Abstract
This current case report describes two rare cases of children with both hearing loss and snoring. Case 1, a 17-month-old male patient, and case 2, an 11-year-old male patient, both presented with nasal obstruction, snoring and hearing loss. Physical examinations showed obvious enlargement of the head circumference and special facial features. The two children underwent otolaryngology examinations, endoscopy, hearing tests, laboratory examinations for bone metabolism markers, cranial computed tomography, X-rays and genome-wide exon sequencing. The first case was diagnosed with craniometaphyseal dysplasia, which was relieved after giving a low-calcium diet. The second case was diagnosed with osteopathia striata with cranial sclerosis by gene sequencing. Snoring improved after medication and the speech and quality of life improved with a hearing aid. Paediatric otolaryngological physicians need to have a deeper understanding of congenital diseases involving the bones. Only by genetic testing to determine the pathogenesis can those children be given the correct treatment, which is of great importance for improving their prognosis.
Keywords: Craniometaphyseal dysplasia, osteopathia striata with cranial sclerosis, snoring, hearing impairment, second-generation sequencing
Introduction
Children with otolaryngological problems often suffer with a stuffy nose, snoring and mouth breathing along with hearing impairment. The most commonly diagnosed condition is obstructive sleep apnoea hypopnoea syndrome (OSAHS). 1 In the diagnosis of this disease, physicians need to carefully exclude maxillofacial abnormalities and neuromuscular diseases. 2 Maxillofacial abnormalities include micrognathic deformity, Down’s syndrome and giant tongue. 1 A clinical understanding of maxillofacial abnormalities and their implications in the diagnosis and treatment of congenital diseases involving the bones is currently insufficient. With the implementation of the precision medicine concept, this current case report describes two rare diseases that presented with similar symptoms, but that yielded very different final diagnoses and ultimate intervention requirements. The reporting of these two cases conforms to CARE guidelines. 3
Case report
Case 1
In November 2015, a 17-month-old male with snoring, nasal obstruction, open-mouth breathing and poor hearing response presented to the Department of Otolaryngology and Head and Neck Surgery, Shanghai Children’s Hospital, Shanghai, China. The child was born by a natural birth. Except for a large head circumference (38 cm), he did not have prenatal or perinatal complications. His length was 49.3 cm at 12 months of age. He was found to have a stuffy nose at 2 months of age, but that did not affect his quality of life. At 6 months of age, the fibre nasal laryngeal mirror showed bilateral posterior naris stenosis, but he was not given special treatment. By the time he was 1 year old, nasal obstruction had become so serious that he had to open his mouth to breath. Physical examination at that time showed the following: head circumference was 51 cm; the forehead was prominent; the occipital bone was prominent; the nasal ridge was swollen and widened; cartilage was low; the distance between the eyes was widened inconspicuously; the alveolar bone was thick; the teeth were small; there were 16 teeth; the fontanelle was not closed; limb muscle tension and muscle force were normal; he had no facial paralysis and no abnormal nerve reflex. Nasolaryngoscopy showed that the bilateral posterior nostril openings were pinhole-sized; the nasal base heaved and the hard palate bone was thickened. The nasal endoscope with a diameter of 1.8 mm could not enter beyond 3.5 cm of the nasal cavity. No abnormalities were found in the bilateral tympanic membranes. Hearing examination showed that tympanometry-acoustic immittance testing was ‘B’ in both ears. Otoacoustic emission was not elicited from both ears. Auditory brainstem response findings were as follows: 1 ∼ V waves of both ears were slightly delayed and V waves were elicited from 50 decibel hearing loss (dBHL). There were no abnormalities in intracranial pressure, vision or intelligence (Figure 1).
