Abstract
Background/Aims
Elobixibat, an ileal bile acid transporter (apical sodium-dependent bile acid transporter) inhibitor, was recently launched in Japan for the treatment of chronic idiopathic constipation. We conducted an interim analysis of post-marketing surveillance to evaluate the safety and efficacy of elobixibat in elderly patients with chronic constipation and compared the efficacy according to administration time.
Methods
Safety and efficacy outcomes were evaluated through patient interviews for 4 weeks.
Results
Adverse drug reactions (ADRs) were observed in 5.24% of the 1049 patients analyzed; diarrhea (2.19%) and abdominal pain (1.81%) were the most common. A serious ADR of death was reported in one patient (0.10%). The incidence of ADRs in the ≥ 65-year old or ≥ 75-year-old subpopulation was similar to that in the total patient population. Mean bowel movements per week significantly increased from 2.9 ± 2.5 at baseline to 5.0 ± 3.1 (P < 0.001) at Week 2 and 5.3 ± 2.6 (P < 0.001) at Week 4. The mean Bristol Stool Form Scale score significantly increased from 2.3 ± 1.4 at baseline to 3.8 ± 1.3 (P < 0.001) at Week 2 and 3.9 ± 1.1 at Week 4 (P < 0.001). Bowel movements significantly increased in the elderly population and subpopulations receiving elobixibat before breakfast, lunch, or dinner. The median time to bowel movement was 5 hours.
Conclusion
The results suggested that elobixibat was well-tolerated and efficacious in elderly patients with chronic constipation and can be administered before any meals.
Keywords: Bile acids; Constipation; Elobixibat; Product surveillance, postmarketing
Introduction
The global prevalence of chronic constipation in the general population is reported to be 14%,1 and the prevalence of self-reported constipation is 16-28%2,3; however, these reports depend on different diagnostic criteria of constipation. Chronic constipation is more common in women than in men in the younger generation, and its prevalence increases with age because of decreased abdominal muscle strength, bowel movements, and food intake. A high prevalence of constipation is observed in both genders aged ≥ 65 years.4 Therefore, the growth in the elderly population is expected to increase the number of patients with chronic constipation. Constipation is often regarded as a trivial condition; however, the symptoms such as bloating, abdominal pain, and difficulty in defecation significantly impair quality of life (QOL),5 to an extent comparable to that seen in inflammatory bowel disease or rheumatoid arthritis.6 In particular, in elderly patients with constipation, straining may increase blood pressure and burden the heart and blood vessels, which can lead to myocardial infarction, heart failure, and stroke.7,8
Recently, a clinical practice guideline for the treatment of chronic constipation was published for the first time in Japan in line with the global classification of constipation and prescribing of evidence-based therapeutic drugs.9 In Japan, magnesium oxide (osmotic laxatives) and senna or sennoside (stimulant laxatives) have been extensively used for a long time and now account for up to 90% of all written prescriptions. Unfortunately, these treatment options often exhibit limited efficacy. Approximately 30-50% of the patients with chronic constipation were not fully satisfied with the efficacy of these treatments, and there are gaps in satisfaction between physicians and patients with regard to treatment10,11 in the selection of drug treatment for constipation and in the definition of efficacy among doctors and patients.12 These results suggest that chronic constipation is not sufficiently managed in a significant number of patients treated with current osmotic and stimulant laxatives.
Elobixibat is a first-in-class selective ileal bile acid transporter (IBAT, also known as apical sodium-dependent bile acid transporter) inhibitor for the treatment of chronic constipation.13-15 IBAT, a seven transmembrane protein expressed in epithelial cells on the luminal side of ileal terminal, co-transports sodium ions and conjugated bile acids and participates in the enterohepatic circulation of bile acids.16,17 Elobixibat reduces active ileal reabsorption of bile acids, increasing the concentration of bile acids entering the colon, which leads to bowel movements via stimulation of colonic secretion and motility.18 A phase 3 study in Japan revealed that orally administered 10 mg elobixibat once daily before breakfast for 2 weeks improved the frequency of spontaneous bowel movements (SBMs), time to the first SBM, and stool form based on the Bristol Stool Form Scale (BSFS) scores in patients with chronic constipation.19 Although elobixibat was safe and generally well-tolerated, adverse drug reactions (ADRs) of abdominal pain (19%), and diarrhea (13%) were frequently observed. In addition to short-term outcomes, elobixibat also showed tolerability in 1-year treatment in patients with chronic constipation.19 The clinical results indicate that elobixibat showed a median time to the first SBM after administration of approximately 5 hours and mean BSFS score of 4, leading to high satisfaction among patients.19 Elobixibat has dual actions on water secretion and colonic motility, indicating that the drug has characteristics of both osmotic and irritant laxatives. In addition to the standard dose of 10 mg (2 tablets), a wide range of doses including 5 mg and 15 mg are available and are prescribed to patients according to the status of their bowel movements. Hence, elobixibat, with a novel mechanism of action, may serve as a new option for the treatment of chronic constipation.
Based on these clinical results, elobixibat was approved for the treatment of chronic constipation in Japan in January 2018. However, in addition to the relatively small sample size in the phase 3 study, no safety or efficacy information is available on the drug in elderly patients, patients with combined diseases that could cause chronic constipation, and patients with previous and concomitant use of other laxatives. Furthermore, the efficacy of elobixibat has been investigated only by dosing before breakfast; therefore, the efficacy of the drug administered before lunch or dinner is unknown. Currently, a post-marketing surveillance for approximately 5 years is ongoing to evaluate the safety and efficacy of elobixibat in patients with chronic constipation in the clinical practice setting. In this report, we conducted an interim analysis of clinical outcomes after a 4-week treatment with elobixibat. In particular, we evaluated the safety and efficacy of the drug in elderly patients and compared the efficacy according to administration time.
