxytocin impacts higher-order forms of social cognition in humans. In an experimental cooperative setting, intranasal oxytocin increases trust and willingness to accept social risks [52], depending on the kind of social information available [53]. Importantly, exogenous oxytocin does not always effect positive valence, as oxytocin administration in other contexts can increase aggression, envy, gloating, and both in- and out-group bias [54–56]. Further, the effect of oxytocin in the brain is modulated by epigenetic regulation of oxytocin receptors [57] and oxytocin can cause sexually dimorphic neuronal and behavioural effects [58]. Overall, oxytocin signalling shapes complex rodent, non-human primate, and human social behaviours and can amplify social salience and reward in specific social contexts [59,60].
However, as a treatment strategy, exogenously administered oxytocin has yielded mixed results in terms of clinical efficacy. Intranasal oxytocin improved emotional recognition in adults and children with ASD [61–63] and facilitated pro-social behaviour [64] and social responsiveness [65] in children with ASD; however, these results are inconsistent [66,67]. Oxytocin also enhanced social interaction in patients with social anxiety disorder, but this effect was specific to a social setting with explicit priming [68]. Overall, multiple clinical studies have been performed, with varying results [69], and studies of oxytocin have not yielded any U.S. Food and Drug Administration (FDA)-approved treatments for the social domain. In fact, oxytocin may be limited as a treatment modality as it does not cross the blood–brain barrier well, must be administered intranasally, and has short-lived effects [70–72]. However, given the effects seen with oxytocin administration across model systems, there remains enthusiasm that manipulating the oxytocin system holds promise to treat characteristic social symptoms in ASD and other psychiatric conditions [73,74]. Understanding how other signalling systems in the brain interact with oxytocin to modulate social behaviour and accordingly applying new technologies towards circuit-based treatment has emerged as a promising approach [75].
3. Endocannabinoids in the control of social behaviour
Another promising signalling target for impacting social function is the endocannabinoid system. While cannabinoids have long been used in social ritual [76,77], only more recently has the important role of endocannabinoid signalling in the control of specific aspects of social behaviour been mechanistically demonstrated. In animal and human studies, evidence for its roles span social stress reactivity, social reward and social development, and its dysregulation is linked to neuropsychiatric social impairment. The molecular and systems neurobiology of endocannabinoid signalling provides evidence of its importance to social behaviour and provide context for the insights from human studies on cannabinoids. For more comprehensive reviews on cannabinoid-based drug development as well as the neurobiology of endocannabinoid signalling in the control of social behaviour, see Wei et al. [77,78]; here we focus on clinical studies and network principles important to inform therapeutic development in the social domain, particularly towards combinatorial approaches. The endocannabinoid system is a modulatory neurotransmitter system consisting of three main components: (i) two lipid-derived local messengers—2-arachidonoyl-sn-glycerol (2-AG) and anandamide; (ii) enzymes and transporters that mediate their formation and elimination; and (iii) cannabinoid receptors that are activated by endocannabinoids and regulate neuronal activity (mainly, albeit not exclusively, via CB1 cannabinoid receptors (CB1) in the brain [79,80].
The endocannabinoid system mediates the effects of the psychoactive molecule in Cannabis sativa, a plant that contains a family of chemically related terpene-like molecules, which include delta-9-tetrahydrocannabinol (Δ-9-THC). Δ-9-THC has a complex set of pharmacological properties stemming from its binding to cannabinoid receptors expressed throughout the human body. The binding of Δ-9-THC to CB1 receptors in the brain causes a characteristic acute mental state often subjectively described as a combination of enhanced sociability, quickened mental associations, heightened sensitivity to certain sensory stimuli (e.g. sounds or colours), alterations in the perception of time and space, and increased appetite for sweet and fatty foods [76]. It has also been shown to lead to states of anxiety or paranoia [81]. These behavioural effects are broadly consistent with the demonstrated roles of the endocannabinoid system.
The brain distribution of components of the endocannabinoid system is consistent with its role in social behaviour. CB1 receptors are highly expressed in associational cortical regions of the frontal lobe and subcortical structures such as the amygdala and hippocampus that underpin human socio-emotional functioning [82,83]. However, the action of CB1 receptors is determined by many factors, including their cellular subtype, activity of nearby interacting proteins such as cannabinoid receptor-interacting protein 1A (CRIP1A), and associations with varying G-coupled protein receptors [84,85]. CB1 receptors are also expressed throughout regions implicated in reward and addiction, including the central and basolateral amygdala, prefrontal cortex (PFC), hippocampus, dorsolateral striatum, ventral tegmental area, and, to a lesser extent, the nucleus accumbens [82,86]. These regions have also been considered key parts of the brain that mediate aspects of social cognition, based on imaging and network studies [83]. The regional distribution of the aforementioned endocannabinoid-synthesizing and -degradative enzymes is associated with that of CB1 receptors [86–88]. The specificity of endocannabinoid signalling likely relies on the convergence of receptor distribution, ligand chemical identity (both endogenous and exogenous), the differential expression of regulatory enzymes, as well as downstream second-messenger cascades [89].
As with oxytocin, multiple lines of research have demonstrated diverging effects on social interaction, likely owing to differential recruitment of circuits such as for social anxiety and social reward. Early studies in rodents demonstrated the ability of Δ-9-THC to modulate aggressive behaviour, fight or flight responses, and sexual behaviour, depending on the sex, dose and behavioural context [90–92]. In an early experiment in rhesus monkeys in which one observer was required to respond based on the facial expression of a ‘stimulus’ conspecific, Δ-9-THC enhanced discrimination of facial expressions in observers while impairing the ability to display facial expressions in ‘stimulus’ conspecifics [93]. Later studies employed genetic approaches to evaluate the role of this system in social behaviour. CB1 receptor knock-out (CB1-KO) mice showed increased aggression in a familiar environment but decreased sociability in an unfamiliar environment [94]. CB1-KO mice also showed increased social anxiety and enhanced levels of social memory [95]. CB1-KO mice also showed increased aggression, but giving a CB1 agonist ACEA was sufficient to decrease aggression in OF1 mice [96]. A more recent study showed altered ultrasonic vocalization communication and deficits in social interest and social interaction in CB1-KO mice [97]. Interestingly, over-expressing the CB1 receptor in the medial prefrontal cortex (mPFC) decreased social interaction with an unfamiliar animal [98]. A recent study on capuchin monkeys found that pharmacological disruption of CB1 signalling reduced spontaneous social interaction, corroborating a conserved role for endocannabinoid signalling in social behaviour [99]. While studies of cellular specificity are lacking, knockout in ventral hippocampus GABA neurons has been shown to enhance social investigation while knockout in glutamatergic neurons reduced interaction [100,101]. While wholesale manipulation of CB1 receptors produce dichotomous results on social interaction, more consistent effects at the ligand- and region-specific level support this hypothesis of circuit selectivity. For example, a direct CB1 receptor agonist reduced social play while an indirect cannabinoid agonist enhanced social play [102].
The role of endocannabinoids has also been elucidated through interruption of the enzymes involved in the synthesis and/or degradation of its signalling molecules. For example, targeted deletion of diacylglycerol lipase α (DGLα), the primary synthetic enzyme of 2-AG, decreased levels of 2-AG and led to deficits in social interaction [103]. Deletion of FAAH (the primary degradative enzyme for anandamide), thereby potentiating anandamide signalling, increased time spent in social interaction and enhanced social reward [104]. This effect was driven by circuit-selective oxytocin signalling and stands in contrast to global reduction of 2-AG signalling, which generalized to impair other natural rewards such as food (and was not driven by oxytocin) [105]. Particularly in contrast to receptor agonism, these studies highlight the flexibility of this system to modulate social behaviour based on ligand-, context- and circuit-specific mechanisms.
Neurodevelopmental studies of the endocannabinoid system also underscore its important role in social development and properly tuned network signalling. Developmental exposure to cannabinoid agents persistently influences the expression of social behaviour later in adulthood, which is particularly salient for addictive and psychotic disorders [106,107]. The expression of CB1 receptors throughout regions of the social brain network is remarkably dynamic—it reaches a peak during adolescence, and decreases into adulthood [106] (though some rodent studies suggest it can increase to adulthood [108]). Rather than simply modulating the development of other social brain systems, endocannabinoid signalling appears to play a central role in mediating the social transition between adolescence and adulthood [109–111]. Although CB1 receptor expression does not necessarily determine its function, this change implies an important role of changing receptor expression in development. Indeed, enhanced CB1 activity in rats prolonged the adolescent behavioural repertoire, including increased impulsivity and social play, into adulthood [112]. While the developmental trajectories of endocannabinoids are less clear, endocannabinoid levels have been shown to be dependent on age as well as region of activity [109]. Lastly, endocannabinoid activity regulates processes such as neuronal genesis, migration and differentiation, as well as in synaptic pruning and glial cell formation [106,113]. Together, these studies suggest that endocannabinoid signalling is crucial for social development and appropriately flexible network signalling.
Human studies also emphasize context dependence and network selectivity. Early studies showed that cannabis significantly decreased feelings of hostility and increased socio-emotional communication, social interaction time, and group problem solving; however, its effect on social cognition was dependent on dose, environment and situation [114–117]. Later studies showed that this effect on reducing threat perception and enhancing socio-emotional regulation is likely mediated through enhancing the connectivity in the mPFC/ACC–amygdala network [118]. Interestingly, functional impairments in this same network have been linked to socio-emotional dysregulation and aggression in borderline personality disorder, a condition characterized by impaired social cognition [119–121]. Borderline personality disorder has also been associated with elevated FAAH binding and decreased endocannabinoid tone in the PFC [122].
Genetic studies show that single nucleotide polymorphisms (SNPs) within the genes for CB1 receptor and FAAH are associated with altered social gaze, reactivity to threat, and altered neuronal signalling in the PFC and amygdala [123–125]. Likewise, decreased serum levels of endocannabinoids have been found in individuals with depression [126,127] and children with ASD [128] while increased levels have been found in schizophrenia [129]. Indeed, dysregulated endocannabinoid signalling has now been implicated in a variety of psychiatric conditions [125,130–133].
Preclinical studies have examined the therapeutic potential of targeting the endocannabinoid system to ameliorate social dysfunction. Consistently, selective potentiation of either anandamide or 2-AG signalling—through pharmacological inhibition of their respective degradative enzymes (FAAH and MGL)—ameliorated social deficits in various monogenic, polygenic, immunogenic and other animal models related to ASD [134–137]. Cannabidiol (CBD), another psychoactive compound found in cannabis, whose mechanism of action is poorly understood, is hypothesized to enhance endocannabinoid signalling through inhibition of the reuptake and enzymatic degradation of anandamide [138] (though it is thought to also influence activity at a number of receptors, including vanilloid receptors and serotonin receptors 5-hydroxytryptamine [139]) and has been shown to enhance social interaction [140] and reduce isolation-induced aggression [141] in rodents.
Supporting therapeutic potential, clinical studies indicate that enhancement of endocannabinoid signalling may treat social anxiety and post-traumatic stress disorder (PTSD) as well as several facets of ASD, and these approaches are undergoing clinical development [142–144]. Findings thus far, while mixed, are promising, as cannabinoids appear to improve ASD-associated symptoms such as caregiver-rated social responsiveness and decrease polypharmacy burden [143]. Recently, administration of a selective FAAH inhibitor resulted in significant improvements in social anxiety over a placebo in a randomized control trial with 149 patients [145]. However, FAAH inhibitors, CBD and compounds like nabiximols (purified plant extract of 1 : 1 ratio of CBD and D9-THC) have controversial results with mixed evidence of efficacy in social anxiety and ASD [139,142,143] and may have different mechanisms of action in brains of patients with ASD [146]. In a related line of work, plasma anandamide concentrations were found to be lower in children with ASD [147].
In sum, multiple lines of evidence suggest that the endocannabinoid system is important in social development and behaviour across rodents, non-human primates and humans. Various molecular and circuit mechanisms of endocannabinoid signalling have been demonstrated in the regulation of social behaviour. A theme in this literature is that endocannabinoid potentiation leads to variable effects, which is likely due to its context-, dose- and circuit-dependence—a better understanding of the mechanisms selective for ameliorating social impairment may be harnessed to design more effective treatment strategies.
4. Designing combination treatments for social impairment
As described, therapeutic engagement of the oxytocin or endocannabinoid system exhibits varying efficacy and specificity based on the social and environmental context. This highlights that multifunctionality and redundancy are continuing challenges of creating targeted therapies for social cognition [148]. One general solution to improve efficacy and enhance specificity of treatment in medicine is well-validated, combination pharmacological approaches [148]. Here, we expand on this principle to encompass multimodal combination therapies beyond dual pharmacology approaches. We propose that pharmacology, brain stimulation and psychosocial treatments can be combined in a network-based, mechanistic framework to enhance treatment specificity and efficacy. Here, we describe biological principles of multisystem signalling, describe current combination treatment approaches used in psychiatry today, and provide examples of modalities that can be used in combination approaches for social impairment and their potential network mechanisms.