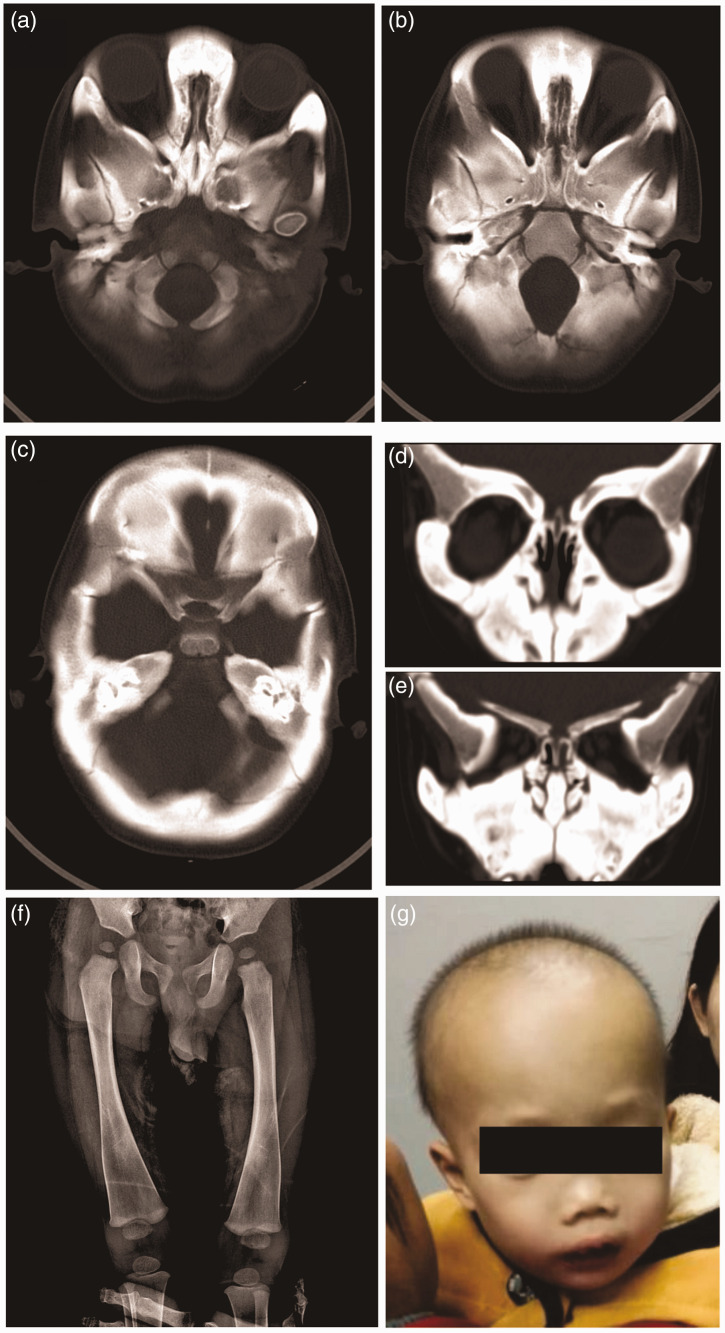
Figure 1.
Computed tomography, digital radiography and facial appearance of a 17-month-old male patient that presented with snoring, nasal obstruction, open-mouth breathing and poor hearing response (case 1): (a and b) the bone density of the skull had increased significantly and the bone plate had thickened; (c) the middle ear cavities were narrowed, while the lumen of the labyrinthine (vestibular, semicircular and cochlear) bones had become sclerotic and the ossicular chain had thickened; (d and e) the sinus cavity was small and the nasal cavity was obviously narrowed. The nasal bone was thickened with abnormal morphology; (f) an X-ray image showed pronounced metaphyseal flaring in the distal femora and ‘Flask deformation’ of the proximal metaphysis on both sides (Erlenmeyer flask configuration) and (g) the facial appearance of the patient showed a wide nasal bridge, paranasal bossing, widely spaced eyes with an increased bizygomatic width and a prominent mandible.
Case 2
In December 2017, an 11-year-old male with snoring, nasal congestion and poor hearing presented to the Department of Otolaryngology and Head and Neck Surgery, Shanghai Children’s Hospital, Shanghai, China. Previously diagnosed with mental retardation and pituitary growth hormone deficiency, he had been receiving growth hormone therapy for 2 years. Physical examination showed the following: a large head circumference of 62 cm; other special features of a prominent forehead, flat face, high cheekbones, low nasal bridge, epicanthus, high palate arch, grade II tonsillar hypertrophy, but with no tympanic membrane abnormality. Audiometry showed (pure tone) the following: moderate-to-severe mixed hearing loss; bone conduction bilateral ears 21 dBHL; air conduction right ear 56 dBHL, left ear 63 dBHL (the mean value of 500, 1k, 2k and 4k Hz octaves). Acoustic reactance was binaural type B. Fibreoptic nasopharyngoscopy revealed adenoid hypertrophy and blocking of 2/3 of the posterior nostril area.