Materials and Methods
Patients and Surveillance Design
This survey was designed as a prospective, multi-center, post-marketing study to evaluate the safety and efficacy of elobixibat. The overall survey period was planned to extend from June 2018 to December 2022 and to enroll 3000 patients. This surveillance was carried out using a centralized registration system for patients with chronic constipation newly treated with elobixibat. Patients with chronic constipation were diagnosed according to the criteria in Japanese 2017 Guideline for the Management of Chronic Constipation, which has been developed in reference to Rome IV criteria for constipation,20 and enrolled in surveillance. For the analysis of interim data, the cutoff date for the receipt of survey forms was July 18, 2019. The data of 1090 patients, which were locked in the study database at that time, were evaluated to determine the early safety and efficacy of elobixibat for 4 weeks after treatment initiation. Elobixibat was prescribed and administered for 4 weeks in accordance with the package insert.
Accordingly, a once-daily oral dose of 10 mg was administered before any meals. A lower dose (5 mg) was permitted at the attending physician’s discretion according to the severity of constipation and administered with caution. A dose increase to 15 mg was also permitted if the treating physician considered the treatment efficacy insufficient, and the patient’s clinical course was carefully monitored. The observation period was set for 4 weeks and extended up to 52 weeks for patients who received the drug for more than 5 weeks. The following patient demographics were evaluated at baseline and during the survey: sex; age; and duration of chronic constipation, combined diseases with irritable bowel syndrome with constipation (IBS-C), other combined diseases, and the use of other drugs for constipation (1 month before baseline and during the treatment period). Dose and timing of administration of elobixibat and the reasons for completion or discontinuation of administration were recorded. The clinical data were entered into the internet-based electronic data capture system.
This survey was conducted in compliance with Good Post-marketing Study Practice for drugs (the Japanese Ministry of Health, Labour and Welfare Ministerial Ordinance). The survey protocol was reviewed and approved by the Pharmaceuticals and Medical Devices Agency prior to initiation. All participants gave informed consent before entering the survey. This survey was registered at the Japan Pharmaceutical Information Center (Japic CTI-184007).
Safety Assessments
The incidence, types, severity, period of occurrence, and outcomes of ADRs were evaluated for 4 weeks after the initiation of treatment with elobixibat. ADRs were defined as adverse events that were considered to be related to elobixibat. Multiple occurrences of the same events in 1 patient were counted only once. The causal relationship of the drug with adverse events and the severities of these events were assessed by investigators at each facility. Individual ADRs were coded according to Japanese translation of the Medical Dictionary for Regulatory Activities version 22.0 and classified according to the System Organ Class and Preferred Term. Subgroup analyses of safety were performed among those of older age and those who used elobixibat monotherapy (no prior or concomitant laxatives).
Efficacy Assessments
Efficacy outcomes were collected by patient interview. The efficacy of elobixibat was evaluated based on mean number of bowel movements, BSFS scores, patient satisfaction with bowel movements (4 grades: satisfaction, slight satisfaction, slight dissatisfaction, and dissatisfaction), abdominal distension (5 grades: no, rarely, occasionally, often, and always), straining during defecation (5 grades: no, rarely, occasionally, often, and always), presence or absence of fecal disimpaction, and time to bowel movement at Weeks 2 and 4. These efficacy parameters were measured during Week 1 of treatment at baseline, Weeks 2 and 4 (ie, the last week of the 2-week, 4-week, and run-in period). In the subgroup analyses, the effects of older age, administration of elobixibat before any meals, elobixibat monotherapy, and severity of constipation on the efficacy outcomes after treatment were evaluated on the same schedule.
A subgroup analysis of efficacy was performed among those with more severe constipation. This criterion was classified into 3 groups based on the occurrence of SBMs and mean BSFS score during the second week of the 2-week run-in period: severe constipation, with SBM ≤ 2 and BSFS score ≤ 3; very severe constipation, with SBM ≤ 1 and BSFS score ≤ 3; or absolute constipation, with SBM = 0.21
Statistical Methods
The efficacy analysis population included patients who had data of all efficacy items of bowel movements excluding those “no evaluation.” The mean differences in the number of bowel movements and BSFS score from baseline to Weeks 2 and 4 were analyzed using the paired t test and Wilcoxon signed-rank test, respectively, both with a two-sided significance level of 5%. The differences in the presence and absence of fecal disimpaction from baseline to Week 2 or Week 4 in patients with paired data at these times were analyzed using the McNemar’s test with a two-sided significance level of 5%. Time to bowel movement after the last administration was summarized at each time, and the summary statistics were calculated. The median time to bowel movement (minimum and maximum) was estimated using the Kaplan-Meier method. The statistical analyses were performed using the statistical analysis software version 9.4 (SAS Institute Japan Ltd, Tokyo, Japan).
Results
Patient Disposition and Demographics
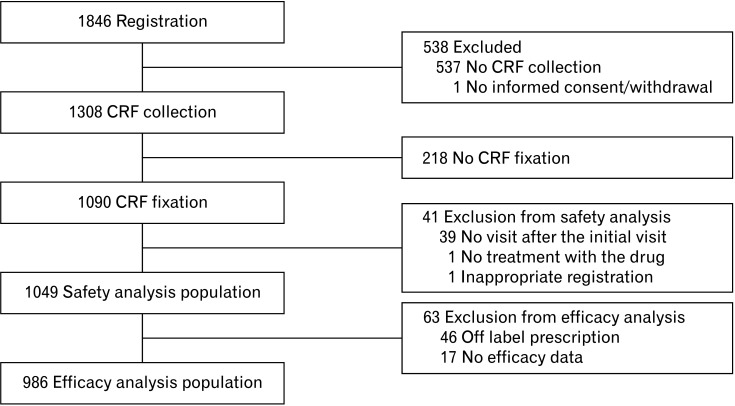
During the survey period, 1846 patients with chronic constipation were registered at 466 study sites in Japan while survey forms were collected and locked from 1090 (Fig. 1). Subsequently, 1049 patients, excluding 41 (39 had no visit after the initial visit and 1 each received no treatment and registered 15 days or longer after the initial treatment), were evaluated as the safety analysis population. In addition, 986 patients, excluding 63 (46 did not adhere to the prescribed regimen, and 17 had no data of bowel movement evaluation), were evaluated as the efficacy analysis population. Patients treated with off label prescription included those who used a drug at times other than before meals (40 patients, mainly after meals and at bedtime), used a drug at doses other than per tablet (5, mainly less than 1 tablet), were aged under 15 years (4), and received a prescription with an excessive dose (1).
