Abstract
Purpose of Review
Invasive fungal infections are a complication of COVID-19 disease. This article reviews literature characterizing invasive fungal infections associated with COVID-19.
Recent Findings
Multiple invasive fungal infections including aspergillosis, candidiasis, pneumocystosis, other non-Aspergillus molds, and endemic fungi have been reported in patients with COVID-19. Risk factors for COVID-19-associated fungal disease include underlying lung disease, diabetes, steroid or immunomodulator use, leukopenia, and malignancy. COVID-19-associated pulmonary aspergillosis (CAPA) and COVID-19-associated mucormycosis (CAM) are the most common fungal infections described. However, there is variability in the reported incidences related to use of differing diagnostic algorithms.
Summary
Fungal pathogens are important cause of infection in patients with COVID-19, and the diagnostic strategies continue to evolve. Mortality in these patients is increased, and providers should operate with a high index of suspicion. Further studies will be required to elucidate the associations and pathogenesis of these diseases and best management and prevention strategies.
Keywords: COVID-19, SARS-CoV-2, Aspergillosis, Pneumocystis, Endemic fungi, Candidiasis
Introduction
Since the emergence of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 in early 2020, there have been more than 500 million recorded cases and over 6 million deaths worldwide [1]. An important complication of COVID-19 in severely ill patients is superinfection, often caused by bacteria, fungi, or other viruses [2•, 3•, 4•, 5••, 6••, 7•].
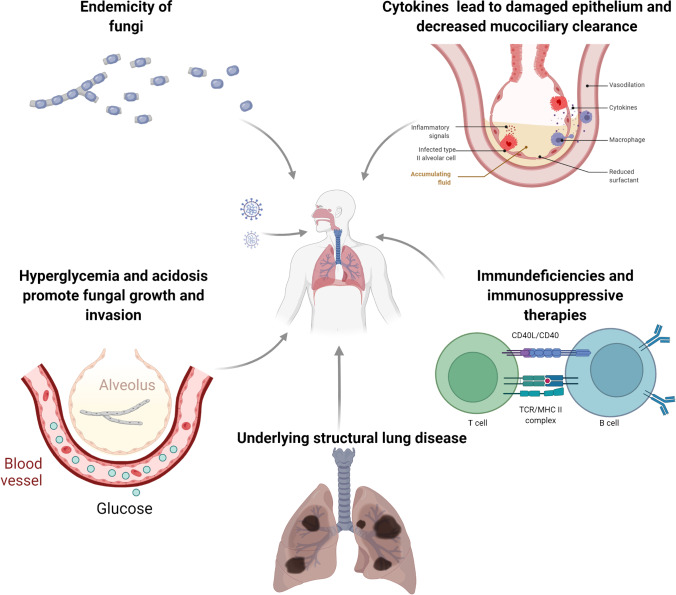
Invasive fungal infections (IFIs), especially invasive pulmonary aspergillosis (IPA), are recognized as causing disease following viral respiratory infections with influenza, referred to as influenza-associated pulmonary aspergillosis (IAPA) [8–11]. Similarly, there has been an emergence of reported fungal infections complicating COVID-19. The pathophysiology of secondary fungal infections in COVID-19 is poorly understood. Analogous to IAPA, SARS-CoV-2 infection is thought to release danger-associated molecular patterns (DAMPs) that set into motion the hyperinflammatory cascade and cytokine storm leading to ARDS. This disrupts the lung epithelial barrier along with concomitant immune dysregulation, compromised host defenses, and impaired muco-ciliary clearance that aids in fungal invasion and pathogenesis (Fig. 1) [12•, 13–15].
Fig. 1.
Proposed factors and mechanisms of invasive fungal disease in COVID-19 patients. Created with BioRender.com
Several predisposing risk factors for COVID-10-associated IFI have been identified, including the viral infection itself, underlying chronic structural lung disease, immunosuppressive therapies such as corticosteroids and immunomodulators, leukopenia, malignancy, longer duration (> 14 days) on mechanical ventilation, prior antibiotic use, cardiovascular disease, liver disease, and uncontrolled diabetes [16••, 17, 18••, 19].
COVID-19-associated pulmonary aspergillosis (CAPA) was initially reported to have high incidence comparable to IAPA and was associated with poor outcomes and prolonged hospital stays. However, the true incidence, associated risk factors, best diagnostic, prevention, and treatment strategies of COVID-19-associated fungal infections continue to evolve despite multiple observational studies and the recent consensus guidelines [20••]. Herein, we review current literature regarding incidence, diagnosis, and management of COVID-19-associated fungal infections (Table 1).
Table 1.
COVID-19-associated fungal Infection
| Invasive fungal disease | Key points |
|---|---|
| COVID-19-associated pulmonary aspergillosis (CAPA) |
• Disease pathogenesis not fully understood • Knowledge gaps in true incidence, risk factors, and outcomes • Wide variability in reported incidence due to lack of consensus on CAPA definition, differing diagnostic algorithms and surveillance practices • Diagnoses are driven by isolation of Aspergillus species on culture or positive tests (Aspergillus galactomannan, Aspergillus PCR) from upper respiratory samples, which could reflect colonization • Diagnostic tests currently used like serum and BAL Aspergillus galactomannan, Aspergillus PCR have not yet been validated in the COVID-19 population • Most studies report an association of corticosteroids or IL-6 inhibitors with CAPA • Role for prophylaxis or pre-emptive treatment needs to be further explored as it has been shown to reduce CAPA incidence rates but not overall mortality • CAPA is associated with worse outcomes compared to non-CAPA patients |
| Mucormycosis |
• Most commonly reported non-Aspergillus mold infection • Uncontrolled diabetes and corticosteroid use appear associated with a higher risk • Rhino-cerebral CAM is most common, but pulmonary CAM with the highest mortality • Surgical intervention is an important part of management |
| Other non-Aspergillus molds |
• Fusariosis reported in immunocompetent patients • All cases involved lung parenchyma • A single case of scedosporiosis reported in Chile • Additional data are needed |
| Pneumocystosis |
• More often in the elderly and immunocompromised (HIV, malignancy, chronic steroid use) • Can be clinically indistinguishable from COVID-19 pneumonia • Key features: elevated LDH, Beta-D Glucan, and lymphopenia |
| Candidiasis |
• Suspected in patients who receive steroids, long ICU stays, indwelling central venous catheters • Immunomodulators may increase risk |
| Cryptococcosis |
• Patients older than 55 years of age with underlying comorbidities may be at risk • High mortality |
| Endemic mycoses |
• Limited data, but no clear association with COVID-19 • Endemicity is the greatest risk factor |
Abbreviations: BAL bronchoalveolar lavage, CAM COVID-19-associated mucormycosis, CKD chronic kidney disease, IL-6 interleukin 6, LDH lactate dehydrogenase, PCR polymerase chain reaction, ICU intensive care unit
CAPA
During the initial phase of the COVID-19 pandemic, several European centers reported an increased incidence of IPA, almost as high as IAPA rates ~ 20–30% [10, 11, 21–25]. However, subsequent studies have shown wide variability in CAPA incidence (2–33%), related in part to lack of consensus on case definition, poor understanding of diagnostic test performance, the clinical relevance of Aspergillus colonization of upper airways, limitations in obtaining lower respiratory samples/biopsies for proven disease, patient socioeconomic factors, and environmental burden of aspergillus spores [4•, 16••, 26, 27].
Neither the revised 2019 European Organization for Research and Treatment of Cancer and Mycosis Study Group Education and Research Consortium (EORTC/MSGERC) IPA guidelines [28] nor the AspICU algorithm formulated for IAPA[29] could appropriately classify cases given the absence of host factors and typical imaging findings for fungal pneumonia. This led the European Confederation for Medical Mycology and the International Society for Human and Animal Mycology (ECMM/ISHAM) to propose a new criteria for CAPA case definition, diagnosis and management. A new category of “possible CAPA” was proposed to include and manage COVID-19 patients with positive Aspergillus tests or culture on samples not yet validated in this patient population, such as upper respiratory samples (bronchial/tracheal aspirate) or non-directed Bronchioalveolar lavage fluid (NBL) [20••].
