Abstract
Currently, people all over the world have been affected by coronavirus disease 2019 (COVID-19). Fighting against COVID-19 is the top priority for all the countries and nations. The development of a safe and effective COVID-19 vaccine is considered the optimal way of ending the pandemic. Three hundred and 44 vaccines were in development, with 149 undergoing clinical research and 35 authorized for emergency use as to March 15 of 2022. Many studies have shown the effective role of COVID-19 vaccines in preventing SARS-CoV-2 infections as well as serious and fatal COVID-19 cases. However, tough challenges have arisen regarding COVID-19 vaccines, including long-term immunity, emerging COVID-19 variants, and vaccine inequalities. A systematic review was performed of recent COVID-19 vaccine studies, with a focus on vaccine type, efficacy and effectiveness, and protection against SARS-CoV-2 variants, breakthrough infections, safety, deployment and vaccine strategies used in the real-world. Ultimately, there is a need to establish a unified evaluation standard of vaccine effectiveness, monitor vaccine safety and effectiveness, along with the virological characteristics of SARS-CoV-2 variants; and determine the most useful booster schedule. These aspects must be coordinated to ensure timely responses to beneficial or detrimental situations. In the future, global efforts should be directed toward effective and immediate vaccine allocations, improving vaccine coverage, SARS-CoV-2 new variants tracking, and vaccine booster development.
Keywords: breakthrough infection, coronavirus disease 2019, emergency use authorization, mass vaccine administration, SARS-CoV-2 variants, vaccine type
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus), first identified in December 2019, has a spectrum of clinical presentations, which vary from asymptomatic or minor flu-like symptoms to acute respiratory distress syndrome, pneumonia and includes morality [1], [2], [3], [4]. At least one third of individuals who become infected with SARS-CoV-2 are asymptomatic but remain capable of spreading the virus to others in vulnerable populations [4], [5], [6]. Mild-to-moderate symptoms were identified in a large cohort of approximately 44,000 COVID-19 confirmed cases in China in which 81% of the subjects were symptomatic, 14% experienced severe symptoms, and 5% had critical symptoms, with the highest severity observed in persons aged ≥65 years [7]. The overall case fatality ratio of 2% related to SARS-CoV-2 is considerably higher than that for seasonal influenza (<1%) [8], but significantly lower than SARS in 2003 (10%) or Middle East Respiratory Syndrome (MERS) (35%) [9]. Notably, a plethora of epidemiologic research has documented SARS-CoV-2 transmission during the period of pre-symptomatic incubation [10]. It is estimated that the elementary reproduction rate (R0) for SARS-CoV-2 is 2.5, compared with 2.0–3.0 for SARS-CoV and the 1918 influenza pandemic, 0.9 for MERS-CoV, and 1.5 for the 2009 influenza pandemic [11, 12]. The traits of SARS-CoV-2, which include early and rapid transmission, have caused COVID-19 to surge exponentially throughout the world.
The outbreak of a novel coronavirus was declared by the World Health Organization (WHO) on March 11, 2020 [13, 14]. The number of confirmed global COVID-19 cases at the time of writing on March 17, 2022 exceeded 462 million, with 6.05 million deaths having been reported in more than 220 countries, areas, and territories; of the total, over 168,000 confirmed cases and 4,638 deaths were reported in China [15]. The COVID-19 outbreak has disastrously impacted the health and lifestyles of people and national economies throughout the world [16]. Without doubt, the COVID-19 pandemic is the worst public threat that humans have encountered in the past 100 years.
The findings of previous research on the control of infectious diseases have emphasized the importance of developing effective vaccines, and this step is considered the only economically viable method of ending the pandemic and restoring normality in societies and economies [17]. Notably, the widespread administration of the 2009 H1N1 Influenza Vaccine ended the 2009 H1N1 pandemic, saving thousands of lives [18]. Prior to the successful development of COVID-19 vaccines, governments implemented nonpharmaceutical interventions (NPIs), including suppression, elimination, and contamination strategies to further inhibit SARS-CoV-2 transmission [19]. However, the NPIs were unsustainable for a long duration because they interrupted the normal order of human life and society [20]. Hence, the development of an effective COVID-19 vaccine was identified as having the greatest potential to provide a long-term viable solution [21].
Worldwide, researchers, clinicians, and pharmaceutical companies have cooperated closely, dedicating research efforts to the development of COVID-19 vaccines, which started with sharing the genetic sequence of SARS-CoV-2 via the Global Initiative on Sharing All Influenza Data in January 2020 [22]. According to the WHO, 344 vaccine candidates were successfully developed, and 35 of these were evaluated by the different authorities for emerging demands or full use [23]. Thereafter, a race for mass vaccine administration was launched globally against the virus—the first in human history. However, numerous questions remain unanswered because the timeline for clinical trials on the long term-protection, safety, and efficacy of various vaccine candidates was drastically restricted. Fortunately, 12,992 articles on COVID-19 vaccines have been published to date, and this has provided a sound opportunity for a synopsis of the recent progress made by COVID-19 vaccine candidates against SARS-CoV-2 [24, 25].
The current study comprised a systematic review of studies on COVID-19 vaccines, with attention being paid to the advantages, disadvantages, safety, and efficacy of differing vaccine types in clinical trials; the identification of breakthrough infections, immunization strategies, and key populations for early COVID-19 immunization; as well as vaccination boosters and progress. The effectiveness of approved COVID-19 vaccines in the real-world and their variants in different countries was evaluated. Thus, the aim of the current study was to provide governments, public healthcare workers, researchers, and vaccine developers with a comprehensive understanding of the features of different COVID-19 vaccines and the issues about the COVID-19 correlates of immune control.
Study objectives
A large number of candidates for the COVID-19 vaccine, of which the efficacy has been confirmed in randomized clinical trial (RCT), have been subjected to further testing in the real-world. However, to the best of our knowledge, a review has not previously been conducted with regard to this issue. Thus, the objective of the current study was to systematically review the features of different vaccine types, vaccine efficacy and effectiveness, breakthrough infections, the degree of protection provided by vaccines against SARS-CoV-2 variants, vaccine safety, administration strategies, vaccine deployment, and vaccination processing, to provide a comprehensive overview of the profile and testing of vaccines undertaken in RCTs to prevent COVID-19 in the real-world context. This study will be useful to governments and medical healthcare systems in the promotion of public health.
Methodology
Data sources
Databases (i.e., PubMed, Embase, Web of Science, and the China National Knowledge Internet Database) were searched to identify published articles in journals and peer-reviewed articles, as well as preprints, and the results of clinical trials and the research of real-world and technical documents in English and Chinese up to March 17, 2022. In addition, reports published on official healthcare websites (i.e., the WHO, the Centers for Disease Control and Prevention [CDC], and the European Centre for Disease Prevention and Control) were searched. The following keywords were applied: “severe acute respiratory syndrome coronavirus 2”, “SARS-CoV-2”, “COVID-19”, “coronavirus”, “vaccines”, “COVID-19 vaccine type”, “COVID-19 vaccine effectiveness”, “COVID-19 vaccine efficacy”, “SARS-CoV-2 variants”, “Delta SARS-CoV-2 variant”, “breakthrough infections”, “vaccine safety”, “vaccine boosters”, and “real world”. Data from pre-clinical trials and experimental studies were excluded. Ultimately, 448 articles up to March 17, 2022 were considered eligible for inclusion in the current study.
Definitions of vaccine efficacy and effectiveness
According to the WHO, vaccine efficacy is determined by how the vaccine performed under ideal conditions (i.e., in controlled clinical trials) based on a comparison of the number of vaccinated individuals who went on to test positive for COVID-19 with the number of people who received the placebo but who experienced the same outcome [26, 27].
Vaccine effectiveness is determined by how a vaccine performed in a real-world setting (i.e., a measure of how well vaccination protected people against outcomes, such as, infection, symptomatic illness, hospitalization, and death), typically measured in an observational study [26, 27].
Vaccine effectiveness in the real-world context may differ to vaccine efficacy determined in a clinical trial because it is not possible to accurately predict how effective vaccination will be in larger, variable real-world populations. Vaccine efficacy and effectiveness is measured by calculating the risk of disease among vaccinated and unvaccinated persons and determining the percentage reduction in the risk of disease among vaccinated persons relative to unvaccinated persons [28]. The greater the percentage reduction in illness in the vaccinated group, the greater the efficacy and effectiveness of the vaccine. The basic formula is written as follows [28]:
Risk among unvaccinated group – risk among vaccinated group/ Risk among unvaccinated group, or: 1 − risk ratio
Vaccine candidate types
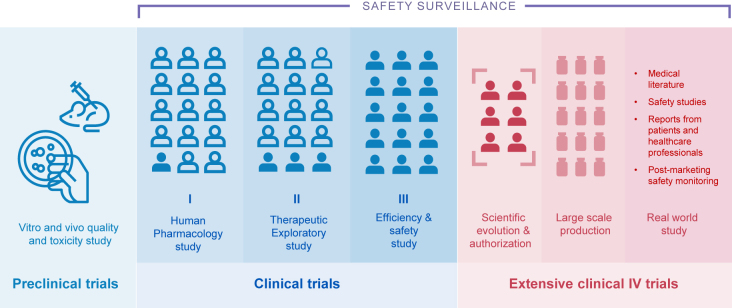
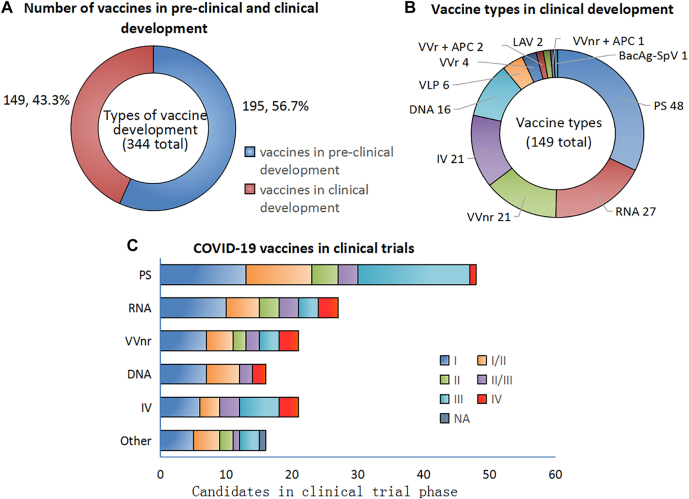
Substantial advances have been made in vaccine development, and a plethora of studies has been performed to assess the safety and efficacy of vaccines, including preclinical and clinical trials (Stage I–IV) (Figure 1). By March 15, 2022 the WHO had reported on the development, via different clinical approaches, of 11 SARS-CoV-2 vaccine formulations still in the preclinical and clinical stages (Figure 2), and these included inactivated viruses, protein subunits, non-replicating viral vectors (VVnr), replicating viral vectors (VVr), DNA, RNA, virus-like particles, VVr + antigen-presenting cells (APCs), live attenuated viruses, and VVnr + APCs and Bacterial antigen-spore expression vector(BacAg-SpV) [23]. The most common approach utilized protein subunits, which accounted for 32% (48/149) of all vaccines types, while 18% (27/149), 14% (21/149), and 14% (21/149) of the vaccines comprised RNA vaccines, VVnr, and inactivated viruses, respectively (Figure 2). Most COVID-19 vaccines that were developed to provide acquired immunity against SARS-CoV-2 were built using a complete or partial portion of the SARS-CoV-2 spike protein [29, 30]. By March 15, 2022, a total of 344 vaccine candidates were being developed. Ninety of these were in clinical Stage I or I–II trials; 48 were in clinical Stage II–III or III trials, and 10 were in clinical Stage IV trials and one in unknown clinical stages among 149 vaccines in trials in total (Figure 2) [23]. March 15, 2022, 35 vaccines (RNA [n=3], DNA [n=1], viral vectors [n=6], protein subunits [n=13], and traditional inactivated vaccines [n=11]) and virus-like particle (VLP, n=1) had been authorized for conditional marketing for public emergency use (Table 1) [23].
Figure 1:
Overview of COVID-19 vaccine development in clinical trials and approval phases.
Figure 2:
Landscape of COVID-19 vaccine candidates in clinical development and authorization across the globe March 15, 2022. Notes: A: A total of 344 vaccine candidates is in pre-clinical and clinical development. B: The percentages and number of different vaccine candidates’ in clinical development. C: The number of different vaccine candidates in clinical testing I-IV phase. Abbreviation: VVnr = Viral vector (No n-replicating); APC= Antigen Presenting Cell; VVr= Viral Vector (replicating); IV= Inactivated Virus; PS= Protein subunit; LAV= Live Attenuated Virus. VLP= virus-like particle; NA=no data available. The vaccine candidate data was sourced from WHO (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines).
