Abstract
Intestinal ischemia/reperfusion (I/R) is a common pathophysiological process in clinical severe patients, and the effect of intestinal I/R injury on the patient's systemic pathophysiological state is far greater than that of primary intestinal injury. In recent years, more and more evidence has shown that intestinal microbiota and its metabolites play an important role in the occurrence, development, diagnosis and treatment of intestinal I/R injury. Intestinal microbiota is regulated by host genes, immune response, diet, drugs and other factors. The metabolism and immune potential of intestinal microbiota determine its important significance in host health and diseases. Therefore, targeting the intestinal microbiota and its metabolites may be an effective therapy for the treatment of intestinal I/R injury and intestinal I/R-induced extraintestinal organ injury. This review focuses on the role of intestinal microbiota and its metabolites in intestinal I/R injury and intestinal I/R-induced extraintestinal organ injury, and summarizes the latest progress in regulating intestinal microbiota to treat intestinal I/R injury and intestinal I/R-induced extraintestinal organ injury.
Keywords: Intestinal microbiota, Metabolites, Intestinal ischemia reperfusion, extraintestinal organ injury
Introduction
Intestinal ischemia/reperfusion (I/R) injury is common in the perioperative period, especially in both surgical and trauma patients, and has high morbidity and mortality associated with it 1-3. Intestinal I/R plays an important role in the pathophysiological evolution of severe infection, trauma, shock, intestinal obstruction, mesenteric artery embolism, abdominal aortic aneurysm surgery, cardiopulmonary bypass surgery, liver transplantation, and small intestine transplantation 4, 5. It not only causes local intestinal injury, but can also disrupt intestinal mucosal barrier, which allows enteric bacterial endotoxin and various locally produced free radicals to penetrate the blood, translocate into the peripheral organs, and cause extraintestinal multiple organ dysfunction or even failure 6-8. Intestinal microbiota refers to the trillions of microorganisms that exist in the gastrointestinal tract, including bacteria, viruses, fungi, archaea and protozoa 9-14. They can interact with the host in a variety of ways and play a critical role in nutrient metabolism, xenobiotics and drug metabolism, maintenance of the intestinal barrier, and the structure and function of the gastrointestinal tract 15-19. In the past few decades, the research on intestinal microbiota and its metabolites and intestinal I/R injury and intestinal I/R-induced extraintestinal organ injury has increased rapidly. In this review, we focus on the role of intestinal microbiota and its metabolites in the occurrence, development, and prevention of intestinal I/R injury and intestinal I/R-induced extraintestinal organ injury, and provide guidance for the prediction, diagnosis and treatment of intestinal I/R injury in the future.
Synopsis of intestinal I/R injury
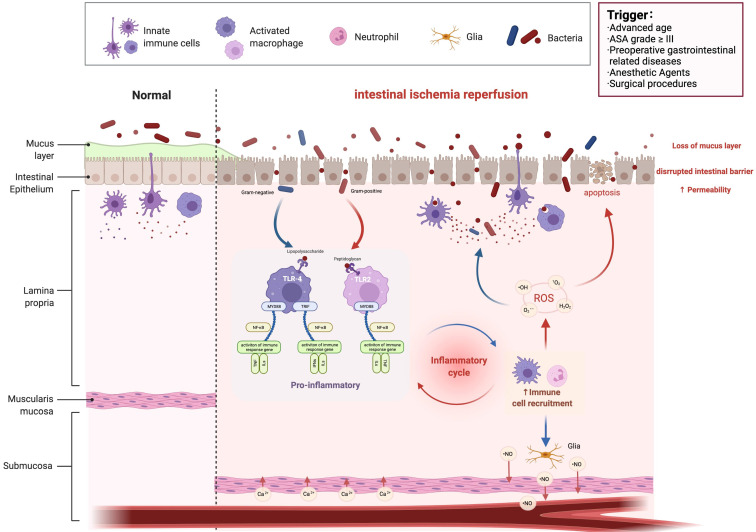
Perioperative acute intestinal I/R injury is a common emergency and critical condition 2. It not only causes damage to the intestinal barrier, but can also lead to multiple organ injury outside the intestine, with a very high complication rate and mortality rate 20. Therefore, prevention and treatment of acute intestinal I/R injury during the perioperative period is of great significance to improve patient outcomes. As shown in Table 1, the factors that cause acute ischemic intestinal injury during the perioperative period can be divided into patients, anesthesia and surgery-related factors. Patient factors include advanced age, American Society of Anaesthesiologists (ASA) grade ≥ III, preoperative gastrointestinal disease or other diseases that lead to impaired gastrointestinal function (such as severe infection, acute severe pancreatitis, trauma, shock, anemia, myocardial infarction, aortic dissection, mesenteric artery embolism, etc.). Anesthetic factors include hypotension and intestinal hypoperfusion caused by anesthetics; contraction of small blood vessels in the gastrointestinal mucosa caused by some vasoconstrictor drugs induces intestinal ischemia; the sympathetic nervous system is excited under stress, intestinal mucosal blood vessels contract strongly and blood perfusion is reduced. Surgical factors include abdominal aortic aneurysm surgery, cardiopulmonary bypass (CPB), abdominal surgery and other intestinal operations that affect intestinal blood perfusion; CO2 pneumoperitoneum can cause stress response, and plasma catecholamine, cortisol, and antidiuretic hormone levels increase during laparoscopic surgery; at the same time, the increase in abdominal pressure affects the blood perfusion of internal organs. The intestine is the body's largest endotoxin reservoir and microbial reservoir. Once intestinal injury occurs, endotoxin and flora shift, secondary to endotoxemia and sepsis, resulting in multiple organs throughout the body (lung, brain, liver, etc.) dysfunction and even failure. However, the prediction of intestinal I/R injury still lacks effective biomarkers; diagnosis lacks uniform standards; treatment lacks effective drugs and measures. In recent years, the intestinal microbiota has been proved to play an important role in the occurrence, development, prediction, diagnosis and treatment of diseases. Therefore, summarizing the changes and potential roles of intestinal microbiota and its metabolites in intestinal I/R injury is of great significance for finding predictive and diagnostic biomarkers, new treatment methods and drugs for intestinal I/R injury.
Table 1.
The risk factors of intestinal I/R injury during perioperative period
| Factor | |
|---|---|
| Patient factors | Advanced age |
| ASA grade ≥ III | |
| Preoperative gastrointestinal disease | |
| Other diseases that lead to impaired gastrointestinal function (such as severe infection, acute severe pancreatitis, trauma, shock, anemia, myocardial infarction, aortic dissection, mesenteric artery embolism, etc.) | |
| Anesthetic factors | Hypotension and intestinal hypoperfusion caused by anesthetics |
| Vasoconstrictor drugs that cause small blood vessels to constrict in the gastrointestinal mucosa | |
| Sympathetic nervous system excitement | |
| Surgical factors | Abdominal aortic aneurysm surgery |
| Cardiopulmonary bypass | |
| Abdominal surgery and laparoscopic surgery | |
| Other intestinal operations that affect intestinal blood perfusion |
Overview of the intestinal microbiota
The human intestinal microbiota refers to the general name of the microorganisms inhabiting the human digestive tract, including bacteria, archaea, viruses, fungi and protists, etc., most of which are bacteria 21-25. There are direct or indirect interactions between them, and they form a complex interaction network with the host through direct contact, secreting proteins or metabolites, forming a dynamically balanced micro-ecosystem, which is closely related to human health and disease 26-29. Existing studies have shown that intestinal microbiota is closely related to the occurrence and development of diabetes, hypertension, cardiovascular disease, tumors, etc., because of its important role in human health, it is also called the human body's forgotten organs 9, 30-36. In addition, the intestinal microbiota is huge, and it contains about 100 times the number of genes in the human genome, so it is also known as the second genome of the human body 17, 37, 38. The main proportion of bacteria are Firmicutes and Bacteroides, which account for more than 90%. The relative abundance of Proteobacteria and Eubacteria is relatively low 39. The overall structure and function of the intestinal microbiota are stable for a period of time, but they are highly sensitive to changes in internal and external environments. Exogenous factors such as diet, exposure to bacterial infections or taking drugs can reduce the diversity of the intestinal microbiota; endogenous factors such as acute body fluid imbalance, chronic intestinal congestion or ischemia hypoxia, acid-base imbalance, gastrointestinal weakened exercise and nutritional deficiencies can potentially change the intestinal microbiota 40-44. Therefore, it is necessary and meaningful to summarize the changes of intestinal microbiota and its metabolites caused by intestinal I/R and the role of intestinal microbiota and its metabolites in intestinal I/R injury.
