Abstract
Extracellular proton concentration is at 40 nM when pH is 7.4. In disease conditions such as brain ischemia, proton concentration can reach µM range. To respond to this increase in extracellular proton concentration, the mammalian brain expresses at least three classes of proton receptors. Acid-sensing ion channels (ASICs) are the main neuronal cationic proton receptor. The proton-activated chloride channel (PAC), which is also known as (aka) acid-sensitive outwardly rectifying anion channel (ASOR; TMEM206), mediates acid-induced chloride currents. Besides proton-activated channels, GPR4, GPR65 (aka TDAG8, T-cell death-associated gene 8), and GPR68 (aka OGR1, ovarian cancer G protein-coupled receptor 1) function as proton-sensitive G protein-coupled receptors (GPCRs). Though earlier studies on these GPCRs mainly focus on peripheral cells, we and others have recently provided evidence for their functional importance in brain injury. Specifically, GPR4 shows strong expression in brain endothelium, GPR65 is present in a fraction of microglia, while GPR68 exhibits predominant expression in brain neurons. Here, to get a better view of brain acid signaling and its contribution to ischemic injury, we will review the recent findings regarding the differential contribution of proton-sensitive GPCRs to cerebrovascular function, neuroinflammation, and neuronal injury following acidosis and brain ischemia.
Keywords: Acid signaling, acidosis, brain pH, ischemia, neuroinflammation, neuronal injury
Introduction
It has long been known that the brain becomes acidic during and following ischemic stroke.1–3 There have been extensive studies on the multiple mechanisms contributing to the regulation of brain pH homeostasis in health and disease. The data have revealed that carbonic anhydrases, sodium-proton exchanger, different proton, bicarbonate, and lactate transporters all contribute to pH regulation in the brain (for reviews, see4–7) On the other hand, it remains underappreciated regarding the complexity of brain acid signaling or its contribution to ischemia-induced cerebrovascular dysfunction. Protons can modulate the activities of multiple membrane proteins, e.g., inhibiting the NMDA receptors.8,9 Moreover, proton can serve as ligands and signal directly through three classes of proton-sensitive receptors. These include the cationic acid-sensing ion channels (ASICs), 10 the proton-activated chloride channel (PAC)/acid-sensitive outward rectifying anion channel (ASOR),11,12 and the family of proton-activated GPCRs, which include GPR4, GPR65 (aka T cell death associated gene-8, TDAG8), and GPR68 (aka ovarian cancer G-protein coupled receptor 1, OGR1). 13 Section ‘An overview of brain pH regulation’ of this review will summarize what the literature has documented regarding brain pH regulation and pH dynamics in brain ischemia. Next, we will discuss acid-induced signaling through proton-sensitive receptors in the brain, with a focus on recent findings of PAC/ASOR and proton-sensitive GPCRs.
An overview of brain pH regulation
Within the brain parenchyma, the main cell types contributing to brain pH homeostasis include neurons, astrocytes, and microglia.4,5 One universal buffer in these cells is the carbon dioxide (CO2)-bicarbonate system. However, the conversion between carbonic acid and bicarbonate is a very slow reaction on its own. For the CO2/HCO3− buffering system to work efficiently, the presence of functional carbonic anhydrase is a key.6,7 Indeed, the brain expresses eleven of the thirteen functionally active carbonic anhydrases, with some exhibit preferential localization to specific domains (intracellular, membrane bond, or extracellular) or cell types. For example, carbonic anhydrase IV and XIV, which are membrane bond, exert their catalytic function on the extracellular side. For a detailed review on carbonic anhydrases in the brain, see reference. 7
Besides the bicarbonate system, non-bicarbonate buffering also contributes to pH regulation in the brain. While most molecules can act as proton donor and receiver and thus contribute to buffering, lactate is one important molecule for brain pH during neural activity or in anaerobic metabolism.6,14 This is in part because lactate levels exhibit over 10 fold change under anaerobic conditions such as ischemia. 15 The regulation of brain pH apparently depends on the crosstalk between neurons and astrocytes.5,6,14 Under physiological conditions, neurons preferentially utilize aerobic respiration for its ATP production. Astrocytes, on the other hand, tend to go through aerobic glycolysis, which converts glucose to lactate even when there is sufficient oxygen supply. 6 Astrocytes also are the main cells for glucose uptake, especially in response to increased activities. These properties together enable the astrocytes to uptake glucose, convert it to lactate, and then shuttle it to neurons as the energy source for aerobic respiration.6,14
The monocarboxylate transporters (MCT2 in neurons; MCT1 and MCT4 in astrocytes) are responsible for transporting lactate in and out of astrocytes and neurons. 6 Multiple other transporters in neurons and astrocytes are important for pH regulation. These include the sodium-hydrogen exchangers, sodium bicarbonate cotransporter, sodium-driven chloride/bicarbonate exchanger, calcium/proton exchangers. For more information on these topics, see reviews.5,6,14
Brain pH dynamics in ischemia
Protons get buffered fast as essentially all biomolecules can act as proton donors/acceptors. Thus, accurate pH measurement, especially in vivo, is challenging. Typical approaches used in previous studies include direct measurement with pH microelectrode, fluorescent imaging with pH sensitive dyes or reporters, and functional pH imaging. It has long been known that ischemia leads to brain acidosis, both during ischemia and after reperfusion. The exact pH values measured differ between studies. In various studies, the resting or physiological extracellular brain pH is in the range of 7.2–7.4, while that of intracellular pH can be slightly lower.16–19 Based on these data, in our discussion here, we consider extracellular “acidotic or acidosis” starts at ∼pH 7.2.
pH changes during the ischemic phase
During ischemia, the stop of blood flow limits the supple of oxygen to brain tissue, and consequently brain cells transit from aerobic respiration to anaerobic glycolysis. 20 This conversion appears to occur within minutes of ischemia. As a result, neurons quickly deplete ATP, creatine, and phosphor-creatine but build up lactate and protons, and the intracellular pH (pHi) becomes acidic.3,15,20 The subsequent exporting of lactate could contribute in part to extracellular acidification. Other mechanisms which can contribute to interstitial pH reduction include the activation of NHE and the import of bicarbonate inside to counteract intracellular acidification.4,5 In several reports, mostly using rodent models, a rapid pH reduction occurs within minutes of ischemia, to the range of 6.5–6.0.17,21–23 At about 30 min after occlusion, brain pH is typically down to 6.2 or even below 6 in some cases. Hyperglycemia further worsens the degree of acidification while hypoglycemia alleviates it.17,19,24–26 When there is no reperfusion, this level of pH reduction is maintained for up to 6 hr. In cats, acidosis persists to the second day, though the degree of acidosis becomes milder. 27 In human, at an average of 6 days after ischemic stroke, the brain exhibits a slight alkaline shift. 28
pH changes following reperfusion
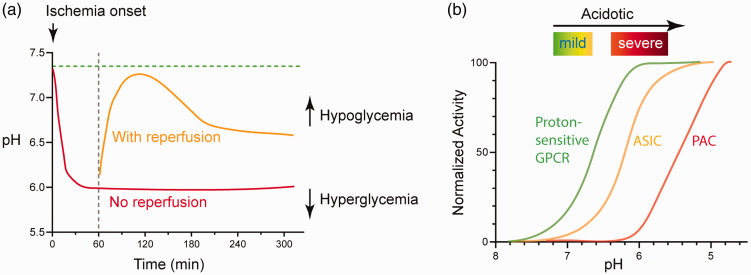
In transient ischemia, reperfusion quickly normalizes the metabolites and brings pH back to the normal range. 15 In another study, Maruiki et al performed 12 min complete ischemia in dogs and found that brain pH returned to pre-ischemia level within 30–60 min of reperfusion. 23 However, if the ischemic event lasts longer, the rise in oxidative stress accompanies disrupted metabolism at the reperfusion stage can lead to another phase of prolonged reduction in brain pH.18,29,30 In one study, following 60 min transient middle cerebral artery occlusion (tMCAO), a transient rebound into the alkaline range occurs at 2-hr after reperfusion, then brain pH reaches ∼6.5 at the 4-hr time point. 18 It is worth noting that while the extracellular brain pH typically maintains in acidic phase for hours following reperfusion, intracellular pH can become slightly alkaline within an hour. 15 Figure 1(a) illustrates qualitatively the dynamics of pH changes in ischemia, either with or without reperfusion.
