Abstract
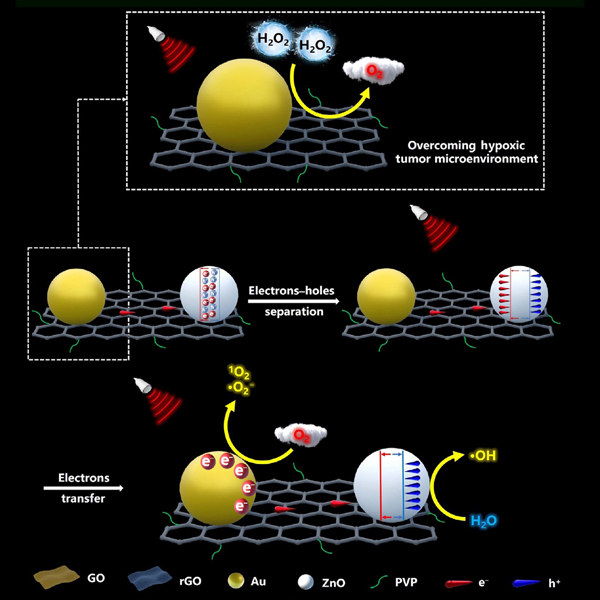
Sonodynamic therapy has attracted widespread attention for cancer treatment because of its noninvasiveness and high tissue-penetration ability. Generally, ultrasound irradiation of sonosensitizers produces separated electrons (e−) and holes (h+), which inhibits cancer by producing reactive oxygen species (ROS). However, the separated electrons (e−) and holes (h+) could easily recombine, lowering the yield of ROS and hindering the application of sonodynamic therapy (SDT). Herein, we present a highly efficient sonosensitizer system for enhanced sonodynamic therapy built on reduced graphene oxide (rGO) nanosheets, bridged ZnO and Au nanoparticles, coated with polyvinyl pyrrolidone (PVP). The ultrasound irradiation activates ZnO nanoparticles to generate separated electron-hole (e−−h+) pairs, and the rGO nanosheets facilitate electron transfer from ZnO to Au nanoparticles because of the narrow band gap of rGO, which could efficiently restrain the recombination of the e−−h+ pairs, thereby significantly augmenting the production of ROS to kill cancer cells, such as U373MG, HeLa, and CT26 cells. Moreover, rGO nanosheets integrated with Au nanoparticles could catalyze the endogenous decomposition of H2O2 into O2, which can alleviate hypoxic tumor microenvironment (TME). Therefore, the rational design of Au−rGO−ZnO@PVP nanomaterials can not only improve the efficiency of sonodynamic therapy, but also mitigate the hypoxic tumor microenvironment, which would provide a new perspective in the development of efficient sonosensitizers.
Electronic Supplementary Material
Supplementary material (the UV-vis-NIR absorption spectra of the DPBF and the RhB, biological effect assessment of the Au−rGO−ZnO@PVP, and the inhibition rate of tumor under different treatments during the animal study) is available in the online version of this article at 10.1007/s12274-022-4599-5.
Keywords: sonodynamic therapy, reactive oxygen species, reduced graphene oxide, tumor
Electronic Supplementary Material
Integrating Au and ZnO nanoparticles onto graphene nanosheet for enhanced sonodynamic therapy
Acknowledgements
We acknowledge founding support from the National Key R&D program of China (Nos. 2017YFA0205600 and 2020YFA0710700), the National Science Funds for Distinguished Yong Scholars (No. 51625305), the National Natural Science Foundation of China (Nos. 52131305, 52073269, 51873202, 22131010, 22101275, 81603339, 81602344, and 31870993), and the Fundamental Research Funds for the Central Universities (Nos. YD2060002016 and WK9110000005).
Contributor Information
Guang Chen, Email: cg1995@mail.ustc.edu.cn.
Ye-Zi You, Email: yzyou@ustc.edu.cn.
