This pooled analysis study examines associations between prenatal urinary biomarkers of phthalate exposure and preterm birth.
Key Points
Question
Is phthalate exposure during pregnancy associated with preterm birth?
Findings
In this pooled analysis of 16 studies in the US including 6045 pregnant individuals, phthalate metabolites were quantified in urine samples collected during pregnancy. Higher urinary metabolite concentrations for several prevalent phthalates were associated with greater odds of delivering preterm, and hypothetical interventions to reduce phthalate exposure levels were associated with fewer preterm births.
Meaning
In this large observational study, urinary biomarkers of common phthalates used in consumer products were a risk factor for preterm birth.
Abstract
Importance
Phthalate exposure is widespread among pregnant women and may be a risk factor for preterm birth.
Objective
To investigate the prospective association between urinary biomarkers of phthalates in pregnancy and preterm birth among individuals living in the US.
Design, Setting, and Participants
Individual-level data were pooled from 16 preconception and pregnancy studies conducted in the US. Pregnant individuals who delivered between 1983 and 2018 and provided 1 or more urine samples during pregnancy were included.
Exposures
Urinary phthalate metabolites were quantified as biomarkers of phthalate exposure. Concentrations of 11 phthalate metabolites were standardized for urine dilution and mean repeated measurements across pregnancy were calculated.
Main Outcomes and Measures
Logistic regression models were used to examine the association between each phthalate metabolite with the odds of preterm birth, defined as less than 37 weeks of gestation at delivery (n = 539). Models pooled data using fixed effects and adjusted for maternal age, race and ethnicity, education, and prepregnancy body mass index. The association between the overall mixture of phthalate metabolites and preterm birth was also examined with logistic regression. G-computation, which requires certain assumptions to be considered causal, was used to estimate the association with hypothetical interventions to reduce the mixture concentrations on preterm birth.
Results
The final analytic sample included 6045 participants (mean [SD] age, 29.1 [6.1] years). Overall, 802 individuals (13.3%) were Black, 2323 (38.4%) were Hispanic/Latina, 2576 (42.6%) were White, and 328 (5.4%) had other race and ethnicity (including American Indian/Alaskan Native, Native Hawaiian, >1 racial identity, or reported as other). Most phthalate metabolites were detected in more than 96% of participants. Higher odds of preterm birth, ranging from 12% to 16%, were observed in association with an interquartile range increase in urinary concentrations of mono-n-butyl phthalate (odds ratio [OR], 1.12 [95% CI, 0.98-1.27]), mono-isobutyl phthalate (OR, 1.16 [95% CI, 1.00-1.34]), mono(2-ethyl-5-carboxypentyl) phthalate (OR, 1.16 [95% CI, 1.00-1.34]), and mono(3-carboxypropyl) phthalate (OR, 1.14 [95% CI, 1.01-1.29]). Among approximately 90 preterm births per 1000 live births in this study population, hypothetical interventions to reduce the mixture of phthalate metabolite levels by 10%, 30%, and 50% were estimated to prevent 1.8 (95% CI, 0.5-3.1), 5.9 (95% CI, 1.7-9.9), and 11.1 (95% CI, 3.6-18.3) preterm births, respectively.
Conclusions and Relevance
Results from this large US study population suggest that phthalate exposure during pregnancy may be a preventable risk factor for preterm delivery.
Introduction
Preterm birth is a leading cause of neonatal mortality and morbidity.1 The societal burden of preterm birth is particularly high in the US,2 with approximately 10% of pregnancies delivered preterm annually.3 While the underlying risk factors for most preterm births are unknown, exposure to environmental chemicals like phthalates may play a role.
Phthalates are synthetic chemicals used in everyday consumer products such as personal care items and food processing or packaging.4 Exposure can occur through many sources, including household dust, diet, and personal care products like cosmetics.5 Consequently, phthalate exposure is ubiquitous among pregnant individuals.6,7 Human and animal studies suggest that prenatal phthalate exposure is associated with adverse effects on children’s neurodevelopment and male reproductive tract development.8,9 While several studies have found positive associations between prenatal biomarkers of phthalate exposure and preterm birth,10,11,12,13,14,15,16 others have shown null17,18,19,20 or inverse21,22,23 associations. This may be partly due to the limited number of preterm births included, differences in exposure assessment methods, and variation in the baseline risk of preterm birth and phthalate exposure.
The purpose of this analysis was to pool individual-level data from 16 prospective studies conducted in the US11,12,14,17,21,22,23,24,25,26,27,28,29,30,31,32 and examine associations between prenatal urinary biomarkers of phthalate exposure and preterm birth. We also considered the potential influence of exposure to an overall phthalate mixture and evaluated how hypothetical interventions to reduce this exposure could impact preterm birth.
Methods
Study Population
In May 2019, we systematically reviewed the literature to identify epidemiologic studies conducted in the US with data on urinary phthalate metabolites quantified during pregnancy and gestational age at delivery (eMethods 1 and 2 in the Supplement). We focused on US studies to facilitate generalizability of results to the US general population, which experiences relatively high levels of phthalate exposure33 and high rates of preterm birth.34 Of 21 unique studies, 17 had sufficient sample size (N > 50) and 16 corresponding authors agreed to collaborate (eFigure 1 in the Supplement). Participating studies received ethics approval from the institutional review board or human research ethics committees from their respective institutions. Participants provided written or verbal informed consent. Analysis of anonymized data sets sent to the National Institute of Environmental Health Sciences was deemed to not be human subjects research by the National Institute of Environmental Health Sciences institutional review board. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Study acronyms and design characteristics are provided in Table 1, and eligibility criteria are described in eTable 1 in the Supplement. All studies prospectively enrolled participants during prepregnancy (North Carolina Early Pregnancy Study [EPS]14 and Environment and Reproductive Health Study [EARTH]27) or pregnancy and all participants had live births between 1983 and 2018. The only case-control study included was LIFECODES,11 a study of preterm birth nested within a prospective cohort. Studies provided gestational age at delivery (defined by last menstrual period, early pregnancy ultrasonography, date of conception in pregnancies using assisted reproductive technologies, or some combination thereof). We defined preterm birth as delivery prior to 37 weeks’ gestation. Our final analytic sample included 6045 participants after excluding 1136 of 7181 participants in the total pooled sample (eFigure 1 and eTable 2 in the Supplement).
Table 1. Study Design Elements Among Cohorts Included in the Pooled Phthalate and Preterm Birth Study Population (N = 6045).
