Abstract
Disorders of gut-brain interaction (DGBIs) are common conditions in community and clinical practice. As specialized enteroendocrine cells, enterochromaffin (EC) cells produce up to 95% of total body serotonin and coordinate luminal and basolateral communication in the gastrointestinal (GI) tract. EC cells affect a broad range of gut physiological processes, such as motility, absorption, secretion, chemo/mechanosensation, and pathologies, including visceral hypersensitivity, immune dysfunction, and impaired gastrointestinal barrier function. We aim to review EC cell and serotonin-mediated physiology and pathophysiology with particular emphasis on DGBIs. We explored the knowledge gap and attempted to suggest new perspectives of physiological and pathophysiological insights of DGBIs, such as (1) functional heterogeneity of regionally distributed EC cells throughout the entire GI tract; (2) potential pathophysiological mechanisms mediated by EC cell defect in DGBIs; (3) cellular and molecular mechanisms characterizing EC cells and gut microbiota bidirectional communication; (4) differential modulation of EC cells through GI segment-specific gut microbiota; (5) uncover whether crosstalk between EC cells and (i) luminal contents; (ii) enteric nervous system; and (iii) central nervous system are core mechanisms modulating gut-brain homeostasis; and (6) explore the therapeutic modalities for physiological and pathophysiological mechanisms mediated through EC cells. Insights discussed in this review will fuel the conception and realization of pathophysiological mechanisms and therapeutic clues to improve the management and clinical care of DGBIs.
Keywords: Enterochromaffin cells, Gastrointestinal microbiome, Gastroparesis, Gut barrier dysfunction, Irritable bowel syndrome
Introduction
Functional gastrointestinal disorders (FGIDs) are currently defined as disorders of gut-brain interactions (DGBIs) to underscore the term “functional” as it has a connotation of being primarily psychologically in origin.1 Moreover, in place of brain-gut interaction, which overestimates the importance of cerebral dysfunction over abnormalities in the gut, in the new Rome classification, these are called DGBIs, overemphasizing the importance of gut over the brain.1 The board of directors of the Rome foundation, with the feedback and final approval from the chairs and co-chairs of the Rome IV committees, created the new definition for DGBI and defined it as a group of disorders classified by gastrointestinal (GI) symptoms related to any combination of the following: motility disturbance, visceral hypersensitivity, altered mucosal and immune function, altered gut microbiota, and altered central nervous system (CNS) processing.2 DGBIs, for instance, irritable bowel syndrome (IBS) and functional dyspepsia (FD), are common conditions needing referral to gastroenterology clinics and are also highly prevalent in the community.3 DGBIs are heterogeneous conditions with intertwined pathophysiological mechanisms, such as altered gut motility, impaired GI barrier function, gut immune dysregulation, gut microbial dysbiosis including small intestinal bacterial overgrowth, low-grade inflammation, visceral hypersensitivity, and dysregulated gut-brain axis.4,5 A recent global epidemiological study of 73 076 participants in 33 countries reported that more than 40% of participants met the Rome IV criteria for at least one DGBIs.6 This study indicated FD and IBS were the top rankers amongst the 22 DGBIs on the pooled prevalence rate.6 Furthermore, DGBIs and specific GI motility disorders often present significant clinical overlaps, for instance, gastroparesis and FD-postprandial distress syndrome (90%); gastroparesis and slow-transit constipation (60%); and FD and IBS (15-42%).7-9 The rising prevalence of DGBIs established a substantial burden and challenge on the patients, families, and the healthcare system.3 Despite extensive research, the underlying pathophysiology of DGBIs remains enigmatic providing the impetus for future research to elucidate the precise pathologies and treatment to optimize patient care.
Serotonin (5-hydroxytryptamine, 5-HT) affects a wide range of gut and brain physiological functions such as gut motility, secretion, visceral sensation, immune regulation, gastrointestinal epithelial integrity, sleep, and mood.10,11 The vast majority of serotonin in our body is produced and secreted from enterochromaffin (EC) cells.11 Serving as a communication circuit between the apical and basolateral interface of the gut epithelium, EC cells sense chemical-mechanical stimuli, transduce signals to enteric nervous system (ENS) and CNS, and neighboring cells; these functions reinforce EC cells as a front-line player in modulating the pathophysiology of DGBIs.12 However, the potential mechanistic link between EC cells/serotonin metabolism and the DGBIs is largely elusive. In this review, we uncovered the most recent discoveries of EC cells and/or serotonin-mediated physiology and pathophysiology in DGBIs. Decoding complex mechanisms will strengthen the understanding of DGBIs, facilitate potential diagnostic tools, and therapeutic modalities to refine the management of these disorders.
Current Challenges of Disorders of Gut-Brain Interactions
To broaden our horizons on pathophysiology of DBGIs, current challenges that need to be addressed include: (1) symptoms may not always reflect the pathophysiological mechanisms. Without knowing the cellular and molecular pathologies, the medications may be ineffective and results in higher healthcare expenditure and poor quality of life.3,13,14 Better pathophysiology-directed therapeutic strategies rather than symptomatic treatment are warranted. (2) The interplay between EC cells/serotonin signaling and gut microbiota and/or its derived metabolites in the pathogenesis of DGBIs are enigmatic. The association between altered serotonin signaling and symptom severity among patients with DGBIs is scanty.11,15,16 (3) Despite gut microbiota having an essential role in gut health, its precise functions in DGBI are cloudy.4 Given the fact that the gut microbiome varies between person, race, ethnicity, nationality, and even neighboring communities, what constitutes a healthy microbiome? What is gut microbial dysbiosis?17,18 (4) The mechanism of EC cells contributing to the intestinal barrier integrity and the fundamental impact of gut microbiota and its derived molecules on EC cell’s function are limited.19 Large-scale and longitudinal studies utilizing multi-omics analysis are needed to dissect the exact contribution of gut microbiota and serotonin signaling alterations in the progression/regression of DGBIs. (5) Research on the role of the vagus nerve innervation in ENS, which connects the gut to the brain, concerning EC cell in DGBI pathophysiology is enigmatic?20 How does the interaction/interplay between EC cells, sensory afferent neurons, and gut immune cells modulate visceral hypersensitivity in DGBIs?21 Further, how does the gut-brain-neuroendocrine axis involve psychological comorbidity or early adverse life events that lead to DGBIs?21 (6) The explicit understanding of the central players, such as EC cells and serotonin, and how they coordinate the gut functions and crosstalk with neighboring cells and luminal content is elusive. New drugs targeting EC cells may offer hope for novel therapeutic approaches in the clinical care of DGBIs. (7) The mode of administration and the pharmacokinetics of therapeutic agents are critical features for clinical outcomes.22,23 The therapeutic modalities of enhancing the selectivity and specificity of the drugs for the gut cells are another approach to achieve better target efficacy.24 A further challenge for achieving the prokinetic effect is still incomplete, which requires activation of both luminal and myenteric circuitry 5-HT receptor 4 (5-HT4R).24 Furthermore, 5-HTR expression is abundant in the GI cells throughout the gut, but differential expression of their subtypes specific to the GI cells is elusive, which is critical for the on-target and off-target side effects associated with 5-HTR agonists and antagonists. (8) To discriminate FD from gastroparesis, which has a similar underlying clinicopathological spectrum, is a significant challenge in clinical practice and research, given that these disorders are indistinguishable by gastric emptying assessment.25 Precise biomarkers for identifying the pathologies are warranted besides the gastric emptying test, which does not correlate with symptoms and their severity.25 (9) The clinical presentation of DGBIs significantly overlaps. For instance, 60-70% of patients with gastroparesis have slow transit constipation (STC), and the overlap between IBS and FD is 15-42%.3,8 These clinical findings raise questions about the causal effect and the origin of the different subtypes DGBIs. Significant clinical overlaps among DGBIs suggest the sharing pathophysiological mechanisms and therapeutic targeting of these cellular and molecular pathologies might provide a better treatment approach.
Gastrointestinal Cells Dynamics in Gut Health and Disorders of Gut-Brain Interactions
Gastrointestinal Cells Dynamics in Gut Physiology
The GI tract is a highly dynamic tubular organ that processes ingested food, assimilates water and nutrients, and eliminates waste to maintain our health.26 A recent human intestinal single-cell resolution spatiotemporal analysis has cataloged 101 cell types and 9 intestinal compartments clusters, including immune, epithelium, muscularis, endothelium, neural, fibroblast, myofibroblast, pericyte, and mesothelium.27 The staggering complexity of intercellular interactions coordinates the directional movement of luminal contents from ingestion to expulsion.28 The pacemaker activity of interstitial cells of Cajal (ICCs), platelet-derived growth factor-alpha cells, smooth muscle cells, enteroendocrine cells, glia cells, and excitatory and nitrergic neurons of the ENS maintains the regular GI motility pattern (Figure).29 The cellular and molecular remodeling in these cells are responsible mechanisms for GI function alterations in FD, gastroparesis, and IBS.30 However, the inconsistent association between altered gut physiology and symptoms warrants further investigations of differential innervating patterns and complexity of enteric and vagal neurons, pacemaker cells, immune and enteroendocrine cells in region-specific GI segments.
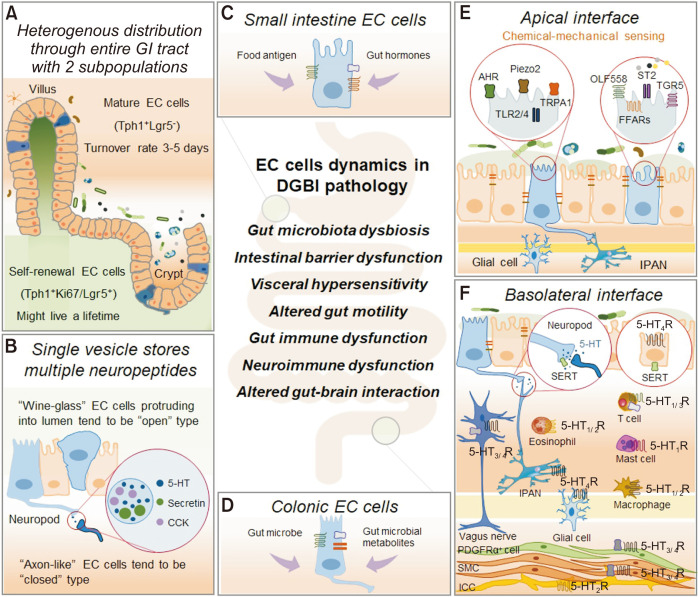
Figure.
