Abstract
Background/Aims
The 3-phase fermentable oligo-, di-, mono-saccharides, and polyols (FODMAP) diet has shown a high level of efficacy in irritable bowel syndrome, largely based on dietitian delivered education. However, access to dietitians can be limited, and challenges exist when applying the diet to a wide range of cultures, such as limited FODMAP analysis of local foods. This review aims to discuss ways to optimally use the FODMAP diet in practice in a wide range of cultures, directed at gastroenterologists from a dietitian’s perspective.
Methods
Recent literature was analysed via search databases including Medline, CINAHL, PubMed and Scopus.
Results
The dietetic process involves detailed assessment and follow-up through the 3 stages of the FODMAP diet (restriction, re-introduction, and long-term maintenance). Emerging evidence suggests the diet can be delivered by other health professionals such as the gastroenterologist or nurse, but training on how to do so successfully would be needed. Self-guided approaches through use of technology or specialised food delivery services may be an alternative when dietitians are not available, but efficacy data is limited. Regardless of delivery mode, nutritional and psychological risks of the diet must be mitigated. Additionally, culturally appropriate education must be provided, with accommodations necessary when the FODMAP content of local foods are unknown.
Conclusion
While the diet has shown improved irritable bowel syndrome outcomes across studies, it is important to acknowledge the essential role of dietitians in implementing, tailoring, and managing the diet to achieve the best outcome for each individual.
Keywords: Diet, carbohydrate-restricted; Diet, food, and nutrition; Diet therapy; Irritable bowel syndrome
Introduction
Irritable bowel syndrome (IBS) is a disorder of the gut-brain interaction (DGBI), characterized by abdominal pain, bloating, constipation, and diarrhea1 with a worldwide prevalence of 4.1% according to the Rome IV criteria.2 Various physiological mechanisms have been suggested to contribute to the development of IBS,3 including alterations to the intestinal microbiome4 and interaction of the central, autonomic, and enteric nervous systems.5,6 A wide variety of treatments are utilized in IBS management. This includes a range of pharmacological and non-pharmacological therapies such as gut-directed hypnotherapy7 and dietary modifications such, as the low fermentable oligo-, di-, mono-saccharides, and polyols (FODMAP) diet.8 Taking an integrated approach through utilizing a range of treatments has recently become the preferred option due to lack of ability to predict response in the individual.9 In this review paper, we analyze recent literature from search databases including Medline, CINAHL, PubMed and Scopus, to discuss how to implement the 3-phase FODMAP diet, paying attention to the role of the dietitian. We also provide recommendations for practice in a range of cultures, and where FODMAP data is scarce.
What Is the 3-Phase FODMAP Diet?
FODMAPs are types of short-chain carbohydrates found in a wide variety of foods. They comprise monosaccharides and polyols that are slowly absorbed in the small intestine, and di- and oligo-saccharides that are not digestible because of the lack of suitable hydrolases in the human small intestine or reduced/absent hydrolase activity in a proportion of the population (such as lactose, sucrose, and trehalase). Mono- and di-saccharides and polyols attract water into the intestinal lumen via their osmotic effect due to their relatively small molecular size.10-12 and those passing into the colon are rapidly fermented by bacteria with the release of gas.13 Acutely, symptoms are generated by the distension of the intestinal lumen with water (small intestine) or gas (proximal colon), although other mechanisms are involved in modulating these responses in the longer term.14-16
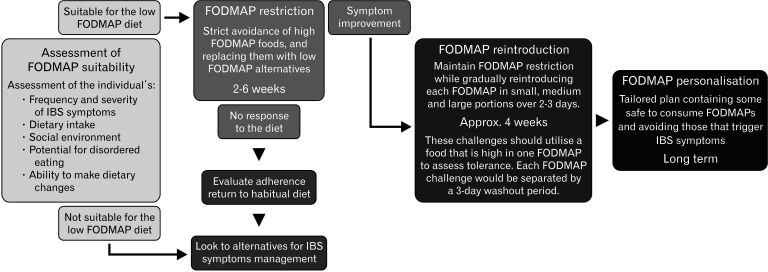
The traditional practice of implementing the FODMAP diet are to initially restrict all FODMAPs, ie, the low FODMAP diet, to determine its effect on symptoms, then, if symptomatic response is achieved, to develop a strategy for maintaining the benefits over the longer term with less restriction. Thus, the FODMAP diet involves a 3-stage strategy, restriction, reintroduction, and personalization, with the ultimate goal of maintaining symptom control while maximizing FODMAP intake, as outlined in Figure 1, which has been described in detail elsewhere.17,18 Other less restrictive variations of the FODMAP diet have been suggested for situations where nutritional adequacy or the ability to adhere to the FODMAP diet may be compromised.18 Such an approach has been variably termed the “bottom-up” or “FODMAP-gentle” approach, which involves restricting a limited number of foods that are highly concentrated sources of FODMAPs, and/or restricting foods high in specific FODMAPs if these are suspected of triggering symptoms.19 However, the efficacy of this approach on IBS symptoms has not been formally studied.
Figure 1.
The stages of the fermentable oligo-, di-, mono-saccharides, and polyols (FODMAP) dietary strategy.
Proving the efficacy of the FODMAP diet poses challenges common to dietary intervention studies.20 The first stage of the diet is amenable to randomized controlled trials (RCTs), which have been heterogeneous in design utilizing feeding or dietary counseling methodologies with a variety of comparator diets. However, these studies consistently show improvements in bloating, pain, and quality of life. When the studies are subjected to a network meta-analysis, the FODMAP diet is superior to other dietary interventions.21 Efficacy of the first stage of the diet in real-world clinical practice has also been demonstrated,22 while prospective observational studies show that mild FODMAP restriction in the third stage of the diet (personalized FODMAP diet), ameliorates symptoms in the majority of patients.23-26
Published analysis of RCTs and real-world experience have nearly all been related to dietitian-led interventions. While the FODMAP diet may not work for all individuals with IBS, as some may achieve better symptom reduction through other interventions, it is possible that, for others who do not experience symptom relief, this may be due to low adherence27 or a poor understanding of the diet. In a retrospective evaluation of 80 patients with IBS, patient-led implementation of a FODMAP diet was linked to more than double the intake of FODMAPs during the restriction phase, than when dietitian-led. Additionally, patients supported by dietitians displayed significantly higher adherence through each stage of the diet.22 This exemplifies the importance of the dietitian in the implementation of the FODMAP diet.
The Dietetic Process—Clinical Management of Irritable Bowel Syndrome
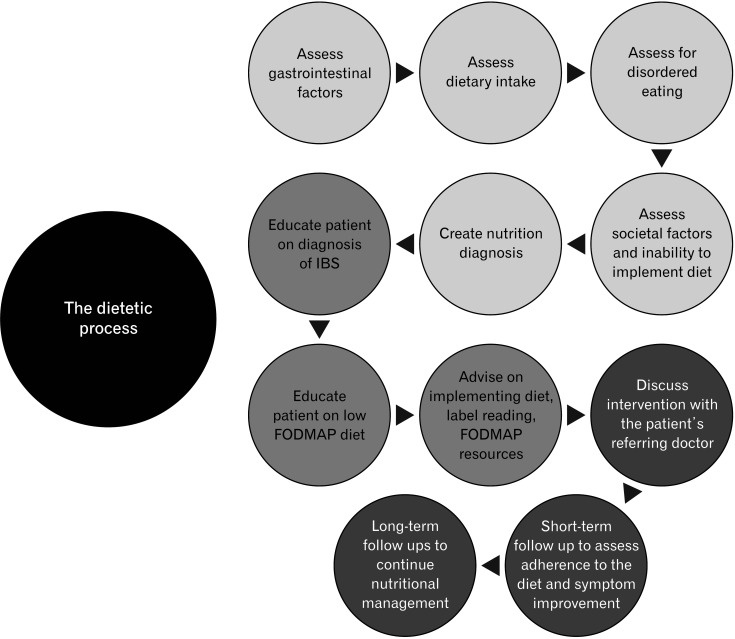
The dietetic management of IBS predominantly takes place in an ambulatory setting, rather than in the acute setting. As illustrated in Figure 2, dietitians can recognize dietary habits that may influence gastrointestinal (GI) function and, following detailed assessment, implement dietetic interventions, such as the FODMAP diet, where appropriate.