Laboratory findings
In both children, the parathyroid hormone, 25-hydroxyvitamin D3, serum calcium, serum phosphorus and 5′-nucleotidase levels were normal (Table 1). Serum osteocalcin, combined β-CrossLaps and serum alkaline phosphatase were significantly increased, indicating abnormal bone metabolism.
Table 1.
Bone metabolism data for two paediatric patients that presented with hearing loss and snoring.
| Parameter | Case 1 |
Case 2 | Reference range | |
|---|---|---|---|---|
| First visit | Second visita | |||
| Parathyroid hormone, pmol/l | – | 3.48 | 4.99 | 1.58–6.83 |
| Serum osteocalcin, ng/ml | – | 95 | 140.80 | 14–16 |
| Combined β-CrossLaps, ng/ml | – | 1.76 | 2.06 | 0.04–0.78 |
| 25-hydroxyvitamin D3, ng/ml | – | 22.3 | 54.35 | 30–100 |
| Serum alkaline phosphatase, U/l | 884 | 673 | 305 | 104–345 (1–3 years old)93–309 (4–6 years old)86–315 (7–9 years old)42–462 (10–12 years old) |
| Serum calcium, mmol/l | 2.44 | 2.43 | 2.35 | 2.2–2.75 |
| Serum phosphorus, mmol/l | 1.67 | 1.36 | 1.59 | 1–2.15 (1 m–3 years old)0.84–1.85 (4–100 years old) |
| 5′-nucleotidase, U/l | 3 | 2 | 2 | 0–11 |
aAfter 2 months on a low-calcium diet.
Radiological examination
In case 1, cranial computed tomography showed that the density of the frontal skull, parietal, skull base and facial bones had increased; the bone plate had thickened; the upper and lower jaw protruded and were thickened and wider. The maxillary sinus had ossified. The middle ear cavity had shrunk and the labyrinthine (vestibular, semicircular and cochlear) bones had become sclerotic. The mastoid cavity had disappeared, ossified and had no tympanic effusion; and the ossicular chain had thickened. The distal femur was significantly enlarged; the metaphysis was flared; and the femur and tibial diaphysis were hypertrophic – ‘flask deformed’. The clavicles and ribs were sclerotic (Table 2). In case 2, cranial computed tomography showed that the skull was obviously thickened; the corpus callosum was small; and the hypophysis was small. The mastoid process of the middle ear was small, the ossicular chain was thickened and there was no effusion in the tympanum (Figure 2). There was no significant abnormality in the femur.
Table 2.
Radiological manifestations observed in two paediatric patients that presented with hearing loss and snoring.
| Case 1 | Case 2 | |
|---|---|---|
| Femur X-ray findings | The distal femur was significantly enlarged, the metaphysis was flared and the femur and tibial diaphysis were hypertrophic – ‘flask deformed’.The clavicles and ribs were sclerotic. | There was no significant abnormality in the femur. |
| Cranial and facial computed tomography findings | Skull frontal, parietal, skull base and facial bone density had increased. The bone plate had thickened. The upper and lower jaw protruded, was thickened and wide. The bone thickness in the forehead was 14.22 mm. The middle/lower turbinate was significantly thickened. The nasal septum was 8.44 mm thick and the nasal cavity and posterior nostrils were significantly narrowed. The maxillary sinus was ossified. The middle ear cavity had shrunk and the labyrinthine (vestibular, semicircular and cochlear) bones had become sclerotic. The mastoid cavity had disappeared, ossified and had no tympanic effusion. The ossicular chain had thickened. The width of the optic canal was normal. | The skull was obviously thickened, the mastoid process of the middle ear was small, the ossicular chain was thickened and there was no effusion in the tympanum. |
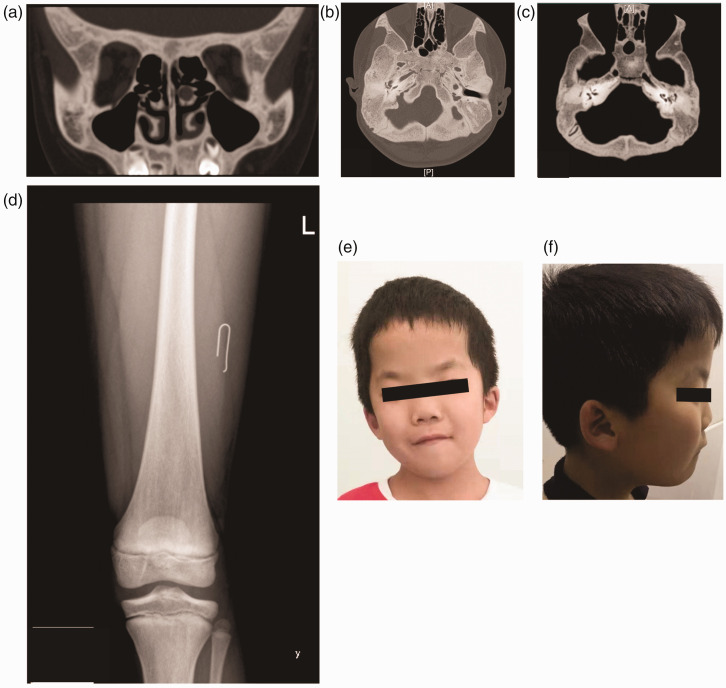
Figure 2.