Figure 1.
pH dynamics and the three classes of acid-sensitive receptors in the brain. (a) Illustration showing brain pH changes during brain ischemia and following reperfusion. The red line illustrates the change in permanent occlusion. The orange line illustrates the approximate change following reperfusion. These curves may shift upward or downward in hypoglycemic or hyperglycemic conditions, respectively. See text for details. (b) Diagram illustrating the pH sensitivity of the three classes of proton receptors. The curves are qualitative representation of the approximate/average pH response curves of a group of receptors within that family. See text for more explanation.
The proton-sensitive receptors
The first receptor identified to functionally mediate acid-sensing in the brain is the acid-sensing ion channel 1a (ASIC1a) . 31 It is now evident that the brain expresses most members in the three families of proton-sensitive receptors, which include the ASICs, the PAC/ASOR, and the proton-sensitive GPCRs. However, these receptors exhibit distinct patterns of expression and downstream signaling. In Table 1, we summarized the genomic location, main downstream signaling of these receptors, and some of the reagents which are relatively specific for the subtypes of the receptors. It is important to note that, especially for ASIC channels, there are additional reagents which are less selective among the subtypes. For more extensive reviews on ASIC modulation, including additional pharmacological reagents, see references.32,33
Table 2.
Expression of proton-sensitive receptors in the brain and genetic tools available.
| Family | Protein | Expression in brain | Mouse models available |
|---|---|---|---|
| ASIC | ASIC1a | Neuron-high expressionAlso reported in astrocyte & microglia | Asic1a−/− (JAX #013733) 133 Tg(Syn1-ASIC1a) (JAX #013734) ASIC1aflox/flox 45 ASIC1aflox/flox 134 |
| ASIC1b | Protein undetected in the brain, though mRNA is present | ASIC1b−/− 135 ASIC1b-Cre 135 | |
| ASIC2a | Neuron-high expressionAlso reported in astrocyte & microglia | Asic2−/− (JAX #013126) 136 Asic2−/− 137 Tg(Syn1-Asic2a) (JAX #012878) 138 Tg(Syn1-Asic2a) (JAX #012877) 138 | |
| ASIC2b | Neuron-high expressionAlso reported in astrocyte & microglia | ||
| ASIC3 | No to limited expression | Asic3−/− (JAX #013127) 139 ASIC3−/− 140 ASIC3−/− 141 ASIC3flox/flox 141 Tg(Syn1-Accn3*) (JAX #012879) 142 Tg(Syn1-Accn3*) (JAX #012880) 142 | |
| PAC/ASOR | PAC/ASOR | NeuronAstrocytePossibly in other cell types | PAC−/− 12 |
| GPCR | GPR4 | Endothelial cell-high expressionNeurons-in some areas | Gpr4−/− (JAX #008580) 71 Gpr4−/− 83 |
| GPR65 (TDAG8) | Microglia or macrophages-limited expression | Gpr65−/− (JAX #008577) 95 Gpr65−/− 85 Gpr65-cre (JAX #029282) 143 | |
| GPR68 (OGR1 | Neuron-high expression Endothelial cell-sporadic expression | GPR68−/− 114 GPR68−/− 144 GPR68flox/flox 114 Gpr68-eGFP reporter (MMRRC #031057) |
In previous studies, multiple groups have generated various kinds of mouse models for these receptors, including global knockout, conditional knockout, and reporter lines. Table 2 summarizes the mice which have been reported in the literature. Table 2 also presents a summary of the overall expression of these receptors in the central nervous system. In the brain, ASICs are mostly present in neurons.32,34 ASIC1a, 2a, and 2 b are the major ASIC subunits expressed in brain neurons. The PAC/ASOR channel are more ubiquitously expressed in both neurons and non-neuronal cells.11,12,35,36 For the GPCRs, GPR4, GPR65, and GPR68 exhibit preferential expression in endothelium, neuron, and microglia, respectively (see text below for more detailed discussion).
Besides differential expression, another important difference exist among these receptors is their pH sensitivity and kinetics. The ASICs, depending on the subunit composition, have a pH50 around 6.8–5. 37 The PAC requires much lower pH to get activated, with pH50 around 5.11,12,35 Compared to the ASICs and PAC, the GPCRs exhibit higher pH sensitivity (Figure 1(b)). All three receptors start to get activated at about pH 7.4 or even higher, and typically reach maximal activation at pH 6.8–6.238–40. One caveat of these studies, however, is that the majority of these studies on pH responses in GPCRs used ectopic expression systems. Nevertheless, it is apparent that the proton-sensitive GPCRs do not exhibit fast desensitization, making them an attractive mediator of acid signaling during persistent acidosis.
Acid-sensing ion channels (ASICs)
The ASICs are a family of proton-gated cation channels. There are four genes encoding ASICs: ASIC1-4.10,32 ASIC1 and ASIC2 have two splice variants a and b. The ASIC subunits have two transmembrane domains with a huge extracellular domain. Three subunits form one functional ASIC channel. 41 ASIC1a, 1 b, 2a, and 3 can conduct acid-activated cation currents. ASIC1 and 3 respond to pH drop with a threshold range of 7.0-7.2 while ASIC2a are much less pH sensitive and starts to get activated at pH close to 5.5.33,37 ASIC channels mostly conduct Na+. However, homomeric ASIC1a, human ASIC1b, as well as ASIC1a/2b heteromers, exhibit a permeability to Ca2+.31,42 Though proton is the only known ligand, various cations, neuropeptides, and oxidants can have important modulatory effects on ASIC function.34,37 As a neuronal and synaptic acid receptor, ASICs contribute to synaptic function, plasticity, and learning.10,32,34 Detailed review of ASIC biophysical properties and regulation can be found in multiple review articles.33,43
ASIC and hypercapnia-induced cerebral vasodilation
Besides its function in neuron physiology, ASIC also contributes to cerebral blood flow regulation. In one study, ASIC1a deletion or local infusion of psalmotoxin (PcTx1), a specific inhibitor of homomeric ASIC1a and heteromeric ASIC1a/2b channels, attenuated hypercapnia-induced vasodilation. 44 The authors further generated a synapsin-cre driven syn-ASIC1a knockout mouse, which had reduced ASIC current in interneurons and principal neurons. 45 The syn-ASIC1a knockout mice had attenuated response to CO2. This result suggests that ASIC1a activation in neurons contributes to hypercapnia-induced vasodilation. While the finding is interesting, it is worth noting that GPR4 in endothelium may play a more direct role in CO2-induced vasodilation (see below).
ASICs in acidotoxicity and ischemic injury
In disease conditions which induce large pH reduction, ASICs are one important mediator of acid-induced neuronal injury. ASIC1a−/− exhibits significant reduction in ischemia-induced brain injury.18,46 Inhibiting ASIC activity by either amiloride, a non-specific ASIC inhibitor, or either PcTx1 or Hi1a, two disulfide-rich spider venom peptides, all have protective effect following tMCAO.46,47 Deleting the ASIC2 gene, through reducing ASIC1a trafficking and possibly biogenesis as well, also reduces tMCAO-induced brain injury.48,49 The protective effect of ASIC inhibition is additive to that of NMDA receptor inhibition.18,50 These data suggest that ASIC targeting can be combined with other approaches to offer enhanced protection against ischemia-induced brain injury. In most of the above studies, the pro-injury function of ASICs correlates with its channel activity. However, there are data showing that ASIC1a can elicit necroptosis independent of its channel activity. 51 This new mechanism requires the ASIC1a C-terminal tail, which elicits cell death through an interaction with serine/threonine kinase receptor interaction protein 1.
Proton-activated chloride channel
Discovery
It has been known for some years that protons induce an acid-sensitive outward rectifying anion channel (ASOR), which passes chloride.52–54 With a cell-based RNA interference screening, two groups recently identified the molecular constituent of this anion current.11,12 The ASOR channel turns out to be a previously uncharacterized protein, TMEM206 (transmembrane protein 206 or C1orf75). One group named it based on its function as “proton-activated chloride channel (PAC)”. 12 PAC/ASOR/TMEM206 is present in multiple organisms, including zebrafish, chicken, rodents, and human. Ectopic expression of PAC in heterologous cells reconstitutes the typical proton-activated ASOR current.11,12
General properties
PAC/ASOR has two transmembrane domains, with the N- and C-termini inside the cell and a large extracellular domain.11,12 A recent Cryo-EM study shows that PAC also forms a trimer. 55 Thus, the overall topology and stoichiometry resemble that of the ASICs, even though the two types of channels do not share any close sequence homology. At room temperature, PAC has an activation threshold of about pH 5.5 and a pH50 of close to pH 5. At 37 °C, PAC starts to get activated at about pH 6.2 with its pH50 shifted to around ∼5.7. These data indicate that PAC is less pH sensitive than ASICs (also see Figure 1(b)). Consequently, PAC activation requires relatively more severe acidosis, i.e., when pH is reduced to 6 or lower.