Table 1:
The vaccination program of authorized and approved COVID-19 vaccines in primary series by WHO or China as to March 21, 2022.
| Vaccine types | Vaccine ID | Vaccine Brand Name | Vaccinated populations | Number of shots | When Are You Fully Vaccinated | References |
|---|---|---|---|---|---|---|
| RNA | Spikevaxa | Moderna | People 18 years and older | 2 doses Given 4–8 weeks apart |
2 weeks after final dose in primary series | https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html |
| Comirnatya | Pfizer-BioNTech | People 5 -11 years old | 2 doses Given 3 weeks apart |
2 weeks after final dose in primary series | https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html | |
| People 12 years and older | 2 doses Given 3–8 weeks apart |
2 weeks after final dose in primary series | https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html | |||
| Viral Vector (non-replicating) | Convideciab | CanSino | People 18 years and older | 1 shot | 2 weeks after 1st dose | [38] |
| Ad26.COV2.Sa | Janssen (Johnson & Johnson’s Janssen) | People 18 years and older | 1 shot | 2 weeks after 1st dose | https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html | |
| Vaxzevriaa | Oxford/AstraZeneca | People 18 years and older | 2 shots Given 8–12 weeks apart |
2 weeks after your second shot | [37] https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1 | |
| Covishield (Oxford/AstraZeneca formulation)a | Serum Institute of India | People 18 years and older | 2 shots Given 12-16 weeks apart |
2 weeks after your second shot | 1. https://www.mohfw.gov.in/pdf/AdministrationofSecondDoseofCovishieldVaccinePriortoPrescribedTimeInterval.pdf2. https://extranet.who.int/pqweb/vaccines/covid-19-vaccine-chadox1-s-recombinant-covishield | |
| Protein subunit | Zifivaxb | Anhui Zhifei Longcom | People 18 years and older | 3 shots Given 30 days apart |
2 weeks after your third shot | [90] |
| COVOVAX (Novavax formulation)a | Serum Institute of India | People 12 years of age and older | 2 shots Given 3 weeks apart |
2 weeks after your second shot | https://www.seruminstitute.com/pdf/COVOVAX_SmPC.pdf | |
| Nuvaxovida | Novavax | People 18 years and older | 2 shots Given 21 days apart |
2 weeks after your second shot | https://www.ema.europa.eu/en/documents/product-information/nuvaxovid-epar-product-information_en.pdf | |
| Inactivated Virus | CoronaVaca,b | Sinovac | People 3 years and older | 2 shots Given 14–28 days apart |
2 weeks after your second shot | [45, 46] https://extranet.who.int/pqweb/vaccines/who-recommendation-sinovac-covid-19-vaccine-vero-cell-inactivated-coronavac |
| Coviloa,b | Sinopharm (Beijing) | People 3 years and older | 2 shots Given 21–28 days apart |
2 weeks after your second shot | https://covid19.trackvaccines.org/vaccines/5/#trial-irct20171122037571n3 | |
| Covaxina | Bharat Biotech | People 18 years and older | 2 shots Given 28 days apart |
2 weeks after your second shot | https://www.bharatbiotech.com/covaxin.html | |
| Inactivated (Vero Cells)b | Sinopharm (Wuhan) | People 3 years and older | 2 shots Given 14–28 days apart |
2 weeks after your second shot | https://www.chictr.org.cn/showprojen.aspx?proj=52227 | |
| KCONVACb | Minhai Biotechnology Co | People 18 years and older | 2 shots Given 28 days apart |
2 weeks after your second shot | https://covid19.trackvaccines.org/vaccines/47/ | |
| IMBCAMSb | The Institute of Medical Biology of the Chinese Academy of Medical Sciences | People 18 years and older | 2 shots Given 14–28 days apart |
2 weeks after your second shot |
https://clinicaltrials.gov/ct2/show/NCT04659239
https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_02April2022.pdf |
Virus-like particle=VLP. aWorld Health Organization (WHO) issued an emergency use listing (EUL); bChina issued an emergency use listing or conditional market approval for public use.
In general, in terms of vaccine development, it is thought that a vaccine that is most similar to the pathogenic virus is most likely to have the best immunological response. However, structurally, vaccine types differ slightly to one another; accordingly, their merits and demerits also vary with respect to immunogenicity, safety, efficacy and effectiveness, ease of use, and storage conditions.
DNA and mRNA vaccines
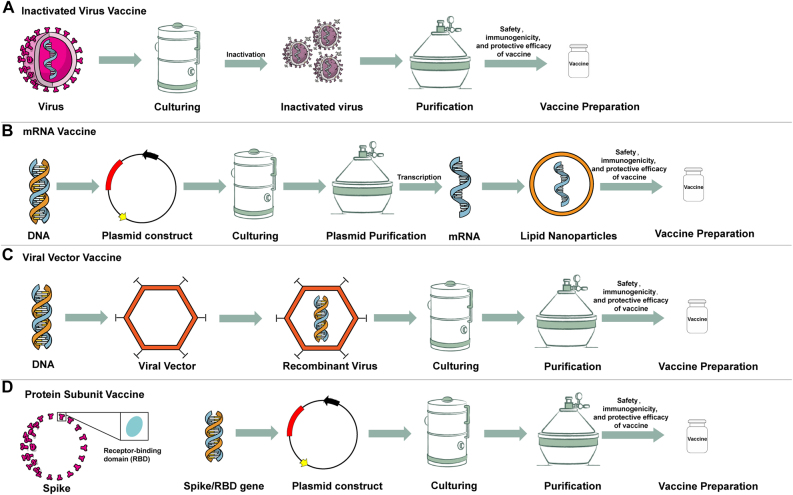
The latest vaccine technology is based on nucleic acids (i.e., DNA and mRNA). DNA, which encodes the antigen of a pathogen into a plasmid (e.g., the antigenic components of SARS-CoV-2, including the spike protein), is introduced to make DNA vaccines. By contrast, the mRNA vaccine comprises the mRNA or self-amplifying RNA that codes for a portion of the SARS-CoV-2 spike protein. The RNA is co-formed into lipid molecules for protection and to aid delivery into the specific immune cells of vaccinated individuals (Figure 3) [31], [32], [33], [34]. The RNA is taken up by the host cells and used by the host’s own protein-making machinery to manufacture the virus protein, which then induces the desired immune response against subsequent infection. Compared to other vaccine types, DNA and mRNA vaccines have several benefits, including the use of non-infectious elements, shorter manufacturing times, and enhanced potential for targeting multiple diseases. In comparison with DNA vaccines, RNA vaccines have an enhanced security profile. With a DNA vaccine, the theoretical possibility is that some of the DNA may insert into the individual’s own DNA. The most significant benefit of these technological advances is that mRNA and DNA vaccines can be developed in laboratories using readily available materials. This means the process can be standardized and scaled up, which ensures more rapid vaccine development than that achieved using traditional methods [24, 25, 29]. However, the development and licensing of vaccines based on RNA and DNA has not previously been awarded for use in humans, meaning that nucleic acid vaccine types are unproven in practice. Some RNA and DNA vaccines also require ultra-cold storage conditions and the potential risks of adverse events (ADEs) when used alone (Table 2) [24, 25, 29]. The vaccination program for authorized and approved mRNA vaccines is depicted in Table 1. The first approved COVID-19 vaccines in the United States (USA), United Kingdom (UK), and European Union (EU) were RNA vaccines.
Figure 3:
Conceptual graph for four different COVID-19 vaccine types. Notes: A: Inactive vaccine; B: mRNA vaccine; C: Viral vector vaccine; D: Protein subunit vaccine.
Table 2:
Comparison of the advantages and disadvantages of different COVID-19 vaccine candidates in clinical phases.
| COVID-19 vaccine candidates | Technology | Composition | Advantages | Disadvantages |
| mRNA | Recombinant RNA technologies | S gene+ Lipid nanoparticles |
|
|
| DNA | Recombinant DNA technologies | S gene |
|
|
| Viral Vector (non-replicating) | Recombinant genetic engine | S gene + Vector (adenovirus or influenza virus) |
|
|
| Viral Vector (replicating) | Recombinant genetic engine | S gene +Vector | Same to Viral Vector (non-replicating) |
|
| Protein subunit | Recombinant DNA and protein technologies | S protein or RBD of S protein +adjuvant |
|
|
| Inactivated Virus | The virus is completely inactivated by high heat or low amounts of formaldehyde | A whole SARS-CoV-2+ adjuvant |
|
|
| Live Attenuated Virus | Repeatedly growing-or passaging—the virus in nonhuman cells, or cells for which the virus does not have optimal tropism | A whole SARS-CoV-2, but the virus has been attenuated, or weakened |
|
|
Viral vector vaccines
The separation of viral vector vaccines into viral vectors that can continuously copy themselves in the body (VVr) and those that can no longer do so (VVnr) has previously been performed. However, the same vaccination principle applies to both cases. In terms of an inactivated virus, including an adenovirus, the given genetic material that encodes the COVID-19 spike protein is introduced into the cells. The infected cells are instructed to produce a large amount of antigen, and this then triggers an immune response (Figure 3). In essence, the vaccine mimics what occurs when certain pathogens, especially viruses, are naturally infected [35]. Viral vector-based vaccines have the merits of inducing a substantial cellular immune response by the T and B cells [25]. Another advantage of this approach is that it can be easily adapted to mass production in different parts of the world. A challenge of this method is that people who have been exposed to the virus vector in the past could have an increased immune response against it, thereby decreasing the effectiveness of the vaccine. Another hidden disadvantage of live vaccines is that they can spread in genetically unstable environments because altered biological properties may be obtained by the carrier vaccine organisms before or after recombination with wild strains [36]. Researchers have less experiences in viral vector vaccines compared to the new approaches, including mRNA vaccines (Table 2). The recombinant vesicular stomatitis virus–Zaire Ebola virus vaccine against Ebola, an authorized and approved vaccination program for VVnr, is an example of a viral vector vaccine (Table) 1 [37, 38].
Protein subunit vaccines
Subunit vaccines contain one or more antigens that trigger an immune response without the need to introduce whole pathogen particles (Figure 3). Nearly all COVID-19 vaccine types target a specific viral protein, known as a spiked protein, specifically selected for its ability to stimulate immune cells. Subunit vaccines have several advantages and disadvantages (Table 2). The most important advantage of recombinant subunit vaccines is that a viral protein or group of proteins is contained, which means that they are incapable of causing disease and are considered very safe. Another advantage of protein subunit vaccines is that they are associated with fewer side-effects compared to those that contain whole viruses (i.e., weaker or inactivated virus vaccines). An additional benefit of protein subunit vaccines is their ability to last longer than novel vaccine technologies. Nevertheless, the use of adjuvants is required by protein subunit vaccines to drive the immune response. An adjuvant is an ingredient that is used in some vaccines to stimulate the body to produce stronger humoral immunity and/or a cellular immunity response in people receiving the vaccine [39]. Many different types of adjuvants are available, for example, aluminum salts, liposomes, and cytosine phosphoguanine, and each has a different mode of action. Adjuvant vaccines are associated with more intense local reactions (i.e., redness, swelling, and pain at the injection site) and systemic reactions (i.e., fever, chills, and body aches) than non-adjuvant vaccines [40]. In all cases, adjuvants have been used safely in vaccines for decades, and many vaccines that contain adjuvants have been tested for safety and effectiveness in clinical trials [41, 42]. The immunity triggered by protein subunit vaccines may not be as long-lasting as that achieved by vaccines that contain the whole virus. A few subunit vaccines are used in widespread applications; examples include the hepatitis B and acellular pertussis vaccines (protein subunits). The vaccination program for authorized and approved protein subunit vaccines is depicted in Table 1.
Inactivated virus vaccines
Inactivated virus vaccines are produced using traditional technologies that involve the cell culture of virus particles. They are then completely inactivated by chemical or physical agents (e.g. heat, formalin, or β-propiolactone) (Figure 3). This causes the viruses to lose infectivity while simultaneously stimulating an immune response. The most significant advantage of inactivated virus vaccines is that they do not contain any live viral particles, so they are noninfectious; in addition, they are also more stable and safer than live attenuated vaccines. Vaccines developed using inactivated virus technologies have been licensed previously, and the technologies have been widely applied in the development of vaccines against viruses, such as hepatitis A; influenza; hand, foot, and mouth disease; and polio. Therefore, with an established manufacturing process, controlled quality norms, and extensive scope for control, inactivated viral vaccines can be utilized for large-scale vaccinations, with internationally accepted standards being used to gauge their security and effectiveness [40].
A drawback to the use of inactivated virus vaccines is that the immunogenicity following vaccination is weak, so an aluminum adjuvant is required to increase immunogenicity, and the vaccinations require further augmentation [25]. Notably, the use of inactivated viruses against COVID-19 has had to take into account the potential risks of incurring antibody-dependent enhancement, as was found with other coronaviruses, such as MERS-CoV and SARS-CoV (Table 2) [43, 44]. The vaccination program for authorized and approved inactivated virus COVID-19 vaccines is depicted in Table 1 [45, 46].
Live attenuated vaccines
A live attenuated vaccine contains a version of the living virus that is transmitted through animal or human cells until mutation occurs in the genome so that the virus is no longer capable of causing disease [42]. Good examples of live attenuated vaccines are those that were developed against tuberculosis (i.e., Bacillus Calmette–Guérin), smallpox, and polio. Generally, the elongated virus reproduces an inborn infection, which stimulates a substantial T and B cell response, leading to long-lasting immunity [47]. The design of single-use vaccines that use live virus vaccines is easier than that of other vaccine types. It is also less likely that such vaccines require the utilization of an extra adjuvant, an agent that boosts the immune response [41].