Fekkar and colleagues noted a significantly decreased probable/possible CAPA incidence of 6.1% after applying the 2021 ECMM/ISHAM consensus criteria to the cohorts cited by the expert panel in the same article [30•]. The results were closer to the incidence in proven autopsy studies early in the pandemic, as low as 0–3% [31, 32]. In contrast, another study from a single German ICU that evaluated autopsy findings after long-term treatment of COVID-19 patients found evidence of angioinvasive fungal infection in 7 out of 8 autopsies [33]. The variation in CAPA prevalence among different centers may be a result of the different diagnostic strategies that have been implemented.
The diagnosis of IPA remains challenging in the non-neutropenic and immunocompetent population. A number of diagnostic tests are currently being utilized for the diagnosis of CAPA, such as histopathology, fungal biomarkers (serum and BALF Aspergillus galactomannan, 1–3-Beta-D-glucan (BDG)) and Aspergillus polymerase chain reaction (PCR) [20••]. However, since test positivity is used to classify CAPA, the diagnostic accuracy of these tests can be overestimated. Additionally, these individual diagnostic test assays lack adequate sensitivity when used by themselves to make a diagnosis. Therefore, the recommendation is to use a more comprehensive screening algorithm that employs a combination of these tests to improve test characteristics like sensitivity to increase the likelihood of CAPA [16••, 20••].
A definitive diagnosis of CAPA requires invasive procedures such as bronchoscopy to show histopathological or direct microscopic evidence of Aspergillus species causing damage or invasion of the lung tissue. Bronchoscopy also aids in the visualization of plaques/eschars indicating Aspergillus tracheobronchitis [20••]. Early in the pandemic, the risk of aerosolization limited bronchoscopies at various centers. Tertiary care centers often made the diagnosis of probable/possible CAPA from suboptimal and unvalidated upper respiratory samples like bronchial or tracheal aspirates on routine surveillance [3•, 34, 35]. Use of NBL samples obtained via a closed-circuit suction catheter reduces transmission risk of SARS-CoV-2; however, these samples are often not reflective of the lower respiratory tract microbiology and are yet to be validated for CAPA diagnosis [2•, 20••, 36, 37].
The clinical relevance of aspergillus PCR/culture from an upper respiratory sample has been questioned as it could reflect colonization [26, 31, 40–45]. While this should prompt a diagnostic workup in a patient with risk factors for CAPA, it may not always indicate invasive disease. Instead, it should be interpreted in conjunction with other mycological evidence, preferably from lower respiratory samples or serum. BALF galactomannan is highly indicative of IPA, particularly with high titers, but does not prove tissue invasion [15]. Invasive disease is more likely if circulating serum galactomannan is present. However, there are reports of proven IPA cases with negative serum galactomannan, supporting findings of the lower sensitivity of serum galactomannan in CAPA ~ 20%, compared to IAPA ~ 65% [2•, 44, 45]. Another recommendation has been to include serum BDG as part of the mycological evidence to increase the sensitivity of the serum Aspergillus galactomannan. Two consecutive positive tests were found to mount a specificity as high as 90% [41]. However, cautious interpretation in the appropriate clinical context after excluding an alternative etiology like candidemia is imperative due to non-specificity in IFI.
The non-specific radiological manifestations of SARS-CoV-2, with ground-glass opacities and nodular/cavitary lesions, are very similar to those seen in classic IPA. Moreover, findings of fungal pneumonia may be masked by the diffuse lung parenchymal damage caused by SARS-CoV-2. When these radiological manifestations are present, the ECMM/ISHAM panel recommends further investigation and workup for CAPA in a patient with high pretest probability, new fevers, or unclear cause of clinical deterioration [20••].
Several studies have investigated potentially modifiable risk factors that could help improve outcomes in patients with CAPA[2•, 17, 18••, 19]. Immunomodulatory agents are routinely administered in patients at risk of respiratory failure secondary to ARDS and cytokine storming. A significant association was established between dexamethasone and CAPA in multiple studies [2•, 3•, 17, 49, 50], while others failed to find an obvious correlation [51•, 52]. Although corticosteroids are known to suppress the body’s natural immunity to fight invasive fungal infections, they might also simultaneously have a protective effect by decreasing the lung parenchymal damage (an independent IPA risk factor) caused by the hyperinflammatory syndrome [51•]. Corticosteroids have become the standard of care in severe COVID-19, given clear mortality benefits, making it difficult to compare to a control group that could have biased the analysis in a few of the studies mentioned above [46].
Additionally, tocilizumab has also been identified as a risk factor for CAPA [4•, 54–57]. One study reported a higher incidence of fungal infections, particularly when multiple doses were administered. CAPA was diagnosed in 2.5% of patients who did not receive tocilizumab, 20% in patients who received a single dose, and 50% in those who received two doses (p < 0.01) [51•]. Other cohorts, however found no increased risk of CAPA in tocilizumab versus non-tocilizumab groups [18••, 52, 53].
Compared to critically ill COVID-19 patients admitted to the ICU without invasive aspergillosis, patients with CAPA have been reported to have higher mortality (30–40% vs 50%), longer duration on mechanical ventilation, and hospital stay [2•, 3•, 4•, 5••, 6••, 7•, 12•, 17, 18••]. Most studies, however, did not see a decreased mortality with use of antifungal therapy [3•]. Of note, patients with possible CAPA in a few observational studies survived despite not receiving antifungal agents, raising the possibility of misclassification of infection, perhaps colonization or less invasive disease, and better outcomes in patients who are immunocompetent [54, 55].
Antifungal prophylaxis or pre-emptive treatment strategies have been suggested as management options in high-risk COVID-19 patients [2•]. Hatzl et al. observed a decrease in CAPA incidence without reducing overall mortality in patients who were on antifungal prophylaxis [61•]. In another study, mechanically ventilated COVID-19 patients who were started on prophylaxis with inhaled amphotericin because of a CAPA outbreak were noted to have reduced CAPA incidence rates [57]. A study of isavuconzole as fungal prophylaxis in COVID-19 patients is currently underway and will hopefully give much needed efficacy information [58].
As per current EORTC/MSGERC guidelines, a triazole antifungal has remained the mainstay therapeutic in the management of invasive aspergillosis [28]. The ECMM/ISHAM panel similarly recommends treatment of CAPA with voriconazole or isavuconazole as first-line treatment. Clinicians should keep in mind the local epidemiology of azole resistant Aspergillus species. Limitations of voriconazole include its narrow therapeutic window, drug-drug interactions, and the need for close therapeutic drug monitoring (TDM). COVID-19 patients with acute renal failure are often unable to receive liposomal amphotericin given its nephrotoxicity [20••].
Non-Aspergillus Mold Infections
While Aspergillus is the most well-established fungal infection associated with COVID-19, many less common fungal infections have since been reported. In contrast to aspergillosis, mucormycosis is historically rare following viral infection [59]. However, COVID-19-associated mucormycosis (CAM) is now the most widely reported non-Aspergillus mold infection [64•, 65••, 66, 67]. Most early cases were reported from India, although cases have since been reported from the Middle East, Australia, Asia, Europe, South America, and the USA [64•, 65••, 67, 68]. There has been much speculation regarding the clustering of cases in India. Though still unclear, the outbreak is suspected to be due to India’s large diabetic population, environmental factors including the tropical and sub-tropical humid climates with the presence of Mucorales spores, and practice variations in use of corticosteroids [65••].
Uncontrolled diabetes and hyperglycemia appear to be key underlying risk factors for CAM. Analyses of CAM cases found that 83–94% of cases had diabetes, of which 67–83% were poorly controlled [68, 69•]. Acidemic states such as diabetic ketoacidosis (DKA) increase unbound iron supporting Mucorales growth [65••]. Hyperglycemia from diabetes and steroids also supports mold growth (Fig. 1) [69•]. Additionally, the by-products of DKA (B-hydroxybutyrate, glucose, and iron) increase both cell expression of GRP78, the receptor by which Rhizopus enters epithelial cells, and CotH, the fungal protein which binds GRP78 [65••]. Other risk factors for CAM include hematologic malignancy, organ and hematopoietic stem cell transplant, end-stage renal disease, and trauma [65••, 68].