Figure 2.
Summary of the steps involved in the dietetic process of patients with irritable bowel syndrome (IBS). FODMAP, fermentable oligo-, di-, mono-saccharides, and polyols.
Initial Dietetic Consultation (60 Minutes)
Assessment (approximately 30 minutes)
During the initial consultation, the dietitian documents the individual’s clinical issues (validated tools such as the Rome IV criteria, which measures frequency and severity of symptoms,28 and the Bristol stool form scale may be used29) as a baseline for future assessment of response, and confirms that a DGBI is likely (especially in cases not referred from gastroenterologists) and that alarm features have been assessed. The dietitian would also assess the individual’s health and food knowledge and social environment, taking note of food preparation skills, personal responsibilities or social systems that may influence their ability to adhere to dietary advice; and dietary intake, taking note of FODMAP intake and potential triggers. Dietary intake may be measured utilising the Monash University Comprehensive Nutrition Assessment Questionnaire (CNAQ), which is a food frequency questionnaire that can specifically measure FODMAP intake.30 The CNAQ is available for use online, however at over 200 items long, it may be too cumbersome for regular usage in clinical practice. Alternatives may include conducting a 24-hour diet history with the patient or asking them to complete a 7-day food diary prior to attending the consult. This nutritional assessment may also involve screening for disordered eating as will be discussed in further detail below, using a validated tool such as the Sick, Control, One Stone, Fat, Food (SCOFF) questionnaire.31 During this session, the dietitian may produce a nutrition diagnosis to identify the nutritional problem that may be related to the individual’s IBS. For example, a nutrition diagnosis may identify that the individual is consuming sufficient quantities of FODMAPs to render a FODMAP diet is a suitable intervention. Another example may be identification of a very low fiber intake that may in itself be a causal component of symptoms. Two of the important assessments made are the appropriateness of the implementation of a restrictive diet and its nature for that individual.32
Education and intervention (approximately 30 minutes)
If deemed to be appropriate for the FODMAP diet, the dietitian would then educate the individual on normal gut physiology and the pathophysiology of DGBI, including relevant concepts of visceral hypersensitivity and altered bowel motility, what FODMAPs are, their mechanisms of action and food sources. They would also provide the patient with information to implement the initial stage of the diet including suitable low FODMAP alternatives to incorporate. The dietitian would then add suitable adjustments to the diet needed for existing dietary restrictions, such as vegetarian or certain uncontrolled environments, such as dining out. This may involve providing printed diet sheets or recommending resources to assist with identifying high FODMAP foods33 including label reading to identify foods likely high in FODMAPs, and recipe modification. While there are many written educational resources on the FODMAP diet available, it is important that these are screened and described to patients by qualified dietitians, as patients have reported a low level of understanding when given these resources by gastroenterologists and general practitioners alone,34 and diet sheets lack personalization to the patients usual diet. Digital resources may also be utilized and recommended to patients including the Monash University FODMAP Diet app, which provides comprehensive and up-to-date information about the FODMAP content of food, and other useful resources such as background education on the role of FODMAPs in DGBIs.
It is important to note that the dietitian uses their clinical knowledge to provide nutrition education that is tailored and appropriate for each patient. This may not always align with the recommendation of the referring doctor. For this reason, it is important for the lines of communication between dietitian and referrer to be open to ensure the best outcome for the patient.
Short-term Dietetic Follow-up (2-8 Weeks Since Initial Appointment, Approximately 30-45 Minutes per Appointment)
Following this first consultation there would be a short-term follow up at the conclusion of the “restriction” phase of the diet, to assist the patient with implementing “reintroduction.”27 Clinical assessment at this stage may involve measuring IBS symptoms and assessing adherence to the dietary modifications. If there has not been adequate symptom reduction by this stage, this may be an indicator that there has been low adherence, or that the diet is ineffective for this individual. If the latter is the case, the best practice is to cease implementation of the diet and look to other potential therapies. The dietitian may then refer the patient back to the gastroenterologist or primary care physician who may recommend alternative therapies such as gut-directed hypnotherapy or pharmacological support for specific symptoms.7 In integrated care, such referrals may occur directly from the dietitian. For those patients continuing the diet, depending on the requirements of the individual, regular short-term follow-ups may occur throughout the “reintroduction” phase to assist with implementation and addressing challenges with reintroduction. For other individuals, only a final follow-up consultation to administer “personalization” may be required.
Long-term Dietetic Follow-up (Approximately 30-45 Minutes per Appointment)
The “personalization” phase involves evaluating the impact of the FODMAP diet on the patient, and tailoring a long-term diet that encourages nutritional adequacy and avoidance of the FODMAPs that trigger IBS symptoms for the individual. Prospective evaluation of patient cohorts in this stage have found adequate symptom relief and quality of life improvement.23,24,35 Considering that the FODMAP diet is restrictive during the initial stages, it is important that the long-term personalised FODMAP diet is more flexible. The patient should be encouraged to continue to reintroduce poorly tolerated high FODMAP foods periodically to re-assess tolerance, as tolerance may change over time. Education may also be centered around application of non-dietary DGBI therapies and how they may be combined with dietary therapy. For example, how peppermint oil, antispasmodic agents, and a-galactosidase36-39 may be used to reduce symptoms and allow more FODMAP flexibility in uncontrolled eating environments. These additional strategies may be targeted to individual requirements, such as the use of α-galactosidase in galacto-oligosaccharides sensitive individuals requiring vegetarian protein sources such as legumes and pulses.
Since the severity of symptoms fluctuates over time, patients are advised that FODMAP restriction can be increased or decreased according to their needs. Using such strategies in the long-term, FODMAP intake generally normalizes to include tolerated high FODMAP foods.25 However, episodes of high FODMAP intake (FODMAP “binges”) are generally avoided.
Mitigating Risks of the FODMAP Diet
A FODMAP diet is a restrictive diet and this poses nutritional and psychological risks for the patient. Strategies to mitigate these risks are highlighted in Table 1.
Table 1.
Summary of Key Recommendations to Minimize the Negative Consequences of Restrictive Diets
|
Nutritional Risks
Since FODMAPs are naturally found in a large variety of commonly consumed foods, strict long-term FODMAP avoidance may result in nutritional deficiency. In a study of over 3000 patients, those who adhered strictly to the FODMAP diet in the “restriction” phase recorded significantly lower intakes of calcium, magnesium, vitamin C, folate, and riboflavin than those who adhered less strictly,40 suggesting that individuals may develop deficiencies if they were to follow this stage of the diet longer than necessary. To reduce the risk of nutritional deficiency, dietitians should encourage the substitution of high FODMAP foods with nutritionally equivalent FODMAP alternatives. For example, substituting a high FODMAP dairy product, such as cow’s milk, with a FODMAP dairy alternative, like lactose free cow’s milk or calcium-fortified soy protein milk. Notably, patients who follow the diet without guidance from a professional record significantly lower intakes of beta-carotene, riboflavin, calcium, magnesium and phosphorus than those who are guided.41 This again exemplifies the importance of the dietitian.