| Study | No. of individuals | Preterm birth, No. (%) | Years of deliverya | Location | Primary method for determining gestational age | Mean gestational age at enrollment, wkb |
|---|---|---|---|---|---|---|
| Puerto Rico Testsite for Exploring Contamination Threats (PROTECT)12 | 1101 | 100 (9.1) | 2011-2018 | Puerto Rico | Last menstrual period and ultrasonography | 11 |
| The Infant Development and the Environment Study (TIDES)24 | 779 | 69 (8.9) | 2011-2013 | California, Minnesota, Washington, and New York | Ultrasonography or physician estimate | 12 |
| LIFECODES11 | 480 | 130 (27.1) | 2007-2009 | Massachusetts | Last menstrual period and ultrasonography | 10 |
| Healthy Start Study (Healthy Start)17 | 444 | 14 (3.2) | 2012-2014 | Colorado | Medical record | 18 |
| Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS)25 | 429 | 27 (6.3) | 1999-2001 | California | Medical record | 14 |
| Columbia Center for Children's Environmental Health (CCCEH)26 | 389 | 14 (3.6) | 1999-2006 | New York | Medical record | 33 |
| Health Outcomes and Measures of the Environment Study (HOME)23 | 389 | 37 (9.5) | 2003-2006 | Ohio, Kentucky | Last menstrual period | 16 |
| Environment and Reproductive Health Study (EARTH)27 | 385 | 27 (7.0) | 2005-2017 | Massachusetts | Medical record and guidelines for medically assisted reproduction | Prepregnancyc |
| Children’s Environmental Health Study at the Mount Sinai School of Medicine (MSSM)22 | 362 | 28 (7.7) | 1998-2002 | New York | Last menstrual period | 31 |
| Study for Future Families (SFF)21 | 353 | 17 (4.8) | 2000-2005 | California, Minnesota, Missouri, and Iowa | Medical record or last menstrual period | 25 |
| Reproductive Development Study (RDS)28 | 318 | 28 (8.8) | 2011-2014 | South Carolina | Ultrasonography | 20 |
| Harvard Epigenetic Birth Cohort (HEBC)30 | 189 | 12 (6.3) | 2007-2009 | Massachusetts | Medical record | 10 |
| Markers of Autism Risk in Babies-Learning Early Signs (MARBLES)29 | 179 | 12 (6.7) | 2007-2014 | California | Medical record | 20 |
| The North Carolina Early Pregnancy Study (EPS)14 | 126 | 5 (4.0) | 1983-1986 | North Carolina | Day of implantation | Prepregnancyc |
| Michigan Mother-Infant Pairs Project (MMIP)31 | 68 | 2 (2.9) | 2010-2013 | Michigan | Medical record | 11 |
| Rutgers University32 | 54 | 17 (31.5) | 2009-2010 | New Jersey | Medical record | 26 |
Data harmonization details for year of delivery data are provided in eMethods 2 in the Supplement.
Mean gestational age at enrollment is based on participants included in this study.
All urine samples analyzed in this study were collected after conception and during pregnancy (at least 1 week prior to delivery).
Phthalate Exposure Assessment
Participants provided urine samples during pregnancy for quantification of phthalate monoester metabolites. Urinary phthalate metabolites are the preferred biomarker of phthalate exposure35 and are highly stable in urine samples stored at ≤20 °C, as they were for all cohorts.36,37 All studies collected spot urine samples, except for EPS14 and Markers of Autism Risk in Babies-Learning Early Sign (MARBLES)29 that pooled multiple samples prior to measurement (eTable 1 in the Supplement). Phthalate metabolite measurements were performed separately by cohort. Most studies measured at the US Centers for Disease Control and Prevention (CDC) or using CDC-developed methods, and targeted the same metabolites as the CDC biomonitoring program. Briefly, after enzymatic hydrolysis of phthalate metabolite conjugates, phthalate metabolites were extracted from urine using online solid phase extraction, separated by high-performance liquid chromatography, and detected by isotope dilution tandem mass spectrometry. The analysis of deidentified specimens at the CDC was determined not to constitute engagement in human subjects research. We included 11 metabolites based on availability in at least 50% of participants (eTable 4 in the Supplement): monoethyl phthalate, mono-n-butyl phthalate (MBP), mono-isobutyl phthalate, monobenzyl phthalate, mono(2-ethylhexyl) phthalate, mono(2-ethyl-5-hydroxyhexyl) phthalate, mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(2-ethyl-5-oxohexyl) phthalate, mono(3-carboxypropyl) phthalate (MCPP), monocarboxy-isooctyl phthalate, and monocarboxy-isononyl phthalate.
Statistical Analyses
Using multiple imputation by chained equations, we simultaneously imputed (1) phthalate biomarker concentrations below the limit of detection without instrument-read values (eMethods 3 in the Supplement) and (2) missing covariates (eTable 5 in the Supplement). We performed all subsequent analyses on the imputed data sets and pooled results using Rubin’s rules.38 Studies measured urinary specific gravity or creatinine to account for urine dilution (eTable 1 in the Supplement). We used covariate-adjusted standardization to correct phthalate metabolite concentrations for urine dilution (eMethods 4 in the Supplement).39,40 Most studies (9 of 16) quantified phthalate metabolites in multiple (range, 2-10) urine samples (eTable 1 in the Supplement). After dilution standardization, we calculated the within-participant geometric mean of phthalate metabolite concentrations across pregnancy. Subsequently, we natural-log–transformed concentrations and standardized concentrations by dividing by the interquartile range (IQR) to facilitate interpretability.
We used multivariable logistic regression to examine associations of mean pregnancy phthalate metabolites with odds of preterm birth. Odds ratios and 95% CIs were interpreted as the change in log-odds of preterm birth per 1-IQR increase in mean phthalate metabolite concentration. Crude models adjusted for study (via fixed effects for each study) and adjusted models included additional covariates that were measured across all 16 studies. We selected primary confounders a priori from the literature, including self-reported maternal race and ethnicity (categorical),18,41,42 education (categorical),12,17,18,28,41 maternal age at enrollment (years),12,18,28,41 and prepregnancy body mass index.17,18,28,41 Race and ethnicity was used as a confounder based on the consistent disparities in preterm birth43 and environmental exposures41 experienced by minoritized racial and ethnic populations in the US, which is driven by social determinants including racism and discrimination.44 We defined race and ethnicity by combining several self-identified categories to maximize sample size and consistency across pooled studies, including non-Hispanic Black, Hispanic/Latina, non-Hispanic White, and other (including American Indian/Alaskan Native, Native Hawaiian, >1 racial identity, or reported as other).
We used 2 complementary methods, quantile g-computation and standard g-computation, to examine the association of an overall mixture of phthalate metabolites and preterm birth. The mixture included all metabolites except monocarboxy-isooctyl phthalate and monocarboxy-isononyl phthalate, which were excluded a priori because fewer participants (n = 3758) and studies (10 total) quantified these biomarkers. This provided 5471 participants (14 studies) for the mixture analyses (eTable 6 in the Supplement). We used quantile g-computation to examine the odds of preterm birth per IQR increase in all phthalate metabolites in the mixture.45 We used standard g-computation to estimate the probability of preterm birth following several hypothetical interventions to reduce concentrations of the phthalate metabolite mixture,46 which provides potentially more interpretable results than model coefficients.47,48 Hypothetical interventions reduced each metabolite in the mixture by 10% to 90% in 10% increments. The 95% CIs were estimated using nonparametric bootstrapping (2.5th and 97.5th percentiles across 2000 iterations).46 We transformed results to be interpreted as the estimated number of preterm births prevented per 1000 live births by contrasting each hypothetical intervention with no intervention.
We conducted several sensitivity analyses. (1) To assess heterogeneity in effect estimates by study, we qualitatively compared estimates from fixed-effect models to mixed models in which we specified study indicator as a random intercept49; used Wald tests of goodness of fit for an interaction term between study and metabolite in the primary model49; and examined differences in effect estimates after we fit models that drop participants from single cohorts. This leave-1-out analysis provides a way to examine how overall results may have been influenced by individual cohorts. (2) We used Wald tests to assess potential differences in confounding across studies by fitting a series of models that additionally included interaction terms between study and each of the following covariates: maternal age, prepregnancy body mass index, race and ethnicity, and education. (3) We fit models additionally adjusted for precision variables associated with phthalate exposure or preterm delivery, including delivery year, smoking, or parity. (4) We assessed potential effect measure modification by fetal sex using model stratification and a nonstratified model with an interaction term between phthalate metabolite and sex.24 (5) We examined nonlinearity in associations by fitting quadratic terms. (6) We examined metabolite associations with gestational age at delivery (continuous) using multivariable linear regression using the same covariates but applied inverse probability of sampling weights to account for the LIFECODES study design.50 We chose not to conduct sensitivity analyses for other pregnancy complications (eg, preeclampsia) because evidence suggests such conditions are potentially on the causal pathway between phthalate exposure and preterm birth.51,52,53 We considered Wald tests or interactions statistically significant if 2-sided P values were less than .05. We performed analyses using R version 4.0.3 (R Foundation).