Figure 1.
Patient disposition. CRF, case report form.
The patient demographics and characteristics at baseline in the safety analysis population are presented in Table 1; of the 1049 patients, 60.6% were women. The mean age ± SD was 70.4 ± 17.2 years; 50.6% aged ≥ 75 years, whereas 11 patients were aged < 20 years. Among the total patients, the duration of constipation was ≥ 5 years in 46.3% and 10.8% had IBS-C. More than half of the patients (66.6%) had been treated with other drugs prescribed for constipation mainly including osmotic laxatives (54.6%), stimulant laxatives (42.6%), and drugs altering epithelial function (chloride channel activator and guanylate cyclase-C receptor agonist) (20.9%). Approximately 40.0% of patients were prescribed elobixibat in combination with other drugs for constipation, including magnesium salts (52.9%), stimulant laxatives (31.9%), and drugs that alter epithelial function (13.0%).
Table 1.
Patient Demographics and Baseline Characteristics
| Item | n (%) |
|---|---|
| Safety analysis population | 1049 |
| Sex | |
| Male | 413 (39.4) |
| Female | 636 (60.6) |
| Age (mean ± SD, yr) | 70.4 ± 17.2 |
| < 65 | 286 (27.3) |
| ≥ 65 | 763 (72.7) |
| < 75 | 518 (49.4) |
| ≥ 75 | 531 (50.6) |
| Duration of chronic constipation (yr) | |
| < 5 | 380 (36.2) |
| ≥ 5 | 486 (46.3) |
| Unknown | 183 (17.4) |
| IBS-C | |
| No | 936 (89.2) |
| Yes | 113 (10.8) |
| Combined disease | |
| No | 285 (27.2) |
| Yes | 764 (72.8) |
| Hypertension | 317 (41.5) |
| Dyslipidemia | 208 (27.2) |
| Diabetes mellitus | 153 (20.0) |
| Gastroesophageal reflux disease | 148 (19.4) |
| Cardiovascular disease | 101 (13.2) |
| Respiratory disease | 67 (8.8) |
| Kidney disease | 60 (7.9) |
| Depression | 40 (5.2) |
| Liver disease | 27 (3.5) |
| Parkinson's disease | 21 (2.7) |
| Biliary disease | 13 (1.7) |
| Other diseases | 454 (59.4) |
| Prior OTC laxative | |
| No | 877 (83.6) |
| Yes | 93 (8.9) |
| Unknown | 79 (7.5) |
| Prior prescribed laxative | |
| No | 350 (33.4) |
| Yes | 699 (66.6) |
| Concomitant drugs for constipation | |
| No | 635 (60.5) |
| Yes | 414 (39.5) |
IBS-C, irritable bowel syndrome with constipation; OTC, over-the-counter.
Laxatives used within 1 month prior to treatment with elobixibat were recorded.
The baseline demographics and characteristics of the efficacy and safety analysis populations were similar (data not shown).
Exposure to Elobixibat
The daily dose (mean ± SD) of elobixibat in the safety analysis population was 9.33 ± 2.24 mg; most patients (813, 77.5%) were prescribed 10 mg (2 tablets) daily. A lower dose of 5 mg (1 tablet) and a higher dose of 15 mg (3 tablets) daily were prescribed to 149 (14.2%) and 84 (8.0%) patients, respectively. Most patients (826, 78.7%) continued elobixibat treatment after 4 weeks, and 223 (21.3%) discontinued or completed treatment by Week 4. The main reasons for treatment discontinuation or termination were lack of efficacy (68, 30.5%), patient choice (48, 21.5%), improvement in symptoms (35, 15.7%), adverse events (35, 15.7%), and no visit (32, 14.3%). Of the 68 patients who experienced lack of efficacy, only 5 (7.4%) had their doses increased to the maximum of 15 mg, whereas most patients (56, 82.4%) did not have their dose changed from 10 mg. Most patients (643, 76.4%) were directed to take elobixibat before breakfast, whereas 26 (3.1%) and 162 (19.2%) took the drug before lunch and dinner, respectively, at Week 4. The mean daily dose (9.39 ± 2.19 mg) in the efficacy analysis population was similar to that in the safety analysis populations.
Safety
Of the 1049 patients in the safety analysis population, ADRs were reported in 55 patients treated for 4 weeks (5.24%, Table 2); the most common ADRs (≥ 3 patients) were gastrointestinal disorders (4.96%), including diarrhea (2.19%), abdominal pain (1.81%), abdominal distension (0.38%), and nausea (0.29%). A serious ADR, death, was reported in 1 female patient (0.10%), aged 82 years old, whose medical history included aortic aneurysm, angina pectoris and Alzheimer’s type dementia. The patient’s death was reported 3 days after enrollment; however, the cause of death and information about the use of elobixibat were not provided. Elderly patients, the incidence of ADRs in those aged ≥ 65 years and ≥ 75 years was 4.46% and 3.58%, respectively. In the 46 patients who did not adhere to the prescribed regimen of elobixibat, diarrhea was observed in 1 patient who received elobixibat after meals.
Table 2.