Some short-term studies have indicated that fiber intake may be reduced during the initial restrictive phase of the FODMAP diet.42,43 However, other studies have shown maintained fiber intake,44,45 and intake is often improved in longer-term studies that include the reintroduction and long-term personalization phases.23,25,42 Constipation did not improve following a physician-led FODMAP diet, possibly due to inadequate education about fiber intake, although dietary intake was not measured.46 Due to the many health benefits of fiber, and the knowledge of potential short-term reductions in intake, regardless of how the FODMAP diet is delivered, it is key that patients are educated on ways to maintain or improve fiber intake. However, considering the time involved and expertise required to adequately educate patients on suitable fiber sources in IBS, dietitians are likely best placed to provide this. The alternative is to use fiber supplements. A recent RCT has indicated that a minimally fermented fiber (sugarcane bagasse) can improve stool characteristics without exacerbation of other symptoms.47,48 If the desire is to add a fermentable fiber such as resistant starch, this can generally be tolerated when combined with a minimally fermented fiber to slow fermentation down in the colon.47,48
Psychological Risks
As the initial stage of the FODMAP diet is restrictive, it has been suggested that it may impact emotional and mental health.32 A population study of nearly 3000 individuals found that those with GI diseases on therapeutic diets displayed lower health-related quality of life, but the results were not statistically significant.49 Specifically, the gluten-free diet in patients with celiac disease has been linked to food-related anxiety and social exclusion, especially upon initial diagnosis of the disease.50 However, these feelings appear to dissipate over time as the individual gains knowledge and the confidence to self-manage their diet.51,52 As a diet that can alter and normalize over time to suit an individual’s lifestyle while still managing their IBS symptoms, it is unclear if the FODMAP diet carries similar social stigma, although a recent study of 205 participants in the long-term personalization stage of the diet experienced lower food related quality of life than controls.53 Unlike celiac disease, in which ingestion of gluten has pathological consequences such as mucosal damage,54 consuming FODMAPs in IBS may be associated with symptoms after the meal, but no evidence has suggested that these symptoms are related to mucosal damage, and they can be managed. This risk of more serious consequences when ingesting gluten may account for the psychological impacts. High quality studies are required to examine the social effects of the FODMAP diet.
On the other hand, it is important to acknowledge the high risk of disordered eating behaviors in this patient group. An individual may have disordered eating if they demonstrate irregular eating behaviours that do not fit the criteria for an eating disorder as defined by the Diagnostic and statistical manual of mental disorders, fifth edition.55 This may include calorie counting or skipping meals to an extent that impacts their health or nutritional status.32,56 Severe IBS symptoms are associated with higher rates of disordered eating in adolescents56 and adults.57 One study of 45 adults with IBS found that 71% were classified as having an actual eating disorder when measured by the SCOFF questionnaire.58 Likewise, when specifically examining the FODMAP diet, it was found that 57% of IBS patients classified as having an eating disorder closely adhered to the “restriction” phase of the diet for 6 weeks, while 36% of those who did not have an eating disorder closely adhered.59 The SCOFF was designed for, and has been validated in, individuals with anorexia and bulimia nervosa.31 However, the eating disorders that are thought to be associated with IBS are those linked to restrictive food choices rather than body dysmorphia, such as avoidant restrictive food intake disorder (ARFID) or orthorexia nervosa. Hence, the accuracy of data generated using the SCOFF is uncertain.
ARFID is defined as the fear of eating specific foods or food groups due to sensory characteristics or the potential adverse consequences of consuming foods.55 Dietitians have suggested that individuals with IBS may be at risk of ARFID, due to their strict avoidance of specific foods that may trigger GI symptoms.60 While an emerging area of research, clinical trials have reported an ARFID prevalence of 13-21% in individuals with DGBIs.61,62 Notably, 44% of individuals with ARFID had previously been prescribed a FODMAP diet by their gastroenterologists to manage their symptoms.62 However, it was not mentioned if these patients had also seen a dietitian for guidance. Moreover, orthorexia is characterized by restricting foods based on perceived health quality.63 Similar to ARFID research, orthorexia is still a novel concept. However, preliminary research has found that IBS symptom severity may be positively correlated with orthorexia.58,64 Various screening tools have been developed to assess risk of ARFID and orthorexia, but none have been validated in IBS. Considering that groundwork research has indicated a relationship between IBS and eating disorders, future research should explore this link further.
As mentioned, patients with IBS should be screened for eating disorders at the initial assessment. Individuals who are found to be classified as having an active eating disorder should not be placed on a FODMAP, or other restrictive diet, and clinicians should instead advise of alternative therapies to manage their symptoms.60 Additionally, clinicians may refer these patients to experienced eating disorder dietitians and psychologists.32 In IBS patients who are not classified as having an eating disorder, but are at risk or exhibit disordered eating behaviours, a discussion of the risks and benefits of restrictive diets is warranted, along with recommendations of alternative diet or non-diet therapies. The FODMAP-Gentle approach may be suitable18 with long-term studies showing that good symptom management is achieved when patients restrict only a small number of very high FODMAP foods.53,65
The Problem of Printed Diet Sheets
IBS patients are often provided with printed diet sheets to educate on the FODMAP diet. The inadequacy of these printed sheets was highlighted in a small qualitative study from the United Kingdom. Eight IBS patients who were handed printed diet sheets by their general practitioner or gastroenterologist were asked about their real-world experience. Patients reported that the sheets were overly simplistic, often just “food lists” with little or no personalization to accommodate social, cultural or family needs, and that the non-personalized nature of these sheets reduced their utility.34
A one-size-fits-all approach to restrictive diets is inadequate for several reasons. First, diet sheets include inaccurate information and often there are discrepancies between different diet sheets, as there are in published papers,66 compounding patient confusion.33 Secondly, food composition data included in these sheets quickly becomes out-dated, unlike data in digital applications that are updated regularly. Thirdly, food lists are limited in the breadth of foods they cover and fail to account for socially and culturally diverse eating habits. Fourthly, lists indicate high vs low FODMAP content on the basis of standard servings and do not consider individuals who consume larger portions, which can alter the FODMAP rating from low to high. Fifthly, supermarkets contain thousands of packaged and processed foods. The FODMAP content of these cannot be communicated via a simple food list and deciphering FODMAP content from food labels is complex and therefore difficult for many patients.67 Furthermore, the actual FODMAP intake of a processed food may not be predictable according to the food label list of ingredients. The last two issues have been partly addressed on the Monash University FODMAP Diet app by using an orange symbol for borderline content and by including some processed and packaged foods where the ingredients remain constant. Lastly, diet sheets provide no context as to why the foods are restricted, how this may be manipulated for individual differences and how the food lists relate to their diagnosis. This may fuel misunderstanding on the use of dietary therapy, diminish compliance and potentially compound the risks outlined above.
The Importance of a Multidisciplinary Team, Including a Dietitian
While gastroenterologists may be the initial contact for patients with GI disorders, treatment response is improved when the patient is managed by a multidisciplinary team. This was highlighted in a study which showed that 84% of GI patients managed by a multidisciplinary team (including a gastroenterologist, dietitian, hypnotherapist, psychiatrist, and physiotherapist), experienced symptom improvement, compared to 57% managed by the gastroenterologist alone.68 Another recent study of 35 patients provided with FODMAP diet education via the physician, rather than dietitian, suggested that, while understanding of the diet was adequate and symptoms were improved, lack of dietitian involvement resulted in poor compliance, with only 52% reporting they followed the diet most of the time.46 Additionally, more than 3 patients out of 4 reported they wished to see a dietitian at least once, highlighting the importance of a multidisciplinary approach including a dietitian. Despite this evidence, clinical practice does not always utilize a multidisciplinary approach.
Addressing the Barriers to Dietetic Involvement
Although the value of dietetic input is now recognized in clinical guidelines, this is not universal.69 For example, while the American College of Gastroenterology recommends use of the FODMAP diet administered only by dietitians,70 the Korean and Japanese guidelines make no mention of dietitians and only recommend limited use of dietary therapies in IBS.71,72 Moreover, a survey of gastroenterologists found that, while 60% agreed that patients associated dietary intake with IBS symptoms, only 21% regularly referred to dietitians. Additionally, over half of gastroenterologists recommended the FODMAP diet to patients,73 suggesting they may appreciate the importance of diet therapies, but not the value of a dietitian. Recent studies conducted in Australia and the United States have reported that the barriers to dietetic referral include a lack of access to specialised dietitians, a poor understanding of their role, and the out-of-pocket costs for patients due to limited insurance coverage of dietetic services.74,75 Therefore, it is essential that both gastroenterologists and dietitians advocate for the role of dietitians in this specialized fields, as 78% of non-complex GI patients could be managed exclusively by a dietitian.76 Simons, Taft, Doerfler, Ruddy, Bollipo, Nightingale, Siau, van Tilburg32 proposed guidelines to assist doctors to determine when they should refer IBS patients to dietitians, as presented in Table 2.