Results
Study Characteristics
The overall study population consisted of 6045 pregnant individuals (mean [SD] age, 29.1 [6.1] years), of whom 539 (9%) delivered preterm (eFigure 1 in the Supplement). Overall participant characteristics are presented in Table 2 and characteristics by study are shown in eTable 3 in the Supplement. A total of 802 individuals (13.3%) were Black, 2323 (38.4%) were Hispanic/Latina, 2576 (42.6%) were White, and 328 (5.4%) had other race and ethnicity (including American Indian/Alaskan Native, Native Hawaiian, >1 racial identity, or reported as other). Participant characteristics were similar between individuals who delivered term vs preterm (Table 2). Concentrations of urinary phthalate metabolites included for analysis were detectable in 96% or more of urine samples, except for mono(2-ethylhexyl) phthalate (83%) and MCPP (90%) (eTable 5 in the Supplement) and were highest for monoethyl phthalate, MBP, and MECPP (eTable 7 in the Supplement). Correlations were highest between metabolites with shared parent chemicals (eFigure 2 in the Supplement). Overall, there was substantial overlap in the distributions of phthalate metabolite concentrations across studies (eFigure 3 in the Supplement). However, concentrations for several metabolites (eg, monobenzyl phthalate, MCPP) were higher for EPS,14 which was the only study to collect samples in the 1980s.
Table 2. Distributions of Participant Characteristics Overall and by Preterm Birth Outcome in the Pooled Phthalate and Preterm Birth Study.
| Characteristica | No. (%) | ||
|---|---|---|---|
| Overall | Term birthb | Preterm birthb | |
| Total | 6045 (100) | 5506 (91) | 539 (9) |
| Gestational age at delivery, mean (SD), wk | 39.1 (1.9) | 39.5 (1.2) | 34.8 (2.5) |
| Missing, No. (%) | 0 | 0 | 0 |
| Maternal age, mean (SD), y | 29.1 (6.1) | 29.0 (6.1) | 30.0 (6.4) |
| Missing, No. (%) | 16 (0.3) | 16 (0.3) | 0 |
| Maternal race and ethnicityc | |||
| Non-Hispanic Black | 802 (13.3) | 710 (88.5) | 92 (11.5) |
| Hispanic/Latina | 2323 (38.4) | 2145 (92.3) | 178 (7.7) |
| Non-Hispanic White | 2576 (42.6) | 2342 (90.9) | 234 (9.1) |
| Other | 328 (5.4) | 297 (90.5) | 31 (9.5) |
| Missing | 16 (0.3) | 12 (75) | 4 (25) |
| Maternal education | |||
| <High school | 1045 (17.3) | 960 (91.9) | 85 (8.1) |
| High school | 706 (11.7) | 633 (89.7) | 73 (10.3) |
| Some college | 1410 (23.3) | 1294 (91.8) | 116 (8.2) |
| College graduate | 1263 (20.9) | 1141 (90.3) | 122 (9.7) |
| Graduate school | 1223 (20.2) | 1109 (90.7) | 114 (9.3) |
| Missing | 398 (6.6) | 369 (92.7) | 29 (7.3) |
| Maternal prepregnancy body mass indexd | 25.7 (6.0) | 25.6 (5.9) | 26.6 (6.5) |
| Missing | 496 (8.2) | 448 (8.1) | 48 (8.9) |
| Delivery year | |||
| 1983-2000 | 919 (15.2) | 858 (93.4) | 61 (6.6) |
| 2001-2010 | 2113 (35.0) | 1865 (88.3) | 248 (11.7) |
| 2011-2020 | 3013 (49.8) | 2783 (92.4) | 230 (7.6) |
| Maternal smoking during pregnancy | |||
| No | 5499 (91.0) | 5012 (91.1) | 487 (8.9) |
| Yes | 463 (7.7) | 419 (90.5) | 44 (9.5) |
| Missing | 83 (1.4) | 75 (90.4) | 8 (9.6) |
| Fetal sex | |||
| Female | 2870 (47.5) | 2631 (91.7) | 239 (8.3) |
| Male | 3109 (51.4) | 2814 (90.5) | 295 (9.5) |
| Missing | 66 (1.1) | 61 (92.4) | 5 (7.6) |
| Parity | |||
| Nulliparous | 3027 (50.1) | 2780 (91.8) | 247 (8.2) |
| Parous | 2940 (48.6) | 2662 (90.5) | 278 (9.5) |
| Missing | 78 (1.3) | 64 (82.1) | 14 (17.9) |
Characteristics represent distributions prior to imputation. Data harmonization details for all characteristics are provided in eMethods 2 in the Supplement.
Preterm birth was defined as <37 weeks of completed gestational age at delivery.
Each race and ethnicity category represents a composite measure to maximize sample size and consistency between pooled studies, including non-Hispanic Black (African American, Black), Hispanic/Latina (Hispanic, Latino, Latin American indigenous heritage), non-Hispanic White, and other (American Indian/Alaskan Native, Native Hawaiian, and/or >1 racial identity).
Body mass index was calculated as weight in kilograms divided by height in meters squared.
Associations With Preterm Birth
Regression analyses showed that higher concentrations of most phthalate metabolites were associated with slightly higher odds of preterm birth (Figure 1). After covariate adjustment, there was a 12% to 16% higher odds of preterm birth associated with an IQR increase in urinary concentrations of MBP (OR, 1.12 [95% CI, 0.98-1.27]), mono-isobutyl phthalate (OR, 1.16 [95% CI, 1.00-1.34]), MECPP (OR, 1.16 [95% CI, 1.00-1.34]), and MCPP (OR, 1.14 [95% CI, 1.01-1.29]). Other phthalate metabolites also displayed positive but nonsignificant associations. An IQR increase in the mixture of 9 phthalate metabolites was associated with 25% higher odds of preterm birth (OR, 1.25 [95% CI, 0.88-1.77]), although the confidence interval included the null. Based on results from g-computation, hypothetical interventions to reduce the phthalate metabolite mixture were estimated to prevent a mean of 2 to 32 preterm births per 1000 live births (Figure 2). For example, reducing the mixture of phthalate metabolite concentrations by 10%, 30%, or 50% was estimated to prevent 1.8 (95% CI, 0.5-3.1), 5.9 (95% CI, 1.7-9.9), and 11.1 (95% CI, 3.6-18.3) preterm births per 1000 live births, respectively.
Figure 1. Forest Plot of Associations Between Urinary Phthalate Metabolite Concentrations and Preterm Birth.