Studies have confirmed that intestinal microbiota and its metabolites play a variety of important roles and functions in our normal life activities, including nutrient absorption, growth and development, biological barrier, immune regulation, fat metabolism, anti-tumor, etc 45-47. The human intestinal microbiota is stimulated by large number of dietary nutrients to produce bioactive compounds such as bile acids, short-chain fatty acids (SCFA), ammonia, phenols, and endotoxins 18, 48-50. These microbial-derived metabolites are the communication medium between the microbe and the host, which is essential to maintain the normal physiological state of the host. As mentioned earlier, most of the human microbiota, especially the intestinal microbiota, cannot be isolated and cultured purely, which makes it difficult for traditional microbiological research methods to carry out research on the human microbiota. With the advent of metagenomics, important breakthroughs have been made in the study of intestinal microbiota. At present, the most direct and efficient method to detect the composition of the microbial community is to use amplicon sequences for target genes, such as 16S rRNA gene sequencing to identify the composition of bacterial communities, and Internally Transcribed Spacer (ITS) sequencing to identify the composition of fungal communities. However, amplicon sequencing cannot provide the genetic information carried by the flora. Currently, metagenomic sequencing is mainly used, that is, to sequence the genomes of various microorganisms including bacteria, fungi, viruses, etc., to identify the genetic information carried by the microbiota, and to analyze the functions of genes and possible metabolic pathways. In order to further examine the functions of these microbial groups, multi-omics research is more used. Metatranscriptome is used to detect the structure of functionally active microbial populations at the transcriptional level 51, 52; metaproteome is used to detect protein information translated by functionally active microbial populations 17, 53; metabolome is used to detect metabolite information produced by functionally active microbial populations 46, 54.
Changes in intestinal microbiota and metabolites induced by intestinal I/R
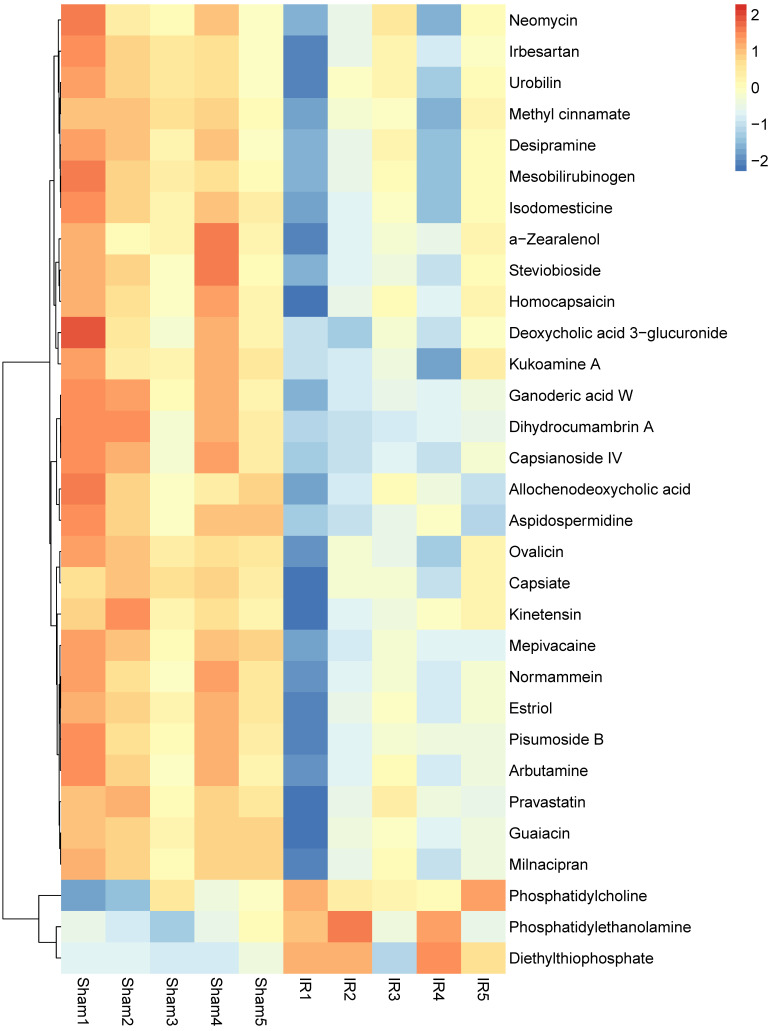
In recent years, the potential role of intestinal microbiota and its metabolites in the development of various human diseases has attracted considerable attention. We summarize the changes in the composition of intestinal microbiota and its metabolites in intestinal I/R injury (Table 2 and Figure 1). Wang et al. found that intestinal I/R affect the bacterial structure of the rat's colon. The colonic microbiota began to change as early as 1 hour after reperfusion, and reached the most obvious bacteria structure change at 6 hours after reperfusion. Among them, the abundance of Escherichia coli significantly increased at 1 hour and 3 hours of reperfusion; the content of Lactobacillus increased significantly after 6 hours of reperfusion; the abundance of Oral Prevotella was significantly increased from 1 hour to 12 hours after reperfusion 55. The research team also reported the imbalance of the ileal bacteria in rats caused by intestinal I/R. The ileal bacteria changed at the beginning of reperfusion, and the most obvious difference appeared at 12 hours of reperfusion, and then gradually returned to normal. Specific ecological disorders are characterized by the proliferation of Escherichia coli and the decrease of Lachnospiraceae and Lactobacillus, and changes in the ileal bacteria follow epithelial changes 56. Deng et al. used 16S rRNA combined with metabonomics to clarify the changes in intestinal bacteria and metabolites induced by mouse intestinal I/R. The study has shown that the composition of the colonic bacteria is significantly disordered after intestinal I/R in mice. The relative abundance of Firmicutes and Bacteroidetes increases significantly, while the relative abundance of Verrucomicrobia was reduced. At the species level, the relative abundance of Bacteroides vulgatus and Parabacteroides distasonis increased after I/R. Metabonomics results showed that the biosynthesis of secondary metabolites and polysaccharides after I/R and the genomic abundance of metabolic pathways are significantly impaired, and the content of metabolites of microbiota such as capsiate and pravastatin occurs change (Figure 2) 57.
Table 2.
Microbiota changes in intestinal I/R injury
| Species | Intestial site | Microbiota changes | Main results | Ref. |
|---|---|---|---|---|
| Rat | Colon |
Diversity ↑ Escherichia coli ↑ Lactobacillus ↑ Prevotella oralis ↑ |
Dysbiosis and tendency of recovery of colonic microbiota after damage and repair of the epithelium. | 55 |
| Rat | Ileum |
Diversity ↑ Escherichia coli ↑ Lactobacilli ↑ Prevotella ↑ Lactobacillus ↓ Lachnospiraceae ↓ Prevotella ↓ |
Earlier damage and repair in the ileal epithelium compared with later dysbiosis and recovery of ileal microbiome. | 56 |
| Mice | Cecum |
Diversity ↑ Bacteroides vulgatus ↑ Parabacteroides distasonis ↑ Verrucomicrobia ↓ Capsiate (One microbiota metabolite) ↓ |
Capsiate enhances Gpx4 expression and inhibits ferroptosis by activating TRPV1 in intestinal I/R injury | 57 |
Figure 1.
Intestinal I/R injury. I/R, ischemia reperfusion; ASA, American Society of Anesthesiologists; TLR, Toll-like receptors; MYD88, Myeloid differentiation factor 88; ROS, Reactive oxygen species; TNF, tumor necrosis factor.
Figure 2.
The changes of metabolites in intestinal I/R.
Intestinal I/R injury will not only involve local intestinal tissue injury, but also the secondary bacterial migration, inflammatory factors and endotoxin release after the intestinal barrier disorder will cause damage to the extraintestinal organs. Therefore, accurately revealing the changes of intestinal microbiota and its metabolites in intestinal I/R injury will not only help clarify the mechanism of intestinal and extraintestinal organ injury induced by intestinal I/R, but also seek the biomarker of intestinal microbiota and metabolites for predicting, diagnosing and preventing intestinal I/R-induced intestinal injury and extraintestinal organ injury; and help to find effective drugs for the treatment of intestinal and extraintestinal organ injury induced by intestinal I/R.
The overall effect of microbiota on intestinal I/R
While the intestinal I/R injury changes the composition of intestinal microbiota and its metabolites, on the contrary, studies have found that overall changes of microbiota, usually by antibiotics and fecal microbiota transplantation (FMT) treatment, also affect the outcome of intestinal I/R injury (Table 3).
Table 3.