Table 2.
When to Refer to a Dietitian (Adapted From Simons et al32)
|
FODMAP, fermentable oligo-, di-, mono-saccharides, and polyols.
Professional Standards for Gastrointestinal-experienced Dietitians
As the role of dietitians in GI disorders involves evaluation of the patient and providing an individualised approach, it should be appreciated that the understanding of, and experience in managing, such disorders, in terms of pathophysiology, differential diagnosis, and therapeutic options is essential to achieve optimal patient outcomes. Unfortunately, there is no formal training program for GI dietitians, and specialization in the field is generally related to the amount of clinical experience and professional development undertaken by the individual. Hence, it is difficult to discern what is a “GI dietitian”. An Australian survey revealed that 30-51% of currently practicing dietitians in the state of Victoria, stated that they were involved in the management of patients with IBS, celiac disease, liver disease, inflammatory bowel disease, food allergies and other disorders associated with the GI tract.77 However, the level of GI training of the dietitians surveyed was not explored, and to describe all those working in GI and food allergy spaces as “GI dietitians” is inaccurate, and could be likened to calling all primary care physicians “gastroenterologists” because they deal with GI problems in some of their practice. Formalization of the specialization in GI dietetics is clearly needed.
Professional development courses are available for dietitians to upskill in the field. In Australia, 2 such courses include the Monash FODMAP online course and the Dietitians Australia Centre for Advanced Learning course on GI nutrition provide specific education to dietitians about IBS and dietary therapies. While these courses exist, there is no accreditation to provide formal recognition of GI dietitians. In other areas of dietetics in Australia such as sports dietetics, completion of an internationally recognised course combined with at least 1 year of practical experience in the field can qualify a dietitian to become an accredited sports dietitian. This type of accreditation could be applied to GI and may be a way forward in the future to provide formal recognition.
How to Deliver the Diet in Areas Where Dietitians Are Not Available?
Numerous papers and clinical guidelines now specifically recommend dietitian-led delivery of the FODMAP diet.65,70,78-81 However, in situations where dietitian availability is limited or patients choose a self-guided approach, several considerations may be made depending upon the scenario.
(1) Access to dietitians is limited: In many parts of the world, face-to-face dietitian consultation is not possible due to lack of trained dietitians in practical proximity. Telehealth may be an appropriate solution. In recent years, telehealth has emerged as a mainstream tool to access healthcare remotely82,83 and recent experience has indicated that guidance through the FODMAP diet by a dietitian online is both feasible and successful (personal observations). However, limited internet access and/or technical skills may prevent some patients from engaging in telehealth.
(2) Availability of dietitians is limited: Dietary consultation/education is a time-consuming process (as outlined above) and, given the prevalence of IBS in the community, too few dietitians may be available to manage the patient load. In this setting, group education sessions on the FODMAP diet is an alternative that may be as effective as one-on-one counselling in reducing IBS symptoms in 54-60% of selected participants, assuming they are pre-screened for suitability (thus excluding patients with atypical symptoms, complex health issues, and/or language barriers).84 However, relevant considerations include that there is limited capacity to individually assess and tailor advice to patients in a group setting. Therefore, in practice, one-on-one counselling should be available alongside group dietetic education, so that patients unsuitable for group education can access the individualized dietetic care they need.
(3) Delivery of FODMAP dietary education by other health professionals: There is no impediment to other health professionals who are properly trained in the FODMAP strategy delivering this diet, although less evidence supports this approach and efficacy data are more mixed.41,85 Suitable health professionals may include GI nurses or gastroenterologists. For example, one study reported that participants guided by IBS and FODMAP-trained nurses reduced their intake of FODMAP-containing foods,85 while others reported improved quality of life and IBS symptoms.41,86 Gastroenterologists may also successfully deliver the diet, with 1 observational study showing that patients with IBS who followed a gastroenterologist-taught FODMAP diet experienced improved IBS symptoms, with apparent ongoing adherence. The gastroenterologist in this study clearly followed a protocol that would have required considerable time and training before such an intervention was made available. Other aspects regarding assessment were not addressed. The learnings from this study are that the doctor (or other health professional) can successfully deliver a FODMAP diet, but that training on how to do so successfully would be needed. Such education is readily available on-line, as outlined above for dietitians wishing to up-skill.87
(4) Self-guided approach: Across the world, this seems a common approach, although the success this strategy is suboptimal according to the limited study of such outcomes.22 Challenges include identifying quality information amidst the masses of inaccurate information available online, and patients are unlikely to mitigate the risks of this diet, as outlined above. While printed diet sheets may be used as a guide, these are often overly simplistic, lacking personalization and nothing but food lists of high and FODMAP foods of questionable accuracy. More detailed guidance on how to follow the 3-phase FODMAP diet is available in books, booklets and digital applications, such as the Monash University FODMAP Diet app, which also provides up-to-date information about the FODMAP content of food. An online course for patients was designed by the Monash University FODMAP team to supplement and reinforce professional advice, and potentially may be used where professional coaching is not available. The success of this course in enabling people to independently follow the 3 stages of the FODMAP diet has not undergone formal evaluation, but at least the information is from a reliable source.
(5) Utilizing specialised food delivery services: The use of ready-made meals delivered to your home has become popular when weight loss is the goal in those with the means to pay. Similarly, services that deliver low-FODMAP meals are available in Australia and the United States and provided by a range of companies. They offer a convenient means of determining the symptomatic success of FODMAP restriction and potentially make the reintroduction phase easier. Ideally however, they would be accompanied by dietetic education to upskill the patient in appropriate food selection and to ensure a suitable diet that both provides symptomatic relief and mitigates risks of nutritional inadequacy and disordered eating practices is established for the long-term. Furthermore, symptoms can still occur with the consumption of these ready-made meals, particularly with those that are not certified for FODMAP content.
Culturally-appropriate FODMAP Diets
Potential benefits of the FODMAP diet for patients with IBS appear to stretch across all continents, with the exception of Africa from which there have been no reports. RCTs of the FODMAP diet have been conducted in Australasia, North America, Europe, Middle East, some countries in Asia,42,88-90 although the number of studies is limited. Uncontrolled observations have suggested efficacy of the FODMAP diet in Central America91 and association of high FODMAP foods with symptoms have been made in South America.92 It does appear that the moderate intake of FODMAPs (sufficient to enable the benefits of their restriction to manifest) stretches across most cultures, with the exception of Japan where efficacy of the diet has yet to be reported, and an association study of perceptions of the relationship of specific food intake and lower GI symptoms suggested that FODMAPs were not a problem, although such interpretation of the findings was highly speculative.93 Table 3 outlines staple high-FODMAP and low-FODMAP alternatives of different cultural diets including Western, Mediterranean, Middle Eastern, East Asian, and South Asian diets. More data are needed for African, Asian, Middle Eastern, and South American diets.
Table 3.