Cells expressing PAC are present in multiple anatomical places, with high expression levels in brain, bone marrow, kidney, lung, lymph node, spleen, and bladder. In the brain, neurons possess robust PAC current.12,35 In addition, PAC is also present in non-neuronal cells in the brain, including astrocytes and microglia. 36
PAC/ASOR and intracellular trafficking
Besides its presence at cell membrane, PAC/ASOR can traffic to endosomes and forms a functional chloride channel there. 56 The trafficking to endosome apparently requires the YXXL motif of PAC/ASOR. PAC regulates endosomal pH and chloride concentration. Deleting PAC abolishes endosomal chloride leakage, which consequently raises chloride concentration and reduces endosomal pH. 56 In PAC deficient HEK293 cells, transferrin uptake exhibits a 30% increase. 56 This finding suggests that, through modulating endosomal pH and ion gradient across endosomal membrane, PAC/ASOR serves as one regulator of vesicle recycling and/or receptor trafficking.
Role in acidotoxicity and ischemic injury
PAC/ASOR activation in acidotic conditions leads to cell swelling, which suggests that its activation contributes to acidosis-induced cellular injury. In addition, its pH sensitivity indicates that the PAC/ASOR pathways contribute to injuries when the acidosis is more severe (e.g. when pH is lower than 6). In HEK293 cells, deleting PAC/ASOR protects the cells from pH 4.5-induced swelling and cell death. 11 In cultured cortical neurons, PAC deletion or shRNA knockdown offers protection against pH 6 and 5.6-induced cell death.12,35 In a permanent model of brain ischemia, PAC null mice exhibit a reduction in MCAO-induced brain infarction. 12
Proton-sensitive GPCRs
Introduction
Around mid-1990s, homologous cloning led to the identification of three proton-sensitive GPCRs: GPR4, GPR65, and GPR68.57–59 These three GPCRs belong to the orphan family of GPCRs. Protons are the only well-established ligand which activates these receptors. 60 Phylogenic analysis shows that GPR4, 65, 68 evolved from a common ancestor, GPR132 (or G2A, G2 accumulation protein). 38 Early studies showed that GPR132 also responds to proton.61,62 However, based on structural modeling and mutagenesis analysis, GPR132 does not contain the key proton-sensing residues which are conserved in the other three receptors. 38 In addition, GPR132 does not exhibit a robust pH-dependent signaling as compared to the other three GPCRs. In qPCR and RNA-Seq analysis, GPR132 had little expression in either mouse or human brain.63,64 For these reasons, we focus here on GPR4, GPR65, and GPR68.
For all three receptors, when ectopically expressed together with various G alpha subunits, they are capable of coupling to most G alpha subunits tested. 38 This result indicates that the exact signaling these receptors conduct will depend on the specific system/cell type and the availability of the G alpha subunits. In the discussion below, we will mainly cover the results obtained from the brain and/or mammalian cells without the overexpression of G alpha. Table 1 presents a summary of the signaling and main pharmacological reagents which are currently available for these receptors.
Table 1.
Summary of signaling and pharmacological reagents for proton-sensitive channels and GPCRs.
| Gene | Location and accession number | Ion selectivity or signaling | Agonist (EC50) | Antagonist (IC50) | Modulator | References for pharmacological reagents |
|---|---|---|---|---|---|---|
| ASIC1 | Human: 12q13.12NM_001095.4 (ASIC1a) HM991481 (ASIC1b) | Na+/Ca2+ | MitTx: ASIC1a (9.4 nM) ASIC1b (23 nM) | PcTx1 (1 nM) Mambalgin ASIC1a (55 nM) Hi1aDiminazene AmilorideA-317567 | Big dynorphin & dynaorphin A (EC50 ∼30 µM) Spermine | 120–124 125,126 |
| Mouse: chr 15NM_009597.2 (ASIC1a) NM_001289791 (ASIC1b) | ||||||
| Identity: ASIC1a 97.9% ASIC1b 93.8% | ||||||
| ASIC2 | Human: 17q11.2-q12NM_001094 (ASIC2a) NM_183377.2 (ASIC2b) | Na+ | Diminazene Amiloride | Zn2+ | 125 , 127 | |
| Mouse: chr 11NM_001034013 (ASIC2a) NM_007384 (ASIC2b) | ||||||
| Identity: ASIC2a 99.2% ASIC2b 97.2% | ||||||
| ASIC3 | Human: 7q36.1NM_004769.4 | Na+ | MitTx (830 nM) | APETx2 (63 nM) AmilorideA-317567 | GMQ (67 µM) Neuropeptide SF (50 µM) lactate | 126,128–130 |
| Mouse: chr 5 NM_183000.2 | ||||||
| Identity: 83.3% | ||||||
| PAC/ASOR | Human: 1q32.3NM_001198862.2 | Cl− | pregnenolone sulfate | 11 | ||
| Mouse: chr 1 NM_025864.4 | ||||||
| Identity: 90.3% | ||||||
| GPR4 | Human: 19q13.32NM_005282 | GsG12/13Gqpossibly Gi | Compound 3b (67 nM) NE 52-QQ 57 (70 nM hGPR4; 1.8 µM rGPR4) | 84 , 131 | ||
| Mouse: Chr 7NM_175668 | ||||||
| Identity: 91.5% | ||||||
| GPR65 (TDAG8) | Human: 14q31.3NM_003608 | Gs | ZINC13684400 (positive) ZINC62678696 (negative) | 106 | ||
| Mouse: chr 12 NM_008152.3 | ||||||
| Identity: 78.5% | ||||||
| GPR68 (OGR1) | Human: 14q32.11NM_003485 | Gq, Gspossibly Giand G12/13 | CARTPT (76–96) 3.2 µMOsteocrin (115–133) 0.4 µMCorticotropin (17–40) 1.8 µM | Cu2+ (µM range) Zn2+ (µM range) | Ogerin (positive modulator for Gs pathway) Lorazepam (non-specific) MS48107 | 72 , 106 , 132 |
| Mouse: Chr 12NM_175493 | ||||||
| Identity: 92.1% |
CARTPT: cocaine- and amphetamine-regulated protein; GMQ: 2-guanidine-4-methylquinazoline; PcTx1: psalmotoxin 1.
GPR4
GPR4 expression: GPR4 mRNA is present in multiple tissues throughout the body, with abundant expression in brain, heart, lung, placenta, spleen, skeletal muscle, testis, kidney, and ovary.39,40,65 Cells expressing GPR4 include immune cells, peripheral and central endothelial cells, kidney epithelial cells, and certain types of neurons.66–70 Compared to other proton-sensitive GPCRs, GPR4 is the only one that exhibits robust expression in multiple types of endothelial cells.
GPR4 signaling: GPR4 exhibits promiscuous signaling in various types of cells. The initial studies show that GPR4 signals through Gs, though it can also couple to G12/13 and Gq.40,67,71–76 Depending on the context, GPR4 recruits multiple downstream effectors, including the activation of cAMP-protein kinase A (PKA), exchange protein directly activated by cAMP (EPAC), and Rho-Rho-associated protein kinase (ROCK) pathways. What determines which pathway is turned on is not clear. Other than the cell type and/or treatment paradigm, the duration of acidosis appears to be one factor. The rise of cAMP occurs within minutes of acidic stimulation while ROCK activation typically associates with a longer (hours) acidosis.
Proinflammatory role in peripheral cells: In peripheral endothelial and epithelial systems, GPR4 activation initiates stress responses and contributes to intestinal inflammation, paracellular gap formation and ischemia-induced renal injury.77–80 In human umbilical vein endothelial cells (HUVEC), lung microvascular endothelial cells, and lung arterial endothelial cells, acid activation of GPR4 increases the expression of pro-inflammatory chemokines, cytokines, and genes in the NF-κB pathway. 81 GPR4 inhibition inhibits acidosis-induced gap formation in endothelial cells. 80 The Rho-ROCK pathway is one mediator of acidosis-induced junctional disruption. Part of the GPR4 effect may be due to a change in cell-cell adhesion, which parallels with an increase in VCAM, E-selectin, and ICAM-1. 82 Consistent with an important role in vascular function, one global GPR4 null mouse line exhibits increased incidence of neonatal hemorrhage. 71 However, vascular malformation or spontaneous hemorrhage is not present with a different GPR4 null mouse line. 83
GPR4 in hypercapnia-induced vascular responses: In the brain, GPR4 exhibits predominant vascular expression.67,68 Systematic inhibition of GPR4 with NE 52-QQ57, a specific inhibitor of GPR4, has no effect on cerebral blood flow or hemodynamics.67,84 However, GPR4 activity in cerebral endothelium is required for hypercapnia-induced vasodilation. 68 GPR4 deletion largely abolishes CO2-induced release of prostacyclin and the vasodilating effect of hypercapnia. This effect does not appear to depend on cAMP but rather requires the activation of Gq/11. These data suggest that endothelial GPR4 activity has little impact on baseline cerebral blood flow but mediates endothelial responses when the brain becomes acidic.