The overall effect of microbiota on intestinal I/R
| Intestinal microbiota treatment | Species | Main effects | References | |
|---|---|---|---|---|
| Antibiotic treatment & germ-free mice | Antibiotic treatment | Mice | Depletion of gut commensal bacteria attenuated intestinal inflammation and injury following I/R. | 58 |
| Antibiotic treatment & germ-free mice | Mice | Gut microbiota suppressed NETing neutrophil hyperreactivity in mesenteric I/R injury, while ensuring immunovigilance by enhancing neutrophil recruitment. | 59 | |
| Rapamycin antibiotic treatment | Mice | Rapamycin treatment improved mortality following intestinal I/R via the inhibition of remote lung inflammation in mice. | 60 | |
| Ampicillin antibiotic treatment | Mice | Depletion of commensal bacteria by ampicillin resulted in inhibition of injury, neutrophil infiltration, and TNF-α expression. | 106 | |
| Antibiotic treatment | Mice | Commensal bacteria deletion improved mice survival in the early phase, but failed to improve the overall survival at 96 h after intestinal I/R. | 61 | |
| Germ-free mice | Mice | The lack of intestinal microbiota is accompanied by a state of active IL-10-mediated inflammatory hyporesponsiveness. | 107 | |
| FMT | Schaedler flora (ASF) | Mice | ASF-colonized mice showed reduced leukocyte adherence in I/R injury. | 62 |
Antibiotics
Intestinal dysbacteriosis and changes in metabolites are causal to each other in intestinal and extraintestinal organ injury induced by intestinal I/R. Because the role of specific bacteria strains and their metabolites in intestinal I/R-induced intestinal and extraintestinal organ injury has not been fully elucidated, and the intervention of specific bacteria strains still lacks effective measures; in addition, intestinal bacterial translocation may increase the level of inflammatory factors and endotoxins in the blood. Therefore, many factors suggest that the use of antibiotics may be an effective measure to reduce the intestinal and extraintestinal organ injury induced by intestinal I/R. Studies have found that using antibiotics to consume intestinal commensal bacteria reduces the expression of B cells, immunoglobulins (Igs) and Toll-like receptors (TLRs) in the intestine, inhibit complement activation, and reduce intestinal inflammation and injury after intestinal I/R 58. During intestinal I/R, symbiotic bacteria activate and attract inflammatory cells such as neutrophils, macrophages and lymphocytes, which cause intestinal inflammation and aggravate I/R-induced intestinal injury. Ascher et al. revealed that antibiotic treatment reduces lymphatic tissue and the deposition of immunoglobulin and complement, reduces intestinal inflammation, and improves intestinal integrity 59. Rapamycin treatment improved the survival rate of mice, and reduced of lung bacteria and increase in phagocytic activity after intestinal I/R 60. However, some studies have also reported that antibiotic treatment aggravated intestinal I/R injury. Ascher et al. found that antibiotic pretreatment reduces leukocyte adhesion, but increases NETing neutrophils, which is because neutrophil TLR4/TRIF signal-mediated intestinal I/R injury is not conducive to the recovery of NETosis 59. Zhang et al. uncovered that the commensal bacteria enhance the proliferation and migration of intestinal epithelial cells, and the absence of commensal bacteria eliminated the inflammatory response in the early stage, but failed to improve the overall survival after intestinal I/R 61. Therefore, the above-mentioned use of antibiotics in intestinal I/R injury is currently controversial, which may be related to the complexity of the intestinal microbiota, the angle of observation and the difference in time.
Fecal microbiota transplantation
Adjusting or improving disease-related microbiota imbalance or disorder through FMT has always been an effective direction and strategy for the treatment of diseases. Franziska Bayer et al. reported that transplanting altered Schaedler flora from C3H/HeNTac mice reduces the adhesion of leukocytes to the endothelium of mesenteric venules before and after intestinal I/R, and reduces the vascular inflammation induced by intestinal I/R 62. Therefore, FMT is a potentially effective treatment for intestinal I/R injury.
The role of intestinal microbiota and its metabolites in intestinal and extraintestinal organ injury induced by intestinal I/R
In recent years, more and more studies have found that intestinal microbiota and its metabolites play an important role in the treatment of intestinal and extraintestinal organ injury induced by intestinal I/R (Table 4).
Table 4.
The role of intestinal microbiota and its metabolites in intestinal and extraintestinal organ injury induced by intestinal I/R
| Intestinal microbiota treatment | Species | Main effects | References | |
|---|---|---|---|---|
| Bacterial strain | B. bifidum PRL2010 | Mice | B. bifidum PRL2010 reduced bacterial translocation, transcription levels of TNFalpha and IL-10 both in liver and kidneys and neutrophil recruitment in the lungs. | 63 |
| Bifidobacteria | Mice | Pretreatment of animals with bifidobacteria prevented I/R-induced bacterial translocation, reduced pro-inflammatory cytokine release, the levels of endotoxin, intestinal epithelial cell apoptosis, disruption of tight junction and increased the concentration of SCFA, resulting in recovered microbiota and mucosal integrity. | 64 | |
| Lactobacillus plantarum L2 | Rats | Lactobacillus plantarum prevented I/R induced bacterial translocation, reduced pro-inflammatory cytokine release, and intestinal epithelial cell apoptosis. | 65 | |
| Probiotic VSL#3 | Mice | VSL#3 reduced local tissue injury from I/R by down-regulating pro-inflammatory mediators and immune cell recruitment. | 66 | |
| Lactobacillus plantarum DSM 9843 and rose hip | Mice | Decreased MDA levels in cecum tissue and Enterobacteriaceae counts in cecun stool. | 68 | |
| Microbiota induced natural Abs | Mice | Microbiota induced natural Abs restored IgG deposition, leukocyte influx, NF-κB activation, and proinflammatory gene expression and concomitantly downregulated annexin-1 and IL-10 production. | 69 | |
| Pseudomonas aeruginosa | Mice | P. aeruginosa potentiates the lethal effect of IR in mice in part due to in vivo virulence activation of its epithelial barrier disrupting protein PA-IL. | 70 | |
| Escherichia coli | Mice | Escherichia coli augmented leukocyte adhesion to the ischemia-reperfusion injured endothelium of mesenteric venules. | 59 | |
| Bifidobacteria | Mice | Bifidobacteria prevented I/R-induced bacterial translocation, reduced pro-inflammatory cytokine release, the levels of endotoxin, intestinal epithelial cell apoptosis, disruption of TJ and increased the concentration of SCFA, resulting in recovered microbiota and mucosal integrity. | 64 | |
| Gut metabolites | SCFA (butyrate, propionate and acetate) | Rats | SCFA protect the distal small bowel mucosa and diminishes infiltration of neutrophils to the gut lamina propria in intestinal I/R. | 108 |
| SCFA (acetate) | Mice | Acetate treatment to WT mice prior to ischemia protected the intestine from I/R-induced damage protective, which were GPR43-independent. | 77 | |
| SCFA (butyrate) | Rats | Butyrate attenuated the inflammatory factor levels and leukocyte infiltration, maintained the intestinal barrier structures, increased the expression of tight junction proteins, and decreased endotoxin translocation. | 78 | |
| FXR agonist (obeticholic acid) | Rats | Obeticholic acid improved survival in a rodent model of intestinal I/R injury, preserved the gut barrier function and suppressed inflammation. | 85 | |
| FXR agonist (INT-747) | Mice | FXR activation enhanced intestinal epithelial cell tolerance to I/R by suppressing the inflammatory response and NF-κB pathway via cystathionine-γ-lyase mediation. | 86 | |
| Capsiate | Mice | Capsiate enhanced Gpx4 expression and inhibited ferroptosis by activating TRPV1 in intestinal I/R injury. | 57 | |
| Pravastatin | Mice | Pravastatin attenuated intestinal I/R injury by promoting the release of IL-13 from type II innate lymphoid cells via IL-33/ST2 signaling. | 98 | |
| Drugs | N-3 polyunsaturated fatty acids (PUFAs) | Rats | N-3 PUFAs protect the intestinal barrier by modifying intracellular I-FABP, activating the PPARg pathway, and then upregulating tight junction protein expression. | 109 |
| Others | Melatonin | Rats | Melatonin significantly reduced the intestinal IR injury and prevented bacterial translocation. | 100 |
| Two-day fasting | Rats | Two-day fasting increased the susceptibility of bacterial translocation to systemic organs in I/R injury. | 101 | |
| Hypoxic preconditioning (HPC) | Rats | Neutrophil priming by HPC protected against I/R-induced BT via direct antimicrobial activity by oxidative respiratory bursts and through promotion of epithelial barrier integrity for luminal confinement of enteric bacteria. | 102 | |
| Glucan and glutamine | Rats | Glucan and glutamine reduced bacterial translocation, intestinal damage, and cytokine levels after I/R. | 103 | |
| Beta-(1-3)-D-glucan | Rats | Beta-(1-3)-D-glucan modulated the production of pro-inflammatory and anti-inflammatory cytokines during bowel ischemia/reperfusion, and attenuated translocation of labelled bacteria. | 104 | |
Intestinal bacteria
With the use of probiotics, more and more intestinal bacteria strains were discovered to have the potential to treat intestinal and extraintestinal organ injury induced by intestinal I/R. Bifidobacterium bifidum PRL2010 was found to reduce intestinal I/R injury, inhibit neutrophil infiltration, especially at the lung level, moderately reduce oxidative stress, significantly reduce bacterial translocation, and down-regulate the transcription level of inflammatory factors in the liver and kidney 63. Wang et al. found that Bifidobacteria inhibits I/R-induced the apoptosis of intestinal epithelial cells, the destruction of tight junctions and bacterial translocation, and reduces the release of pro-inflammatory cytokines and endotoxins, and increases the concentration of SCFA, restores the microbial community structure and the integrity of the mucosa 64. Lactobacillus plantarum reduces intestinal I/R injury and inflammation, prevents intestinal epithelial cell apoptosis, and effectively prevents bacterial translocation 65. Long-term feeding of probiotic VSL#3 improves intestinal I/R injury by reducing leukocyte recruitment and pro-inflammatory cytokines 66. Lactobacillus murinus relieves intestinal I/R injury by activating TLR2 signal and promotes the release of Interleukin-10 (IL-10) from M2 macrophages, which can significantly prevent intestinal I/R injury. In addition, correlation analysis showed that clinically, the abundance of Lactobacillus murinus in the feces of patients undergoing cardiopulmonary bypass surgery is closely related to the degree of postoperative intestinal I/R injury 67. The combined use of L. plantarum DSM 9843 and rose hip reduces cecal I/R injury, the oxidative stress level of cecal tissue and the abundance of enterobacter 68. Serum transfer from young wild mice with bacteria reverses the sterile inflammatory injury caused by the intestinal I/R of sterile mice, which may be related to the restoration of IgG deposition, leukocyte influx, NF-κB activation and pro-inflammatory gene expression in inflamed tissues, while down-regulating the production of Annexin-1 and IL-10 69.
