Abstract
Parkinson’s disease (PD) is a common neurodegenerative disorder, yet little is known about cerebral haemodynamics in this patient population. Previous studies assessing dynamic cerebral autoregulation (dCA), neurovascular coupling (NVC) and vasomotor reactivity (VMR) have yielded conflicting findings. By using multi-variate modelling, we aimed to determine whether cerebral blood flow (CBF) regulation is impaired in PD patients.
55 healthy controls (HC) and 49 PD patients were recruited. PD subjects underwent a second recording following a period of abstinence from their anti-Parkinsonian medication. Continuous bilateral transcranial Doppler in the middle cerebral arteries, beat-to-beat mean arterial blood pressure (MAP; Finapres), heart rate (HR; electrocardiogram), and end-tidal CO2 (EtCO2; capnography) were measured. After a 5-min baseline period, a passive motor paradigm comprising 60 s of elbow flexion was performed. Multi-variate modelling quantified the contributions of MAP, ETCO2 and neural stimulation to changes in CBF velocity (CBFV). dCA, VMR and NVC were quantified to assess the integrity of CBF regulation.
Neural stimulation was the dominant input. dCA, NVC and VMR were all found to be impaired in the PD population relative to HC (p < 0.01, p = 0.04, p < 0.01, respectively). Our data suggest PD may be associated with depressed CBF regulation. This warrants further assessment using different neural stimuli.
Keywords: Cerebral autoregulation, multi-variate modelling, neurovascular coupling, Parkinson’s disease, vasomotor reactivity
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that affects 2-3% of those over the age of 65. 1 It is characterised pathologically by the degeneration of dopaminergic neurones in the substantia nigra and the presence of Lewy bodies.1,2 Despite advancements in functional and molecular imaging, PD primarily remains a clinical diagnosis based on the presence of the cardinal symptoms of bradykinesia, tremor, rigidity and postural instability. 3 Non-motor symptoms are common, including cognitive dysfunction which is the leading cause of reduced quality of life, carer burden and economic cost associated with the disease.4–6 Autonomic dysfunction is also common, with clinical symptoms including orthostatic hypotension. 7
Cerebral autoregulation (CA) describes the ability of the cerebrovasculature to maintain a relatively constant blood supply to the brain, despite fluctuations in blood pressure (BP). 8 The autoregulation of cerebral blood supply can be described as either static (sCA) or dynamic (dCA). sCA describes the response of the cerebrovasculature to long term changes in BP (minutes), while dCA refers to the response to acute fluctuations in BP (seconds). 9 dCA can be quantified using the autoregulation index (ARI) as described by Tiecks et al., 9 which allows for the objective comparison of autoregulatory capacity between individuals and patient populations.
Vasomotor reactivity (VMR) is the ability of arterioles in the cerebrovascular tree to alter their diameter in response to changes in the partial pressure of carbon dioxide (pCO2). 10 Hypercapnia causes vasodilation, while hypocapnia causes vasoconstriction. Hypercapnia is known to impair CA, while conversely hypocapnia is associated with improved metrics of autoregulation. 11 The mechanisms underlying VMR remain uncertain. Recent work suggests that the arterial partial pressure of CO2 plays a role independent of pH; 12 the autonomic nervous system has also been implicated. 13
During times of increased cognitive activity, there is an associated increase in cerebral blood flow (CBF), termed neurovascular coupling (NVC). 14 This is mediated through a complex series of interactions by cells surrounding the microcirculation, including smooth muscle cells, astrocytes, neurones and vascular endothelial cells. Together, these cells comprise the neurovascular unit.15,16 Through the neurovascular unit, NVC provides a temporal and regional link between blood flow and cognitive load, with the dual purpose of substrate provision and the removal of metabolic by-products from the active tissue. 17
NVC can be assessed using a variety of stimuli to create a local or global cerebral blood flow (CBF) response. These include cognitive assessment tools,18,19 writing, 20 visual stimuli, 19 speaking 21 and sensori-motor tasks. 22 The changes in CBF can be quantified using imaging techniques such as functional MRI (fMRI) and near infrared spectroscopy (NIRS). 23 An alternative to these techniques is transcranial Doppler ultrasound (TCD), which allows for the non-invasive measurement of CBF velocity (CBFV) as a surrogate measure for CBF.24,25 Its ease of use, excellent temporal resolution and low cost make it a suitable and reliable bedside alternative to fMRI or NIRS. 26
Previous studies assessing cerebral haemodynamics in PD are heterogenous and of varying quality. A variety of paradigms and stimuli have been employed to assess CA in this population, including head-up tilt, 27 the cold-pressor test, 28 breath holding 29 and acetazolamide. 30 Some studies have found CA to be abnormal,28,29,31 while others report it to be preserved in this population.32–34 Only one study employed transfer function analysis (TFA), 33 which is a widely accepted and verified technique for the reliable assessment of dCA.35,36 Studies have found VMR to be both impaired 29 and intact,37–39 while the general consensus is that NVC is intact in PD patients when assessed by TCD.31,32,34
However, the majority of these studies have reported changes in CBFV as a direct reflection of NVC, without considering the influence of the co-variates of pCO2 and BP on the cerebrovascular response to the stimulus. While pCO2 and BP are usually measured and reported as baseline values, there is rarely a quantification of their contribution to the CBFV response.18–22 This can be addressed with the use of multivariate modelling, whereby CBFV is modelled as the output, with BP, pCO2, and a neural stimulation function, s(t), as inputs.40,41 The added benefit of this approach is that it allows for the simultaneous assessment of dCA, VMR and NVC through the quantification of the contributions of BP, pCO2 and s(t), respectively, all through the use of a sole stimulus, and at the same period of time. 42 Additionally, the majority of these studies have been performed in relatively small populations of PD patients, which further diminishes the reliability of the conclusions that can be drawn from their data.
