Abstract
Behavioural responses to hypoglycaemia require coordinated recruitment of broadly distributed networks of interacting brain regions. We investigated hypoglycaemia-related changes in brain connectivity in people without diabetes (ND) and with type 1 diabetes with normal (NAH) or impaired (IAH) hypoglycaemia awareness. Two-step hyperinsulinaemic hypoglycaemic clamps were performed in 14 ND, 15 NAH and 22 IAH participants. BOLD timeseries were acquired at euglycaemia (5.0 mmol/L) and hypoglycaemia (2.6 mmol/L), with symptom and counter-regulatory hormone measurements. We investigated hypoglycaemia-related connectivity changes using established seed regions for the default mode (DMN), salience (SN) and central executive (CEN) networks and regions whose activity is modulated by hypoglycaemia: the thalamus and right inferior frontal gyrus (RIFG). Hypoglycaemia-induced changes in the DMN, SN and CEN were evident in NAH (all p < 0.05), with no changes in ND or IAH. However, in IAH there was a reduction in connectivity between regions within the RIFG (p = 0.001), not evident in the ND or NAH groups. We conclude that hypoglycaemia induces coordinated recruitment of the DMN and SN in diabetes with preserved hypoglycaemia awareness which is absent in IAH and ND. Changes in connectivity in the RIFG, a region associated with attentional modulation, may be key in impaired hypoglycaemia awareness.
Keywords: Hypoglycaemia, neuroimaging, resting state networks, impaired awareness of hypoglycaemia, type 1 diabetes
Introduction
Impaired awareness of hypoglycaemia (IAH), the inability to detect symptoms of hypoglycaemia, remains the largest risk factor for development of severe hypoglycaemia (SH) in diabetes treatments.1,2 In type 1 diabetes (T1D), IAH is associated with a 6-fold increase in SH and is reported by up to 33% of people.3,4
Responses to insulin-induced hypoglycaemia are complex. In addition to their metabolic effects, counter-regulatory neuro-endocrine responses provide sensations, such as tremors or sweating, that people perceive as hypoglycaemia. 5 Behavioural responses to these sensations, including prompt ingestion of carbohydrate, are critical protection from severe hypoglycaemia, particularly in insulin treated diabetes.
In IAH, there is a well-described loss of counter-regulatory response resulting in the onset of neuroglycopenia before appreciable symptoms are detected and acted upon. 6 Qualitative studies have identified key thought processes in people with IAH that can prevent the avoidance or prompt treatment of hypoglycaemia and increase the risk of further hypoglycaemia. 7 Some of these unhelpful thoughts (e.g. “I can function OK with low (below 3 mmol/L) blood glucose”) may predispose to delayed 8 or absent self-treatment of hypoglycaemia and prevent recovery from IAH. Other thoughts (e.g. wanting to “get on with life”) involve the minimisation of symptoms. In a cohort study, people with recurrent SH were more likely to express beliefs such as lack of concern about asymptomatic hypoglycaemia or fear of hyperglycaemia. 9 These observations raise the possibility that in some people, IAH does not occur simply as a result of loss of counter-regulatory responses but occurs and is maintained by maladaptive cognitive responses surrounding hypoglycaemia. In support of this, we have previously described alterations in blood flow of regions involved in aversion, salience detection, recall and drive to eat in neuroimaging studies during hypoglycaemia in people with IAH, compared to people with preserved awareness of hypoglycaemia. 10 In this work, we address the question of how these crucial brain regions are functionally connected and how they interact during the hypoglycaemic stimulus.
The present analysis was designed to investigate changes in connectivity between specific resting state networks during early hypoglycaemia. Resting state networks are groups of spatially distinct brain regions that activate and deactivate contemporaneously indicating sharing of information and therefore functional connectivity. 11 We first investigated the change in connectivity of well-established resting state networks that we expected to be involved in the response to hypoglycaemia: the default mode network (DMN), salience network (SN) and central executive network (CEN). These networks have been shown to interact when switching from rest to engaging in complex activity, with the SN activity associated with transitioning between rest and cognitive functioning due to behaviourally significant internal or external stimuli.12,13
We then sought to explore the connectivity of the thalamus10,14–20 and right inferior frontal gyrus (RIFG), 21 two brain regions which have been shown to be activated during hypoglycaemia. The thalamus has a role in processing of sensory data and relaying to other parts of the brain.10,14–20 The RIFG, has been shown to be more active during hypoglycaemia in IAH 21 and is known to modulate attention towards salient sensory stimuli.22–24
Materials and methods
Study participants
This analysis was performed on neuroimaging data obtained in people without diabetes (ND), in people with T1D and normal awareness of hypoglycaemia (NAH) and also at baseline from participants with T1D and IAH in two intervention studies (HypoAware and HARPdoc) conducted at King’s College London using the same imaging protocol. HypoAware was designed to understand differences in regional cerebral blood flow in response to experimental hypoglycaemia between ND and participants with NAH or IAH, defined as a gold score of ≥4. This study went on to evaluate the impact of restoration of awareness with diabetes technology (Medtronic 640 G with predictive low glucose management system) and frequent contact on these neuroimaging changes. The cerebral blood flow data from HypoAware have been published.19,20 HARPdoc is a randomised controlled trial of a novel psychoeducational course with cognitive behavioural and motivational strategies targeting unhelpful thoughts surrounding hypoglycaemia whose participants were also invited for neuroimaging studies at baseline. In both studies, inclusion criteria included age 18–75 years and absence of history consistent with neurological disease or injury that would be expected to produce changes on MRI, diagnosis of psychological or psychiatric disorder, impaired renal function (estimated glomerular filtration rate >30 ml/min/1.73 m2) or contraindications to MRI. Those with diabetes were assessed for hypoglycaemia awareness status at the recruitment visit using the Gold score, a seven-part Likert scale in which participants rank their awareness of hypoglycaemic events from 1 (I am always aware) to 7 (I am never aware). 25 IAH was diagnosed with Gold score ≥4, those with Gold score ≤3 were designated NAH. 26 Throughout recruitment, age, sex and diabetes duration were monitored to achieve groups matched for these characteristics. In this post-hoc analysis, all participants in either study with complete data sets were included and their pre-intervention neuroimaging data analysed. The research was approved by the Dulwich (HypoAware; 13/LO/1821) and Surrey (HARPscan, 18/LO/1464) Research Ethics Committees (National Research Ethics Service, London, UK) in accordance with the Declaration of Helsinki. Each participant gave written informed consent. Perfusion data from HypoAware have been published.19,20
Protocol
The neuroimaging protocol has been described.19,20 In brief, those with T1D stayed overnight in the National Institute for Health Research and Wellcome Trust King’s Clinical Research Facility following their evening meal on the day before the study. Basal insulins or continuous subcutaneous insulin infusions were replaced with variable rate intravenous (IV) insulin (Actrapid; Novo-Nordisk, UK) overnight to maintain glucose between 5–9 mmol/L without hypoglycaemia. Participants fasted from 10 pm, allowing oral glucose if venous glucose fell below 4.5 mmol/L.