Not only that, some strains have also been discovered to promote intestinal I/R injury. The presence of Pseudomonas aeruginosa in the distal intestine may enhance the lethal effect of intestinal I/R on mice, partly due to the in vivo virulence activation of the epithelial barrier destroying protein PA 1 lectin 70. Single colonization of Escherichia coli strain JP313 enhanced the degree of leukocyte adhesion to the mesenteric venules damaged by I/R 59. What's more, certain strains, like E. coli, affect the level of injury to extra-intestinal organs induced by intestinal I/R. Wen et al. found that the gram-negative gut pathobiont E. coli translocated into livers, as a result of barrier disruption, and was present in the hepatic sinusoid and close to the endothelium, suggesting bacterial translocation-induced hepatic damage after intestinal I/R injury 71. Xu et al. found that brain ischemia rapidly induced intestinal ischemia and Enterobacteriaceae expansion, exacerbating brain infarction in turn by enhancing systemic inflammation 72.
Metabolites of intestinal microbiota
In addition to specific intestinal bacteria strains, intestinal microbiota metabolites have also been confirmed to play an important role in intestinal and extraintestinal organ injury induced by intestinal I/R.
Short-chain fatty acids
SCFA are the main bacterial metabolites produced by specific colonic anaerobes after fermenting dietary fiber and resistant starch, mainly including acetate, propionate and butyrate 73. SCFA are signaling molecules that mediate the interaction between diet, intestinal microbiota and the host, and play an important role in the body's immunity, metabolism, and endocrine 74. SCFA regulates intestinal and host metabolism by activating G protein-coupled cell surface receptors G protein-coupled receptor 41 (GPR41) and GPR43 75. These two SCFA receptors are not only expressed in the intestine, but also in human fat, muscle and liver tissues, indicating that SCFA can directly regulate substrate and energy metabolism in peripheral tissues. SCFA protects the distal small intestinal mucosa during intestinal I/R and reduces the infiltration of neutrophils into the intestinal lamina propria 76. Schofield et al. reported that acetate reduces intestinal I/R injury through GPR43 77. Qiao et al. revealed that butyrate administration reduces intestinal I/R injury, which is related to protecting the intestine tight junction barrier and inhibiting the infiltration of inflammatory cells in the intestinal mucosa 78. Therefore, SCFA plays an important role in maintaining intestinal barrier homeostasis and reducing mucosal inflammation during intestinal I/R.
Secondary bile acids
Bile acids play an important role in the body's lipid metabolism 79, 80. After a meal, primary bile acids enter the intestinal tract along with bile. In the upper part of the intestine, bile acids can regulate the digestion and absorption of lipids; primary bile acids can be converted to secondary bile acids, including deoxycholic acid and lithocholic acid, by removing hydroxyl groups under the action of intestinal bacteria in the lower part of the intestine (ileum and proximal colon), some of which will also be reabsorbed into liver. Bile acids have a great influence on the structural composition of the intestinal flora. Bile acids can combine with the phospholipids on the bacterial cell membrane to play a destructive effect, and resist bacterial adhesion and neutralize endotoxins. The high concentration of bound bile acid has a direct antibacterial effect 81. At the same time, the intestinal flora also plays a key role in the bile acid cycle. The bacteria in the intestine dissociate the conjugates of taurine, glycine, sulfate, etc. in the primary bile acid through bile salt hydrolase, change its chemical properties, and regulate the body's lipid metabolism. Bile acid receptors include nuclear receptors and membrane receptors, the former includes farnesoid X receptor (FXR), pregnane X receptor (PXR), and vitamin D receptor (VDR), while the latter refers to G protein-coupled bile acid receptor 1 (GPBAR1), also known as Takeda G-protein-coupled receptor 5 (TGR5) 82, 83. After intestinal I/R, the expression levels of FXR and PXR in the intestinal tissue and liver are significantly reduced, and IL-6 is one of the main reasons for the decreased expression of these receptors 84. Pretreatment with FXR agonist OCA improves the survival rate of caries animal intestinal I/R model, protects the intestinal barrier function and inhibits inflammation 85. The FXR agonist CSE reduces intestinal I/R injury by inhibiting the inflammatory response and the NF-κb pathway 86.
Tryptophan metabolites
In many aspects such as the intestinal barrier, intestinal immunity and endocrine function, and intestinal motility, intestinal tryptophan and its metabolites interact closely with the intestinal microbiota 87, 88. There are three metabolic pathways of tryptophan in the human intestine: first, in intestinal epithelial cells and immune cells, about 90% of tryptophan is metabolized to kynurenine by indoleamine 2,3-dioxygenase 1 (IDO1); secondly, in the intestinal lumen, the intestinal flora directly metabolizes about 4--6% tryptophan; thirdly, about 3% tryptophan in enterochromaffin cells is metabolized to serotonin via tryptophan hydroxylase 1, the serotonin (5-HT) pathway, which produces more than 90% of the body's 5-HT 89, 90. Intestinal tryptophan metabolites also affect various intestinal functions such as barrier, peristalsis, digestion and absorption, secretion, immunity, etc. There have been some studies reporting the role of 5-HT in intestinal I/R injury. Potentiation of 5-HT signaling is associated with mucosal protection from intestinal I/R injury without alterations in villus cell distribution, possibly via increased rates of enterocyte renewal 91. Knock-out of serotonin re-uptake transporters (SERT) or use of selective serotonin re-uptake inhibitors (SSRIs) significantly decreased mucosal injury and inflammation after intestinal I/R 91. However, sumatriptan may regulate inflammation by activating 5-HT1B/1D receptors, thereby inhibiting I/R-induced intestinal injury 92. Intestinal I/R induces continuous disturbance of liver microcirculation, leading to liver dysfunction. 5-HT may be one of the mediators of liver dysfunction after intestinal I/R 93. Therefore, the role of 5-HT in intestinal I/R injury is still controversial.
Furthermore, Aryl hydrocarbon receptors (AHR), a ligand-dependent transcription factor and widely expressed in immune, epithelial, endothelial and stromal cells in barrier tissues, mediate the regulation of intestinal immunity and barrier homeostasis by tryptophan metabolites. Jing et al. found that regulating AHR expression may reduce liver injury induced by intestinal I/R 94. AHR activation improves epithelial barrier dysfunction following intestinal I/R 95, 96. Furthermore, AHR has been proved to be key receptors that promote host defense and enhance disease tolerance of endotoxemia 97. Therefore, AHR is a potentially effective target for the treatment of intestinal I/R injury.