References
- [1].Grivennikov S I, Greten F R, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou Z J, Song J B, Nie L M, Chen X Y. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016;45:6597–6626. doi: 10.1039/C6CS00271D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu J T, Zhang L, Lei J P, Shen H, Ju H X. Multifunctional metal-organic framework nanoprobe for cathepsin b-activated cancer cell imaging and chemo-photodynamic therapy. ACS Appl. Mater. Interfaces. 2017;9:2150–2158. doi: 10.1021/acsami.6b14446. [DOI] [PubMed] [Google Scholar]
- [4].Zhu Y, Shi H D, Li T W, Yu J N, Guo Z X, Cheng J J, Liu Y Z. A dual functional nanoreactor for synergistic starvation and photodynamic therapy. ACS Appl. Mater. Interfaces. 2020;12:18309–18318. doi: 10.1021/acsami.0c01039. [DOI] [PubMed] [Google Scholar]
- [5].He G L, Xu N, Ge H Y, Lu Y, Wang R, Wang H X, Du J J, Fan J L, Sun W, Peng X J. Red-light-responsive Ru complex photosensitizer for lysosome localization photodynamic therapy. ACS Appl. Mater. Interfaces. 2021;13:19572–19580. doi: 10.1021/acsami.0c22551. [DOI] [PubMed] [Google Scholar]
- [6].Zhong S, Chen C, Yang G L, Zhu Y C, Cao H L, Xu B J, Luo Y Q, Gao Y, Zhang W A. Acid-triggered nanoexpansion polymeric micelles for enhanced photodynamic therapy. ACS Appl. Mater. Interfaces. 2019;11:33697–33705. doi: 10.1021/acsami.9b12620. [DOI] [PubMed] [Google Scholar]
- [7].Liu C H, Dong H F, Wu N Q, Cao Y, Zhang X J. Plasmonic resonance energy transfer enhanced photodynamic therapy with Au@SiO2@Cu2O/perfluorohexane nanocomposites. ACS Appl. Mater. Interfaces. 2018;10:6991–7002. doi: 10.1021/acsami.8b00112. [DOI] [PubMed] [Google Scholar]
- [8].Zhang Y, Wang F M, Liu C Q, Wang Z Z, Kang L H, Huang Y Y, Dong K, Ren J S, Qu X G. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano. 2018;12:651–661. doi: 10.1021/acsnano.7b07746. [DOI] [PubMed] [Google Scholar]
- [9].Agostinis P, Berg K, Cengel K A, Foster T H, Girotti A W, Gollnick S O, Hahn S M, Hamblin M R, Juzeniene A, Kessel D, et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tang H L, Zheng Y Y, Chen Y. Materials chemistry of nanoultrasonic biomedicine. Adv. Mater. 2017;29:1604105. doi: 10.1002/adma.201604105. [DOI] [PubMed] [Google Scholar]
- [11].Qian X Q, Zheng Y Y, Chen Y. Micro/nanoparticle-augmented sonodynamic therapy (SDT): Breaking the depth shallow of photoactivation. Adv. Mater. 2016;28:8097–8129. doi: 10.1002/adma.201602012. [DOI] [PubMed] [Google Scholar]
- [12].Pan X T, Bai L X, Wang H, Wu Q Y, Wang H Y, Liu S, Xu B L, Shi X H, Liu H Y. Metal-organic-framework-derived carbon nanostructure augmented sonodynamic cancer therapy. Adv. Mater. 2018;30:1800180. doi: 10.1002/adma.201800180. [DOI] [PubMed] [Google Scholar]
- [13].Zhu P, Chen Y, Shi J L. Nanoenzyme-augmented cancer sonodynamic therapy by catalytic tumor oxygenation. ACS Nano. 2018;12:3780–3795. doi: 10.1021/acsnano.8b00999. [DOI] [PubMed] [Google Scholar]
- [14].Chen H J, Zhou X B, Gao Y, Zheng B Y, Tang F X, Huang J D. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov. Today. 2014;19:502–509. doi: 10.1016/j.drudis.2014.01.010. [DOI] [PubMed] [Google Scholar]