Enterochromaffin (EC) cells dynamics in gut physiology and disorders of gut-brain interactions. (A) Heterogenous distribution of EC cells. (B-D) Functional heterogeneity of EC cells. Small intestine EC cells are specialized for sensing nutrients and colonic EC cells for gut microbiota and its derived molecules. (E, F) Apical and basolateral interface of EC cells. EC cells coordinate bilateral communication through crosstalk between dietary, microbial, and inflammatory factors to which their apical surface is exposed and to enteric neurons with afferent endings close to their basolateral surface, coordinating gut-brain communication. GI, gastrointestinal; Tph1, tryptophan hydroxylase 1; Lgr5, leucine-rich repeat-containing G-protein coupled receptor 5; 5-HT, 5-hydroxytryptamine; CCK, cholecystokinin; DGBI, disorders of gut-brian interaction; AHR, aryl hydrocarbon receptor; TRPA1, transient receptor potential ankyrin 1; TLR, toll-like receptor; OLF558, olfactory receptor 558; ST2, suppressing the tumorigenicity 2 receptor; TGR5, Takeda G protein-coupled receptor 5; FFARs, free fatty acid receptor genes; SERT, serotonin reuptake transporter; 5-HTR, 5-hydroxytryptamine receptor; IPAN, intrinsic primary afferent neuron; PDGFRα, platelet-derived growth factor receptor α; SMC, smooth muscle cell; ICC, interstitial cells of Cajal.
As the second-largest epithelium in our body, the GI tract constitutes a surface area of > 30 m2.31 A large surface area of GI epithelium in direct contact with luminal contents is responsible not only for nutrient absorption but also protection against environmental pathogens.32 The gut epithelium functions as a protective barrier that withstands extreme pH variations, mechanical abrasion, and bacteria colonization. Enteroendocrine cells (< 1% of gut epithelium) are primary secretory lineages that serve vital functions that rely on hormones and neurotransmitters to integrate signals arising in the gut to the brain.33 EC cell is a unique subset of enteroendocrine cells, scattered among epithelium throughout the GI tract, synthesize and secrete 90-95% of the serotonin in the human body (Figure).16 As a specialized intestinal epithelium, EC cells closely coordinate with neighboring cells to adopt various strategies balancing the intestinal barrier functions with secretion, chemo-and mechanosensation, nutrient absorption, and crosstalk with gut microbiota.34 Serotonin is a versatile biogenic amine, neurotransmitter, and hormone, which is primarily responsible for diarrhea associated with carcinoid tumors.35 It regulates pivotal peripheral and central effects, including secretion, visceral sensation, gut motility, energy homeostatis, inflammation, sleep, food intake, aggression, depression, anxiety, and psychosis critical for gut health and alternations responsible for DGBIs.11
Gastrointestinal Cells Dynamics in Disorders of Gut-Brain Interactions Pathophysiology
State-of-the-art studies suggest impaired GI barrier function as a central pathological mechanism that regulates gut-brain interactions due to immune dysregulation via microbial alterations and food antigens.36,37 Gut immune dysregulation drives a breach in the intestinal barrier and functional deficits in GI pacemaker cells, leading to alerted visceral sensation, secretion, and gut motility.38 As a result, the activated mast cells and eosinophils induce tissue damage and interferes with enteric nerve function. In addition, dysregulation of the neuroimmune crosstalk has been proposed as the leading cause of visceral hypersensitivity responsible for abdominal pain in subsets of DGBIs.39 Studies suggest EC cells serve as a communication circuit for the gut-brain interactions. The interplay between host and gut microbiota modulates serotonin signaling/metabolism, affecting gut neurohormonal-immunoregulation, intestinal barrier function, and visceral sensation.40
Enterochromaffin Cells Mediating Gut Physiology
Owing to their extensive heterogeneity and variability, the functional roles of EC cells and serotonin are still in large part undetermined concerning the scope of its contribution to gut-brain homeostasis.
Functional Heterogeneity
EC cells secrete cholecystokinin (CCK), secretin, and peptide YY (PYY), even though serotonin is the primary hormone synthesized and secreted by these cells.41 Twenty-five percent of serotonin-containing cells also secrete PYY in the human colon, whereas CCK and serotonin colocalize in the murine small intestine.42 More intriguingly, researchers identified serotonin, CCK, and PYY inside one single vesicle of EC cells (Figure).43 The dynamic functions of EC cells are not restricted to secretion; spontaneously, the tasting/sensing function is due to the expression of nutrient receptors, and transporters differ considerably between EC cells in gut region-specific manner.44 Small intestine EC cells are specialized for sensing nutrients and colonic EC cells for gut microbiota and its derived molecules.44 Small intestine EC cells are devoid of nutrient metabolites sensing receptors such as G protein-coupled bile acid receptor 1 (GPBAR1), G protein-coupled receptor 119 (GPR119), free fatty acid receptor 1 (FFAR 1), calcium-sensing receptor, and GPR142 (Figure).44 Colonic EC cells express GPCR sensors of microbial molecules, comprising of receptors for short chain fatty acids (SCFAs), for instance, FFAR2, olfactory receptor 558 (OLF558), OLF78; aromatic acids receptors, GPR35; secondary bile acids receptors, GPBAR1; and acyl-amides and lactate, GPR132 (Figure).44
Life Cycle of Enterochromaffin Cells: Self-renewal and Mature Enterochromaffin Cells
The turnover rate of the gut epithelial cell is 3-5 days.45 The epithelium starts its journey from the crypt base and migrates to the villus until it reaches the top edge and has been “squeezed out” while undergoing apoptosis. During its short but magnificent life, the epithelial cells not only “witness” a great war between host-pathogenic microbes, and the harmonious mutualistic relationship between host and commensal microbiota, but also deliver its substantial duties as the major subtype of enteroendocrine cells. EC cells have different cell morphologies and heterogeneous distribution throughout the entire GI tract (Figure).42,46 The typical “wine-glass” type of EC cells are located in the small intestine, whereas the “axon-like” EC cells are predominantly found in the colon.47 The “wine-glass” shaped EC cells are believed to be “open type” and directly taste luminal contents. The “axon-like” EC cells tend to function as “closed type” enteroendocrine cells. A recent murine study demonstrated 2 subpopulations of EC cells. This study clarified their positions, with the mature cells positioned in the apical surface and the self-renewal cells (expressing leucine-rich repeat-containing G-protein coupled receptor 5 or Ki67) may serve as precursor cells to keep up EC cell populations in the basolateral surface (Figure).16 This discovery challenges the stereotype that the epithelium has a short life and showed EC cells could sustain their cell features for a long time, and some even last a lifetime.
Apical and Basolateral Interface of Enterochromaffin Cells
EC cells interact with neighboring enterocytes, other enteroendocrine cells, enteric neurons, gut microbiota, dietary contents, and chemical- and mechanical-stimuli, as autocrine, paracrine, synaptic, and endocrine moderators (Figure).33,48 Activation of EC cell sensory receptors by food antigens, acidity, invading pathogens, and mechanical stimuli triggers serotonin release.12 Although there was recently a breakthrough in understanding EC cell sensory pathway, it is well-accepted that the luminal sensing signaling involves a complex of autocrine, neurocrine, and paracrine actions.40 For instance, EC cells are mechanosensitive; upon activation, Piezo2 channels generate an ionic current essential for the intracellular Ca2+ resulting in serotonin release spontaneously.49 Meanwhile, EC cells are chemosensitive and recruit transient receptor potential ankyrin 1 channel (TRPA1) to detect irritants leading to the activation of voltage-gated Ca2+ channel-dependent serotonin release.50,51 In addition, neighboring enteroendocrine L-cells express nutrient metabolite receptors, which trigger glucagon-like peptide secretion to stimulate serotonin secretion from EC cells suggesting that EC cells sense nutrients indirectly via the L-cell and relay the signal luminal nutrient metabolites to basolateral vagal afferent neurons.44 Once serotonin is released, the signals travel to the local ENS and distant organs, including the pancreas and the brain, thus regulating food intake, digestive enzyme secretion, and bowel movement.52
Not long ago, researchers thought EC cells interact with nerves indirectly through paracrine or endocrine signals.53 Using transgenic and advanced optical tools, EC cells received a better description as having several anatomic features observed in neurons, including dendritic-like spines and axon-like processes, suggesting EC cells may communicate with ENS through synaptic structure, known as neuropod.48,54 Through neuropod, serotonin released from EC cells acts on extrinsic primary afferent neurons (EPANs) and intrinsic primary afferent neurons in a paracrine and synaptic manner.40 EPANs stimulated via serotonin modulate nausea and abdominal discomfort. We believe that the mature EC cells positioned in the luminal surface serve to act as paracrine modulators; self-renewal EC cells are primarily positioned in the basolateral crypt base with neuropod sever to connect the gut-brain axis.
Taken together, EC cells not only communicate with neighboring epithelium and enteric neurons as paracrine and synaptic modulators, as well as “tasting” and “feeling” gut microbiota and food particles via chemo- and mechanosensing receptors.55 Thus, as a flexible and resourceful communication circuit, the EC cell is crucial in GI health and diseases.
Enterochromaffin Cells Mediating Pathophysiology Mechanisms of Disorders of Gut-Brain Interactions
The regulation of GI functions entails complex coordinated interactions between gut and brain, which necessitates the proper functioning of GI pacemaking cells, epithelial cells, and immune cells.16,29,30 Any functional defects in these cells will potentially hinder gut function and generate symptoms of DGBIs.30
Apical Interface of Enterochromaffin Cell
Gut microbiota dysbiosis
Gut microbiota dysbiosis is a notable pathophysiological mechanism of DGBIs.17,36,56 The fecal microbiota profile of IBS patients differs significantly from that of healthy subjects and influences gut physiology, for instance, motility, GI barrier function, visceral sensation, and gut immune function.4,57 In a recent meta-analysis, IBS patients had a reduced number of commensal bacteria of genera Bifidobacterium and Lactobacillus and an increased number of pathogenic bacteria of genera Enterobacter as compared to the healthy subjects.58 Studies reported overrepresentation of Methanobravibacter smithii in stool samples from patients with FD, functional constipation, and constipation-predominant IBS (IBS-C).59,60 A microbiome signature linked with symptom severity of IBS has been suggested with reduced diversity of commensal bacteria and the presence of pathogenic bacteria such as methanogenic or Clostridiales species.59 Fecal microbiota transplantation (FMT) may be beneficial; however, a meta-analysis of randomized-controlled trials in IBS patients reported no significant improvement in symptoms after FMT compared to placebo treatment.61 On the contrary, a double-blind, randomized controlled trial in IBS patients suggested FMT was beneficial with a significant improvement in symptoms compared with placebo.62 Still, the clinical outcome of gut microbiota intervention with FMT is hazy, particularly due to the unclearness of the healthy microbiome constitution as well as the interplay between host and gut microbiota.
