Abstract
Acute encephalitis is a syndromic diagnosis. In the last two decades, a unique clinico-radiological entity, named acute encephalopathy with biphasic seizures and late restricted diffusion (AESD), has been reported in children from Asia. It is characterised by an acute febrile illness with seizures and encephalopathy, with some initial improvement followed by a second flurry of seizures and deep encephalopathy, 3–4 days later. MRI may show a pattern of ‘bright tree appearance’. An aetiological agent may not always be identified but an infectious trigger is proposed. Immunomodulatory therapy has been tried with variable results. The prognosis is variable, and children are usually left with neurological sequelae including epilepsy and cognitive impairment. We describe a female infant who presented with the typical clinico-radiological syndrome of AESD and human bocavirus was identified in the stool. She received steroids and antiepileptic drugs. She has persistent cognitive impairment at follow-up but remained seizure free.
Keywords: Neuroimaging, Epilepsy and seizures, Neonatal and paediatric intensive care
Background
Acute encephalopathy with biphasic seizures and late restricted diffusion (AESD) is a parainfectious entity, characterised by febrile onset of seizures followed by a flurry of subsequent seizures, with an intervening seizure-free interval (biphasic seizures).1 In almost half of the cases, viral infections are identified as triggers.2 Children usually remain encephalopathic for a prolonged period with variable recovery in sensorium and neurological function. AESD is a rare disorder, probably underreported due to lack of awareness. AESD is reported mainly from East Asian countries, with limited literature from India.2 3 Here, we report an infant with AESD associated with human bocavirus (HBoV) infection.
Case presentation
A female infant was brought to the paediatric emergency department of a referring hospital with a history of fever and diarrhoea for 3 days and 3 episodes of generalised tonic-clonic seizures (GTCS) followed by encephalopathy. A syndromic diagnosis of acute febrile encephalopathy was considered, and she was treated with ceftriaxone, acyclovir, and phenytoin. Over the next 2 days, there was no recurrence of seizures, her encephalopathy abated, and she started accepting direct breast feeds. On day 6 of illness, there was recurrence of multiple episodes of GTCS without regaining consciousness in between seizures, for which she received levetiracetam and continuous midazolam infusion and was referred to our centre for further management.
At triage, she had an unstable airway, bradypnoea and encephalopathy with a Glasgow Coma Scale of 8, normal equal-sized pupils with normal reaction to light and no clinical seizures. She had generalised appendicular hypertonia, normal deep tendon reflexes, and no signs of meningeal irritation. The rest of the systemic examination was normal.
Investigations
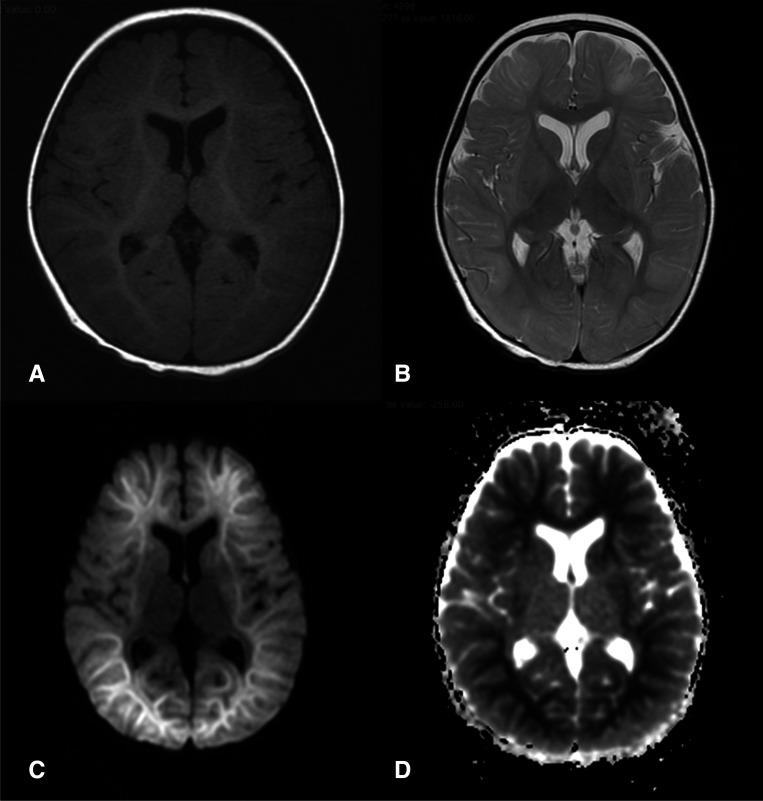
Blood glucose, renal and liver function tests, complete blood counts and C reactive proteins were normal. Cerebrospinal fluid (CSF) examination revealed lymphocytic pleocytosis (22 cells/mm3 with 96% lymphocytes), normal glucose and proteins, and sterile culture. MRI of the brain, done on day 5 of illness, revealed bilateral diffusion restriction in subcortical and deep white matter in the frontal, temporal and parieto-occipital areas (figure 1).