GPR4 in brain neurons: The overall expression of GPR4 is low in most types of brain neurons.63,67 However, neurons in specific regions, including the retrotrapezoid nucleus, dorsal raphe, and lateral septum, express detectable GPR4 levels.67,85 Consistent with GPR4 expression in retrotrapezoid nucleus, CO2 induces Fos expression in these neurons while deleting GPR4 abolishes CO2-induced hyperventilation.65,85 This finding is consistent with the observation that systematic GPR4 inhibition reduces CO2-induced hyperventilation. 67 Though the inhibitor and global knockout do not determine the site of action, lentiviral-mediated re-expression of GPR4 in retrotrapezoid nucleus is sufficient to rescue the deficit of CO2 sensing in the global GPR4−/− mice. 85 This result indicates that the retrotrapezoid nucleus neurons are one key determinant of the central response to CO2.
GPR4 and acidotoxicity: As discussed above, most brain neurons do not express detectable levels of GPR4. In our recent study, we examined organotypic cortical slices and found that WT and GPR4−/− slices exhibit comparable acidosis-induced neuronal injury. 63 In another study, following 30 min tMCAO, GPR4−/− mice did not exhibit statistically significant changes in brain infarct volume as compared to the wild-type mice. 86 These data suggest that GPR4 does not have a direct effect on acidotoxicity in neurons or acute brain injury following a mild stroke. However, GPR4 activation shows a consistent pro-inflammatory or pro-injury role in peripheral endothelial and epithelial cells. In SG-SY5Y neuroblastoma cell line, GPR4 silencing is also protective and reduces neurotoxin-induced cell injury. 87 These data suggest that GPR4 inhibition or silencing can have a protective effect. It will be of interest to assess this speculation in cells or regions which exhibit robust GPR4 expression.
GPR65
GPR65 expression: GPR65 has limited expression in the brain.64 One report showed that GPR65 is present in a limited number of microglia within the sensory circumventricular organs. 88 Given that the circumventricular organs do not have a blood-brain barrier, it raises a possibility that infiltrating macrophages, which express GPR65, account for some of the GPR65-positive microglia in brain. Consistent with the limited number of GPR65 positive cells, baseline mRNA level of GPR65 is over one order of magnitude lower as compared to GPR4 or GPR68.64,86 However, following ischemia-reperfusion, GPR65 expression is increased.64,86,89 Immunostaining shows that these GPR65 positive cells are IBA1 positive. Since ischemia reperfusion compromises the blood-brain barrier, it remains unclear whether these post-stroke GPR65/IBA1 positive cells reflect an upregulation of GPR65 in microglia or ischemia-induced macrophage infiltration.
GPR65 signals through cAMP: In CHO and HEK 293 T cells, acid activation of transfected GPR65 activates the Gs-cAMP pathway.90–93 In macrophages, thymocytes, splenocytes, and microglia, acidosis activates cAMP through GPR65.94,95 In dorsal root ganglion neurons, GPR65 mediates pH 6.4-induced cAMP increases following hyperalgesia.93 These data demonstrate that, for both endogenous and overexpressed GPR65, cAMP is the major downstream effector.
GPR65 in acidotic injury: In heterologous cells, GPR65 overxpression reduces acidosis-induced LDH release. 90 In heart, GPR65 expression is elevated following myocardial infarction and GPR65 deletion leads to larger infarction. 96 In retina, GPR65 deletion accelerates the degeneration of photoreceptor cells in both a genetic and a light-induced injury in a mouse model. 97 In the rodent EAE model of multiple sclerosis, GPR65 null mice show worsened clinical outcome. 98 All these studies suggest that GPR65-dependent signaling elicits protection in acidotic and/or injurious paradigms. In rodent models of brain ischemia, GPR65 deletion worsens brain infarction. 86 In this case, GPR65 signaling in microglia/macrophage likely attenuates post-ischemia inflammatory responses and leads to post-ischemia protection in the brain.
GPR68
GPR68 expression: GPR68 exhibits widespread expression in multiple types of cells, including immune cells, muscle cell, neurons, and some types of endothelial cells.39,63,99–103 In the brain, GPR68 mRNA is detected in multiple regions.59,63,70 Cerebellar granule cells express GPR68. 104 In brain neurons isolated by Thy1 labeling and FACS sorting, RT-PCR reveals robust GPR68 expression and diminished GPR4 level. 63 Using a GFP reporter mouse, which expresses GFP under the control of the GPR68 promoter, we recently showed that, in cortex, hippocampus, and striatum, GPR68 positive cells were NeuN positive.63,102 Within the hippocampus, GFP expression is apparent throughout pyramidal neurons, with higher expression in CA3 neurons. These mRNA and reporter data clearly demonstrate that GPR68 is widely expressed in the brain and neurons are the main GPR68-expressing cells. Besides neurons, in situ hybridization reveals the presence of GPR68 mRNA in a fraction of endothelial cells in the small diameter arterioles in the brain. 103 Some studies have further examined the expression/localization of GPR68 at the protein level. However, we and others found that, using GPR68−/− tissue as the negative control, current GPR68 antibodies do not generate specific signals.63,103 In organotypic hippocampal slices, ectopically expressed GPR68 exhibits a relatively ubiquitous distribution in CA1 neuronal cell body, dendrites, dendritic spines, and axons. 102
GPR68 signaling: GPR68 primarily couples to Gq and its activation by acidosis elevates IP3 and DAG.72,105 Depending on the cell type, and typically associated with ectopic expression, GPR68 activation can increase cAMP level and/or recruit the G12/13 effectors.38,99,106,107 In cerebral granule cells, GPR68 mediates acidosis-induced calcium increase. 108 However, under the culture condition studied, revealing this GPR68-dependent mechanism requires the inhibition of calcium sensing receptors (CaSR). Reducing extracellular calcium and magnesium to close to 0 mM also largely alleviates the inhibitory effect of CaSR and facilitates acid-induced activation of GPR68. 108 Interestingly, another study in HEK293T cells shows that divalent metal ions can enhance GPR68 signaling. 109 In organotypic hippocampal and cortical slices, we found that acidosis induced the activation of PKC, and this effect requires GPR68. 63 These results suggest that, in both organotypic brain slices and cerebellar granule cells, GPR68 activation by acidosis activates the Gq-DAG-PKC axis.
Peripheral function of GPR68: GPR68 contributes to signaling and gene regulation in multiple peripheral cells, including smooth muscle cell, immune cell, and airway epithelial cells. In human airway smooth muscle cells, extracellular acidification elevates intracellular calcium levels, induces cell rounding, and stimulates IL-6 production. 100 siRNA-mediated knockdown of either GPR68 or Gq/11, or application of YM-254890, a Gq inhibitor, attenuates acidosis-induced changes.100,101 In a mouse asthma model, GPR68 in dendritic cells is required for ovalbumin-induced asthma. 110 GPR68 also contributes to bone and cartilage function. GPR68 inhibition or silencing attenuates acid-induced calcium increase and acidification induced survival.111,112 GPR68 deletion alters osteoclast number in mice, although the direction of change differs between two different knockouts.113,114 For detailed review of GPR68 function in non-neuronal cells, see.39,40,115
GPR68 in synaptic function and learning: At the Schaffer collateral-CA1 synapse, deleting GPR68 does not alter paired-pulse facilitation but attenuates hippocampal long-term potentiation (LTP). 102 Consistent with a deficit in LTP, GPR68 null mice show a reduced dark-entry latency in the step-through passive avoidance test. In another study, however, GPR68−/− does not exhibit difference in a fear conditioning assay or the Morris water maze test. 106 However, Ogerin, a positive allosteric modulator of GPR68, attenuates context fear memory without affecting cued fear memory. 106 These data suggest that GPR68 likely regulates synaptic physiology and contributes to learning and memory. However, its exact effect depends on the training and testing paradigm.