Other
In addition to several typical types of intestinal microbiota metabolites, other proven intestinal microbiota metabolites have also been observed to have a significant effect on intestinal I/R injury. We have uncovered that gut microbiota metabolite capsiate (CAT) enhances glutathione peroxidase 4 (GPX4) expression and inhibits ferroptosis by activating transient receptor potential cation channel subfamily V member 1 (TRPV1) in intestinal I/R injury, providing a potential avenue for the management of intestinal I/R injury. Pravastatin (PA), a metabolite of intestinal microbiota, promotes the release of IL-13 from type II innate lymphoid cells (ILC2s) through IL-33/ST2 signals. And IL-13 promotes the self-renewal of intestinal stem cells by activating Notch1 and Wnt signals, and ultimately reduces intestinal I/R injury. In addition, the PA content in the feces of patients before CPB surgery promotes patients to resist postoperative intestinal I/R injury 98. Tian et al. reported that polyunsaturated fatty acids (PUFAs), especially n-3 PUFAs, improve the function of the intestinal barrier by regulating the innate immunity after intestinal I/R 99. Sileri et al. confirmed that a single injection of exogenous Melatonin significantly reduces intestinal I/R injury and prevents bacterial translocation 100. Furthermore, fasting for two days without I/R injury will not cause mucosal changes and bacterial translocation, but in the case of intestinal I/R injury, fasting for two days will increase the susceptibility of bacterial translocation to the rat's systemic organs 101. Neutrophils induced by hypoxic pretreatment prevent I/R-induced bacterial translocation through antibacterial activity and promotion of epithelial barrier integrity 102. Glutamine and glucan reduce bacterial translocation and cytokine release levels, thereby reducing intestinal injury 103. Beta-(1-3)-D-glucan regulates the production of pro-inflammatory and anti-inflammatory cytokines in the intestinal I/R and reduces bacterial translocation 104. Anti-CINC antibody treatment reduces the infiltration of small intestinal neutrophils and the degree of mucosal damage, inhibits inflammation, reduces bacterial translocation, and protects the small intestine from I/R injury 105. Therefore, maintaining intestinal barrier homeostasis and reducing intestinal barrier permeability are effective measures to reduce bacterial translocation.
Conclusion
As emphasized in this review, intestinal I/R can cause imbalance of the intestinal microflora; the intestinal microbiome is also involved in the development of intestinal I/R and affects the level of injury to extraintestinal organs. Strengthening the intestinal barrier function is a good way to reduce the translocation of bacteria and bacterial metabolites and reduce the damage of extraintestinal organs induced by intestinal I/R. The intestinal microbiota includes bacteria, fungi, viruses, and archaea. Although more than 90% of the intestinal flora are bacteria, the role of viruses, fungi and archaea in intestinal I/R damage cannot be limited. Since the studies on the role of metabolites of intestinal microbiota cited in the review were mainly animal studies, more clinical studies are needed to confirm these findings. The detailed role of these microorganisms in the damage of intestinal I/R and its extraintestinal organs needs further study. It remains to be seen whether the composition and changes of the intestinal microbiome can be used as biomarkers of intestinal I/R and its extraintestinal organ damage. This review hopes that a more detailed understanding of the changes, effects and mechanisms of the intestinal microbiota in intestinal I/R injury will help to develop effective methods to reduce the incidence and mortality of intestinal I/R injury.
Acknowledgments
Funding
This work was supported by grants from Key Program of National Natural Science Foundation, Beijing, China (81730058 to Ke-Xuan Liu); China Postdoctoral Science Foundation, Beijing, China (2021M701611 to Fan Deng); President Foundation of Nanfang Hospital (2021C048 to Fan Deng).
Author Contributions
DF and Liu KX contributed equally to the writing of this manuscript; DF, Lin ZB, Hu JJ and Liu KX wrote the manuscript; Sun QS, MY, and ZY designed the illustrations; Sun QS,CY and Chen WT analyzed the data; DF, Hu JJ and Liu KX revised the manuscript.
Data Availability Statements
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
- I/R
ischemia/reperfusion
- ASA
American Society of Anaesthesiologists
- CPB
cardiopulmonary bypass
- ITS
Internally Transcribed Spacer
- Igs
immunoglobulins
- TLRs
Toll-like receptors
- FMT
Fecal microbiota transplantation
- IL-10
Interleukin-10
- SCFA
Short-chain fatty acids
- GPR41
G protein-coupled receptor 41
- FXR
farnesoid X receptor
- PXR
pregnane X receptor
- VDR
vitamin D receptor
- GPBAR1
G protein-coupled bile acid receptor 1
- TGR5
Takeda G-protein-coupled receptor 5
- IDO1
indoleamine 2,3-dioxygenase 1
- SERT
serotonin re-uptake transporters
- SSRIs
selective serotonin re-uptake inhibitors
- AHR
Aryl hydrocarbon receptors
- CAT
capsiate
- GPX4
glutathione peroxidase 4
- TRPV1
transient receptor potential cation channel subfamily V member 1
- PA
Pravastatin
- ILC2s
type II innate lymphoid cells
- PUFAs
polyunsaturated fatty acids
References
- 1.Ma Y, Zabell T, Creasy A, Yang X, Chatterjee V, Villalba N. et al. Gut Ischemia Reperfusion Injury Induces Lung Inflammation via Mesenteric Lymph-Mediated Neutrophil Activation. Front Immunol. 2020;11:586685. doi: 10.3389/fimmu.2020.586685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu M, Rowe JM, Fleming SD. Complement Initiation Varies by Sex in Intestinal Ischemia Reperfusion Injury. Front Immunol. 2021;12:649882. doi: 10.3389/fimmu.2021.649882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu J, Deng F, Zhao B, Lin Z, Sun Q, Yang X. et al. Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome. 2022;10:38. doi: 10.1186/s40168-022-01227-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu KX, Li YS, Huang WQ, Chen SQ, Wang ZX, Liu JX. et al. Immediate postconditioning during reperfusion attenuates intestinal injury. Intensive Care Med. 2009;35:933–42. doi: 10.1007/s00134-009-1428-1. [DOI] [PubMed] [Google Scholar]
- 5.Chassin C, Hempel C, Stockinger S, Dupont A, Kubler JF, Wedemeyer J. et al. MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol Med. 2012;4:1308–19. doi: 10.1002/emmm.201201298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia Y, Cui R, Wang C, Feng Y, Li Z, Tong Y. et al. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biology. 2020;32:101534. doi: 10.1016/j.redox.2020.101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Cao Y, Xiao J, Shang J, Tan Q, Ping F, Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. 2020. [DOI] [PMC free article] [PubMed]
- 8.Han SJ, Li H, Kim M, D'Agati V, Lee HT. Intestinal Toll-like receptor 9 deficiency leads to Paneth cell hyperplasia and exacerbates kidney, intestine, and liver injury after ischemia/reperfusion injury. Kidney Int. 2019;95:859–79. doi: 10.1016/j.kint.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Wu IW, Lin CY, Chang LC, Lee CC, Chiu CY, Hsu HJ. et al. Gut Microbiota as Diagnostic Tools for Mirroring Disease Progression and Circulating Nephrotoxin Levels in Chronic Kidney Disease: Discovery and Validation Study. International journal of biological sciences. 2020;16:420–34. doi: 10.7150/ijbs.37421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nayfach S, Paez-Espino D, Call L, Low SJ, Sberro H, Ivanova NN. et al. Metagenomic compendium of 189,680 DNA viruses from the human gut microbiome. Nat Microbiol. 2021;6:960–70. doi: 10.1038/s41564-021-00928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanahan F, Ghosh TS, O'Toole PW. The Healthy Microbiome-What Is the Definition of a Healthy Gut Microbiome? Gastroenterology. 2021;160:483–94. doi: 10.1053/j.gastro.2020.09.057. [DOI] [PubMed] [Google Scholar]