Staple Fermentable Oligo-, Di-, Mono-saccharides, and Polyols–containing Foods Across Different Cultural Diets
| FODMAP diets | Galacto-oligosaccharides | Fructo-oligosaccharides | Excess fructose | Lactose | Polyols | Staple low FODMAP foods |
|---|---|---|---|---|---|---|
| Western diet96-98 | Lentils Chickpeas Broad beans Kidney beans Pistachio Almonds |
Wheat Onion Garlic Spring onion Pomegranate Watermelon Zucchini |
Banana Mango Watermelon Honey Jam |
Cow’s milk | Sweet potato Avocado Mushroom Watermelon Eggplant Apple |
Rice Peanuts Tomato Fish/meat/chicken Cucumber Spinach Red capsicum Potato |
| Mediterranean diet99,100 | Lentils Chickpeas Broad beans Kidney beans Pistachio Almonds |
Wheat Onion Garlic Pomegranate Watermelon Zucchini Pistachio |
Watermelon Fig Honey |
Cow’s milk | Mushroom Watermelon Eggplant Green capsicum |
Peanuts Tomato Fish/meat/chicken Cucumber Spinach Red capsicum Olives Olive oil Potato Wine |
| Middle Eastern diet101,102 | Lentils Chickpeas Broad beans Kidney beans Tahini Pistachio |
Okra Wheat Couscous Bulgur Onion Garlic Spring onion Dates Pomegranate Watermelon Zucchini Molasses |
Banana Mango Watermelon Molasses Fig |
Cow’s milk Goat’s milk |
Watermelon Eggplant Green capsicum |
Pumpkin seeds Sesame Rice Peanuts Tomato Fish/meat/chicken Spinach Pine nuts Cucumber Red capsicum Olives Olive oil Ghee Camel’s milk |
| East Asian diet93,95 | Lentils Chickpeas Soy bean milk Soy beans Bitter melon Silken tofu Wasabi Taro |
Jackfruit Silken tofu Onion/shallot Garlic Wheat Watermelon |
Banana Mango Jackfruit Watermelon Nashi pear |
Cow’s milk | Wasabi Mushroom Watermelon Coconut Nashi pear |
Soy protein milk Mung bean Rice Rice noodles Peanuts Sesame Soy sauce Fish sauce Hard tofu Bok choy Guava Plantain Durian Fish/meat/chicken Rice bran oil |
| South Asian diet 94 | Lentils Chickpeas Beetroot Soy bean milk Almonds Silken tofu |
Onion/shallot Wheat Beetroot Okra Spring onion Dates Pomegranate Silken tofu Watermelon |
Banana Mango Jackfruit Watermelon |
Cow’s milk Goat’s milk |
Sweet potato Avocado Coconut Watermelon Eggplant |
Soy protein milk Rice noodles Pumpkin seeds Sesame seeds Peanuts Tomato Fish/meat/chicken Ghee Hard tofu |
FODMAP, fermentable oligo-, di-, mono-saccharides, and polyols.
Despite evidence revealing its efficacy, there are challenges in implementing the FODMAP diet into different cultures. Some cultures hold specific food beliefs that can differ from the FODMAP diet. For instance, Chinese Food Therapy classifies foods based on the perceived reaction they have in the body and recommends treating GI conditions using Traditional Chinese Medicine, which may not align well with FODMAP principles.103 Additionally, in many cultures, such as South Asian, it is not common to measure ingredients and portion sizes, and meals are commonly served with side dishes that are shared amongst a table.94 This can make it difficult to measure an individual’s consumption of FODMAPs. Also, detailed review of food content in South and East Asia have revealed many foods of unknown FODMAP content.94,95 Some foods that are staples in Asian cuisine are high in FODMAP content, such as garlic and legumes/pulses, which can make it challenging to adequately implement the diet. However, strategies such as using garlic alternatives eg, chives, ginger or garlic-infused oil rather than whole garlic can be applied.95 Additionally, both food processing and cooking methods have been shown to alter the FODMAP content of foods,104 which may be a useful strategy to reduce the effects of some high FODMAP foods. For example, the use of legumes in condiments are prevalent in Asian cooking and may have reduced FODMAP content, such as fermented beans, depending on the length and type of fermentation process utilised. Alternatively, a trial-and-error approach to test for individual tolerance can be used for recipes that utilize ingredients whom FODMAP content is uncertain. Such region-specific differences require ongoing research and thought.
Implementing the Diet When the FODMAP Content of Food is Unknown
The success of the FODMAP diet strategy is contingent on patients and clinicians having access to accurate FODMAP composition data, derived from laboratory analyses using well established techniques.96,97 Monash University has a large database describing the FODMAP content of food, made accessible via the Monash University FODMAP Diet app. While Monash University has sourced and tested food from all over the world, the majority of food listed in the app are from Western nations, including Australia, Europe and the United States, with a smaller fraction of food sourced from Asian countries.
Despite this, some foods listed in the Monash App are common to both Asian and Western countries, many of which can be consumed in a low FODMAP serve. Examples include breads and cereals such as rice and rice noodles; fruits such as durian, dragon fruit, and pawpaw; vegetables, legumes and nuts such as bok choy, tofu, and peanuts; herbs and spices such as curry leaves and saffron; and dairy/alternatives such as soy milk. Foods containing minimal carbohydrates will also have little in the way of FODMAP content. Examples include protein-rich foods such as plain red meat, fish, poultry and eggs, and fats and oils such as peanut oil, sesame oil, and ghee.
The FODMAP diet differs markedly in different geographic locations, so dietitians must use the available food composition data to construct nutritionally adequate, culturally appropriate FODMAP diets. Where food composition data are lacking, dietitians can take one of two approaches. The first approach would be to assume foods with unknown FODMAP content are suitable to consume and only restrict foods known to be high in FODMAPs initially. Restriction of foods with unknown FODMAP content would only be needed if symptom response is inadequate. The second approach would be to initially restrict both foods known to be high in FODMAPs and foods of unknown FODMAP content. The usual reintroduction approach can be used to determine tolerance of foods with known FODMAP content.17 For foods of unknown FODMAP content, a “test to tolerance” approach can be used, whereby the patient would wait until symptoms are well controlled, then include a small amount (about one-third usual serving size) daily for 3 days. If the food is tolerated it would be considered suitable to include for that individual.
Conclusion
The structure of, and considerations around implementing the FODMAP diet are now well described and understood. While the diet has consistently shown improved IBS outcomes across clinical studies, it is important to acknowledge the essential role of dietitians in implementing, tailoring and managing the diet to achieve the best patient outcome. Dietitians are indispensable members of the GI multidisciplinary team, and more attention should be given to creating an internationally recognised accreditation for GI-specialized dietitians. In limited scenarios where dietitian access or availability is lacking, a combination of approaches may be considered including telehealth or group education with a dietitian, utilisation of accurate FODMAP resources, or undertaking specialized training for non-dietetic health professionals. Whichever approach is used, care should be taken to mitigate any risks arising with long-term use of a FODMAP diet.
Footnotes
Financial support: None.
Conflicts of interest: The Department of Gastroenterology, Monash University financially benefits from the sales of a digital application, booklets, and online courses on the FODMAP diet. The authors have no other conflicts of interest to declare.
Author contributions: Nessmah Sultan, Jane E Varney, Peter R Gibson, and Caroline J Tuck planned the review. All authors were involved in the writing, and approval of the final manuscript.