GPR68 in neuroprotection: In organotypic hippocampal slices, GPR68 deletion worsened acidosis-induced neuronal injury. 63 At 24 hr following a 45-min tMCAO, we showed that GPR68 deletion exacerbated ischemia-induced brain injury. 63 However, using a 30 min tMCAO protocol, another study reported no difference between WT and GPR68−/− mice. 86 We speculate that the technical differences, including tMCAO duration and criteria for inclusion/exclusion (e.g. cerebral blood flow monitoring), contribute to the differences in outcome between the two studies. In our study, we further examined the outcome at day 3 after 45 min tMCAO. We found that GPR68−/− mice exhibited an increase in left (ipsilateral) rotation, which indicates a worsened left:right imbalance. Similar to the 24 hr result, brain infarction was larger in the knockout at 72 hr. The effect of GPR68 deletion on brain injury appeared to correlate with the incidence of hemorrhagic transformation (HT). When we quantified HT incidence following tMCAO, the difference in brain injury was apparent when there existed a diffuse, or moderate level, of HT. 116 It remains unclear whether this correlation with HT involves GPR68 in endothelial cells. Supporting a protective role of GPR68 in ischemia, AAV-mediated overexpression reduced tMCAO-induced brain injury. 63 In summary, our data obtained from both slice and animal studies, and with both knockout and overexpression, are in agreement to suggest that GPR68 activation offers protection in acidotic and ischemic conditions.
A recent study examined the contribution of GPR68 in a sevoflurane-induced neurotoxicity. 117 In this model, 4.9% sevoflurane for 2 hrs induces neuronal loss in hippocampus in neonatal rats. This change correlates with a reduction in GPR68 expression. AAV-mediate GPR68 overexpression reverted sevoflurane-induced loss of neurons. This effect correlates with a reduced neuronal apoptosis with GPR68 overexpression. Functionally, GPR68 overexpression improves the behavioral performance in the Morris water maze test. Compared to the group injected with control AAV, the group receiving AAV-GPR68 had reduced escape latency, increased time in target quadrant, and increased number of platform crossings. 117 The study further shows that GPR68 overexpressed group exhibits an increase in oligodendrocyte proliferation and myelination. Another interesting observation here is that sevoflurane treatment reduces BDNF expression while GPR68 overexpression partially rescues the reduction in BDNF. Though the mechanism is unclear, the finding is consistent with a neuroprotective role of GPR68 in the brain, and suggests a potential link to neurotrophic signaling pathways.
In organotypic cortical slices, inhibiting PKC activities with Go6983 worsened acidosis-induced neuronal injury in WT slices, but had no significant effect in GPR68−/− slices. 63 In our RNA-Seq analysis, GPR68 deletion reduced the expression of Hsp70 and Grp78, 64 suggesting a link to protein misfolding/ER stress function. In previous studies, ischemia leads to upregulation of neuronal hemoglobin or neuroglobin, which is linked to increased antioxidant activities.118,119 Interestingly, we found that deleting GPR68 abolished this increase. 64 These data suggest that GPR68 may contribute to neuroprotection through modulating chaperone and/or ER function, and PKC-mediated signaling likely mediates part of the effect in acidotic conditions. The exact downstream effector of GPR68 in ischemia or neuronal injury in vivo warrants further investigation.
Summary and speculations
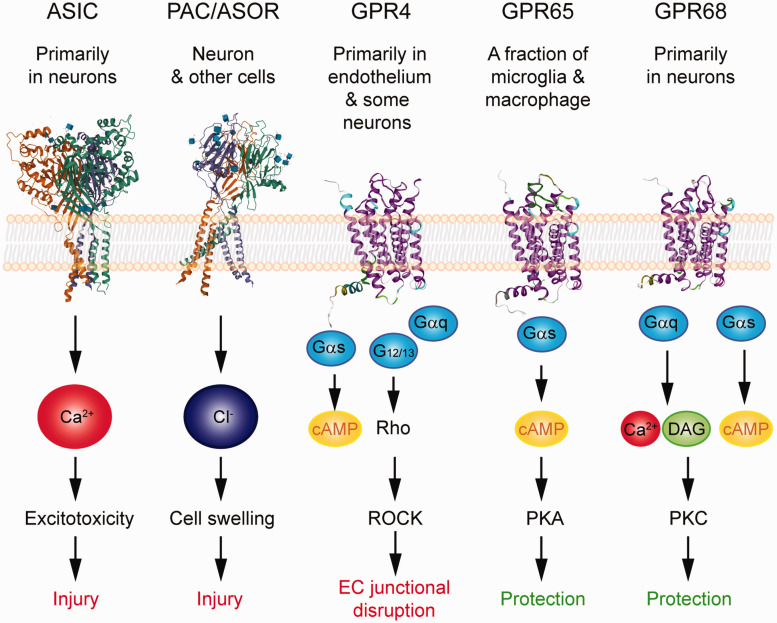
We have gained much knowledge on brain acid signaling from the ASICs. With the emerging evidence regarding metabotropic proton receptor function in the brain, together with the discovery of PAC/ASOR anion channel, it is apparent that brain acid signaling is more complex than what was initially thought. As discussed above, the three classes of proton receptors differ in their pH sensitivity. The proton-sensitive GPCRs are most sensitive to acidification and are activated at ∼pH 7.438–40. The ASICs are in the middle and start to open at ∼7.34,37 The PAC is least pH sensitive and only activates when pH is at or below 6.11,12,35 Together, these receptors cover a wide range of pH changes, starting from the resting pH (∼7.3–7.4) down to ∼5. Besides their differences in pH sensitivity, these receptors differ in their expression and signaling (Figure 2). This combination suggests that these receptors work in concert to determine the outcome of cerebrovascular function, neuroinflammation, and brain injuries. Given that some receptors mediate protection while others elicit injury, discovering specific pharmacological reagents will be a key step and offer new opportunities for therapeutic targeting of these receptors in brain ischemia or other neurological diseases which involve acidotoxicity.
Figure 2.
Summary of the expression, signaling, and impact on ischemic injury of the acid-sensitive receptors. 3D illustration of ASIC and PAC/ASOR was based on crystal structure deposited in NCBI PDB database (PBD ID 3S3W and 7JNA) and created with the NGL viewer.145–147 Note that these structures do not contain most of the intracellular tails. For GPCRs, the 3D illustrations were generated using GPCR homology modeling located on GPCRdb.148,149 The signaling illustrates the key pathways which have been either demonstrated or implicated in the receptor’s contribution to neuronal injury, vascular dysfunction, or neuroinflammation.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Xiangming Zha is supported by the NIH/NINDS R01NS102495 and startup funds from University of Missouri-Kansas City.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions: XMZ reviewed the literature and wrote the manuscript. ZGX and RPS provided important discussions. All authors reviewed the manuscript.