Recent findings highlighted the crosstalk between host and microbiota in gut homeostasis.34,63 One study demonstrated the critical role of indigenous spore-forming bacteria promote serotonin biosynthesis from colonic EC cells modulating GI motility.34 On the other hand, elevating luminal serotonin increases the number of Turicibacter sanguinis, a spore-forming bacteria, which expresses a neurotransmitter sodium symporter protein with structural and sequence homology to mammalian serotonin reuptake transporter (SERT).63 Treating T. sanguinis with serotonin or fluoxetine enhanced its colonization in the murine gut.63 Furthermore, one study showed serotonin was capable of decreasing virulence gene expression of Citrobacter rodentium and enterohemorrhagic Echerichia coli by inactivating bacterial serotonin receptor transcription.64 Together, these findings suggested that the selective indigenous gut bacteria crosstalk with the host serotonergic pathways to boost their colonization in the gut, also sparked interest in whether modulating EC cell and gut microbiota interaction may be an effective treatment strategy for DGBIs.
Gut microbial-derived molecules
One of the fundamental regulations of serotonin biogenesis is host-microbiota crosstalk.65 Gut microbial-derived molecules communicate signals to modulate the targeted cells’ function, eventually affect the physiological and pathophysiological mechanisms, influencing health status; still, the precise molecular mechanisms in DGBIs are elusive.66
Bile acids. Gut microbiota dysbiosis induces alternations in the bile acid (BA) pool.67,68 On the contrary, reduction in BA levels influences gut microbial diversity leading to gut microbiota dysbiosis.69 A higher amount of BAs results in increased intestinal fluid secretion.10 Excess fecal BAs in diarrhea-predominant IBS (IBS-D) lead to altered gut microbiota profile enriched in Clostridia.70 Higher amounts of BA signatures, chenodeoxycholic acid and cholic acid, were reported in stool samples from patients with IBS-D and lower amounts in patients with IBS-C.71 The authors also found a positive correlation between a higher amount of BAs and abdominal pain severity. Furthermore, BAs can activate Takeda G-protein-coupled receptor 5 (TGR5) localized on EC cells and promote gut motility.72 TGR5-deficient mice displayed prolonged colonic transit and reduced fecal pellet frequency compared to control mice advocating TGR5-dependent improvement in gut motility mediated by EC cell serotonin secretion.73 The aforementioned findings suggest that the interplay between BAs, EC cells, and gut microbiota modulates DGBIs pathogenesis.
Short chain fatty acids. SCFAs such as butyrate, propionate, and acetate are the prime fermentation metabolites produced from gut microbiota-mediated degradation of dietary fiber.74 SCFAs are essential to sustain EC cell and serotonin production via activation of GPCRs (FFAR2).44 Compared to the germ-free (GF) mice, conventionally raised and humanized mice had higher colonic tryptophan hydroxylase 1 (TPH1) mRNAs due to butyrate-related increase in EC cell TPH1 expression and serotonin production.75 Furthermore, butyrate alleviates impaired barrier function through increasing claudin-2 expression. Bifidobacterium dentium-derived acetate stimulate serotonin release from EC cells.76 Meanwhile, this bacterium colonized mice had upregulated expression of 5-HT2AR in the hippocampus leading to normalized anxiolytic behavior.76 Moreover, isovalerate, a volatile fatty acid metabolite generated by gut microbiota, evoked serotonin release from EC cells, through voltage-gated Ca2+ channel modulated 5-HT3R sensory neurons.12 These studies showed that gut homeostasis relies on microbiota-derived SCFA, which plays an important role in GI motility, visceral sensation, secretion, and intestinal barrier function regulation, further reinforcing EC cells as central modulators in the gut-brain axis.
Aryl hydrocarbon receptor and serotonin signaling. The AhR has a fundamental role in host defense against bacteria in the intestine and functions as a biosensor connecting gut microbiota to program ENS and immune cells affecting gut homeostasis.77,78 Microbiota-directed AhR expression in neurons of the colon tissue enables these neurons to communicate with luminal contents and to trigger the expression of neuron-specific effector mechanisms.79 Depleting neuron-specific AhR, or overexpression of AhR negative feedback regulator cytochrome P450 1A1 (CYP1A1) causes colonic dysmotility in mice.79 Intriguingly, serotonin serves as a ligand/activator of AhR in intestinal epithelial cells through increasing CYP1A1 expression of the canonical gene target of AhR.80,81 Furthermore, this serotonin-AhR-CYP1A1 pathway was confirmed in SERT knockout mice.80 These studies demonstrated a novel mechanistic link between serotonergic and AhR signaling, which have implications in modulating gut immune function and motility.
Tryptophan metabolites. Tryptophan (Trp) metabolism is crucial in host-microbial communication to fine-tune gut physiology.10,57,71,82 Trp metabolism comprises 3 critical pathways in modulating gut functions: (1) indole pathway (direct transformation into metabolites by the gut microbiota, such as AhR and indole derivatives ligands); (2) kynurenine pathway (transformation into kynurenine and its derivatives by immune and epithelial cells); and (3) serotonin pathway (transformation into serotonin and its derivatives by EC cells). Impaired Trp metabolism contributes to DGBIs pathogenesis, particularly IBS.10,82
Tryptamine. Tryptamine is a monoamine metabolite product of tryptophan metabolism, and the gut bacteria involved in this process are Clostridium sporogenes and Ruminococcus gnavus.83 Tryptamine relay its signal in the gut primarily via the 5-HT4R pathway.84 Tryptamine participates in regulating intestinal secretion. Tryptamine generated in both GF and humanized mice showed increased epithelium fluid secretions in colonoids. Furthermore, gut motility improved in GF mice colonized with Bacteroides thetaiotaomicron, a tryptamine-producing engineered bacteria.84 Tryptamine also activates 5-HT4R on goblet cells to release mucus.19 In a murine inflammatory bowel disease model, GF mice colonized with B. thetaiotaomicron exhibited increased mucus release with improved intestinal barrier function and reduced aggressive phenotypes.19 Tryptamine levels were elevated in the stools of IBS-D patients and reduced in IBS-C patients.71 Tryptamine can modulate gut motility by triggering the release of serotonin by enteric neurons or acting as an AhR ligand.79 A recent study showed gut microbiota induces AhR expression in enteric neurons, enabling them more responsive/excitable to microbiota-derived AhR ligands, such as tryptamine, and contributing to gut motility.79
Kynurenine. Patients with IBS often have altered kynurenine pathway with elevated kynurenine levels in the serum.85,86 Indoleamine 2,3-dioxygenase, a key metabolite in the kynurenine pathway, positively correlated with IBS symptoms severity.
Serotonin. Alterations in the serotonin pathway are one of the crucial features in DGBIs.10 Whereas the patients with IBS-C have reduced serotonin activity, those with IBS-D have increased serotonin expression. Studies have shown serotonin metabolisms in the physiology and pathophysiology of IBS, FD, and gastroparesis.10,16 Clostridiales species trigger EC cells to produce serotonin by inducing TPH1 expression.34 This effect is sustained by gut microbiota-derived metabolites (propionate, butyrate, tyramine, cholate, and deoxycholate).34 One recent study showed the role of TRPA1 in the regulation of serotonin secretion by EC cells through Trp metabolites.51 Indoles are metabolite products of the tryptophan metabolism of Edwardsiella tarda. Furthermore, authors showed indoles activate TRPA1 in EC cells to release serotonin that excites enteric neurons to accelerate gut motility and stimulate vagal afferents for CNS modulation.51
Sleep disturbances are also a major contributing factor in IBS development and positively correlated with worsening IBS symptoms.87,88 Monoamine oxidase A converts serotonin to 5-hydroxyindoleacetic acid in the brain and gut.89 In the brain, serotonin is converted into melatonin, the primary hormone of the body’s sleep-wake cycle.89 Indoleamine 2,3-dioxygenase might shunt Trp to kynurenine in the brain, leading to sleep disturbances.57 Thus, these findings demonstrated that Trp metabolites are crucially important contributing to different facets of gut physiology and brain function.
Gut microbial metabolites are key molecules relaying signals to fill a specific niche and maintain bidirectional gut and brain interactions. Taken together, gut microbiota and their host may share a “common language” and harmonies as a mutualistic system. Deciphering this “common language” will offer therapeutic perspectives in DGBIs.
Intestinal barrier dysfunction
In susceptible conditions, GI infection or intake of particular foods weakens the intestinal barrier integrity through damage to the tight junctions, resulting from proteasome-mediated degradation activated by inflammatory molecules including proteases, histamine, and eicosanoids.37,90 Because of impaired barrier function, food antigens and pathogens cross the gut epithelium and activate a T helper 2 cell response that results in immune activation mediated via mast cells and eosinophils.91,92 In patients with IBS, an elevated number of colonic mast cells correlates with impaired intestinal barrier function and visceral hypersensitivity.93,94 Duodenal mucosa of patients with IBS challenged with food antigens have increased intervillous spaces and epithelial breaks compared to healthy controls.95 The cell-to-cell adhesion protein, zonula occludens-1, and occludin expression are significantly reduced in duodenal tissues from patients with DGBIs compared to healthy subjects.96,97 Duodenal biopsy samples from patients with DGBIs more often have decreased transepithelial electrical resistance resulting in intestinal barrier dysfunction with immune cell infiltration.36,37,98 One study examined whether serotonergic signaling alterations may commence a chain of events leading to changes in epithelial integrity in healthy individuals and patients with IBS.85 The authors found increased serotonergic metabolic activity, which happened after intake of the serotonin precursor, 5-hydroxytryptophan, and reinforced intestinal mucosal barrier function through redistribution of zonula occludens-1 in healthy individuals; such regulation was absent in IBS patients.85 A commensal bacterium, B. dentium, stimulates EC cells to release serotonin, which interacts with 5-HT4R on goblet cells to promote Mucin 2 and Trefoil factor 3 release, resulting in the activation of C-X-C motif chemokine receptor 4 to trigger actin cytoskeleton rearrangement and restitution mediating intestinal epithelial repair.99 Overall, these studies point to the broad implications of a potential protective mechanism involving serotonin signaling for impaired intestinal barrier function.