- [15].Feng Q H, Zhang W X, Yang X M, Li Y Z, Hao Y W, Zhang H L, Hou L, Zhang Z Z. pH/ultrasound dual-responsive gas generator for ultrasound imaging-guided therapeutic inertial cavitation and sonodynamic therapy. Adv. Healthc. Mater. 2018;7:1700957. doi: 10.1002/adhm.201700957. [DOI] [PubMed] [Google Scholar]
- [16].Sun D, Pang X, Cheng Y, Ming J, Xiang S J, Zhang C, Lv P, Chu C C, Chen X L, Liu G, et al. Ultrasound-switchable nanozyme augments sonodynamic therapy against multidrug-resistant bacterial infection. ACS Nano. 2020;14:2063–2076. doi: 10.1021/acsnano.9b08667. [DOI] [PubMed] [Google Scholar]
- [17].Ma A Q, Chen H Q, Cui Y H, Luo Z Y, Liang R J, Wu Z H, Chen Z, Yin T, Ni J, Zheng M B, et al. Metalloporphyrin complex-based nanosonosensitizers for deep-tissue tumor theranostics by noninvasive sonodynamic therapy. Small. 2019;15:1804028. doi: 10.1002/smll.201804028. [DOI] [PubMed] [Google Scholar]
- [18].Cao Y, Wu T T, Dai W H, Dong H F, Zhang X J. TiO2 nanosheets with the Au nanocrystal-decorated edge for mitochondria-targeting enhanced sonodynamic therapy. Chem. Mater. 2019;31:9105–9114. doi: 10.1021/acs.chemmater.9b03430. [DOI] [Google Scholar]
- [19].Deepagan V G, You D G, Um W, Ko H, Kwon S, Choi K Y, Yi G R, Lee J Y, Lee D S, Kim K, et al. Long-circulating Au-TiO2 nanocomposite as a sonosensitizer for ROS-mediated eradication of cancer. Nano Lett. 2016;16:6257–6264. doi: 10.1021/acs.nanolett.6b02547. [DOI] [PubMed] [Google Scholar]
- [20].Son S, Kim J H, Wang X W, Zhang C L, Yoon S A, Shin J, Sharma A, Lee M H, Cheng L, Wu J S, et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020;49:3244–3261. doi: 10.1039/C9CS00648F. [DOI] [PubMed] [Google Scholar]
- [21].Wang X W, Zhong X Y, Bai L X, Xu J, Gong F, Dong Z L, Yang Z J, Zeng Z J, Liu Z, Cheng L. Ultrafine titanium monoxide (TiO1+x) nanorods for enhanced sonodynamic therapy. J. Am. Chem. Soc. 2020;142:6527–6537. doi: 10.1021/jacs.9b10228. [DOI] [PubMed] [Google Scholar]
- [22].Liang S, Deng X R, Xu G Y, Xiao X, Wang M F, Guo X S, Ma P A, Cheng Z Y, Zhang D, Lin J. A novel Pt−TiO2 heterostructure with oxygen-deficient layer as bilaterally enhanced sonosensitizer for synergistic chemo-sonodynamic cancer therapy. Adv. Funct. Mater. 2020;30:1908598. doi: 10.1002/adfm.201908598. [DOI] [Google Scholar]
- [23].Liu Y, Wang Y, Zhen W Y, Wang Y H, Zhang S T, Zhao Y, Song S Y, Wu Z J, Zhang H J. Defect modified zinc oxide with augmenting sonodynamic reactive oxygen species generation. Biomaterials. 2020;251:120075. doi: 10.1016/j.biomaterials.2020.120075. [DOI] [PubMed] [Google Scholar]
- [24].Dai C, Zhang S J, Liu Z, Wu R, Chen Y. Two-dimensional graphene augments nanosonosensitized sonocatalytic tumor eradication. ACS Nano. 2017;11:9467–9480. doi: 10.1021/acsnano.7b05215. [DOI] [PubMed] [Google Scholar]
- [25].Xu H Y, Zhang X, Han R B, Yang P M, Ma H F, Song Y, Lu Z C, Yin W D, Wu X X, Wang H. Nanoparticles in sonodynamic therapy: State of the art review. RSC Adv. 2016;6:50697–50705. doi: 10.1039/C6RA06862F. [DOI] [Google Scholar]
- [26].He W W, Zhou Y T, Wamer W G, Hu X N, Wu X C, Zheng Z, Boudreau M D, Yin J J. Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging. Biomaterials. 2013;34:765–773. doi: 10.1016/j.biomaterials.2012.10.010. [DOI] [PubMed] [Google Scholar]