The following morning, a hyperinsulinaemic glucose clamp was initiated with a primed 1.5 mU kg−1 min−1 IV insulin infusion and plasma glucose stabilised at 5 mmol/l by varying the rate of a 20% wt/vol glucose infusion (Baxter, UK) 30 minutes before transfer to the MRI room. Plasma glucose was sampled every 5 minutes through an IV cannula placed in a dorsal hand vein and arterialisation was achieved using a heated gel pad applied around the arm.19,20
Participants were placed in the supine position and wore earplugs and earphones to reduce perception of noise. Movement artefact was minimised using padding inside the scanner receiver head coil and by asking the participants to remain still. Glucose was clamped at 5.0 mmol/L for approximately 30 minutes during which localisation sequences and resting Blood Oxygen Level Dependent (BOLD) fMRI timeseries were acquired to permit the study of resting state network connectivity. Following euglycaemic imaging, plasma glucose was lowered over 15–20 mins to 2.6 mmol/L. A further resting-state BOLD fMRI timeseries was acquired once hypoglycaemia was achieved. During the same study visit, participants underwent six arterial spin labelling sequences which have been reported separately.19,20 A schematic of the imaging timings is available online (Supplementary Figure 1). Immediately before each BOLD timeseries, blood was drawn for measurement of counterregulatory hormones and participants completed an autonomic (anxiety, pounding heart, shaking, tingling, sweating, hunger and nausea) and neuroglycopenic (drowsiness, irritability, visual disturbance and confusion) symptom questionnaire on a 7-point visual analogue Likert scale, using a button box. We classified adrenergic symptoms as anxiety, pounding heart and shaking. 5 Participants were blinded to their glucose.
Following completion of the scanning protocol, IV insulin was stopped, and IV glucose infused until the measured blood glucose was >7 mmol/L. Participants were given a meal and supported until discharge, whereupon they were given emergency contact details and advice to minimise risk of hypoglycaemia for 48 hours.
Biochemical analysis
Arterialised venous plasma glucose was sampled at 5-minute intervals and measured with a glucose oxidase analyser (YSI 2300 STAT PLUS, Yellow Springs Instruments). Adrenaline was analysed by high-performance liquid chromatography with electrochemical detection. 27
Biochemical statistical analysis
Statistical analysis was conducted using jamovi (version 1.0; www.jamovi.org). Demographic data were compared between group using one-way ANOVA or independent samples t-tests for data relevant only to the diabetes groups following assessment of normality using the Shapiro-Wilk test. Within group symptom scores, mean glucose and counterregulatory hormone responses were analysed using paired t-tests and one-way ANOVA following assessment for normality using the Shapiro-Wilk test. The Games-Howell post hoc test was implemented for non-parametric data. A threshold of p < 0.05 was taken to assume statistical significance. Data are presented as mean ± standard deviation.
MRI parameters
MRI images were acquired using a 3 Tesla GE Healthcare MR750 scanner (GE Medical Systems, Milwaukee, WI, USA). After an initial localiser scan and T1-weighted image for registration, a six-minute BOLD timeseries were acquired with the following parameters: 180 functional volumes using gradient-recalled Echo Planar Imaging, repetition time 2000 ms, ECHO time 30 ms, flip angle 75°, 39 axial slices and a 64 × 64 acquisition matrix and an isotropic 3.3 mm voxel size.
T1-weighted structural imaging used a 3D Magnetisation Prepared Rapid Acquisition Gradient Recalled ECHO (MP-RAGE) with the following acquisition parameters: slice thickness 1.2 mm, 196 slices, TR 7.312 ms, TE 3.016 ms, inversion time 400 ms, flip angle 11°, matrix size 256 × 256 and a field of view 27 cm.
Pre-processing
Pre-processing was carried out in the CONN toolbox. 28 The images were motion- and slice time-corrected prior to normalisation via unified segmentation of the T1-weighted image. The resulting images were spatially smoothed with an 8 mm Gaussian kernel. Data denoising was carried out in CONN by means of their CompCor procedure. To minimise the potential contribution of head motion to the observed connectivity, we identified outlier scans as either those with >3 sd from the mean global BOLD signal or those where volume-to-volume head movements (i.e. framewise displacement) was >0.5 mm. Scan nulling regressors were combined with the original 12 realignment parameter regressors and these were regressed out of the data. The resultant residual time-courses were band pass filtered to retain frequencies between 0.009 to 0.08 Hz for denoising. Furthermore, we excluded the data of participants with a mean framewise displacement of greater than 0.5 mm over that run.