References
- 1.Adams HL, Basude D, Kyle A, Sandmann S, Paul SP. Managing irritable bowel syndrome in children. Nurs Stand. 2016;31:42–52. doi: 10.7748/ns.2016.e10439. [DOI] [PubMed] [Google Scholar]
- 2.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology. 2021;160:99–114. e3. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ. What causes functional gastrointestinal disorders? a proposed disease model. Am J Gastroenterol. 2020;115:41–48. doi: 10.14309/ajg.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 4.Menees S, Chey W. The gut microbiome and irritable bowel syndrome. F1000Res. 2018;7:F1000 Faculty Rev-1029. doi: 10.12688/f1000research.14592.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. 2014;20:14105–14125. doi: 10.3748/wjg.v20.i39.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grad S, Dumitrascu DL. Irritable bowel syndrome subtypes: new names for old medical conditions. Dig Dis. 2020;38:122–127. doi: 10.1159/000505287. [DOI] [PubMed] [Google Scholar]
- 7.Peters SL, Yao CK, Philpott H, Yelland GW, Muir JG, Gibson PR. Randomised clinical trial: the efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:447–459. doi: 10.1111/apt.13706. [DOI] [PubMed] [Google Scholar]
- 8.Paine P. Review article: current and future treatment approaches for pain in IBS. Aliment Pharmacol Ther. 2021;54(suppl 1):S75–S88. doi: 10.1111/apt.16550. [DOI] [PubMed] [Google Scholar]
- 9.Chey WD, Keefer L, Whelan K, Gibson PR. Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. Gastroenterology. 2021;160:47–62. doi: 10.1053/j.gastro.2020.06.099. [DOI] [PubMed] [Google Scholar]
- 10.Barrett JS, Gearry RB, Muir JG, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 11.Murray K, Wilkinson-Smith V, Hoad C, et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109:110–119. doi: 10.1038/ajg.2013.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Major G, Pritchard S, Murray K, et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology. 2017;152:124–133. e2. doi: 10.1053/j.gastro.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 13.Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 14.Gibson PR, Halmos EP. The FODMAP diet: more than just a symptomatic therapy? Gut. 2021:gutjnl-2021-326284. doi: 10.1136/gutjnl-2021-326284. [DOI] [PubMed] [Google Scholar]
- 15.Gibson PR, Halmos EP, Muir JG. Review article: FODMAPS, prebiotics and gut health-the FODMAP hypothesis revisited. Aliment Pharmacol Ther. 2020;52:233–246. doi: 10.1111/apt.15818. [DOI] [PubMed] [Google Scholar]
- 16.Vervier K, Moss S, Kumar N, et al. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut. 2021;0:1–10. doi: 10.1136/gutjnl-2021-325177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuck C, Barrett J. Re-challenging FODMAPs: the low FODMAP diet phase two. J Gastroenterol Hepatol. 2017;32(suppl 1):11–15. doi: 10.1111/jgh.13687. [DOI] [PubMed] [Google Scholar]
- 18.Halmos EP, Gibson PR. Controversies and reality of the FODMAP diet for patients with irritable bowel syndrome. J Gastroenterol Hepatol. 2019;34:1134–1142. doi: 10.1111/jgh.14650. [DOI] [PubMed] [Google Scholar]
- 19.Wang XJ, Camilleri M, Vanner S, Tuck C. Review article: biological mechanisms for symptom causation by individual FODMAP subgroups - the case for a more personalised approach to dietary restriction. Aliment Pharmacol Ther. 2019;50:517–529. doi: 10.1111/apt.15419. [DOI] [PubMed] [Google Scholar]
- 20.Yao CK, Gibson PR, Shepherd SJ. Design of clinical trials evaluating dietary interventions in patients with functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:748–758. doi: 10.1038/ajg.2013.77. [DOI] [PubMed] [Google Scholar]
- 21.Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2022;71:1117–1126. doi: 10.1136/gutjnl-2021-325214. [DOI] [PubMed] [Google Scholar]
- 22.Tuck CJ, Reed DE, Muir JG, Vanner SJ. Implementation of the low FODMAP diet in functional gastrointestinal symptoms: a real-world experience. Neurogastroenterol Motil. 2020;32:e13730. doi: 10.1111/nmo.13730. [DOI] [PubMed] [Google Scholar]
- 23.Staudacher HM, Rossi M, Kaminski T, et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterol Motil. 2022:e14241. doi: 10.1111/nmo.14241. [DOI] [PubMed] [Google Scholar]
- 24.Harvie RM, Chisholm AW, Bisanz JE, et al. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J Gastroenterol. 2017;23:4632–4643. doi: 10.3748/wjg.v23.i25.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Keeffe M, Jansen C, Martin L, et al. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30:e13154. doi: 10.1111/nmo.13154. [DOI] [PubMed] [Google Scholar]
- 26.Bellini M, Rossi A. Is a low FODMAP diet dangerous? Tech Coloproctol. 2018;22:569–571. doi: 10.1007/s10151-018-1835-9. [DOI] [PubMed] [Google Scholar]
- 27.Whelan K, Martin LD, Staudacher HM, Lomer MCE. The low FODMAP diet in the management of irritable bowel syndrome: an evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J Hum Nutr Diet. 2018;31:239–255. doi: 10.1111/jhn.12530. [DOI] [PubMed] [Google Scholar]
- 28.Palsson OS, Whitehead WE, van Tilburg MA, et al. Development and validation of the rome IV diagnostic questionnaire for adults. Gastroenterology. 2016;150:1481–1491. doi: 10.1053/j.gastro.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Blake MR, Raker JM, Whelan K. Validity and reliability of the bristol stool form scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693–703. doi: 10.1111/apt.13746. [DOI] [PubMed] [Google Scholar]
- 30.Healey G, Brough L, Murphy R, Hedderley D, Butts C, Coad J. Validity and reproducibility of a habitual dietary fibre intake short food frequency questionnaire. Nutrients. 2016;8:558. doi: 10.3390/nu8090558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: a new screening tool for eating disorders. West J Med. 2000;172:164–165. doi: 10.1136/ewjm.172.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simons M, Taft TH, Doerfler B, et al. Narrative review: risk of eating disorders and nutritional deficiencies with dietary therapies for irritable bowel syndrome. Neurogastroenterol Motil. 2022;34:e14188. doi: 10.1111/nmo.14188. [DOI] [PubMed] [Google Scholar]
- 33.McMeans AR, King KL, Chumpitazi BP. Low FODMAP dietary food lists are often discordant. Am J Gastroenterol. 2017;112:655–656. doi: 10.1038/ajg.2016.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trott N, Aziz I, Rej A, Surendran Sanders D. How patients with IBS use low FODMAP dietary information provided by general practitioners and gastroenterologists: a qualitative study. Nutrients. 2019;11:1313. doi: 10.3390/nu11061313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kortlever TL, Ten Bokkel Huinink S, Offereins M, et al. Low-FODMAP diet is associated with improved quality of life in IBS patients-a prospective observational study. Nutr Clin Pract. 2019;34:623–630. doi: 10.1002/ncp.10233. [DOI] [PubMed] [Google Scholar]
- 36.Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi: 10.1186/s12906-018-2409-0.5d768ce383694ac1b2d6039fcca38816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford AC, Talley NJ, Spiegel BM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chumpitazi BP, Kearns GL, Shulman RJ. Review article: the physiological effects and safety of peppermint oil and its efficacy in irritable bowel syndrome and other functional disorders. Aliment Pharmacol Ther. 2018;47:738–752. doi: 10.1111/apt.14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuck CJ, Taylor KM, Gibson PR, Barrett JS, Muir JG. Increasing symptoms in irritable bowel symptoms with ingestion of galacto-oligosaccharides are mitigated by α-galactosidase treatment. Am J Gastroenterol. 2018;113:124–134. doi: 10.1038/ajg.2017.245. [DOI] [PubMed] [Google Scholar]
- 40.Pourmand H, Keshteli AH, Saneei P, Daghaghzadeh H, Esmaillzadeh A, Adibi P. Adherence to a low FODMAP diet in relation to symptoms of irritable bowel syndrome in Iranian adults. Dig Dis Sci. 2018;63:1261–1269. doi: 10.1007/s10620-018-4986-7. [DOI] [PubMed] [Google Scholar]