Visceral hypersensitivity
The modulators for visceral sensation comprise transient receptor potential vanilloid subtype 1 (TRPV1), protease-activated receptors, histamine, serotonin, tachykinin, cannabinoid, acid-sensing ion channels, voltage-gated sodium and calcium channels, and TRPA1.12,100 The TRPV1 activation by capsaicin, thermal stimulus, acidic pH, inflammatory mediators, prostaglandins, nerve growth factors, and microbes trigger the release of neuropeptides, such as substance P and calcitonin gene-related peptide-1.100 These neuropeptides are responsible for enhanced visceral sensitivity and induce painful and/or non-painful sensations.101 The hypersensitivity mediated through chemicals such as elevated visceral sensation to acid associates with nausea. The increased TRPV1 expression in colonic tissues often correlates with abdominal pain in patients with IBS-D.102,103 Visceral hypersensitivity in patients with gastroparesis associates with abdominal pain, anorexia, and early satiety.104
The expression level of TRPA1 in EC cell-enriched fraction is 16 times higher than that in the whole mucosal layer.105 Upon TRPA1 receptor stimulation, Ca2+ channel-dependent serotonin release is activated in EC cell.12 These data suggest that TRPA1 receptors on EC cells may serve as the primary detector of luminal contents prior to mucosal damage and a potential therapeutic target for the management of IBS.12 Furthermore, serotonin signaling mediates visceral hypersensitivity by activation of 5-HT3R on extrinsic sensory neurons in IBS patients.106 Activation of 5-HT4R via tegaserod and naronapride attenuates colonic hypersensitivity.107 In contrast, acute oral administration of 5-hydroxytryptophan elevates visceral nociceptive responses and abdominal distension, resulting in increased pain sensation in a subset of IBS patients.108
Taken together, serotonergic modulation through selective serotonin reuptake inhibitor (SSRI) and 5-HTR agonists/antagonists of visceral sensation appear promising to the development of novel therapeutic modalities for DGBIs.
Basolateral Interface of Enterochromaffin Cell
Altered gut motility
Functional defects of particular GI cells, for instance, ICCs, smooth muscle cells, EC cells, immune cells, and enteric neurons, have the potential to hamper gut motility.13 The normal antral motility, essential for triturating solid food and maintaining the gastric emptying rhythm, is mediated by intrinsic nitrergic and cholinergic neurons and extrinsic vagal innervation that facilitate pyloric motor functions and intragastric contractions.109 Abnormalities of the proximal stomach’s intrinsic inhibitory innervation (nitrergic pathway), inhibition of cholinergic (5-HT1AR on cholinergic neurons) and 5-HT2BR pathways, and an impaired antro-fundic reflex are involved in defective gastric accommodation and delayed gastric emptying.13,109,110 5-HT2BR antagonist normalized the impaired gastric accommodation; in contrast, 5-HT2BR agonist exacerbated the inhibition of gastric accommodation.111 A recent study reports reduced EC cell numbers in the antral biopsy in patients with idiopathic gastroparesis.16 Furthermore, depletion of EC cells in a murine model of gastroparesis and STC led to delayed gastric emptying and colonic motility. This study provided possible pathogenesis of alteration in EC cell numbers and mucosal serotonin in gastroparesis and STC.16 Further studies are warranted to underpin how and to what extent alternations of each cell type contribute to symptom generation and severity. Furthermore, to elucidate underlying neuromuscular and neuroimmune mechanisms mediated via EC cells of DGBIs are currently unclear and warrants further research.
Gut immune dysfunction
The increased recruitment of mast cells and eosinophils to gut inflammation induces tissue damage, leading to impaired gut homeostasis amongst DGBIs patients.91,112 Breach of intestinal barrier allows infiltration of luminal contents, further cascading the immune events and contributing to the development of symptoms in DGBIs.113 FD patients show elevated proinflammatory cytokine production and enhanced numbers of α4β7 integrin CCR9 T lymphocytes.114 Macrophage-driven immune dysregulation is the primary factor associated with gastroparesis in a recent transcriptomic analysis of gastric body biopsies.115 Genes associated with specific macrophages are enriched in gastric tissues from gastroparesis patients compared with controls.115 A reduction of CD206+ macrophages in the gastric antrum of patients with diabetic gastroparesis correlates with the loss of ICCs.116
A wide range of immune cells, including macrophages, mast cells, dendritic cells, and T cells, have extensive 5-HTR expression.117 5-HT1AR mediates the chemotactic influence of serotonin on mast cells, 5-HT2AR on eosinophils, 5-HT1BR, and 5-HT2AR on dendritic cells, and 5-HT2BR and 5-HT7R on macrophages.117 In a murine postoperative ileus model, 5-HT4R agonist mosapride accelerated acetylcholine release from myenteric cholinergic neurons, subsequently activated α7-nicotinic acetylcholine receptor on macrophages to inhibit their inflammatory reactions and improved the intestinal motility.118 Increased serotonin release mediating gut immune dysfunction contributes to the development of IBS symptoms.119 Spontaneous release of serotonin correlates with the severity of abdominal pain and increased mast cells in patients with IBS.119 These findings suggest a possible new paradigm for the development of DGBIs initiated through gut immune dysregulation via serotonin metabolisms.
Neuroimmune dysfunction
Immune cells interact with ENS to modulate the gut function.120 Intestinal mast cell infiltration and its close proximity to submucosal plexus neurons leads to altered neuronal responsiveness in patients with DGBIs.121-123 Further, the close proximity of activated mast cells to colonic nerves correlated with abdominal pain in patients with IBS.94,124 Sprouting of fine nerve fibers was more often noted in the duodenal tissues of patients with IBS compared to healthy controls.123,125 The grade of fine nerve fiber sprouting was positively correlated with the degree of gut immune alterations in patients with IBS.125 Moreover, the mucosal mediators from IBS patients trigger the activation of visceral pain pathways.
Not only 5-HTRs are enriched in gut immune cells, but also EC cells are capable of “sensing” immune regulators through specific receptors.126 One state-of-the-art study demonstrated neuroimmune interaction mediating through EC cells.50 IL-33 sensed by EC cells induces release of serotonin, which mediates activation of enteric neurons resulting in accelerated gut motility.50 Furthermore, histamine and serotonin regulate enteric neuron functions in patients with IBS.119 These studies revealed an immune-neuroendocrine axis in calibrating serotonin release for intestinal homeostasis.
Dysregulated gut-brain interaction
Dysregulation of the gut-brain axis is a critical pathophysiological mechanism of DGBIs.127 Brain modulates gut physiology, for instance, motility, visceral sensations mediating symptoms of DGBIs.128 Similarly, alterations in gut physiology feedback to the brain sustain psychological health.128 In a meta-analysis, the prevalence of anxiety and depressive disorders is 23% in IBS patients.129 The presence of depression and anxiety symptoms are overrepresented in IBS patients with a frequency of 39% and 29%, respectively. Psychological comorbidity and corticotropin-releasing hormone pathways contribute to the development of impaired gut barrier function.130 In response to stress, eosinophils release corticotropin-releasing hormone and substance P, which lead to activation of mast cells, ultimately resulting in impaired gut barrier function.91 Functional brain MRI studies in patients with DGBIs demonstrate abnormalities of structural and functional networks in areas of the brain responsible for processing information of vasovagal reflux and visceral motor system.131 Gut microbiota dysbiosis alters the function of neurotransmitters, such as serotonin, acetylcholine, dopamine, and γ-aminobutyric acid, either by the production or consumption of these substances, leading to changes in behavior and emotional state.21 The identification of BAs and their receptors in the brain reinforces the gut-brain axis.132 In addition, EC cells throughout the entire gut axis sense luminal contents and generate electrical signals bridging the mucosal barrier through interface with the CNS via extrinsic afferent neurons and the ENS via intrinsic afferent neurons.50,51 Thus, EC cells play a fundamental role in linking the gut connectome to the brain connectome.
As the primary source of body serotonin, EC cell-derived serotonin impacts its circulating levels and has the power to modulate brain function directly or indirectly. The information about luminal microenvironment is broadcasted to the brain by EC cells through the super-highway “vagus nerve.”21 Vagal afferents nerve terminals express 5-HT3R and locate in proximity to the EC cells.11 A direct interaction of EC cell signaling to vagal afferents in response to gut luminal chemical contents with the vagal neurocircuits is involved in reflexes, such as, vomiting.11 Probiotic intervention with Bifidobacterium spp. increases serotonin levels in the brain leading to improved behavior in the depression murine model.133 Oral administration of an SSRI elevates the gut serotonin bioavailability, alleviates depressive-like behavior in mice, possibly through vagus nerve activation.133 Overall, these findings suggest that EC cells and their derived serotonin have the potential to relay signals to the brain. Further, identifying and characterizing the specific gut microbiota and their metabolites for enhancing gut-brain physiological functions via EC cells/serotonin signaling may offer a treatment strategy for extrinsic neuron modulation.
Post-infection Disorders of Gut-Brain Interactions and Serotonin Signaling
Post-infection disorders of gut-brain interactions
Post-infection DGBIs (PI-DGBIs) develops in about 10% of patients after a bout of gut infections such as bacterial, protozoal, or viral gastroenteritis.68,134 Transient non-specific and specific GI inflammation are often associated with long-lasting DGBIs symptoms, for instance, bacterial, parasitic, viral gastroenteritis, and after resolution of a flareup of inflammatory bowel disease (IBD).68 The pathophysiological mechanism of PI-DGBIs include gut dysmotility, microbial dysbiosis, intestinal barrier dysfunction, visceral hypersensitivity, altered serotonin metabolism, bile acid malabsorption, increased density of EC cells, and T lymphocytes.10,68 A meta-analysis of 45 studies including 21 421 participants with acute gastroenteritis showed a pooled prevalence of 10.1% of PI-DGBIs at 12 months after the episode.135 A subgroup of patients with gastroparesis has an onset of symptoms after prior gastroenteritis.13 Various viral agents such as Epstein-Barr virus, enterovirus, and cytomegalovirus could cause PI-gastroparesis.13 Clinical presentation of patients with PI-gastroparesis is similar to patients with gastroparesis without prior gastroenteritis but is associated with an acute onset and severe symptoms. Acute gastroenteritis also leads to the onset of FD, known as PI-FD.68 Furthermore, approximately one-third of patients with IBD after resolution of a flareup of IBD experience symptoms of IBS.136 Additionally, acute infective gastroenteritis leads to the onset of IBS, termed PI-IBS. A broad range of bacterial pathogens has been reported in PI-IBS, such as Salmonella enterica, Clostridioides difficile, Campylobacter jejuni, Vibrio cholerae, and Escherichia coli.137 Reduced gene expression of SERT has been reported in patients with PI-IBS, suggesting increased bioavailability of luminal serotonin may trigger stimulation of 5-HTRs on afferent neurons leading to enhanced visceral sensation.68 In colonic tissue of patients with Shigella and Campylobacter PI-IBS had increased EC cell density.138,139 However, patients with post-Giardia IBS had decreased EC cell density in colonic tissue compared with controls.140
Post-corona virus disease 19 disorders of gut-brain interactions
Previous studies on GI symptoms (nausea, abdominal pain, diarrhea, anorexia, and vomiting) overlapping in patients with corona virus disease 19 (COVID-19) showed diarrhea symptoms as the most prevalent associated with the severity of COVID-19.134,141 PI-IBS, including IBS-D, is associated with increased serotonergic activity.68 These observations highlighted the importance of alterations in serotonin metabolism. The overrepresentation of GI symptoms in patients with COVID-19 suggests that post-SARS-CoV-2 infection may lead to persistent abnormal gut functions underpinning certain pathophysiological aspects in PI-DGBIs. Hence, regulating serotonin signaling might be a potential therapeutic modality for the clinical care in a subset of COVID-19 patients.