CAM generally presents with fever and symptoms of rhino-orbital or rhino-orbital-cerebral mucormycosis (orbital swelling, nasal symptoms, discoloration, and necrosis) within 2 weeks of diagnosis of COVID-19 [64•, 65••, 67, 68]. Most cases of CAM are rhino-cerebral though lung; musculoskeletal, gastrointestinal, and disseminated cases have also been reported [65••, 68].
Diagnosis of CAM is challenging and requires a high index of suspicion. Nearly all cases of CAM are confirmed via biopsy with histopathology or RT-PCR [61•]. Additionally, up to 30% of cases have been reported to also have CAPA [62].
Treatment of CAM does not differ from previously established guidelines for mucormycosis [64•]. CAM patients require systemic antifungal treatment — most commonly amphotericin — and 78% require surgical debridement [61•, 62]. Mortality ranges from 37 to 80% with pulmonary CAM portending a worse prognosis than rhino-orbital [59, 61•, 62].
Several cases of COVID-19-associated fusariosis have been reported [65••, 66, 67]. Given the small number of case reports, broad conclusions cannot be drawn about the risk factors and presentation. However, in the cases reviewed, no patients had significant immunocompromising conditions other than COVID-19, and all involved lung parenchyma [65••, 66, 67]. All patients were diagnosed by cultures of either blood or sputum and were treated with systemic antifungal therapy; one patient died [65••, 66, 67]. One suspected case was never critically ill and treated on an outpatient basis [66].
COVID-associated Scedosporium or Lomentaspora infections appear rare. In a series of 16 cases of invasive mold infection among COVID-19 patients in Chile, only one had infection with Scedosporium [68]. The dearth of reports may stem from limitations in diagnostics [69•]. Nonetheless, Scedosporium or Lomentaspora are important opportunistic pathogens in immunocompromised patients and should be considered in such patients with COVID-19 [69•].
Pneumocystosis
Several cases of COVID-19-associated Pneumocystis jirovecii have been reported [76, 77•]. Generally, cases have been in older patients (mean age 78) and have an underlying immunocompromising condition such as HIV, malignancy, or chronic steroid use [76, 77•]. The presentation of severe COVID-19 can be clinically indistinguishable from Pneumocystis pneumonia (PCP), as patients present with bilateral ground-glass opacities and lymphopenia [76, 77•]. All reported cases of PJP coinfection had increased lactate dehydrogenase levels and beta-D-glucan [76, 77•]. Lymphopenia is reportedly severe with absolute lymphocyte counts below 900 cells/mm and CD4+ T-cell counts below 200 cells/mm [77•]. Patients are generally diagnosed via PCR of respiratory specimens from 2 to 21 days after initial COVID-19 presentation [77•]. A prospective study that tested all severe COVID-19 patients admitted to a single unit over 1 month found that 17% had positive respiratory samples for Pneumocystis [72]. However, given the clinical similarities between severe COVID-19 and PCP, it is speculated that contamination or colonization rather than true infection may contribute to this high prevalence [77•, 78, 79].
Treatment of PJP in COVID-19 is with use of use of the standard regimen of trimethoprim-sulfamethoxazole and steroids, with reported mortality ranging from 42 to 100% [76, 77•]. The use of prophylactic trimethoprim-sulfamethoxazole in patients with COVID-19 who have a positive Pneumocystis assay and unclear PJP diagnosis remains controversial [72, 73].
Candidiasis
Comparative reports suggested a higher incidence of IC among COVID-19 patients vs non-COVID-19 patients, occurring earlier in hospitalization and associated with higher mortality rates [74–76]. No distinctive risk factors for COVID-19-associated IC have been identified, though generally, these patients appear more likely to have received steroids, have longer ICU stays, and have longer central venous catheter dwell times [77•]. Additionally, steroid use, sepsis, and age over 65 were identified as independent risk factors for mortality in COVID-19 associated candidemia [75]. A COVID-19 predisposition to candidemia due to immune paralysis, intestinal translocation, and altered microbiota may also contribute [81•].
Early reports implicated immunomodulators such as tocilizumab as a risk factor for IC, particularly in the critically ill and those receiving renal replacement therapy [78, 79]. A recent meta-analysis examining IL-6 pathway inhibitors found that while sarilumab and anakinra did not affect the risk of secondary infections, tocilizumab specifically increased the risk of fungal infections [87••]. Additionally, baricitinib does not appear to be associated with increased risk fungal infections since being incorporated into the management of COVID-19 [81•].
COVID-19 patients particularly the elderly, critically ill, and those receiving immunomodulating therapy appear likely to be at increased risk for IC. Providers should maintain a high index of suspicion in such patients and obtain blood and site-specific cultures and start appropriate antifungal therapy per available guidelines [82].
Cryptococcosis
Cryptococcosis is historically seen in patients with AIDS, malignancy, transplants, and other sources of immunosuppression. To date, several cases of COVID-19-associated cryptococcosis have been reported [83•, 84–86, 87••, 88–91]. Of the cases reported, all patients older than 55 years of age and had an underlying comorbidity (diabetes, hypertension, CKD). Only one had a history of organ transplant, and none had HIV [90]. All but one of the cases were male [83•]. Cryptococcosis was diagnosed from culture growth in blood, CSF, or bronchoalveolar lavage, and treated with amphotericin and flucytosine. All cases died, except one who remained in a vegetative state six weeks after appropriate therapy was initiated [84]. Traver et al. also reported a case of CAPA and cryptococcal coinfection [85]. Given the low number of reports, it is unclear if COVID-19 creates any specific predisposition to cryptococcal infection. However, clinicians should retain a high index of suspicion for cryptococcosis in patients with severe COVID-19 who have suspected meningitis.
Endemic Fungi
There have been limited reports of COVID-19-associated infection with endemic fungi.
Several cases of histoplasmosis in COVID-19 patients have been reported [92–97]. However, three of these cases were diagnosed with COVID-19 after already being treated for histoplasmosis [92, 94, 95]. In one of these cases, the patient was on immunosuppressants [92] for a renal transplant, while two other had AIDS [94, 95]. Additionally, one case developed pulmonary histoplasmosis 4 months after recovering from COVID-19 [96]. These cases responded well to standard therapy of amphotericin and/or itraconazole. More information is needed to better understand the association of COVID-19 and histoplasmosis.
Like histoplasmosis, several cases of COVID-19 and coccidioidomycosis have been reported [98–104]. While one case was reportedly mild and did not require hospitalization, another case experienced rapid disease dissemination shortly after infection with SARS-CoV-2 [99, 100]. Additionally, one report describes a case of possible reactivation Coccidiodes from COVID-19 [98]. Other than geography, risk factors for coinfection appear to be older age, diabetes, immunosuppression, and minority status [101].
To date, only one case of COVID-19-associated blastomycosis has been reported in a 24-year-old pregnant woman with severe COVID-19, although additional cases have been noted anecdotally [105].
Overall, the association of endemic mycoses with COVID-19 needs is unclear; however, endemic mycoses should remain as a differential diagnosis in patients with severe COVID-19 who have symptoms consistent with endemic mycoses and appropriate geographic exposures. Management guidelines are available for these infections, and they are typically treated with triazole antifungals or amphotericin [106–108]
Conclusion
Given the extensive pulmonary tissue damage and the immunosuppression related to SARS-CoV-2 infection and its management, fungal pathogens are important to consider in the assessment of suspected superinfection in COVID-19 patients. Aspergillosis, candidiasis, non-aspergillus mold infections, endemic fungi, and PJP have all been reported, and data will continue to emerge. Steroids and other immunomodulatory therapies appear to increase the risk for invasive fungal disease, as do pre-existing comorbidities such as diabetes, chronic kidney disease, and baseline immunosuppression. In many cases, the appropriate use of diagnostic studies and clinically similar syndromes has made diagnosis difficult, leading to a wide range of disease incidence or prevalence. Therefore, a high index of suspicion combined with selective diagnostics from a differential honed by clinical presentation and exposure history is necessary to best assess these patients. Further studies with larger cohorts will be required to elucidate the true associations and pathogenesis of these diseases and best management and prevention strategies.
Declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
AAS: No conflicts.
MM: No conflicts.
JWB: has received consulting fees from Pfizer and Lilly for serving on data safety committees.
Footnotes
This article is part of the Topical Collection on COVID-19 and Fungal Infections.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.COVID-19 Map - Johns Hopkins Coronavirus Resource Center [Internet]. [cited 2022 May 14]. Available from: https://coronavirus.jhu.edu/map.html
- 2.• White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, et al. A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clinical Infectious Diseases [Internet]. Oxford Academic; 2021 [cited 2022 Apr 24];73:e1634–44. Available from: https://academic.oup.com/cid/article/73/7/e1634/5899192. A national, multicenter, prospective cohort which implemented routine Aspergillus surveillance using NBL samples and antifungal therapy as empiric therapy [DOI] [PMC free article] [PubMed]
- 3.• Bartoletti M, Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, et al. Clinical infectious diseases epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clinical Infectious Diseases ® [Internet]. 2021 [cited 2022 Apr 24];73:3606–20. Available from: https://academic.oup.com/cid/article/73/11/e3606/5876990. One of the initial European prospective, multicenter studies which reported a high prevalence of CAPA and lower mortality with antifungal therapy [DOI] [PMC free article] [PubMed]
- 4.• Prattes J, Wauters J, Giacobbe DR, Salmanton-García J, Maertens J, Bourgeois M, et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients—a multinational observational study by the European Confederation of Medical Mycology. Clinical Microbiology and Infection [Internet]. Elsevier; 2022 [cited 2022 May 10];28:580–7. Available from: http://www.clinicalmicrobiologyandinfection.com/article/S1198743X21004742/fulltext. A multinational study which found varied prevalence between centers as per ECMM/ISHAM guidelines and increased mortality among CAPA patients. [DOI] [PMC free article] [PubMed]
- 5.•• Singh S, Verma N, Kanaujia R, Chakrabarti A, Rudramurthy SM. Mortality in critically ill patients with coronavirus disease 2019-associated pulmonary aspergillosis: a systematic review and meta-analysis. Mycoses [Internet]. John Wiley & Sons, Ltd; 2021 [cited 2022 May 12];64:1015–27. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/myc.13328. Systemic review and metanalysis of 20 studies which revealed high mortality rates in CAPA patients and no clear mortality benefit from antifungal therapy [DOI] [PubMed]
- 6.•• Mitaka H, Kuno T, Takagi H, Patrawalla P. Incidence and mortality of COVID-19-associated pulmonary aspergillosis: a systematic review and meta-analysis. Mycoses [Internet]. John Wiley & Sons, Ltd; 2021 [cited 2022 May 12];64:993–1001. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/myc.13292. Systemic review with high CAPA incidence and mortality rate [DOI] [PMC free article] [PubMed]
- 7.• Er B, Er AG, Gülmez D, Şahin TK, Halaçlı B, Durhan G, et al. A screening study for COVID-19-associated pulmonary aspergillosis in critically ill patients during the third wave of the pandemic. Mycoses [Internet]. John Wiley & Sons, Ltd; 2022 [cited 2022 May 12]; Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/myc.13466. Prospective study which implemented routine surveillance with high incidence of CAPA and mortality [DOI] [PMC free article] [PubMed]
- 8.Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza. Curr Opin Infect Dis. 2018;31:471–480. doi: 10.1097/QCO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 9.Verweij PE, Rijnders BJA, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Medicine Springer. 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, van Tienen C, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. The Lancet Respiratory Medicine [Internet]. Elsevier; 2018 [cited 2022 Apr 23];6:782–92. Available from: http://www.thelancet.com/article/S2213260018302741/fulltext [DOI] [PubMed]
- 11.Crum-Cianflone N. Invasive aspergillosis associated with severe influenza infections. Open Forum Infect Dis. 2016;3:ofw171. doi: 10.1093/ofid/ofw171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.• Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. Journal of Fungi [Internet]. Multidisciplinary Digital Publishing Institute (MDPI); 2020 [cited 2022 May 14];6:1–17. Available from: /pmc/articles/PMC7346000/. Fungal immunology and pathogenesis in COVID-19 patients [DOI] [PMC free article] [PubMed]
- 13.Tolle LB, Standiford TJ. Danger-associated molecular patterns (DAMPs) in acute lung injury. J Pathol [Internet]. J Pathol; 2013 [cited 2022 May 14];229:145–56. Available from: https://pubmed.ncbi.nlm.nih.gov/23097158/ [DOI] [PubMed]
- 14.Cunha C, Carvalho A, Esposito A, Bistoni F, Romani L. DAMP signaling in fungal infections and diseases. Frontiers in Immunology [Internet]. Frontiers Media SA; 2012 [cited 2022 May 14];3. Available from: /pmc/articles/PMC3437516/ [DOI] [PMC free article] [PubMed]
- 15.Rutsaert L, Steinfort N, van Hunsel T, Bomans P, Naesens R, Mertes H, et al. COVID-19-associated invasive pulmonary aspergillosis. Annals of Intensive Care [Internet]. Springer; 2020 [cited 2022 May 12];10:1–4. Available from: https://annalsofintensivecare.springeropen.com/articles/10.1186/s13613-020-00686-4 [DOI] [PMC free article] [PubMed]
- 16.•• Baddley JW, Thompson GR, Chen SC-A, White PL, Johnson MD, Nguyen MH, et al. Coronavirus disease 2019–associated invasive fungal infection. Open Forum Infectious Diseases. 2021;8. Comprehensive review article on COVID-19 associated fungal infections. [DOI] [PMC free article] [PubMed]
- 17.Gangneux J-P, Dannaoui E, Fekkar A, Luyt C-E, Botterel F, de Prost N, et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med. 2022;10:180–190. doi: 10.1016/S2213-2600(21)00442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.•• Permpalung N, Chiang TPY, Massie AB, Zhang SX, Avery RK, Nematollahi S, et al. Coronavirus disease 2019–associated pulmonary aspergillosis in mechanically ventilated patients. Clinical Infectious Diseases [Internet]. Oxford Academic; 2022 [cited 2022 Apr 24];74:83–91. Available from: https://academic.oup.com/cid/article/74/1/83/6164950. Retrospective cohort study which evaluated various CAPA risk factors and outcomes [DOI] [PMC free article] [PubMed]
- 19.Costantini C, van de Veerdonk FL, Romani L. Covid-19-associated pulmonary aspergillosis: the other side of the coin. [cited 2022 May 15]; Available from: www.mdpi.com/journal/vaccines [DOI] [PMC free article] [PubMed]
- 20.•• Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. The Lancet Infectious Diseases [Internet]. Lancet Publishing Group; 2021 [cited 2022 Apr 23];21:e149–62. Available from: www.thelancet.com/infectionCAPA guidelines outlining definition/classification, diagnosis and management by an expert international panel
- 21.Wauters J, Baar I, Meersseman P, Meersseman W, Dams K, De R, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. 2012; [DOI] [PMC free article] [PubMed]