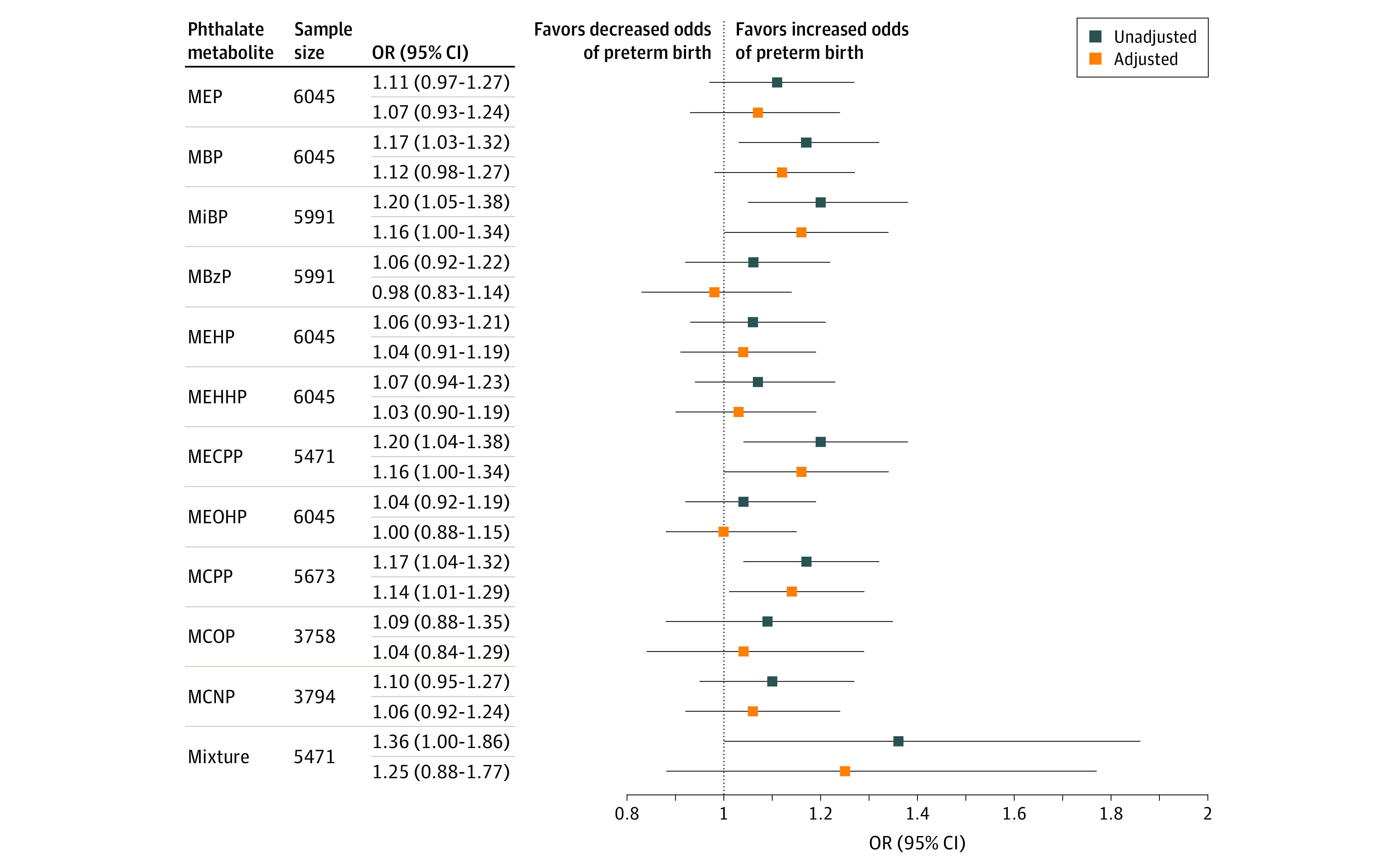
Associations represent the odds ratios (ORs) and 95% CIs of preterm birth per interquartile range increase in mean pregnancy urinary phthalate metabolite concentration in the Pooled Phthalate and Preterm Birth Study (N = 6045). The interquartile range (ng/mL) of each metabolite is as follows: monoethyl phthalate (MEP), 168.2; mono-n-butyl phthalate (MBP), 21.4; mono-isobutyl phthalate (MiBP), 8.6; monobenzyl phthalate (MBzP), 11.0; mono(2-ethylhexyl) phthalate (MEHP), 5.0; mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), 17.3; mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), 26.8; mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), 12.4; mono(3-carboxypropyl) phthalate (MCPP), 2.5; monocarboxy-isooctyl phthalate (MCOP), 18.5; and monocarboxy-isononyl phthalate (MCNP), 2.2 (eTable 7 in the Supplement). Single metabolite results were estimated by multivariable logistic regression models and mixture results were produced by quantile g-computation models. Unadjusted models adjusted for study as a fixed effect. Adjusted models were adjusted for study, maternal age, race and ethnicity, education, and prepregnancy body mass index. Missing covariate values were multiply imputed for all models. The metabolites MCOP and MCNP were excluded from the mixtures analysis owing to limited sample size across cohorts.
Figure 2. Estimated Number of Prevented Preterm Births per 1000 Live Births Under Hypothetical Interventions to Reduce the Overall Mixture of Phthalate Metabolite Concentrations in Maternal Urine.
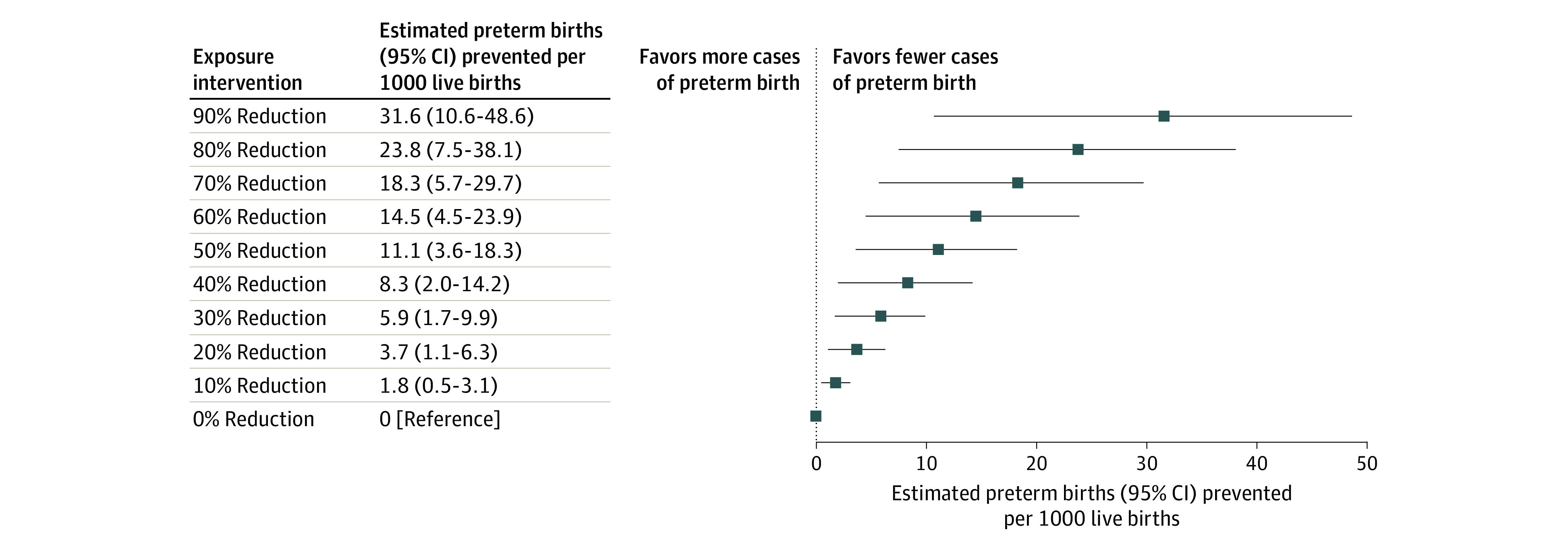
Estimates represent the difference in mean probability of preterm birth following a series of hypothetical interventions to proportionally reduce concentrations of 9 phthalate metabolites in the pooled study population (n = 5471), including monoethyl phthalate, mono-n-butyl phthalate, mono-isobutyl phthalate, monobenzyl phthalate, mono(2-ethylhexyl) phthalate, mono(2-ethyl-5-carboxypentyl) phthalate, mono(2-ethyl-5-oxohexyl) phthalate, mono(2-ethyl-5-hydroxyhexyl) phthalate, and mono(3-carboxypropyl) phthalate. G-computation was implemented to estimate probabilities from a multivariable logistic regression model, which adjusted for study, maternal age, race and ethnicity, education, and prepregnancy body mass index. Differences were multiplied by 1000 to estimate the rate per 1000 live births. The 95% CIs were estimated using quantiles of the nonparametric bootstrap distribution across 2000 iterations. Estimations were performed on a single randomly chosen imputed data set.