Pathophysiology-directed Therapeutic Approach for Disorders of Gut-Brain Interactions
Pathophysiological mechanisms, both peripheral (intestinal barrier dysfunction, altered motility, visceral hypersensitivity, gut microbiota dysbiosis, gut immune dysfunction, bile acid malabsorption, and enteric neuroendocrine abnormality) and central (psychological comorbidity, cognitive dysfunctions, and neuroendocrine dysregulation), may be a better strategy to be targeted by therapeutic agents to treat patients with DGBIs (Refer to Tables 1, 2, and 3 with key references cited). Based on the significant overlaps due to common pathogenesis between the different DGBIs, pathophysiology-directed therapeutic modalities may provide precise clinical outcomes in DGBIs. Furthermore, studies are warranted on personalized treatment of different DGBIs employing a robust study design incorporating longitudinal multicentric studies integrating multi-omics data of gut physiology and pathophysiology. Such comprehensive approaches may lead to novel therapeutic modalities, resulting in targeted restoration of abnormal physiological functions in DGBIs patients.
Table 1.
Summary of Pathophysiology-directed Therapeutic Approach for Disorders for Gut-Brain Interactions
| Drug name | Function/pathway | Pathophysiological mechanisms | Clinical outcome | DGBI types | |
|---|---|---|---|---|---|
| Prokinetics | Prucalopride | 5-HT4R agonist | Altered gut motility | Improved GI symptoms as assessed by the GCSI. | Gastroparesis, FD, chronic constipation |
| Improved solid gastric emptying. | |||||
| Felcisetrag | Selective 5-HT4R agonist | Altered gut motility | Accelerated transit of solids throughout the gut in patients with idiopathic or diabetic gastroparesis. | Gastroparesis | |
| Stimulate motility and secretion through enhanced release of acetylcholine from excitatory motor neurons and interneurons. | |||||
| Alosetron | 5-HT3R antagonist | Altered gut motility | Improved stool consistency and bowel movements. | IBS-D | |
| Ondasetron | |||||
| Ramosetron | |||||
| Cilansetron | |||||
| Buspirone | 5-HT1R agonist | Altered gut motility | Fundus relaxation, improve gastric accommodation. | FD | |
| Tandospirone | |||||
| Relamorelin | Ghrelin receptor agonist | Altered gut motility | Stimulates nodose afferents and DMV neurons and accelerates gastric emptying. | Gastroparesis | |
| Metoclopramide | Dopamine-2 receptor antagonists | Altered gut motility | Improved gut motility. | FD, gastroparesis | |
| Domperidone | |||||
| Itopride | |||||
| Acotiamide | Muscarinic receptor antagonists | Altered gut motility | Inhibits acetylcholinesterase and antagonizes the presynaptic muscarinic receptors, present on cholinergic nerve endings, which leads to an increase in acetylcholine levels in the synaptic cleft. | FD | |
| Antibiotics | Neomycin | Modulating gut microbiota profile | Gut microbiota dysbiosis | Shifting the microbial community composition. | IBS |
| Rifaximin | Improvement in constipation, SIBO and dyspeptics symptoms. | ||||
| Probiotics | Bifidobacterium animalis DN-173010154, Bifidobacterium lactis DN-173 | Modulating gut microbiota profile | Gut microbiota dysbiosis | Shifting the microbial community composition. | IBS |
| Lactobacillus gasseri | Improved symptoms and gut transit. | ||||
| Bile acid sequestrants | Obeticholic acid | FXR agonist | Bile acid alterations | Stimulate the synthesis and subsequent release of FGF-19 from ileal epithelial cells and inhibit bile acid synthesis by hepatocytes. | IBS-D |
| Tropifexor | |||||
| Cholestyramine | Improve stool form and symptoms of diarrhea. | ||||
| Bile acid transporter inhibitor | Elobixibat | IBAT antagonist | Bile acid alterations | Efficacious treatment for constipation, improving gut transit and symptoms via increasing colonic bile acids. | IBS-C |
| Cholestipol | |||||
| Anti-inflammatory agents | Mesalazine | Gut immune homeostasis | Low grade inflammation | Sustained therapy response and benefits for a subgroup of patients with IBS. | IBS |
| Visceral hypersensitivity | |||||
| Ketotifen | Mast cell stabilizer | Visceral hypersensitivity | Increased the threshold for discomfort in patients with IBS with visceral hypersensitivity, and reduced IBS symptoms. | IBS | |
| Ebastine | Histamine receptor-1 antagonist | Visceral hypersensitivity | Reduced visceral hypersensitivity and abdominal pain in patients with IBS. | IBS | |
| Acid suppressive therapy | Pantoprazole | Proton pump inhibitors | Gut immune dysfunction | Reduced duodenal eosinophilia, mast cells, and intestinal permeability in patients with FD. | FD |
| Epithelial barrier dysfunction | |||||
| Visceral hypersensitivity | |||||
| Neuromodulators | Amitriptyline | TCA | Gut-brain dysregulation | Affect gastrointestinal motility through anticholinergic and serotonergic mechanisms. TCAs reduce visceral hypersensitivity. Antidepressant therapy might lead to neurogenesis. | IBS, FD |
| Mirtazapine | Tetracyclic antidepressants | Gut-brain dysregulation | Upregulates the levels of orexigenic hormones and downregulated the levels of anorexigenic hormones. | FD | |
| Escitalopram | SSRI | Gut-brain dysregulation | Treating depressive symptoms with these molecules modulates the severity of GI symptoms indirectly via a positive effect on depression. | IBS | |
| Venlafaxine | SNRI | ||||
| Duloxetine | |||||
| Intestinal Secretagogues | Lubiprostone | CCl2 agonist | Abnormal secretion | Increased fecal water content. | IBS-C, chronic constipation |
| Creates an ion gradient that promotes water and sodium secretion into the intestinal lumen. | |||||
| Linaclotide | Guanylate cyclase-C receptor agonist | Abnormal secretion | Increase water secretion via targeting cGMP leads to the secretion of chloride and bicarbonate into the intestinal lumen. | IBS-C | |
| Visceral Analgesics | Oliceridine | Biased μ-Opioid receptor ligands | Visceral hypersensitivity | Management of moderate to severe acute pain in adults for whom alternative treatments other than opioids had failed. | IBS |
| Olorinab | Cannabinoid type 2 receptor agonist | Visceral hypersensitivity | Potential analgesic effects in patients with IBS. | IBS | |
DGBIs, disorders of gut-brain interactions; 5-HTR, 5-hydroxytriptamine receptor; GI, gastrointestinal; GCSI, gastroparesis cardinal symptom index; FD, functional dyspepsia; DMV, dorsal motor nucleus of the vagus; IBS-D, diarrhea-predominant irritable bowel syndrome; SIBO, small intestinal bacterial overgrowth; IBS, irritable bowel syndrome; FXR, farnesoid X receptor; FGF-19, fibroblast growth factor 19; IBAT, ileal bile acid transporter; IBS-C, constipation-predominant irritable bowel syndrome; TCA, tricyclic antidepressant; SSRI, serotonin reuptake inhibitor; SNRI, serotonin norepinephrine reuptake inhibitor; CCl2, type 2 chloride channel; cGMP, cyclic guanosine monophosphate.
Table 2.
Gut Microbiota-derived Molecules Modulating Pathogenesis of Disorders for Gut-Brain Interactions
| Metabolites | Targeting cell types | Pathophysiological mechanisms | Key findings |
|---|---|---|---|
| Butyrate and acetate | EC cells | Altered gut motility | These metabolites increase 5-HT biosynthesis from EC cells through stimulatory activities.75 |
| SCFAs | EC cells and 5-HT3R+ vagal nerves | Altered gut motility | Release of 5-HT from EC cells in response to SCFAs stimulates 5-HT3R located on the vagal sensory fibers. The sensory information is transferred to the vagal efferent and stimulates the release of acetylcholine from the colonic myenteric plexus, resulting in muscle contraction.142 |
| Butyrate | EC cells and TRPV1+ cells | Visceral hypersensitivity | Repetitive stimulation of TRPV1 receptor via butyrate-induced 5-HT release desensitize TRPV1+ neurons resulting in less pain sensation.143 |
| Butyrate | Enterocytes and immune cells | Altered gut immune function and impaired barrier function | Butyrate regulates neutrophil function and migration, inhibits inflammatory cytokine induced expression of vascular cell adhesion molecule-1, increases expression of tight junction proteins in colon epithelia, and exhibits anti-inflammatory effects by reducing cytokine and chemokine release from immune cells. Butyrate or specific species of butyrate producing gut bacteria may be a new target for restoring host immune function and barrier integrity.144 |
| Acetate | Enterocytes and EC cells | Abnormal secretion | Gut microbiota alters 5-HT-evoked intestinal secretion in a 5-HT3R-dependent mechanism. Acetate alters 5-HT3R expression in colonoids.145 |
| BAs | EC cells | Altered gut motility | TGR5 receptor on EC cells mediates the effects of BAs on colonic motility. |
| Deficiency of TGR5 causes constipation in mice.73 | |||
| Tryptamine | Enterocytes | Altered gut motility and secretion | Tryptamine accelerates gut transit and increases colonic secretion by activating epithelial 5-HT4R. Genetically engineered bacteria Bacteroides thetaiotaomicron produce tryptamine.84 |
| Methane | EC cells and nitrergic neurons | Altered gut motility | Methane derived from Methanobravibacter smithii in the colon depletes gut 5-HT resulting in slowed gut transit and constipation.146 |
| Visceral hypersensitivity | |||
| Isovalerate | EC cells | Altered gut motility | Isovalerate evoked 5-HT release from EC cells through voltage-gated Ca2+ channel and modulated 5-HT3R neurons in visceral sensation and gut motility.12 |
| Visceral hypersensitivity | |||
| Indole | EC cells | Altered gut motility | Edwardsiella tarda metabolized tryptophan to produce indoles that activate TRPA1 on EC cells to produce 5-HT that stimulates enteric neurons and induces gut motility.51 |
EC, enterochromaffin; 5-HT, 5-hydroxytryptamine; SCFA, short chain fatty acid; 5-HTR, 5-hydroxytryptamine receptor; TRPA1, transient receptor potential ankyrin 1 channel; BAs, bile acids; TGR5, Takeda G protein-coupled receptor 5; TRPV1, transient receptor potential vanilloid 1.