Neuroimaging data analysis
Participants were pseudonymised with a study ID which was used throughout the neuroimaging analyses. The BOLD neuroimaging data analysis was completed using the Conn toolbox. 28 For each individual, we created a Fisher-transformed, seed-to-voxel bivariate Pearson’s correlation map based on the seed ROI’s BOLD time-courses. Pre-specified seed regions included in the seed-to-voxel analyses were conducted with seed regions defined using 20% probabilistic threshold using the Brainnetome atlas: the posterior cingulate cortex (PCC; core node of the DMN), anterior agranular insula (core node of the salience network), Brodman’s areas 9 and 46 (dlPFC; core node of the central executive network) and thalamus. Based on a previous publication, we were also interested in studying the connectivity of a region in the vicinity of the RIFG and consequently for this seed only we used a 10 mm sphere based on published MNI co-ordinates [36, 32, −13]. 21
Our primary objective was to identify between group differences in the effect of hypoglycaemia induction. Between group comparisons were performed twice (ND vs NAH or NAH vs IAH) for each network using a 2 × 2 mixed group by condition (euglycemia vs hypoglycaemia) ANOVA interaction design. This was preferred to a single 2 × 3 design as the comparison between ND and IAH was not considered useful for interpretation. Between group comparisons are represented graphically. The figure beta scores reflect group estimates of correlation in the activity timeseries for the seed region and the observed cluster. In line with many studies in the field we went on to investigate within group comparisons using paired t-tests as a secondary objective.14–16,18,20,21,29
To enhance power to detect important between group differences we performed region of interest (ROI) analyses for the core components of our networks of interest using probabilistic masks generated in FSLeyes (https://git.fmrib.ox.ac.uk/fsl/fsleyes/fsleyes/) (Supplemental Table 1). Each of the networks was defined a priori. Results were considered significant if the clusters survived familywise error correction (pFWE-Corrected <0.05) based on cluster-extent, with the default cluster-forming voxel threshold of p < 0.001.
Regression analyses
Regression analyses were performed in SPM12 (www.fil.ion.ucl.ac.uk/spm/software/spm12). We constructed multiple regression models with differences between the hypoglycaemic and euglycaemic contrast maps as the dependent variable and the change in adrenergic symptom score as the explanatory variables. Given that this was a post-hoc exploratory analysis we have implemented an additional Bonferroni multiple comparisons correction for the regression analyses. With 5 ROIs we considered a result significant if and only if the associated p-value was below a critical α of p < 0.01.
Results
Participant characteristics
Fifty-two individuals were considered for this analysis (15 ND, 15 T1D NAH, 22 T1D IAH), however one ND individual was excluded due to loss of the hypoglycaemic BOLD data. No participants were excluded for excessive movement with the group mean framewise displacement being 0.24 ± 0.13 mm. We therefore analysed data from 51 individuals, 14 ND, 15 T1D NAH and 22 T1D IAH (Table 1). The three groups were well matched for age, body mass index and sex distribution. The groups of participants with diabetes were well matched for HbA1c and diabetes duration, which was long, with no significant difference between the groups.
Table 1.
Participant characteristics.
| ND (n = 14) | NAH (n = 15) | IAH (n = 22) | p-value | |
|---|---|---|---|---|
| Age (y) | 40.1 ± 11.7 | 39.1 ± 13.5 | 39.2 ± 11.1 | 0.989† |
| Sex (n female) | 8 | 9 | 13 | 0.859† |
| BMI (kg/m2) | 25.0 ± 2.8 | 24.7 ± 4.0 | 24.6 ± 4.6 | 0.774† |
| HbA1c (mmol/mol) | – | 59.7 ± 11.2 | 61.6 ± 10.8 | 0.276* |
| T1D duration (y) | – | 24.0 ± 12.8 | 22.2 ± 7.2 | 0.346* |
| Gold score | – | 1.5 ± 0.5 | 5.8 ± 1.3 | <0.001* |
*t-test.†ANOVA.
Plasma glucose results
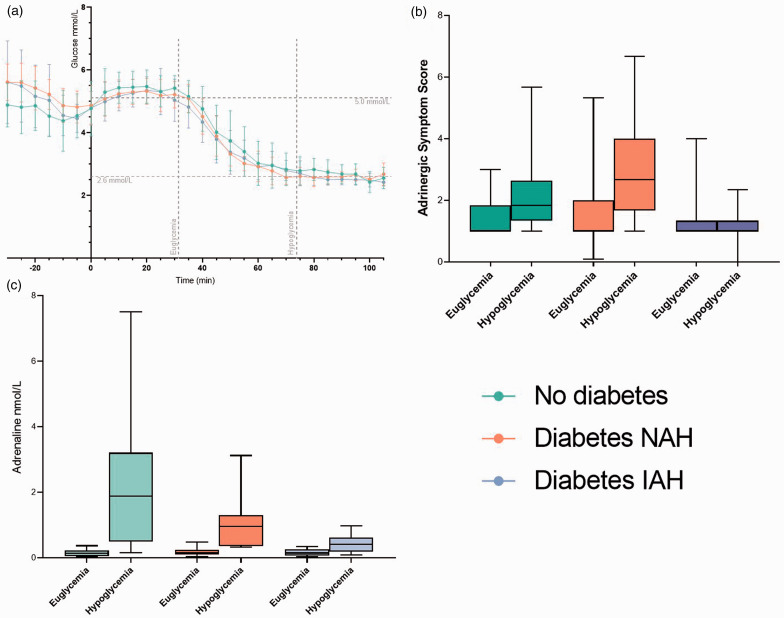
Mean plasma glucose levels for the euglycaemic and hypoglycaemic timepoints were 5.16 ± 0.58 mmol/L (92.9 ± 10.5 mg/dL) and 2.67 ± 0.58 mmol/L (48.1 ± 10.5 mg/dL) respectively (Figure 1(a)). There were no differences in measured glucose level between the groups at each timepoint (one-way ANOVA, euglycaemia p = 0.74, hypoglycaemia p = 0.46).
Figure 1.
Biochemical and symptom responses during the clamp studies. People without diabetes are in green, people with T1D and NAH in orange and people with T1D and IAH in purple. a – Plasma glucose concentrations during the clamp studies presented as mean ± SD. Time from initiation of MRI sequences is on the x axis. b – A boxplot of adrenergic symptom responses immediately before the euglycaemic and hypoglycaemic resting state network scans. Error bars indicate the 95% confidence interval. c – A boxplot ofdrenaline concentrations measured immediately before the euglycaemic and hypoglycaemic resting state network scans. Error bars indicate the 95% confidence interval.