Figure 1.
MRI of the child showing normal T1 (A) and T2 (B) images. Diffusion weighted images (C), show restricted diffusion (appearing hyperintense) in bilateral frontal, parietal and occipital subcortical areas (‘bright tree appearance’) while sparing the basal ganglia. The same areas appear hypointense in apparent diffusion coefficient (ADC) images (D). This pattern of involvement with the associated clinical findings were suggestive of a diagnosis of acute encephalopathy with biphasic seizures and late restricted diffusion (AESD).
Stool sample was positive for HBoV DNA and negative for rotavirus, enterovirus, norovirus and astrovirus RNA by multiplex PCR assay. CSF HSV DNA PCR was negative. However, we could not perform extended viral panel on CSF and respiratory samples.
Differential diagnosis
Based on the clinical features and radiological investigations, the possibilities of rotaviral encephalitis or AESD were considered.
Due to the biphasic seizures, typical febrile presentation and deep encephalopathy, AESD was considered more likely.
HBoV could be the cause of preceding acute gastroenteritis like illness, which probably triggered the AESD in this child. However, we could suggest only an association of AESD with HBoV infection rather than causation.
Treatment
At presentation to our centre (day 6 of illness), she was intubated for airway protection and started on mechanical ventilation and continued on intravenous ceftriaxone and acyclovir.
Her seizures were controlled on three antiepileptic drugs, that is, phenytoin (8 mg/kg/day), levetiracetam (60 mg/kg/day) and midazolam infusion (maximum up to 12 microgram/kg/min).
She received pulse dose of intravenous methylprednisolone (30 mg/kg/day) (started on day 4 of illness in the referring hospital) for a total of 3 days.
On day 7 of illness, ceftriaxone and acyclovir were stopped as the CSF culture was sterile and HSV DNA PCR was negative.
On day 7 of illness, midazolam infusion was tapered and stopped following a 12-hour seizure-free interval and phenytoin and levetiracetam were continued.
On day 10 of illness, she was extubated and discharged (back-referred to the referring hospital) on oral phenytoin (8 mg/kg/day) and levetiracetam (60 mg/kg/day).
Outcome and follow-up
At the time of discharge, she was afebrile, had stable vitals and spontaneous movements of all limbs. However, she was unable to recognise her parents or respond to surroundings. As she was not able to feed herself, she was discharged on nasogastric feeding.
At 2 months follow-up, she had started indicating hunger by crying, responding to the mother’s voice and was able to take direct breast feeds. She remains seizure free on two antiseizure medications. However, there was no visual fixation and is due for visual evoked potential assessment.