Computed tomography, digital radiography and facial appearance of an 11-year-old male patient that presented with snoring, nasal congestion and poor hearing (case 2): (a and b) the skull bone plate was thickened and increased density osteosclerosis was clearly observed; (c) the mastoid process of the middle ear was small, the ossicular chain was thickened and there was no effusion in the tympanum. The tympanic cavity and the tympanic antrum were well inflated. There was no stenosis or expansion of the internal auditory canal; (d) an X-ray of the femur showed no significant abnormality and (e and f) the facial appearance of the patient showed special features of a prominent forehead, flat face, high cheekbones, low nasal bridge, epicanthus and a high palate arch.
Genetic testing
Both patients were tested using second-generation sequencing by the Institute of Genetics, Shanghai, China. In the first case, a heterozygous mutation in the ANKH inorganic pyrophosphate transport regulator (ANKH) gene was detected, so he was diagnosed with craniometaphyseal dysplasia (CMD) (OMIM 123000). The second case was found to have a c.228delA (exon 2) mutation in the APC membrane recruitment protein 1 (AMER1) gene, so he was diagnosed with osteopathia striata with cranial sclerosis (OSCS) (OMIM 300373).
Treatment
Case 1 received a low-calcium diet that contained approximately 150 mg per day achieved by restricting calcium-rich foods such as milk, beans or bean soup, oysters, shrimp and cauliflower for 3 months. His nasal congestion and snoring were significantly improved and biochemical indicators were also improved, but the hearing and appearance remained unchanged. Nasolaryngoscopy showed that the bilateral posterior nostril openings were still pinhole-sized, the nasal base was elevated and the hard palate bone was thickened at 4 years of age. Case 2 was treated by a physician at another hospital for ‘snoring for several years, hearing loss’ when he was 11 years old. He was previously incorrectly diagnosed with ‘OSAHS and secretory otitis media, mixed hearing loss’ and was recommended for surgery in that hospital . When he presented at our hospital for surgery, our department found that the child had special features. He was given mometasone furoate aqueous nasal spray (one spray per nostril, once a day for 2 months) and 5 mg/day montelukast oral, once a day for 2 months. He was then advised to undergo genetic testing. After 2 months, the patient underwent endoscopy, which showed grade II° tonsillar hypertrophy, adenoidal hypertrophy mildly and obstruction of 1/2 of the posterior nostril. Snoring had been improved. Then the child underwent polysomnography and hypoxia was not obvious. After wearing hearing aids, his speech ambiguity improved, but comprehensibility was still not good.
These case reports were approved by the Ethics Committee of Jiahui International Hospital, Shanghai China (no. A-CR-2022001) and written informed consent was obtained from the parents of the paediatric patients for the publication of their case reports and any accompanying images.
Discussion
Abnormal facial skull development can lead to nasal congestion, open-mouth breathing and snoring. In this current case report, both children presented with nasal obstruction, snoring and hearing loss. Physical examination showed obvious enlargement of their head circumference and special features of their general appearance. No tympanic effusion was found in either case, but hearing loss was observed in both. Therefore, genetic testing was recommended.