Sensitivity Analyses
Fixed-effects and random-effects models produced nearly equivalent estimates and metabolite by study interactions were not statistically significant (eTable 8 in the Supplement), indicating minimal heterogeneity by study. Magnitudes of associations were similar after excluding participants from individual study populations (eFigure 4 in the Supplement). However, associations were attenuated for MBP, MECPP, and MCPP after exclusion of LIFECODES participants.11 Heterogeneity in confounding was not detected (eTable 9 in the Supplement). We did not observe differences in associations when models were additionally adjusted for precision variables (delivery year, smoking, or parity) (eTable 10 in the Supplement) or evidence of effect measure modification by fetal sex (eTable 11 in the Supplement). We did not find evidence of nonlinear associations (eTable 12 in the Supplement). Importantly, direction of associations was consistent when gestational age at delivery was evaluated continuously (eTable 13 in the Supplement).
Discussion
In this pooled analysis of more than 6000 pregnancies from 16 prospective studies in the US, we observed that higher maternal pregnancy concentrations of several urinary phthalate metabolites, particularly MBP, mono-isobutyl phthalate, MECPP, and MCPP, were associated with higher odds of preterm birth. While ORs were seemingly small in magnitude, g-computation estimates suggested that joint reductions in phthalate metabolites could produce significant population-level reductions in preterm births. Our findings suggest that exposure to multiple phthalates is associated with an increased risk of preterm birth.
At the population-level, modest effect sizes can be important when exposures are widespread and the outcome is prevalent.54 The imprecision of our estimates, as reflected by our confidence intervals, may be related to inconsistencies of methods used across pooled studies. Several studies quantified phthalates using spot urine samples collected at single time points in different periods of pregnancy,17,21,22,26,30,31,32 and such isolated measures are not ideal estimators of long-term exposure to be attributable to short half-life.55 Further, we did not have the data to subdivide preterm births into those that were spontaneous vs indicated, which may be important for assessing risk.11,12
Our results are important to consider in the context of the literature. As in our study, urinary metabolites of di-n-butyl phthalate, di-isobutyl phthalate, and di(2-ethylhexyl) phthalate have been associated with reduced gestational age at delivery or increased likelihood of preterm birth in several prospective US studies included here11,12,13,14,26,31,32 as well as studies from China16 and Mexico.15 Although null17,18,19,20,56 or contradictory14,21,22,23 associations have also been observed, associations between metabolites of these parent chemicals and preterm birth appear to be more consistent than other phthalate metabolites. Variation across studies with respect to magnitudes of association and statistical significance is expected owing to differences in (1) sample size and preterm birth prevalence, (2) metabolite distributions, (3) exposure assessment approaches, (4) gestational age at exposure assessment, and (5) geographic location, where some populations may have different underlying susceptibilities or patterns of exposure.33,34 While pooling data cannot address all systematic biases, our study directly addressed several limitations by achieving larger sample size and examining associations across wide distributions of phthalate biomarkers.
The mechanistic pathway between phthalate exposure and preterm birth is unclear, but several lines of evidence provide biologic plausibility for a relationship. Associations of phthalate metabolites with preterm birth may be mediated by oxidative stress and inflammation at the maternal-fetal interface.57,58 Additional mechanisms may include dysregulated trophoblast differentiation and endocrine disruption, as phthalate biomarkers have been associated with downregulated expression of placental genes responsible for these processes.59
Our findings provide additional evidence of the need to reduce phthalate exposures among pregnant individuals, which could take the form of behavioral interventions or regulations. Although phthalate exposure can occur through many sources and environments,4,7,60,61 there has been a long-standing scientific effort to accurately determine whether a single source drives the majority of human exposure.62 The US Consumer Product Safety Commission attempted to estimate exposure by source and found food and medications, not children’s toys, were the primary sources of exposure.63 Unfortunately, there is still substantial uncertainty in the primary source of exposure. In the US, phthalate exposure varies widely by sociodemographic factors,64 including whether a person is pregnant,65 at a disadvantaged socioeconomic status,64,66 or is of a particular marginalized race or ethnicity.66
Targeted interventions may help modify consumer behaviors that lead to phthalate exposures, such as altering the type of personal care products purchased.67,68 However, behavioral approaches are difficult to implement on a population scale because of the vast number of available consumer products containing phthalates and the limited ability of US consumers to access accurate ingredient lists.69 For example, the US Food and Drug Administration does not require phthalates to be listed as ingredients when designated as part of the fragrance. Alternatively, interventions to reduce exposures through diet have had mixed results.68,70 Compounding these difficulties, economic disparities may make access to phthalate-free products and diet more difficult for certain populations.28,41 Past public health efforts have successfully led to federally mandated restrictions on the use of certain phthalates in consumer products intended for children,4,71 but few restrictions exist for products intended for people who are pregnant. The US Food and Drug Administration also has the power to regulate phthalates in food, but 28 phthalates are currently allowed as food additives or in food contact materials.72 Given this reality, Project TENDR (Targeting Environmental Neuro-Development Risks) recommends a multipronged approach to reducing human exposure to multiple phthalates, including regulations at the federal and state levels, as well as voluntary action on the part of retailers and manufacturers.8
Our analysis of hypothetical interventions to reduce exposure to the phthalate mixture, regardless of whether reductions occur via behavioral or regulatory mechanisms, helps to highlight the potential magnitude of effect that population-level phthalate exposure may have on preterm birth, meanwhile addressing the fact that realistic interventions will change exposure to multiple phthalates simultaneously, rather than one at a time. Based on the rate of about 90 preterm births per 1000 live births birth in the pooled study population, hypothetical interventions of 10% to 50% would correspond with an estimated mean of 2% to 12% reduction in preterm births. Given that most individuals are exposed to multiple phthalates, regulatory approaches to mitigate population-level health effects from phthalates would be most effective when considering phthalates as a class, rather than as individual chemicals.8 We took an approach used by previous studies48,73,74 and evaluated a range of possible decrements in exposure. This approach allowed us to evaluate whether any reductions, large or small, in phthalate exposure would be worth pursuing based on the potential to result in fewer preterm births in community settings. Our results are consistent with the hypothesis that modest, but potentially feasible, reductions in phthalate exposure could reduce rates of preterm birth. However, our results should be interpreted cautiously in light of the assumptions required for causality (eMethods 5 in the Supplement).48 Although g-computation is often used to facilitate causal inference,75 it is still a statistical model and thus we opt for associational rather than causal language. Regardless, “preterm births prevented” uses causal language because there is not useful associational language for this statistic.