- 41.Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep. 2012;5:1382–1390. doi: 10.3892/mmr.2012.843. [DOI] [PubMed] [Google Scholar]
- 42.Goyal O, Batta S, Nohria S, et al. Low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet in patients with diarrhea-predominant irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol Hepatol. 2021;36:2107–2115. doi: 10.1111/jgh.15410. [DOI] [PubMed] [Google Scholar]
- 43.Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149:1399–1407. e2. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 44.Staudacher HM, Lomer MCE, Farquharson FM, et al. Diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and probiotic restores bifidobacterium species: a randomized controlled trial. Gastroenterology. 2017;153:936–947. doi: 10.1053/j.gastro.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol. 2016;111:1824–1832. doi: 10.1038/ajg.2016.434. [DOI] [PubMed] [Google Scholar]
- 46.Van Ouytsel P, Szalai A, Van Gossum A, Arvanitakis M, Louis H. Feasibility of a low FODMAPs diet without initial dietician intervention in the management of patients with irritable bowel syndrome: a prospective study. Acta Gastroenterol Belg. 2021;84:593–600. doi: 10.51821/84.4.010. [DOI] [PubMed] [Google Scholar]
- 47.So D, Yao CK, Ardalan ZS, et al. Supplementing dietary fibers with a low FODMAP diet in irritable bowel syndrome: a randomized controlled crossover trial. Clin Gastroenterol Hepatol Published Online First. 2021 Dec 17;:doi: 10.1016/j.cgh.2021.12.016. doi: 10.1016/j.cgh.2021.12.016. [DOI] [PubMed] [Google Scholar]
- 48.So D, Yao CK, Gibson PR, Muir JG. Evaluating tolerability of resistant starch 2, alone and in combination with minimally fermented fibre for patients with irritable bowel syndrome: a pilot randomised controlled cross-over trial. J Nutr Sci. 2022;11:e15. doi: 10.1017/jns.2022.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stocks NP, Gonzalez-Chica D, Hay P. Impact of gastrointestinal conditions, restrictive diets and mental health on health-related quality of life: cross-sectional population-based study in Australia. BMJ Open. 2019;9:e026035. doi: 10.1136/bmjopen-2018-026035.c9449f8712f44cc0892a435a9c8530c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rocha S, Gandolfi L, Santos JE. [The psychosocial impacts caused by diagnosis and treatment of coeliac disease.]. Rev Esc Enferm USP. 2016;50:65–70. doi: 10.1590/S0080-623420160000100009. [Portuguese] [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez Almagro J, Rodríguez Almagro D, Solano Ruiz C, Siles González J, Hernández Martínez A. The experience of living with a gluten-free diet: an integrative review. Gastroenterol Nurs. 2018;41:189–200. doi: 10.1097/SGA.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 52.Busby E, Bold J, Fellows L, Rostami K. Mood disorders and gluten: it's not all in your mind! a systematic review with meta-analysis. Nutrients. 2018;10:1708. doi: 10.3390/nu10111708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rej A, Shaw CC, Buckle RL, et al. The low FODMAP diet for IBS; a multicentre UK study assessing long term follow up. Dig Liver Dis. 2021;53:1404–1411. doi: 10.1016/j.dld.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Schuppan D, Mäki M, Lundin KEA, et al. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N Engl J Med. 2021;385:35–45. doi: 10.1056/NEJMoa2032441. [DOI] [PubMed] [Google Scholar]
- 55.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders : DSM-5. Vol. 5. American Psychiatric Association; Washington DC: 2013. [DOI] [Google Scholar]
- 56.Reed-Knight B, Squires M, Chitkara DK, van Tilburg MA. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28:1915–1920. doi: 10.1111/nmo.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kayar Y, Agin M, Dertli R, et al. Eating disorders in patients with irritable bowel syndrome. Gastroenterol Hepatol. 2020;43:607–613. doi: 10.1016/j.gastrohep.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Tuck C, Sultan N, Tonkovic M, Biesiekierski J. Su572 comparing the prevalence of orthorexia nervosa and psychosocial sensations in patients with irritable bowel syndrome, eating disorders and healthy controls. Gastroenterology. 2021;160:S-744. doi: 10.1016/S0016-5085(21)02489-6. [DOI] [Google Scholar]
- 59.Mari A, Hosadurg D, Martin L, Zarate-Lopez N, Passananti V, Emmanuel A. Adherence with a low-FODMAP diet in irritable bowel syndrome: are eating disorders the missing link? Eur J Gastroenterol Hepatol. 2019;31:178–182. doi: 10.1097/MEG.0000000000001317. [DOI] [PubMed] [Google Scholar]
- 60.Scarlata K, Catsos P, Smith J. From a dietitian's perspective, diets for irritable bowel syndrome are not one size fits all. Clin Gastroenterol Hepatol. 2019;18:543–545. doi: 10.1016/j.cgh.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Zia JK, Riddle M, DeCou CR, McCann BS, Heitkemper M. Prevalence of eating disorders, especially DSM-5's avoidant restrictive food intake disorder, in patients with functional gastrointestinal disorders: a cross-sectional online survey. Gastroenterology. 2017;152:S715–S716. doi: 10.1016/S0016-5085(17)32490-3. [DOI] [Google Scholar]
- 62.Harer KN, Jagielski CH, Riehl ME, Chey WD. 272-avoidant/restrictive food intake disorder among adult gastroenterology behavioral health patients: demographic and clinical characteristics. Gastroenterology. 2019;156:S–53. doi: 10.1016/S0016-5085(19)36916-1. [DOI] [Google Scholar]
- 63.Dunn TM, Bratman S. On orthorexia nervosa: a review of the literature and proposed diagnostic criteria. Eat Behav. 2016;21:11–17. doi: 10.1016/j.eatbeh.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Gajdos P, Román N, Tóth-Király I, Rigó A. Functional gastrointestinal symptoms and increased risk for orthorexia nervosa. Eat Weight Disord. 2022;27:1113–1121. doi: 10.1007/s40519-021-01242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Keeffe M, Lomer MC. Who should deliver the low FODMAP diet and what educational methods are optimal: a review. J Gastroenterol Hepatol. 2017;32(suppl 1):23–26. doi: 10.1111/jgh.13690. [DOI] [PubMed] [Google Scholar]
- 66.San Mauro Martín I, Garicano Vilar E, López Oliva S, Sanz Rojo S. Existing differences between available lists of FODMAP containing foods. Rev Esp Enferm Dig Published Online First. 2022 Feb 1;:doi: 10.17235/reed.2022.8463/2021. doi: 10.17235/reed.2022.8463/2021. [DOI] [PubMed] [Google Scholar]
- 67.Chumpitazi BP, Lim J, McMeans AR, Shulman RJ, Hamaker BR. Evaluation of FODMAP carbohydrates content in selected foods in the united states. J Pediatr. 2018;199:252–255. doi: 10.1016/j.jpeds.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basnayake C, Kamm MA, Stanley A, et al. 409 randomised trial of multi-disciplinary versus standard gastroenterologist care for functional gastrointestinal disorders. Gastroenterology. 2020;158:S–77. doi: 10.1016/S0016-5085(20)30874-X. [DOI] [PubMed] [Google Scholar]
- 69.Li H, Xing X, Yao L, et al. Assessment of the quality and content of clinical practice guidelines on irritable bowel syndrome using the AGREE II instrument. Digestion. 2020;101:355–365. doi: 10.1159/000499838. [DOI] [PubMed] [Google Scholar]
- 70.Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol. 2021;116:17–44. doi: 10.14309/ajg.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 71.Fukudo S, Okumura T, Inamori M, et al. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J Gastroenterol. 2021;56:193–217. doi: 10.1007/s00535-020-01746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song KH, Jung HK, Kim HJ, et al. Clinical practice guidelines for irritable bowel syndrome in Korea, 2017 revised edition. J Neurogastroenterol Motil. 2018;24:197–215. doi: 10.5056/jnm17145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lenhart A, Ferch C, Shaw M, Chey WD. Use of dietary management in irritable bowel syndrome: results of a survey of over 1500 united states gastroenterologists. J Neurogastroenterol Motil. 2018;24:437–451. doi: 10.5056/jnm17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siopis G, Colagiuri S, Allman-Farinelli M. Doctors identify regulatory barriers for their patients with type 2 diabetes to access the nutritional expertise of dietitians. Aust J Prim Health. 2021;27:312–318. doi: 10.1071/PY20228. [DOI] [PubMed] [Google Scholar]