- 12.Kohn N, Szopinska-Tokov J, Llera Arenas A, Beckmann CF, Arias-Vasquez A, Aarts E. Multivariate associative patterns between the gut microbiota and large-scale brain network connectivity. Gut Microbes. 2021;13:2006586. doi: 10.1080/19490976.2021.2006586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaroque C, Chervy M, Gewirtz AT, Chassaing B. Social overcrowding impacts gut microbiota, promoting stress, inflammation, and dysglycemia. Gut Microbes. 2021;13:2000275. doi: 10.1080/19490976.2021.2000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Q, Huang JF, Cheng Y, Dai MY, Zhu WF, Yang XW. et al. Polyamine metabolism links gut microbiota and testicular dysfunction. Microbiome. 2021;9:224. doi: 10.1186/s40168-021-01157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy U, de Oliveira RS, Galvez EJC, Gronow A, Basic M, Perez LG. et al. Induction of IL-22-Producing CD4+ T Cells by Segmented Filamentous Bacteria Independent of Classical Th17 Cells. Front Immunol. 2021;12:671331. doi: 10.3389/fimmu.2021.671331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogatyrev SR, Rolando JC, Ismagilov RF. Self-reinoculation with fecal flora changes microbiota density and composition leading to an altered bile-acid profile in the mouse small intestine. Microbiome. 2020;8:19. doi: 10.1186/s40168-020-0785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Den Bossche T, Arntzen M, Becher D, Benndorf D, Eijsink VGH, Henry C. et al. The Metaproteomics Initiative: a coordinated approach for propelling the functional characterization of microbiomes. Microbiome. 2021;9:243. doi: 10.1186/s40168-021-01176-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregor A, Pignitter M, Trajanoski S, Auernigg-Haselmaier S, Somoza V, König J. et al. Microbial contribution to the caloric restriction-triggered regulation of the intestinal levels of glutathione transferases, taurine, and bile acid. Gut Microbes. 2021;13:1992236. doi: 10.1080/19490976.2021.1992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao T, Su CW, Ji Q, Chen CY, Wang R, Vijaya Kumar D. et al. Hyaluronan-induced alterations of the gut microbiome protects mice against Citrobacter rodentium infection and intestinal inflammation. Gut Microbes. 2021;13:1972757. doi: 10.1080/19490976.2021.1972757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarborough JE, Schumacher J, Kent KC, Heise CP, Greenberg CC. Associations of Specific Postoperative Complications With Outcomes After Elective Colon Resection: A Procedure-Targeted Approach Toward Surgical Quality Improvement. JAMA Surg. 2017;152:e164681. doi: 10.1001/jamasurg.2016.4681. [DOI] [PubMed] [Google Scholar]
- 21.Wu WH, Zegarra-Ruiz DF, Diehl GE. Intestinal Microbes in Autoimmune and Inflammatory Disease. Front Immunol. 2020;11:597966. doi: 10.3389/fimmu.2020.597966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong F, Perdew GH. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes. 2020;12:1859812. doi: 10.1080/19490976.2020.1859812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foley SE, Tuohy C, Dunford M, Grey MJ, De Luca H, Cawley C. et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis. Microbiome. 2021;9:183. doi: 10.1186/s40168-021-01137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hullar MAJ, Jenkins IC, Randolph TW, Curtis KR, Monroe KR, Ernst T. et al. Associations of the gut microbiome with hepatic adiposity in the Multiethnic Cohort Adiposity Phenotype Study. Gut Microbes. 2021;13:1965463. doi: 10.1080/19490976.2021.1965463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrizales-Sánchez AK, García-Cayuela T, Hernández-Brenes C, Senés-Guerrero C. Gut microbiota associations with metabolic syndrome and relevance of its study in pediatric subjects. Gut Microbes. 2021;13:1960135. doi: 10.1080/19490976.2021.1960135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markandey M, Bajaj A, Ilott NE, Kedia S, Travis S, Powrie F. et al. Gut microbiota: sculptors of the intestinal stem cell niche in health and inflammatory bowel disease. Gut Microbes. 2021;13:1990827. doi: 10.1080/19490976.2021.1990827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pothuraju R, Chaudhary S, Rachagani S, Kaur S, Roy HK, Bouvet M. et al. Mucins, gut microbiota, and postbiotics role in colorectal cancer. Gut Microbes. 2021;13:1974795. doi: 10.1080/19490976.2021.1974795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan A, Ding Z, Ishaq M, Bacha AS, Khan I, Hanif A. et al. Understanding the Effects of Gut Microbiota Dysbiosis on Nonalcoholic Fatty Liver Disease and the Possible Probiotics Role: Recent Updates. International journal of biological sciences. 2021;17:818–33. doi: 10.7150/ijbs.56214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruellman R, Llorente C. A Perspective Of Intestinal Immune-Microbiome Interactions In Alcohol-Associated Liver Disease. International journal of biological sciences. 2021;17:307–27. doi: 10.7150/ijbs.53589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mokhtari P, Metos J, Anandh Babu PV. Impact of type 1 diabetes on the composition and functional potential of gut microbiome in children and adolescents: possible mechanisms, current knowledge, and challenges. Gut Microbes. 2021;13:1–18. doi: 10.1080/19490976.2021.1926841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia WJ, Xu ML, Yu XJ, Du MM, Li XH, Yang T. et al. Antihypertensive effects of exercise involve reshaping of gut microbiota and improvement of gut-brain axis in spontaneously hypertensive rat. Gut Microbes. 2021;13:1–24. doi: 10.1080/19490976.2020.1854642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy CL, Barrett M, Pellanda P, Killeen S, McCourt M, Andrews E. et al. Mapping the colorectal tumor microbiota. Gut Microbes. 2021;13:1–10. doi: 10.1080/19490976.2021.1920657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Que Y, Cao M, He J, Zhang Q, Chen Q, Yan C. et al. Gut Bacterial Characteristics of Patients With Type 2 Diabetes Mellitus and the Application Potential. Front Immunol. 2021;12:722206. doi: 10.3389/fimmu.2021.722206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang M, Yang Y, He Q, Zhu P, Liu M, Xu J. et al. Intestinal Microbiota-A Promising Target for Antiviral Therapy? Front Immunol. 2021;12:676232. doi: 10.3389/fimmu.2021.676232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Lu D, Zhuo J, Lin Z, Yang M, Xu X. The Gut-liver Axis in Immune Remodeling: New insight into Liver Diseases. International journal of biological sciences. 2020;16:2357–66. doi: 10.7150/ijbs.46405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selma-Royo M, Calatayud Arroyo M, García-Mantrana I, Parra-Llorca A, Escuriet R, Martínez-Costa C. et al. Perinatal environment shapes microbiota colonization and infant growth: impact on host response and intestinal function. Microbiome. 2020;8:167. doi: 10.1186/s40168-020-00940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body. Protein Cell. 2010;1:718–25. doi: 10.1007/s13238-010-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li NN, Li W, Feng JX, Zhang WW, Zhang R, Du SH. et al. High alcohol-producing Klebsiella pneumoniae causes fatty liver disease through 2,3-butanediol fermentation pathway in vivo. Gut Microbes. 2021;13:1979883. doi: 10.1080/19490976.2021.1979883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okwelogu SI, Ikechebelu JI, Agbakoba NR, Anukam KC. Microbiome Compositions From Infertile Couples Seeking In vitro Fertilization, Using 16S rRNA Gene Sequencing Methods: Any Correlation to Clinical Outcomes? Front Cell Infect Microbiol. 2021;11:709372. doi: 10.3389/fcimb.2021.709372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X, Xia Y, He F, Zhu C, Ren W. Intestinal mycobiota in health and diseases: from a disrupted equilibrium to clinical opportunities. Microbiome. 2021;9:60. doi: 10.1186/s40168-021-01024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Bai Y, Pi Y, Gerrits WJJ, de Vries S, Shang L. et al. Xylan alleviates dietary fiber deprivation-induced dysbiosis by selectively promoting Bifidobacterium pseudocatenulatum in pigs. Microbiome. 2021;9:227. doi: 10.1186/s40168-021-01175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du Y, Gao Y, Zeng B, Fan X, Yang D, Yang M. Effects of anti-aging interventions on intestinal microbiota. Gut Microbes. 2021;13:1994835. doi: 10.1080/19490976.2021.1994835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chi L, Tu P, Ru H, Lu K. Studies of xenobiotic-induced gut microbiota dysbiosis: from correlation to mechanisms. Gut Microbes. 2021;13:1921912. doi: 10.1080/19490976.2021.1921912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu M, Alimujiang M, Hu L, Liu F, Bao Y, Yin J. Berberine alleviates lipid metabolism disorders via inhibition of mitochondrial complex I in gut and liver. International journal of biological sciences. 