Adverse Drug Reactions
| Event | Total | ≥ 65 yr | ≥ 75 yr |
|---|---|---|---|
| Safety analysis population | 1049 | 763 | 531 |
| Patients with ADRs | 55 (5.24) | 34 (4.46) | 19 (3.58) |
| Gastrointestinal disorders | 52 (4.96) | 31 (4.06) | 16 (3.01) |
| Diarrhea | 23 (2.19) | 16 (2.10) | 7 (1.32) |
| Abdominal pain | 19 (1.81) | 11 (1.44) | 5 (0.94) |
| Abdominal distension | 4 (0.38) | 2 (0.26) | 2 (0.38) |
| Nausea | 3 (0.29) | 1 (0.13) | 1 (0.19) |
| Abdominal discomfort | 2 (0.19) | 0 (0.00) | 0 (0.00) |
| Constipation | 2 (0.19) | 2 (0.26) | 0 (0.00) |
| Eructation | 1 (0.10) | 0 (0.00) | 0 (0.00) |
| Vomiting | 1 (0.10) | 1 (0.13) | 1 (0.19) |
| Soft feces | 1 (0.10) | 1 (0.13) | 1 (0.19) |
| Anal incontinence | 1 (0.10) | 1 (0.13) | 1 (0.19) |
| Skin and subcutaneous tissue disorders | 1 (0.10) | 1 (0.13) | 1 (0.19) |
| Rash | 1 (0.10) | 1 (0.13) | 1 (0.19) |
| Nervous system disorders | 1 (0.10) | 1 (0.13) | 1 (0.19) |
| Headache | 1 (0.10) | 1 (0.13) | 1 (0.19) |
| General disorders and administration site conditions | 1 (0.10) | 1 (0.13) | 1 (0.19) |
| Death | 1 (0.10) | 1 (0.13) | 1 (0.19) |
ADR, adverse drug reaction.
Data are presented as n (%) of patients who experienced each event.
Efficacy
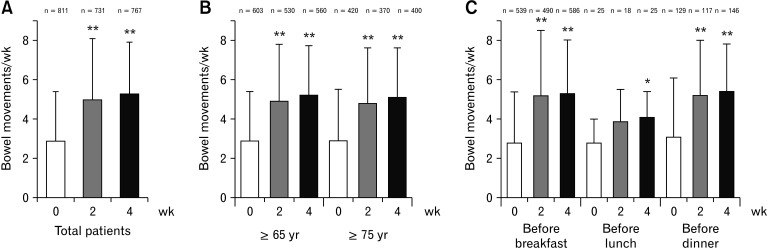
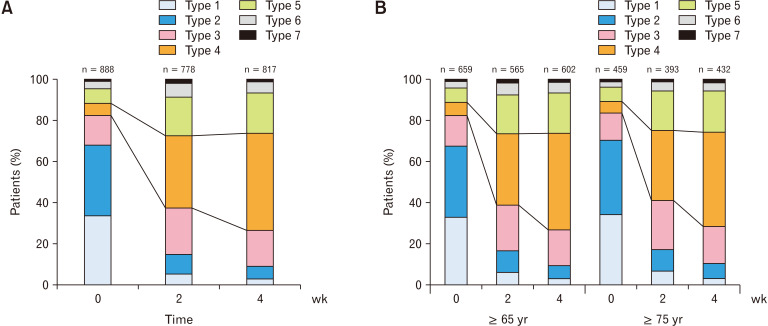
The mean bowel movements per week significantly increased from 2.9 ± 2.5 at baseline to 5.0 ± 3.1 and 5.3 ± 2.6 at Weeks 2 (P < 0.001) and 4 (P < 0.001), respectively (Fig. 2A). The BSFS score was close to ideal stool form (Type 4) at Weeks 2 and 4 (Fig. 3A). The mean BSFS score significantly increased from 2.3 ± 1.4 at baseline to 3.8 ± 1.3 and 3.9 ± 1.1 at Weeks 2 (P < 0.001) and 4 (P < 0.001), respectively. The proportion of patients with satisfaction was higher, and those with bloating or straining during bowel movement was lower at Weeks 2 and 4 than baseline. The time to bowel movement was 6.1 ± 6.0 hours and 6.4 ± 5.9 hours (mean ± SD) at Weeks 2 and 4, respectively. Moreover, the proportion of patients with fecal disimpaction at Week 2 (4.1%) and Week 4 (3.4%) was lower than that at baseline (11.4%) (Table 3). Statistically significant decreases from baseline were observed at Weeks 2 and 4 in the limited number of patients who had paired data for baseline and Week 2 or 4 (P < 0.001, Supplementary Table 1).
Figure 2.
Bowel movements after treatment with elobixibat. Bowel movements per week in (A) total patients, (B) patients aged ≥ 65 years and ≥ 75 years, and (C) patients administered elobixibat before breakfast, lunch, or dinner. Columns represent the mean ± SD. n, number of patients with bowel movements evaluation at each visit. *P < 0.01 and **P < 0.001 vs baseline (Week 0).
Figure 3.
Bristol Stool Form Scale score after treatment with elobixibat. Stool form of (A) total patients and (B) patients aged ≥ 65 years and ≥ 75 years. n, number of patients with stool form evaluation at each visit. Stool form was evaluated using Bristol Stool Form Scale: types 1 and 2 are hard, 3-5 are within the normal range, and 6 and 7 are muddy or liquid stools, respectively.
Table 3.