Discussion
AESD is a parainfectious phenomenon under the umbrella term of acute leucoencephalopathy with restricted diffusion (ALERD) in children.4 The description of AESD is mainly in the form of retrospective case series from Southeast Asian countries. A causative agent is not always identified, but viruses such as influenza, rotavirus, adenovirus, human herpes virus 6, enterovirus and coxsackie virus A6; and bacteria such as Mycoplasma and Escherichia coli O157:H7 have been implicated.5 It has also been reported as a complication of meningoencephalitis caused by Streptococcus pneumoniae.6 7 Kamate and colleagues reported 16 cases of ALERD from India, out of which only one had a typical AESD like presentation.3 A detailed aetiological workup was not carried out in this case series. The exact pathophysiology of AESD is yet to be elucidated, but the possible proposed mechanism is immune mediated excitotoxic injury leading to neuronal death.4 AESD has typical MRI findings which appear at the time of second flurry of seizures (biphasic illness), characterised by diffusion restriction in the subcortical areas of bilateral hemispheres while sparing the basal ganglia (as ‘bright tree appearance’). Diffuse and central-sparing patterns have also been described.8 Okumura and colleagues tried to compare the outcomes between these radiologically distinct subtypes and found that children with diffuse involvement may have a severe disease and worse outcomes.9 The index case had a central sparing pattern as described. Hypoxic ischaemic encephalopathy or non-accidental trauma might present with similar imaging, which may be excluded by history and examination. A similar symmetrical but patchy involvement is also described in rotaviral encephalitis, which was considered in the differential diagnosis of the index case.10 11
HBoV, a member of the parvoviridae family, has been associated with respiratory tract infections.12 Extrapulmonary infections have also been reported with HBoV including gastroenteritis and encephalitis.13 14 The isolation of HBoV from stool specimens in the index case is not sufficient to establish causation but adds to the list of possible infectious triggers of AESD. Thus, inclusion of HBoV testing in viral panels is warranted while evaluating for the triggers for AESD. In addition, initiation of multicentric studies would be ideal to establish the aetiology.
As the pathogenesis appears to be immune mediated, immunomodulatory therapies such as intravenous methyl-prednisolone and intravenous immunoglobulins have been tried in AESD but there is no strong evidence from randomised trials.9 These are usually started only after ruling out infectious aetiology. Other agents like cyclosporine A, dextromethorphan and N-methyl-D-aspartate receptor antagonists have been tried with variable results.15 There are no established guidelines to use any of these agents and prospective randomised trials are the need of the hour.
Prognosis of AESD is highly variable. Only 2 children out of the 16 reported from India had neurologically intact survival, indicating that this is an exception rather than the norm.3 Diffuse pattern of involvement on neuroimaging, intractable seizures and development of post-encephalitis epilepsy (PEE) are poor prognostic markers.
A large proportion of children will continue to have cognitive impairment, speech impairment, and behavioural problems. These residual neurological issues have been reported in up to 87.5% of children in the Indian series and 55% in the East Asian studies.3 9 PEE, characterised by the onset of a new variety of seizures or recurrence of similar seizures, more than 3 months after the initial presentation, is seen in 23%–44% of cases.5 The index child did not have recurrence of seizures or new onset of seizures, but she is under close follow-up to monitor for the PEE and other neurological sequalae.
Learning points.
Acute encephalopathy with biphasic seizures and late-restricted diffusion (AESD) is a recently described parainfectious entity which has characteristic clinico-radiological features.
A typical biphasic presentation of febrile onset seizures with typical MRI findings can clinch the diagnosis.
Viral infections are the most common triggers for AESD.
Human bocavirus should be included in viral panels for AESD.
Prognosis is not always favourable and children may develop epilepsy, cognitive impairment, speech impairment and behavioural problems.
Acknowledgments
Authors thank the Regional Virus Research and Diagnostic Laboratory (RVRDL), Department of Health Research (DHR), Ministry of Health and Family Welfare (MoHFW), Government of India. Authors also acknowledge the role of Dr. Sathwik who was involved in the clinical management of the child.