Strengths and Limitations
Our study represents the largest prospective investigation of phthalate exposure in pregnancy and preterm birth, to date and to our knowledge, and includes individual-level data from almost all US studies that have quantified phthalate metabolites in pregnancy. Thus, we were not restricted to studies that only published on associations with preterm birth or gestational age at birth25,27,30 and avoided publication bias. Pooled participant characteristics (eg, exposure distributions, geographic locations, education, and race and ethnicities) were more diverse than any single prior study, which provided better representation of the US population. Further, our mixtures approach helped reflect the reality that pregnant individuals are exposed to a variety of phthalates in their environments, which should be a central consideration for any future policies intended to reduce phthalate exposures.8
Several limitations in our study are important to acknowledge. First, there was variation in exposure assessment methods across studies. This may have produced measurement error of metabolites, which could have contributed to observed exposure differences and could not be disentangled from true differences in exposure levels across the study populations. However, there was large overlap in distributions across studies, and we adjusted for known confounders. Although calculating mean values across multiple spot urine samples can improve characterization of exposure,76 single spot urine samples may provide lower accuracy.77 Second, ORs from our statistical approach will tend to overestimate risk ratios, which are arguably more interpretable. We selected a logistic model to ensure that the model predictions remain within logical bounds without placing constraints on the phthalate distribution, and we use g-computation to allow easier interpretation of results. Third, we were also unable to examine potentially important confounders, such as diet.78 Concentrations of certain phthalate biomarkers are higher in individuals who have diets high in ultraprocessed food, fast food, or meat and dairy.60,61,79 Because some parameterizations of poor diet that include these foods are also associated with increased risk of preterm birth,80 residual confounding may exist in our analysis. However, phthalate exposure can come from many dietary pathways,70 so the role of diet in this relationship is uncertain.
Conclusions
In this pooled analysis of 16 prospective US studies, higher concentrations of several urinary phthalate metabolites in pregnancy were associated with preterm birth. These findings highlight the need for public health and policy measures to reduce phthalate exposures among pregnant individuals.
eMethods 1.
eMethods 2.
eMethods 3.
eMethods 4.
eMethods 5.
eFigure 1. Flow diagram of study participant selection and exclusion in the Pooled Phthalate and Preterm Birth Study
eFigure 2. Spearman correlations between pregnancy-averaged concentrations of urinary phthalate metabolites
eFigure 3. Distributions of pregnancy-averaged phthalate metabolite concentrations (a-k) in the Pooled Phthalate and Preterm Birth Study (overall) and by study
eFigure 4. Comparison of main effects (odds ratios) when excluding individual studies
eTable 1. Additional study design elements of cohorts included in the Pooled Phthalate and Preterm Birth Study population
eTable 2. Description of participant exclusions and final sample size in the Pooled Phthalate and Preterm Birth Study population
eTable 3. Participant characteristics (n [%] or mean [SD]) by study (a-p)
eTable 4. Urinary metabolites of phthalate and phthalate alternative compounds measured in the Pooled Phthalate and Preterm Birth study
eTable 5. Limits of detection (LOD) for phthalate metabolites and distribution of samples with concentrations above and below LOD
eTable 6. Sample size for each urinary phthalate metabolite across studies
eTable 7. Distribution of pregnancy-averaged urinary phthalate metabolite concentrations (ng/mL)
eTable 8. Heterogeneity by study in main effects using fixed effect, random effect, and interaction models
eTable 9. Effect estimates and Wald tests for tests of heterogeneity in confounding by study
eTable 10. Comparison of odds ratio (OR) estimates for preterm birth with additional adjustment for year of delivery, maternal smoking, and parity
eTable 11. Odds ratio (OR) for preterm birth in the overall study population and stratified by fetal sex
eTable 12. Urinary phthalate metabolite specified using non-linear term
eTable 13. Estimated change (β) in length of gestation (weeks) per IQR increase in urinary phthalate biomarkers
eReferences
References
- 1.Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol. 2017;41(7):387-391. doi: 10.1053/j.semperi.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 2.Beam AL, Fried I, Palmer N, et al. Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008-2016. J Perinatol. 2020;40(7):1091-1099. doi: 10.1038/s41372-020-0635-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2019. Natl Vital Stat Rep. 2021;70(2):1-51. [PubMed] [Google Scholar]
- 4.U.S. Environmental Protection Agency (EPA) . Phthalates: Action Plan. Revised March 14, 2012. Accessed June 2, 2022. https://www.epa.gov/sites/default/files/2015-09/documents/phthalates_actionplan_revised_2012-03-14.pdf [Google Scholar]
- 5.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210(5):623-634. doi: 10.1016/j.ijheh.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 6.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011;119(6):878-885. doi: 10.1289/ehp.1002727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitro SD, Dodson RE, Singla V, et al. Consumer product chemicals in indoor dust: a quantitative meta-analysis of U.S. studies. Environ Sci Technol. 2016;50(19):10661-10672. doi: 10.1021/acs.est.6b02023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel SM, Patisaul HB, Brody C, et al. Neurotoxicity of ortho-phthalates: recommendations for critical policy reforms to protect brain development in children. Am J Public Health. 2021;111(4):687-695. doi: 10.2105/AJPH.2020.306014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lioy PJ, Hauser R, Gennings C, et al. Assessment of phthalates/phthalate alternatives in children’s toys and childcare articles: review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J Expo Sci Environ Epidemiol. 2015;25(4):343-353. doi: 10.1038/jes.2015.33 [DOI] [PubMed] [Google Scholar]
- 10.Radke EG, Glenn BS, Braun JM, Cooper GS. Phthalate exposure and female reproductive and developmental outcomes: a systematic review of the human epidemiological evidence. Environ Int. 2019;130:104580. doi: 10.1016/j.envint.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61-67. doi: 10.1001/jamapediatrics.2013.3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson KK, Rosen EM, Rosario Z, et al. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ Int. 2019;132:105099. doi: 10.1016/j.envint.2019.105099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson KK, Rosen EM, Barrett ES, et al. Joint impact of phthalate exposure and stressful life events in pregnancy on preterm birth. Environ Int. 2019;133(pt B):105254. doi: 10.1016/j.envint.2019.105254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin HB, Jukic AM, Wilcox AJ, et al. Association of urinary concentrations of early pregnancy phthalate metabolites and bisphenol A with length of gestation. Environ Health. 2019;18(1):80. doi: 10.1186/s12940-019-0522-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meeker JD, Hu H, Cantonwine DE, et al. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect. 2009;117(10):1587-1592. doi: 10.1289/ehp.0800522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao H, Wang YF, Huang K, et al. Prenatal phthalate exposure in relation to gestational age and preterm birth in a prospective cohort study. Environ Res. 2019;176:108530. doi: 10.1016/j.envres.2019.108530 [DOI] [PubMed] [Google Scholar]