Although it is thought that live attenuated vaccines have optimal biological immunity, they do have major limitations, one of which is that, since they are replicating viruses, the possibility always exists that the attenuated virus may revert to a fully virulent wild-type strain [47]. Another limitation is that the use of a live attenuated vaccines is not suitable for people with compromised immune systems. In addition, the vaccine is dependent on cold chain distribution (Table 2).
Vaccine efficacy in clinical trials
The efficacy of new vaccines is described in terms of the protection offered by immunization in a specific population. Efficacy is determined by comparing the relative risk of contracting the disease in vaccinated vs. unvaccinated individuals. A vaccine is subject to three assessment rounds (Phase I–III trials) to assess efficacy. Each phase includes objectives and endpoints. Phase III clinical trials are necessary for all vaccine candidates to demonstrate their effectiveness in the greater population. Following Phase III trials, each vaccine must receive the approval of an authorized institution before licensing and distribution can take place. Phase IV comprises the final assessment phase and includes ongoing studies of risks and side-effects (Figure 1). As the number of vaccines and clinical trials increases, it can be difficult to determine how much efficacy is required for a vaccine to attain herd immunity [40].
The approval agencies (i.e., the US Food and Drug Administration [FDA]) have set 50% efficacy as the lowest value required for COVID-19 vaccine authorization, and the same standard is required by the European Medicines Agency [EMA]). The value of the basic reproduction number (R0) varies with different SARS-CoV- 2 variants and, consequently, in response to the herd immunity threshold and vaccination coverage. For example, the herd immunity threshold for COVID-19 would be 60% with an R0 value of 2.5 for the original strain and 80% with an R0 value of 5.0 for Delta variants. Health professionals have estimated that 70%–90% of the population must be immune to achieve herd immunity against COVID-19 [48], [49], [50], [51], [52].
Presently, three mRNA COVID-19 vaccines are under consideration for emergency use authorization (Table 1). The first, the BNT162b2 mRNA COVID-19 vaccine (Pfizer–BioNTech), was evaluated in Stage III trials performed in Argentina, the USA, Brazil, Germany, South Africa, and Turkey, and it demonstrated efficacy of 95% against symptomatic, laboratory-confirmed COVID-19 in people with no evidence of past SARS-CoV-2 infection following the administration of two doses [53], [54], [55], [56], [57], [58]. Estimated vaccine efficacy for severe COVID-19 was 89% over the entire study period.
The mRNA-1273 vaccine (Moderna) was the second vaccine to gain approval from the FDA and the EMA. The mRNA-1273 vaccine was shown to have high efficacy, with 94% of vaccinated persons achieving full immunization two weeks after administration of the second dose, along with 100% control against severe infections [59]. In addition, its efficacy did not vary according to age, gender, ethnicity, or co-morbidities. These findings suggest that its efficacy is as high as that of the BNT162b2 mRNA COVID-19 vaccine (Pfizer–BioNTech). The TAK-919 was the third vaccine, which included the Moderna formulation.
Six VVnr were licensed and authorized for emergency use. One of VVnr, namely the AZD1222 vaccine (Oxford University–AstraZeneca), which was shown to have 81% efficacy against mild and moderate symptoms and 100% efficacy against serious infection and death, according to the preliminary findings of a Phase III trial on approximately 40,000 subjects [60]. Another VVnr, namely, Ad26.COV2.S vaccine (Johnson & Johnson), a single-dose, adenovirus-vectored vaccine, along with the Ad5-nCoV vaccine (CanSino Biologics), were shown to have 66% efficacy in preventing moderate COVID-19 symptoms and 91% efficacy against severe SARS-CoV-2 infection during clinical trials [61].
Thirteen protein subunit (i.e., NVX-CoV2373, EpiVacCorona, RBD-Dimer, and MVC-COV1901, etc.) were licensed and authorized by the local government for emergency use as March 15, 2022. A recent study confirmed total efficacy of 90% at seven days after the administration of the second dose of the NVX-CoV2373 vaccine [62, 63]. However, a South African trial indicated that it had reduced effectiveness of NVX-CoV2373 against the Beta variant, according to preliminary findings.
Eleven inactivated vaccines have received authorization for emergency use in at least one country and area. CoronaVac, also known as the Sinovac COVID-19 vaccine (Sinovac Biotech), has efficacy of 51%–84%, 80%, and 85%–100% in preventing symptoms of infection, hospitalization, and serious illness, respectively, according to the results of peer-reviewed Stage III trials from Brazil, Chile, and Turkey [64]. Similar efficacy of 78% against symptomatic infection and 100% against serious infection was reported for the BBIBP-CorV vaccine, also known as the Sinopharm COVID-19 or BIBP vaccine (Sinopharm’s Beijing Institute of Biological Products) in Stage III trials in Bahrain and the United Arab Emirates (UAE) [65]. Bharat Biotech International (India) reported that the vaccine was 64% effective against asymptomatic cases, 78% effective against symptomatic cases, 93% effective against severe COVID-19 infection, and 65% effective against the Delta variant.
In general, it is difficult comparing the efficacy of different vaccines since the clinical trials covered different populations, regions, and virus-predominant variants at different stages of the COVID-19 pandemic.
Vaccine effectiveness in the real-world
By March 15, 2022, a total of 35 vaccines had been approved, based on clinical research, for emergency use in at least one region across the globe. COVID-19 vaccinations were launched at the end of 2020. With the massive global vaccination campaign against COVID-19, the effectiveness of vaccines had to be evaluated to determine a range of outcomes under real-world conditions [66]. Clinical tests are considered to be the “gold standard” for assessing vaccine efficacy, but they cannot replicate mass vaccination owing to the smaller sample sizes, limited subgroups, and strict inclusion criteria. Real-world studies can confirm the effectiveness of a certain vaccine in vaccinated people in a large population, and this has proven crucial in controlling COVID-19 infection, hospitalization, and mortality in clinical trials. To date, several real-world studies have reported on the efficacy of different vaccines, and this has varied by age, population, race, environment, and exposure to different variants strains in different countries during various stages of the pandemic.
mRNA vaccines
Three RNA vaccines (Pfizer–BioNTech and Moderna) Takeda TAK-919 (Moderna formulation) were approved for emergency use authorization in 181 countries after the development of the first Pfizer–BioNTech vaccine dose in December 2020 in the UK. The effectiveness of the mRNA vaccine against SARS-CoV-2 in the real-world context has been assessed in the general population, healthcare workers, and older populations [67], [68], [69].
In the general population, the effectiveness of the BNT162b vaccine (Pfizer–BioNTech) and the mRNA-1273 vaccine (Moderna) in fully vaccinated people ranged from 77%–98% and 93%–99%, respectively. These results are similar to the clinical Phase III trial results for these two mRNA vaccines, both established to have efficacy of 95%. However, the effectiveness of the vaccines in fully vaccinated individuals was much higher than that in partially vaccinated individuals (i.e., 42%–78% for BNT162b and 67%–93% for mRNA-1273, respectively) [70]. The mRNA vaccine was demonstrated to be 95% effective in preventing symptomatic infections in a real-world study in Demark [70]. In addition, the effectiveness of the vaccines against infection was reported to be 92% vs. 46% against symptomatic infection, 94% vs. 57% against hospital admission, 87% vs. 74% against severe infection, and 92% vs. 62% in fully vaccinated individuals in a study in Israel with a large sample size [66]. However, vaccine effectiveness decreased by 86% in individuals with underlying medical conditions [57]. In addition, the effectiveness of the vaccines in the general population was confirmed in real-world studies performed in England and Scotland [71].
In terms of the elderly, a study in England indicated that people aged ≥70 years (over 7.5 million) who were vaccinated with the BNT162b2 vaccine experienced initial effectiveness of 61% following vaccination; however, this then plateaued. One dose of the BNT162b2 vaccine was associated with an additional lower risk of emergency hospitalization and death of 43% and 51%, respectively.
From January 1, 2021, to March 26, 2021, a multistate network of USA hospitals received Pfizer–BioNTech or Moderna COVID-19 vaccines, and the CDC reported vaccine effectiveness, using either, of 94% and 64%, against hospitalization due to COVID-19 in fully vaccinated adults aged ≥65 years old and in partially vaccinated adults of the same age, respectively [72]. A USA study reported on the incidence of SARS-CoV-2 infection in vaccinated and unvaccinated residents living in 280 nursing homes across 21 states. The findings showed that cases identified with SARS-CoV-2 infection in the first- (5%) and second-dose vaccine groups (1%) were much lower than those in the unvaccinated group (4%) [73].This finding was also consistent with the vaccine efficacy identified in previous clinical tests on a group of adults aged ≥65 years, thereby providing evidence of the effectiveness of mRNA vaccines against infection with COVID-19 in the real-world context in adults, particularly older individuals [54], [55], [56], [57], [58], [59].
The mRNA vaccine is particularly useful in frontline workers who may spread the virus to colleagues and the public through frequent and intimate exposure to patients. Estimates of mRNA vaccine effectiveness at preventing SARS-CoV-2 infection in healthcare personnel have been reported in Israel, the USA, and England. Data from 5,297 healthcare workers in Israel demonstrated vaccine effectiveness of 75% in reducing symptomatic infections 2–3 weeks after the administration of the first dose of the BNT162b2 vaccine [54], [55], [56], [57], [58]. In England, real-time PCR experiments were conducted twice a week from December 7, 2020, to February 5, 2021, on 15,121 medical staff members in 104 hospitals. On average, the use of the Pfizer–BioNTech vaccine led to an reduction in all infections (including asymptomatic disease) by 72% (a range of 58%–86%) 21 days after the administration of the first dose and to a reduction of 86% (a range of 76%–97%) seven days after the administration of the second dose [74].
A similar study, conducted by the CDC from December 14, 2020 to March 13, 2021 on 3,950 medical staff members, first responders, and basic/frontline workers from eight different institutions reported rates of 1.38% per 1,000 person-days for SARS-CoV-2 infections in unvaccinated participants, with respective figures of 0.04% in fully immunized participants and 0.19% in partially immunized participants at least 14 days after the first dose and prior to the second dose [75]. Substantial effectiveness derived from full immunization against SARS-CoV-2 infections (90%) was confirmed with the use of the mRNA vaccine regardless of symptom status, along with 80% vaccine effectiveness in individuals who were partially immunized [75].
A medical center in the USA recorded a marked reduction in infections in healthcare workers following full immunization in non-vaccinated (2.61% [234/8,969]) vs. fully vaccinated personnel (0.05% [4/8,121]) [62, 63]. Cohort research on 3,975 healthcare personnel, first responders, and other essential and frontline workers conducted by the CDC from December 14, 2020 to April 10, 2021, detected SARS-CoV-2 in 204 participants (5%); five of the study subjects had been completely vaccinated at least 14 days after the administration of dose 2, 11 had been partially vaccinated at least 14 days after the administration of dose 1 but less than 14 days after the administration of dose 2, and 156 were unvaccinated. Modified vaccine effectiveness was 91% after full vaccination and 81% after partial vaccination. In participants infected with SARS-CoV-2, the average viral RNA load was 40% lower in partially or completely vaccinated individuals, compared to unvaccinated individuals. The vaccinated participants also demonstrated a 58% lower risk of fever and a shorter duration of illness, with 2.3 fewer days spent in bed [76].