Various aspects of social behaviour have been shown to rely on an overlapping network of signalling involving molecules such as oxytocin, dopamine, serotonin, opioids and endocannabinoids acting across multiple brain regions (which are also involved in non-social states such as general anxiety) [3,24,33,36]. This combinatorial biology suggests that effective treatment of social dysfunction may require combination approaches that parallel social brain physiology. Neural networks for motivated behaviours, including brain regions such as the amygdala, hypothalamus, ventral tegmental area and nucleus accumbens, also process social information [149], and advances in connectomics and brain-wide imaging reveal diverse multisystem connectivity for social cognition [150,151]. This overlapping connectivity has been demonstrated in principle to result in synergistic effects in potentially clinically relevant treatments targeting the social domain. For example, in an experiment in which non-human primates were given oxytocin alone or oxytocin in combination with an opioid antagonist, the combination treatment was found to enhance social attention in a supralinear manner when compared with oxytocin alone [152]. This provides one example of combining oxytocin with engagement of other signalling networks in order to enhance specific effects on social cognition [153].
In fact, combination pharmacological approaches are widely used in psychiatry for depression, anxiety, schizophrenia and other conditions in cases in which the use of one agent is ineffective [154–157]. In some cases, treatment resistance to a pharmacological agent with a particular molecular mechanism is thought to arise as a result of compensatory mechanisms that engage a second molecular mechanism [158]. In this case combination approaches can (a) target such alternative mechanisms, (b) potentiate an existing mechanism of action or (c) mitigate potential adverse or dependence effects of the primary pharmacological agent to allow its titration [159–162]. More broadly, treatment resistance in this context can be expanded to also include psychological resistance, which is an expected part of the therapeutic process [163,164]. In this multi-factorial context, a network-based view for convergent combination therapy would provide a grounding framework to account for and overcome such resistance holistically and enhance treatment strategies (figure 1).
Figure 1.
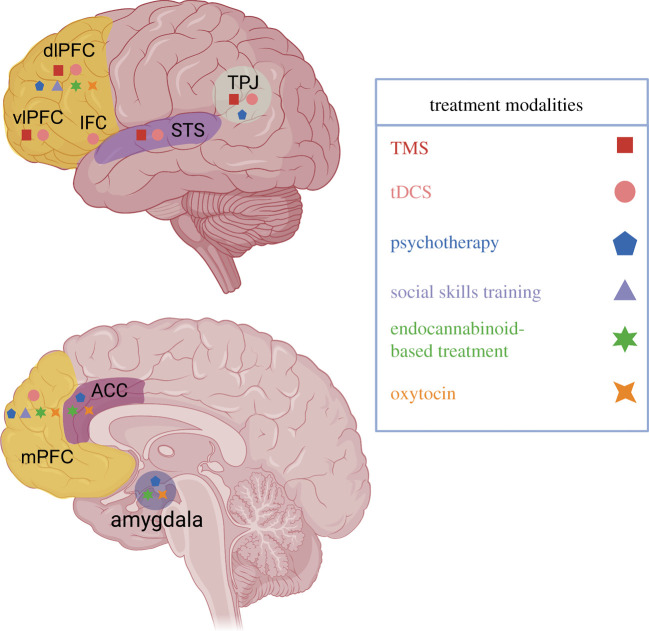
Target regions for network-based combinatorial treatment strategies. Selected treatment modalities act at overlapping and distinct brain regions to drive activity in networks that influence social behaviour. Regions in which these treatment modalities have shown the ability to drive social effects include subregions of the prefrontal cortex (dorsolateral prefrontal cortex (dlPFC), vlPFC, inferior frontal cortex (IFC) and medial prefrontal cortex (mPFC)), as well as the anterior cingulate cortex (ACC), superior temporal sulcus (STS), temporoparietal junction (TPJ) and amygdala. We propose that different modalities can be combined using their profile in this framework in order to achieve more specific treatment of social symptoms. This figure was made with Biorender. (Online version in colour.)
5. Non-invasive brain stimulation for treating social impairment
Other tools in psychiatry can also be incorporated in a combinatorial approach such as brain stimulation. Non-invasive brain stimulation (NIBS) is an example of a demonstrably effective non-pharmacological option for conditions such as depression, bipolar disorder, schizophrenia, schizoaffective disorder, catatonia, neuroleptic malignant syndrome, obsessive compulsive disorder and smoking cessation [165,166]. While general non-invasive interventional approaches include transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), magnetic seizure therapy (MST), electroconvulsive therapy (ECT) and photobiomodulation, none of these interventions is FDA-approved for the treatment of symptoms in the social domain [167–169]. However, there is evidence that some of these approaches have the potential to improve social functioning [170,171]. Here, we will focus on the NIBS methods TMS and tDCS as they are pain-free, non-invasive, well-tolerated and have high acceptance from patients [169,172].
TMS is a non-invasive tool that applies changing magnetic fields to induce an electric current that modulates neuronal excitability in a localized area of the brain. TMS has demonstrated its ability to disrupt and enhance function of neural circuitry and alter social behaviour. TMS applied to the superior temporal sulcus (STS) alters social gaze [173] while application to the ventrolateral prefrontal cortex (vlPFC) reduces social pain [174]. TMS application to the temporoparietal junction (TPJ) enhanced perspective-taking ability [175] and disrupted moral judgements [176], while application to the dorsolateral prefrontal cortex (dlPFC) altered reciprocal fairness [177] and moral decision-making [178] in healthy participants. Thus, TMS has a demonstrated ability to modulate complex aspects of social cognition in healthy adults. As a treatment, TMS applied to the dlPFC and STS has been used to improve social and executive function and reduce repetitive behaviours in individuals with ASD [171,179–181]. For instance, TMS applied to the dlPFC successfully reduced impairment in social relating and social anxiety in individuals with ASD [182]. Additionally, TMS administered to the TPJ improved associative learning in ASD patients [183], and a multisite randomized control trial is currently assessing TMS in the TPJ to treat ASD [184]. However, results using TMS as a treatment modality for some domains of ASD are heterogeneous and vary based on stimulus location and stimulation parameters adjusted to modulate inhibitory or excitatory networks [185].
tDCS uses a direct low-intensity current flowing between a pair of scalp electrodes to modulate cortical activity in a localized area of the brain. tDCS has also shown potential as a treatment for social dysfunction [186]. tDCS applied to the vlPFC bidirectionally modulated social pain [187] and reduced aggression after social exclusion. tDCS applied to the dlPFC reduced or enhanced emotional contagion [188,189], while tDCS applied to the inferior frontal cortex (IFC) enhanced imitation during social interaction [190]. tDCS applied to the TPJ has also been shown to improve sociocognitive processing in healthy participants [191] and application to the lPFC bi-directionally modulates compliance to social sanctions [192]. As a treatment, tDCS applied to the TPJ led to lasting improvements in social function in a patient with ASD [193] while tDCS stimulation of the ventromedial prefrontal cortex (vmPFC) significantly improved theory of mind in children with ASD. Lastly, stimulation to the dlPFC significantly decreased social attentional bias in patients with social anxiety disorder [194].
Medications have also been shown to modulate the efficacy of the intervention techniques described above [195] and informed combinatorial approaches could be used to augment pharmacological approaches with stimulation and vice versa. For example, TMS was found to augment and accelerate the onset of action and response to amitriptyline in severe depression [196]. Likewise, there is evidence that tDCS might be most effective when combined with pharmacological agents or cognitive behavioural therapies [197]. To this end, one study suggested that tDCS is most effective for treatment response in depression when combined with selective serotonin reuptake inhibitors [198].
While these techniques hold promise and have shown varying effects that enhance aspects of social behaviour in conditions like ASD, the results are heterogeneous and reflect varying protocols, study design and a lack of longitudinal experimental protocols with a sufficient follow-up period after treatment [171,199]. This underscores the need for more research in this area to inform combined therapeutic approaches. To that end, new stimulation parameters are demonstrating improved efficacy, shortened protocol times and improved circuit precision [200], which may improve these interventions. The current research indicates that the candidate target brain regions for TMS or tDCS that will likely play a role in treatments of social behaviour are the various subregions of the PFC (mPFC, dlPFC, vlPFC), the TPJ and the STS [184,201] (figure 1).
6. Psychological approaches for treating social impairment
While NIBS and pharmacotherapy can address aspects of social cognition and behaviour, one of the most powerful tools in psychiatry for addressing specific aspects of social behaviour is psychotherapy. Psychotherapy itself is inherently a social phenomenon that depends on the dynamic social cognition of both the therapist and patient; group therapy further relies on social interaction, altruism, and attachment processes and related brain networks [202–204]. Combination approaches have shown psychotherapy can enhance the effects of pharmacological approaches and vice versa [205]. In fact, psychotherapy has been shown to specifically improve social function in patients with depression when added to pharmacological approaches [206,207]. In social anxiety disorder, psychotherapy in combination with phenelzine was found to be more effective than phenelzine alone [208]. More recently, the resurgence of psychedelics as therapeutic agents within psychiatry has also led to studies illustrating the improved benefits of psychedelic-assisted psychotherapy for social anxiety in ASD, PTSD, anxiety, and depression when compared with psychotherapy alone [209,210]; similar approaches have also enhanced the results of group psychotherapy [211,212]. The prominence of such enhancement effects would suggest mechanistic specificity beyond coincident or summative effects.
Moreover, the use of the right pharmacological agent could significantly enhance the social dynamics of therapy. For instance, the entactogen 3,4-methylenedioxymethamphetamine (MDMA), in combination with psychotherapy, has been shown to rapidly improve symptoms of PTSD in phase III trials [213]. Translational studies have investigated possible mechanisms by which MDMA might enhance the therapeutic process and have found that MDMA enhances emotional empathy and prosociality [214], attention to social cues [215], and responses to social reward [216], and leads to a sense of social connection and ‘openness’ characteristic of mystical experience [217,218]. In treatment, MDMA-assisted psychotherapy has also shown to lead to rapid and durable improvement in social anxiety symptoms in adults with ASD [219]. MDMA-assisted therapy has illustrated the power of combining a psychotherapeutic context with the acute administration of a pharmacological agent [220].
These ligand-, anatomical- and augmentation-based approaches echo the combinatorial systems neurobiology described for the oxytocin and endocannabinoid systems. This parallel can be harnessed to derive more network-selective and effective treatments. For instance, oxytocin and endocannabinoids could evoke a neuromodulatory physiology that primes for more targeted psychotherapeutic interventions. Experiential therapies focused on overcoming resistance could use the anxiolytic properties of endocannabinoids, and transference-focused therapies could utilize the rewarding properties of oxytocin to build trust. TMS or tDCS can induce plasticity in networks that could then facilitate the impact of psychotherapy. For instance, psychotherapy has been shown to alter neuronal network activity in brain regions relevant to social behaviour such as the mPFC, TPJ, amygdala, hippocampus and anterior cingulate cortex [221–223] and also leads to synchrony in the TPJ between therapist and patient [224]. Some of these changes have been correlated with symptom improvement [225].
In addition to the modalities described above, several other approaches may have potential as well. For example, social cognitive interventions such as social skills training helps teach individuals how to interact with peers by providing explicit instructions and opportunities to develop or improve social interaction, social performance, and interpersonal skills and has been effective in schizophrenia and ASD [226–229]. Administering social cognitive interventions with recovery-oriented individual and group therapy that targets dysfunctional socialization attitudes or behaviours [230–232] could have further benefits than psychotherapy alone. Proof-of-concept pharmacotherapy-aided approaches have been extended to other practices such as mindfulness as well with promising results [233–235]. In addition, aerobic exercise has pro-social cognitive effects [236] and music therapy activates cortical regions and improves social cognition and behaviour in children with ASD and might be usefully combined with social cognitive interventions [237–240].
7. Trans-diagnostic social domains for targeting combinatorial strategies
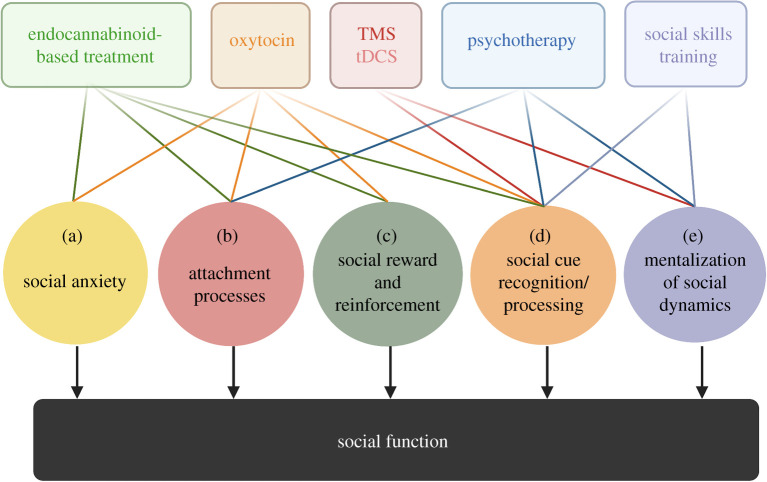
Using multiple pharmacological agents or combining pharmacological agents with stimulation, psychotherapy, skills-based work, music therapy and mindfulness all hold promise. Based on the available literature and the postulated circuit neurobiology of oxytocin and endocannabinoid signalling as detailed, we hypothesize that these combination approaches in social settings can be distinguished in mechanism, which has important implications for designing treatment approaches. We propose the following trans-diagnostic social domains as a framework to guide combinatorial treatment: modulation of (a) social anxiety, (b) attachment processes that result in potentiating or protective effects, (c) reward processes that reinforce social engagement, (d) recognition of social cues and (e) mentalization of ongoing social dynamics (figure 2). Previous models have posited that deficits in processes underlying social cognition and motivation could be targeted for treatment [241–243]. This framework provides more specificity by relying on available circuit-based evidence and is also centred on the social processes in which oxytocin and endocannabinoid signalling are likely to play an important role. We also recognize that these are broad and interrelated mechanisms—for example, empathy requires tempering of social anxiety, recognition of social cues and mentalization of social dynamics. However, we believe that conceptual distinction of these processes is important to inform combinatorial treatment approaches.