In case 1, genome-wide exon sequencing revealed that there was a heterozygous mutation in the ANKH gene. The ANKH gene is the only known gene related to autosomal dominant CMD. 4 The clinical manifestations caused by this mutation were consistent with the clinical manifestations of case 1 and they were diagnosed CMD (OMIM 123000). CMD is a rare hereditary bone dysplasia disease characterized by skull hyperplastic sclerosclerosis with an abnormally long diaphyseal bone. 5 It was first reported in 1954. 6 It is an autosomal recessive or dominant genetic disease. 7 CMD is characterized by progressive diffusion of skull bone hypertrophy with distinct clinically wide nasal bridge, lateral nasal eminence, bilateral zygomatic width increase and a prominent mandible. Tooth eruption may be delayed or even impossible due to bone hypertrophy and alveolar bone hardening. Enlargement and hardening of the skull can cause compression of the cranial nerves, leading to hearing loss and facial paralysis, and it can even reduce life expectancy of patients with CMD. There is a requirement for regular neurological and ophthalmic examinations and hearing testing. The risks of decompression surgery are high. Currently, calcitonin therapy or calcitriol supplementation is used for low-calcium diet therapy. 8 In case 1, a low-calcium diet was administered and his nasal congestion and snoring were significantly improved; and the biochemical indicators were also significantly improved, but the hearing and appearance remained unchanged.
In the second case, after whole-genome exon sequencing, a c.228delA (exon2) mutation in the AMER1 gene was found that resulted in an amino acid change. This gene mutation can lead to OSCS (OMIM 300373). This disease is rare and mainly manifests with special features of general appearance like those observed in case 1. X-ray examinations of the long bones and the metaphyseal area of the pelvis were often associated with linear striated osteosclerosis, accompanied by craniosclerosis (Table 2). The mode of inheritance is X-linked dominant inheritance and the pathogenic gene is AMER1, also known as WTX. 9 The second case was diagnosed with OSCS. The disease is an X-linked dominant inheritance and is rare in males, usually resulting in fetal or postnatal death. 10 Females with this condition generally manifest with characteristic facial features. The mother of this patient was not affected, but the mother’s sister has similar features such as a prominent forehead, wide eye spacing, conductive hearing loss and slightly lower intelligence than the general population, but she was not willing to undergo a genetic examination. Case 2 was administered drugs to speed up adenoid atrophy to improve upper airway obstruction and his snoring was clearly under control. The hearing aid helped him improve his quality of life.
The parathyroid hormone, 25-hydroxyvitamin D3, serum calcium, serum phosphorus and 5′-nucleotidase levels were normal in both cases (Table 1). An increase of 25-hydroxyvitamin D3 caused by pathological factors could be excluded. However, serum osteocalcin, combined β-CrossLaps and serum alkaline phosphatase were significantly increased, indicating abnormal bone metabolism. Alkaline phosphatase and osteocalcin can be sensitive biochemical indicators of bone growth and development in children. 11 Alkaline phosphatase is a marker of the early differentiation of osteoblasts. 12 Serum osteocalcin can directly reflect the status of bone formation. 13 Serum osteocalcin mainly appears during the mineralization and formation stage, so it is a marker of osteoblast maturation. 11 The expression of alkaline phosphatase and osteocalcin during the process of new bone formation parallels that of the development of osteoblasts, with their levels increasing with the differentiation of osteoblasts and then decreasing with the maturation of osteoblasts. 14 The reciprocal relationship between the expression pattern of alkaline phosphatase and osteocalcin and the development of osteoblasts helps to maintain homeostasis. 14 Serum combined β-CrossLaps is one of the most valuable methods for evaluating osteoclast activity and bone resorption. 15 In the current two cases, alkaline phosphatase, serum osteocalcin and serum combined β-CrossLaps levels were increased, which was suggestive of enhanced osteogenesis and active osteoclasts. The increased levels of alkaline phosphatase and osteocalcin (suggesting osteogenesis) were more pronounced than the increased serum combined β-CrossLaps (suggesting osteogenesis), so both children showed significant signs of osteosclerosis, such as increased head circumference and skull thickness.