Bristol Stool Form Scale Score, Patient Satisfaction, and Symptoms of Constipation, Fecal Disimpaction, and Time to Bowel Movements After Treatment With Elobixibat
| Item | Baseline (n = 986) | Week 2 (n = 986) | Week 4 (n = 986) |
|---|---|---|---|
| Bristol Stool Form Scale score | 2.3 ± 1.4 (888) | 3.8 ± 1.3a (778) | 3.9 ± 1.1a (817) |
| Patient satisfaction | |||
| Satisfaction | 6 (0.7) | 222 (28.1) | 324 (38.9) |
| Slight satisfaction | 51 (5.6) | 300 (38.0) | 317 (38.1) |
| Slight dissatisfaction | 297 (32.7) | 164 (20.8) | 118 (14.2) |
| Dissatisfaction | 553 (61.0) | 103 (13.1) | 73 (8.8) |
| No evaluation | 79 (−) | 197 (−) | 154 (−) |
| Abdominal distension | |||
| No | 64 (7.2) | 175 (22.4) | 232 (28.3) |
| Rarely | 165 (18.7) | 309 (39.5) | 355 (43.2) |
| Occasionally | 326 (36.9) | 231 (29.5) | 193 (23.5) |
| Often | 229 (25.9) | 49 (6.3) | 28 (3.4) |
| Always | 99 (11.2) | 18 (2.3) | 13 (1.6) |
| No evaluation | 103 (−) | 204 (−) | 165 (−) |
| Straining during defecation, n (%) | |||
| No | 42 (4.9) | 148 (19.2) | 190 (23.5) |
| Rarely | 102 (11.9) | 289 (37.5) | 318 (39.3) |
| Occasionally | 266 (31.0) | 221 (28.7) | 212 (26.2) |
| Often | 278 (32.4) | 69 (8.9) | 55 (6.8) |
| Always | 171 (19.9) | 44 (5.7) | 34 (4.2) |
| No evaluation | 127 (−) | 215 (−) | 177 (−) |
| Fecal disimpaction | |||
| No | 805 (88.6) | 757 (95.9) | 803 (96.6) |
| Yes | 104 (11.4) | 32 (4.1) | 28 (3.4) |
| No evaluation | 77 (−) | 197 (−) | 155 (−) |
| Time to bowel movement (hr) | |||
| Mean ± SD (n) | ND | 6.1 ± 6.0 (415) | 6.4 ± 5.9 (439) |
| Median | 6.0 | 6.0 | |
aP < 0.001 vs baseline, Wilcoxon signed-rank test for Bristol Stool Form Scale score.
ND, not determined; n, number of patients with each item at each visit.
Data are presented as mean ± SD (n) or n (%).
In the elderly subpopulations aged ≥ 65 years and ≥ 75 years, the weekly mean number of bowel movements was also significantly higher at Weeks 2 (P < 0.001) and 4 (P < 0.001) than at baseline (Fig. 2B). The BSFS score changed to stool 4 at Weeks 2 and 4 in both populations (Fig. 3B). The mean BSFS score in patients aged ≥ 65 years significantly increased from 2.3 ± 1.4 at baseline to 3.7 ± 1.3 and 4.0 ± 1.1 at Weeks 2 (P < 0.001) and 4 (P < 0.001), respectively. Similarly, the mean BSFS score in the patients aged ≥ 75 years significantly increased from 2.3 ± 1.4 at baseline to 3.7 ± 1.3 and 3.9 ± 1.1 at Weeks 2 (P < 0.001) and 4 (P < 0.001), respectively.
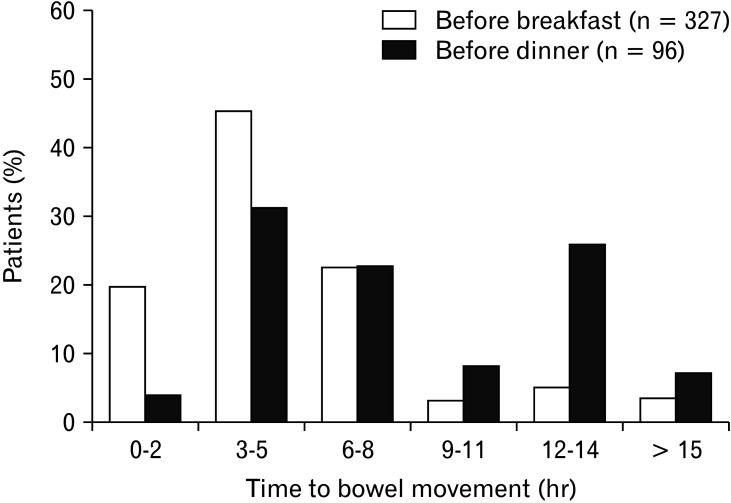
In another subpopulation analysis, the mean number of bowel movements per week significantly increased at Weeks 2 and 4 in patients who were prescribed elobixibat before breakfast and dinner and at Week 4 in those who were prescribed the drug before lunch (Fig. 2C). The mean BSFS score also significantly increased at Week 4 in all subpopulations (P < 0.001). There was no difference in the increase of patient satisfaction among these patients (Table 4). The patients who received the drug before breakfast most commonly showed a time to bowel movement of 3-5 hours, whereas those who received the drug before dinner showed 3-5 hours and 12-14 hours (Fig. 4).
Table 4.
Bristol Stool Form Scale Score, Patient Satisfaction, and Time to Bowel Movement After Treatment With Elobixibat Before Breakfast, Lunch, or Dinner
| Item | Before breakfast (n = 643) | Before lunch (n = 26) | Before dinner (n = 162) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Baseline | Week 4 | Baseline | Week 4 | |||
| Bristol Stool Form Scale score | 2.3 ± 1.5 (582) | 3.9 ± 1.1a (624) | 2.6 ± 1.1 (26) | 4.0 ± 0.9a (26) | 2.3 ± 1.3 (143) | 3.9 ± 1.0a (158) | ||
| Patient satisfaction | ||||||||
| Satisfaction | 3 (0.5) | 256 (40.2) | 0 | 8 (30.8) | 0 | 58 (36.5) | ||
| Slight satisfaction | 26 (4.4) | 233 (36.6) | 4 (16.0) | 13 (50.0) | 12 (8.3) | 68 (42.8) | ||
| Slight dissatisfaction | 183 (30.8) | 86 (13.5) | 10 (40.0) | 4 (15.4) | 55 (38.2) | 24 (15.1) | ||
| Dissatisfaction | 383 (64.4) | 62 (9.7) | 11 (44.0) | 1 (3.8) | 77 (53.5) | 9 (5.7) | ||
| No evaluation | 48 (−) | 6 (−) | 1 (−) | 0 | 18 (−) | 3 (−) | ||
| Time to bowel movement (hr) | ||||||||
| Mean ± SD (n) | ND | 5.6 ± 5.7 (327) | ND | 9.9 ± 6.1 (14) | ND | 8.5 ± 6.0 (96) | ||
| Median | 5.0 | 8.0 | 8.0 | |||||
aP < 0.001 vs baseline, Wilcoxon signed-rank test for Bristol Stool Form Scale score.