2021;17:1693–707. doi: 10.7150/ijbs.54604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jansma J, El Aidy S. Understanding the host-microbe interactions using metabolic modeling. Microbiome. 2021;9:16. doi: 10.1186/s40168-020-00955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan X, Chen B, Duan Z, Xia Z, Ding Y, Chen T. et al. Depression and anxiety in patients with active ulcerative colitis: crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes. 2021;13:1987779. doi: 10.1080/19490976.2021.1987779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wan X, Song M, Wang A, Zhao Y, Wei Z, Lu Y. Microbiome Crosstalk in Immunotherapy and Antiangiogenesis Therapy. Front Immunol. 2021;12:747914. doi: 10.3389/fimmu.2021.747914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome. 2021;9:140. doi: 10.1186/s40168-021-01101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nogal A, Valdes AM, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13:1–24. doi: 10.1080/19490976.2021.1897212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuang P, Li H, Jia W, Shou Q, Zhu Y, Mao L. et al. Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs axis in db/db mice. Microbiome. 2021;9:185. doi: 10.1186/s40168-021-01126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian Y, Gui W, Rimal B, Koo I, Smith PB, Nichols RG. et al. Metabolic impact of persistent organic pollutants on gut microbiota. Gut Microbes. 2020;12:1–16. doi: 10.1080/19490976.2020.1848209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiang S, Ye K, Li M, Ying J, Wang H, Han J. et al. Xylitol enhances synthesis of propionate in the colon via cross-feeding of gut microbiota. Microbiome. 2021;9:62. doi: 10.1186/s40168-021-01029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nalpas N, Hoyles L, Anselm V, Ganief T, Martinez-Gili L, Grau C. et al. An integrated workflow for enhanced taxonomic and functional coverage of the mouse fecal metaproteome. Gut Microbes. 2021;13:1994836. doi: 10.1080/19490976.2021.1994836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muller E, Algavi YM, Borenstein E. A meta-analysis study of the robustness and universality of gut microbiome-metabolome associations. Microbiome. 2021;9:203. doi: 10.1186/s40168-021-01149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan Wang QL, Chenyang Wang, Chun Tang, Jieshou Li. Dynamic Alteration of the Colonic Microbiota in Intestinal Ischemia-Reperfusion Injury. PLoS One. 2012;7(7):e42027. doi: 10.1371/journal.pone.0042027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang F, Li Q, He Q, Geng Y, Tang C, Wang C. et al. Temporal variations of the ileal microbiota in intestinal ischemia and reperfusion. Shock (Augusta, Ga) 2013;39:96–103. doi: 10.1097/SHK.0b013e318279265f. [DOI] [PubMed] [Google Scholar]
- 57.Deng F, Zhao BC, Yang X, Lin ZB, Sun QS, Wang YF. et al. The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes. 2021;13:1–21. doi: 10.1080/19490976.2021.1902719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kazuhisa Yoshiya, Peter H. Lapchak, To-Ha Thai, Lakshmi Kannan, Poonam Rani, Jurandir J. Dalle Lucca, et al. Depletion of gut commensal bacteria attenuates intestinal ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1020–G30. doi: 10.1152/ajpgi.00239.2011. [DOI] [PubMed] [Google Scholar]
- 59.Ascher S, Wilms E, Pontarollo G, Formes H, Bayer F, Muller M. et al. Gut Microbiota Restricts NETosis in Acute Mesenteric Ischemia-Reperfusion Injury. Arterioscler Thromb Vasc Biol. 2020;40:2279–92. doi: 10.1161/ATVBAHA.120.314491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iida T, Takagi T, Katada K, Mizushima K, Fukuda W, Kamada K. et al. Rapamycin Improves Mortality Following Intestinal Ischemia-Reperfusion via the Inhibition of Remote Lung Inflammation in Mice. Digestion. 2015;92:211–9. doi: 10.1159/000439300. [DOI] [PubMed] [Google Scholar]
- 61.Zhang HY, Wang F, Chen X, Meng X, Feng C, Feng JX. Dual roles of commensal bacteria after intestinal ischemia and reperfusion. Pediatr Surg Int. 2020;36:81–91. doi: 10.1007/s00383-019-04555-5. [DOI] [PubMed] [Google Scholar]
- 62.Bayer F, Ascher S, Kiouptsi K, Kittner JM, Stauber RH, Reinhardt C. Colonization with Altered Schaedler Flora Impacts Leukocyte Adhesion in Mesenteric Ischemia-Reperfusion Injury. Microorganisms. 2021. 9. [DOI] [PMC free article] [PubMed]
- 63.Duranti S, Vivo V, Zini I, Milani C, Mangifesta M, Anzalone R. et al. Bifidobacterium bifidum PRL2010 alleviates intestinal ischemia/reperfusion injury. PLoS One. 2018;13:e0202670. doi: 10.1371/journal.pone.0202670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H, Zhang W, Zuo L, Zhu W, Wang B, Li Q. et al. Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury. Br J Nutr. 2013;109:1990–8. doi: 10.1017/S0007114512004308. [DOI] [PubMed] [Google Scholar]
- 65.Wang B, Huang Q, Zhang W, Li N, Li J. Lactobacillus plantarum prevents bacterial translocation in rats following ischemia and reperfusion injury. Dig Dis Sci. 2011;56:3187–94. doi: 10.1007/s10620-011-1747-2. [DOI] [PubMed] [Google Scholar]
- 66.Salim SY, Young PY, Lukowski CM, Madsen KL, Sis B, Churchill TA. et al. VSL#3 probiotics provide protection against acute intestinal ischaemia/reperfusion injury. Benef Microbes. 2013;4:357–65. doi: 10.3920/BM2013.0026. [DOI] [PubMed] [Google Scholar]
- 67.Hu J, Deng F, Zhao B, Lin Z, Sun Q, Yang X, Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome. 2022. 10. [DOI] [PMC free article] [PubMed]
- 68.Hakansson A, Stene C, Mihaescu A, Molin G, Ahrne S, Thorlacius H. et al. Rose hip and Lactobacillus plantarum DSM 9843 reduce ischemia/reperfusion injury in the mouse colon. Dig Dis Sci. 2006;51:2094–101. doi: 10.1007/s10620-006-9170-9. [DOI] [PubMed] [Google Scholar]
- 69.Cisalpino D, Fagundes CT, Brito CB, Ascencao FR, Queiroz-Junior CM, Vieira AT. et al. Microbiota-Induced Antibodies Are Essential for Host Inflammatory Responsiveness to Sterile and Infectious Stimuli. Journal of immunology (Baltimore, Md: 1950) 2017;198:4096–106. doi: 10.4049/jimmunol.1600852. [DOI] [PubMed] [Google Scholar]
- 70.Fink D, Romanowski K, Valuckaite V, Babrowski T, Kim M, Matthews JB. et al. Pseudomonas aeruginosa potentiates the lethal effect of intestinal ischemia-reperfusion injury: the role of in vivo virulence activation. J Trauma. 2011;71:1575–82. doi: 10.1097/TA.0b013e31821cb7e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wen S, Li X, Ling Y, Chen S, Deng Q, Yang L. et al. HMGB1-associated necroptosis and Kupffer cells M1 polarization underlies remote liver injury induced by intestinal ischemia/reperfusion in rats. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2020;34:4384–402. doi: 10.1096/fj.201900817R. [DOI] [PubMed] [Google Scholar]
- 72.Xu K, Gao X, Xia G, Chen M, Zeng N, Wang S, Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut. 2021. [DOI] [PubMed]
- 73.Cholan PM, Han A, Woodie BR, Watchon M, Kurz AR, Laird AS. et al. Conserved anti-inflammatory effects and sensing of butyrate in zebrafish. Gut Microbes. 2020;12:1–11. doi: 10.1080/19490976.2020.1824563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun N, Meng F, Zhao J, Li X, Li R, Han J. et al. Aurka deficiency in the intestinal epithelium promotes age-induced obesity via propionate-mediated AKT activation. International journal of biological sciences. 2021;17:1302–14. doi: 10.7150/ijbs.56477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dai X, Guo Z, Chen D, Li L, Song X, Liu T. et al. Maternal sucralose intake alters gut microbiota of offspring and exacerbates hepatic steatosis in adulthood. Gut Microbes. 2020;11:1043–63. doi: 10.1080/19490976.2020.1738187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.José Eduardo de Aguilar-Nascimento, Alberto Bicudo Salomão, Rubens Jardim Nochi Jr, Mariana Nascimento, Neves. JdS. Intraluminal injection of short chain fatty acids diminishes intestinal mucosa injury in experimental ischemia-reperfusion. Acta Cir Bras. 2006;21(1):21–5. doi: 10.1590/s0102-86502006000100006. [DOI] [PubMed] [Google Scholar]
- 77.Schofield ZV, Wu MCL, Hansbro PM, Cooper MA, Woodruff TM. Acetate protects against intestinal ischemia-reperfusion injury independent of its cognate free fatty acid 2 receptor. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2020;34:10418–30. doi: 10.1096/fj.202000960R. [DOI] [PubMed] [Google Scholar]