In addition, differential diagnosis is required in these types of case. Craniodiaphyseal dysplasia is a rare sclerosing bone dysplasia with skull and long tubular bone dysplasia, which is characterized by craniofacial bone and long bone hypertrophy. 16 The main clinical manifestations are malformations that include short stature, skull deformity, widened eye distance, protrusion of the jaw and lateral posterior protrusion of the spine. 16 Mental retardation is also common. 17 X-ray examination shows obvious thickening of the metaphyseal cortex, widespread osteosclerosis and hyperplasia of the craniofacial bone and the limb long bone, and frequent involvement of the vertebrae. 18 Stem epiphyseal end dysplasia (metaphyseal dysplasia) is a congenital and familial bone dysplasia. 19 Its clinical manifestations include the following: knock knees, mild limited elbow extension, no tenderness of the wider metaphysis and wider collarbone, mandibular protrusion, dental caries and disordered tooth arrangement. 20 A small number of children have spinal deformity and bone fragility. 17 X-ray examination shows that the long bone end is symmetrically widened and the metaphysis of the long diaphysis has a flask shape with osteosclerosis, manifestations that are often accompanied by swelling of the pubic ramus. 21 Stony bone disease, 21 also known as marble bone, primary brittle bone sclerosis or sclerosing proliferative bone disease, is a rare disorder of bone development and metabolism, whose main characteristics are increased whole-body bone density, brittle and hard bone, narrow or even lost marrow cavity and serious anaemia. 22 This disease is an autosomal dominant or autosomal recessive genetic disorder and the vast majority of cases have the recessive form. 22 The human skull is composed of 29 bones; 20 of which form the human facial skull, another mandible belongs to the irregular bones and the other eight belong to the flat bones. There are paired parietal and temporal bones, as well as an unpaired frontal bone, sphenoid bone, occipital bone and ethmoid bone. Together, these bones form the skull to protect brain tissue and form the facial features. The common feature of metaphyseal dysplasia and osteopathia striata cranial sclerosis is that the flat bones of the skull are obviously involved, so the impact on the facial features is obvious.
Children that have a stuffy nose, special facial features, a large head and snore should be considered to have a bone metabolism disease. In addition to the skull, the long bones of children with craniodiaphyseal dysplasia are also affected, so they will be short. The lesions of children with metaphyseal dysplasia mainly involve the long bones. Although the mandible will also be affected as an irregular bone, it has little effect on other flat bones of the face and skull, so the abnormal face is different to that observed in case 1. Sclerosis of the skull base and the reduction of nasal sinuses are particularly significant in children with osteopetrosis. Computed tomography findings are very similar to those of case 1, but the parietal, frontal and facial bones can be unchanged or have only slight changes, so changes to the facial features are not as obvious as those observed in case 1.
In conclusion, paediatric otolaryngological physicians need to have a deeper understanding of congenital diseases involving the bones. More attention should be paid to the presence of congenital diseases where children have symptoms of nasal obstruction, mouth breathing, snoring, special facial features or abnormal growth. Only by genetic testing to determine the pathogenesis can those children be given the correct treatment, which is of great importance for improving their prognosis.
Acknowledgements
We thank our colleagues at the Department of Otolaryngology and Head and Neck Surgery, Shanghai Children’s Hospital, Shanghai, China. We also thank Professor Xiaoyan Li, Vice President of Shanghai Children's Hospital; Wei Wang from the Institute of Genetics, Shanghai, China; Jingjing Chen from the Department of Child Health Care, Shanghai Children’s Hospital, Shanghai, China; Tong Qiao from the Department of Ophthalmology, Shanghai Children’s Hospital, Shanghai, China; Bo Xiao from the Department of Neurosurgery, Shanghai Children’s Hospital, Shanghai, China; and Sun Wang from the Department of Paediatric Orthopaedics, Shanghai Children’s Hospital, Shanghai, China for their continuous help and support.
Footnotes
Author contributions: J.W. and S.C. designed the study and wrote the manuscript. S.C. performed the clinical evaluation of the patients and determined the clinical diagnosis. X.L. performed the diagnostic imaging. All authors have read and approved the final version of the manuscript.