Hormonal and symptomatic responses
Adrenaline concentrations rose significantly from baseline in all three groups (Figure 1(b)): absolute values were ND 0.16 ± 0.09 nmol/L vs. 2.22 ± 2.03 nmol/L (p < 0.001), NAH 0.19 ± 0.12 nmol/L vs. 1.01 ± 0.79 nmol/L (p < 0.001), and IAH 0.17 ± 0.10 nmol/L vs. 0.43 ± 0.28 nmol/L (p < 0.001). The magnitude of response was different between all groups (one-way ANOVA, euglycaemia p = 0.74, hypoglycaemia p < 0.005), with the greatest response being in ND, and the smallest in IAH (Games-Howell post-hoc test).
Adrenergic symptom scores (Figure 1(c)) rose with hypoglycaemia as expected in ND (+0.77 ± 0.34, paired t-test p < 0.05) and NAH (+1.36 ± 0.39, p < 0.005) but not in IAH (−0.15 ± 0.19, p = 0.278). In a one-way ANOVA with Games-Howell post-hoc test there were significant differences in hypoglycaemia induced adrenergic symptom difference between NAH and IAH (p = 0.005) but not between NAH and ND (p = 0.509). There was no difference in hypoglycaemia-induced visual disturbance reported in each of the 3 groups (one-way ANOVA p = 0.186).
Functional connectivity data (hypothesis led network analysis at baseline – ND group at euglycaemia)
In line with our hypothesis and given that the thalamic and RIFG seeds have not been studied previously, we present the baseline resting state networks associated with each of these seeds as context for the subsequent analyses.
In the ND group the thalamic seed was functionally connected at euglycaemia with a large region encompassing the right and left thalamus, large portions of the putamen and caudate nuclei as well as parts of the insular and opercular cortices (Supplemental Fig 2). Additionally, thalamic activity was negatively correlated with the inferior and middle temporal gyri bilaterally, the left occipital fusiform gyrus and the right frontal pole. There were no between-group differences in the thalamic network at euglycaemia.
In the ND group at euglycaemia the right inferior frontal gyrus seed was functionally connected with large areas of the frontal cortex bilaterally, including the frontal pole, frontal orbital cortex, superior frontal gyrus and frontal insula, as well as the left cerebellum and middle temporal gyrus (Supplemental Fig 3). The RIFG activity timecourse was negatively correlated with those of the precuneus, left post-central gyrus and right lingual gyrus. There were no between-group differences in the right inferior frontal gyrus network at euglycaemia.
Functional connectivity data (between-group comparisons of hypoglycaemia-induced connectivity changes)
Default mode network (using PCC as seed)
ND had a significantly different change in connectivity within the components of the DMN compared with the NAH group during hypoglycaemia using small volume correction (cluster size = 24, pFWE = 0.032, MNIxyz [54,−54,48], Figure 2). In this hypothesis-led ROI analysis, the PCC seed showed increased positive correlation with the right angular gyrus in the ND group at hypoglycaemia whilst the NAH group had a higher baseline connectivity which reduced at hypoglycaemia. In exploratory whole brain analyses, there were no between group differences in hypoglycaemia-related to the modulation of PCC connectivity.
Figure 2.
Between group differences in connectivity of the PCC seed. a – The between group effect of hypoglycaemia induction projected on a representative T1-weight brain image. The 2 × 2 repeated measures ANOVA using the PCC seed with ND > NAH and hypoglycaemia > euglycaemia. The right angular gyrus displays significantly greater increase in connectivity with the PCC in ND compared to the NAH group. Cluster forming threshold p < 0.001, pFWE < 0.05. b – Beta-scores representing group estimates of correlation in BOLD signal between the PCC seed and the right angular gyrus in all groups. There is a reduction in connectivity in the NAH group not seen in the other groups which is significantly different from the change in ND.
There were no differences in the NAH vs IAH 2 × 2 ANOVA in any of our ROIs or exploratory whole brain analyses.
Thalamic network
There were no differences in the ND vs NAH 2 × 2 ANOVA in any of our ROIs or exploratory whole brain analyses.
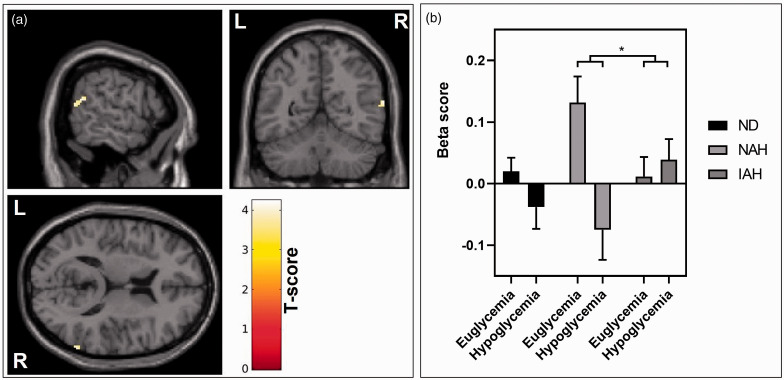
In the 2 × 2 ANOVA analyses of the thalamic network in NAH and IAH, the NAH group demonstrated a reduction in thalamic functional connectivity with a cluster in the right angular gyrus at hypoglycaemia. This was not seen in IAH, and the between group interaction was significant when using small volume correction (cluster size = 33, pFWE = 0.019, MNIxyz [64, −56, 16], Figure 3). There were no significant differences in the other ROIs or corresponding exploratory whole brain analysis.
Figure 3.