Figure 2.
Target trans-diagnostic domains for the treatment of social impairment. Deficits in social behaviour occur in many psychiatric conditions, including depression, anxiety, schizophrenia and ASD. A transdiagnostic model that targets domains of social function rather than a specific diagnosis will provide a mechanistically based approach for developing combined treatment strategies. We propose the following trans-diagnostic social domains: (a) social anxiety, (b) attachment processes, (c) reward processes that reinforce social engagement, (d) recognition of social cues and (e) mentalization of ongoing social dynamics. We provide examples of putative combination approaches utilizing endocannabinoids, oxytocin, non-invasive brain stimulation (NIBS), psychotherapy and social skills training. (Online version in colour.)
Ultimately, in order to design more targeted treatments for social impairments, the optimal approach may be more holistic and/or multimodal, combining pharmacology, psychotherapy, skills, music and mindfulness with stimulation. Neurostimulation (e.g. tDCS) has shown potential additive value in combination with cognitive remediation [244], and might also be usefully combined with social cognitive treatments [245]. This comprehensive approach would provide top-down cognitive effects through interventions like psychotherapy, tDCS and TMS acting on cortical networks [246], while pharmacological agents provide a bottom-up effect through altering the levels of neuromodulatory signalling molecules that shape network activity (figure 1). For example, individuals with an impaired ability for recognizing social cues and social anxiety could use oxytocin and endocannabinoids to enhance the salience of social information and reduce social stress, respectively. This increase in salience and reduction in social stress, in turn, would increase the ability of subjects to learn higher-level social cognitive skills during psychotherapy or social skills interventions. Evidence for the viability of this approach can already be seen as studies have shown improvements of several symptoms, including social anxiety, when CBD-based medicine is given as an adjunct to pharmacotherapy or psychotherapy [247]. In addition, rTMS delivered to the dlPFC in depressed patients led to changes in peripheral 2-AG which were correlated to symptom improvements, but this was not seen for anandamide [248]. Although these types of early findings require further elaboration, they indirectly support our hypothesis that brain stimulation and pharmacology could be combinatorially designed to converge on circuits (specific to anandamide versus 2-AG, or differential oxytocin/vasopressin receptor expression) in a mechanism that is fundamentally not possible via each modality alone.
8. Oxytocinergic and endocannabinoid system-based combination treatments for social impairment
We have discussed several different approaches that together suggest combination therapy may improve efficacy. Yet, not all combinatorial approaches result in higher efficacy, but rather consist of redundant or burdensome effects. One driving force of a desired efficacy may be specificity. While there has been active thought into the principles of such treatment approaches individually, we suggest applying tools that converge upon functional connectivity within specific neuronal populations and networks. Here, we describe approaches centred on oxytocin and endocannabinoids to inform these types of potential approaches.
It has been postulated that socially driven oxytocinergic signalling interacts with neuromodulatory systems such as the endocannabinoid system to enhance functional plasticity and connectivity [51]; preclinical mechanistic evidence as well as clinical translational evidence support a role for driving combinatorial oxytocin and endocannabinoid signalling to modulate specific aspects of behaviour.
Beyond previously described studies on within-system context specificity, circuit-selective recruitment can also be achieved across neuromodulator systems. In animal models, chemically distinct endocannabinoid signalling has been found to be involved in distinct aspects of oxytocin-dependent and -independent signalling pathways. For instance, disrupting endocannabinoid signalling alters expression of the oxytocin receptor [249] and using a CB1 receptor antagonist reduces oxytocin-induced analgesic behaviour [250]. Anandamide-mediated endocannabinoid signalling was found to mediate the effects of oxytocin to enhance and consolidate the rewarding properties of social interactions [78], but, by contrast, 2-AG-mediated endocannabinoid signalling was found to be oxytocin-independent and generalized to other non-social natural rewards [105]. In prairie voles, oxytocin's effect on pair bonding was shown to be dependent on endocannabinoid signalling through the CB1 receptor. It was also shown that blocking CB1 receptors after pair bonding led to a specific form of social rejection towards a partner but not an unfamiliar individual [251]. That these effects can range widely from the proposed facets of social behaviour (e.g. social reward, attachment) to analgesic behaviour suggests this type of across-system recruitment could be a powerful approach to circuit-selective therapeutic development.
Further, while global cannabinoid receptor activation causes dichotomous effects on social behaviour, on-demand potentiation of endocannabinoid signalling consistently corrects social impairment in ASD-related animal models [77,134,135,137]. Based on these results, oxytocin-driven signalling is likely the result of social stimulation driving a hypothalamic–accumbens circuit that recruits endocannabinoid signalling. We would hypothesize that chemically distinct endocannabinoid mediators would be recruited to control distinct aspects of a given circuit, which may or may not be oxytocin-driven. This hypothesis would support a treatment approach that emphasizes selective and on-demand endocannabinoid mobilization in response to specific social settings or activation of oxytocin signalling.
Clinical translational evidence also supports the possibility that circuit-selective endocannabinoid signalling would be more responsible for specific aspects of processing social information and controlling related behaviour. For instance, a randomized control trial was designed to treat patients with social phobia or panic disorder by combining CBD with exposure therapy, with the specific hypothesis that CBD's role in fear extinction and social behaviour would enhance exposure therapy (a central mechanism for which is promotion of fear extinction) [252]. Lastly, a preliminary study for ASD suggested that a neuroendocrine treatment using melatonin, oxytocin and CBD led to specific social effects through combined targeting of the oxytocin and endocannabinoid system [253].
Taken together, these lines of evidence on oxytocin and endocannabinoid neurobiology, clinical effects and combinatorial treatment approaches suggest that endocannabinoid augmentation of oxytocin-driven or socially driven therapies could be selectively recruited, mobilized on demand, and thus be more likely to act in a state- and context-dependent manner. Harnessing such physiological properties with combinatorial treatment approaches could improve selective targeting of disease entities or trans-diagnostic social domains (figure 2). A design that considers oxytocin or endocannabinoid signalling specific to social settings and brain networks would represent an evolution of current treatment approaches, which typically use mono-modal, context-independent or non-synergistic polypharmacy implementation of treatments.
In conclusion, targeted treatments for social impairment in neuropsychiatric conditions are critically needed. While clinical development of neuromodulators such as oxytocin and endocannabinoids are promising, we have proposed a framework grounded in systems neurobiology (figure 1) and categorization of trans-diagnostic social processes based on known endocannabinoid and oxytocin physiology (figure 2) to guide immediately actionable development of combinatorial treatment approaches. We have described how these approaches may be related to wider multimodal approaches as well as psychosocial considerations (box 1). We believe that these considerations can aid mechanistically driven strategies that target various components of social behaviour to improve the specificity and efficacy of treatment.
Box 1. Biopsychosocial considerations for future cannabinoid-based treatments.
Overall, therapeutically targeting the endocannabinoid system with THC, CBD, nabiximols or CB1 agonists has proven clinical benefit for a wide variety of conditions including chronic pain, chemotherapy-induced nausea/vomiting and spasticity in multiple sclerosis [254,255]. However, despite the clear medical benefits that have been established, the products used in clinical studies typically have little comparison to products available on the commercial market [81]. After the long history of criminalization that has impacted commercial markets and disproportionately affected Black, Indigenous and People of Colour [256–258], new legalization policies have led to an increase in the availability and potency of cannabis products. This has been thought to contribute to an increase in cannabis use disorders, cannabis withdrawal, and emergency room visits related to cannabis use [259]. The increased risk of psychosis with cannabis use is an additional concern, though cannabis use is thought to neither be neither necessary nor sufficient for psychosis and instead dependent on age of onset of cannabis use, genetic vulnerability and psychosocial stressors [260–262]. These possible harmful effects will need to be considered when evaluating the potential benefits of novel treatment strategies targeting the endocannabinoid system. In addition, understanding how chronic cannabis use affects treatment strategies will also be necessary. For instance, in humans, brain CB1 receptors are downregulated in cannabis and alcohol abusers [263,264], and thus treatment strategies would need to be adjusted in these populations. To that end, administration of oxytocin in conjunction with motivational enhancement therapy leads to reductions in daily cannabis use and illustrates a role for combinatorial oxytocin in potential strategies for modulating cannabis use [265].
Future research should be aimed at building a mechanistic understanding of multi-system approaches for social cognition that allow us to personalize endocannabinoid-based combinatorial treatment. Genetic testing is one form of personalization that can provide information to potentially guide combinatorial treatment strategies. For example, variation in SNPs in cnr1 (CB1 receptor) was associated with either a favourable or a poor response to cognitive behavioural treatment [266]. Additionally, preclinical and clinical studies have consistently shown sex differences in the endocannabinoid system due to differences in metabolism, cannabinoid receptor expression and ovarian hormone influence [106,267]. Data suggest that women are more susceptible to unwanted effects of cannabis use such as abuse, dependence, withdrawal and relapse [268]. In addition, oxytocin administration led to greater stress reactivity in recreational cannabis users who were women [269]. Understanding how sex differences impact treatment strategies that use the endocannabinoid system is critical to advancing these treatment approaches.
Further personalization should also be informed by a patient's psycho-social history. For instance, the mechanism of endocannabinoids seems to be dependent on stress and trauma history and can be affected by epigenetic modulation that may be transmitted through trans-generational epigenetic inheritance [270]. Individuals with a trauma history may then in fact self-medicate with cannabis [271,272] in order to treat an endocannabinoid-deficient state that impacts stress susceptibility and may be especially amenable to treatment enhancing endocannabinoid tone [127]. Populations with significant trauma history may also be uniquely impacted by modulation of the endocannabinoid system. For instance, Native Americans have altered 2-AG levels in the cerebrospinal fluid compared with Caucasians [273]. In addition, people of African descent have a higher prevalence of polymorphisms in the cnr1 (CB1 receptor) and faah (degradative enzyme of anandamide) genes and polymorphisms in these genes have been linked to aberrant endocannabinoid signalling [274].
Data accessibility
This article has no additional data.
Authors' contributions
D.W.: conceptualization, writing—original draft, writing—review and editing; S.T.: conceptualization, writing—review and editing; J.C.M.: conceptualization, supervision, writing—review and editing; A.Z.A.S.A.A.: conceptualization, data curation, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interest.
Funding
We received no funding for this study.