ND, not determined; n, number of patients with each item at each visit.
Data are presented as mean ± SD (n) or n (%).
Figure 4.
Time to bowel movement after administration of elobixibat before breakfast or dinner. n, number of patients with bowel movement evaluation at Week 4.
Moreover, in the subgroups of patients administered with elobixibat monotherapy (no prior or concomitant administration of drugs for constipation), patients with severe constipation, and those with very severe constipation, the mean number of bowel movements per week was significantly higher at Weeks 2 and 4 than at baseline (P < 0.001, Supplementary Tables 2-4). The BSFS score was close to the ideal score (Type 4) at Weeks 2 and 4. Furthermore, the mean BSFS score was significantly higher at Weeks 2 and 4 than at baseline (P < 0.001, Supplementary Tables 2-4). No sufficient data were obtained for the subgroup of patients with absolute constipation (n = 3).
Discussion
The results of the interim analysis of the post-marketing surveillance data of 1049 patients with chronic constipation who were administered elobixibat orally once daily for 4 weeks demonstrated an ADR incidence of 5.24%, with diarrhea (2.19%) and abdominal pain (1.81%) as the most commonly reported ADRs. The subgroup analysis for ADR in the patients treated with no prior or concomitant drugs suggested that there was a similar incidence of ADRs in patients treated with elobixibat alone and those treated with prior or concomitant laxatives. No new safety concerns were observed in this survey. In the clinical trial conducted prior to elobixibat approval,19 the ADR incidence was 32.89% after 2 weeks of treatment. Additionally, more patients in the phase 3 trial experienced diarrhea (13%) and abdominal pain (19%) than those in this survey. A direct comparison of the incidences would be difficult because of a difference in the ADR detection rate between the survey and controlled trial. Nonetheless, the results of this survey suggest that elobixibat could be used safely in clinical practice. Abdominal pain induced by elobixibat appears to be associated with a propagating contraction in the colon.22 Patients with chronic constipation experience fewer transmission systolic waves in the large intestine than healthy individuals.23 Bile acids have a stimulatory effect on water secretion in the large intestine and colonic motility.18 However, no serious events of abdominal pain were reported in this survey, and the outcomes of all abdominal pains were recovering or recovered. Additionally, elobixibat appeared to be well-tolerated in elderly patients with chronic constipation as the incidence of ADRs in patients aged ≥ 65 years and ≥ 75 years was similar to that in the total patient population. Moreover, although the reporting physician reported that a causal relationship between the death and elobixibat could not be ruled out, we consider that the event was mainly owing to the patient’s old age and underlying co-morbidities. In this survey, 1 patient who received elobixibat after meals exhibited an ADR of diarrhea. Elobixibat is minimally absorbed after oral administration and is likely to affect ileum IBATs locally.14 As a negative feedback mechanism to the decreased enterohepatic circulation of bile acids by elobixibat, a reduction in plasma low-density lipoprotein-cholesterol levels and the low-density lipoprotein/ high-density lipoprotein ratio was observed in patients with chronic constipation.13,19 Additionally, the increased secretion of bile acids into the colon induced by elobixibat may affect epithelial cell function in the colon17 and explain the potential side effects, such as bile acid malabsorption accompanied by watery stool, changes in resident microbiota, and even susceptibility to colon cancer. Although elobixibat was well-tolerated in a 52-week open-label trial,19 the influence of long-term treatment with elobixibat on these side effects requires further investigation.
This survey showed that elobixibat significantly increased the number of bowel movements at Weeks 2 and 4 compared to that at baseline. It is noteworthy that elobixibat improved the BSFS score from 2.3 at baseline to approximately 4, which is considered an ideal “sausage-like” normal stool.24 Recently, patients with BSFS score 4 type stool showed significantly higher QOL than those with other types of stool.25 In addition, the BSFS score has been shown to correlate with colon transit time26 and is a useful tool for the prediction of delayed colonic transit time in patients with chronic constipation; mean 5-day BSFS score (≤ 3) and stool frequency (≤ 2) predicts delayed colonic transit time.27 However, numerous patients with chronic constipation have shown a normal transit type of constipation.28,29 The most important bothersome symptom associated with chronic constipation is excess straining to have a bowel movement, especially in elderly patients. This symptom is critical because it may be associated with an increased risk of aggravating cardiovascular and cerebrovascular complications.7,8 Other symptoms of constipation include abdominal discomfort, bloating, and a feeling of incomplete evacuation.30 In this survey, in addition to improving bowel movements, elobixibat improved straining and abdominal distension significantly at Weeks 2 and 4, according to patient claims, and decreased the rate of fecal disimpaction. These improvements induced by elobixibat may have a significant impact on the QOL of the patients. In this survey, more than 60% of the patients showed satisfaction or slight satisfaction after the administration of elobixibat. In contrast, the number of unsatisfied patients decreased from ≥ 60% at baseline to < 10% at Week 4. Collectively, coinciding with the results of the phase 3 study, the present results suggested that elobixibat effectively improved bowel movements in patients with chronic constipation in clinical practice settings and was particularly beneficial for constipation in elderly patients aged ≥ 65 and ≥ 75 years. The subgroup analysis by the severity of constipation suggested that elobixibat was efficient in patients with severe constipation and very severe constipation.21 Moreover, elobixibat may also be safe and efficacious in patients with diabetes mellitus, renal disease, depression, or Parkinson’s disease who are known to be more likely to experience chronic constipation. Further evaluation of the efficacy is needed in a large number of patients with such diseases.
Elobixibat is taken before a meal to ensure that the drug exerts its action efficiently at the target site of IBAT and increases bile acid flow into the colon. In the phase 3 study,19 the drug was administered only before breakfast. In this survey, elobixibat improved bowel movements and the BSFS score similarly in patients who took the drug before breakfast, lunch, or dinner, while the number of patients who took the drug before lunch was relatively small. There was some difference in the time to bowel movements between the patients who received the drug before breakfast and those who received the drug before dinner. Nonetheless, elobixibat was shown to improve patient satisfaction in a similar manner in both patient groups. The reason for the prolonged time to bowel movement in patients administered elobixibat before dinner is unknown, but it may be related to decreased gastrointestinal motility during the night. These results suggest that elobixibat can be administered before a meal at any time of the day according to the patient’s lifestyle.