Table 3.
Pharmacological Agents Modulating Serotonin-mediated Mechanisms in Disorders of Gut-Brain Interactions
| Drug name | Mechanisms of action | Clinical outcome |
|---|---|---|
| TCA | TCA has 5-HT and NA reuptake inhibition properties, primarily used for anti-depressant and analgesic. | Affect gut motility through anticholinergic and serotonergic mechanisms. Reduces visceral hypersensitivity, intestinal pain sensitivity, mediated either in peripheral nerves or the CNS.147 |
| Their mode of action involves mechanisms beyond 5-HT and NA, like blockage of voltage-gated ion channels, opioid receptor activation and also modulates neuroimmune anti-inflammatory effects. | ||
| Tetracyclic antidepressant | Boosts both 5-HT and NA neurotransmission, not by blocking their reuptake pumps, but by blocking presynaptic α2-noradrenergic receptors on NA and 5-HT neurons, resulting in an increased noradrenergic and serotonergic activity | Upregulates the levels of orexigenic hormones and downregulated the levels of anorexigenic hormones. |
| Reduce colonic hypersensitivity and improve gastric emptying.148 | ||
| SSRI | SSRIs are characterized by selective blockade of the presynaptic 5-HT transporter, boosting 5-HT neurotransmission. | Increase colonic contractility and reduce colonic tone during fasting conditions and reduce the colonic tone increase. Decrease IBS scores for abdominal pain and bloating independent of anxiety, depression and colonic sensorimotor function.149 |
| SNRI | Primarily block both 5-HT and NA reuptake, boosting 5-HT and NA neurotransmission. | Increase compliance, relax tone and reduce the postprandial colonic contraction and increase sensory thresholds in response to balloon distensions.149 |
| Considering the central roles of 5-HT and NA in the descending modulatory nerve pathways, SNRIs are better pharmacological agents to modulate pain sensation. | ||
| 5-HT4R agonist | 5-HT4R agonist target 5-HT4R to promote peristalsis and secretion through enhanced release of acetylcholine from excitatory motor neurons and interneurons. | Improves GI motility.57,150 |
| 5-HT3R antagonist | Abnormal neurotransmission of 5-HT via the 5-HT3R has been reported in IBS-D patients. Blocking 5-HTR receptors is of clinical relevance in chronic diarrhea as this leads to reduced contractility, slows colonic transit, and increases fluid absorption. | Global improvement in IBS symptoms and relieve abdominal pain and discomfort, improve stool consistency and bowel movements.150,151 |
| 5-HT1AR agonist | Activation of 5-HT1AR at the level of the CNS increases gastric tone and decrease gastric sensitivity to distension. | Enhances fundus relaxation, gastric accommodation and improves postprandial symptoms independently from its anxiolytic effect.152 |
| Peripheral inhibitory effect exerted by the 5-HT1AR agonist improve gastric accommodation. |
TCA, tricyclic antidepressant; 5-HT, 5-hydroxytriptamine; NA, noradrenaline; CNS, central nervous system; SSRI, serotonin reuptake inhibitor; IBS, irritable bowel syndrome; SNRI, serotonin norepinephrine reuptake inhibitor; 5-HTR, 5-hydroxytriptamine receptor; GI, gastrointestinal; IBS-D, diarrhea-predominant IBS.
Conclusion and Further Directions
Serotonin metabolism has a central role in gut physiology and pathophysiology. The aforementioned EC cell-mediated physiological and pathophysiological mechanisms are differentially affected in DGBIs but remain tightly interconnected.12,16,50-52 Moreover, serotonin metabolism is directly or indirectly modulated through the gut microbiota, reinforcing the essential roles of EC cells in the regulation of gut-brain axis.153-156 Therefore, EC cell is a potential therapeutic target that may usher the emergence of pathophysiology-modifying therapies, using pharmacological agents targeting serotonin signaling or exploiting gut microbiota interventions. The pathophysiological mechanisms for similar symptoms may differ between patients with DGBIs. Therefore, the pathophysiological basis may be more relevant than clinical symptom profiles in differentiating, characterizing, and defining different DGBIs. The current challenges of DGBIs warrant a holistic approach to better characterize the patients based on the multi-omics data on the transcriptome, host epigenome, dietary profiles, gut microbiome, and metabolome from longitudinal studies. However, owing to the heterogeneous nature of DGBIs, further research is warranted to gain an advanced understanding of pathophysiological mechanisms for better clinical care. To discover the precise biomarkers discriminating the disorders with overlapping symptoms profile and refining pathophysiology-directed therapeutics targets, may assist with the hope for the future to relieve symptoms and restore gut-brain homeostasis.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Lai Wei, Rajan Singh, and Uday C Ghoshal made substantial contributions to the conception and design of the manuscript; Lai Wei and Rajan Singh reviewed the literature and drafted the original manuscript; Uday C Ghoshal revised the manuscript critically and provided important intellectual directives; and Lai Wei and Rajan Singh contributed equally as co-first authors to this study.
References
- 1.Drossman DA, Hasler WL. Rome IV-functional GIdisorders: disorders of gut-brain interaction. Gastroenterology. 2016;150:1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and rome IV. Gastroenterology. 2016;150:1262–1279. e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Black CJ, Drossman DA, Talley NJ, Ruddy J, Ford AC. Functional gastrointestinal disorders: advances in understanding and management. Lancet. 2020;396:1664–1674. doi: 10.1016/S0140-6736(20)32115-2. [DOI] [PubMed] [Google Scholar]
- 4.Mars RAT, Frith M, Kashyap PC. Functional gastrointestinal disorders and the microbiome-what is the best strategy for moving microbiome-based therapies for functional gastrointestinal disorders into the clinic? Gastroenterology. 2021;160:538–555. doi: 10.1053/j.gastro.2020.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a rome foundation working team report. Gastroenterology. 2018;154:1140–1171. e1. doi: 10.1053/j.gastro.2017.11.279. [DOI] [PubMed] [Google Scholar]
- 6.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology. 2021;160:99–114. e3. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–115. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zikos TA, Kamal AN, Neshatian L, et al. High prevalence of slow transit constipation in patients with gastroparesis. J Neurogastroenterol Motil. 2019;25:267–275. doi: 10.5056/jnm18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473–486. doi: 10.1038/s41575-020-0286-8. [DOI] [PubMed] [Google Scholar]
- 10.Barbara G, Feinle-Bisset C, Ghoshal UC, et al. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology. 2016;150:1305–1318. e8. doi: 10.1053/j.gastro.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellono NW, Bayrer JR, Leitch DB, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170:185–198. e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grover M, Farrugia G, Stanghellini V. Gastroparesis: a turning point in understanding and treatment. Gut. 2019;68:2238–2250. doi: 10.1136/gutjnl-2019-318712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396:1675–1688. doi: 10.1016/S0140-6736(20)31548-8. [DOI] [PubMed] [Google Scholar]
- 15.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Wei L, Singh R, Ha SE, et al. Serotonin deficiency is associated with delayed gastric emptying. Gastroenterology. 2021;160:2451–2466. e19. doi: 10.1053/j.gastro.2021.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei L, Singh R, Ro S, Ghoshal UC. Gut microbiota dysbiosis in functional gastrointestinal disorders: underpinning the symptoms and pathophysiology. JGH Open. 2021;5:976–987. doi: 10.1002/jgh3.12528.18949b32040c445aabbe4b6c17bc2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danne C, Rolhion N, Sokol H. Recipient factors in faecal microbiota transplantation: one stool does not fit all. Nat Rev Gastroenterol Hepatol. 2021;18:503–513. doi: 10.1038/s41575-021-00441-5. [DOI] [PubMed] [Google Scholar]
- 19.Bhattarai Y, Jie S, Linden DR, et al. Bacterially derived tryptamine increases mucus release by activating a host receptor in a mouse model of inflammatory bowel disease. iScience. 2020;23:101798. doi: 10.1016/j.isci.2020.101798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaelberer MM, Buchanan KL, Klein ME, et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361:eaat5236. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Needham BD, Kaddurah-Daouk R, Mazmanian SK. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci. 2020;21:717–731. doi: 10.1038/s41583-020-00381-0. [DOI] [PubMed] [Google Scholar]
- 22.Konen JR, Haag MM, Guseva D, et al. Prokinetic actions of luminally acting 5-HT4 receptor agonists. Neurogastroenterol Motil. 2021;33:e14026. doi: 10.1111/nmo.14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chedid V, Brandler J, Arndt K, et al. Randomised study: effects of the 5-HT4 receptor agonist felcisetrag vs placebo on gut transit in patients with gastroparesis. Aliment Pharmacol Ther. 2021;53:1010–1020. doi: 10.1111/apt.16304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers BD, Sayuk GS. Editorial: felcisetrag-forward movement as a novel prokinetic for gastroparesis. Aliment Pharmacol Ther. 2021;53:1158–1159. doi: 10.1111/apt.16334. [DOI] [PubMed] [Google Scholar]
- 25.Pasricha PJ, Grover M, Yates KP, et al. Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathologic features. Gastroenterology. 2021;160:2006–2017. doi: 10.1053/j.gastro.2021.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633–645. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fawkner-Corbett D, Antanaviciute A, Parikh K, et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell. 2021;184:810–826. e23. doi: 10.1016/j.cell.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao M. An increasingly complex view of intestinal motility. Nat Rev Gastroenterol Hepatol. 2020;17:72–73. doi: 10.1038/s41575-019-0249-0. [DOI] [PubMed] [Google Scholar]
- 29.Singh R, Ha SE, Wei L, et al. miR-10b-5p rescues diabetes and gastrointestinal dysmotility. Gastroenterology. 2021;160:1662–1678. e18. doi: 10.1053/j.gastro.2020.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh R, Wei L, Ghoshal UC. Micro-organic basis of functional gastrointestinal (GI) disorders: role of microRNAs in GI pacemaking cells. Indian J Gastroenterol. 2021;40:102–110. doi: 10.1007/s12664-021-01159-7. [DOI] [PubMed] [Google Scholar]
- 31.Helander HF, Fändriks L. Surface area of the digestive tract - revisited. Scand J Gastroenterol. 2014;49:681–689. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 32.Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latorre R, Sternini C, De Giorgio R, Greenwood-Van Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil. 2016;28:620–630. doi: 10.1111/nmo.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naraev BG, Halland M, Halperin DM, Purvis AJ, O'Dorisio TM, Halfdanarson TR. Management of diarrhea in patients with carcinoid syndrome. Pancreas. 2019;48:961–972. doi: 10.1097/MPA.0000000000001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh R, Zogg H, Wei L, et al. Gut microbial dysbiosis in the pathogenesis of gastrointestinal dysmotility and metabolic disorders. J Neurogastroenterol Motil. 2021;27:19–34. doi: 10.5056/jnm20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–1526. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbara G, Cremon C, Carini G, et al. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349–359. doi: 10.5056/jnm.2011.17.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boeckxstaens GE, Wouters MM. Neuroimmune factors in functional gastrointestinal disorders: a focus on irritable bowel syndrome. Neurogastroenterol Motil. 2017;29:e13007. doi: 10.1111/nmo.13007. [DOI] [PubMed] [Google Scholar]