References
- 1.Adolphs R. 2001. The neurobiology of social cognition. Curr. Opin. Neurobiol. 11, 231-239. ( 10.1016/S0959-4388(00)00202-6) [DOI] [PubMed] [Google Scholar]
- 2.Donaldson ZR, Young LJ. 2008. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900-904. ( 10.1126/science.1158668) [DOI] [PubMed] [Google Scholar]
- 3.Adolphs R. 2009. The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693-716. ( 10.1146/annurev.psych.60.110707.163514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frith CD, Frith U. 2012. Mechanisms of social cognition. Annu. Rev. Psychol. 63, 287-313. ( 10.1146/annurev-psych-120710-100449) [DOI] [PubMed] [Google Scholar]
- 5.Bicks LK, Koike H, Akbarian S, Morishita H. 2015. Prefrontal cortex and social cognition in mouse and man. Front. Psychol. 6, 1805. ( 10.3389/fpsyg.2015.01805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P, Hong W. 2018. Neural circuit mechanisms of social behavior. Neuron 98, 16-30. ( 10.1016/j.neuron.2018.02.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews GA, Tye KM. 2019. Neural mechanisms of social homeostasis. Ann. N. Y. Acad. Sci. 1457, 5-25. ( 10.1111/nyas.14016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokolowski MB. 2010. Social interactions in ‘simple’ model systems. Neuron 65, 780-794. ( 10.1016/j.neuron.2010.03.007) [DOI] [PubMed] [Google Scholar]
- 9.de Waal FBM, Suchak M. 2010. Prosocial primates: selfish and unselfish motivations. Phil. Trans. R. Soc. B 365, 2711-2722. ( 10.1098/rstb.2010.0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weitekamp CA, Hofmann HA. 2014. Evolutionary themes in the neurobiology of social cognition. Curr. Opin. Neurobiol. 28, 22-27. ( 10.1016/j.conb.2014.06.005) [DOI] [PubMed] [Google Scholar]
- 11.House JS, Landis KR, Umberson D. 1988. Social relationships and health. Science 241, 540-545. ( 10.1126/science.3399889) [DOI] [PubMed] [Google Scholar]
- 12.Cacioppo JT, Hawkley LC. 2003. Social isolation and health, with an emphasis on underlying mechanisms. Perspect. Biol. Med. 46, S39-S52. ( 10.1353/pbm.2003.0049) [DOI] [PubMed] [Google Scholar]
- 13.Berkman LF, Syme SL. 1979. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am. J. Epidemiol. 109, 186-204. ( 10.1093/oxfordjournals.aje.a112674) [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Williamson G. 1988. Perceived stress in a probability sample of the United States. In The social psychology of health: the Claremont Symposium on Applied Social Psychology (eds Spacapan S, Oskamp S), pp. 31-67. London, UK: Sage Publications. [Google Scholar]
- 15.Cole SW, et al. 2007. Social regulation of gene expression in human leukocytes. Genome Biol. 8, R189. ( 10.1186/gb-2007-8-9-r189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt-Lunstad J, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7, e1000316. ( 10.1371/journal.pmed.1000316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perissinotto CM, Stijacic Cenzer I, Covinsky KE. 2012. Loneliness in older persons: a predictor of functional decline and death. Arch. Intern. Med. 172, 1078-1083. ( 10.1001/archinternmed.2012.1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkley LC, Cacioppo JT. 2010. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 40, 218-227. ( 10.1007/s12160-010-9210-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dohrenwend BP. 1966. Social status and psychological disorder: an issue of substance and an issue of method. Am. Sociol. Rev. 31, 14-34. ( 10.2307/2091276) [DOI] [PubMed] [Google Scholar]
- 20.Fryers T, Melzer D, Jenkins R. 2003. Social inequalities and the common mental disorders: a systematic review of the evidence. Soc. Psychiatry Psychiatr. Epidemiol. 38, 229-237. ( 10.1007/s00127-003-0627-2) [DOI] [PubMed] [Google Scholar]
- 21.Chiarotti F, Viglione A, Giuliani A, Branchi I. 2017. Citalopram amplifies the influence of living conditions on mood in depressed patients enrolled in the STAR*D study. Transl. Psychiatry 7, e1066. ( 10.1038/tp.2017.35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viglione A, Chiarotti F, Poggini S, Giuliani A, Branchi I. 2019. Predicting antidepressant treatment outcome based on socioeconomic status and citalopram dose. Pharmacogenomics J. 19, 538-546. ( 10.1038/s41397-019-0080-6) [DOI] [PubMed] [Google Scholar]
- 23.Kennedy DP, Adolphs R. 2012. The social brain in psychiatric and neurological disorders. Trends Cogn. Sci. 16, 559-572. ( 10.1016/j.tics.2012.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allsop SA, Vander Weele CM, Wichmann R, Tye KM. 2014. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front. Behav. Neurosci. 8, 241. ( 10.3389/fnbeh.2014.00241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein MB, Stein DJ. 2008. Social anxiety disorder. Lancet 371, 1115-1125. ( 10.1016/S0140-6736(08)60488-2) [DOI] [PubMed] [Google Scholar]
- 26.Losh M, Adolphs R, Poe MD, Couture S, Penn D, Baranek GT, Piven J. 2009. Neuropsychological profile of autism and the broad autism phenotype. Arch. Gen. Psychiatry 66, 518-526. ( 10.1001/archgenpsychiatry.2009.34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McPartland JC, Pelphrey KA. 2012. The implications of social neuroscience for social disability. J. Autism Dev. Disord. 42, 1256-1262. ( 10.1007/s10803-012-1514-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brothers L. 1990. The neural basis of primate social communication. Motiv. Emot. 14, 81-91. ( 10.1007/BF00991637) [DOI] [Google Scholar]
- 29.Eisenberger NI, Cole SW. 2012. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat. Neurosci. 15, 669-674. ( 10.1038/nn.3086) [DOI] [PubMed] [Google Scholar]
- 30.Carter CS, Williams JR, Witt DM, Insel TR. 1992. Oxytocin and social bonding. Ann. N. Y. Acad Sci 652, 204-211. ( 10.1111/j.1749-6632.1992.tb34356.x) [DOI] [PubMed] [Google Scholar]
- 31.Ross HE, Young LJ. 2009. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 30, 534-547. ( 10.1016/j.yfrne.2009.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang SWC, Platt ML. 2014. Oxytocin and social cognition in rhesus macaques: implications for understanding and treating human psychopathology. Brain Res. 1580, 57-68. ( 10.1016/j.brainres.2013.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caldwell HK. 2017. Oxytocin and vasopressin: powerful regulators of social behavior. Neuroscientist 23, 517-528. ( 10.1177/1073858417708284) [DOI] [PubMed] [Google Scholar]
- 34.Pedersen CA, Prange AJ. 1979. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl Acad. Sci. USA 76, 6661-6665. ( 10.1073/pnas.76.12.6661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Insel TR, Winslow JT, Wang Z, Young LJ. 1998. Oxytocin, vasopressin, and the neuroendocrine basis of pair bond formation. Adv. Exp. Med. Biol. 449, 215-224. ( 10.1007/978-1-4615-4871-3_28) [DOI] [PubMed] [Google Scholar]
- 36.Dolen G, Darvishzadeh A, Huang KW, Malenka RC. 2013. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179-184. ( 10.1038/nature12518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNeilly AS, Robinson IC, Houston MJ, Howie PW. 1983. Release of oxytocin and prolactin in response to suckling. Br. Med. J. Clin. Res. Educ. 286, 257-259. ( 10.1136/bmj.286.6361.257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. 1996. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl Acad. Sci. USA 93, 11 699-11 704. ( 10.1073/pnas.93.21.11699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young IR. 2001. The comparative physiology of parturition in mammals. Front. Horm. Res. 27, 10-30. ( 10.1159/000061036) [DOI] [PubMed] [Google Scholar]
- 40.Hung LW, et al. 2017. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406-1411. ( 10.1126/science.aan4994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resendez SL, et al. 2020. Social stimuli induce activation of oxytocin neurons within the paraventricular nucleus of the hypothalamus to promote social behavior in male mice. J. Neurosci. 40, 2282-2295. ( 10.1523/JNEUROSCI.1515-18.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang Y, et al. 2020. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat. Neurosci. 23, 1125-1137. ( 10.1038/s41593-020-0674-y) [DOI] [PubMed] [Google Scholar]
- 43.Knobloch HS, et al. 2012. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553-566. ( 10.1016/j.neuron.2011.11.030) [DOI] [PubMed] [Google Scholar]
- 44.Jiang Y, Platt ML. 2018. Oxytocin and vasopressin flatten dominance hierarchy and enhance behavioral synchrony in part via anterior cingulate cortex. Scient. Rep. 8, 8201. ( 10.1038/s41598-018-25607-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burkett JP, Andari E, Johnson ZV, Curry DC, De Waal FBM, Young LJ. et al. 2016. Oxytocin-dependent consolation behavior in rodents. Science 351, 375-378. ( 10.1126/science.aac4785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. 2012. Inhaled oxytocin amplifies both vicarious reinforcement and self-reinforcement in rhesus macaques (Macaca mulatta). Proc. Natl Acad. Sci. USA 109, 959-964. ( 10.1073/pnas.1114621109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamagishi A, Okada M, Masuda M, Sato N. 2020. Oxytocin administration modulates rats’ helping behavior depending on social context. Neurosci. Res. 153, 56-61. ( 10.1016/j.neures.2019.04.001) [DOI] [PubMed] [Google Scholar]
- 48.Marlin BJ, Froemke RC. 2017. Oxytocin modulation of neural circuits for social behavior. Dev. Neurobiol. 77, 169-189. ( 10.1002/dneu.22452) [DOI] [PubMed] [Google Scholar]
- 49.Gimpl G, Fahrenholz F. 2001. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 81, 629-683. ( 10.1152/physrev.2001.81.2.629) [DOI] [PubMed] [Google Scholar]
- 50.Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki CJ, Chao MV, Froemke RC. 2016. A distributed network for social cognition enriched for oxytocin receptors. J. Neurosci. 36, 2517-2535. ( 10.1523/JNEUROSCI.2409-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Froemke RC, Young LJ. 2021. Oxytocin, neural plasticity, and social behavior. Annu. Rev. Neurosci. 44, 359-381. ( 10.1146/annurev-neuro-102320-102847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. 2005. Oxytocin increases trust in humans. Nature 435, 673-676. ( 10.1038/nature03701) [DOI] [PubMed] [Google Scholar]
- 53.Declerck CH, Boone C, Kiyonari T. 2010. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Horm. Behav. 57, 368-374. ( 10.1016/j.yhbeh.2010.01.006) [DOI] [PubMed] [Google Scholar]
- 54.Ne'eman R, Perach-Barzilay N, Fischer-Shofty M, Atias A, Shamay-Tsoory SG. 2016. Intranasal administration of oxytocin increases human aggressive behavior. Horm. Behav. 80, 125-131. ( 10.1016/j.yhbeh.2016.01.015) [DOI] [PubMed] [Google Scholar]
- 55.Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. 2009. Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biol. Psychiatry 66, 864-870. ( 10.1016/j.biopsych.2009.06.009) [DOI] [PubMed] [Google Scholar]
- 56.Schiller B, Domes G, Heinrichs M. 2020. Oxytocin changes behavior and spatio-temporal brain dynamics underlying inter-group conflict in humans. Eur. Neuropsychopharmacol. 31, 119-130. ( 10.1016/j.euroneuro.2019.12.109) [DOI] [PubMed] [Google Scholar]
- 57.Puglia MH, Connelly JJ, Morris JP. 2018. Epigenetic regulation of the oxytocin receptor is associated with neural response during selective social attention. Transl. Psychiatry 8, 116. ( 10.1038/s41398-018-0159-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rilling JK, Chen X, Chen X, Haroon E. 2018. Intranasal oxytocin modulates neural functional connectivity during human social interaction. Am. J. Primatol. 80, e22740. ( 10.1002/ajp.22740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tillman R, Gordon I, Naples A, Rolison M, Leckman JF, Feldman R, Pelphrey KA, Mcpartland JC. et al. 2019. Oxytocin enhances the neural efficiency of social perception. Front. Hum. Neurosci. 13, 71. ( 10.3389/fnhum.2019.00071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckstein M, Bamert V, Stephens S, Wallen K, Young LJ, Ehlert U, Ditzen B. 2019. Oxytocin increases eye-gaze towards novel social and non-social stimuli. Social Neurosci. 14, 594-607. ( 10.1080/17470919.2018.1542341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. 2007. Oxytocin increases retention of social cognition in autism. Biol. Psychiatry 61, 498-503. ( 10.1016/j.biopsych.2006.05.030) [DOI] [PubMed] [Google Scholar]
- 62.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. 2010. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol. Psychiatry 67, 692-694. ( 10.1016/j.biopsych.2009.09.020) [DOI] [PubMed] [Google Scholar]