- 75.Sastre LR, Van Horn LT. Family medicine physicians' report strong support, barriers and preferences for registered dietitian nutritionist care in the primary care setting. Fam Pract. 2021;38:25–31. doi: 10.1093/fampra/cmaa099. [DOI] [PubMed] [Google Scholar]
- 76.Ryan D, Pelly F, Purcell E. The activities of a dietitian-led gastroenterology clinic using extended scope of practice. BMC health serv Res. 2016;16:604. doi: 10.1186/s12913-016-1845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Department of Health and Human Services, author. Victorian allied health: dietetics workforce report. Victorian Government; Melbourne: 2018. [Google Scholar]
- 78.Vasant DH, Paine PA, Black CJ, et al. British society of gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70:1214–1240. doi: 10.1136/gutjnl-2021-324598. [DOI] [PubMed] [Google Scholar]
- 79.McKenzie YA, Bowyer RK, Leach H, et al. British dietetic association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update) J Hum Nutr Diet. 2016;29:549–575. doi: 10.1111/jhn.12385. [DOI] [PubMed] [Google Scholar]
- 80.Hookway C, Buckner S, Crosland P, Longson D. Irritable bowel syndrome in adults in primary care: summary of updated NICE guidance. BMJ. 2015;350:h701. doi: 10.1136/bmj.h701. [DOI] [PubMed] [Google Scholar]
- 81.Gibson PR, Halmos EP, So D, Yao CK, Varney JE, Muir JG. Diet as a therapeutic tool in chronic gastrointestinal disorders: lessons from the FODMAP journey. J Gastroenterol Hepatol. 2022;37:644–652. doi: 10.1111/jgh.15772. [DOI] [PubMed] [Google Scholar]
- 82.Kelly JT, Allman-Farinelli M, Chen J, et al. Dietitians australia position statement on telehealth. Nutr Diet. 2020;77:406–415. doi: 10.1111/1747-0080.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tuckson RV, Edmunds M, Hodgkins ML. Telehealth. N Engl J Med. 2017;377:1585–1592. doi: 10.1056/NEJMsr1503323. [DOI] [PubMed] [Google Scholar]
- 84.Whigham L, Joyce T, Harper G, et al. Clinical effectiveness and economic costs of group versus one-to-one education for short-chain fermentable carbohydrate restriction (low FODMAP diet) in the management of irritable bowel syndrome. J Hum Nutr Diet. 2015;28:687–696. doi: 10.1111/jhn.12318. [DOI] [PubMed] [Google Scholar]
- 85.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Effects of dietary guidance on the symptoms, quality of life and habitual dietary intake of patients with irritable bowel syndrome. Mol Med Rep. 2013;8:845–852. doi: 10.3892/mmr.2013.1565. [DOI] [PubMed] [Google Scholar]
- 86.Moore JS, Gibson PR, Perry RE, Burgell RE. Endometriosis in patients with irritable bowel syndrome: specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust N Z J Obstet Gynaecol. 2017;57:201–205. doi: 10.1111/ajo.12594. [DOI] [PubMed] [Google Scholar]
- 87.Gravina AG, Dallio M, Romeo M, et al. Adherence and effects derived from FODMAP diet on irritable bowel syndrome: a real life evaluation of a large follow-up observation. Nutrients. 2020;12:928. doi: 10.3390/nu12040928.907be1c3e58b4442becf867d9a58f954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patcharatrakul T, Juntrapirat A, Lakananurak N, Gonlachanvit S. Effect of structural individual low-FODMAP dietary advice vs. brief advice on a commonly recommended diet on IBS symptoms and intestinal gas production. Nutrients. 2019;11:2856. doi: 10.3390/nu11122856.360f02f50cae44cb8881f92b8849a91b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Feng L, Wang X, et al. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet compared with traditional dietary advice for diarrhea-predominant irritable bowel syndrome: a parallel-group, randomized controlled trial with analysis of clinical and microbiological factors associated with patient outcomes. Am J Clin Nutr. 2021;113:1531–1545. doi: 10.1093/ajcn/nqab005. [DOI] [PubMed] [Google Scholar]
- 90.Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. J Gastroenterol Hepatol. 2018;33:1192–1199. doi: 10.1111/jgh.14051. [DOI] [PubMed] [Google Scholar]
- 91.Pérez y López N, Torres-López E, Zamarripa-Dorsey F. [Clinical response in Mexican patients with irritable bowel syndrome treated with a low diet low in fermentable carbohydrates (FODMAP).]. Rev Gastroenterol Mex. 2015;80:180–185. doi: 10.1016/j.rgmxen.2015.08.011. [Spanish] [DOI] [PubMed] [Google Scholar]
- 92.Aufieri MC, Morimoto JM, Viebig RF. Severity of irritable bowel syndrome symptome and FODMAPS intake in university students. Arq Gastroenterol. 2021;58:461–467. doi: 10.1590/s0004-2803.202100000-84. [DOI] [PubMed] [Google Scholar]
- 93.Kaneko H, Tsuboi H, Yamamoto S, Konagaya T. Observational study on knowledge and eating habits with respect to low- and high-FODMAP foods in medical heckup populations in Japan. Nutrients. 2019;11:2436. doi: 10.3390/nu11102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hewawasam SP, Iacovou M, Muir JG, Gibson PR. Dietary practices and FODMAPs in South Asia: applicability of the low FODMAP diet to patients with irritable bowel syndrome. J Gastroenterol Hepatol. 2018;33:365–374. doi: 10.1111/jgh.13885. [DOI] [PubMed] [Google Scholar]
- 95.Iacovou M, Tan V, Muir JG, Gibson PR. The low FODMAP diet and its application in east and southeast Asia. J Neurogastroenterol Motil. 2015;21:459–470. doi: 10.5056/jnm15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muir JG, Shepherd SJ, Rosella O, Rose R, Barrett JS, Gibson PR. Fructan and free fructose content of common Australian vegetables and fruit. J Agric Food Chem. 2007;55:6619–6627. doi: 10.1021/jf070623x. [DOI] [PubMed] [Google Scholar]
- 97.Muir JG, Rose R, Rosella O, et al. Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography (HPLC) J Agric Food Chem. 2009;57:554–565. doi: 10.1021/jf802700e. [DOI] [PubMed] [Google Scholar]
- 98.Biesiekierski JR, Rosella O, Rose R, et al. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J Hum Nutr Diet. 2011;24:154–176. doi: 10.1111/j.1365-277X.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 99.Romaguera D, Bamia C, Pons A, Tur JA, Trichopoulou A. Food patterns and Mediterranean diet in western and eastern Mediterranean islands. Public Health Nutr. 2009;12:1174–1181. doi: 10.1017/S1368980008003674. [DOI] [PubMed] [Google Scholar]
- 100.de Lorgeril M, Salen P, Rabaeus M. New and traditional foods in a modernized Mediterranean diet model. Eur J Clin Nutr. 2019;72(suppl 1):47–54. doi: 10.1038/s41430-018-0308-6. [DOI] [PubMed] [Google Scholar]
- 101.Hoteit M, Zoghbi E, Al Iskandarani M, et al. Nutritional value of the middle eastern diet: analysis of total sugar, salt, and iron in Lebanese traditional dishes. F1000Res. 2020;9:1254. doi: 10.12688/f1000research.26278.1.2c21891732a6439ca2918613b63b1c63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoteit M, Zoghbi E, Rady A, Shankiti I, Al-Jawaldeh A. Fatty acids quality in middle eastern traditional dishes, arabic sweets and market foods frequently consumed in Lebanon. Nutrients. 2021;13:2462. doi: 10.3390/nu13072462.6136c60077ae4a7d85c5101f6ec924df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chu NHS, Yao CK, Tan VPY. Food therapy in sinosphere Asia. J Clin Gastroenterol. 2018;52:105–113. doi: 10.1097/MCG.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 104.Tuck C, Ly E, Bogatyrev A, et al. Fermentable short chain carbohydrate (FODMAP) content of common plant-based foods and processed foods suitable for vegetarian- and vegan-based eating patterns. J Hum Nutr Diet. 2018;31:422–435. doi: 10.1111/jhn.12546. [DOI] [PubMed] [Google Scholar]