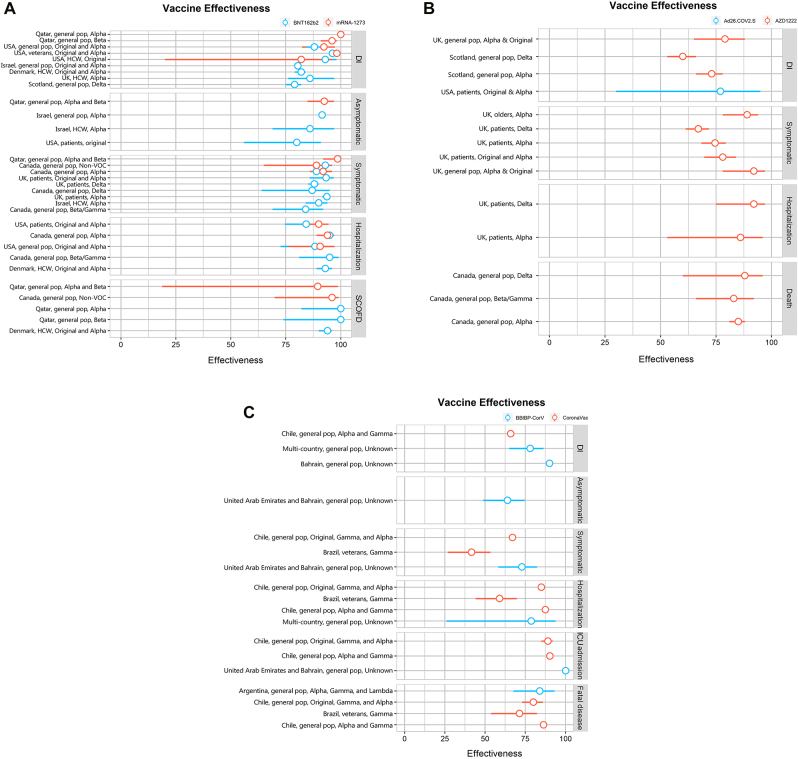
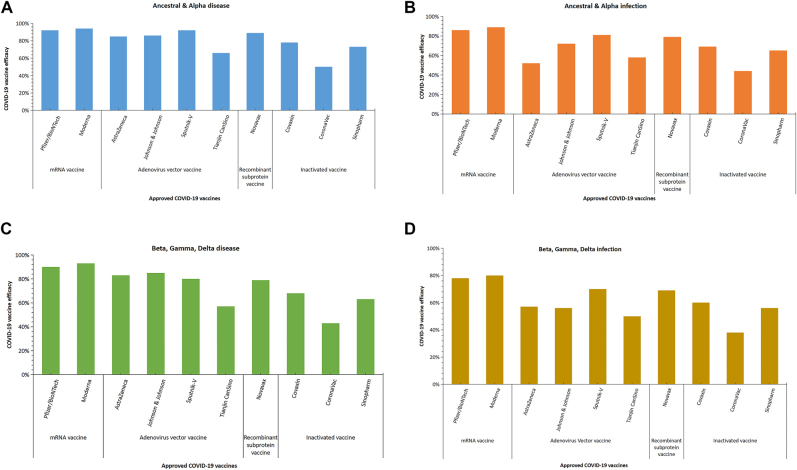
The number of vaccines administered to people in the real-world greatly exceeds the number of vaccines assessed during the Phase III clinical trials of Pfizer and Moderna combined. The mRNA vaccine has been demonstrated to be highly effective in preventing SARS-CoV-2 infection, serious illness, hospitalization, and morality the general population, elderly populations, and healthcare workers. Increasingly, the evidence is that people who have been fully vaccinated with an mRNA vaccine (Pfizer–BioNTech or Moderna) at are less risk of acquiring SARS-CoV-2 than those who have not been vaccinated. In addition, the vaccine helps attenuate viral RNA load, the risk of fever, and disease duration in the context of a vaccine breakthrough [49]. Real-world applications of the mRNA vaccine offer strong reassurance of the validity of the benefits observed during RCTs [76]. The level of effectiveness afforded by full vaccination with the mRNA vaccine is depicted in Figure 4A [77].
Figure 4:
The differenct COVID-19 vaccine effectiveness of full immunization was assessed in real world study. Notes: A: mRNA vaccine; B: Adenoviral vector vaccine (non-replicating); C: Inactive vaccine. DI = Documented infection; SCOFD = Severe, critical or fatal disease; ICU = Intensive Care Unit.
Non-replicating viral vector vaccines
The AZD1222 vaccine (Oxford University–AstraZeneca) is the most representative of the VVnr vaccines, and it was authorized for public use in the UK on December 30, 2020. At the time of writing, the AZD1222 vaccine was licensed in 183 countries and regions [78].
Real-world studies have been conducted on the AZD1222 vaccine in the general and senior populations of Brazil, Scotland, and England. The study in Brazil indicated a 71% decrease in symptomatic infections following its administration. Between December 2020 and February 2021, 1.3 million of 5.4 million people included in a study in Scotland had been vaccinated over the study period, and, of these, 620,154 had received the Oxford University–AstraZeneca vaccine.
The mass roll-out of the first doses of the ChAdOx1 vaccine was reported to provide 88% protection against admission to hospital due to COVID-19 28–34 days after vaccination [71]. In a study with a large cohort (10,400 long-term care patients aged ≥65 years old residing in 310 long-term institutional care facilities across England), which covered a period between December 8, 2020, to March 15, 2021, vaccine effectiveness was shown to be 56% at 28%–34 days and 62% (a range of 23%–81%) at 35%–48 days after the administration of a single dose of the ChAdOx1 or BNT162b2 vaccine. The average PCR cycle threshold (Ct) value was greater for infectious disease identified at least 28 days after vaccination than that identified prior to vaccination (i.e., 26.6 in 552 PCR-positive experiments vs. 31.3 in 107 PCR-positive experiments) (p≤0.000) [78]. This suggests that substantial control against infection, along with a reduction in SARS-CoV-2 transmission, was provided 4–7 weeks following the administration of a single ChAdOx1 vaccination dose to seniors [78]. This is one of the earliest examples of evidence of the real-world efficacy of the ChAdOx1 vaccine in patients in long-term institutional care.
The Janssen Ad26.COV2.S vaccine (Johnson & Johnson) is another example of a VVnn vaccine, and it has been included in the WHO Emergency Use Listing. In a real-world study conducted in the USA, the rate of infection in 2,195 people who received a dose of the Ad26.COV2.S vaccine was compared with the rate of infection in 21,950 unvaccinated individuals. Of 1,779 vaccinated people, only three individuals (0.17%) tested positive for SARS-CoV-2, compared to 128 of 17,744 unvaccinated individuals (0.72%), representing a 4.34-fold decrease, and this corresponded to a preventive effect, through vaccination, of 77% against infection with SARS-CoV-2, with the identification of disease at least two weeks following vaccination. This information confirms the level of effectiveness (i.e., of 67% ) of the Ad26.COV2 vaccine against moderate to severe COVID-19 that was previously determined in the clinical trial on the Ad26.COV2 vaccine, with vaccine effectiveness beginning at least 14 days after vaccination [73]. The effectiveness of viral vector vaccines against infection with SARS-CoV-2 after full vaccination is depicted in Figure 4B [77].
Inactivated virus vaccines
Of the ten vaccines approved for use by the WHO, three of them, the CoronaVac and Covilo and Covaxin vaccines, are inactivated virus vaccines. The primary focus of several real-world studies has been on the CoronaVac vaccine. In their real-world study from February 2, 2021, to May 1, 2021, conducted using a cohort of 10.2 million Chileans, Alejandro et al. reported modified vaccine effectiveness of 66%, 88%, 90%, and 86% against symptomatic COVID-19 infection, hospitalization, intensive care unit hospitalization, COVID-19-associated mortality, respectively, in fully immunized individuals [79]. The results of this study correlated with those of the Sinovac Phase II trials, which suggests that the findings on the effectiveness of inactivated virus vaccines were valid and reliable. Cesar et al. evaluated the impact of early immunization against COVID-19 on mortality in the elderly in Brazil under real conditions. CoronaVac and AstraZeneca comprised 77 and 16% of the administered doses. The rate of first-dose vaccination increased rapidly in people aged ≥80 years, with 49% of this age group having been vaccinated by weeks 5–6 and 90% having been vaccinated after week 9. The percentage of deaths in individuals aged ≥80 years was 25% in weeks 1–6, decreasing rapidly to 13% in weeks 13–14. The rate of mortality up to week 6 was 13 times greater in individuals aged ≥80 years, compared to those aged 0–79 years, decreasing to 6.9 times greater in weeks 13–14. This shows that the rapid expansion of vaccination coverage among the elderly in Brazil related to a significant decline in relative mortality, compared with mortality in young people. The effectiveness of inactivated virus vaccines in individuals who have been fully vaccinated against COVID-19 is illustrated in Figure 4C [77].
In a meta-analysis of 58 studies that evaluated vaccine effectiveness, Liu et al. reported that two-dose vaccination was 85%, 97%, 93%, 96%, and 95% (a range of 92%–98%) effective in preventing SARSCoV-2 infections, symptomatic COVID-19, hospitalization, ICU admission, and COVID-19-related deaths, respectively [77].
In general, real-world studies on vaccine effectiveness inform estimations of vaccine efficacy in clinical trials and show that ongoing vaccination efforts have resulted in substantial preventive benefits for the general population, seniors, and healthcare workers. Thus, it is hoped that the mass vaccination of approved vaccines will have a far-reaching impact on global efforts to crush the COVID-19 pandemic.
Vaccine protection against SARS-CoV-2 variants
All viruses, including SARS-CoV-2, mutate with the passage of time, leading to the emergence of novel variants, for example, a novel SARS-CoV-2 variant, the Alpha variant (lineage B.1.1.7), in the UK in September 2020, the Beta variant (lineage B.1.351) in South Africa in May 2020, the Gamma variant (lineage P.1) in Brazil in November 2020, and the Delta variant (lineage B.1.617) in India in October 2020, and Omicron variant (lineage B.1.1.529) in South Africa in November 2021 [80], [81], [82]. All of these variants have spread globally. Subsequently, changes have taken place in relation to the transmission of the virus or features of the illness, which have impacted vaccination approaches, diagnostics, therapeutics, and the efficacy of public health and social methods globally, raising global concerns about the continued effectiveness of vaccination against certain variants.
Alpha (lineage B.1.1.7)
Initial research has shown that the most widely distributed vaccines retain antibody neutralization against the Alpha variant. A case–control study on mRNA vaccines in Qatar, published in May 2021, reported effectiveness of 90% by the Pfizer–BioNTech vaccine against the Alpha variant, in support of early research that the Pfizer and Moderna vaccines had a 10-percentage point reduction in their effectiveness from their peak values against the B.1.1.7 variant [83, 84]. As a non-replicating vector vaccine, the research has demonstrated that the efficacy of the ChAdOx1 vaccine (Oxford University–AstraZeneca) against the B.1.1.7 variant of SARS-CoV-2 is similar to that against non-B.1.1.7 variants (75% vs. 84%, respectively); however, recipients of the ChAdOx1 CoV-19 vaccine had a significantly lower viral load than the study participants who did not receive the vaccine [85]. A study in China demonstrated that the B.1.1.7 variant had a reduced ability to resist the neutralizing activity of convalescent or inactivated virus vaccine serum [86]. The Novavax vaccine was shown to have 86% effectiveness against the Alpha variant, according to primary data [87]. These findings demonstrate a reduction in the effectiveness of vaccines in the context of Alpha variants of concern (VOCs) following a comparison of vaccine effectiveness in a non-VOC context. Nevertheless, a decrease in vaccine effectiveness does not inevitably imply the loss of control as shown by the vaccine-mediated protection provided against the B.1.1.7 spectrum.
Beta (lineage B.1.351)
Early reports based on an analysis of South African convalescent serum samples have indicated that Beta is an immune-evading variant [88, 89]. A series of studies have confirmed a decrease in the effectiveness of the AZD1222 vaccine (substantial), Novavax vaccine (moderate), BNT162b2 vaccine, and the Ad26.COV2.S vaccine (minor) against illness due to the Beta variant. A report issued on February 6, 2021, documented a decrease in the effectiveness of the AZD1222 vaccine against the Beta variant, 83 and another study indicated effectiveness of only 60% provided by the Novavax vaccine against the B.1.351 variant [87]. Several other studies have subsequently verified a decline in neutralizing titers against the Beta variant in individuals vaccinated with the Moderna and Pfizer–BioNTech vaccines. In Qatar, the Pfizer–BioNTech vaccine was found to be only 75% effective against the Beta variant [84]. Similarly, the CoronaVac, BBIBP-CorVac, and ZF2001 vaccines from China induced a minimal to moderate reduction in the counteractive antibody response against Beta. For example, one study demonstrated that the B.1.351 variant resisted the counteractive activity of two convalescent serum samples (by an element of 2) and inactivated virus vaccine serum (by an element of 2.5–3.3) to a greater extent than the wild virus [86]. In a study performed by Gao et al. in China, BBIBP-CorV, an inactivated vaccine, and RBD-Dimer, the ZF2001 vaccine, were shown to largely preserve the neutralizing titers, with a 1.6-times reduction in vaccine effectiveness against the B.1.351 virus, compared with its level of effectiveness against the original SARS-CoV-2 [90].
Gamma (lineage P.1)
Most vaccines are likely to achieve minimal to moderate neutralizing antibody levels against the Gamma variant. The Pfizer–BioNtech vaccine was demonstrated to have slightly lower effectiveness against the Gamma P1 variant, compared with wild-type viruses [91]. Elsewhere, it was confirmed that the Brazilian P.1 variant was intractable to various counteractive monoclonal antibodies and more resistant to counteraction by convalescent plasma (6.5-fold) and vaccine serum (2.2–2.8 folds) [92, 93]. These findings support reports that current vaccines have less protective effectiveness against the P.1 variant [94]. In another study on the Gamma variant in Brazil, it was found that 81% of the population had been vaccinated with the ADZ1222 vaccine, with reports of a 71% reduction in symptomatic infection [92, 93].
Delta (lineage B.1.617)
Lineage B.1.617 was first discovered in India, and it went on to become the dominant strain globally, spreading to at least 130 countries throughout the world. The spike protein in B.1.617 has several mutations, such as E484Q, and L452R, and it is possible that these have induced the production of less antibodies. The impact of Delta variants on COVID-19 vaccine performance has been assessed in several studies.
In a study in India, the effectiveness of the AstraZeneca–Vaxzevria vaccine was assessed in a context in which the Delta variant was predominant, and two doses of the AstraZeneca–Vaxzevria vaccine were established to be 63 and 82% effective, respectively, in controlling infection and moderate severe illness.