Between group differences in connectivity of the thalamic seed. a – The between group effect of hypoglycaemia induction projected on a representative T1-weight brain image. The 2 × 2 repeated measures ANOVA using the thalamic seed with IAH > NAH and hypoglycaemia > euglycaemia. The right angular gyrus displays significantly greater increase in connectivity with the thalamus in the IAH group compared to the NAH group. Cluster forming threshold p < 0.001, pFWE < 0.05. b – Beta scores representing group estimates of correlation in BOLD signal between the thalamus seed and the right angular gyrus in all groups. There is an increase in connectivity seen in IAH at hypoglycaemia that is not seen in the other groups and is significantly different from the NAH group.
Other networks
There were no significant differences in connectivity due to hypoglycaemia between any of the groups in the salience, central executive or RIFG analyses on either the ROI or whole brain analyses.
Functional connectivity data (within-group comparisons of hypoglycaemia-induced connectivity changes))
Default mode network (using PCC as seed)
In the within-group analyses the ND group had no significant hypoglycaemia-related changes in functional connectivity of the DMN.
In contrast the NAH group showed a significant hypoglycaemia induced reduction in functional connectivity between the DMN seed a region in the post central gyrus (cluster size = 404, clusterwise pFWE < 0.001, MNIxyz [−28, −28, 66]) and an increase in functional connectivity between the DMN seed and the right frontal pole (cluster size = 156, clusterwise pfwe < 0.05, MNIxyz [50, 42, −08]) (Table 2).
Table 2.
Regions with significantly altered connectivity at hypoglycaemia in the within group analyses. Increased reflects increased connectivity between the seed and region at hypoglycaemia and reduced indicates a reduction in connectivity at hypoglycaemia.
| Seed | Group | Size | Location | Direction of change | Significance (cluster pFWE) | MNIxyz Co-ordinates | ||
|---|---|---|---|---|---|---|---|---|
| DMN | ND | -- | -- | -- | -- | -- | ||
| NAH | 404 | Postcentral gyrus | Reduced | <0.001 | −28 | −28 | +66 | |
| 156 | Right frontal pole | Increased | <0.05 | +50 | +42 | −08 | ||
| IAH | -- | -- | -- | -- | -- | |||
| SN | ND | -- | -- | -- | -- | -- | ||
| NAH | 241 | Central operculum | Increased | 0.005 | +62 | +2 | −4 | |
| IAH | -- | -- | -- | -- | -- | |||
| CEN | ND | -- | -- | -- | -- | -- | -- | -- |
| NAH | -- | -- | -- | -- | -- | -- | -- | |
| IAH | -- | -- | -- | -- | -- | |||
| Thalamus | ND | 253 | R Frontal pole | Increased | 0.006 | +35 | +24 | +56 |
| 176 | Precuneus | Increased | <0.05 | +12 | −44 | +46 | ||
| NAH | -- | -- | -- | -- | -- | |||
| IAH | 184 | Precuneus | Reduced | <0.05 | −6 | −62 | +52 | |
| 248 | Lateral occipital cortex | Increased | <0.05 | −36 | −74 | −8 | ||
| Right inferior frontal gyrus | ND | -- | -- | -- | -- | -- | ||
| NAH | -- | -- | -- | -- | -- | |||
| IAH | 346 | R Inferior frontal gyrus | Reduced | 0.0020 | +50 | +20 | +4 | |
There were no significant within-group functional connectivity changes of the DMN in IAH.
Salience network (using right insula cortex seed)
In the within-group analyses the ND group had no significant hypoglycaemia-related changes in functional connectivity of the SN.
In the NAH group, at hypoglycaemia the SN seed showed increased connectivity with a cluster centred over the central opercular cortex (cluster size = 241, clusterwise pFWE = 0.005, MNIxyz [64, 2, −4]) (Table 2).
There were no significant within-group functional connectivity changes of the SN due to hypoglycaemia in IAH.
Central executive network (using dorsolateral pre-frontal cortex seed)
There were no significant within-group functional connectivity changes of the CEN due to hypoglycaemia in any group.
Thalamus
In the ND group the thalamus was more strongly coupled with a region in the right frontal pole and middle frontal gyrus at hypoglycaemia (cluster size = 253, clusterwise pFWE < 0.005, MNIxyz [30, 42, 40]) and a decrease in functional connectivity with a region in the precuneus (cluster size= 176, clusterwise pfwe <0.05, MNIxyz [12, −44, 46]) (table 2).
In the within-group analyses, in the NAH group there were no significant changes in functional connectivity of the thalamus due to hypoglycaemia.
In the IAH group there was an increase in functional connectivity with the lateral occipital cortex (cluster size = 248, clusterwise pFWE < 0.05, MNIxyz [−36, −74, −8]) and a decrease in functional connectivity with a cluster in the precuneus (cluster size = 184, clusterwise pFWE < 0.05, MNIxyz [−6, −62, 52]) (Table 2).
Right inferior frontal gyrus
There were no significant within group functional connectivity changes of inferior frontal gyrus connectivity due to hypoglycaemia in either ND or NAH.
In IAH there was reduced functional connectivity with a separate region within the RIFG (cluster size = 346, clusterwise pFWE = 0.002, MNIxyz [50, 20, 4]) (Table 2).
Connectivity and symptom regression analyses
To explore the relationship between resting state network connectivity and symptoms of hypoglycaemia we performed a post-hoc regression analysis.
Default mode network
There were no correlations between symptoms and DMN connectivity in the ND group.
In the NAH group there was a significant negative correlation between adrenergic symptoms and change in connectivity with the postcentral gyrus on a whole brain analysis (cluster size = 353, clusterwise pFWE < 0.001, MNIxyz [10, −34, 76]). There was also a positive correlation between adrenergic symptoms and increased connectivity with the right anterior insula using a small volume correction (cluster size = 26, pFWE < 0.01, MNIxyz [30, 28, 2]) but not on whole brain analysis.
There were no correlations between symptoms and DMN connectivity in the IAH groups.
Salience and Central executive networks
There were no significant correlations between in change in functional connectivity and adrenergic symptoms in any group in the SN or CEN.