- 40.Liddle RA. Neuropods. Cell Mol Gastroenterol Hepatol. 2019;7:739–747. doi: 10.1016/j.jcmgh.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martins P, Fakhry J, de Oliveira EC, et al. Analysis of enteroendocrine cell populations in the human colon. Cell Tissue Res. 2017;367:161–168. doi: 10.1007/s00441-016-2530-7. [DOI] [PubMed] [Google Scholar]
- 42.Diwakarla S, Fothergill LJ, Fakhry J, Callaghan B, Furness JB. Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol Motil. 2017;29:e13101. doi: 10.1111/nmo.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fothergill LJ, Furness JB. Diversity of enteroendocrine cells investigated at cellular and subcellular levels: the need for a new classification scheme. Histochem Cell Biol. 2018;150:693–702. doi: 10.1007/s00418-018-1746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund ML, Egerod KL, Engelstoft MS, et al. Enterochromaffin 5-HT cells - a major target for GLP-1 and gut microbial metabolites. Mol Metab. 2018;11:70–83. doi: 10.1016/j.molmet.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan DW, Barker N. Intestinal stem cells and their defining niche. Curr Top Dev Biol. 2014;107:77–107. doi: 10.1016/B978-0-12-416022-4.00003-2. [DOI] [PubMed] [Google Scholar]
- 46.Gehart H, van Es JH, Hamer K, et al. Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell. 2019;176:1158–1173. e16. doi: 10.1016/j.cell.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 47.Gustafsson BI, Bakke I, Tømmerås K, Waldum HL. A new method for visualization of gut mucosal cells, describing the enterochromaffin cell in the rat gastrointestinal tract. Scand J Gastroenterol. 2006;41:390–395. doi: 10.1080/00365520500331281. [DOI] [PubMed] [Google Scholar]
- 48.Liddle RA. Interactions of gut endocrine cells with epithelium and neurons. Compr Physiol. 2018;8:1019–1030. doi: 10.1002/cphy.c170044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcaino C, Knutson KR, Treichel AJ, et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci USA. 2018;115:E7632–E7641. doi: 10.1073/pnas.1804938115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z, Luo J, Li J, et al. Interleukin-33 promotes serotonin release from enterochromaffin cells for intestinal homeostasis. Immunity. 2021;54:151–163. e6. doi: 10.1016/j.immuni.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye L, Bae M, Cassilly CD, et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021;29:179–196. e9. doi: 10.1016/j.chom.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gershon MD. 5-hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gribble FM, Reimann F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- 54.Kaelberer MM, Rupprecht LE, Liu WW, Weng P, Bohórquez DV. Neuropod cells: the emerging biology of gut-brain sensory transduction. Annu Rev Neurosci. 2020;43:337–353. doi: 10.1146/annurev-neuro-091619-022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beumer J, Clevers H. How the gut feels, smells, and talks. Cell. 2017;170:10–11. doi: 10.1016/j.cell.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 56.Shin A, Preidis GA, Shulman R, Kashyap PC. The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clin Gastroenterol Hepatol. 2019;17:256–274. doi: 10.1016/j.cgh.2018.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghoshal UC. Marshall and warren lecture 2019: a paradigm shift in pathophysiological basis of irritable bowel syndrome and its implication on treatment. J Gastroenterol Hepatol. 2020;35:712–721. doi: 10.1111/jgh.15032. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Alammar N, Singh R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-control studies. J Acad Nutr Diet. 2020;120:565–586. doi: 10.1016/j.jand.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 59.Ghoshal U, Shukla R, Srivastava D, Ghoshal UC. Irritable bowel syndrome, particularly the constipation-predominant form, involves an increase in Methanobrevibacter smithii, which is associated with higher methane production. Gut Liver. 2016;10:932–938. doi: 10.5009/gnl15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghoshal UC, Srivastava D, Misra A. A randomized double-blind placebo-controlled trial showing rifaximin to improve constipation by reducing methane production and accelerating colon transit: a pilot study. Indian J Gastroenterol. 2018;37:416–423. doi: 10.1007/s12664-018-0901-6. [DOI] [PubMed] [Google Scholar]
- 61.Ianiro G, Eusebi LH, Black CJ, Gasbarrini A, Cammarota G, Ford AC. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:240–248. doi: 10.1111/apt.15330. [DOI] [PubMed] [Google Scholar]
- 62.El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69:859–867. doi: 10.1136/gutjnl-2019-319630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fung TC, Vuong HE, Luna CDG, et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol. 2019;4:2064–2073. doi: 10.1038/s41564-019-0540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar A, Russell RM, Pifer R, et al. The serotonin neurotransmitter modulates virulence of enteric pathogens. Cell Host Microbe. 2020;28:41–53. e8. doi: 10.1016/j.chom.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cryan JF, O'Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 66.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 67.Beeckmans D, Riethorst D, Augustijns P, et al. Altered duodenal bile salt concentration and receptor expression in functional dyspepsia. United European Gastroenterol J. 2018;6:1347–1355. doi: 10.1177/2050640618799120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbara G, Grover M, Bercik P, et al. Rome foundation working team report on post-infection irritable bowel syndrome. Gastroenterology. 2019;156:46–58. e7. doi: 10.1053/j.gastro.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101:47–64. doi: 10.1007/s00253-016-8006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao L, Yang W, Chen Y, et al. A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J Clin Invest. 2020;130:438–450. doi: 10.1172/JCI130976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mars RAT, Yang Y, Ward T, et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell. 2020;182:1460–1473. e17. doi: 10.1016/j.cell.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ticho AL, Malhotra P, Dudeja PK, Gill RK, Alrefai WA. Bile acid receptors and gastrointestinal functions. Liver Res. 2019;3:31–39. doi: 10.1016/j.livres.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 75.Reigstad CS, Salmonson CE, Rainey JF, 3rd, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Engevik MA, Luck B, Visuthranukul C, et al. Human-derived Bifidobacterium dentium modulates the mammalian serotonergic system and gut-brain axis. Cell Mol Gastroenterol Hepatol. 2021;11:221–248. doi: 10.1016/j.jcmgh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scott SA, Fu J, Chang PV. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2020;117:19376–19387. doi: 10.1073/pnas.2000047117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hindson J. Enteric neuron regulation of gut motility by the microbiota. Nat Rev Gastroenterol Hepatol. 2020;17:194–195. doi: 10.1038/s41575-020-0283-y. [DOI] [PubMed] [Google Scholar]
- 79.Obata Y, Castaño Á, Boeing S, et al. Neuronal programming by microbiota regulates intestinal physiology. Nature. 2020;578:284–289. doi: 10.1038/s41586-020-1975-8. [DOI] [PubMed] [Google Scholar]
- 80.Manzella C, Singhal M, Alrefai WA, Saksena S, Dudeja PK, Gill RK. Serotonin is an endogenous regulator of intestinal CYP1A1 via AhR. Sci Rep. 2018;8:6103. doi: 10.1038/s41598-018-24213-5.519824b2f4d143929624a61385e070f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manzella CR, Ackerman M, Singhal M, et al. Serotonin modulates AhR activation by interfering with CYP1A1-mediated clearance of AhR ligands. Cell Physiol Biochem. 2020;54:126–141. doi: 10.33594/000000209.8e41b12155e94945a0945bf4fc245d2f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benech N, Rolhion N, Sokol H. Tryptophan metabolites get the gut moving. Cell Host Microbe. 2021;29:145–147. doi: 10.1016/j.chom.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 83.Williams BB, Van Benschoten AH, Cimermancic P, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhattarai Y, Williams BB, Battaglioli EJ, et al. Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe. 2018;23:775–785. e5. doi: 10.1016/j.chom.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keszthelyi D, Troost FJ, Jonkers DM, Kruimel JW, Leue C, Masclee AA. Decreased levels of kynurenic acid in the intestinal mucosa of IBS patients: relation to serotonin and psychological state. J Psychosom Res. 2013;74:501–504. doi: 10.1016/j.jpsychores.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 86.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Ghoshal UC, Singh R. Frequency and risk factors of functional gastro-intestinal disorders in a rural Indian population. J Gastroenterol Hepatol. 2017;32:378–387. doi: 10.1111/jgh.13465. [DOI] [PubMed] [Google Scholar]
- 88.Tu Q, Heitkemper MM, Jarrett ME, Buchanan DT. Sleep disturbances in irritable bowel syndrome: a systematic review. Neurogastroenterol Motil. 2017;29:e12946. doi: 10.1111/nmo.12946. [DOI] [PubMed] [Google Scholar]
- 89.Fitzgerald P, Cassidy Eugene M, Clarke G, et al. Tryptophan catabolism in females with irritable bowel syndrome: relationship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol Motil. 2008;20:1291–1297. doi: 10.1111/j.1365-2982.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- 90.Albert-Bayo M, Paracuellos I, González-Castro AM, et al. Intestinal mucosal mast cells: key modulators of barrier function and homeostasis. Cells. 2019;8:135. doi: 10.3390/cells8020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 92.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Braak B, Klooker TK, Wouters MM, et al. Mucosal immune cell numbers and visceral sensitivity in patients with irritable bowel syndrome: is there any relationship? Am J Gastroenterol. 2012;107:715–726. doi: 10.1038/ajg.2012.54. [DOI] [PubMed] [Google Scholar]
- 94.Aguilera-Lizarraga J, Florens MV, Viola MF, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature. 2021;590:151–156. doi: 10.1038/s41586-020-03118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2014;147:1012–1020. e4. doi: 10.1053/j.gastro.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 96.Lee JY, Kim N, Choi YJ, et al. Expression of tight junction proteins according to functional dyspepsia subtype and sex. J Neurogastroenterol Motil. 2020;26:248–258. doi: 10.5056/jnm19208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bertiaux-Vandaële N, Youmba SB, Belmonte L, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165–2173. doi: 10.1038/ajg.2011.257. [DOI] [PubMed] [Google Scholar]