ORCID iD: Xiang-ming Zha https://orcid.org/0000-0001-9490-2731
References
- 1.Ljunggren B, Norberg K, Siesjo BK. Influence of tissue acidosis upon restitution of brain energy metabolism following total ischemia. Brain Res 1974; 77: 173–186. [DOI] [PubMed] [Google Scholar]
- 2.Astrup J, Symon L, Branston NM, et al. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 1977; 8: 51–57. [DOI] [PubMed] [Google Scholar]
- 3.Kalimo H, Rehncrona S, Soderfeldt B, et al. Brain lactic acidosis and ischemic cell damage: 2. Histopathology. J Cereb Blood Flow Metab 1981; 1: 313–327. [DOI] [PubMed] [Google Scholar]
- 4.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 2003; 83: 1183–1221. [DOI] [PubMed] [Google Scholar]
- 5.Song S, Luo L, Sun B, et al. Roles of glial ion transporters in brain diseases. Glia 2020; 68: 472–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magistretti PJ, Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci 2018; 19: 235–249. [DOI] [PubMed] [Google Scholar]
- 7.Ruusuvuori E, Kaila K. Carbonic anhydrases and brain pH in the control of neuronal excitability. Subcell Biochem 2014; 75: 271–290. [DOI] [PubMed] [Google Scholar]
- 8.Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc Natl Acad Sci U S A 1990; 87: 6445–6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature 1990; 345: 347–350. [DOI] [PubMed] [Google Scholar]
- 10.Wemmie JA Taugher RJ, andKreple CJ. Acid sensing ion channels in pain and disease. Nat Rev Neurosci 2013; 14: 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullrich F, Blin S, Lazarow K, et al. Identification of TMEM206 proteins as pore of PAORAC/ASOR acid-sensitive chloride channels. Elife 2019; 8: e49187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Chen J, Del Carmen Vitery M, et al. PAC, an evolutionarily conserved membrane protein, is a proton-activated chloride channel. Science 2019; 364: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okajima F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal 2013; 25: 2263–2271. [DOI] [PubMed] [Google Scholar]
- 14.Deitmer JW. A role for CO(2) and bicarbonate transporters in metabolic exchanges in the brain. J Neurochem 2002; 80: 721–726. [DOI] [PubMed] [Google Scholar]
- 15.Mabe H, Blomqvist P, Siesjo BK. Intracellular pH in the brain following transient ischemia. J Cereb Blood Flow Metab 1983; 3: 109–114. [DOI] [PubMed] [Google Scholar]
- 16.Siesjo BK, von Hanwehr R, Nergelius G, et al. Extra- and intracellular pH in the brain during seizures and in the recovery period following the arrest of seizure activity. J Cereb Blood Flow Metab 1985; 5: 47–57. [DOI] [PubMed] [Google Scholar]
- 17.Smith ML, von Hanwehr R, Siesjo BK. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J Cereb Blood Flow Metab 1986; 6: 574–583. [DOI] [PubMed] [Google Scholar]
- 18.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain 2007; 130: 151–158. [DOI] [PubMed] [Google Scholar]
- 19.Nedergaard M, Kraig RP, Tanabe J, et al. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol 1991; 260: R581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siesjo BK. Cerebral circulation and metabolism. J Neurosurg 1984; 60: 883–908. [DOI] [PubMed] [Google Scholar]
- 21.Nemoto EM, Frinak S. Brain tissue pH after global brain ischemia and barbiturate loading in rats. Stroke 1981; 12: 77–82. [DOI] [PubMed] [Google Scholar]
- 22.Widmer H, Abiko H, Faden AI, et al. Effects of hyperglycemia on the time course of changes in energy metabolism and pH during global cerebral ischemia and reperfusion in rats: correlation of 1H and 31P NMR spectroscopy with fatty acid and excitatory amino acid levels. J Cereb Blood Flow Metab 1992; 12: 456–468. [DOI] [PubMed] [Google Scholar]
- 23.Maruki Y, Koehler RC, Eleff SM, et al. Intracellular pH during reperfusion influences evoked potential recovery after complete cerebral ischemia. Stroke 1993; 24: 697–703; discussion 704. [DOI] [PubMed] [Google Scholar]
- 24.Bolas NM, Rajagopalan B, Mitsumori F, et al. Metabolic changes during experimental cerebral ischemia in hyperglycemic rats, observed by 31P and 1H magnetic resonance spectroscopy. Stroke 1988; 19: 608–614. [DOI] [PubMed] [Google Scholar]
- 25.Chopp M, Welch KM, Tidwell CD, et al. Global cerebral ischemia and intracellular pH during hyperglycemia and hypoglycemia in cats. Stroke 1988; 19: 1383–1387. [DOI] [PubMed] [Google Scholar]
- 26.Chopp M, Frinak S, Walton DR, et al. Intracellular acidosis during and after cerebral ischemia: in vivo nuclear magnetic resonance study of hyperglycemia in cats. Stroke 1987; 18: 919–923. [DOI] [PubMed] [Google Scholar]
- 27.Morikawa S, Inubushi T, Takahashi K, et al. Dissociation between lactate accumulation and acidosis in middle cerebral artery-occluded rats assessed by 31P and 1H NMR metabolic images under a 2-T magnetic field. Magn Reson Imaging 1996; 14: 1197–1204. [DOI] [PubMed] [Google Scholar]
- 28.Zollner JP, Hattingen E, Singer OC, et al. Changes of pH and energy state in subacute human ischemia assessed by multinuclear magnetic resonance spectroscopy. Stroke 2015; 46: 441–446. [DOI] [PubMed] [Google Scholar]
- 29.Hertz L. Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology 2008; 55: 289–309. [DOI] [PubMed] [Google Scholar]
- 30.Siesjo BK, Katsura K, Mellergard P, et al. Acidosis-related brain damage. Prog Brain Res 1993; 96: 23–48. [PubMed] [Google Scholar]
- 31.Waldmann R, Champigny G, Bassilana F, et al. A proton-gated cation channel involved in acid-sensing. Nature 1997; 386: 173–177. [DOI] [PubMed] [Google Scholar]
- 32.Storozhuk M, Cherninskyi A, Maximyuk O, et al. Acid-sensing ion channels: focus on physiological and some pathological roles in the brain. Curr Neuropharmacol 2021; 19: 1570–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vullo S, Kellenberger S. A molecular view of the function and pharmacology of acid-sensing ion channels. Pharmacol Res 2020; 154: 104166. [DOI] [PubMed] [Google Scholar]
- 34.Zha XM. Acid-sensing ion channels: trafficking and synaptic function. Mol Brain 2013; 6: 1. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osei-Owusu J, Yang J, Del Carmen Vitery M, et al. PAC proton-activated chloride channel contributes to acid-induced cell death in primary rat cortical neurons. Channels (Austin) 2020; 14: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okada Y, Sato-Numata K, Sabirov RZ, et al. Cell death induction and protection by activation of ubiquitously expressed anion/cation channels. Part 2: functional and molecular properties of ASOR/PAC channels and their roles in cell volume dysregulation and acidotoxic cell death. Front Cell Dev Biol 2021; 9: 702317–20210709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grunder S, Pusch M. Biophysical properties of acid-sensing ion channels (ASICs). Neuropharmacology 2015; 94: 9–18. [DOI] [PubMed] [Google Scholar]
- 38.Rowe JB, Kapolka NJ, Taghon GJ, et al. The evolution and mechanism of GPCR proton sensing. J Biol Chem 2021; 296: 100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Justus CR, Dong L, Yang LV. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol 2013; 4: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomura H, Mogi C, Sato K, et al. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell Signal 2005; 17: 1466–1476. [DOI] [PubMed] [Google Scholar]
- 41.Jasti J, Furukawa H, Gonzales EB, et al. Structure of acid-sensing ion channel 1 at 1.9 a resolution and low pH. Nature 2007; 449: 316–323. [DOI] [PubMed] [Google Scholar]
- 42.Sherwood TW, Lee KG, Gormley MG, et al. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci 2011; 31: 9723–9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gründer S, Chen X. Function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASI. C1a. Int J Physiol Pathophysiol Pharmacol 2010; 2: 73–94. review. [PMC free article] [PubMed] [Google Scholar]
- 44.Faraci FM, Taugher RJ, Lynch C, et al. Acid-sensing ion channels: novel mediators of cerebral vascular responses. Circ Res 2019; 125: 907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreple CJ, Lu Y, Taugher RJ, et al. Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nat Neurosci 2014; 17: 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong ZG, Chu XP, Simon RP. Acid sensing ion channels–novel therapeutic targets for ischemic brain injury. Front Biosci 2007; 12: 1376–1386. [DOI] [PubMed] [Google Scholar]
- 47.Chassagnon IR, McCarthy CA, Chin YK, et al. Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a. Proc Natl Acad Sci U S A 2017; 114: 3750–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang N, Wu J, Leng T, et al. Region specific contribution of ASIC2 to acidosis-and ischemia-induced neuronal injury. J Cereb Blood Flow Metab 2017; 37: 528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zha XM, Costa V, Harding AM, et al. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci 2009; 29: 8438–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mishra V, Verma R, Singh N, et al. The neuroprotective effects of NMDAR antagonist, ifenprodil and ASIC1a inhibitor, flurbiprofen on post-ischemic cerebral injury. Brain Res 2011; 1389: 152–160. [DOI] [PubMed] [Google Scholar]