Thalamus
In NAH there was a positive correlation between adrenergic symptoms and functional connectivity change at hypoglycaemia between the thalamus and left frontal pole (cluster size = 148, clusterwise pFWE < 0.05, MNIxyz −22, 56, 28). This did not survive Bonferroni correction for multiple ROIs. There was no correlation between symptoms and thalamic connectivity in the ND or IAH groups.
Right inferior frontal gyrus
There were no significant correlations between in change in RIFG functional connectivity with adrenergic symptoms in any group.
Discussion
In this study, we have investigated the impact of type 1 diabetes (T1D) and hypoglycaemia awareness status on connectivity between key brain regions known to be affected during hypoglycaemia. Studies focussing on regional cerebral blood flow responses to hypoglycaemia are increasingly described.10,14–21,29–33 The thalamus, anterior cingulate cortex, right insular cortex, medial prefrontal cortex and the orbitofrontal cortex have been identified in many of these. There have been fewer studies investigating the impact of diabetes and hypoglycaemia awareness status on the way these different brain areas interact with each other during hypoglycaemia. In the present work, we find that hypoglycaemia does alter such functional connectivity in several established brain networks and that loss of awareness of hypoglycaemia alters the connectivity in the right inferior frontal gyrus.
A recent study has shown important differences in the DMN connectivity response in people with diabetes and normal awareness of hypoglycaemia that is lost in impaired awareness. 34 The DMN has been widely studied and is known to be disrupted in numerous disease states. 35 The impact of awareness status on the effect of hypoglycaemia induction in resting state networks, other than the DMN, has not been explored in previous connectivity studies.
Our main focus was the impact of hypoglycaemia awareness status on brain connectivity responses during hypoglycaemia in people with T1D. In the NAH group, we observed strong symptomatic responses at the time of hypoglycaemia. This was expected and confirmed the clinical diagnosis of normal hypoglycaemia awareness. It is of interest that the symptom responses were strong in this group despite lower adrenaline responses compared to the ND group and may represent an adaptive response to heighten symptomatic appreciation in NAH following historical experience that is subsequently lost in IAH.
We observed changes in connectivity due to hypoglycaemia in two of our networks of interest which survived between-group comparison. In our investigations of the effect of diabetes, we observed that the PCC and right angular gyrus connectivity significantly reduced at hypoglycaemia in NAH and this was not seen in ND. Together with this, we found a significant negative correlation between the change in DMN connectivity in NAH due to hypoglycaemia and the symptomatic response. We postulate that activity and connectivity of the DMN reduces as the brain responds to symptom generation in hypoglycaemia. Similar responses have been seen previously where sensory detection negatively correlated with DMN activation. 36 Furthermore, DMN activation has been shown to be anticorrelated with reaction times 37 which mirrors our observation that NAH has a rapid symptomatic response but ND take longer to achieve the same symptomatic response. Intriguingly, our second between-group difference showed that in NAH the thalamus had high baseline connectivity also with the angular gyrus which became anticorrelated with induction of hypoglycaemia and was significantly different from in the IAH group where there was no change in connectivity due to hypoglycaemia.
Our within-group analyses also demonstrated results that may be important for understanding the symptomatology of hypoglycaemia unawareness; however, we did not have the statistical power to confirm the significance of these differences in between-group studies. In NAH the DMN was negatively correlated with regions in the sensory cortex, whilst the SN showed positive connectivity with the central opercular cortex. These regions are involved in receiving and processing input from somatosensory stimuli. 38 This suggests that processed somatosensory signals may have greater salience in the NAH group, in keeping with their prior experience of symptomatic hypoglycaemia. Such a change has been interpreted as an individual evolving from rest, to preparing to act in response to the physiological stress of incipient hypoglycaemia. Grecius and colleagues have previously described stimulus-induced attenuation of the DMN with activation of the central executive network.39,40 We were not able to confirm corresponding enhanced connectivity of the CEN in NAH, perhaps because we only captured resting state data at a single hypoglycaemic timepoint.
Together, the ND and NAH data, suggest that the observed differential symptomatic responses may be an important part of a learned response to hypoglycaemia. Repeated excitation of neuronal pathways is known to modify cortical responsiveness through cortical plasticity dependent on differential release of neuromodulatory transmitters. 41 Whilst it is interesting to note that the symptom response is brisk in people in the NAH group, we are unable to comment on causality: i.e. whether the symptoms drive the changes in connectivity or if the awareness of symptoms depends on changes in functional connectivity between these key areas. However, the correlation of symptoms and alteration in PCC functional connectivity in people without diabetes suggests a link in network disruption and symptomatic experience. What remains intriguing is that even though we recognise that those with IAH have a greater exposure to hypoglycaemia than those with NAH, given the duration of diabetes and level of glucose control in this study, those with NAH were able to maintain appropriate responses to hypoglycaemia despite likely having experienced many episodes of hypoglycaemia over the years.
Our IAH group differed from our other groups in having no connectivity changes seen in the DMN or SN induced by hypoglycaemia in the within group analyses. In a recent study that investigated changes in regional cerebral perfusion during a hypoglycaemic challenge, 20 the IAH group seemed to have little or no response to the hypoglycaemic stimulus. There are several possible interpretations for loss of connectivity responses in the DMN and SN in IAH. The first is that the absent counterregulatory response means there is no central neural representation of their effect. Second, neuronal circuits involved in the detection or relay of hypoglycaemia signals may have become adapted to recurrent exposure to low glucose states in IAH, such that there are no downstream cortical responses during hypoglycaemia. In these habituation scenarios, people with IAH are not consciously aware of hypoglycaemia because of the absence of a signal. Third, it may be that people with IAH subconsciously use strategies to modulate their awareness of hypoglycaemic symptoms so that the conscious appreciation of hypoglycaemia is suppressed. Downregulation of stress responses to recurrent exposure to that stress is well-described in the psychology literature, a phenomenon known as habituation. Previously, the right inferior frontal gyrus has been proposed as a gateway for the top-down control of attention.22–24 Our results are compatible with hypoglycaemia changing the co-ordinated activity between separate regions within the right inferior frontal gyrus as a neural correlate for IAH. It is conceivable that in IAH, there is a maladaptive reorganisation which leads to the inhibition of hypoglycaemia relevant behaviours.