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Shumei Chen https://orcid.org/0000-0002-5811-2393
References
- 1.Lo Bue A, Salvaggio A, and Insalaco G. Obstructive sleep apnea in developmental age. A narrative review. Eur J Pediatr 2020; 179: 357.–. [DOI] [PubMed] [Google Scholar]
- 2.Chan KC, Au CT, Hui LL, et al. How OSA Evolves From Childhood to Young Adulthood: Natural History From a 10-Year Follow-up Study. Chest 2019; 156: 120.–. [DOI] [PubMed] [Google Scholar]
- 3.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 4.Reichenberger E, Tiziani V, Watanabe S, et al. Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. Am J Hum Genet 2001; 68: 1321.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiFrancesco JC, Isimbaldi G, Bedeschi MF, et al. Biopsy-proven multiple sclerosis in an adult patient with atypical craniometaphyseal dysplasia. BMJ Case Rep 2018; 2018: bcr2017223390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson WP, Albright F, Drewry G, et al. Metaphyseal dysplasia, epiphyseal dysplasia, diaphyseal dysplasia, and related conditions. I. Familial metaphyseal dysplasia and craniometaphyseal dysplasia; their relation to leontiasis ossea and osteopetrosis; disorders of bone remodeling. AMA Arch Intern Med 1954; 94: 871–885. [DOI] [PubMed] [Google Scholar]
- 7.Yeom HG. Craniometaphyseal dysplasia: Report of 2 cases with an emphasis on panoramic imaging features. Imaging Sci Dent 2018; 48: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin K, Nathwani S, Bunyan R. Craniometaphyseal Dysplasia: A review and novel oral manifestation. J Oral Biol Craniofac Res 2017; 7: 134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gear R, and Savarirayan R. Osteopathia Striata with Cranial Sclerosis. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A (eds). GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. –2022. PMID: 33856753. [PubMed]
- 10.Perdu B, de Freitas F, Frints SG, et al. Osteopathia striata with cranial sclerosis owing to WTX gene defect. J Bone Miner Res 2010; 25: 82–90. [DOI] [PubMed] [Google Scholar]
- 11.Kusumi T, and Kusumi A. Osteocalcin/bone Gla protein(BGP). Nihon Rinsho 2004; 62 Suppl 2: 136.–. [Article in Japanese]. [PubMed] [Google Scholar]
- 12.Li J, Fan L, Yu Z, et al. The effect of deferoxamine on angiogenesis and bone repair in steroid-induced osteonecrosis of rabbit femoral heads. Exp Biol Med (Maywood) 2015; 240: 273.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komori T. What is the function of osteocalcin? J Oral Biosci 2020; 62: 223–227. [DOI] [PubMed] [Google Scholar]
- 14. Chang X, Hou ZM, Yasuaki S, et al. Quantitative study of alkaline phosphatase and osteocalcin in the process of new bone. Hua Xi Kou Qiang Yi Xue Za Zhi 2005; 23: 424–426 [Article in Chinese, English abstract]. [PubMed] [Google Scholar]
- 15.Chen IP, Wang L, Jiang X, et al. A Phe377del mutation in ANK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD). Hum Mol Genet 2011; 20: 948–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnevale A, Grether P, del Castillo V, et al. Autosomal dominant craniometaphyseal dysplasia. Clinical variability. Clin Genet 1983; 23: 17–22. [DOI] [PubMed] [Google Scholar]
- 17.Costa JR, Santos M, Bebiano Coutionho M, et al. Craniodiaphyseal dysplasia: A Rare And Successful Bone-Anchored Hearing Aid Implantation. Int J Pediatr Otorhinolaryngol 2019; 123: 202–205. [DOI] [PubMed] [Google Scholar]
- 18.Kaissi AA, Csepan R, Hofstaetter JG, et al. Fractures in connection with an atypical form of craniodiaphyseal dysplasia: case report of a boy and his mother. Clinics (Sao Paulo) 2012; 67: 1505–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SR, Han YS. Craniometaphyseal dysplasia. Arch Plast Surg 2013; 40: 157–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balasubramaniyan M, Kaur A, Sinha A, et al. Metaphyseal dysplasia, Spahr type: a mimicker of rickets. BMJ Case Rep 2019; 12: e230257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu CC, Econs MJ, DiMeglio LA, et al. Diagnosis and Management of Osteopetrosis: Consensus Guidelines From the Osteopetrosis. Working Group. J Clin Endocrinol Metab 2017; 102: 3111–3123. [DOI] [PubMed] [Google Scholar]
- 22.Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis 2009; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]