- 22.van de Veerdonk FL, Kolwijck E, Lestrade PPA, Hodiamont CJ, Rijnders BJA, van Paassen J, et al. Influenza-associated aspergillosis in critically ill patients. American Journal of Respiratory and Critical Care Medicine. American Thoracic Society; 2017;196:524–7. [DOI] [PubMed]
- 23.van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19–associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202:132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koehler P, Cornely OA, Böttiger BW, Dusse F, Eichenauer DA, Fuchs F, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. The Lancet Respiratory Medicine [Internet]. Lancet Publishing Group; 2020 [cited 2022 Apr 23];8:e48–9. Available from:10.1016/S1473- [DOI] [PMC free article] [PubMed]
- 26.Richardson M, Bowyer P, Sabino R. The human lung and Aspergillus: you are what you breathe in? Med Mycol. 2019;57:S145–S154. doi: 10.1093/mmy/myy149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fournel I, Sautour M, Lafon I, Sixt N, L’Ollivier C, Dalle F, et al. Airborne Aspergillus contamination during hospital construction works: efficacy of protective measures. American Journal of Infection Control [Internet]. Elsevier; 2010 [cited 2022 May 13];38:189–94. Available from: http://www.ajicjournal.org/article/S0196655309008359/fulltext [DOI] [PubMed]
- 28.Peter Donnelly J, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clinical Infectious Diseases [Internet]. Oxford Academic; 2020 [cited 2022 May 14];71:1367–76. Available from: https://academic.oup.com/cid/article/71/6/1367/5645434 [DOI] [PMC free article] [PubMed]
- 29.Blot SI, Taccone FS, van den Abeele A-M, Bulpa P, Meersseman W, Brusselaers N, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. [cited 2022 May 14]; Available from: http://ajrccm.atsjournals.org [DOI] [PubMed]
- 30.• Fekkar A, Neofytos D, Nguyen M-H, Clancy CJ, Kontoyiannis DP, Lamoth F. COVID-19-associated pulmonary aspergillosis (CAPA): how big a problem is it? 2021 [cited 2022 Apr 23]; Available from:10.1016/j.cmi.2021.06.025More accurate prevalence based on the ECMM/ISHAM guidelines [DOI] [PMC free article] [PubMed]
- 31.Flikweert AW, Grootenboers MJJH, Yick DCY, du Mée AWF, van der Meer NJM, Rettig TCD, et al. Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. 2020;59:149–155. doi: 10.1016/j.jcrc.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kula BE, Clancy CJ, Hong Nguyen M, Schwartz IS. Invasive mould disease in fatal COVID-19: a systematic review of autopsies. The Lancet Microbe. 2021;2:e405–e414. doi: 10.1016/S2666-5247(21)00091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evert K, Dienemann T, Brochhausen C, Lunz D, Lubnow M, Ritzka M, et al. Autopsy findings after long-term treatment of COVID-19 patients with microbiological correlation. Virchows Archiv [Internet]. Springer Science and Business Media Deutschland GmbH; 2021 [cited 2022 Apr 24];479:97–108. Available from: https://link.springer.com/article/10.1007/s00428-020-03014-0 [DOI] [PMC free article] [PubMed]
- 34.Segrelles-Calvo G, Araújo GRS, Llopis-Pastor E, Carrillo J, Hernández-Hernández M, Rey L, et al. Prevalence of opportunistic invasive aspergillosis in COVID-19 patients with severe pneumonia. Mycoses [Internet]. Mycoses; 2021 [cited 2022 May 19];64:144–51. Available from: https://pubmed.ncbi.nlm.nih.gov/33217071/ [DOI] [PMC free article] [PubMed]
- 35.Gangneux J-P, Reizine F, Guegan H, Pinceaux K, le Balch P, Prat E, et al. Is the COVID-19 pandemic a good time to include aspergillus molecular detection to categorize aspergillosis in ICU patients? A monocentric experience. 2020 [cited 2022 Apr 24]; Available from: www.mdpi.com/journal/jof [DOI] [PMC free article] [PubMed]
- 36.Lahmer Id T, Kriescher S, Herner A, Rothe K, Spinner CD, Schneider J, et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: results from the prospective AspCOVID-19 study. 2021 [cited 2022 May 8]; Available from: 10.1371/journal.pone.0238825 [DOI] [PMC free article] [PubMed]
- 37.van Biesen S, Kwa D, Bosman RJ, Juffermans NP. Detection of invasive pulmonary aspergillosis in COVID-19 with non-directed bronchoalveolar lavage. Am J Respir Crit Care Med [Internet]. Am J Respir Crit Care Med; 2020 [cited 2022 May 12];202:1171–3. Available from: https://pubmed.ncbi.nlm.nih.gov/32668167/ [DOI] [PMC free article] [PubMed]
- 38.Brown LAK, Ellis J, Gorton R, De S, Stone N. Surveillance for COVID-19-associated pulmonary aspergillosis. The Lancet Microbe. Elsevier. 2020;1:e152. doi: 10.1016/S2666-5247(20)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamoth F, Glampedakis E, Boillat-Blanco N, Oddo M, Pagani JL. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clinical Microbiology and Infection [Internet]. Elsevier B.V.; 2020 [cited 2022 Apr 24];26:1706–8. Available from: 10.1016/j.cmi.2020.07.010 [DOI] [PMC free article] [PubMed]
- 40.Fekkar A, Poignon C, Blaize M, Lampros A. Fungal infection during COVID-19: Does aspergillus mean secondary invasive aspergillosis? American Journal of Respiratory and Critical Care Medicine [Internet]. American Thoracic Society; 2020 [cited 2022 Apr 24];202:902–3. Available from: /pmc/articles/PMC7491399/ [DOI] [PMC free article] [PubMed]
- 41.Wang J, Yang Q, Zhang P, Sheng J, Zhou J, Qu T, et al. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: a retrospective case series. Critical Care [Internet]. BioMed Central; 2020 [cited 2022 Apr 24];24:1–4. Available from: https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03046-7 [DOI] [PMC free article] [PubMed]
- 42.Bounhiol A, Pasquier G, Novara A, Bougnoux M-E, Dannaoui E. Aspergillus detection in airways of ICU COVID-19 patients: to treat or not to treat? Journal of Medical Mycology [Internet]. Elsevier Masson; 2022 [cited 2022 May 10];101290. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1156523322000476 [DOI] [PMC free article] [PubMed]
- 43.Yusuf E, Vonk A, van den Akker JPC, Bode L, Sips GJ, Rijnders BJA, et al. Frequency of positive aspergillus tests in COVID-19 patients in comparison to other patients with pulmonary infections admitted to the intensive care unit. Journal of Clinical Microbiology [Internet]. American Society for Microbiology 1752 N St., N.W., Washington, DC ; 2020 [cited 2022 Apr 23];59. Available from: 10.1128/JCM.02278-20 [DOI] [PMC free article] [PubMed]
- 44.Prattes J, Wauters J, Giacobbe DR, Lagrou K, Hoenigl M, Koehler P, et al. Diagnosis and treatment of COVID-19 associated pulmonary apergillosis in critically ill patients: results from a European confederation of medical mycology registry. Intensive Care Medicine [Internet]. Nature Publishing Group; 2021 [cited 2022 May 12];47:1. Available from: /pmc/articles/PMC8284037/ [DOI] [PMC free article] [PubMed]
- 45.Blaize M, Mayaux J, Nabet C, Nabet C, Lampros A, Marcelin AG, et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis [Internet]. Emerg Infect Dis; 2020 [cited 2022 May 14];26:1636–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32343223/ [DOI] [PMC free article] [PubMed]