We speculate that the lifetime response to hypoglycaemia in T1D is triphasic. During the first episode of hypoglycaemia, there is no previous reference for it and there is slow responsiveness and understanding of what the symptoms indicate. This is the situation for our ND group. During subsequent hypoglycaemic episodes, memory, salience and neuromodulation allow a more adapted response. For some people, potentially after high frequency of repeated stimulation, habituation to the stimulus occurs, with loss of adaptations that allowed rapid recognition and response.
In order to eliminate the potential for age, sex, BMI, HbA1c and duration of diabetes to influence connectivity we matched our participants for these factors. Matching for diabetes duration is particularly important, due to its known association with IAH and for which adjustment as a covariate may not provide adequate control, given the many physiological and psychological features that accompany it. Importantly, our study investigated glucose lowering to 2.6 mmol/L which is important for investigating effects in IAH due to the absence of counter-regulation and symptoms at higher thresholds.
The study does have some limitations. Previous resting state analyses have had, at most, 12 participants with IAH 34 and this is the largest resting state functional neuroimaging study investigating hypoglycaemia in diabetes of which we are aware. Despite this, we may have lacked power and missed important changes in brain connectivity due to hypoglycaemia. A further limitation is the absence of a task-based paradigm which limits the ability to identify functional connectivity changes that relate to change in self-treatment behaviours. Future studies probing task-based performance in these domains may help confirm whether these early changes in connectivity enhance the ability of these individuals to move towards executive control and planning of self-treatment of hypoglycaemia. Participant selection may also have been a confounder. People with T1D IAH, recruited from a hospital which specialises in treatment of problematic hypoglycaemia, who are willing to participant in trials to restore symptoms of hypoglycaemia may not represent the whole population of people with IAH.
To conclude, our data show that people with a greater symptomatic response to hypoglycaemia show alterations in network connectivity typically associated with change from rest to executive function. This seems to be key for a rapid behavioural response to the stimulus when encountered during insulin therapy by people with T1D. However, in those with loss of awareness, we found evidence of co-ordinated reorganisation of core neural circuits. We believe there may be alterations in attentional control that explains the loss of these protective behavioural responses that predispose to ongoing hypoglycaemia risk.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221082911 for Altered functional connectivity during hypoglycaemia in type 1 diabetes by Peter Jacob, Munachiso Nwokolo, Sally M Cordon, Ian A Macdonald, Fernando O Zelaya, Stephanie A Amiel, Owen O’Daly and Pratik Choudhary in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors thank the participants; the diabetes research nurses, Andrew Pernet, Bula Wilson, Marcia Henderson-Wilson; the clinical research nurses at the NIHR and Wellcome Trust Clinical Research Facility, Louisa Green and John Lord Villajin; and the radiographers and administrative staff at the Centre for Neuroimaging Sciences, King’s College London.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Hypoaware was funded by Diabetes UK (grant number 13/0004653). HARPscan (the neuroimaging study around the HARPdoc study) and HARPdoc were funded by the Juvenile Diabetes Research Foundation (HARPscan grant number 3-SRA-2017-484-S-B, HARPdoc grant number 4- SRA-2017-266-M-N). The funder had no role in the study design, data collection, analysis, decision to publish or preparation of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: All authors made a substantial contribution to the study concept and design, the article and revising it for intellectual content. PJ, MN, FOZ, SAA, OOD and PC contributed to the conception and design of the research and interpreting the results. PJ, MN and PC collected the data. SMC and IM analysed the catecholamine data. PJ, FOZ and OOD analysed the neuroimaging data. PJ, MN, SMC, IM, FOZ, SAA, OOD and PC reviewed and edited the manuscript. PC is the guarantor of this work, had access to the data and accepts full responsibility for the work and conduct of the study and the decision to publish.
ORCID iDs: Peter Jacob https://orcid.org/0000-0001-6684-7173
Munachiso Nwokolo https://orcid.org/0000-0001-7200-6004
Sally M Cordon https://orcid.org/0000-0001-5034-5507
Supplemental material: Supplemental material for this article is available online.
References
- 1.Pedersen-Bjergaard U, Kristensen PL, Beck-Nielsen H, et al. Effect of insulin analogues on risk of severe hypoglycaemia in patients with type 1 diabetes prone to recurrent severe hypoglycaemia (HypoAna trial): a prospective, randomised, open-label, blinded-endpoint crossover trial. Lancet Diabetes Endocrinol 2014; 2: 553–561. [DOI] [PubMed] [Google Scholar]
- 2.Lin YK, Hung M, Sharma A, et al. Impaired awareness of hypoglycemia continues to be a risk factor for severe hypoglycemia despite the use of continuous glucose monitoring system in type 1 diabetes. Endocr Pract 2019; 25: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geddes J, Schopman JE, Zammitt NN, et al. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabetic Med 2008; 25: 501–504. [DOI] [PubMed] [Google Scholar]
- 4.Choudhary P, Geddes J, Freeman JV, et al. Frequency of biochemical hypoglycaemia in adults with type 1 diabetes with and without impaired awareness of hypoglycaemia: no identifiable differences using continuous glucose monitoring. Diabet Med 2010; 27: 666–672. [DOI] [PubMed] [Google Scholar]
- 5.Towler DA, Havlin CE, Craft S, et al. Mechanism of awareness of hypoglycaemia: perception of neurogenic (predominantly cholinergic) rather than neuroglycopaenic symptoms. Diabetes 1993; 42: 1791–1798. [DOI] [PubMed] [Google Scholar]
- 6.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 1993; 91: 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers HA, de Zoysa N, Amiel SA. Patient experience of hypoglycaemia unawareness in Type1 diabetes: are patients appropriately concerned? Diabet Med 2012; 29: 321–327. [DOI] [PubMed] [Google Scholar]