- [27].Tao Y, Lin Y H, Huang Z Z, Ren J S, Qu X G. Incorporating graphene oxide and gold nanoclusters: A synergistic catalyst with surprisingly high peroxidase-like activity over a broad ph range and its application for cancer cell detection. Adv. Mater. 2013;25:2594–2599. doi: 10.1002/adma.201204419. [DOI] [PubMed] [Google Scholar]
- [28].Aboulaich A, Tilmaciu C M, Merlin C, Mercier C, Guilloteau H, Medjahdi G, Schneider R. Physicochemical properties and cellular toxicity of (poly)aminoalkoxysilanes-functionalized ZnO quantum dots. Nanotechnology. 2012;23:335101. doi: 10.1088/0957-4484/23/33/335101. [DOI] [PubMed] [Google Scholar]
- [29].Chen Z W, Li Z H, Wang J S, Ju E G, Zhou L, Ren J S, Qu X G. A multi-synergistic platform for sequential irradiation-activated high-performance apoptotic cancer therapy. Adv. Funct. Mater. 2011;24:522–529. doi: 10.1002/adfm.201301951. [DOI] [Google Scholar]
- [30].Wang C C, Shieu F S, Shih H C. Enhanced photodegradation by RGO/ZnO core-shell nanostructures. J. Environ. Chem. Eng. 2020;8:103589. doi: 10.1016/j.jece.2019.103589. [DOI] [Google Scholar]
- [31].Roy P, Periasamy A P, Liang C T, Chang H T. Synthesis of graphene-ZnO-Au nanocomposites for efficient photocatalytic reduction of nitrobenzene. Environ. Sci. Technol. 2013;47:6688–6695. doi: 10.1021/es400422k. [DOI] [PubMed] [Google Scholar]
- [32].Kim S H, Lee J E, Sharker S M, Jeong J H, In I, Park S Y. In vitro and in vivo tumor targeted photothermal cancer therapy using functionalized graphene nanoparticles. Biomacromolecules. 2015;16:3519–3529. doi: 10.1021/acs.biomac.5b00944. [DOI] [PubMed] [Google Scholar]
- [33].Lv K L, Fang S, Si L L, Xia Y, Ho W, Li M. Fabrication of TiO2 nanorod assembly grafted rGO (rGO@TiO2−NR) hybridized flake-like photocatalyst. Appl. Surf. Sci. 2017;391:218–227. doi: 10.1016/j.apsusc.2016.03.195. [DOI] [Google Scholar]
- [34].Li Q, Guo B D, Yu J G, Ran J R, Zhang B H, Yan H J, Gong J R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011;133:10878–10884. doi: 10.1021/ja2025454. [DOI] [PubMed] [Google Scholar]
- [35].Wang P F, Zhan S H, Xia Y G, Ma S L, Zhou Q X, Li Y. The fundamental role and mechanism of reduced graphene oxide in rGO/Pt-TiO2 nanocomposite for high-performance photocatalytic water splitting. Appl. Catal. B Environ. 2017;207:335–346. doi: 10.1016/j.apcatb.2017.02.031. [DOI] [Google Scholar]
- [36].Zhang W J, Hu X L, Shen Q, Xing D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat. Commun. 2019;10:1704. doi: 10.1038/s41467-019-09566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhu W J, Chen Q, Jin Q T, Chao Y, Sun L L, Han X, Xu J, Tian L L, Zhang J L, Liu T, et al. Sonodynamic therapy with immune modulatable two-dimensional coordination nanosheets for enhanced anti-tumor immunotherapy. Nano Res. 2021;14:212–221. doi: 10.1007/s12274-020-3070-8. [DOI] [Google Scholar]
- [38].Pan X T, Wang W W, Huang Z J, Liu S, Guo J, Zhang F R, Yuan H J, Li X, Liu F Y, Liu H Y. MOF-derived double-layer hollow nanoparticles with oxygen generation ability for multimodal imaging-guided sonodynamic therapy. Angew. Chem., Int. Ed. 2020;59:13557–13561. doi: 10.1002/anie.202004894. [DOI] [PubMed] [Google Scholar]