- 63.Anagnostou E, et al. 2012. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol. Autism 3, 16. ( 10.1186/2040-2392-3-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. 2010. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl Acad. Sci. USA 107, 4389-4394. ( 10.1073/pnas.0910249107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. 2016. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol. Psychiatry 21, 1225-1231. ( 10.1038/mp.2015.162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sikich L, et al. 2021. Intranasal oxytocin in children and adolescents with autism spectrum disorder. N. Engl. J. Med. 385, 1462-1473. ( 10.1056/NEJMoa2103583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamasue H, et al. 2022. Effect of a novel nasal oxytocin spray with enhanced bioavailability on autism: a randomized trial. Brain 145, 490-499. ( 10.1093/brain/awab291) [DOI] [PubMed] [Google Scholar]
- 68.Voncken MJ, Dijk C, Stohr F, Niesten IJM, Schruers K, Kuypers KPC. 2021. The effect of intranasally administered oxytocin on observed social behavior in social anxiety disorder. Eur. Neuropsychopharmacol. 53, 25-33. ( 10.1016/j.euroneuro.2021.07.005) [DOI] [PubMed] [Google Scholar]
- 69.Keech B, Crowe S, Hocking DR. 2018. Intranasal oxytocin, social cognition and neurodevelopmental disorders: a meta-analysis. Psychoneuroendocrinology 87, 9-19. ( 10.1016/j.psyneuen.2017.09.022) [DOI] [PubMed] [Google Scholar]
- 70.Erdozain AM, Peñagarikano O. 2020. Oxytocin as treatment for social cognition, not there yet. Front. Psychiatry 10, 930. ( 10.3389/fpsyt.2019.00930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernaerts S, Boets B, Bosmans G, Steyaert J, Alaerts K. 2020. Behavioral effects of multiple-dose oxytocin treatment in autism: a randomized, placebo-controlled trial with long-term follow-up. Mol. Autism 11, 6. ( 10.1186/s13229-020-0313-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quintana DS, Lischke A, Grace S, Scheele D, Ma Y, Becker B. 2021. Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research. Mol. Psychiatry 26, 80-91. ( 10.1038/s41380-020-00864-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamasue H, Yee JR, Hurlemann R, Rilling JK, Chen FS, Meyer-Lindenberg A, Tost H. et al. 2012. Integrative approaches utilizing oxytocin to enhance prosocial behavior: from animal and human social behavior to autistic social dysfunction. J. Neurosci. 32, 14 109-14 117. ( 10.1523/JNEUROSCI.3327-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young LJ, Barrett CE. 2015. Can oxytocin treat autism? Science 347, 825-826. ( 10.1126/science.aaa8120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ford CL, Young LJ. 2021. Translational opportunities for circuit-based social neuroscience: advancing 21st century psychiatry. Curr. Opin. Neurobiol. 68, 1-8. ( 10.1016/j.conb.2020.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pertwee R. 2009. The science of marijuana, 2nd edn. Br. J. Clin. Pharmacol. 67, 268. ( 10.1111/j.1365-2125.2008.03355.x) [DOI] [Google Scholar]
- 77.Wei D, Allsop S, Tye K, Piomelli D. 2017. Endocannabinoid signaling in the control of social behavior. Trends Neurosci. 40, 385-396. ( 10.1016/j.tins.2017.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei D, Lee DY, Cox CD, Karsten CA, Peñagarikano O, Geschwind DH, Gall CM, Piomelli D. et al. 2015. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc. Natl Acad. Sci. USA 112, 14084-14089. ( 10.1073/pnas.1509795112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mechoulam R, Parker LA. 2013. The endocannabinoid system and the brain. Annu. Rev. Psychol. 64, 21-47. ( 10.1146/annurev-psych-113011-143739) [DOI] [PubMed] [Google Scholar]
- 80.Piomelli D. 2014. More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology 76, 228-234. ( 10.1016/j.neuropharm.2013.07.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Volkow ND, Swanson JM, Evins AE, Delisi LE, Meier MH, Gonzalez R, Bloomfield MAP, Curran HV, Baler R. et al. 2016. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry 73, 292-297. ( 10.1001/jamapsychiatry.2015.3278) [DOI] [PubMed] [Google Scholar]
- 82.Glass M, Dragunow M, Faull RL. 1997. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77, 299-318. ( 10.1016/S0306-4522(96)00428-9) [DOI] [PubMed] [Google Scholar]
- 83.Stanley DA, Adolphs R. 2013. Toward a neural basis for social behavior. Neuron 80, 816-826. ( 10.1016/j.neuron.2013.10.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Breivogel CS, Sim LJ, Childers SR. 1997. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J. Pharmacol. Exp. Ther. 282, 1632-1642. [PubMed] [Google Scholar]
- 85.Busquets-Garcia A, Bains J, Marsicano G. 2018. CB1 receptor signaling in the brain: extracting specificity from ubiquity. Neuropsychopharmacology 43, 4-20. ( 10.1038/npp.2017.206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parsons LH, Hurd YL. 2015. Endocannabinoid signaling in reward and addiction. Nat. Rev. Neurosci. 16, 579-594. ( 10.1038/nrn4004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viveros MP, Llorente R, Suarez J, Llorente-Berzal A, Lopez-Gallardo M, Rodriguez De Fonseca F. et al. 2012. The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. J. Psychopharmacol. 26, 164-176. ( 10.1177/0269881111408956) [DOI] [PubMed] [Google Scholar]
- 88.Suárez J, Ortiz O, Puente N, Bermudez-Silva FJ, Blanco E, Fernandez-Llebrez P, Grandes P, De Fonseca FR, Moratalla R. 2011. Distribution of diacylglycerol lipase alpha, an endocannabinoid synthesizing enzyme, in the rat forebrain. Neuroscience 192, 112-131. ( 10.1016/j.neuroscience.2011.06.062) [DOI] [PubMed] [Google Scholar]
- 89.Bosier B, Muccioli GG, Hermans E, Lambert DM. 2010. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem. Pharmacol. 80, 1-12. ( 10.1016/j.bcp.2010.02.013) [DOI] [PubMed] [Google Scholar]
- 90.Dorr M, Steinberg H. 1976. Effects of Δ9-tetrahydrocannabinol on social behaviour in mice. Psychopharmacology (Berl.) 47, 87-91. ( 10.1007/BF00428707) [DOI] [PubMed] [Google Scholar]
- 91.Miczek KA. 1978. Δ9-Tetrahydrocannabinol: antiaggressive effects in mice, rats, and squirrel monkeys. Science 199, 1459-1461. ( 10.1126/science.415367) [DOI] [PubMed] [Google Scholar]
- 92.Cutler MG, Mackintosh JH. 1984. Cannabis and delta-9-tetrahydrocannabinol. Effects on elements of social behaviour in mice. Neuropharmacology 23, 1091-1097. ( 10.1016/0028-3908(84)90134-5) [DOI] [PubMed] [Google Scholar]
- 93.Miller RE, Deets AC. 1976. Delta-9-THC and nonverbal communication in monkeys. Psychopharmacology (Berl.) 48, 53-58. ( 10.1007/BF00423306) [DOI] [PubMed] [Google Scholar]
- 94.Haller J, Varga B, Ledent C, Barna I, Freund TF. 2004. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur. J. Neurosci. 19, 1906-1912. ( 10.1111/j.1460-9568.2004.03293.x) [DOI] [PubMed] [Google Scholar]
- 95.Litvin Y, Phan A, Hill MN, Pfaff DW, McEwen BS. 2013. CB1 receptor signaling regulates social anxiety and memory. Genes Brain Behav. 12, 479-489. ( 10.1111/gbb.12045) [DOI] [PubMed] [Google Scholar]
- 96.Rodriguez-Arias M, Navarrete F, Daza-Losada M, Navarro D, Aguilar MA, Berbel P, Minarro J, Manzanares J. 2013. CB1 cannabinoid receptor-mediated aggressive behavior. Neuropharmacology 75, 172-180. ( 10.1016/j.neuropharm.2013.07.013) [DOI] [PubMed] [Google Scholar]
- 97.Fyke W, et al. 2021. Communication and social interaction in the cannabinoid-type 1 receptor null mouse: implications for autism spectrum disorder. Autism Res. 14, 1854-1872. ( 10.1002/aur.2562) [DOI] [PubMed] [Google Scholar]
- 98.Klugmann M, Goepfrich A, Friemel CM, Schneider M. 2011. AAV-mediated overexpression of the CB1 receptor in the mPFC of adult rats alters cognitive flexibility, social behavior, and emotional reactivity. Front. Behav. Neurosci. 5, 37. ( 10.3389/fnbeh.2011.00037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonczarowska N, Tomaz C, Caixeta FV, Malcher-Lopes R, Barros M, Nishijo H, Maior RS. 2019. CB1 receptor antagonism in capuchin monkeys alters social interaction and aversive memory extinction. Psychopharmacology (Berl.) 236, 3413-3419. ( 10.1007/s00213-019-05305-0) [DOI] [PubMed] [Google Scholar]
- 100.Loureiro M, Kramar C, Renard J, Rosen LG, Laviolette SR. 2016. Cannabinoid transmission in the hippocampus activates nucleus accumbens neurons and modulates reward and aversion-related emotional salience. Biol. Psychiatry 80, 216-225. ( 10.1016/j.biopsych.2015.10.016) [DOI] [PubMed] [Google Scholar]
- 101.Terzian ALB, Micale V, Wotjak CT. 2014. Cannabinoid receptor type 1 receptors on GABAergic vs. glutamatergic neurons differentially gate sex-dependent social interest in mice. Eur. J. Neurosci. 40, 2293-2298. ( 10.1111/ejn.12561) [DOI] [PubMed] [Google Scholar]
- 102.Trezza V, Vanderschuren LJMJ. 2008. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology (Berl.) 197, 217-227. ( 10.1007/s00213-007-1025-3) [DOI] [PubMed] [Google Scholar]
- 103.Shonesy BC, et al. 2018. Role of striatal direct pathway 2-arachidonoylglycerol signaling in sociability and repetitive behavior. Biol. Psychiatry 84, 304-315. ( 10.1016/j.biopsych.2017.11.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cassano T, et al. 2011. Evaluation of the emotional phenotype and serotonergic neurotransmission of fatty acid amide hydrolase-deficient mice. Psychopharmacology (Berl.) 214, 465-476. ( 10.1007/s00213-010-2051-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei D, Lee DY, Li D, Daglian J, Jung K-M, Piomelli D. 2016. A role for the endocannabinoid 2-arachidonoyl-sn-glycerol for social and high-fat food reward in male mice. Psychopharmacology (Berl.) 233, 1911-1919. ( 10.1007/s00213-016-4222-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rubino T, Parolaro D. 2015. Sex-dependent vulnerability to cannabis abuse in adolescence. Front. Psychiatry 6, 56. ( 10.3389/fpsyt.2015.00056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cossio D, Stadler H, Michas Z, Johnston C, Lopez HH. 2020. Disrupting the endocannabinoid system in early adolescence negatively impacts sociability. Pharmacol. Biochem. Behav. 188, 172832. ( 10.1016/j.pbb.2019.172832) [DOI] [PubMed] [Google Scholar]
- 108.Verdurand M, Nguyen V, Stark D, Zahra D, Gregoire M-C, Greguric I, Zavitsanou K. 2011. Comparison of cannabinoid CB1 receptor binding in adolescent and adult rats: a positron emission tomography study using [18F]MK-9470. Int. J. Mol. Imaging 2011, e548123. ( 10.1155/2011/548123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Devi LA, Hurd YL. 2008. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur. Neuropsychopharmacol. 18, 826-834. ( 10.1016/j.euroneuro.2008.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Renard J, Krebs M-O, Pen GL, Jay TM. 2014. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front. Neurosci. 8, 361. ( 10.3389/fnins.2014.00361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garcia-Mompo C, Curto Y, Carceller H, Gilabert-Juan J, Rodriguez-Flores E, Guirado R, Nacher J. 2020. Δ-9-Tetrahydrocannabinol treatment during adolescence and alterations in the inhibitory networks of the adult prefrontal cortex in mice subjected to perinatal NMDA receptor antagonist injection and to postweaning social isolation. Transl. Psychiatry 10, 177. ( 10.1038/s41398-020-0853-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schneider M, et al. 2015. Enhanced functional activity of the cannabinoid type-1 receptor mediates adolescent behavior. J. Neurosci. 35, 13 975-13 988. ( 10.1523/JNEUROSCI.1937-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fernández-Ruiz J, Berrendero F, Hernández ML, Ramos JA. 2000. The endogenous cannabinoid system and brain development. Trends Neurosci. 23, 14-20. ( 10.1016/S0166-2236(99)01491-5) [DOI] [PubMed] [Google Scholar]
- 114.Salzman C, Kochansky GE, Van Der Kolk BA, Shader RI. 1977. The effect of marijuana on small group process. Am. J. Drug Alcohol Abuse 4, 251-255. ( 10.3109/00952997709002763) [DOI] [PubMed] [Google Scholar]
- 115.Tart CT. 1970. Marijuana intoxication : common experiences. Nature 226, 701-704. ( 10.1038/226701a0) [DOI] [PubMed] [Google Scholar]
- 116.Foltin RW, Brady JV, Fischman MW, Emurian CS, Dominitz J. 1987. Effects of smoked marijuana on social interaction in small groups. Drug Alcohol Depend. 20, 87-93. ( 10.1016/0376-8716(87)90079-2) [DOI] [PubMed] [Google Scholar]
- 117.Foltin RW, Fischman MW. 1988. Effects of smoked marijuana on human social behavior in small groups. Pharmacol. Biochem. Behav. 30, 539-541. ( 10.1016/0091-3057(88)90494-7) [DOI] [PubMed] [Google Scholar]