- 8.Moser O, Ziko H, Elsayed H, et al. People with type 1 diabetes and impaired awareness of hypoglycaemia have a delayed reaction to performing a glucose scan during hypoglycaemia: a prospective observational study. Diabet Med 2020; 37: 2153–2159. [DOI] [PMC free article] [PubMed]
- 9.Cook AJ, DuBose SN, Foster N, et al. Cognitions associated with hypoglycemia awareness status and severe hypoglycemia experience in adults with type 1 diabetes. Diabetes Care 2019; 42: 1854–1864. [DOI] [PubMed] [Google Scholar]
- 10.Dunn JT, Choudhary P, Teh MM, et al. The impact of hypoglycaemia awareness status on regional brain responses to acute hypoglycaemia in men with type 1 diabetes. Diabetologia 2018; 61: 1676–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 2010; 20: 519–534. [DOI] [PubMed] [Google Scholar]
- 12.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 2008; 105: 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teves D, Videen TO, Cryer PE, et al. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci U S A 2004; 101: 6217–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teh MM, Dunn JT, Choudhary P, et al. Evolution and resolution of human brain perfusion responses to the stress of induced hypoglycemia. Neuroimage 2010; 53: 584–592. [DOI] [PubMed] [Google Scholar]
- 16.Mangia S, Tesfaye N, De Martino F, et al. Hypoglycemia-induced increases in thalamic cerebral blood flow are blunted in subjects with type 1 diabetes and hypoglycemia unawareness. J Cereb Blood Flow Metab 2012; 32: 2084–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbeláez AM, Su Y, Thomas JB, et al. Comparison of regional cerebral blood flow responses to hypoglycemia using pulsed arterial spin labeling and positron emission tomography. PLoS One 2013; 8: e60085–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiegers EC, Becker KM, Rooijackers HM, et al. Cerebral blood flow response to hypoglycemia is altered in patients with type 1 diabetes and impaired awareness of hypoglycemia. J Cereb Blood Flow Metab 2017; 37: 1994–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nwokolo M, Amiel SA, O’Daly O, et al. Hypoglycemic thalamic activation in type 1 diabetes is associated with preserved symptoms despite reduced epinephrine. J Cereb Blood Flow Metab 2019; 40: 787–798. [DOI] [PMC free article] [PubMed]
- 20.Nwokolo M, Amiel SA, O’Daly O, et al. Impaired awareness of hypoglycemia disrupts blood flow to brain regions involved in arousal and decision making in Type 1 diabetes. Diabetes Care 2019; 42: 2127–2135. [DOI] [PubMed]
- 21.Dunn JT, Cranston I, Marsden PK, et al. Attenuation of amydgala and frontal cortical responses to low blood glucose concentration in asymptomatic hypoglycemia in type 1 diabetes: a new player in hypoglycemia unawareness? Diabetes. 2007; 56: 2766–2773. [DOI] [PubMed] [Google Scholar]
- 22.Sharp DJ, Bonnelle V, Boissezon X, De, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A 2010; 107: 6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampshire A, Chamberlain SR, Monti MM, et al. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 2010; 50: 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci 2006; 18: 1843–1849. [DOI] [PubMed] [Google Scholar]
- 25.Gold AE, Macleod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994; 17: 697–703. [DOI] [PubMed] [Google Scholar]
- 26.Bosi E, Choudhary P, de Valk HW, et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol 2019; 7: 462–472. [DOI] [PubMed] [Google Scholar]
- 27.Forster CD, Macdonald IA. The assay of the catecholamine content of small volumes of human plasma. Biomed Chromatogr 1999; 13: 209–215. [DOI] [PubMed] [Google Scholar]
- 28.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012; 2: 125–141. [DOI] [PubMed] [Google Scholar]
- 29.Arbelaez AM, Powers WJ, Videen TO, et al. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition a mechanism for hypoglycemia-associated autonomic failure. Diabetes 2008; 57: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cranston I, Reed LJ, Marsden PK, et al. Changes in regional brain 18 F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and Counter-Regulatory failure. Diabetes 2001; 50: 2329–2366. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal JM, Amiel SA, Yágüez L, et al. The effect of acute hypoglycemia on brain function and activation. Diabetes 2001; 50: 1618–1626. [DOI] [PubMed] [Google Scholar]
- 32.Bingham EM, Dunn JT, Smith D, et al. Differential changes in brain glucose metabolism during hypoglycaemia accompany loss of hypoglycaemia awareness in men with type 1 diabetes mellitus. An [11C]-3-O-methyl-d-glucose PET study. Diabetologia 2005; 48: 2080–2089. [DOI] [PubMed] [Google Scholar]
- 33.Page KA, Arora J, Qiu M, et al. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes 2009; 58: 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parikh L, Seo D, Lacadie C, et al. Differential Resting State Connectivity Responses to Glycemic State in Type 1 Diabetes. J Clin Endocrinol Metab; 105. Epub ahead of print 1 January 2020. DOI: 10.1210/clinem/dgz004. [DOI] [PMC free article] [PubMed]
- 35.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008; 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- 36.Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage 2008; 41: 100–112. [DOI] [PubMed] [Google Scholar]
- 37.Hinds O, Thompson TW, Ghosh S, et al. Roles of default-mode network and supplementary motor area in human vigilance performance: evidence from real-time fMRI. J Neurophysiol 2013; 109: 1250–1258. [DOI] [PubMed] [Google Scholar]
- 38.Eickhoff SB, Schleicher A, Zilles K, et al. The Human Parietal Operculum. I. Cytoarchitectonic Mapping of Subdivisions. DOI: 10.1093/cercor/bhi105. [DOI] [PubMed]
- 39.Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 2003; 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci 2004; 16: 1484–1492. [DOI] [PubMed] [Google Scholar]
- 41.Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 2002; 111: 815–835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221082911 for Altered functional connectivity during hypoglycaemia in type 1 diabetes by Peter Jacob, Munachiso Nwokolo, Sally M Cordon, Ian A Macdonald, Fernando O Zelaya, Stephanie A Amiel, Owen O’Daly and Pratik Choudhary in Journal of Cerebral Blood Flow & Metabolism