- [39].Chen W, Liu C, Ji X Y, Joseph J, Tang Z M, Ouyang J, Xiao Y F, Kong N, Joshi N, Farokhzad O C, et al. Stanene-based nanosheets for β-elemene delivery and ultrasound-mediated combination cancer therapy. Angew. Chem., Int. Ed. 2021;60:7155–7164. doi: 10.1002/anie.202016330. [DOI] [PubMed] [Google Scholar]
- [40].Li G Z, Wang S P, Deng D S, Xiao Z S, Dong Z L, Wang Z P, Lei Q F, Gao S, Huang G X, Zhang E P, et al. Fluorinated chitosan to enhance transmucosal delivery of sonosensitizer-conjugated catalase for sonodynamic bladder cancer treatment post-intravesical instillation. ACS Nano. 2020;14:1586–1599. doi: 10.1021/acsnano.9b06689. [DOI] [PubMed] [Google Scholar]
- [41].Ouyang J, Tang Z M, Farokhzad N, Kong N, Kim N Y, Feng C, Blake S, Xiao Y F, Liu C, Xie T, et al. Ultrasound mediated therapy: Recent progress and challenges in nanoscience. Nano Today. 2020;35:100949. doi: 10.1016/j.nantod.2020.100949. [DOI] [Google Scholar]
- [42].Lei B, Li B, Zhang H, Lu S, Zheng Z, Li W, Wang Y. Mesostructured silica chemically doped with RuII as a superior optical oxygen sensor. Adv. Funct. Mater. 2006;16:1883–1891. doi: 10.1002/adfm.200500737. [DOI] [Google Scholar]
- [43].Zhu W J, Yang Y, Jin Q T, Chao Y, Tian L L, Liu J J, Dong Z L, Liu Z. Two-dimensional metal-organic-framework as a unique theranostic nano-platform for nuclear imaging and chemophotodynamic cancer therapy. Nano Res. 2019;12:1307–1312. doi: 10.1007/s12274-018-2242-2. [DOI] [Google Scholar]
- [44].Costley D, McEwan C, Fowley C, McHale A P, Atchison J, Nomikou N, Callan J F. Treating cancer with sonodynamic therapy: A review. Int. J. Hyperthermia. 2015;31:107–117. doi: 10.3109/02656736.2014.992484. [DOI] [PubMed] [Google Scholar]
- [45].Wang C, Cao F J, Ruan Y D, Jia X D, Zhen W Y, Jiang X E. Specific generation of singlet oxygen through the russell mechanism in hypoxic tumors and GSH depletion by Cu-TCPP nanosheets for cancer therapy. Angew. Chem., Int. Ed. 2019;58:9846–9850. doi: 10.1002/anie.201903981. [DOI] [PubMed] [Google Scholar]
- [46].Liang S, Deng X R, Chang Y, Sun C Q, Shao S, Xie Z X, Xiao X, Ma P, Zhang H Y, Cheng Z Y, et al. Intelligent hollow Pt−CuS janus architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy. Nano Lett. 2019;19:4134–4145. doi: 10.1021/acs.nanolett.9b01595. [DOI] [PubMed] [Google Scholar]
- [47].Yue W W, Chen L, Yu L D, Zhou B G, Yin H H, Ren W W, Liu C, Guo L H, Zhang Y F, Sun L P, et al. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat. Commun. 2019;10:2025. doi: 10.1038/s41467-019-09760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maisonnasse P, Guedj J, Contreras V, Behillil S, Solas C, Marlin R, Naninck T, Pizzorno A, Lemaitre J, Gonçalves A, et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- [49].Wang Z Z, Zhang Y, Ju E G, Liu Z, Cao F F, Chen Z W, Ren J S, Qu X G. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 2018;9:3334. doi: 10.1038/s41467-018-05798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Integrating Au and ZnO nanoparticles onto graphene nanosheet for enhanced sonodynamic therapy