- 78.Qiao Y, Qian J, Lu Q, Tian Y, Chen Q, Zhang Y. Protective effects of butyrate on intestinal ischemia-reperfusion injury in rats. The Journal of surgical research. 2015;197:324–30. doi: 10.1016/j.jss.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 79.Streidl T, Karkossa I, Segura Munoz RR, Eberl C, Zaufel A, Plagge J. et al. The gut bacterium Extibacter muris produces secondary bile acids and influences liver physiology in gnotobiotic mice. Gut Microbes. 2021;13:1–21. doi: 10.1080/19490976.2020.1854008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumari A, Pal Pathak D, Asthana S. Bile acids mediated potential functional interaction between FXR and FATP5 in the regulation of Lipid Metabolism. International journal of biological sciences. 2020;16:2308–22. doi: 10.7150/ijbs.44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian Y, Gui W, Koo I, Smith PB, Allman EL, Nichols RG. et al. The microbiome modulating activity of bile acids. Gut Microbes. 2020;11:979–96. doi: 10.1080/19490976.2020.1732268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiang J, Zhang Z, Xie H, Zhang C, Bai Y, Cao H. et al. Effect of different bile acids on the intestine through enterohepatic circulation based on FXR. Gut Microbes. 2021;13:1949095. doi: 10.1080/19490976.2021.1949095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hosseinkhani F, Heinken A, Thiele I, Lindenburg PW, Harms AC, Hankemeier T. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes. 2021;13:1–22. doi: 10.1080/19490976.2021.1882927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogura J, Terada Y, Tsujimoto T, Koizumi T, Kuwayama K, Maruyama H. et al. The decrease in farnesoid X receptor, pregnane X receptor and constitutive androstane receptor in the liver after intestinal ischemia-reperfusion. J Pharm Pharm Sci. 2012;15(5):616–31. doi: 10.18433/j38c88. [DOI] [PubMed] [Google Scholar]
- 85.Ceulemans LJ, Verbeke L, Decuypere JP, Farre R, De Hertogh G, Lenaerts K. et al. Farnesoid X Receptor Activation Attenuates Intestinal Ischemia Reperfusion Injury in Rats. PLoS One. 2017;12:e0169331. doi: 10.1371/journal.pone.0169331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Li S, Chen M, Liu J, Dong R, Wang H. et al. Activation of the Nuclear Receptor Fxr Improves Intestinal Cell Tolerance to Ischemia-Reperfusion Injury. Shock (Augusta, Ga) 2018;50:316–23. doi: 10.1097/SHK.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 87.Dong F, Hao F, Murray IA, Smith PB, Koo I, Tindall AM. et al. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes. 2020;12:1–24. doi: 10.1080/19490976.2020.1788899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown J, Robusto B, Morel L. Intestinal Dysbiosis and Tryptophan Metabolism in Autoimmunity. Front Immunol. 2020;11:1741. doi: 10.3389/fimmu.2020.01741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng Y, Zhou M, Wang J, Yao J, Yu J, Liu W. et al. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes. 2021;13:1–16. doi: 10.1080/19490976.2020.1869501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim M, Tomek P. Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO. Front Immunol. 2021;12:636081. doi: 10.3389/fimmu.2021.636081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tackett JJ, Gandotra N, Bamdad MC, Muise ED, Cowles RA. Potentiation of serotonin signaling protects against intestinal ischemia and reperfusion injury in mice. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2018: e13498. [DOI] [PubMed]
- 92.Gharishvandi F, Abdollahi A, Shafaroodi H, Mohammad Jafari R, Pasalar P, Dehpour AR. Involvement of 5-HT1B/1D receptors in the inflammatory response and oxidative stress in intestinal ischemia/reperfusion in rats. Eur J Pharmacol. 2020;882:173265. doi: 10.1016/j.ejphar.2020.173265. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura N, Hamada N, Murata R, Kobayashi A, Ishizaki N, Taira A. et al. Contribution of serotonin to liver injury following canine small-intestinal ischemia and reperfusion. The Journal of surgical research. 2001;99:17–24. doi: 10.1006/jsre.2001.6119. [DOI] [PubMed] [Google Scholar]
- 94.Jing H, Shen G, Wang G, Zhang F, Li Y, Luo F. et al. MG132 alleviates liver injury induced by intestinal ischemia/reperfusion in rats: involvement of the AhR and NFkappaB pathways. The Journal of surgical research. 2012;176:63–73. doi: 10.1016/j.jss.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Z, Pu A, Yu M, Xiao W, Sun L, Cai Y. et al. Aryl hydrocarbon receptor activation modulates gammadelta intestinal intraepithelial lymphocytes and protects against ischemia/reperfusion injury in the murine small intestine. Molecular medicine reports. 2019;19:1840–8. doi: 10.3892/mmr.2019.9823. [DOI] [PubMed] [Google Scholar]
- 96.Liu Z, Li L, Chen W, Wang Q, Xiao W, Ma Y, Aryl hydrocarbon receptor activation maintained the intestinal epithelial barrier function through Notch1 dependent signaling pathway. International journal of molecular medicine. 2017. [DOI] [PMC free article] [PubMed]
- 97.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C. et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–90. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deng F, Hu JJ, Yang X, Sun QS, Lin ZB, Zhao BC. et al. Gut Microbial Metabolite Pravastatin Attenuates Intestinal Ischemia/Reperfusion Injury Through Promoting IL-13 Release From Type II Innate Lymphoid Cells via IL-33/ST2 Signaling. Front Immunol. 2021;12:704836. doi: 10.3389/fimmu.2021.704836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian F, Gao X, Zhang L, Wang X, Wan X, Jiang T, Effects of n-3 PUFAs on Intestinal Mucosa Innate Immunity and Intestinal Microbiota in Mice after Hemorrhagic Shock Resuscitation. Nutrients. 2016. 8. [DOI] [PMC free article] [PubMed]
- 100.Sileri P, Sica GS, Gentileschi P, Venza M, Benavoli D, Jarzembowski T. et al. Melatonin reduces bacterial translocation after intestinal ischemia-reperfusion injury. Transplant Proc. 2004;36:2944–6. doi: 10.1016/j.transproceed.2004.10.085. [DOI] [PubMed] [Google Scholar]
- 101.Park JJ, Chung KY, Nam YS. Two-day fasting prior to intestinal ischemia-reperfusion injury on bacterial translocation in rats. J Invest Surg. 2011;24:262–6. doi: 10.3109/08941939.2011.589886. [DOI] [PubMed] [Google Scholar]
- 102.Lu YZ, Wu CC, Huang YC, Huang CY, Yang CY, Lee TC. et al. Neutrophil priming by hypoxic preconditioning protects against epithelial barrier damage and enteric bacterial translocation in intestinal ischemia/reperfusion. Lab Invest. 2012;92:783–96. doi: 10.1038/labinvest.2012.11. [DOI] [PubMed] [Google Scholar]
- 103.Medeiros AC, Chacon DA, Sales VS, Egito ES, Brandao-Neto J, Pinheiro LA. et al. Glucan and glutamine reduce bacterial translocation in rats subjected to intestinal ischemia-reperfusion. J Invest Surg. 2006;19:39–46. doi: 10.1080/08941930500444453. [DOI] [PubMed] [Google Scholar]
- 104.Araújo-Filho I, Rêgo AC, Pinheiro LA, Azevedo IM, Medeiros VB, Brandão-Neto J. et al. Prevention of bacterial translocation using beta-(1-3)-D-glucan in small bowel ischemia and reperfusion in rats. Acta Cir Bras. 2006;21:18–22. doi: 10.1590/s0102-86502006001000005. [DOI] [PubMed] [Google Scholar]
- 105.Kaneko H, Tamura A, Ishii T, Maeda T, Katagiri T, Ishii J. et al. Bacterial translocation in small intestinal ischemia-reperfusion injury and efficacy of Anti-CINC antibody treatment. Eur Surg Res. 2007;39:153–9. doi: 10.1159/000100328. [DOI] [PubMed] [Google Scholar]
- 106.Watanabe T, Kobata A, Tanigawa T, Nadatani Y, Yamagami H, Watanabe K. et al. Activation of the MyD88 signaling pathway inhibits ischemia-reperfusion injury in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303:G324–34. doi: 10.1152/ajpgi.00075.2012. [DOI] [PubMed] [Google Scholar]
- 107.Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ. et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. Journal of immunology (Baltimore, Md: 1950) 2004;173:4137–46. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- 108.Aguilar-Nascimento JE, Salomão AB, Nochi RJ Jr, Nascimento M, S NJ. Intraluminal injection of short chain fatty acids diminishes intestinal mucosa injury in experimental ischemia-reperfusion. Acta Cir Bras. 2006;21(1):21–5. doi: 10.1590/s0102-86502006000100006. [DOI] [PubMed] [Google Scholar]
- 109.Wang X, Pan L, Lu J, Li N, Li J. N-3 PUFAs attenuate ischemia/reperfusion induced intestinal barrier injury by activating I-FABP-PPARgamma pathway. Clin Nutr. 2012;31:951–7. doi: 10.1016/j.clnu.2012.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.