- 51.Wang JJ, Liu F, Yang F, et al. Disruption of auto-inhibition underlies conformational signaling of ASIC1a to induce neuronal necroptosis. Nat Commun 2020; 11: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato-Numata K, Numata T, Okada Y. Temperature sensitivity of acid-sensitive outwardly rectifying (ASOR) anion channels in cortical neurons is involved in hypothermic neuroprotection against acidotoxic necrosis. Channels (Austin) 2014; 8: 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato-Numata K, Numata T, Okada T, et al. Acid-sensitive outwardly rectifying (ASOR) anion channels in human epithelial cells are highly sensitive to temperature and independent of ClC-3. Pflugers Arch 2013; 465: 1535–1543. [DOI] [PubMed] [Google Scholar]
- 54.Capurro V, Gianotti A, Caci E, et al. Functional analysis of acid-activated Cl(-) channels: properties and mechanisms of regulation. Biochim Biophys Acta 2015; 1848: 105–114. [DOI] [PubMed] [Google Scholar]
- 55.Deng Z, Zhao Y, Feng J, et al. Cryo-EM structure of a proton-activated chloride channel TMEM206. Sci Adv 2021; 7: eabe5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osei-Owusu J, Yang J, Leung KH, et al. Proton-activated chloride channel PAC regulates endosomal acidification and transferrin receptor-mediated endocytosis. Cell Rep 2021; 34: 108683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heiber M, Docherty JM, Shah G, et al. Isolation of three novel human genes encoding G protein-coupled receptors. DNA Cell Biol 1995; 14: 25–35. [DOI] [PubMed] [Google Scholar]
- 58.Choi JW, Lee SY, Choi Y. Identification of a putative G protein-coupled receptor induced during activation-induced apoptosis of T cells. Cell Immunol 1996; 168: 78–84. [DOI] [PubMed] [Google Scholar]
- 59.Xu Y, Casey G. Identification of human OGR1, a novel G protein-coupled receptor that maps to chromosome 14. Genomics 1996; 35: 397–402. [DOI] [PubMed] [Google Scholar]
- 60.Weiß KT, Fante M, Köhl G, et al. Proton-sensing G protein-coupled receptors as regulators of cell proliferation and migration during tumor growth and wound healing. Exp Dermatol 2017; 26: 127–132. [DOI] [PubMed] [Google Scholar]
- 61.Weng Z, Fluckiger AC, Nisitani S, et al. A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc Natl Acad Sci U S A 1998; 95: 12334–12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murakami N, Yokomizo T, Okuno T, et al. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J Biol Chem 2004; 279: 42484–42491. [DOI] [PubMed] [Google Scholar]
- 63.Wang T, Zhou G, He M, et al. GPR68 is a neuroprotective proton receptor in brain ischemia. Stroke 2020; 51: 3690–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou G, Wang T, Zha XM. RNA-Seq analysis of knocking out the neuroprotective proton-sensitive GPR68 on basal and acute ischemia-induced transcriptome changes and signaling in mouse brain. Faseb J 2021; 35: e21461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 2015; 87: 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown D, Wagner CA. Molecular mechanisms of acid-base sensing by the kidney. J Am Soc Nephrol 2012; 23: 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hosford PS, Mosienko V, Kishi K, et al. CNS distribution, signalling properties and Central effects of G-protein coupled receptor 4. Neuropharmacology 2018; 138: 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wenzel J, Hansen CE, Bettoni C, et al. Impaired endothelium-mediated cerebrovascular reactivity promotes anxiety and respiration disorders in mice. Proc Natl Acad Sci U S A 2020; 117: 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guyenet PG, Bayliss DA, Stornetta RL, et al. Proton detection and breathing regulation by the retrotrapezoid nucleus. J Physiol 2016; 594: 1529–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang CW, Tzeng JN, Chen YJ, et al. Nociceptors of dorsal root ganglion express proton-sensing G-protein-coupled receptors. Mol Cell Neurosci 2007; 36: 195–210. [DOI] [PubMed] [Google Scholar]
- 71.Yang LV, Radu CG, Roy M, et al. Vascular abnormalities in mice deficient for the G protein-coupled receptor GPR4 that functions as a pH sensor. Mol Cell Biol 2007; 27: 1334–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ludwig MG, Vanek M, Guerini D, et al. Proton-sensing G-protein-coupled receptors. Nature 2003; 425: 93–98. [DOI] [PubMed] [Google Scholar]
- 73.Huang F, Mehta D, Predescu S, et al. A novel lysophospholipid- and pH-sensitive receptor, GPR4, in brain endothelial cells regulates monocyte transmigration. Endothelium 2007; 14: 25–34. [DOI] [PubMed] [Google Scholar]
- 74.Justus CR, Yang LV. GPR4 decreases B16F10 melanoma cell spreading and regulates focal adhesion dynamics through the G13/rho signaling pathway. Exp Cell Res 2015; 334: 100–113. [DOI] [PubMed] [Google Scholar]
- 75.Kotake M, Sato K, Mogi C, et al. Acidic pH increases cGMP accumulation through the OGR1/phospholipase C/Ca(2+)/neuronal NOS pathway in N1E-115 neuronal cells. Cell Signal 2014; 26: 2326–2332. [DOI] [PubMed] [Google Scholar]
- 76.Tobo M, Tomura H, Mogi C, et al. Previously postulated “ligand-independent” signaling of GPR4 is mediated through proton-sensing mechanisms. Cell Signal 2007; 19: 1745–1753. [DOI] [PubMed] [Google Scholar]
- 77.Sanderlin EJ, Marie M, Velcicky J, et al. Pharmacological inhibition of GPR4 remediates intestinal inflammation in a mouse colitis model. Eur J Pharmacol 2019; 852: 218–230. [DOI] [PMC free article] [PubMed]
- 78.Wang Y, de Valliere C, Imenez Silva PH, et al. The proton-activated receptor GPR4 modulates intestinal inflammation. J Crohns Colitis 2018; 12: 355–368. [DOI] [PubMed] [Google Scholar]
- 79.Dong L, Krewson E, Yang L. Acidosis activates endoplasmic reticulum stress pathways through GPR4 in human vascular endothelial cells. IJMS 2017; 18: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krewson EA, Sanderlin EJ, Marie MA, et al. The Proton-Sensing GPR4 receptor regulates paracellular gap formation and permeability of vascular endothelial cells. iScience 2020; 23: 100848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong L, Li Z, Leffler NR, et al. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS One 2013; 8: e61991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen A, Dong L, Leffler NR, et al. Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/epac pathway. PLoS One 2011; 6: e27586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wyder L, Suply T, Ricoux B, et al. Reduced pathological angiogenesis and tumor growth in mice lacking GPR4, a proton sensing receptor. Angiogenesis 2011; 14: 533–544. [DOI] [PubMed] [Google Scholar]
- 84.Velcicky J, Miltz W, Oberhauser B, et al. Development of selective, orally active GPR4 antagonists with modulatory effects on nociception, inflammation, and angiogenesis. J Med Chem 2017; 60: 3672–3683. [DOI] [PubMed] [Google Scholar]
- 85.Kumar NN, Velic A, Soliz J, et al. PHYSIOLOGY. Regulation of breathing by CO(2) requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 2015; 348: 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sato K, Tobo A, Mogi C, et al. The protective role of proton-sensing TDAG8 in the brain injury in a mouse ischemia reperfusion model. Sci Rep 2020; 10: 17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haque ME, Akther M, Azam S, et al. GPR4 knockout improves the neurotoxin-induced, caspase-dependent mitochondrial apoptosis of the dopaminergic neuronal cell. IJMS 2020; 21: 7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vollmer LL, Ghosal S, McGuire JL, et al. Microglial acid sensing regulates carbon dioxide-evoked fear. Biol Psychiatry 2016; 80: 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma XD, Hang LH, Shao DH, et al. TDAG8 activation attenuates cerebral ischaemia-reperfusion injury via akt signalling in rats. Exp Neurol 2017; 293: 115–123. [DOI] [PubMed] [Google Scholar]
- 90.McGuire J, Herman JP, Ghosal S, et al. Acid-sensing by the T cell death-associated gene 8 (TDAG8) receptor cloned from rat brain. Biochem Biophys Res Commun 2009; 386: 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang JQ, Kon J, Mogi C, et al. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J Biol Chem 2004; 279: 45626–45633. [DOI] [PubMed] [Google Scholar]
- 92.Ishii S, Kihara Y, Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem 2005; 280: 9083–9087. [DOI] [PubMed] [Google Scholar]
- 93.Chen YJ, Huang CW, Lin CS, et al. Expression and function of proton-sensing G-protein-coupled receptors in inflammatory pain. Mol Pain 2009; 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mogi C, Tobo M, Tomura H, et al. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J Immunol 2009; 182: 3243–3251. [DOI] [PubMed] [Google Scholar]
- 95.Radu CG, Nijagal A, McLaughlin J, et al. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc Natl Acad Sci U S A 2005; 102: 1632–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nagasaka A, Mogi C, Ono H, et al. The proton-sensing G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) shows cardioprotective effects against myocardial infarction. Sci Rep 2017; 7: 7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ail D, Rufenacht V, Caprara C, et al. Increased expression of the proton-sensing G protein-coupled receptor Gpr65 during retinal degeneration. Neuroscience 2015; 301: 496–507. [DOI] [PubMed] [Google Scholar]
- 98.Wirasinha RC, Vijayan D, Smith NJ, et al. GPR65 inhibits experimental autoimmune encephalomyelitis through CD4(+) T cell independent mechanisms that include effects on iNKT cells. Immunol Cell Biol 2018; 96: 128–136. [DOI] [PubMed] [Google Scholar]
- 99.Tomura H, Wang JQ, Komachi M, et al. Prostaglandin I(2) production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J Biol Chem 2005; 280: 34458–34464. [DOI] [PubMed] [Google Scholar]