The effectiveness of vaccination using any COVID-19 vaccine was approximated in the UK between June 24, 2021, and July 12, 2021, when the Delta variant was widespread. Compared with the period from May 20, 2021, to June 7, 2021, a reduction in symptomatic infection, relative to the predominance of the Delta variant in the two periods (59% vs. 83%), was reported, and the results were supported by a similar study conducted in Israel [95]. Significantly, a reduction in viral load (greater cycle, limited values) was observed in vaccinated COVID-19 cases, compared to unvaccinated cases, shortly after the Delta outbreak in the UK [95]. This finding was also supported by the results of a study that evaluated Delta breakthrough infections in Singapore where it was found that people who had been fully vaccinated with a mRNA vaccine experienced a rapid decrease in viral load [96].
The effectiveness of vaccination in COVID-19 cases was evaluated in Mesa County, Colorado, USA, from April to June 2021, following a rapid increase in Delta variant cases. Crude vaccine effectiveness of 78 and 89% against symptomatic infection was reported in Mesa County and the rest of the country, respectively. During the study period, Delta was associated with nearly 100% of order samples in Mesa County, compared with ∼50% in all other states [97]. The different levels of efficacy provided by COVID-19 vaccines against SARS-CoV-2 VOC variants are depicted in Figure 5A,B,C,D.
Figure 5:
COVID-19 vaccine efficacy at preventing infection for SARS-CoV-2 variants Alpha, Beta, Gamma, and Delta.
Following a systematic review and meta-analysis of SARS-CoV-2 vaccines in real-world studies, in fully vaccinated individuals, Liu et al. reported pooled vaccine effectiveness of 85% in protecting against infection with the Alpha variant of SARS-CoV-2, with respective figures of 75% for the Beta variant, 54% for the Gamma variant, and 74% for the Delta variant. Of all vaccine types, the BNT162b2 vaccine demonstrated the highest level of vaccine effectiveness: 92%, 62%, and 84% against the Alpha, Gamma, and Delta variants, respectively [77].
Taken together, the findings indicated that the variants influenced the effectiveness of commodity-given vaccines, and they helped to quantify the decrease in the effectiveness of the vaccines in the context of VOCs, compared to that in the non-VOC context. However, a decrease in the effectiveness of a given vaccine does not necessarily refer to a loss of control, as evidenced by the effectiveness of full vaccination. Vaccines have been shown to provide substantial protection against serious illness; therefore, a small decrease in vaccine effectiveness against severe illness in the context of a VOC may still represent adequate control.
Omicron (lineage B.1.1.529)
A new variant of SARS-CoV-2, B.1.1.529 (Omicron), was first reported to the WHO by South Africa on November 24, 2021, and the WHO declared the Omicron (B.1.1.529) a VOC on November 26, 2021 [98, 99]. As at December 21, 2021, the Omicron variant has been confirmed in 106 countries [100]. The Omicron variant contains a large number of mutations in the S gene, compared with previous VOCs; S mutations have the potential to increase transmissibility, confer resistance to therapeutic treatment, and partially evade infection- or vaccine-induced immunity [98, 99]. Preliminary evidence suggests that there may be a reduction in vaccine effectiveness against infection and transmission with the Omicron variant, as well as an increased risk of reinfection.
In a South African with a small study sample (just 12 people), the findings of which were released as a preprint, there was evidence of a significant reduction in the effectiveness of the Pfizer–BioNTech vaccine against the Omicron variant. A 41-fold reduction in geometric mean titer (GMT) was observed with the Omicron variant, compared to the ancestral strain in the early stages of the pandemic [98, 99].
Another study reported a substantial decrease in the neutralization titers of the Omicron variant in recipients of both the homologous ChAd and BNT primary COVID-19 immunization courses. Neutralizing titers in the sera of participants who received a homologous ChAd regimen dropped from 100 (Victoria strain) to 10 (Omicron variant). Similarly, the median neutralizing titer in the sera of participants who received a homologous BNT regimen reduced 29.8-fold from 1,609 (Victoria strain) to 54 (Omicron variant) [101].
A 25.8-fold reduction, relative to the ancestral strain, was reported in a Pfizer study, in approximately 20 samples collected three weeks after completion of the Pfizer–BioNTech (Comirnaty) primary series. A 2.6-fold reduction was identified in samples collected from persons who received a third dose of the Pfizer–BioNTech vaccine a month prior to sample collection [101].
Preliminary evidence has indicated that sera obtained from previously infected individuals had lower neutralization activity against Omicron than any other circulating SARS-CoV-2 [70, 102].
Thus, the Omicron variant has been shown to reduce vaccine effectiveness against symptomatic infection, and, as a result, increased breakthrough infections in previously infected or double-vaccinated individuals, suggesting that it has the potential to drive a further wave of infection [101, 103], [104], [105], [106], [107]. More data are needed to better understand the extent to which the Omicron variant is able to evade vaccine- and/or infection-derived immunity and the degree to which current vaccines are continuing to protect against severe disease and death due to the Omicron variant.
In the future, five important actions need to be taken. Firstly, global monitoring of the emergence of new variants, vaccine effectiveness, and the impacts of vaccination should be assessed. Secondly, more evidence is needed of the methods by which COVID-19 variants influence how COVID-19 vaccines play a role in practice, with a view to changing the composition of antigens and vaccines where necessary. Thirdly, it will be important to determine how the immune response to variants is affected by prior infection and vaccination against wild-type strains. Fourthly, it is also vital that research on vaccine efficacy and effectiveness follows standardized procedures and testing methods to ensure the quality of research and comparability of the findings. Finally, globally coordinated regulatory mechanisms are warranted to support the rapid growth, evaluation, and deployment of modified vaccines to target variants, should this be required.
Breakthrough infections
The administration of COVID-19 vaccines is a key tool that is being used to mitigate the COVID-19 pandemic. Thirty-five different vaccine types have been approved for emergency use globally. Each vaccine has been shown to have a high level of efficacy in RCTs and real-world studies; however, symptomatic or asymptomatic infectious SARS-CoV-2 disease is likely to manifest in a small percentage of fully vaccinated people. Causes of breakthrough infections remain unclear, and they might be dependent on the virus itself, as well as on individual biological causes (including age, sex, and neutralizing antibody titer levels), vaccine types, and coverage. Therefore, complete effectiveness (100%) is not possible with any vaccine. The CDC has defined breakthrough vaccine infection as a test of SARS-CoV-2 RNA or antigens in an respiratory sample obtained from an individual at least 14 days after he or she has received all suggested doses of an FDA-authorized COVID-19 vaccine [108].
Generally, the incidence of breakthrough infection was very rare in large-scale CDC studies. From January 1, 2021, to April 30, 2021, a large number of SARS-CoV-2 vaccine breakthrough infections (n=10,262) were disclosed in 46 USA states and territories. By comparison, 11.8 million COVID-19 diagnoses were made during the same period, which translates to ≤0.01%. From May 1, 2021, the CDC stopped monitoring vaccine breakthrough cases unless they resulted in hospitalization or death. By August 2, 2021, of 164 million completely vaccinated individuals nationwide, 7,525 patients had COVID-19 vaccine breakthrough infections and were admitted to hospital or died in the USA. This finding was confirmed in other small-scale research; for example, a USA study reported that, following vaccination, the rate of ground-breaking SARS-CoV-2 infectious disease in prison was only 1% (27/2,380) [108].
Only 410 of 258,716 veterans (0.16%) who received two doses of the Pfizer or Moderna vaccine were reported to experience breakthrough infections in a study conducted from December 15, 2020, to March 31, 2021. Eighty-six cases of COVID-19 breakthrough infections were identified between February 1, 2021, and April 30, 2021, representing 1.20% of total COVID-19 cases and 0.07% of fully vaccinated individuals in a New York study. Elsewhere, the rate of breakthrough infection was only 1.60% (i.e., 48 of 3,000 healthcare workers who had received both doses) [109], [110], [111], [112].
Breakthrough infections are associated with individual and clinical features. According to the CDC, breakthrough cases have occurred in people of all ages who have been vaccinated; however, just over 60% of them were females. According to preliminary data, there have been 2,725 asymptomatic infections (27%), 995 cases of hospital admissions (10%), and 160 deaths (2%); with the average age of patients who demised being 82 years [104]. Of 469 cases of breakthrough infections observed in Barnstable County, Massachusetts, USA, only 1% were hospitalized, and no deaths were reported [113]. Typically, hospitalization and death were associated with individuals with medical conditions (i.e., immunocompromised), including cancer and organ transplants. Rana et al. tracked a large number of fully vaccinated medical staff with breakthrough infections and determined that the median time between receiving the second vaccine dose and the breakthrough infection was 29.5 days [109], [110], [111], [112].
Causes of breakthrough infections include mutations in viruses. In April 2021, USA scientists identified two women with vaccine breakthrough infections (5%) out of 417 employees at Rockefeller University who had been fully vaccinated with either the Pfizer or Moderna vaccine. Viral sequencing revealed potentially clinically important variants, including E484K, in one woman, and three mutations (T95I, Del142-144, and D614g) in two women. E484k is referred to as an “escape mutant” because it has demonstrated a capacity to evade several antibodies generated by COVID-19 vaccines [109], [110], [111], [112].
The CDC has reported the available sequence data obtained from 555 (5%) breakthrough cases, 356 (64%) of which were identified as SARS-CoV-2 VOCs, including B.1.429 (n=88, 25%), B.1.1.7 (n=199, 56%), P.1 (n=28, 8%), B.1.427 (n=28, 8%), and B.1.351 (n=13, 4%) [104, 114]. Four hundred and 69 cases of COVID-19 were detected in individuals in Massachusetts, USA, between July 3, 2021, and July 17, 2021, 346 of whom were fully vaccinated. Ninety per cent of variant specimens obtained from 133 patients, identified by testing, were the Delta variant [99]. A case–control study in Israel suggested that the distribution of B.1.351 and B.1.1.7 variants and infections in vaccinated individuals (“breakthrough cases”) was considerably higher than that in unvaccinated individuals (OR of 8:10; OR of 26:10, respectively), which suggests that there are frequent vaccine breakthrough infections with both VOCs [115]. This might relate to the fact that the mRNA vaccine has decreased effectiveness against the Alpha, Beta, Gamma, and Delta variants [116].
Most studies indicated that breakthrough infections were associated with lower viral loads. The CDC reported that the viral load in breakthrough infections had declined significantly in infections that occurred 12–37 days after the administration of the first dose of the BNT162b2 mRNA vaccine. The decline in viral load suggests potentially lower infectivity, which further highlights the role of the vaccine in virus transmission [104]. Nonetheless, the Ct values in the samples of fully vaccinated patients and unvaccinated patients were reported to be similar [113].
The development of ground-breaking SARS-CoV-2 infectious diseases is associated with a drop in the level of counteractive antibodies. Elsewhere, 39 SARS-CoV-2 breakthrough infections were documented in 1,497 fully vaccinated healthcare workers. The level of neutralizing antibody titers in patients during the peri-infection period was reported to be less than that in the uninfected control group (0.165–0.787). Increased peri-infection and a decrease in the level of counteractive antibodies was associated with less infectivity (greater Ct values) [117], [118], [119]. A study in China indicated that a mild breakthrough infection was confirmed in a healthcare worker who received an inactivated COVID-19 vaccine with a high cycle limit (Ct value of 34) for the N gene and 35.27 for ORF1ab chip 7; in addition, the healthcare worker had weakly positive immunoglobulin G (IgG) in the early period [120].
There is mounting evidence that vaccination is extremely effective at preventing COVID-19 infection. Breakthrough infections in vaccinated people remain very rare, as does the development of severe illness. Overall, COVID-19 infections cause mild or no symptoms and are of a short duration; they are also characterized by a low viral load and low antibody levels. However, breakthrough cases of COVID-19 are primarily caused by vaccine “escape” mutations. In the future, continuous monitoring of breakthrough infections will be particularly important, and this should extend to epidemiological data, clinical features, vaccine boosters, immune responses, and viral sequences to determine which of these variables are linked to mutations.
Homologous prime-boost vaccination
The need for COVID-19 booster shots could be triggered by weakening of the immune response, derived from the initial vaccination, over time, as well as the potential that viral variants have to render COVID-19 vaccines less effective, or a combination of the two.
Previous studies have indicated that immunocompromised populations have a low antibody response to vaccines; they are also at high risk of transmitting the virus. To address this, Pfizer performed a clinical trial on a homologous prime-boost vaccine schedule in July 2021. The research demonstrated that a booster Pfizer vaccine increased antibody levels 5–10 times higher than those achieved with the prior two doses. In November 19, 2021, the US FDA amended its emergency use authorization to allow the administration of an additional dose of either the Pfizer–BioNTech COVID-19 or the Moderna COVID-19 vaccines to a small group of people with compromised immune systems. The third dose should be administered at least 28 days following the two-dose regimen of the same vaccine to individuals aged ≥18 years [121]. Israel, France, and Germany administered third doses of the vaccine to individuals aged ≥60 years, and the UK plans to do the same in September 2022.