- 46.Antinori S, Rech R, Galimberti L, Castelli A, Angeli E, Fossali T, et al. Invasive pulmonary aspergillosis complicating SARS-CoV-2 pneumonia: a diagnostic challenge. Travel Medicine and Infectious Disease [Internet]. Elsevier; 2020 [cited 2022 May 14];38:101752. Available from: /pmc/articles/PMC7255262/ [DOI] [PMC free article] [PubMed]
- 47.Talento AF, Dunne K, Joyce EA, Palmer M, Johnson E, White PL, et al. A prospective study of fungal biomarkers to improve management of invasive fungal diseases in a mixed specialty critical care unit. Journal of Critical Care. W.B. Saunders; 2017;40:119–27. [DOI] [PubMed]
- 48.Borman AM, Palmer MD, Fraser M, Patterson Z, Mann C, Oliver D, et al. COVID-19-associated invasive aspergillosis: data from the UK National Mycology Reference Laboratory. 2020 [cited 2022 May 14]; Available from: 10.1128/JCM.02136-20 [DOI] [PMC free article] [PubMed]
- 49.Loughlin L, Hellyer TP, White PL, Mcauley DF, Morris AC, Posso RB, et al. Pulmonary aspergillosis in patients with suspected ventilator-associated pneumonia in UK ICUs. Am J Respir Crit Care Med [Internet]. 2020;202:1125–32. Available from: www.atsjournals.org [DOI] [PMC free article] [PubMed]
- 50.Dellière S, Dudoignon E, Fodil S, Voicu S, Collet M, Oillic PA, et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect [Internet]. Clin Microbiol Infect; 2020 [cited 2022 May 19];27:790.e1–790.e5. Available from: https://pubmed.ncbi.nlm.nih.gov/33316401/ [DOI] [PMC free article] [PubMed]
- 51.• Janssen NAF, Nyga R, Vanderbeke L, Jacobs C, Ergün M, Buil JB, et al. Multinational observational cohort study of COVID-19–associated pulmonary aspergillosis 1. Emerging Infectious Diseases. 2021;27:2892–8. Multinational, observational study — lower CAPA prevalence ~15% noted and no association with corticosteroids [DOI] [PMC free article] [PubMed]
- 52.Vélez Pintado M, Camiro-Zúñiga A, Aguilar Soto M, Cuenca D, Mercado M, Crabtree-Ramirez B. COVID-19-associated invasive pulmonary aspergillosis in a tertiary care center in Mexico City. Medical Mycology [Internet]. Oxford Academic; 2021 [cited 2022 May 7];59:828–33. Available from: https://academic.oup.com/mmy/article/59/8/828/6174029 [DOI] [PMC free article] [PubMed]
- 53.Egger M, Bussini L, Hoenigl M, Bartoletti M. Prevalence of COVID-19-associated pulmonary aspergillosis: critical review and conclusions. J Fungi. 2022;8:390. doi: 10.3390/jof8040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segrelles-Calvo G, Araújo GRS, Llopis-Pastor E, Carrillo | Javier, Hernández-Hernández M, Rey L, et al. Prevalence of opportunistic invasive aspergillosis in COVID-19 patients with severe pneumonia. (2020) [DOI] [PMC free article] [PubMed]
- 55.Nasir N, Mahmood F, Habib K, Khanum I, Jamil B. Tocilizumab for COVID-19 acute respiratory distress syndrome: outcomes assessment Using the WHO Ordinal Scale. (2020) [DOI] [PMC free article] [PubMed]
- 56.Machado M, Valerio M, Álvarez-Uría A, Olmedo M, Veintimilla C, Padilla B, et al. Invasive pulmonary aspergillosis in the COVID-19 era: an expected new entity. Mycoses. 2021;64:132–143. doi: 10.1111/myc.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marta GC, Lorena FE, Laura MV, Angela LM, Blanca LG, Rodrigo AA, et al. COVID-19-associated pulmonary aspergillosis in a tertiary hospital. Journal of Fungi 2022, Vol 8, Page 97 [Internet]. Multidisciplinary Digital Publishing Institute; 2022 [cited 2022 May 8];8:97. Available from: https://www.mdpi.com/2309-608X/8/2/97/htm [DOI] [PMC free article] [PubMed]
- 58.Burger B, Epps S, Cardenas VM, Meena NK, Jagana R, Atchley WT. Tocilizumab is associated with increased risk of fungal infections among critically ill patients with COVID-19. [cited 2022 May 8]; Available from: www.atsjournals.org [DOI] [PMC free article] [PubMed]
- 59.Wu K-L, Chang C-Y, Sung H-Y, Hu T-Y, Kuo L-K. Association of tocilizumab and invasive aspergillosis in critically ill patients with severe COVID-19 pneumonia and acute respiratory distress syndrome. Journal of Fungi [Internet]. Multidisciplinary Digital Publishing Institute (MDPI); 2022 [cited 2022 May 8];8:339. Available from: /pmc/articles/PMC9026544/ [DOI] [PMC free article] [PubMed]
- 60.Angriman F, Ferreyro BL, Burry L, Fan E, Ferguson ND, Husain S, et al. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. The Lancet Respiratory Medicine [Internet]. Elsevier; 2021 [cited 2022 May 8];9:655. Available from: /pmc/articles/PMC8078877/ [DOI] [PMC free article] [PubMed]
- 61.• Hatzl S, Reisinger AC, Posch F, Prattes J, Stradner M, Pilz S, et al. Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: an observational study. Crit Care [Internet]. Crit Care; 2021 [cited 2022 May 13];25. Available from: https://pubmed.ncbi.nlm.nih.gov/34526087/. Role of antifungal prophylaxis in COVID-19 patients with risk factors [DOI] [PMC free article] [PubMed]
- 62.Soriano MC, Narváez-Chávez G, López-Olivencia M, Fortún J, de Pablo R. Inhaled amphotericin B lipid complex for prophylaxis against COVID-19-associated invasive pulmonary aspergillosis. Intensive Care Medicine [Internet]. Springer Science and Business Media Deutschland GmbH; 2022 [cited 2022 May 13];48:360–1. Available from: https://link.springer.com/article/10.1007/s00134-021-06603-y [DOI] [PMC free article] [PubMed]
- 63.• Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia [Internet]. Mycopathologia; 2021 [cited 2022 Feb 1];186:289–98. Available from: https://pubmed.ncbi.nlm.nih.gov/33544266/. An early case report and review of CAM. [DOI] [PMC free article] [PubMed]
- 64.•• Chao CM, Lai CC, Yu WL. COVID-19 associated mucormycosis — an emerging threat. J Microbiol Immunol Infect [Internet]. J Microbiol Immunol Infect; 2022 [cited 2022 Feb 1]; Available from: https://pubmed.ncbi.nlm.nih.gov/35074291/. An early case report and review of CAM. [DOI] [PMC free article] [PubMed]
- 65.Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux J-P, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe [Internet]. Lancet Microbe; 2022 [cited 2022 Feb 1]; Available from: https://pubmed.ncbi.nlm.nih.gov/35098179/ [DOI] [PMC free article] [PubMed]
- 66.Riad A, Shabaan AA, Issa J, Ibrahim S, Amer H, Mansy Y, et al. COVID-19-associated mucormycosis (CAM): case-series and global analysis of mortality risk factors. J Fungi (Basel) [Internet]. J Fungi (Basel); 2021 [cited 2022 Feb 1];7. Available from: https://pubmed.ncbi.nlm.nih.gov/34682258/ [DOI] [PMC free article] [PubMed]
- 67.Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux J-P, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe [Internet]. Elsevier BV; 2022 [cited 2022 Feb 1]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/35098179 [DOI] [PMC free article] [PubMed]
- 68.• John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel) [Internet]. J Fungi (Basel); 2021 [cited 2022 Feb 1];7. Available from: https://pubmed.ncbi.nlm.nih.gov/33920755/. A review of proposed pathophysiology for CAM. [DOI] [PMC free article] [PubMed]
- 69.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis [Internet]. Lancet Infect Dis; 2019 [cited 2022 Feb 1];19:e405–21. Available from: https://pubmed.ncbi.nlm.nih.gov/31699664/ [DOI] [PMC free article] [PubMed]