- 17.Polinski KJ, Dabelea D, Hamman RF, et al. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ Res. 2018;162:308-317. doi: 10.1016/j.envres.2018.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloom MS, Wenzel AG, Brock JW, et al. Racial disparity in maternal phthalates exposure; association with racial disparity in fetal growth and birth outcomes. Environ Int. 2019;127:473-486. doi: 10.1016/j.envint.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Niwa M, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Prenatal exposure to phthalate esters and PAHs and birth outcomes. Environ Int. 2010;36(7):699-704. doi: 10.1016/j.envint.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 20.Hu JMY, Arbuckle TE, Janssen P, et al. Associations of prenatal urinary phthalate exposure with preterm birth: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Can J Public Health. 2020;111(3):333-341. doi: 10.17269/s41997-020-00322-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adibi JJ, Hauser R, Williams PL, et al. Maternal urinary metabolites of Di-(2-Ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol. 2009;169(8):1015-1024. doi: 10.1093/aje/kwp001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092-1097. doi: 10.1289/ehp.11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoaff JR, Romano ME, Yolton K, Lanphear BP, Calafat AM, Braun JM. Prenatal phthalate exposure and infant size at birth and gestational duration. Environ Res. 2016;150:52-58. doi: 10.1016/j.envres.2016.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sathyanarayana S, Barrett E, Nguyen R, Redmon B, Haaland W, Swan SH. First trimester phthalate exposure and infant birth weight in the Infant Development and Environment Study. Int J Environ Res Public Health. 2016;13(10):E945. doi: 10.3390/ijerph13100945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger K, Eskenazi B, Balmes J, et al. Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatr Allergy Immunol. 2019;30(1):36-46. doi: 10.1111/pai.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whyatt RM, Adibi JJ, Calafat AM, et al. Prenatal di(2-ethylhexyl)phthalate exposure and length of gestation among an inner-city cohort. Pediatrics. 2009;124(6):e1213-e1220. doi: 10.1542/peds.2009-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messerlian C, Braun JM, Mínguez-Alarcón L, et al. ; Environment and Reproductive Health (EARTH) Study Team . Paternal and maternal urinary phthalate metabolite concentrations and birth weight of singletons conceived by subfertile couples. Environ Int. 2017;107:55-64. doi: 10.1016/j.envint.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenzel AG, Brock JW, Cruze L, et al. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere. 2018;193:394-402. doi: 10.1016/j.chemosphere.2017.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin HM, Schmidt RJ, Tancredi D, et al. Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ Health. 2018;17(1):85. doi: 10.1186/s12940-018-0428-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaRocca J, Binder AM, McElrath TF, Michels KB. The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ Res. 2014;133:396-406. doi: 10.1016/j.envres.2014.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watkins DJ, Milewski S, Domino SE, Meeker JD, Padmanabhan V. Maternal phthalate exposure during early pregnancy and at delivery in relation to gestational age and size at birth: A preliminary analysis. Reprod Toxicol. 2016;65:59-66. doi: 10.1016/j.reprotox.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberger B, Vetrano AM, Archer FE, et al. Effects of maternal exposure to phthalates and bisphenol A during pregnancy on gestational age. J Matern Fetal Neonatal Med. 2014;27(4):323-327. doi: 10.3109/14767058.2013.815718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Zhu H, Kannan K. A review of biomonitoring of phthalate exposures. Toxics. 2019;7(2):E21. doi: 10.3390/toxics7020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDorman MF, Matthews TJ, Mohangoo AD, Zeitlin J. International comparisons of infant mortality and related factors: United States and Europe, 2010. Natl Vital Stat Rep. 2014;63(5):1-6. [PubMed] [Google Scholar]
- 35.Calafat AM, Koch HM, Swan SH, et al. Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast Cancer Res. 2013;15(5):403. doi: 10.1186/bcr3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samandar E, Silva MJ, Reidy JA, Needham LL, Calafat AM. Temporal stability of eight phthalate metabolites and their glucuronide conjugates in human urine. Environ Res. 2009;109(5):641-646. doi: 10.1016/j.envres.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 37.Baird DD, Saldana TM, Nepomnaschy PA, et al. Within-person variability in urinary phthalate metabolite concentrations: measurements from specimens after long-term frozen storage. J Expo Sci Environ Epidemiol. 2010;20(2):169-175. doi: 10.1038/jes.2009.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; 1987:258. [Google Scholar]
- 39.O’Brien KM, Upson K, Cook NR, Weinberg CR. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect. 2016;124(2):220-227. doi: 10.1289/ehp.1509693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuiper JR, O’Brien KM, Ferguson KK, Buckley JP. Urinary specific gravity measures in the U.S. population: implications for the adjustment of non-persistent chemical urinary biomarker data. Environ Int. 2021;156:106656. doi: 10.1016/j.envint.2021.106656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan M, Mita C, Bellavia A, Parker M, James-Todd T. Racial/ethnic disparities in pregnancy and prenatal exposure to endocrine-disrupting chemicals commonly used in personal care products. Curr Environ Health Rep. 2021;8(2):98-112. doi: 10.1007/s40572-021-00317-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James-Todd TM, Meeker JD, Huang T, et al. Racial and ethnic variations in phthalate metabolite concentration changes across full-term pregnancies. J Expo Sci Environ Epidemiol. 2017;27(2):160-166. doi: 10.1038/jes.2016.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hedderson MM, Xu F, Dayo OM, et al. Contribution of maternal cardiometabolic risk factors to racial-ethnicity disparities in preterm birth subtypes. Am J Obstet Gynecol MFM. 2022;4(3):100608. doi: 10.1016/j.ajogmf.2022.100608 [DOI] [PubMed] [Google Scholar]
- 44.Beck AF, Edwards EM, Horbar JD, Howell EA, McCormick MC, Pursley DM. The color of health: how racism, segregation, and inequality affect the health and well-being of preterm infants and their families. Pediatr Res. 2020;87(2):227-234. doi: 10.1038/s41390-019-0513-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A quantile-based G-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect. 2020;128(4):47004. doi: 10.1289/EHP5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahern J, Hubbard A, Galea S. Estimating the effects of potential public health interventions on population disease burden: a step-by-step illustration of causal inference methods. Am J Epidemiol. 2009;169(9):1140-1147. doi: 10.1093/aje/kwp015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snowden JM, Rose S, Mortimer KM. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am J Epidemiol. 2011;173(7):731-738. doi: 10.1093/aje/kwq472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keil AP, Buckley JP, Kalkbrenner AE. Bayesian G-computation for estimating impacts of interventions on exposure mixtures: demonstration with metals from coal-fired power plants and birth weight. Am J Epidemiol. 2021;190(12):2647-2657. doi: 10.1093/aje/kwab053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basagaña X, Pedersen M, Barrera-Gómez J, et al. ; ESCAPE Birth Outcomes working group . Analysis of multicentre epidemiological studies: contrasting fixed or random effects modelling and meta-analysis. Int J Epidemiol. 2018;47(4):1343-1354. doi: 10.1093/ije/dyy117 [DOI] [PubMed] [Google Scholar]