Footnotes
Twitter: @MSRandhawa192, @Sureshangurana
Contributors: MSR and SKA conceptualised and wrote the manuscript. TSR, MSR and SKA were involved in clinical care of the patient. RKR provided laboratory support for the virological testing. All authors approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1.Tada H, Takanashi J-i, Terada H, et al. Severe form of acute influenza encephalopathy with biphasic seizures and late reduced diffusion. Neuropediatrics 2008;39:134–6. 10.1055/s-2008-1081459 [DOI] [PubMed] [Google Scholar]
- 2.Yadav SS, Lawande MA, Kulkarni SD, et al. Acute encephalopathy with biphasic seizures and late reduced diffusion. J Pediatr Neurosci 2013;8:64–6. 10.4103/1817-1745.111429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamate M, Detroja M, Hattiholi V. Acute Leucoencephalopathy with Restricted Diffusion in Children - A case series. Neurol India 2021;69:466. 10.4103/0028-3886.314577 [DOI] [PubMed] [Google Scholar]
- 4.Kamate M. Acute leukoencephalopathy with restricted diffusion. Indian J Crit Care Med 2018;22:519–23. 10.4103/ijccm.IJCCM_139_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito Y, Natsume J, Kidokoro H, et al. Seizure characteristics of epilepsy in childhood after acute encephalopathy with biphasic seizures and late reduced diffusion. Epilepsia 2015;56:1286–93. 10.1111/epi.13068 [DOI] [PubMed] [Google Scholar]
- 6.Kuwata S, Senzaki H, Urushibara Y, et al. A case of acute encephalopathy with biphasic seizures and late reduced diffusion associated with Streptococcus pneumoniae meningoencephalitis. Brain Dev 2012;34:529–32. 10.1016/j.braindev.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi H, Tanaka T, Maruyama A, et al. Septic encephalopathy characterized by acute encephalopathy with biphasic seizures and late reduced diffusion and early nonconvulsive status epilepticus. Case Rep Neurol Med 2016;2016:1–5. 10.1155/2016/7528238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranjan RS, Arya G, Yadav VK. Acute encephalopathy with biphasic seizures and late reduced diffusion (central sparing type)-MRI and MR spectroscopy findings. Indian J Radiol Imaging 2019;29:426–30. 10.4103/ijri.IJRI_235_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okumura A, Kidokoro H, Tsuji T, et al. Differences of clinical manifestations according to the patterns of brain lesions in acute encephalopathy with reduced diffusion in the bilateral hemispheres. AJNR Am J Neuroradiol 2009;30:825–30. 10.3174/ajnr.A1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamate M, Naik S, Torse S, et al. Neonatal rotaviral encephalitis. Indian J Pediatr 2017;84:865–6. 10.1007/s12098-017-2385-2 [DOI] [PubMed] [Google Scholar]
- 11.Mori T, Morii M, Kuroiwa Y, et al. Rotavirus encephalitis and cerebellitis with reversible magnetic resonance signal changes. Pediatr Int 2011;53:252–5. 10.1111/j.1442-200X.2010.03221.x [DOI] [PubMed] [Google Scholar]
- 12.Allander T. Human bocavirus. J Clin Virol 2008;41:29–33. 10.1016/j.jcv.2007.10.026 [DOI] [PubMed] [Google Scholar]
- 13.Akturk H, Sık G, Salman N, et al. Atypical presentation of human bocavirus: severe respiratory tract infection complicated with encephalopathy. J Med Virol 2015;87:1831–8. 10.1002/jmv.24263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albuquerque MCM, Rocha LN, Benati FJ, et al. Human bocavirus infection in children with gastroenteritis, Brazil. Emerg Infect Dis 2007;13:1756–8. 10.3201/eid1311.070671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo M, Maeda T, Ono N, et al. Efficacy of dextromethorphan and cyclosporine A for acute encephalopathy. Pediatr Neurol 2013;48:200–5. 10.1016/j.pediatrneurol.2012.11.003 [DOI] [PubMed] [Google Scholar]