The findings of this surveillance indicated that approximately 70% of patients had used prescribed drugs for constipation in the past, and approximately 40% of patients used other drugs for constipation concomitantly during the survey. Therefore, the results suggest that elobixibat was also effective in patients who took other drugs concomitantly. Additionally, in the phase 3 study,19 elobixibat was shown to be effective and safe in patients with chronic constipation who concomitantly used rescue medication (10 mg bisacodyl suppositories). In clinical practice, elobixibat is considered to have a novel mechanism of action and may be used concomitantly with other drugs for constipation. Accordingly, stratified analysis by previous and concomitant use of other drugs for constipation is needed to assess the efficacy and safety of elobixibat in more patients with chronic constipation in the survey.
The low patient satisfaction with conventional constipation treatment seems to be related to the focus on promoting rapid bowel movements. However, the therapeutic goal of treatment for constipation is currently changing to softening the stool to make it pass through the colon easily. Specifically, it is not only important to increase the frequency of bowel movements but also to enhance the quality and comfort of defecation. Consequently, a BSFS score of 4 is regarded as a very important index because stool with this score induces a comfortable feeling with no sensation of incomplete evacuation. However, more than half of the current prior prescribed laxative users are not completely satisfied with their chronic treatment.10 Stimulant laxatives, such as senna and sennosides, which are very commonly used in Japan, had no clinical evidence of improving stool form, and these drugs appear to be ineffective in meeting patient requirements. Moreover, their long-term use is not recommended. Recently, in a placebo-controlled study, magnesium oxide was shown to significantly improve bowel movements, BSFS score, and QOL.30 However, periodic monitoring of serum magnesium levels is strongly recommended for its safe use because the risk of developing hypermagnesemia has been shown in elderly patients and those with renal failure prescribed magnesium oxide after long-term use.31 This survey shows that elobixibat treatment provided high levels of satisfaction with bowel movements to the patient with chronic constipation, which may be due to the dual actions on water secretion and colonic motility.
There are some limitations to this study that are worth mentioning. First, these are results of an interim analysis of short-term treatment (4 weeks) during the first year of the special drug use-results survey and may not reflect the final analysis results. However, the results obtained in this study are expected to contribute to promoting the appropriate use of elobixibat by early provision of information on this drug in actual clinical practice. Second, because there is no placebo control, it is difficult to accurately evaluate the efficacy and adverse reactions of this drug as well as the other factors, such as concomitant drugs, that may have an influence. In the future, it would be necessary to evaluate the influence of each factor including using a final stratified analysis. Third, as this survey was conducted in Japanese patients with constipation, it may not be applicable to non-Japanese patients. However, the efficacy and safety results of this drug have been confirmed to be consistent with those of clinical studies of this drug in patients with constipation already reported overseas.18,32
In conclusion, the interim results suggested that elobixibat was well-tolerated and effective in elderly patients with chronic constipation and can be used before any meals.
Supplementary Materials
Note: To access the supplementary tables mentioned in this article, visit the online version of Journal of Neurogastroenterology and Motility at http://www.jnmjournal.org/, and at https://doi.org/10.5056/jnm20263.
Acknowledgements
These trials were funded by EA Pharma Co, Ltd and Mochida Pharmaceutical Co, Ltd, and designed by EA Pharma Co, Ltd in collaboration with all authors listed. We thank the collaborating clinics and hospitals that participated in this study, Kiyoshi Oketani (Honyaku Center Inc) for providing medical writing support, which was funded by EA Pharma Co, Ltd.
Footnotes
Financial support: This work was supported by EA Pharma Co, Ltd and Mochida Pharmaceutical Co, Ltd.
Conflicts of interest: Atsushi Nakajima has served as a medical adviser to EA Pharma Co, Ltd and received lecture fees and a research grant from EA Pharma Co, Ltd. Mio Fujimaki, Yuki Arai, and Kento Emori are employees of EA Pharma Co, Ltd.
Author contributions: Atsushi Nakajima, Mio Fujimaki, and Yuki Arai were involved in designing and interpretation of the post hoc analysis, wrote the first draft, and edited subsequent drafts; and Mio Fujimaki and Kento Emori were involved in performing statistical post hoc analysis and edited subsequent drafts. All authors had complete access to the data and reviewed and approved the final draft of the manuscript for submission.