- 70.Barberis F, Benedetti MF, de Abreu MS, Pola SJ, Posse G, Capece P, et al. Invasive fusariosis in a critically ill patient with severe COVID-19 pneumonia: a case report. Med Mycol Case Rep Elsevier. 2022;35:5–8. doi: 10.1016/j.mmcr.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damani J. COVID-19-associated pulmonary fusarium infection in a non-critically ill immunocompetant patient. Chest [Internet]. Elsevier; 2021 [cited 2022 Feb 1];160:A286. Available from: http://journal.chestnet.org/article/S0012369221017438/fulltext
- 72.Poignon C, Blaize M, Vezinet C, Lampros A, Monsel A, Fekkar A. Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID-19. Clinical Microbiology and Infection [Internet]. Elsevier; 2020 [cited 2022 Feb 1];26:1582–4. Available from: http://www.clinicalmicrobiologyandinfection.com/article/S1198743X20303724/fulltext [DOI] [PMC free article] [PubMed]
- 73.Rabagliati R, Rodríguez N, Núñez C, Huete A, Bravo S, Garcia P. COVID-19–associated mold infection in critically ill patients, Chile. Emerging Infectious Diseases [Internet]. Centers for Disease Control and Prevention; 2021 [cited 2022 Feb 1];27:1454. Available from: /pmc/articles/PMC8084475/ [DOI] [PMC free article] [PubMed]
- 74.Basile K, Halliday C, Kok J, Chen SCA. Fungal infections other than invasive aspergillosis in COVID-19 patients. Journal of Fungi [Internet]. Multidisciplinary Digital Publishing Institute (MDPI); 2022 [cited 2022 Feb 1];8. Available from: /pmc/articles/PMC8779574/ [DOI] [PMC free article] [PubMed]
- 75.Gerber V, Ruch Y, Chamaraux-Tran TN, Oulehri W, Schneider F, Lindner V, et al. Detection of pneumocystis jirovecii in patients with severe COVID-19: diagnostic and therapeutic challenges. Journal of Fungi 2021, Vol 7, Page 585 [Internet]. Multidisciplinary Digital Publishing Institute; 2021 [cited 2022 Feb 1];7:585. Available from: https://www.mdpi.com/2309-608X/7/8/585/htm [DOI] [PMC free article] [PubMed]
- 76.• Chong WH, Saha BK, Chopra A. Narrative review of the relationship between COVID-19 and PJP: does it represent coinfection or colonization? Infection [Internet]. Infection; 2021 [cited 2022 Feb 1];49:1079–90. Available from: https://pubmed.ncbi.nlm.nih.gov/34059997/. A review of pneumocystis in COVID-19 patients. [DOI] [PMC free article] [PubMed]
- 77.Alanio A, Voicu S, Dellière S, Mégarbane B, Bretagne S. Do COVID-19 patients admitted to the ICU require anti-pneumocystis Jirovecii prophylaxis? SSRN Electronic Journal. 2020;
- 78.Alanio A, Dellière S, Voicu S, Bretagne S, Mégarbane B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J Infect [Internet]. J Infect; 2021 [cited 2022 Feb 1];82:84–123. Available from: https://pubmed.ncbi.nlm.nih.gov/33157150/ [DOI] [PMC free article] [PubMed]
- 79.Kelly S, Waters L, Cevik M, Collins S, Lewis J, Wu MS, et al. Pneumocystis pneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clinical Medicine [Internet]. Royal College of Physicians; 2020 [cited 2022 Feb 1];20:590. Available from: /pmc/articles/PMC7687333/ [DOI] [PMC free article] [PubMed]
- 80.• Mastrangelo A, Germinario BN, Ferrante M, Frangi C, Li Voti R, Muccini C, et al. Candidemia in coronavirus disease 2019 (COVID-19) patients: incidence and characteristics in a prospective cohort compared with historical non–COVID-19 controls. Clinical Infectious Diseases [Internet]. Oxford Academic; 2021 [cited 2022 Feb 1];73:e2838–9. Available from: https://academic.oup.com/cid/article/73/9/e2838/5943480. A large cohort examining candidemia in COVID-19 patients. [DOI] [PMC free article] [PubMed]
- 81.Kayaaslan B, Eser F, Kaya Kalem A, Bilgic Z, Asilturk D, Hasanoglu I, et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses. John Wiley and Sons Inc; 2021;64:1083–91. [DOI] [PMC free article] [PubMed]
- 82.• Riche CVW, Cassol R, Pasqualotto AC. Is the frequency of Candidemia increasing in COVID-19 patients receiving corticosteroids? Journal of Fungi 2020, Vol 6, Page 286 [Internet]. Multidisciplinary Digital Publishing Institute; 2020 [cited 2022 Feb 1];6:286. Available from: https://www.mdpi.com/2309-608X/6/4/286/htm. An assessment of steroids as a risk factor for candidiasis in COVID-19 patients. [DOI] [PMC free article] [PubMed]
- 83.Macauley P, Epelbaum O. Epidemiology and Mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses. John Wiley and Sons Inc; 2021;64:634–40. [DOI] [PMC free article] [PubMed]
- 84.Antinori S, Bonazzetti C, Gubertini G, Capetti A, Pagani C, Morena V, et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev [Internet]. Autoimmun Rev; 2020 [cited 2022 Feb 1];19. Available from: https://pubmed.ncbi.nlm.nih.gov/32376396/ [DOI] [PMC free article] [PubMed]
- 85.Burger BJ, Epps SM, Cardenas VM, Jagana R, Meena NK, Atchley WT. Tocilizumab is associated with increased risk of fungal infections among critically ill patients with COVID-19 and acute renal failure: an observational cohort study. 2021 [cited 2022 Feb 1]; Available from: 10.21203/rs.3.rs-611037/v1 [DOI] [PMC free article] [PubMed]
- 86.•• Peng J, Fu M, Mei H, Zheng H, Liang G, She X, et al. Efficacy and secondary infection risk of tocilizumab, sarilumab and anakinra in COVID-19 patients: a systematic review and meta-analysis. Reviews in Medical Virology [Internet]. John Wiley & Sons, Ltd; 2021 [cited 2022 Feb 1];e2295. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/rmv.2295. A review of IL-6 inhibitors effect on secondary infection in COVID-19 patients. [DOI] [PMC free article] [PubMed]
- 87.Winthrop K, Harigai M, Genovese MC, lindsey stephen, Takeuchi T, Fleischmann R, et al. Infections in baricitinib clinical trials for patients with active rheumatoid arthritis. Ann Rheum Dis [Internet]. 2020 [cited 2022 Apr 3];0:1–8. Available from: http://ard.bmj.com/ [DOI] [PubMed]
- 88.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases Society of America. Clin Infect Dis [Internet]. Clin Infect Dis; 2016 [cited 2022 Apr 3];62:409–17. Available from: https://pubmed.ncbi.nlm.nih.gov/26810419/ [DOI] [PubMed]
- 89.Karnik K, Wu Y, Ruddy S, Quijano-Rondan B, Urban C, Turett G, et al. Fatal case of disseminated cryptococcal infection and meningoencephalitis in the setting of prolonged glucocorticoid use in a Covid-19 positive patient IDCases. Elsevier. 2022;27:e01380. doi: 10.1016/j.idcr.2022.e01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thota DR, Ray B, Hasan M, Sharma K. Cryptococcal meningoencephalitis during convalescence from severe COVID-19 pneumonia: 101177/19418744211009766 [Internet]. SAGE PublicationsSage CA: Los Angeles, CA; 2021 [cited 2022 Feb 1];12:96–9. Available from: 10.1177/19418744211009766 [DOI] [PMC free article] [PubMed]