Clinical trials in Bahrain, the UAE, Egypt, Jordan, and China have indicated that individuals with co-morbidities and older adults (aged ≥60 years) who have received two doses of the BBIBP-CorV vaccine have little confidence in its efficacy to prevent COVID-19. However, an enhanced humoral response has been observed with homologous vaccination. To date, a few countries, namely Bahrain, the UAE, and Turkey, have introduced a booster dose following the primary two-dose COVID-19 immunization series.
Heterologous prime-boost vaccination
Positive immunization acts as the keystone of the worldwide health policy response to COVID-19. However, research has shown that new SARS-CoV-2 variants have reduced the efficacy of vaccinations and that they are highly transmissible and infective. Identifying ways to improve the effectiveness of vaccines has become the most important issue. Heterologous prime-boost vaccination is a potential option as it has been reported to boost immunity. Several studies have evaluated heterologous prime-boost vaccination schedules in pre-clinical studies and clinical trials [122].
Pre-clinical studies support the further evaluation of heterologous prime-boost vaccine schedules (i.e., ChAd/BNT), especially since the cellular response was demonstrated to be considerably higher in the ChAd/BNT cohort than in the BNT/BNT cohort in a study in Germany [122], [123], [124]. The approach used in the studies in preclinical trials was consistent with the allogeneic protocols applied in animal models based on the use of ChAdOx1-S or BNT162b2 as initiators or enhancers [125]. Clinical studies have also supported the above finding. The immunogenicity and reactogenicity of the BNT162b2 vaccine, managed as a second dose in individuals primed with ChAdOx1-S, was evaluated by Borobia et al. in a Stage II trial in Spain. The GMT of RBD antibodies increased from 71.46 binding antibody units (BAU)/mL at baseline to 7,756.68 BAU/mL at day 14 (p≤0.000). The level of IgG antibodies against SARS-CoV-2 trimeric spike protein increased from 98.40 BAU/mL to 3,684.87 BAU/mL, which suggests that the management of a BNT162b2 vaccine dose, following the injection of a first dose of ChAdOx1-S, induces a robust immune response [125, 126].
Liu et al. also reported that GMC (Geometric mean concentration) in both heterologous vaccination schedules (ChAd/BNT and BNT/ChAd) was greater than those of an approved vaccine schedule (ChAd/ChAd), with verified effectiveness against COVID-19 illness and hospitalization [123]. In view of the unusual thrombotic disorders associated with the ChAdOx1 nCoV-19 vaccine, only France, Canada, Germany, Norway Denmark, and Sweden advocated the use of mixed vaccination in their citizens prior to August 10, 2021 [127].
In terms of adenovirus heterologous prime-boost vaccine schedules, the Sputnik V COVID-19 vaccine program deployed an alternative heterologous prime-boost schedule using COVID-19 vaccines vectored by Ad26 and Ad5, and it was shown to induce a healthy humoral and cellular response, with 92% efficacy against symptomatic disease [128], [129], [130].
Only a few studies have reported on the vaccine effectiveness of inactivated/adenovirus heterologous administrations. A study in India on 98 people, 18 of whom inadvertently received two vaccines—an adenovirus vector platform-based vaccine, Covishield, and a whole-virion inactivated SARS-CoV-2 vaccine, Covaxin (BBV152)—determined that the combination of these two COVID-19 vaccines elicited better immunogenicity than two doses of the same vaccine. Continuous immunization with an adenovirus vector vaccine followed by the administration of an inactivated/reorganized subunit/mRNA vaccine was shown to significantly increase the level of neutralizing antibodies and enhance the modulation of the primary neutralizing antibody response in a mouse model in a study in China [122]. In addition to effectiveness, safety has been identified as a core feature in the use of allergenic vaccines.
Hidden risks are associated when vaccines are combined, as evidenced by an increasing number of ADEs following immunization. Previously, studies disclosed that a non-standardized approach, for example, the administration of an adenoviral-vectored vaccine (ChAd, Vaxzevria, or AstraZeneca) and an mRNA vaccine (i.e., BNT, Comirnaty, or Pfizer) at the four-week interval was more reactogenic than that reported with a standardized approach. An evaluation of the combination of the AZD1222 and BNt162b2 vaccines demonstrated the increased reactogenicity associated with heterologous prime-boost schedules, compared with a homologous vaccination schedule [131, 132]. The incidence of ADEs may even be higher in younger populations due to increased systemic reactogenicity. However, the ADEs of BNT/ChAd vaccines have been associated with acceptable and manageable reactogenicity and short-term reactogenicity [124].
Thus, COVID-19 allergenic regimens have been developed to trigger integrated antibody and cellular responses, which result in greater, wider, or enduring immunity. In addition, the use of an enhanced heterogonous start-up COVID-19 vaccine program would alleviate supply shocks or shortages, accelerate the global vaccination campaign, and maximize control of the pandemic. However, mass vaccination should be tested in ongoing research on thousands or millions of people, and a comprehensive assessment must be performed of the security and effectiveness of any proposed vaccination program.
Vaccine safety
With the development and mass administration of COVID-19 vaccines, the focus of public, governments, and healthcare workers has been on safety, which has become critical in the global battle against COVID-19. The safety of each type of COVID-19 vaccine must be evaluated, first in animals, then in clinical trials, and finally in real-world studies.
The clinical trial data have indicated that pain at the injection site, fever, and headache are the most common ADEs related to mRNA vaccines [133]. Unusual ADEs or worrying outcomes have not been reported in several real-world studies. The largest study conducted in the USA between December 2020 and January 2021 documented data on the administration of 13.8 million mRNA-1273 and BNT162b2 vaccines. Only 6,994 studies reported ADEs following vaccination; 6,354 (91%) of the ADEs were non-severe, and 640 (9%) were severe and included 113 deaths. However, a causal association was not determined between vaccination against COVID-19 and mortality. In addition, the WHO authorities reported the absence of an unexpected or unusual increase in the number of deaths in frail, elderly individuals and the absence of an unusual ADE profile following the use of the BNT162b2 vaccine [134]. Other real-world observations from the USA revealed that 4,041,396 first doses of Moderna’s COVID-19 vaccine have been administered; of these, 1,266 (0.03%) were associated with ADEs. Anaphylaxis was linked to 4.7 cases/1.0 million administered Pfizer-BioNTech vaccine doses (9,943,247 doses) and 2.5 cases/1.0 million administered Moderna vaccine doses (7,581,429 doses) between December 14, 2020, and January 18, 2021. The incidence of allergic reactions after receiving a COVID-19 vaccine has been within the range of those reported following the administration of other infectious vaccines and, fortunately, anaphylaxis is treatable [135]. The side-effects associated with the mRNA COVID-19 vaccine may have been influenced by the composition of lipid nanoparticles, formulation components, or sequence selection [136].
In terms of vaccines, the AZD1222 vaccine (Oxford University–AstraZeneca) was evaluated for safety in clinical trials on 11.7 million participants up to March 7, 2021. Approximately 10% of the recipients reported ADEs [137]. Most of the reported side-effects linked to this vaccine have been mild to medium in severity, often resolving within a few days of vaccination. Typically, the side-effects in older people (aged ≥65 years) have been mild, with a lower reported incidence, compared to younger individuals. Severe ADEs have rarely been reported in the UK, with 234 cases of anaphylaxis identified in relation to 11.7 million vaccinations. Recently, the focus has been on thrombotic incidents linked to the AstraZeneca vaccine; up to March 8, 2021, the EU and the UK identified 15 deep vein thrombosis (DVT) and 22 pulmonary embolism events in 17 million individuals to whom the vaccine was administered [138]. The EMA carefully reviewed the available vaccine safety data for 17 million people to whom the AstraZeneca COVID-19 vaccine had been given in the EU and the UK and determined that the risk of thrombotic events accompanied by thrombocytopenia was rare [139]. Evidence was not found of an increased risk of pulmonary embolism, DVT, or thrombocytopenia based on gender, vaccine batch, age, or unique circumstances. The WHO and the EMA calculated that the benefits associated with the vaccine far outweighed the risks; however, both agencies advised continued surveillance for thrombocytopenia. It has been suggested that vaccination with the ChAdOx1 nCov-19 vaccine may lead to rare immune thrombocytopenia mediated by platelet-activating antibodies [140], [141], [142].
Subunit protein vaccines, like inactivated vaccines, do not contain live components and are considered very safe. Yang et al. evaluated the safety of a protein subunit vaccine, ZF2001, in a Phase I/II vaccine trial on 950 study subjects. This vaccine, developed by China for use in adults, is based on a RBD-Dimer [90]. In the majority of cases, the ADEs disclosed within 30 days of administration were mild or moderate (Grade 1 or 2). During Stage II clinical trials, Grade 3 or severe ADEs were reported by 47 (31%) of 150 participants in the three-dose placebo group and by 72 (48%) of 150 participants in the three-dose experimental group (i.e., 25 μg) [90]. The study demonstrated that the protein subunit vaccine, ZF2001, appeared to be well-tolerated and safe in the Phase I and II trials in a small study sample, but it needs to undergo Phase I clinical trials using a large sample.
Seven-day ADEs occurred in 3 (13%), 5 (21%), 4 (17%), and 6 (25%) of participants to whom an inactivated COVID-19 vaccine, the WIBP-CorV, was administered in the vaccine-only, minimal-dose, standard-dose, and large-dose groups, respectively, during Stage I clinical trials [143]. ADEs were identified in 5 (6%) and 4 (14%) people injected on days 0 and 14 and in 16 (19%) and 5 (18%) individuals who were injected on days 0 and 21 in the Stage II trial [143]. Severe ADEs were not reported in either of these clinical trials.
In a Stage III clinical trial on the BBIBP-CorV vaccine, conducted in Brazil on 12,396 participants, the most common adverse local reactions in the vaccination group were pain at the injection site (60%), swelling (6%), and itching (4%), and the most common systemic adverse reactions were headache (34%), fatigue (16%), and muscle pain (12%). A Stage I/II clinical trial evaluated the ADEs associated with the administration of CoronaVac to healthy people aged ≥60 years up to 28 days after the administration of the injection. ADEs were identified in 20 of 100 recipients (20%) in the group that received a 1.5 μg dose, 25 of 125 recipients (20%) in the group that received a 3 μg dose, 27 of 123 recipients (22%) in the group that received a 6 μg dose, and 15 of 73 recipients (21%) in the placebo group [126]. All the ADEs were mild or moderate in severity, and the most common ADE was pain at the injection site (39 of 421 participants [9%]). By August 28, 2020, eight severe ADEs, believed to be unassociated with the vaccination, were disclosed by 7 participants (2%).
Elsewhere, a Stage III clinical trial on CoronaVac was conducted on the outskirts of China on 45,000 participants aged ≥18 years. The incidence of ADEs was 16%, of which 0.23% was a level III reaction, according to internal data. The data from Phase III clinical trials were consistent with those obtained the Stage I and II trials; inactivated COVID-19 vaccines were observed to have minimal side-effects, and Stage IV vaccine administration was safe and tolerable. In the future, more data are required from Phase III clinical trials and real-world studies to assess the ADEs of inactivated virus vaccines.
To date, millions of people worldwide have received COVID-19 vaccines. In general, they have been shown to have similar ADEs to non-COVID-19 vaccines, and they have been demonstrated to be completely safe in studies. Further validation of these initial discoveries is required so that medical providers and vaccine receivers can increase the level of trust by the public in the safety and efficacy of COVID-19 vaccines. Enhanced monitoring of the safety of COVID-19 vaccines should continue, and this information should be shared in a timely manner with public health officials, healthcare providers, and the public.
Immunization strategy and prioritized populations
After the first vaccine products were licensed, all countries were faced with decisions regarding the prioritization of which populations would receive the COVID-19 vaccine. Many countries implemented phased distribution plans that identified priority populations, considered targets as vaccine availability increased, and compiled detailed information on exposure and transmission risks. In the USA, healthcare workers, residents, and nursing homes staff were defined as prioritized populations; the second distribution phase (Phase 1b) was performed for individuals aged ≥75 years and frontline workers [144], [145], [146], [147]. The EU recommended prioritizing vaccination for healthcare staff, individuals at high risk of exposure, the elderly, and immunocompromised individuals. In the UK, priority was given to residents and care staff in elder care facilities and persons aged ≥80 years. China commenced its phased distribution plan by prioritizing high-risk groups, which included frontline healthcare workers, elderly populations, and people abroad, for COVID-19 vaccination. Secondary vaccination priority was afforded to individuals at high risk of hospitalization and exposure, including those providing public services (i.e., bus and taxi drivers) and the elderly. Finally, priority was given to populations at high risk of transmission, which included students and teachers.
Vaccine deployment
One year after the onset of the COVID-19 pandemic, 200 research groups rose to the challenge of successfully developing vaccines against SARS-CoV-2. However, an ongoing challenge is determining how to make licensed vaccines available and affordable to people worldwide, especially those in impoverished regions. The WHO, vaccine developers, governments, and industry are identifying additional funding for this purpose and continue to assess the delivery of globally licensed vaccines based on country and population needs.