- 118.Gorka SM, Fitzgerald DA, de Wit H, Phan KL. 2015. Cannabinoid modulation of amygdala subregion functional connectivity to social signals of threat. Int. J. Neuropsychopharmacol. 18, pyu104. ( 10.1093/ijnp/pyu104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roepke S, Vater A, Preißler S, Heekeren H, Dziobek I. 2013. Social cognition in borderline personality disorder. Front. Neurosci. 6, 195. ( 10.3389/fnins.2012.00195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fineberg SK, Leavitt J, Landry CD, Neustadter ES, Lesser RE, Stahl DS, Deutsch-Link S, Corlett PR. et al. 2018. Individuals with borderline personality disorder show larger preferred social distance in live dyadic interactions. Psychiatry Res. 260, 384-390. ( 10.1016/j.psychres.2017.11.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lis S, Bohus M. 2013. Social interaction in borderline personality disorder. Curr. Psychiatry Rep. 15, 338. ( 10.1007/s11920-012-0338-z) [DOI] [PubMed] [Google Scholar]
- 122.Kolla NJ, et al. 2020. Elevated fatty acid amide hydrolase in the prefrontal cortex of borderline personality disorder: a [11C]CURB positron emission tomography study. Neuropsychopharmacology 45, 1834-1841. ( 10.1038/s41386-020-0731-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chakrabarti B, Baron-Cohen S. 2011. Variation in the human cannabinoid receptor CNR1 gene modulates gaze duration for happy faces. Mol. Autism 2, 10. ( 10.1186/2040-2392-2-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hariri ARGorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. et al. 2009. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol. Psychiatry 66, 9-16. ( 10.1016/j.biopsych.2008.10.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hillard CJ, Weinlander KM, Stuhr KL. 2012. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience 204, 207-229. ( 10.1016/j.neuroscience.2011.11.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hill MN, Carrier EJ, Mclaughlin RJ, Morrish AC, Meier SE, Hillard CJ, Gorzalka BB. et al. 2008. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J. Neurochem. 106, 2322-2336. ( 10.1111/j.1471-4159.2008.05567.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. 2009. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 34, 1257-1262. ( 10.1016/j.psyneuen.2009.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aran A, et al. 2019. Lower circulating endocannabinoid levels in children with autism spectrum disorder. Mol. Autism 10, 2. ( 10.1186/s13229-019-0256-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koethe D, Giuffrida A, Schreiber D, Hellmich M, Schultze-Lutter F, Ruhrmann S, Klosterkötter J, Piomelli D, Leweke FM. 2009. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br. J. Psychiatry J. Ment. Sci. 194, 371-372. ( 10.1192/bjp.bp.108.053843) [DOI] [PubMed] [Google Scholar]
- 130.Karhson DS, Hardan AY, Parker KJ. 2016. Endocannabinoid signaling in social functioning: an RDoC perspective. Transl. Psychiatry 6, e905. ( 10.1038/tp.2016.169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garani R, Watts JJ, Mizrahi R. 2021. Endocannabinoid system in psychotic and mood disorders, a review of human studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 106, 110096. ( 10.1016/j.pnpbp.2020.110096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fakhoury M. 2017. Role of the endocannabinoid system in the pathophysiology of schizophrenia. Mol. Neurobiol. 54, 768-778. ( 10.1007/s12035-016-9697-5) [DOI] [PubMed] [Google Scholar]
- 133.Chadwick VL, Rohleder C, Koethe D, Leweke FM. 2020. Cannabinoids and the endocannabinoid system in anxiety, depression, and dysregulation of emotion in humans. Curr. Opin. Psychiatry 33, 20-42. ( 10.1097/YCO.0000000000000562) [DOI] [PubMed] [Google Scholar]
- 134.Wei D, Dinh D, Lee DY, Li D, Anguren A, Moreno-Sanz G, Gall CM, Piomelli D. et al. 2016. Enhancement of anandamide-mediated endocannabinoid signaling corrects autism-related social impairment. Cannabis Cannabinoid Res. 1, 81-89. ( 10.1089/can.2015.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Servadio M, et al. 2016. Targeting anandamide metabolism rescues core and associated autistic-like symptoms in rats prenatally exposed to valproic acid. Transl. Psychiatry 6, e902. ( 10.1038/tp.2016.182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Folkes OM, et al. 2020. An endocannabinoid-regulated basolateral amygdala–nucleus accumbens circuit modulates sociability. J. Clin. Invest. 130, 1728-1742. ( 10.1172/JCI131752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu H-F, Lu T-Y, Chu M-C, Chen PS, Lee C-W, Lin H-C. et al. 2020. Targeting the inhibition of fatty acid amide hydrolase ameliorate the endocannabinoid-mediated synaptic dysfunction in a valproic acid-induced rat model of autism. Neuropharmacology 162, 107736. ( 10.1016/j.neuropharm.2019.107736) [DOI] [PubMed] [Google Scholar]
- 138.Poleg S, Golubchik P, Offen D, Weizman A. 2019. Cannabidiol as a suggested candidate for treatment of autism spectrum disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 89, 90-96. ( 10.1016/j.pnpbp.2018.08.030) [DOI] [PubMed] [Google Scholar]
- 139.Khan R, Naveed S, Mian N, Fida A, Raafey MA, Aedma KK. et al. 2020. The therapeutic role of cannabidiol in mental health: a systematic review. J. Cannabis Res. 2, 2. ( 10.1186/s42238-019-0012-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Almeida V, Levin R, Peres FF, Niigaki ST, Calzavara MB, Zuardi A, Hallak JE, Crippa JA, Abílio VC. 2013. Cannabidiol exhibits anxiolytic but not antipsychotic property evaluated in the social interaction test. Prog. Neuropsychopharmacol. Biol. Psychiatry 41, 30-35. ( 10.1016/j.pnpbp.2012.10.024) [DOI] [PubMed] [Google Scholar]
- 141.Hartmann A, Lisboa SF, Sonego AB, Coutinho D, Gomes FV, Guimarãesab FS. 2019. Cannabidiol attenuates aggressive behavior induced by social isolation in mice: involvement of 5-HT1A and CB1 receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 94, 109637. ( 10.1016/j.pnpbp.2019.109637) [DOI] [PubMed] [Google Scholar]
- 142.Bar-Lev Schleider L, Mechoulam R, Saban N, Meiri G, Novack V. 2019. Real life experience of medical cannabis treatment in autism: analysis of safety and efficacy. Scient. Rep. 9, 200. ( 10.1038/s41598-018-37570-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fusar-Poli L, Cavone V, Tinacci S, Concas I, Petralia A, Signorelli MS, Díaz-Caneja CM, Aguglia E. et al. 2020. Cannabinoids for people with ASD: a systematic review of published and ongoing studies. Brain Sci. 10, 572. ( 10.3390/brainsci10090572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Agarwal R, Burke SL, Maddux M. 2019. Current state of evidence of cannabis utilization for treatment of autism spectrum disorders. BMC Psychiatry 19, 328. ( 10.1186/s12888-019-2259-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schmidt ME, et al. 2021. The effects of inhibition of fatty acid amide hydrolase (FAAH) by JNJ-42165279 in social anxiety disorder: a double-blind, randomized, placebo-controlled proof-of-concept study. Neuropsychopharmacology 46, 1004-1010. ( 10.1038/s41386-020-00888-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pretzsch CM, et al. 2019. Effects of cannabidiol on brain excitation and inhibition systems; a randomised placebo-controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacology 44, 1398-1405. ( 10.1038/s41386-019-0333-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karhson DS, Krasinska KM, Dallaire JA, Libove RA, Phillips JM, Chien AS, Garner JP, Hardan AY, Parker KJ. et al. 2018. Plasma anandamide concentrations are lower in children with autism spectrum disorder. Mol. Autism 9, 18. ( 10.1186/s13229-018-0203-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK. 2006. Systems biology and combination therapy in the quest for clinical efficacy. Nat. Chem. Biol. 2, 458-466. ( 10.1038/nchembio817) [DOI] [PubMed] [Google Scholar]
- 149.Volkow ND, Hampson AJ, Baler RD. 2017. Don't worry, be happy: endocannabinoids and cannabis at the intersection of stress and reward. Annu. Rev. Pharmacol. Toxicol. 57, 285-308. ( 10.1146/annurev-pharmtox-010716-104615) [DOI] [PubMed] [Google Scholar]
- 150.Alcalá-López D, et al. 2018. Computing the social brain connectome across systems and states. Cereb. Cortex 28, 2207-2232. ( 10.1093/cercor/bhx121) [DOI] [PubMed] [Google Scholar]
- 151.Wang Y, Metoki A, Xia Y, Zang Y, He Y, Olson IR. 2021. A large-scale structural and functional connectome of social mentalizing. Neuroimage 236, 118115. ( 10.1016/j.neuroimage.2021.118115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Monte OD, et al. 2017. Oxytocin under opioid antagonism leads to supralinear enhancement of social attention. Proc. Natl Acad. Sci. USA 114, 5247-5252. ( 10.1073/pnas.1702725114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fan S, Weinberg-Wolf H, Piva M, Dal Monte O, Chang SWC. 2020. Combinatorial oxytocin neuropharmacology in social cognition. Trends Cogn. Sci. 24, 8-12. ( 10.1016/j.tics.2019.10.004) [DOI] [PubMed] [Google Scholar]
- 154.Schuckit M, Robins E, Feighner J. 1971. Tricyclic antidepressants and monoamine oxidase inhibitors: combination therapy in the treatment of depression. Arch. Gen. Psychiatry 24, 509-514. ( 10.1001/archpsyc.1971.01750120025005) [DOI] [PubMed] [Google Scholar]
- 155.Freudenreich O, Goff DC. 2002. Antipsychotic combination therapy in schizophrenia. A review of efficacy and risks of current combinations. Acta Psychiatr. Scand. 106, 323-330. ( 10.1034/j.1600-0447.2002.01331.x) [DOI] [PubMed] [Google Scholar]
- 156.Souery D, Papakostas GI, Trivedi MH. 2006. Treatment-resistant depression. J. Clin. Psychiatry 67(Suppl. 6), 16-22. [PubMed] [Google Scholar]
- 157.Dunlop BW, Davis PG. 2008. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim. Care Companion J. Clin. Psychiatry 10, 24373. ( 10.4088/PCC.v10n0307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Howes OD, Thase ME, Pillinger T. 2021. Treatment resistance in psychiatry: state of the art and new directions. Mol. Psychiatry 27, 58-72. ( 10.1038/s41380-021-01200-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boyer WF, Bakalar NH, Lake CR. 1987. Anticholinergic prophylaxis of acute haloperidol-induced acute dystonic reactions. J. Clin. Psychopharmacol. 7, 164-166. ( 10.1097/00004714-198706000-00008) [DOI] [PubMed] [Google Scholar]
- 160.Goff DC, et al. 1991. The effect of benztropine on haloperidol induced dystonia, clinical efficacy and pharmacokinetics: a prospective, double-blind trial. J. Clin. Psychopharmacol. 11, 106-112. ( 10.1097/00004714-199104000-00006) [DOI] [PubMed] [Google Scholar]
- 161.Stoller KB, Bigelow GE, Walsh SL, Strain EC. 2001. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology (Berl.) 154, 230-242. ( 10.1007/s002130000637) [DOI] [PubMed] [Google Scholar]
- 162.Yoon G, Petrakis IL, Krystal JH. 2019. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry 76, 337-338. ( 10.1001/jamapsychiatry.2018.3990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Messer SB. 2002. A psychodynamic perspective on resistance in psychotherapy: vive la résistance. J. Clin. Psychol. 58, 157-163. ( 10.1002/jclp.1139) [DOI] [PubMed] [Google Scholar]
- 164.Plakun, E. Treatment resistance and psychodynamic psychiatry: concepts psychiatry needs from psychoanalysis. Psychodyn. Psychiatry 40, 183-209. ( 10.1521/pdps.2012.40.2.183) [DOI] [PubMed] [Google Scholar]
- 165.Lefaucheur J-P, et al. 2020. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin. Neurophysiol. 131, 474-528. ( 10.1016/j.clinph.2019.11.002) [DOI] [PubMed] [Google Scholar]
- 166.Hyde J, et al. 2022. Efficacy of neurostimulation across mental disorders: systematic review and meta-analysis of 208 randomized controlled trials. Mol. Psychiatry 27, 2709-2719. ( 10.1038/s41380-022-01524-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Holtzheimer PE, Mayberg HS. 2012. Neuromodulation for treatment-resistant depression. F1000 Med. Rep 4, 22. ( 10.3410/M4-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Boggio P, Asthana M, Costa TL, Valasek C, Osório A. 2015. Promoting social plasticity in developmental disorders with non-invasive brain stimulation techniques. Front. Neurosci. 9, 294. ( 10.3389/fnins.2015.00294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marini M, Banaji MR, Pascual-Leone A. 2018. Studying implicit social cognition with noninvasive brain stimulation. Trends Cogn. Sci. 22, 1050-1066. ( 10.1016/j.tics.2018.07.014) [DOI] [PubMed] [Google Scholar]
- 170.Hoffman RE, Boutros NN, Hu S, Berman RM, Krystal JH, Charney DS. et al. 2000. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet 355, 1073-1075. ( 10.1016/S0140-6736(00)02043-2) [DOI] [PubMed] [Google Scholar]
- 171.Khaleghi A, Zarafshan H, Vand SR, Mohammadi MR. 2020. Effects of non-invasive neurostimulation on autism spectrum disorder: a systematic review. Clin. Psychopharmacol. Neurosci. Neuropsychopharmacol. 18, 527-552. ( 10.9758/cpn.2020.18.4.527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Polanía R, Nitsche MA, Ruff CC. 2018. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 21, 174-187. ( 10.1038/s41593-017-0054-4) [DOI] [PubMed] [Google Scholar]