- 98.Hanning N, Edwinson AL, Ceuleers H, et al. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol. 2021;14:1756284821993586. doi: 10.1177/1756284821993586.948a5793b5cb42018f4877a867660e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Engevik MA, Chang-Graham A, Hyser JM, Versalovic J. Serotonin promotes epithelial restitution through goblet cell mediated secretion of Muc2 and TFF3. FASEB J. 2019;33(S1):869.1. doi: 10.1096/fasebj.2019.33.1_supplement.869.1. [DOI] [Google Scholar]
- 100.Farzaei MH, Bahramsoltani R, Abdollahi M, Rahimi R. The role of visceral hypersensitivity in irritable bowel syndrome: pharmacological targets and novel treatments. J Neurogastroenterol Motil. 2016;22:558–574. doi: 10.5056/jnm16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vandenberghe J, Vos R, Persoons P, Demyttenaere K, Janssens J, Tack J. Dyspeptic patients with visceral hypersensitivity: sensitisation of pain specific or multimodal pathways? Gut. 2005;54:914–919. doi: 10.1136/gut.2004.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou Q, Yang L, Larson S, et al. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut. 2016;65:797–805. doi: 10.1136/gutjnl-2013-306464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nguyen LA, Snape WJ., Jr Clinical presentation and pathophysiology of gastroparesis. Gastroenterol Clin North Am. 2015;44:21–30. doi: 10.1016/j.gtc.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 105.Nozawa K, Kawabata-Shoda E, Doihara H, et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci USA. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spiller RC. Targeting the 5-HT3 receptor in the treatment of irritable bowel syndrome. Curr Opin Pharmacol. 2011;11:68–74. doi: 10.1016/j.coph.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 107.Hoffman JM, Tyler K, MacEachern SJ, et al. Activation of colonic mucosal 5-HT4 receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854. e4. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Keszthelyi D, Troost FJ, Jonkers DM, et al. Visceral hypersensitivity in irritable bowel syndrome: evidence for involvement of serotonin metabolism--a preliminary study. Neurogastroenterol Motil. 2015;27:1127–1137. doi: 10.1111/nmo.12600. [DOI] [PubMed] [Google Scholar]
- 109.Camilleri M. Relationship of motor mechanisms to gastroparesis symptoms: toward individualized treatment. Am J Physiol Gastrointest Liver Physiol. 2021;320:G558–G563. doi: 10.1152/ajpgi.00006.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bekkelund M, Sangnes DA, Gunnar Hatlebakk J, Aabakken L. Pathophysiology of idiopathic gastroparesis and implications for therapy. Scand J Gastroenterol. 2019;54:8–17. doi: 10.1080/00365521.2018.1558280. [DOI] [PubMed] [Google Scholar]
- 111.Miwa H, Koseki J, Oshima T, et al. Impairment of gastric accommodation induced by water-avoidance stress is mediated by 5-HT2B receptors. Neurogastroenterol Motil. 2016;28:765–778. doi: 10.1111/nmo.12775. [DOI] [PubMed] [Google Scholar]
- 112.Matricon J, Meleine M, Gelot A, et al. Review article: associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009–1031. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 113.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liebregts T, Adam B, Bredack C, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 2011;106:1089–1098. doi: 10.1038/ajg.2010.512. [DOI] [PubMed] [Google Scholar]
- 115.Grover M, Gibbons SJ, Nair AA, et al. Transcriptomic signatures reveal immune dysregulation in human diabetic and idiopathic gastroparesis. BMC Med Genomics. 2018;11:62. doi: 10.1186/s12920-018-0379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grover M, Bernard CE, Pasricha PJ, et al. Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol Motil. 2017;29:e13018. doi: 10.1111/nmo.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, et al. Immunomodulatory effects mediated by serotonin. J Immunol Res. 2015;2015:354957. doi: 10.1155/2015/354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsuchida Y, Hatao F, Fujisawa M, et al. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut. 2011;60:638–647. doi: 10.1136/gut.2010.227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cremon C, Carini G, Wang B, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290–1298. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- 120.Stakenborg N, Viola MF, Boeckxstaens GE. Intestinal neuro-immune interactions: focus on macrophages, mast cells and innate lymphoid cells. Curr Opin Neurobiol. 2020;62:68–75. doi: 10.1016/j.conb.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dothel G, Barbaro MR, Boudin H, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011. e4. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 122.Patman G. IBS: mast cells cause nerves to sprout in patients with IBS. Nat Rev Gastroenterol Hepatol. 2015;12:189. doi: 10.1038/nrgastro.2015.30. [DOI] [PubMed] [Google Scholar]
- 123.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 124.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 125.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–1434. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 126.Manocha M, Shajib MS, Rahman MM, et al. IL-13-mediated immunological control of enterochromaffin cell hyperplasia and serotonin production in the gut. Mucosal Immunol. 2013;6:146–155. doi: 10.1038/mi.2012.58. [DOI] [PubMed] [Google Scholar]
- 127.Ghoshal UC. Gutmicrobiota-brain axis modulation by a healthier microbiological microenvironment : facts and fictions. J Neurogastroenterol Motil. 2018;24:4–6. doi: 10.5056/jnm17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160:1486–1501. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zamani M, Alizadeh-Tabari S, Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:132–143. doi: 10.1111/apt.15325. [DOI] [PubMed] [Google Scholar]
- 130.Rodiño-Janeiro BK, Alonso-Cotoner C, Pigrau M, Lobo B, Vicario M, Santos J. Role of corticotropin-releasing factor in gastrointestinal permeability. J Neurogastroenterol Motil. 2015;21:33–50. doi: 10.5056/jnm14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ford AC, Mahadeva S, Carbone MF, Lacy BE, Talley NJ. Functional dyspepsia. Lancet. 2020;396:1689–1702. doi: 10.1016/S0140-6736(20)30469-4. [DOI] [PubMed] [Google Scholar]
- 132.McMillin M, DeMorrow S. Effects of bile acids on neurological function and disease. FASEB J. 2016;30:3658–3668. doi: 10.1096/fj.201600275R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McVey Neufeld KA, Bienenstock J, Bharwani A, et al. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling. Sci Rep. 2019;9:14290. doi: 10.1038/s41598-019-50807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schmulson M, Ghoshal UC, Barbara G. Managing the inevitable surge of post-COVID-19 functional gastrointestinal disorders. Am J Gastroenterol. 2021;116:4–7. doi: 10.14309/ajg.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 135.Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology. 2017;152:1042–1054. e1. doi: 10.1053/j.gastro.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barbara G, Cremon C, Stanghellini V. Inflammatory bowel disease and irritable bowel syndrome: similarities and differences. Curr Opin Gastroenterol. 2014;30:352–358. doi: 10.1097/MOG.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 137.Ghoshal UC, Gwee KA. Post-infectious IBS, tropical sprue and small intestinal bacterial overgrowth: the missing link. Nat Rev Gastroenterol Hepatol. 2017;14:435–441. doi: 10.1038/nrgastro.2017.37. [DOI] [PubMed] [Google Scholar]
- 138.El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. Changes in the symptom pattern and the densities of large-intestinal endocrine cells following Campylobacter infection in irritable bowel syndrome: a case report. BMC Res Notes. 2013;6:391. doi: 10.1186/1756-0500-6-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim HS, Lim JH, Park H, Lee SI. Increased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection--an observation in a small case control study. Yonsei Med J. 2010;51:45–51. doi: 10.3349/ymj.2010.51.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dizdar V, Spiller R, Singh G, et al. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883–891. doi: 10.1111/j.1365-2036.2010.04251.x. [DOI] [PubMed] [Google Scholar]
- 141.Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fukumoto S, Tatewaki M, Yamada T, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 143.Vanhoutvin SA, Troost FJ, Kilkens TO, et al. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. 2009;21:952–e76. doi: 10.1111/j.1365-2982.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 144.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 145.Bhattarai Y, Schmidt BA, Linden DR, et al. Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-HT3 receptor expression via acetate production. Am J Physiol Gastrointest Liver Physiol. 2017;313:G80–G87. doi: 10.1152/ajpgi.00448.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Triantafyllou K, Chang C, Pimentel M. Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil. 2014;20:31–40. doi: 10.5056/jnm.2014.20.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mertz H, Fass R, Kodner A, Yan-Go F, Fullerton S, Mayer EA. Effect of amitriptyline on symptoms, sleep, and visceral perception in patients with functional dyspepsia. Am J Gastroenterol. 1998;93:160–165. doi: 10.1111/j.1572-0241.1998.00160.x. [DOI] [PubMed] [Google Scholar]
- 148.Tack J, Ly HG, Carbone F, et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin Gastroenterol Hepatol. 2016;14:385–392. e4. doi: 10.1016/j.cgh.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 149.Törnblom H, Drossman DA. Centrally targeted pharmacotherapy for chronic abdominal pain. Neurogastroenterol Motil. 2015;27:455–467. doi: 10.1111/nmo.12509. [DOI] [PubMed] [Google Scholar]
- 150.Simrén M, Tack J. New treatments and therapeutic targets for IBS and other functional bowel disorders. Nat Rev Gastroenterol Hepatol. 2018;15:589–605. doi: 10.1038/s41575-018-0034-5. [DOI] [PubMed] [Google Scholar]
- 151.Rokkas T, Ekmektzoglou K, Niv Y. Comparative effectiveness of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: a network meta-analysis of randomized controlled studies. Ann Gastroenterol. 2021;34:535–546. doi: 10.20524/aog.2021.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tack J, Janssen P, Masaoka T, Farré R, Van Oudenhove L. Efficacy of buspirone, a fundus- relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012;10:1239–1245. doi: 10.1016/j.cgh.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 153.Dodds KN, Travis L, Kyloh MA, et al. The gut-brain axis: spatial relationship between spinal afferent nerves and 5-HT-containing enterochromaffin cells in mucosa of mouse colon. Am J Physiol Gastrointest Liver Physiol. 2022:G523–G533. doi: 10.1152/ajpgi.00019.2022. [DOI] [PubMed] [Google Scholar]
- 154.Treichel AJ, Finholm I, Knutson KR, et al. Specialized mechanosensory epithelial cells in mouse gut intrinsic tactile sensitivity. Gastroenterology. 2022:535–547.:e13. doi: 10.1053/j.gastro.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martin AM, Jones LA, Wei L, et al. Distinguishing the contributions of neuronal and mucosal serotonin in the regulation of colonic motility. Neurogastroenterol Motil. 2022;00:e14361. doi: 10.1111/nmo.14361. [DOI] [PubMed] [Google Scholar]
- 156.Mercado-Perez A, Beyder A. Gut feelings: mechanosensing in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2022:283–296. doi: 10.1038/s41575-021-00561-y. [DOI] [PMC free article] [PubMed] [Google Scholar]