- 91.Traver EC, Malavé SM. Pulmonary aspergillosis and cryptococcosis as a complication of COVID-19. Medical Mycol Case Rep Elsevier. 2022;35:22–25. doi: 10.1016/j.mmcr.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghanem H, Sivasubramanian G. Cryptococcus neoformans Meningoencephalitis in an immunocompetent patient after COVID-19 infection. Case reports in infectious diseases. Hindawi Limited. 2021;2021:1–3. doi: 10.1155/2021/5597473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chastain DB, Henao-Martínez AF, Dykes AC, Steele GM, Stoudenmire LL, Thomas GM, et al. Missed opportunities to identify cryptococcosis in COVID-19 patients: a case report and literature review: 101177/20499361211066363 [Internet]. SAGE PublicationsSage UK: London, England; 2022 [cited 2022 Feb 1];9. Available from: 10.1177/20499361211066363 [DOI] [PMC free article] [PubMed]
- 94.Thyagarajan Rv, Mondy KE, Rose DT. Cryptococcus neoformans blood stream infection in severe COVID-19 pneumonia. Elsevier. 2021;26:01274. doi: 10.1016/j.idcr.2021.e01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alegre-González D, Herrera S, Bernal J, Soriano A, Bodro M. Disseminated Cryptococcus neoformans infection associated to COVID-19. Med Mycol Case Rep Elsevier. 2021;34:35–37. doi: 10.1016/j.mmcr.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Passarelli VC, Perosa AH, Kleber L, Luna S, Conte DD, Nascimento OA, et al. Detected SARS-CoV-2 in ascitic fluid followed by Cryptococcemia: a case report. SN Comprehensive Clinical Medicine 2020 2:11 [Internet]. Springer; 2020 [cited 2022 Feb 1];2:2414–8. Available from: https://link.springer.com/article/10.1007/s42399-020-00574-9 [DOI] [PMC free article] [PubMed]
- 97.Khatib MY, Ahmed AA, Shaat SB, Mohamed AS, Nashwan AJ. Cryptococcemia in a patient with COVID-19: a case report. Clinical Case Reports [Internet]. John Wiley & Sons, Ltd; 2021 [cited 2022 Feb 1];9:853–5. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ccr3.3668 [DOI] [PMC free article] [PubMed]
- 98.Maldonado I, Elisiri ME, Fernández-Canigia L, Sánchez AV, López L, Toranzo AI, et al. COVID-19 associated with disseminated histoplasmosis in a kidney transplant patient. Revista Argentina de Microbiologia [Internet]. Asociacion Argentina de Microbiologia; 2021 [cited 2022 Feb 1]; Available from: https://europepmc.org/articles/PMC8683274 [DOI] [PMC free article] [PubMed]
- 99.Taylor M, Ghodasara A, Ismail A, Gauhar U, El-Kersh K. Disseminated histoplasmosis in an immunocompetent patient after COVID-19 pneumonia. Cureus [Internet]. Cureus; 2021 [cited 2022 Feb 1];13. Available from: https://www.cureus.com/articles/64569-disseminated-histoplasmosis-in-an-immunocompetent-patient-after-covid-19-pneumonia [DOI] [PMC free article] [PubMed]
- 100.Messina FA, Marin E, Caceres DH, Romero M, Depardo R, Priarone MM, et al. Coronavirus disease 2019 (COVID-19) in a patient with disseminated histoplasmosis and HIV—a case report from Argentina and literature review. Journal of Fungi 2020, Vol 6, Page 275 [Internet]. Multidisciplinary Digital Publishing Institute; 2020 [cited 2022 Feb 1];6:275. Available from: https://www.mdpi.com/2309-608X/6/4/275/htm [DOI] [PMC free article] [PubMed]
- 101.Basso RP, Poester VR, Benelli JL, Stevens DA, Zogbi HE, Vasconcellos IC da S, et al. COVID-19-associated histoplasmosis in an AIDS patient. Mycopathologia [Internet]. Springer Science and Business Media B.V.; 2021 [cited 2022 Feb 1];186:109–12. Available from: https://link.springer.com/article/10.1007/s11046-020-00505-1 [DOI] [PMC free article] [PubMed]
- 102.de Macedo PM, Freitas AD, Bártholo TP, Bernardes-Engemann AR, Almeida M de A, Almeida-Silva F, et al. Acute pulmonary histoplasmosis following COVID-19: novel laboratorial methods aiding diagnosis. Journal of Fungi 2021, Vol 7, Page 346 [Internet]. Multidisciplinary Digital Publishing Institute; 2021 [cited 2022 Feb 1];7:346. Available from: https://www.mdpi.com/2309-608X/7/5/346/htm [DOI] [PMC free article] [PubMed]
- 103.Krishnamurthy P, Sharma B, Deepak D, Shukla S, Arya V, Chowdhary A. Disseminated histoplasmosis post-IL6 inhibitor use in a COVID-19 patient. Journal of Microbiology and Infectious Diseases [Internet]. Association of Health Investigations; 2021 [cited 2022 Feb 1];11:170–3. Available from: https://dergipark.org.tr/en/pub/jmid/issue/64866/994001
- 104.Moradi N, Rivero-Moragrega P. Reactivation of pulmonary coccidioides in the setting of COVID-19 infection. Chest [Internet]. Elsevier; 2021 [cited 2022 Apr 3];160:A342. Available from: http://journal.chestnet.org/article/S0012369221017955/fulltext
- 105.Krauth DS, Jamros CM, Rivard SC, Olson NH, Maves RC. Accelerated progression of disseminated coccidioidomycosis following SARS-CoV-2 infection: a case report. Military Medicine [Internet]. Oxford Academic; 2021 [cited 2022 Apr 3];186:1254–6. Available from: https://academic.oup.com/milmed/article/186/11-12/1254/6214331 [DOI] [PMC free article] [PubMed]
- 106.Chang CC, Senining R, Kim J, Goyal R. An acute pulmonary coccidioidomycosis coinfection in a patient presenting with multifocal pneumonia with COVID-19. J Investig Med High Impact Case Rep [Internet]. J Investig Med High Impact Case Rep; 2020 [cited 2022 Apr 3];8. Available from: https://pubmed.ncbi.nlm.nih.gov/33167717/ [DOI] [PMC free article] [PubMed]
- 107.Heaney AK, Head JR, Broen K, Click K, Taylor J, Balmes JR, et al. Coccidioidomycosis and COVID-19 co-infection, United States, 2020. Emerg Infect Dis [Internet]. Emerg Infect Dis; 2021 [cited 2022 Feb 1];27:1266–73. Available from: https://pubmed.ncbi.nlm.nih.gov/33755007/ [DOI] [PMC free article] [PubMed]
- 108.Shah AS, Heidari A, Civelli VF, Sharma R, Clark CS, Munoz AD, et al. The coincidence of 2 epidemics, coccidioidomycosis and SARS-CoV-2: a case report. J Investig Med High Impact Case Rep [Internet]. J Investig Med High Impact Case Rep; 2020 [cited 2022 Apr 3];8. Available from: https://pubmed.ncbi.nlm.nih.gov/32493147/ [DOI] [PMC free article] [PubMed]
- 109.Nielsen MC, Reynoso D, Ren P. The brief case: a fatal case of SARS-CoV-2 coinfection with coccidioides in Texas—another challenge we face. J ClinMicrobiol [Internet]. NLM (Medline); 2021 [cited 2022 Apr 3];59:e0016321. Available from: 10.1128/JCM [DOI] [PMC free article] [PubMed]
- 110.Luoma K, Crouch DR. Opportunistic coccidioides pulmonary infection following COVID-19 pneumonia. Am J Respir Crit Care Med [Internet]. 2021 [cited 2022 Apr 3];203. Available from: www.atsjournals.org
- 111.Nasim R, Prasad A, Nasim H. Postpartum COVID-19 complicated by blastomycosis infection. Chest [Internet]. Elsevier; 2021 [cited 2022 Feb 1];160:A274. Available from: http://journal.chestnet.org/article/S0012369221017335/fulltext
- 112.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, et al. 2016 Infectious Diseases Society of America (IDSA) Clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis [Internet]. Clin Infect Dis; 2016 [cited 2022 Apr 3];63:e112–46. Available from: https://pubmed.ncbi.nlm.nih.gov/27470238/ [DOI] [PubMed]
- 113.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis [Internet]. Clin Infect Dis; 2007 [cited 2022 Apr 3];45:807–25. Available from: https://pubmed.ncbi.nlm.nih.gov/17806045/ [DOI] [PubMed]
- 114.Chapman SW, Dismukes WE, Proia LA, Bradsher RW, Pappas PG, Threlkeld MG, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis [Internet]. Clin Infect Dis; 2008 [cited 2022 Apr 3];46:1801–12. Available from: https://pubmed.ncbi.nlm.nih.gov/18462107/ [DOI] [PubMed]