- 173.Saitovitch A, et al. 2016. Tuning eye-gaze perception by transitory STS inhibition. Cereb. Cortex 26, 2823-2831. ( 10.1093/cercor/bhw045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.He Z, Zhao J, Shen J, Muhlert N, Elliott R, Zhang D. 2020. The right VLPFC and downregulation of social pain: a TMS study. Hum. Brain Mapp. 41, 1362. ( 10.1002/hbm.24881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gooding-Williams G, Wang H, Kessler K. 2017. THETA-rhythm makes the world go round: dissociative effects of TMS theta versus alpha entrainment of right pTPJ on embodied perspective transformations. Brain Topogr. 30, 561-564. ( 10.1007/s10548-017-0557-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Young L, Camprodon JA, Hauser M, Pascual-Leone A, Saxe R. 2010. Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proc. Natl Acad. Sci. USA 107, 6753-6758. ( 10.1073/pnas.0914826107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. 2006. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 314, 829-832. ( 10.1126/science.1129156) [DOI] [PubMed] [Google Scholar]
- 178.Jeurissen D, Sack AT, Roebroeck A, Russ B, Pascual-Leone A. 2014. TMS affects moral judgment, showing the role of DLPFC and TPJ in cognitive and emotional processing. Front. Neurosci. 8, 18. ( 10.3389/fnins.2014.00018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sokhadze EM, El-Baz AS, Sears LL, Opris I, Casanova MF. 2014. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front. Syst. Neurosci. 8, 134. ( 10.3389/fnsys.2014.00134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ni H-C, et al. 2021. Intermittent theta burst stimulation over the posterior superior temporal sulcus for children with autism spectrum disorder: a 4-week randomized blinded controlled trial followed by another 4-week open-label intervention. Autism 25, 1279-1294. ( 10.1177/1362361321990534) [DOI] [PubMed] [Google Scholar]
- 181.Ni H-C, et al. In press. 5-day multi-session intermittent theta burst stimulation over bilateral posterior superior temporal sulci in adults with autism-a pilot study. Biomed. J. 45. ( 10.1016/j.bj.2021.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Enticott PG, Fitzgibbon BM, Kennedy HA, Arnold SL, Elliot D, Peachey A, Zangen A, Fitzgerald PB. et al. 2014. A double-blind, randomized trial of deep repetitive transcranial magnetic stimulation (rTMS) for autism spectrum disorder. Brain Stimulat. 7, 206-211. ( 10.1016/j.brs.2013.10.004) [DOI] [PubMed] [Google Scholar]
- 183.Fujino J, et al. 2021. A single session of navigation-guided repetitive transcranial magnetic stimulation over the right anterior temporoparietal junction in autism spectrum disorder. Brain Stimul. 14, 682-684. (doi:10.1016j.brs.2021.04.009) [DOI] [PubMed] [Google Scholar]
- 184.Enticott PG, et al. 2021. Protocol: repetitive transcranial magnetic stimulation (rTMS) in autism spectrum disorder: protocol for a multicentre randomised controlled clinical trial. BMJ Open 11, e046830. ( 10.1136/bmjopen-2020-046830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rubens MT, Zanto TP. 2012. Parameterization of transcranial magnetic stimulation. J. Neurophysiol. 107, 1257. ( 10.1152/jn.00716.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sellaro R, Nitsche MA, Colzato LS. 2016. The stimulated social brain: effects of transcranial direct current stimulation on social cognition. Ann. N. Y. Acad. Sci. 1369, 218-239. ( 10.1111/nyas.13098) [DOI] [PubMed] [Google Scholar]
- 187.Riva P, Romero Lauro LJ, Vergallito A, DeWall CN, Bushman BJ. 2015. Electrified emotions: modulatory effects of transcranial direct stimulation on negative emotional reactions to social exclusion. Social Neurosci. 10, 46-54. ( 10.1080/17470919.2014.946621) [DOI] [PubMed] [Google Scholar]
- 188.Wang J, Wang Y, Hu Z, Li X. 2014. Transcranial direct current stimulation of the dorsolateral prefrontal cortex increased pain empathy. Neuroscience 281, 202-207. ( 10.1016/j.neuroscience.2014.09.044) [DOI] [PubMed] [Google Scholar]
- 189.Boggio PS, Zaghi S, Fregni F. 2009. Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS). Neuropsychologia 47, 212-217. ( 10.1016/j.neuropsychologia.2008.07.022) [DOI] [PubMed] [Google Scholar]
- 190.Hogeveen J, Obhi SS, Banissy MJ, Santiesteban I, Press C, Catmur C, Bird G. et al. 2015. Task-dependent and distinct roles of the temporoparietal junction and inferior frontal cortex in the control of imitation. Social Cogn. Affect. Neurosci. 10, 1003-1009. ( 10.1093/scan/nsu148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Santiesteban I, Banissy MJ, Catmur C, Bird G. 2012. Enhancing social ability by stimulating right temporoparietal junction. Curr. Biol. 22, 2274-2277. ( 10.1016/j.cub.2012.10.018) [DOI] [PubMed] [Google Scholar]
- 192.Ruff CC, Ugazio G, Fehr E. 2013. Changing social norm compliance with noninvasive brain stimulation. Science 342, 482-484. ( 10.1126/science.1241399) [DOI] [PubMed] [Google Scholar]
- 193.Wilson JE, Quinn DK, Wilson JK, Garcia CM, Tesche CD. 2018. Transcranial direct current stimulation to the right temporoparietal junction for social functioning in autism spectrum disorder: a case report. J. ECT 34, e10. ( 10.1097/YCT.0000000000000445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Heeren A, Billieux J, Philippot P, De Raedt R, Baeken C, De Timary P, Maurage P, Vanderhasselt M-A. et al. 2017. Impact of transcranial direct current stimulation on attentional bias for threat: a proof-of-concept study among individuals with social anxiety disorder. Social Cogn. Affect. Neurosci. 12, 251-260. ( 10.1093/scan/nsw119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.McLaren ME, Nissim NR, Woods AJ. 2018. The effects of medication use in transcranial direct current stimulation: a brief review. Brain Stimulat. 11, 52-58. ( 10.1016/j.brs.2017.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rumi DO, et al. 2005. Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled study. Biol. Psychiatry 57, 162-166. ( 10.1016/j.biopsych.2004.10.029) [DOI] [PubMed] [Google Scholar]
- 197.Stein DJ, Medeiros LF, Caumo W, Torres IL. 2020. Transcranial direct current stimulation in patients with anxiety: current perspectives. Neuropsychiatr. Dis. Treat. 16, 161. ( 10.2147/NDT.S195840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang J, et al. 2021. Is transcranial direct current stimulation, alone or in combination with antidepressant medications or psychotherapies, effective in treating major depressive disorder? A systematic review and meta-analysis. BMC Med. 19, 319. ( 10.1186/s12916-021-02181-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bell SB, DeWall N. 2018. Does transcranial direct current stimulation to the prefrontal cortex affect social behavior? A meta-analysis. Social Cogn. Affect. Neurosci. 13, 899-906. ( 10.1093/scan/nsy069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cole EJ, et al. 2020. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am. J. Psychiatry 177, 716-726. ( 10.1176/appi.ajp.2019.19070720) [DOI] [PubMed] [Google Scholar]
- 201.Donaldson P, et al. 2019. A clinical trial comparing intermittent theta burst stimulation to dorsomedial prefrontal cortex and right temporoparietal junction in autism spectrum disorder. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 12, 395. ( 10.1016/j.brs.2018.12.268) [DOI] [Google Scholar]
- 202.Corsini RJ, Rosenberg B. 1955. Mechanisms of group psychotherapy: processes and dynamics. J. Abnorm. Social Psychol. 51, 406-411. ( 10.1037/h0048439) [DOI] [PubMed] [Google Scholar]
- 203.Vinogradov S, Cox PD, Yalom ID. 2003. Group therapy. In The American psychiatric publishing textbook of clinical psychiatry (eds Hales RE, Yudofsky SC), pp. 1333-1371. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- 204.Yuan M, et al. 2016. Group cognitive behavioral therapy modulates the resting-state functional connectivity of amygdala-related network in patients with generalized social anxiety disorder. BMC Psychiatry 16, 198. ( 10.1186/s12888-016-0904-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Guidi J, Fava GA. 2021. Sequential combination of pharmacotherapy and psychotherapy in major depressive disorder: a systematic review and meta-analysis. JAMA Psychiatry 78, 261-269. ( 10.1001/jamapsychiatry.2020.3650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Molenaar PJ, Dekker J, Van R, Hendriksen M, Vink A, Schoevers RA. et al. 2007. Does adding psychotherapy to pharmacotherapy improve social functioning in the treatment of outpatient depression? Depress. Anxiety 24, 553-562. ( 10.1002/da.20254) [DOI] [PubMed] [Google Scholar]
- 207.Renner F, Cuijpers P, Huibers MJH. 2014. The effect of psychotherapy for depression on improvements in social functioning: a meta-analysis. Psychol. Med. 44, 2913-2926. ( 10.1017/S0033291713003152) [DOI] [PubMed] [Google Scholar]
- 208.Blanco C, et al. 2010. A placebo-controlled trial of phenelzine, cognitive behavioral group therapy, and their combination for social anxiety disorder. Arch. Gen. Psychiatry 67, 286-295. ( 10.1001/archgenpsychiatry.2010.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Reiff CM, Richman EE, Nemeroff CB, Carpenter LL, Widge AS, Rodriguez CI, Kalin NH, Mcdonald WM. et al. 2020. Psychedelics and psychedelic-assisted psychotherapy. Am. J. Psychiatry 177, 391-410. ( 10.1176/appi.ajp.2019.19010035) [DOI] [PubMed] [Google Scholar]
- 210.Luoma JB, Chwyl C, Bathje GJ, Davis AK, Lancelotta R. 2020. A meta-analysis of placebo-controlled trials of psychedelic-assisted therapy. J. Psychoactive Drugs 52, 289-299. ( 10.1080/02791072.2020.1769878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Trope A, Anderson BT, Hooker AR, Glick G, Stauffer C, Woolley JD. et al. 2019. Psychedelic-assisted group therapy: a systematic review. J. Psychoactive Drugs 51, 174-188. ( 10.1080/02791072.2019.1593559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Stauffer CS, Anderson BT, Ortigo KM, Woolley J. 2021. Psilocybin-assisted group therapy and attachment: observed reduction in attachment anxiety and influences of attachment insecurity on the psilocybin experience. ACS Pharmacol. Transl. Sci. 4, 526-532. ( 10.1021/acsptsci.0c00169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mitchell JM, et al. 2021. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 27, 1025-1033. ( 10.1038/s41591-021-01336-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hysek CM, et al. 2014. MDMA enhances emotional empathy and prosocial behavior. Social Cogn. Affect. Neurosci. 9, 1645-1652. ( 10.1093/scan/nst161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bershad AK, Mayo LM, Van Hedger K, Mcglone F, Walker SC, De Wit H. 2019. Effects of MDMA on attention to positive social cues and pleasantness of affective touch. Neuropsychopharmacology 44, 1698-1705. ( 10.1038/s41386-019-0402-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bedi G, Phan KL, Angstadt M, de Wit H. 2009. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl.) 207, 73. ( 10.1007/s00213-009-1635-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Forstmann M, Yudkin DA, Prosser AMB, Heller SM, Crockett MJ. 2020. Transformative experience and social connectedness mediate the mood-enhancing effects of psychedelic use in naturalistic settings. Proc. Natl Acad. Sci. USA 117, 2338-2346. ( 10.1073/pnas.1918477117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wagner MT, Mithoefer MC, Mithoefer AT, Macaulay RK, Jerome L, Yazar-Klosinski B, Doblin R. et al. 2017. Therapeutic effect of increased openness: investigating mechanism of action in MDMA-assisted psychotherapy. J. Psychopharmacol. (Oxf.) 31, 967-974. ( 10.1177/0269881117711712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Danforth AL, Grob CS, Struble C, Feduccia AA, Walker N, Jerome L, Yazar-Klosinski B, Emerson A. et al. 2018. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacology (Berl.) 235, 3137-3148. ( 10.1007/s00213-018-5010-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sessa B, Higbed L, Nutt D. 2019. A review of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front. Psychiatry 10, 138. ( 10.3389/fpsyt.2019.00138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. et al. 2004. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch. Gen. Psychiatry 61, 34-41. ( 10.1001/archpsyc.61.1.34) [DOI] [PubMed] [Google Scholar]
- 222.Winter L, et al. 2019. Neurobiological mechanisms of metacognitive therapy–an experimental paradigm. Front. Psychol. 10, 660. ( 10.3389/fpsyg.2019.00660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Huang X, Huang P, Li D, Zhang Y, Wang T, Mu J, Li Q, Xie P. 2014. Early brain changes associated with psychotherapy in major depressive disorder revealed by resting-state fMRI: evidence for the top-down regulation theory. Int. J. Psychophysiol. 94, 437-444. ( 10.1016/j.ijpsycho.2014.10.011) [DOI] [PubMed] [Google Scholar]