- 50.Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect. 2015;123(3):210-216. doi: 10.1289/ehp.1307996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF. Urinary concentrations of bisphenol A and phthalate metabolites measured during pregnancy and risk of preeclampsia. Environ Health Perspect. 2016;124(10):1651-1655. doi: 10.1289/EHP188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: the HOME Study. Environ Health. 2015;14:75. doi: 10.1186/s12940-015-0062-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosen EM, Muñoz MI, McElrath T, Cantonwine DE, Ferguson KK. Environmental contaminants and preeclampsia: a systematic literature review. J Toxicol Environ Health B Crit Rev. 2018;21(5):291-319. doi: 10.1080/10937404.2018.1554515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellinger DC. Interpretation of small effect sizes in occupational and environmental neurotoxicology: individual versus population risk. Neurotoxicology. 2007;28(2):245-251. doi: 10.1016/j.neuro.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 55.Adibi JJ, Whyatt RM, Williams PL, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116(4):467-473. doi: 10.1289/ehp.10749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casas M, Valvi D, Ballesteros-Gomez A, et al. Exposure to bisphenol A and phthalates during pregnancy and ultrasound measures of fetal growth in the INMA-Sabadell Cohort. Environ Health Perspect. 2016;124(4):521-528. doi: 10.1289/ehp.1409190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferguson KK, Chen YH, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B. Mediation of the relationship between maternal phthalate exposure and preterm birth by oxidative stress with repeated measurements across pregnancy. Environ Health Perspect. 2017;125(3):488-494. doi: 10.1289/EHP282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aung MT, Song Y, Ferguson KK, et al. Application of an analytical framework for multivariate mediation analysis of environmental data. Nat Commun. 2020;11(1):5624. doi: 10.1038/s41467-020-19335-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adibi JJ, Whyatt RM, Hauser R, et al. Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure. Environ Health Perspect. 2010;118(2):291-296. doi: 10.1289/ehp.0900788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zota AR, Phillips CA, Mitro SD. Recent fast food consumption and bisphenol A and phthalates exposures among the U.S. population in NHANES, 2003-2010. Environ Health Perspect. 2016;124(10):1521-1528. doi: 10.1289/ehp.1510803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serrano SE, Karr CJ, Seixas NS, et al. Dietary phthalate exposure in pregnant women and the impact of consumer practices. Int J Environ Res Public Health. 2014;11(6):6193-6215. doi: 10.3390/ijerph110606193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29(1):134-139. doi: 10.1111/j.1365-2605.2005.00567.x [DOI] [PubMed] [Google Scholar]
- 63.CPSC Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives Final Report. US Consumer Product Safety Commission; 2014. [Google Scholar]
- 64.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect. 2014;122(3):235-241. doi: 10.1289/ehp.1306681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shin HM, Dhar U, Calafat AM, Nguyen V, Schmidt RJ, Hertz-Picciotto I. Temporal trends of exposure to phthalates and phthalate alternatives in California pregnant women during 2007-2013: comparison with other populations. Environ Sci Technol. 2020;54(20):13157-13166. doi: 10.1021/acs.est.0c03857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDonald JA, Llanos AAM, Morton T, Zota AR. The environmental injustice of beauty products: toward clean and equitable beauty. Am J Public Health. 2022;112(1):50-53. doi: 10.2105/AJPH.2021.306606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harley KG, Kogut K, Madrigal DS, et al. Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: findings from the HERMOSA Intervention Study. Environ Health Perspect. 2016;124(10):1600-1607. doi: 10.1289/ehp.1510514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rudel RA, Gray JM, Engel CL, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914-920. doi: 10.1289/ehp.1003170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.U.S. Food and Drug Administration . Phthalates in cosmetics. FDA. Updated May 19, 2022. Accessed June 2, 2022. https://www.fda.gov/cosmetics/cosmetic-ingredients/phthalates-cosmetics
- 70.Sathyanarayana S, Alcedo G, Saelens BE, et al. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Environ Epidemiol. 2013;23(4):378-384. doi: 10.1038/jes.2013.9 [DOI] [PubMed] [Google Scholar]
- 71.Prohibition of children's toys and child care articles containing specified phthalates. 82 FR 49982 (2018). [PubMed]
- 72.Edwards L, McCray NL, VanNoy BN, et al. Phthalate and novel plasticizer concentrations in food items from U.S. fast food chains: a preliminary analysis. J Expo Sci Environ Epidemiol. 2022;32(3):366-373. doi: 10.1038/s41370-021-00392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia E, Urman R, Berhane K, McConnell R, Gilliland F. Effects of policy-driven hypothetical air pollutant interventions on childhood asthma incidence in southern California. Proc Natl Acad Sci U S A. 2019;116(32):15883-15888. doi: 10.1073/pnas.1815678116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gennings C, Svensson K, Wolk A, Lindh C, Kiviranta H, Bornehag CG. Using metrics of a mixture effect and nutrition from an observational study for consideration towards causal inference. Int J Environ Res Public Health. 2022;19(4):2273. doi: 10.3390/ijerph19042273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Westreich D, Edwards JK, Rogawski ET, Hudgens MG, Stuart EA, Cole SR. Causal impact: epidemiological approaches for a public health of consequence. Am J Public Health. 2016;106(6):1011-1012. doi: 10.2105/AJPH.2016.303226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harley KG, Berger K, Rauch S, et al. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatr Res. 2017;82(3):405-415. doi: 10.1038/pr.2017.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environ Int. 2015;85:27-39. doi: 10.1016/j.envint.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferguson KK, Lan Z, Yu Y, Mukherjee B, McElrath TF, Meeker JD. Urinary concentrations of phenols in association with biomarkers of oxidative stress in pregnancy: assessment of effects independent of phthalates. Environ Int. 2019;131:104903. doi: 10.1016/j.envint.2019.104903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013-2014. Environ Int. 2019;131:105057. doi: 10.1016/j.envint.2019.105057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chia AR, Chen LW, Lai JS, et al. Maternal dietary patterns and birth outcomes: a systematic review and meta-analysis. Adv Nutr. 2019;10(4):685-695. doi: 10.1093/advances/nmy123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1.
eMethods 2.
eMethods 3.
eMethods 4.
eMethods 5.
eFigure 1. Flow diagram of study participant selection and exclusion in the Pooled Phthalate and Preterm Birth Study
eFigure 2. Spearman correlations between pregnancy-averaged concentrations of urinary phthalate metabolites
eFigure 3. Distributions of pregnancy-averaged phthalate metabolite concentrations (a-k) in the Pooled Phthalate and Preterm Birth Study (overall) and by study
eFigure 4. Comparison of main effects (odds ratios) when excluding individual studies
eTable 1. Additional study design elements of cohorts included in the Pooled Phthalate and Preterm Birth Study population
eTable 2. Description of participant exclusions and final sample size in the Pooled Phthalate and Preterm Birth Study population
eTable 3. Participant characteristics (n [%] or mean [SD]) by study (a-p)
eTable 4. Urinary metabolites of phthalate and phthalate alternative compounds measured in the Pooled Phthalate and Preterm Birth study
eTable 5. Limits of detection (LOD) for phthalate metabolites and distribution of samples with concentrations above and below LOD
eTable 6. Sample size for each urinary phthalate metabolite across studies
eTable 7. Distribution of pregnancy-averaged urinary phthalate metabolite concentrations (ng/mL)
eTable 8. Heterogeneity by study in main effects using fixed effect, random effect, and interaction models
eTable 9. Effect estimates and Wald tests for tests of heterogeneity in confounding by study
eTable 10. Comparison of odds ratio (OR) estimates for preterm birth with additional adjustment for year of delivery, maternal smoking, and parity
eTable 11. Odds ratio (OR) for preterm birth in the overall study population and stratified by fetal sex
eTable 12. Urinary phthalate metabolite specified using non-linear term
eTable 13. Estimated change (β) in length of gestation (weeks) per IQR increase in urinary phthalate biomarkers
eReferences