References
- 1.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:1582–1591. doi: 10.1038/ajg.2011.164. [DOI] [PubMed] [Google Scholar]
- 2.Wald A, Mueller-Lissner S, Kamm MA, et al. Survey of laxative use by adults with self-defined constipation in South America and Asia: a comparison of six countries. Aliment Pharmacol Ther. 2010;31:274–284. doi: 10.1111/j.1365-2036.2009.04169.x. [DOI] [PubMed] [Google Scholar]
- 3.Tamura A, Tomita T, Oshima T, et al. Prevalence and self-recognition of chronic constipation: results of an internet survey. J Neurogastroenterol Motil. 2016;22:677–685. doi: 10.5056/jnm15187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Health, Labour and Welfare, author. Overview of the comprehensive survey of living conditions in 2016. [accessed 26 Jun, 2020]. Available from URL: https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa16/dl/16.pdf . [Japanese]
- 5.Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25:599–608. doi: 10.1111/j.1365-2036.2006.03238.x. [DOI] [PubMed] [Google Scholar]
- 6.Belsey J, Greenfield S, Candy D, Geraint M. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010;31:938–949. doi: 10.1111/j.1365-2036.2010.04273.x. [DOI] [PubMed] [Google Scholar]
- 7.Engler TM, Dourado CC, Amâncio TG, Farage L, de Mello PA, Padula MP. Stroke: bowel dysfunction in patients admitted for rehabilitation. Open Nurs J. 2014;8:43–47. doi: 10.2174/1874434601408010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishiyama Y, Hoshide S, Mizuno H, Kario K. Constipation-induced pressor effects as triggers for cardiovascular events. J Clin Hypertens (Greenwich) 2019;21:421–425. doi: 10.1111/jch.13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Research society for the diagnosis and treatment of chronic constipation/affiliated to the Japanese society of gastroenterology, author. Evidence-based clinical practice guideline for chronic constipation. Nankodo; Japanese. Tokyo: 2017. [DOI] [Google Scholar]
- 10.Harris LA, Horn J, Kissous-Hunt M, Magnus L, Quigley EMM. The better understanding and recognition of the disconnects, experiences, and needs of patients with chronic idiopathic constipation (BURDEN-CIC) study: results of an online questionnaire. Adv Ther. 2017;34:2661–2673. doi: 10.1007/s12325-017-0633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller-Lissner S, Tack J, Feng Y, Schenck F, Specht Gryp R. Levels of satisfaction with current chronic constipation treatment options in Europe - an internet survey. Aliment Pharmacol Ther. 2013;37:137–145. doi: 10.1111/apt.12124. [DOI] [PubMed] [Google Scholar]
- 12.Carter D, Bardan E, Dickman R. Comparison of strategies and goals for treatment of chronic constipation among gastroenterologists and general practitioners. Ann Gastroenterol. 2018;31:71–76. doi: 10.20524/aog.2017.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta A, Camilleri M. Elobixibat and its potential role in chronic idiopathic constipation. Therap Adv Gastroenterol. 2014;7:167–175. doi: 10.1177/1756283X14528269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chedid V, Vijayvargiya P, Camilleri M. Elobixibat for the treatment of constipation. Expert Rev Gastroenterol Hepatol. 2018;12:951–960. doi: 10.1080/17474124.2018.1522248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miner PB., Jr Elobixibat, the first-in-class ileal bile acid transporter inhibitor, for the treatment of chronic idiopathic constipation. Expert Opin Pharmacother. 2018;19:1381–1388. doi: 10.1080/14656566.2018.1508450. [DOI] [PubMed] [Google Scholar]
- 16.Xiao L, Pan G. An important intestinal transporter that regulates the enterohepatic circulation of bile acids and cholesterol homeostasis: the apical sodium-dependent bile acid transporter (SLC10A2/ASBT) Clin Res Hepatol Gastroenterol. 2017;41:509–515. doi: 10.1016/j.clinre.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Hegyi P, Maléth J, Walters JR, Hofmann AF, Keely SJ. Guts and gall: bile acids in regulation of intestinal epithelial function in health and disease. Physiol Rev. 2018;98:1983–2023. doi: 10.1152/physrev.00054.2017. [DOI] [PubMed] [Google Scholar]
- 18.Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A 3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol. 2011;106:2154–2164. doi: 10.1038/ajg.2011.285. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima A, Seki M, Taniguchi S, et al. Safety and efficacy of elobixibat for chronic constipation: results from a randomised, double-blind, placebo-controlled, phase 3 trial and an open-label, single-arm, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:537–547. doi: 10.1016/S2468-1253(18)30123-7. [DOI] [PubMed] [Google Scholar]
- 20.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and rome IV. Gastroenterology. 2016;150:1262–1279. e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima A, Taniguchi S, Kurosu S, Gillberg PG, Mattsson JP, Camilleri M. Efficacy, long-term safety, and impact on quality of life of elobixibat in more severe constipation: post hoc analyses of two phase 3 trials in Japan. Neurogastroenterol Motil. 2019;31:e13571. doi: 10.1111/nmo.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 23.Rao SS, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol. 2004;99:2405–2416. doi: 10.1111/j.1572-0241.2004.40453.x. [DOI] [PubMed] [Google Scholar]
- 24.Blake MR, Raker JM, Whelan K. Validity and reliability of the bristol stool form scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693–703. doi: 10.1111/apt.13746. [DOI] [PubMed] [Google Scholar]
- 25.Ohkubo H, Yoshihara T, Misawa N, et al. Relationship between stool form and quality of life in patients with chronic constipation: an internet questionnaire survey. Digestion. 2019;102:147–154. doi: 10.1159/000502815. [DOI] [PubMed] [Google Scholar]
- 26.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 27.Jaruvongvanich V, Patcharatrakul T, Gonlachanvit S. Prediction of delayed colonic transit using bristol stool form and stool frequency in Eastern constipated patients: a difference from the West. J Neurogastroenterol Motil. 2017;23:561–568. doi: 10.5056/jnm17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyam DC, Pemberton JH, Ilstrup DM, Rath DM. Long-term results of surgery for chronic constipation. Dis Colon Rectum. 1997;40:273–279. doi: 10.1007/BF02050415. [DOI] [PubMed] [Google Scholar]
- 29.Black CJ, Ford AC. Chronic idiopathic constipation in adults: epidemiology, pathophysiology, diagnosis and clinical management. Med J Aust. 2018;209:86–91. doi: 10.5694/mja18.00241. [DOI] [PubMed] [Google Scholar]
- 30.Mori S, Tomita T, Fujimura K, et al. A randomized double-blind placebo-controlled trial on the effect of magnesium oxide in patients with chronic constipation. J Neurogastroenterol Motil. 2019;25:563–575. doi: 10.5056/jnm18194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakai E, Ikemura K, Sugimoto H, Iwamoto T, Okuda M. Risk factors for the development of hypermagnesemia in patients prescribed magnesium oxide: a retrospective cohort study. J Pharm Health Care Sci. 2019;5:4. doi: 10.1186/s40780-019-0133-7.1e70e6bd60cf42219ad00eaac3fca703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chey WD, Camilleri M, Chang L, Rikner L, Graffner H. A randomized placebo-controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol. 2011;106:1803–1812. doi: 10.1038/ajg.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.