- 224.Zhang Y, Meng T, Yang Y, Hu Y. 2020. Experience-dependent counselor-client brain synchronization during psychological counseling. eNeuro 7, ENEURO.0236-20.2020. ( 10.1523/ENEURO.0236-20.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Straub J, Plener PL, Sproeber N, Sprenger L, Koelch MG, Groen G, Abler B. et al. 2015. Neural correlates of successful psychotherapy of depression in adolescents. J. Affect. Disord. 183, 239-246. ( 10.1016/j.jad.2015.05.020) [DOI] [PubMed] [Google Scholar]
- 226.Combs ML, Slaby DA. 1977. Social-skills training with children. In Advances in clinical child psychology, vol. 1 (eds Lahey BB, Kazdin AE), pp. 161-201. New York, NY: Springer. [Google Scholar]
- 227.Spence SH. 2003. Social skills training with children and young people: theory, evidence and practice. Child Adolesc. Ment. Health 8, 84-96. ( 10.1111/1475-3588.00051) [DOI] [PubMed] [Google Scholar]
- 228.Turner DT, Mcglanaghy E, Cuijpers P, Van Der Gaag M, Karyotaki E, Macbeth A. et al. 2018. A meta-analysis of social skills training and related interventions for psychosis. Schizophr. Bull. 44, 475-491. ( 10.1093/schbul/sbx146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Soares EE, Bausback K, Beard CL, Higinbotham M, Bunge EL, Gengoux GW. et al. 2021. Social skills training for autism spectrum disorder: a meta-analysis of in-person and technological interventions. J. Technol. Behav. Sci. 6, 166-180. ( 10.1007/s41347-020-00177-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Herbert JD, Gaudiano BA, Rheingold AA, Myers VH, Dalrymple K, Nolan EM. 2005. Social skills training augments the effectiveness of cognitive behavioral group therapy for social anxiety disorder. Behav. Ther. 36, 125-138. ( 10.1016/S0005-7894(05)80061-9) [DOI] [Google Scholar]
- 231.Grant PM, Beck AT. 2010. Asocial beliefs as predictors of asocial behavior in schizophrenia. Psychiatry Res. 177, 65-70. ( 10.1016/j.psychres.2010.01.005) [DOI] [PubMed] [Google Scholar]
- 232.Fowler D, et al. 2018. Social recovery therapy in combination with early intervention services for enhancement of social recovery in patients with first-episode psychosis (SUPEREDEN3): a single-blind, randomised controlled trial. Lancet Psychiatry 5, 41-50. ( 10.1016/S2215-0366(17)30476-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Heuschkel K, Kuypers KPC. 2020. Depression, mindfulness, and psilocybin: possible complementary effects of mindfulness meditation and psilocybin in the treatment of depression. A review. Front. Psychiatry 11, 224. ( 10.3389/fpsyt.2020.00224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Madsen MK, et al. 2020. A single psilocybin dose is associated with long-term increased mindfulness, preceded by a proportional change in neocortical 5-HT2A receptor binding. Eur. Neuropsychopharmacol. 33, 71-80. ( 10.1016/j.euroneuro.2020.02.001) [DOI] [PubMed] [Google Scholar]
- 235.Smigielski L, Scheidegger M, Kometer M, Vollenweider FX. 2019. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. Neuroimage 196, 207-215. ( 10.1016/j.neuroimage.2019.04.009) [DOI] [PubMed] [Google Scholar]
- 236.Firth J, Carney R, French P, Elliott R, Yung A. 2017. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr. Bull. 43, 546-556. ( 10.1093/schbul/sbx024.102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pater M, Spreen M, van Yperen T. 2021. The developmental progress in social behavior of children with autism spectrum disorder getting music therapy. A multiple case study. Child. Youth Serv. Rev. 120, 105767. ( 10.1016/j.childyouth.2020.105767) [DOI] [Google Scholar]
- 238.Gooding LF. 2011. The effect of a music therapy social skills training program on improving social competence in children and adolescents with social skills deficits. J. Music Ther. 48, 440-462. ( 10.1093/jmt/48.4.440) [DOI] [PubMed] [Google Scholar]
- 239.Liégeois-Chauvel C, Peretz I, Babaï M, Laguitton V, Chauvel P. 1998. Contribution of different cortical areas in the temporal lobes to music processing. Brain 121, 1853-1867. ( 10.1093/brain/121.10.1853) [DOI] [PubMed] [Google Scholar]
- 240.LaGasse AB. 2017. Social outcomes in children with autism spectrum disorder: a review of music therapy outcomes. Patient Relat. Outcome Meas. 8, 23-32. ( 10.2147/PROM.S106267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Derntl B, Habel U. 2011. Deficits in social cognition: a marker for psychiatric disorders? Eur. Arch. Psychiatry Clin. Neurosci. 261, 145-149. ( 10.1007/s00406-011-0244-0) [DOI] [PubMed] [Google Scholar]
- 242.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. 2012. The social motivation theory of autism. Trends Cogn. Sci. 16, 231-239. ( 10.1016/j.tics.2012.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fulford D, Campellone T, Gard DE. 2018. Social motivation in schizophrenia: how research on basic reward processes informs and limits our understanding. Clin. Psychol. Rev. 63, 12-24. ( 10.1016/j.cpr.2018.05.007) [DOI] [PubMed] [Google Scholar]
- 244.Nienow TM, MacDonald AW, Lim KO. 2016. TDCS produces incremental gain when combined with working memory training in patients with schizophrenia: a proof of concept pilot study. Schizophr. Res. 172, 218-219. ( 10.1016/j.schres.2016.01.053) [DOI] [PubMed] [Google Scholar]
- 245.Rassovsky Y, Dunn W, Wynn J, Wu AD, Iacoboni M, Hellemann G, Green MF. et al. 2015. The effect of transcranial direct current stimulation on social cognition in schizophrenia: a preliminary study. Schizophr. Res. 165, 171-174. ( 10.1016/j.schres.2015.04.016) [DOI] [PubMed] [Google Scholar]
- 246.Challis C, Berton O. 2015. Top-down control of serotonin systems by the prefrontal cortex: a path towards restored socioemotional function in depression. ACS Chem. Neurosci. 6, 1040. ( 10.1021/acschemneuro.5b00007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hoch E, Niemann D, Von Keller R, Schneider M, Friemel CM, Preuss UW, Hasan A, Pogarell O. et al. 2019. How effective and safe is medical cannabis as a treatment of mental disorders? A systematic review. Eur. Arch. Psychiatry Clin. Neurosci. 269, 87-105. ( 10.1007/s00406-019-00984-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lazary J, et al. 2021. Peripheral endocannabinoid serum level in association with repetitive transcranial magnetic stimulation (rTMS) treatment in patients with major depressive disorder. Scient. Rep. 11, 8867. ( 10.1038/s41598-021-87840-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Schechter M, Weller A, Pittel Z, Gross M, Zimmer A, Pinhasov A. 2013. Endocannabinoid receptor deficiency affects maternal care and alters the dam's hippocampal oxytocin receptor and brain-derived neurotrophic factor expression. J. Neuroendocrinol. 25, 898-909. ( 10.1111/jne.12082) [DOI] [PubMed] [Google Scholar]
- 250.Russo R, D'Agostino G, Mattace Raso G, Avagliano C, Cristiano C, Meli R, Calignano A. et al. 2012. Central administration of oxytocin reduces hyperalgesia in mice: implication for cannabinoid and opioid systems. Peptides 38, 81-88. ( 10.1016/j.peptides.2012.08.005) [DOI] [PubMed] [Google Scholar]
- 251.Borie AM, et al. et al. 2022. Social experience alters oxytocinergic modulation in the nucleus accumbens of female prairie voles. Curr. Biol. 32, 1026-1037. ( 10.1016/j.cub.2022.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.van der Flier FE, Kwee CMB, Cath DC, Batelaan NM, Groenink L, Duits P, Van Der Veen DC, Van Balkom AJLM, Baas JMP. et al. 2019. Cannabidiol enhancement of exposure therapy in treatment refractory patients with phobias: study protocol of a randomized controlled trial. BMC Psychiatry 19, 69. ( 10.1186/s12888-019-2022-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Di Fede G. 2020. A neuroendocrine therapeutic approach with the pineal hormone melatonin, cannabidiol and oxytocin (MCO regimen) in the treatment of the autism spectrum disorders. J. Immunol. Allergy. 1, 1-7. ( 10.37191/Mapsci-2582-6549-1(2)-007) [DOI] [Google Scholar]
- 254.Kaur R, Ambwani SR, Singh S. 2016. Endocannabinoid system: a multi-facet therapeutic target. Curr. Clin. Pharmacol. 11, 110-117. ( 10.2174/1574884711666160418105339) [DOI] [PubMed] [Google Scholar]
- 255.Cristino L, Bisogno T, Di Marzo V. 2020. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 16, 9-29. ( 10.1038/s41582-019-0284-z) [DOI] [PubMed] [Google Scholar]
- 256.Firth CL, Maher JE, Dilley JA, Darnell A, Lovrich NP. 2019. Did marijuana legalization in Washington State reduce racial disparities in adult marijuana arrests? Subst. Use Misuse 54, 1582-1587. ( 10.1080/10826084.2019.1593007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jordan A, Allsop AS, Collins PY. 2021. Decriminalising being Black with mental illness. Lancet Psychiatry 8, 8-9. ( 10.1016/S2215-0366(20)30519-8) [DOI] [PubMed] [Google Scholar]
- 258.Willits DW, Solensten B, Meize M, Stohr MK, Makin DA, Hemmens C, Stanton DL, Lovrich NP. et al. 2022. Racial disparities in the wake of cannabis legalization: documenting persistence and change. Race Justice, 21533687221087356. ( 10.1177/21533687221087355) [DOI] [Google Scholar]
- 259.Hasin DS. 2018. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology 43, 195. ( 10.1038/npp.2017.198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.D'Souza DC, et al. 2016. Cannabinoids and psychosis. Curr. Pharm. Design 22, 6380-6391. [DOI] [PubMed] [Google Scholar]
- 261.Gage SH, Hickman M, Zammit S. 2016. Association between cannabis and psychosis: epidemiologic evidence. Biol. Psychiatry 79, 549-556. ( 10.1016/j.biopsych.2015.08.001) [DOI] [PubMed] [Google Scholar]
- 262.Shah D, Chand P, Bandawar M, Benegal V, Murthy P. 2017. Cannabis induced psychosis and subsequent psychiatric disorders. Asian J. Psychiatry 30, 180-184. ( 10.1016/j.ajp.2017.10.003) [DOI] [PubMed] [Google Scholar]
- 263.Hirvonen J, et al. 2012. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry 17, 642-649. ( 10.1038/mp.2011.82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S, Van Laere K. et al. 2014. Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J. Neurosci. 34, 2822. ( 10.1523/JNEUROSCI.0849-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sherman BJ, Baker NL, McRae-Clark AL. 2017. Effect of oxytocin pretreatment on cannabis outcomes in a brief motivational intervention. Psychiatry Res. 249, 318-320. ( 10.1016/j.psychres.2017.01.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lester KJ, et al. 2017. Genetic variation in the endocannabinoid system and response to cognitive behavior therapy for child anxiety disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 174, 144-155. ( 10.1002/ajmg.b.32467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Blanton HL, Barnes RC, Mchann MC, Bilbrey JA, Wilkerson JL, Guindon J. et al. 2021. Sex differences and the endocannabinoid system in pain. Pharmacol. Biochem. Behav. 202, 173107. ( 10.1016/j.pbb.2021.173107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Craft RM, Marusich JA, Wiley JL. 2013. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 92, 476-481. ( 10.1016/j.lfs.2012.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Reed SC, Haney M, Manubay J, Campagna BR, Reed B, Foltin RW, Evans SM. et al. 2019. Sex differences in stress reactivity after intranasal oxytocin in recreational cannabis users. Pharmacol. Biochem. Behav. 176, 72-82. ( 10.1016/j.pbb.2018.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Meccariello R, Santoro M, D'angelo J, Morrone BR, Fasano B, Viggiano RW, Pierantoni SM. et al. 2020. The epigenetics of the endocannabinoid system. Int. J. Mol. Sci. 21, E1113. ( 10.3390/ijms21031113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Surkin PN, Gallino SL, Luce V, Correa F, Fernandez-Solari J, De Laurentiis A. et al. 2018. Pharmacological augmentation of endocannabinoid signaling reduces the neuroendocrine response to stress. Psychoneuroendocrinology 87, 131-140. ( 10.1016/j.psyneuen.2017.10.015) [DOI] [PubMed] [Google Scholar]
- 272.Danan D, Todder D, Zohar J, Cohen H. 2021. Is PTSD-phenotype associated with HPA-axis sensitivity?: the endocannabinoid system in modulating stress response in rats. Int. J. Mol. Sci. 22, 6416. ( 10.3390/ijms22126416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Jumpertz R, Guijarro A, Pratley RE, Piomelli D, Krakoff J. 2011. Central and peripheral endocannabinoids and cognate acylethanolamides in humans: association with race, adiposity, and energy expenditure. J. Clin. Endocrinol. Metab. 96, 787-791. ( 10.1210/jc.2010-2028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Thethi TK, et al. 2020. Racial and sex differences in the polymorphisms of the endocannabinoid receptor genes in obesity. J. Diabetes Complications 34, 107682. ( 10.1016/j.jdiacomp.2020.107682) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.