COVID-19 Vaccines Global Access (COVAX), a WHO-backed international program for vaccine distribution in developing states, was designed to meet emergent demands for vaccination, and its objective is to drive the equitable allocation of donor-funded doses of COVID-19 vaccines globally. COVAX aims to ensure that 92 low- and middle-income countries will be able to secure access to COVID-19 vaccines at the same time as high-income countries. For example, the first round of allocation provided 237 million does the AstraZeneca to COVAX institution participants through May 2021 [96]. In addition, in 2021, the Pfizer–BioNTech Reach Agreement with COVAX for the Advance Purchase of Vaccine to Help Combat COVID-19 provided up to 40 million doses to COVAX on a not-for-profit basis. The doses were delivered throughout 2021. China officially collaborated with COVAX in 2020 and offered 10 million COVID-19 vaccine doses to COVAX to drive equal access and affordability in developing states.
Another option has been for countries that produce vaccines to expand their production capacity to service their own citizens, in addition to the donation of vaccines to other countries; for example, Pakistan was the first country to receive Sinopharm vaccines donated by China on February 1, 2021. Since then, China has provided vaccine donations to 69 countries and two international organizations.
In the future, governments, vaccine developers, policymakers, and funders should enhance international cooperation to ensure the rapid development of vaccines, sufficient production, and the equal deployment of vaccines to all countries.
Vaccination progress
By 17 March 2022, globally, 462,758,117 confirmed cases of COVID-19 and 6,056,725 deaths, affecting more than 220 countries and territories, had been reported to the WHO [11, 12]. The most affected areas during the pandemic have been the Europe (41% of total global cases), Americas (32%), SouthEast Asia (12%), Western Pacific countries (8%), Eastern Mediterranean countries (5%),and Africa (2%).
All affected countries are competing to deploy and manage safe and efficient vaccines to end the COVID-19 pandemic. Since the first dose of licensed COVID-19 vaccines was produced by Pfizer–BioNTech in the UK, a total of 7.66 billion doses have been administered globally, with 27.25 million being administered daily. March 16, 2022, 63.8% of the world’s population had received at least one dose of a COVID-19 vaccine. However, only 14.1% of people in low-income countries had received at least one dose Figure 6.
Figure 6:
COVID-19 vaccine doses administered by country income group (March 16, 2022). Notes: Data from our world in data: https://ourworldindata.org/covid-vaccinations. The size of bubble represented the population of different countries, and the marked countries had the most people fully vaccinated per hundred at corresponding income levels.
By the same date, 35 different vaccine types against COVID-19 had been developed in 197 locations worldwide. The most predominant vaccine use has been the Oxford University–AstraZeneca and Pfizer–BioNTech vaccines, distributed in 179 and 153 countries and areas, respectively. The Sinopharm and Moderna vaccines have been administered in 90 and 86 countries and areas, respectively, and the Janssen Ad 26 and Sputnik V vaccines have been administered in 89 and 58 locations, respectively.
As to March 16, 2022, China, India, and the USA were the clear leaders in terms of mass vaccinations globally (i.e., the total number of COVID-19 vaccination doses administered). China had administered 1.27 billion COVID-19 doses to citizens and 1.24 billion people had been fully vaccinated. India had administered 969.28 million doses to people and 816.47 million people with a complete vaccination. The USA has administered 254. 823 million doses, 81.6% of people 5+ have a least one vaccination (https://covid.cdc.gov/covid-data-tracker/#vaccinations).
As to March 16, 2022, measured as a percentage of the total population that had received at least one vaccine dose, the UAE was the highest, with 99% of the population having received at least one dose of a COVID-19 vaccine (96% of the population was fully vaccinated), followed by Portuga and Cuba, with respective figures of 95% (93% of the population was fully vaccinated) and 94% (87% of the population was fully vaccinated).
In terms of the total number of vaccination doses managed per 100 people in the total population, Cuba topped the global table (310.83 doses per 100 people), followed by Chile (257.14), Singapore (251.6), and China 222.2) per 100 people.
In general, developed countries have vaccinated their populations at a much faster rate than less developed countries [21] (Figure 6). Following mass vaccination, the daily number of confirmed cases of COVID-19-associated hospitalization and mortality in most countries (except Chile) has decreased significantly.
Conclusions
The SARS-CoV-2 pandemic is ongoing, and its prevalence remains at moderate to high levels globally. At the international level, it has led to substantial economic and social upheaval. Therefore, the development of safe and effective vaccines, to restore normalcy to daily life and end the pandemic, remains the most important strategy. Scientists around the globe have combined their efforts in response to this threat, and this has led to the successful development of 11 approved vaccines based on different clinical technologies. To date, there are a total of 344 vaccine candidates; 149 of these vaccines are in clinical trials, and 35 have been approved in at least one country. Current evidence indicates that the vaccines that have been developed to date are safe and possess adequate efficacy to protect people against SARS-CoV-2 infection, disease, transmission, hospitalization, and death, and there is support for this finding in both clinical trials and real-world studies [148]. However, vaccine effectiveness has decreased as a result of the emergence of new SARS-CoV-2 variants. COVID-19 vaccination is rapidly being deployed around the globe, and over 80% of the global population is expected to achieve active immunity against SARS-CoV-2.
Perspectives and future challenges
In the future, several challenges remain regarding COVID-19 vaccine development and administration. Firstly, and importantly, a universal standard is required to assess the efficacy and efficiency of SARS-CoV-2 candidate vaccines. Secondly, although COVID-19 vaccine immunity appears to last for at least 6–9 months, the long-term effects of vaccination are unknown. Immunization booster schedules should be further explored to improve vaccine effectiveness. Thirdly, most vaccines are only used in high-income nations, and this accounts for only 14% of the global population. The question of how to rapidly allocate and disseminate approved vaccines to low- and middle-income countries remains; the inequality of vaccine deployment may continue in the long term [114]. In addition, some variants of the SARS-CoV-2 virus have emerged and have the potential to cause COVID-19 breakthrough infection in humans, and this may negatively impact vaccine effectiveness. Thus, new-generation vaccines must be developed and manufactured in response to emerging variants of the SARS-CoV-2 virus [149]. Finally, mass vaccination in the real-world requires consideration in terms of the development of surveillance systems to record all vaccination-related data, including that related to breakthrough infections and variants of the SARS-CoV-2 virus [150, 151]. While populations in more than 200 countries have been vaccinated against COVID-19, the continued use of public health-prevention strategies (i.e., the universal and proper use of masks, social distancing, and hand washing) will assist in preventing the transmission of SARS-CoV-2 in the future. In the future, global efforts should be directed toward effective and immediate vaccine allocations, improving vaccine coverage, SARS-CoV-2 new variants tracking, and vaccine booster development.
Footnotes
Author contributions: Shelan Liu did the literature search and drafted the manuscript. Tie Song designed the study, Min Kang, Na Zhao and Yali Zhuang and Shijian Li performed the statistical analyses and data interpretations, and revised the manuscript. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Research funding: This work was supported by the National Natural Science Foundation of China (Grant No. 82041030) and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents.
Competing interests: Authors state no conflict of interest.
Informed consent: All initial information identifying patients was anonymized in this study and this study donot need the informed consent of individuals.
Ethical approval: This study was done according to the principles and guidelines of the Declaration of Helsinki, and was approved by the Research Ethics Committee of the Zhejiang Provincial Center for Disease Control and Prevention (Grant No. 2020-24).
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–77. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Wu X, Zhou H, Xu F. Clinical characteristics and infectivity of asymptomatic carriers of SARS-CoV-2 (Review) Exp Ther Med. 2021;21:115. doi: 10.3892/etm.2020.9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann Intern Med. 2021;174:655–62. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syangtan G, Bista S, Dawadi P, Rayamajhee B, Shrestha LB, Tuladhar R, et al. Asymptomatic SARS-CoV-2 carriers: a systematic review and meta-analysis. Front Public Health. 2020;8:587374. doi: 10.3389/fpubh.2020.587374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Jernigan DB, Cox NJ. H7N9: preparing for the unexpected in influenza. Annu Rev Med. 2015;66:361–71. doi: 10.1146/annurev-med-010714-112311. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Chan TC, Chu YT, Wu JT, Geng X, Zhao N, et al. Comparative epidemiology of human infections with Middle East respiratory syndrome and severe acute respiratory syndrome coronaviruses among healthcare personnel. PLoS One. 2016;11:e0149988. doi: 10.1371/journal.pone.0149988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, Harrich D, Li Z, Hu D, Li D. The unique features of SARS-CoV-2 transmission: comparison with SARS-CoV, MERS-CoV and 2009 H1N1 pandemic influenza virus. Rev Med Virol. 2021;31:e2171. doi: 10.1002/rmv.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20:e238–44. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Standl F, Jockel KH, Brune B, Schmidt B, Stang A. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2021;21:e77. doi: 10.1016/S1473-3099(20)30648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 14.WHO Director-General’s opening remarks at the media briefing on COVID-19. World Health Organization; 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 cited 2020. Available from: [Google Scholar]
- 15.WHO . WHO coronavirus (COVID-19) dashboard overview. World Health Organization; 2021. https://covid19.who.int/ Available from: [Google Scholar]
- 16.Chamorro-Petronacci C, Martin Carreras-Presas C, Sanz-Marchena A, M AR-F, Maria Suarez-Quintanilla J, Rivas-Mundina B, et al. Assessment of the economic and health-care impact of COVID-19 (SARS-CoV-2) on public and private dental surgeries in Spain: a pilot study. Int J Environ Res Public Health. 2020;17:5139. doi: 10.3390/ijerph17145139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–23. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Chen Q, Feng L, Rodewald L, Xia Y, Yu H, et al. Active case finding with case management: the key to tackling the COVID-19 pandemic. Lancet. 2020;396:63–70. doi: 10.1016/S0140-6736(20)31278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore S, Hill EM, Tildesley MJ, Dyson L, Keeling MJ. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis. 2021;21:793–802. doi: 10.1016/S1473-3099(21)00143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21:73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabaan AA, Al-Ahmed SH, Sah R, Al-Tawfiq JA, Al-Qaaneh AM, Al-Jamea LH, et al. Recent advances in vaccine and immunotherapy for COVID-19. Hum Vaccin Immunother. 2020;16:3011–22. doi: 10.1080/21645515.2020.1825896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-19 – landscape of novel coronavirus candidate vaccine development worldwide. World Health Organization; 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines Available from: [Google Scholar]
- 24.Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5:237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashte S, Gulbake A, El-Amin Iii SF, Gupta A. COVID-19 vaccines: rapid development, implications, challenges and future prospects. Hum Cell. 2021;34:711–33. doi: 10.1007/s13577-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Vaccine efficacy, effectiveness and protection. 2021. https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection [cited 2021 14 July 2021] Available from:
- 27.GAVI . What is the difference between efficacy and effectiveness? 2020. [Google Scholar]
- 28.CDC . An introduction to applied epidemiology and biostatistics. 3rd edn. 2012. Principles of epidemiology in public health practice.https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section6.html [cited 2012 May 18, 2012 ] Available from: [Google Scholar]
- 29.Le TT, Cramer JP, Chen R, Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:667–8. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 30.Kang YF, Sun C, Zhuang Z, Yuan RY, Zheng Q, Li JP, et al. Rapid development of SARS-CoV-2 spike protein receptor-binding domain self-assembled nanoparticle vaccine candidates. ACS Nano. 2021;15:2738–52. doi: 10.1021/acsnano.0c08379. [DOI] [PubMed] [Google Scholar]
- 31.Triggle CR, Bansal D, Farag E, Ding H, Sultan AA. COVID-19: learning from lessons to guide treatment and prevention interventions. mSphere. 2020;5:e00317–20. doi: 10.1128/mSphere.00317-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–27. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 33.Deming ME, Michael NL, Robb M, Cohen MS, Neuzil KM. Accelerating development of SARS-CoV-2 vaccines – the role for controlled human infection models. N Engl J Med. 2020;383:e63. doi: 10.1056/NEJMp2020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park KS, Sun X, Aikins ME, Moon JJ. Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev. 2021;169:137–51. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–9. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–93. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S, Zhong G, Zhang J, Shuai L, Zhang Z, Wen Z, et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat Commun. 2020;11:4081. doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CDC . Adjuvants and vaccines. Atlanta, GA, USA: Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html Available from. [Google Scholar]
- 40.Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11:585354. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petkar KC, Patil SM, Chavhan SS, Kaneko K, Sawant KK, Kunda NK, et al. An overview of nanocarrier-based adjuvants for vaccine delivery. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13040455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah RR, Hassett KJ, Brito LA. Overview of vaccine adjuvants: introduction, history, and current status. Methods Mol Biol. 2017;1494:1–13. doi: 10.1007/978-1-4939-6445-1_1. [DOI] [PubMed] [Google Scholar]
- 43.Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7:e35421. doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85:12201–15. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637–46. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–92. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Natrajan MS, Rouphael N, Lai L, Kazmin D, Jensen TL, Weiss DS, et al. Systems vaccinology for a live attenuated tularemia vaccine reveals unique transcriptional signatures that predict humoral and cellular immune responses. Vaccines (Basel) 2019;8:4. doi: 10.3390/vaccines8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubin R. Difficult to determine herd immunity threshold for COVID-19. JAMA. 2020;324:732. doi: 10.1001/jama.2020.14778. [DOI] [PubMed] [Google Scholar]