- 100.Ichimonji I, Tomura H, Mogi C, et al. Extracellular acidification stimulates IL-6 production and Ca(2+) mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2010; 299: L567–577. [DOI] [PubMed] [Google Scholar]
- 101.Saxena H, Deshpande DA, Tiegs BC, et al. The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br J Pharmacol 2012; 166: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu Y, Lin MT, Zha XM. GPR68 deletion impairs hippocampal long-term potentiation and passive avoidance behavior. Mol Brain 2020; 13: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu J, Mathur J, Vessieres E, et al. GPR68 senses flow and is essential for vascular physiology. Cell 2018; 173: 762–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei WC, Bianchi F, Wang YK, et al. Coincidence detection of membrane stretch and extracellular pH by the proton-sensing receptor OGR1 (GPR68). Curr Biol 2018; 28: 3815–3823. [DOI] [PubMed] [Google Scholar]
- 105.Huang WC, Swietach P, Vaughan-Jones RD, et al. Extracellular acidification elicits spatially and temporally distinct Ca2+ signals. Curr Biol 2008; 18: 781–785. [DOI] [PubMed] [Google Scholar]
- 106.Huang XP, Karpiak J, Kroeze WK, et al. Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature 2015; 527: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li J, Guo B, Wang J, et al. Ovarian cancer G protein coupled receptor 1 suppresses cell migration of MCF7 breast cancer cells via a Galpha12/13-Rho-Rac1 pathway. J Mol Signal 2013; 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wei WC, Jacobs B, Becker EB, et al. Reciprocal regulation of two G protein-coupled receptors sensing extracellular concentrations of Ca2+ and H. Proc Natl Acad Sci U S A 2015; 112: 10738–10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang XP, Kenakin TP, Gu S, et al. Differential roles of extracellular histidine residues of GPR68 for proton-sensing and allosteric modulation by divalent metal ions. Biochemistry 2020; 59: 3594–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aoki H, Mogi C, Hisada T, et al. Proton-sensing ovarian cancer G protein-coupled receptor 1 on dendritic cells is required for airway responses in a murine asthma model. PLoS One 2013; 8: e79985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pereverzev A, Komarova SV, Korcok J, et al. Extracellular acidification enhances osteoclast survival through an NFAT-independent, protein kinase C-dependent pathway. Bone 2008; 42: 150–161. [DOI] [PubMed] [Google Scholar]
- 112.Tomura H, Wang JQ, Liu JP, et al. Cyclooxygenase-2 expression and prostaglandin E2 production in response to acidic pH through OGR1 in a human osteoblastic cell line. J Bone Miner Res 2008; 23: 1129–1139. [DOI] [PubMed] [Google Scholar]
- 113.Krieger NS, Yao Z, Kyker-Snowman K, et al. Increased bone density in mice lacking the proton receptor OGR1. Kidney Int 2016; 89: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li H, Wang D, Singh LS, et al. Abnormalities in osteoclastogenesis and decreased tumorigenesis in mice deficient for ovarian cancer G protein-coupled receptor 1. PLoS One 2009; 4: e5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wiley SZ, Sriram K, Salmeron C, et al. GPR68: an emerging drug target in cancer. IJMS 2019; 20: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang T, He M, Zha XM. Time-dependent progression of hemorrhagic transformation after transient ischemia and its association with GPR68-dependent protection. Brain Hemorrhages 2020; 1: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao D, Zhang M, Yang L, et al. GPR68 improves nerve damage and myelination in an immature rat model induced by sevoflurane anesthesia by activating cAMP/CREB to mediate BDNF. ACS Chem Neurosci 2022; 13: 423–431. [DOI] [PubMed] [Google Scholar]
- 118.He Y, Hua Y, Liu W, et al. Effects of cerebral ischemia on neuronal hemoglobin. J Cereb Blood Flow Metab 2009; 29: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun Y, Jin K, Peel A, et al. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U S A 2003; 100: 3497–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bohlen CJ, Chesler AT, Sharif-Naeini R, et al. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 2011; 479: 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Escoubas P, De Weille JR, Lecoq A, et al. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem 2000; 275: 25116–25121. [DOI] [PubMed] [Google Scholar]
- 122.Diochot S, Baron A, Salinas M, et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature 2012; 490: 552–555. [DOI] [PubMed] [Google Scholar]
- 123.Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci 2009; 29: 14371–14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Babini E, Paukert M, Geisler HS, et al. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J Biol Chem 2002; 277: 41597–41603. [DOI] [PubMed] [Google Scholar]
- 125.Chen X, Qiu L, Li M, et al. Diarylamidines: high potency inhibitors of acid-sensing ion channels. Neuropharmacology 2010; 58: 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 2001; 4: 869–870. [DOI] [PubMed] [Google Scholar]
- 127.Chu XP, Wemmie JA, Wang WZ, et al. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci 2004; 24: 8678–8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diochot S, Baron A, Rash LD, et al. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J 2004; 23: 1516–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu Y, Chen Z, Li WG, et al. A nonproton ligand sensor in the acid-sensing ion channel. Neuron 2010; 68: 61–72. [DOI] [PubMed] [Google Scholar]
- 130.Deval E, Baron A, Lingueglia E, et al. Effects of neuropeptide SF and related peptides on acid sensing ion channel 3 and sensory neuron excitability. Neuropharmacology 2003; 44: 662–671. [DOI] [PubMed] [Google Scholar]
- 131.Fukuda H, Ito S, Watari K, et al. Identification of a potent and selective GPR4 antagonist as a drug lead for the treatment of myocardial infarction. ACS Med Chem Lett 2016; 7: 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Foster SR, Hauser AS, Vedel L, et al. Discovery of human signaling systems: pairing peptides to G protein-coupled receptors. Cell 2019; 179: 895–908. e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wemmie JA, Chen J, Askwith CC, et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 2002; 34: 463–477. [DOI] [PubMed] [Google Scholar]
- 134.Wu PY, Huang YY, Chen CC, et al. Acid-sensing ion channel-1a is not required for normal hippocampal LTP and spatial memory. J Neurosci 2013; 33: 1828–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chang CT, Fong SW, Lee CH, et al. Involvement of acid-sensing ion channel 1b in the development of acid-induced chronic muscle pain. Front Neurosci 2019; 13: 1247–20191122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Price MP, Lewin GR, McIlwrath SL, et al. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 2000; 407: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 137.Ettaiche M, Guy N, Hofman P, et al. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J Neurosci 2004; 24: 1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wemmie JA, Coryell MW, Askwith CC, et al. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci U S A 2004; 101: 3621–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Price MP, McIlwrath SL, Xie J, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 2001; 32: 1071–1083. [DOI] [PubMed] [Google Scholar]
- 140.Wultsch T, Painsipp E, Shahbazian A, et al. Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach-brainstem axis. Pain 2008; 134: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lin SH, Cheng YR, Banks RW, et al. Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nat Commun 2016; 7: 11460–20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vralsted VC, Price MP, Du J, et al. Expressing acid-sensing ion channel 3 in the brain alters acid-evoked currents and impairs fear conditioning. Genes Brain Behav 2011; 10: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang RB, Strochlic DE, Williams EK, et al. Vagal sensory neuron subtypes that differentially control breathing. Cell 2015; 161: 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mohebbi N, Benabbas C, Vidal S, et al. The proton-activated G protein coupled receptor OGR1 acutely regulates the activity of epithelial proton transport proteins. Cell Physiol Biochem 2012; 29: 313–324. [DOI] [PubMed] [Google Scholar]
- 145.Dawson RJ, Benz J, Stohler P, et al. Structure of the acid-sensing ion channel 1 in complex with the gating modifier Psalmotoxin 1. Nat Commun 2012; 3: 936–20120703. [DOI] [PubMed] [Google Scholar]
- 146.Ruan Z, Osei-Owusu J, Du J, et al. Structures and pH-sensing mechanism of the proton-activated chloride channel. Nature 2020; 588: 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rose AS, Bradley AR, Valasatava Y, et al. NGL viewer: web-based molecular graphics for large complexes. Bioinformatics 2018; 34: 3755–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kooistra AJ, Mordalski S, Pandy-Szekeres G, et al. GPCRdb in 2021: integrating GPCR sequence, structure and function. Nucleic Acids Res 2021; 49: D335–D343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pandy-Szekeres G, Munk C, Tsonkov TM, et al. GPCRdb in 2018: adding GPCR structure models and ligands. Nucleic Acids Res 2018; 46: D440–D446. [DOI] [PMC free article] [PubMed] [Google Scholar]