- 49.Fontanet A, Cauchemez S. COVID-19 herd immunity: where are we? Nat Rev Immunol. 2020;20:583–4. doi: 10.1038/s41577-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burki TK. Herd immunity for COVID-19. Lancet Respir Med. 2021;9:135–6. doi: 10.1016/S2213-2600(20)30555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aschwanden C. Five reasons why COVID herd immunity is probably impossible. Nature. 2021;591:520–2. doi: 10.1038/d41586-021-00728-2. [DOI] [PubMed] [Google Scholar]
- 52.Aschwanden C. The false promise of herd immunity for COVID-19. Nature. 2020;587:26–8. doi: 10.1038/d41586-020-02948-4. [DOI] [PubMed] [Google Scholar]
- 53.Makhoul M, Ayoub HH, Chemaitelly H, Seedat S, Mumtaz GR, Al-Omari S, et al. Epidemiological impact of SARS-CoV-2 vaccination: mathematical modeling analyses. Vaccines (Basel) 2020;8:668. doi: 10.3390/vaccines8040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vergnes JN. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384:1577. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 55.Skowronski DM, De Serres G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384:1576–7. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 56.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Absalon J, Koury K, Gruber WC. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. Reply. N Engl J Med. 2021;384:1578. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 58.Wang X. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384:1577–8. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 59.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–91. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadoff J, Gray G, Vandebosch A, Cardenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;384:1899–909. [Google Scholar]
- 63.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172–83. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanriover MD, Doganay HL, Akova M, Guner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–22. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–23. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875–7. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdool Karim SS, de Oliveira T. New SARS-CoV-2 variants – clinical, public health, and vaccine implications. N Engl J Med. 2021;384:1866–8. doi: 10.1056/NEJMc2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–12. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng CJ, Lu CY, Chang YH, Sun Y, Chu HJ, Lee CY, et al. Effectiveness of the WHO-authorized COVID-19 vaccines: a rapid review of global reports till 30 June 2021. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9121489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–57. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, et al. Effectiveness of pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged >/=65 years – United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674–9. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White EM, Yang X, Blackman C, Feifer RA, Gravenstein S, Mor V. Incident SARS-CoV-2 infection among mRNA-vaccinated and unvaccinated nursing home residents. N Engl J Med. 2021;385:474–6. doi: 10.1056/NEJMc2104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al. Effectiveness of BNT162b2 mRNA vaccine against infection and COVID-19 vaccine coverage in healthcare workers in England, multicentre prospective cohort study. Lancet. 2021;397:1725–35. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers – eight U.S. Locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385:320–9. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Q, Qin C, Liu M, Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10:132. doi: 10.1186/s40249-021-00915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021;21:1529–38. doi: 10.1016/S1473-3099(21)00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jara A, Undurraga EA, Gonzalez C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–84. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384:1466–8. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, et al. Neutralizing activity of BNT162b2-elicited serum – preliminary report. N Engl J Med. 2021;384:1466–8. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384:1466–8. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muik A, Wallisch AK, Sanger B, Swanson KA, Muhl J, Chen W, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science. 2021;371:1152–3. doi: 10.1126/science.abg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–5. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 85.Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–62. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang GL, Wang ZY, Duan LJ, Meng QC, Jiang MD, Cao J, et al. Susceptibility of circulating SARS-CoV-2 variants to neutralization. N Engl J Med. 2021;384:2354–6. doi: 10.1056/NEJMc2103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahase E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. BMJ. 2021;372:n296. doi: 10.1136/bmj.n296. [DOI] [PubMed] [Google Scholar]
- 88.Moore JP, Offit PA. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325:821–2. doi: 10.1001/jama.2021.1114. [DOI] [PubMed] [Google Scholar]
- 89.Karim SSA. Vaccines and SARS-CoV-2 variants: the urgent need for a correlate of protection. Lancet. 2021;397:1263–4. doi: 10.1016/S0140-6736(21)00468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang S, Li Y, Dai L, Wang J, He P, Li C, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21:1107–19. doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang P, Wang M, Yu J, Cerutti G, Nair MS, Huang Y, et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747–51. doi: 10.1016/j.chom.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoffmann M, Arora P, Groß R, Seidel A, Hörnich BF, Hahn AS, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184:2384–93. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoffmann M, Arora P, Gross R, Seidel A, Hornich BF, Hahn AS, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184:2384–93. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Q, Nie J, Wu J, Zhang L, Ding R, Wang H, et al. SARS-CoV-2 501Y.V2 variants lack higher infectivity but do have immune escape. Cell. 2021;184:2362–71. doi: 10.1016/j.cell.2021.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Organization WH . Weekly epidemiological update on COVID-19 – 10 August 2021. World Health Organization; 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---10-august-2021 [cited 10 August 2021 10 August 2021] Available from: [Google Scholar]
- 96.Chia PY, Ong SWX, Chiew CJ, Li WA, Chavatte J-M, Mak T-M, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. Clin Microbiol Infect. 2021;21:S1198–743X. 00638–8. doi: 10.1016/j.cmi.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herlihy R, Bamberg W, Burakoff A, Alden N, Severson R, Bush E, et al. Rapid increase in circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant – Mesa County, Colorado, April-June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1084–7. doi: 10.15585/mmwr.mm7032e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cele S, Jackson L, Khan K, Khoury DS, Moyo-Gwete T, Tegally H, et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv. 2021 2021.12.08.21267417. [Google Scholar]
- 99.Mahase E. Covid-19: do vaccines work against omicron-and other questions answered. BMJ. 2021;375:n3062. doi: 10.1136/bmj.n3062. [DOI] [PubMed] [Google Scholar]
- 100.WHO COVID-19 weekly epidemiological update 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---21-december-2021 [updated 21 December 2021 21 December 2021] Available from.
- 101.Dejnirattisai W, Shaw RH, Supasa P, Liu C, Stuart AS, Pollard AJ, et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2021;399:234–6. doi: 10.1016/S0140-6736(21)02844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen EC, Gilchuk P, Zost SJ, Suryadevara N, Winkler ES, Cabel CR, et al. Convergent antibody responses to the SARS-CoV-2 spike protein in convalescent and vaccinated individuals. Cell Rep. 2021;36:109604. doi: 10.1016/j.celrep.2021.109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thakur V, Kanta Ratho R. OMICRON (B.1.1.529): a new SARS-CoV-2 Variant of Concern mounting worldwide fear. J Med Virol. 2021 doi: 10.1002/jmv.27541. [DOI] [PubMed] [Google Scholar]
- 104.CDC COVID-19 Vaccine Breakthrough Case Investigations Team COVID-19 vaccine breakthrough infections reported to CDC – United States, January 1–April 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:792–3. doi: 10.15585/mmwr.mm7021e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Islam R, Hossain J. Detection of Omicron (B.1.1.529) variant has created panic among the people across the world: what should we do right now? J Med Virol. 2021 doi: 10.1002/jmv.27546. https://doi.org/ 10.1002/jmv.27546 [DOI] [PubMed] [Google Scholar]
- 106.Gao SJ, Guo H, Luo G. Omicron variant (B.1.1.529) of SARS-CoV-2, a global urgent public health alert. J Med Virol. 2021;94:1255–6. doi: 10.1002/jmv.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen X, Wang H. On the rise of the new B.1.1.529 variant: five dimensions of access to a COVID-19 vaccine. Vaccine. 2021;40:403–5. doi: 10.1016/j.vaccine.2021.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.CDC . COVID-19 vaccine breakthrough case investigation and reporting. CDC; 2021. https://www.cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html Available from: [Google Scholar]
- 109.Rana K, Mohindra R, Pinnaka L. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;385:e7. doi: 10.1056/NEJMc2107808. [DOI] [PubMed] [Google Scholar]
- 110.Nixon DF, Ndhlovu LC. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;385:e7. doi: 10.1056/NEJMc2107808. [DOI] [PubMed] [Google Scholar]
- 111.Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384:2212–8. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blachere NE, Hacisuleyman E, Darnell RB. Vaccine breakthrough infections with SARS-CoV-2 variants. Reply. N Engl J Med. 2021;385:e7. doi: 10.1056/NEJMc2107808. [DOI] [PubMed] [Google Scholar]
- 113.Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings – Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059–62. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Levine-Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, Schreiber L, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27:790–2. doi: 10.1038/s41591-021-01316-7. [DOI] [PubMed] [Google Scholar]
- 115.Kustin T, Harel N, Finkel U, Perchik S, Harari S, Tahor M, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379–84. doi: 10.1038/s41591-021-01413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abu-Raddad LJ, Chemaitelly H, Butt AA. National study group for C-V. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385:187–9. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jung J, Sung H, Kim SH. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1629–30. doi: 10.1056/NEJMc2113497. [DOI] [PubMed] [Google Scholar]
- 118.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–84. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amit S, Gonen T, Regev-Yochay G. Covid-19 breakthrough infections in vaccinated health care workers. Reply. N Engl J Med. 2021;385:1630–1. doi: 10.1056/NEJMc2113497. [DOI] [PubMed] [Google Scholar]
- 120.Chaofeng Ma SX, Yao Y, Yu P, Xu Y, Wu R, Chen H, et al. Mild breakthrough infection in a healthcare professional working in the isolation area of a hospital designated for treating COVID-19 patients — Shaanxi Province, China, March, 202. China CDC Weekly. 2021;3:397–401. doi: 10.46234/ccdcw2021.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.(FDA) USFaDA . Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. U.S. Food and Drug Administration (FDA); 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised [updated August 12, 2021. Available from: [Google Scholar]
- 122.He Q, Mao Q, An C, Zhang J, Gao F, Bian L, et al. Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg Microbes Infect. 2021;10:629–37. doi: 10.1080/22221751.2021.1902245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–69. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hillus D, Schwarz T, Tober-Lau P, Vanshylla K, Hastor H, Thibeault C, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;398:856–69. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Borobia AM, Carcas AJ, Perez-Olmeda M, Castano L, Bertran MJ, Garcia-Perez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–30. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Duarte-Salles T, Prieto-Alhambra D. Heterologous vaccine regimens against COVID-19. Lancet. 2021;398:94–5. doi: 10.1016/S0140-6736(21)01442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kunal S, Sakthivel P, Gupta N, Ish P. Mix and match COVID-19 vaccines: potential benefit and perspective from India. Postgrad Med J. 2022;98:e99–101. doi: 10.1136/postgradmedj-2021-140648. [DOI] [PubMed] [Google Scholar]
- 128.Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Shcheblyakov DV, Dzharullaeva AS, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–97. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–81. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–81. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021:1–11. doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD, et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–6. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blumenthal KG, Freeman EE, Saff RR, Robinson LB, Wolfson AR, Foreman RK, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384:1273–7. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.GACVS . COVID-19 vaccine safety subcommittee meeting to review reports of deaths of very frail elderly individuals vaccinated with Pfizer BioNTech COVID-19 vaccine, BNT162b2. World Health Organization; 2021. https://www.who.int/news/item/22-01-2021-gacvs-review-deaths-pfizer-biontech-covid-19-vaccine-bnt162b2 Available from: [Google Scholar]
- 135.McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–78. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–50. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baker N, Callaway E. Coronapod: the Oxford-AstraZeneca COVID vaccine – what you need to know. Nature. 2021 doi: 10.1038/d41586-021-00847-w. [DOI] [PubMed] [Google Scholar]
- 138.Mahase E. Covid-19: WHO says rollout of AstraZeneca vaccine should continue, as Europe divides over safety. BMJ. 2021;372:n728. doi: 10.1136/bmj.n728. [DOI] [PubMed] [Google Scholar]
- 139.Ostergaard SD, Schmidt M, Horvath-Puho E, Thomsen RW, Sorensen HT. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: side-effect or coincidence? Lancet. 2021;397:1441–3. doi: 10.1016/S0140-6736(21)00762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schultz NH, Sorvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–30. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–60. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oliver SE, Gargano JW, Scobie H, Wallace M, Hadler SC, Leung J, et al. The advisory committee on immunization practices’ interim recommendation for use of Janssen COVID-19 vaccine – United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:329–32. doi: 10.15585/mmwr.mm7009e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The advisory committee on immunization practices’ interim recommendation for use of Moderna COVID-19 vaccine – United States, december 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653–6. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.FDA authorizes Moderna COVID-19 vaccine. Med Lett Drugs Ther. 2021;63:9–10. [PubMed] [Google Scholar]
- 147.Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine – United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–4. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel) 2021;9:467–88. doi: 10.3390/vaccines9050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eaton L. Covid-19: WHO warns against “vaccine nationalism” or face further virus mutations. BMJ. 2021;372:n292. doi: 10.1136/bmj.n292. [DOI] [PubMed] [Google Scholar]
- 150.Kaur SP, Gupta V. COVID-19 Vaccine: a comprehensive status report. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Khuroo MS, Khuroo M, Khuroo MS, Sofi AA, Khuroo NS. COVID-19 vaccines: a race against time in the middle of death and devastation. J Clin Exp Hepatol. 2020;10:610–21. doi: 10.1016/j.jceh.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]