Through the use of a multi-variate modelling approach in a comparatively larger study population, we aimed to address the heterogeneity, variable quality and contradictory findings of the existing literature by testing the hypothesis that CBF regulation is depressed in PD, as manifested by alterations in dCA, VMR or NVC. Furthermore, we aimed to ascertain whether anti-parkinsonian medication had an effect on cerebral haemodynamics in PD patients.
Materials and methods
Patients with PD were recruited from specialist clinics at the University Hospitals of Leicester NHS Trust, or by direct invitation from Parkinson’s Disease UK. All patients had received a diagnosis of PD from a specialist physician. Age matched healthy controls (HC) were defined as those with stable and well-controlled medical co-morbidities and were recruited locally or from the partners of PD patients. We excluded patients with diabetes mellitus, dementia, peripheral neuropathy, ischaemic heart disease or cerebrovascular disease. Patients with PD whose swallowing was dependent on their medication or who had undergone deep brain stimulation were also excluded.
The study had ethical approval from a UK Ethics Committee (ref: 11/EM/0369). All procedures were conducted in accordance with the Declaration of Helsinki 1975. All participants provided written informed consent.
Data collection was undertaken in the Cerebral Haemodynamics in Ageing and Stroke Medicine (CHIASM) laboratory, which is kept at a constant temperature and free from distraction to provide a suitable environment for uninterrupted data collection. Participants were asked to abstain from caffeine, alcohol, chocolate, large meals and smoking in the 4 hours prior to data collection. Upon arrival, baseline parameters of age, sex and handedness, as assessed by the Edinburgh Handedness Inventory, were collected. 43
Experimental methods
Following a 10 minute period of rest and stable recordings, a series of recordings were obtained. First, we obtained a 5 minute recording of the patient at rest, lying supine, awake and with their eyes open. Next, participants underwent a respiratory paradigm; the results of which are reported elsewhere. 37 Finally, participants underwent a passive motor paradigm, which is the focus of the present study. Following a 90 s period of rest, the researcher passively flexed the participants dominant arm at the elbow for 60 s, at a frequency of 1 Hz, as guided by a metronome. This was followed by a 90 s period of silent recovery. The passive elbow flexion manoeuvre was selected due to its proven validity and reproducibility in older adults. 44
PD patients were invited to attend the laboratory on two occasions, no more than two weeks apart. On the second visit they attended after a period of abstinence from their anti-Parkinsonian medications for either 12 or 24 hours depending on the drugs and their preparations.
Instrumentation
Heart rate (HR) was measured using 3-lead electrocardiography. Bilateral insonation of the middle cerebral artery (MCA) was performed using 2 MHz TCD probes (Viasys Companion III). Once the MCA was identified and a good trace obtained, probes were held in a fixed position by a custom-built headset. Continuous beat-to-beat estimates of blood pressure (BP) were obtained through arterial clamping of the digital artery (Finometer, Finapres Medical Systems, Amsterdam). End-tidal CO2 (EtCO2) was measured with nasal capnography (Salter labs, ref. 4000) Intermittent brachial BP was measured using a validated electrosphygmomanometer (Omron UA 767) to calibrate recordings from the Finometer.
Data processing
Data were visually inspected, and non-physiological spikes in CBFV were removed through linear interpolation. Files that contained poor quality or unilateral TCD signals were excluded from further analysis. Narrow spikes and artefacts in other physiological parameters in remaining files were removed through linear interpolation. Data were filtered in the forward and reverse direction using a low-pass Butterworth filter with a cut-off frequency of 20 Hz. The beginning and end of each cardiac cycle were detected in the ECG signal, and mean values of BP, CBFV and HR were obtained for each cardiac cycle. End-expiratory values were detected in the EtCO2 signal, linearly interpolated, and resampled at each cardiac cycle.
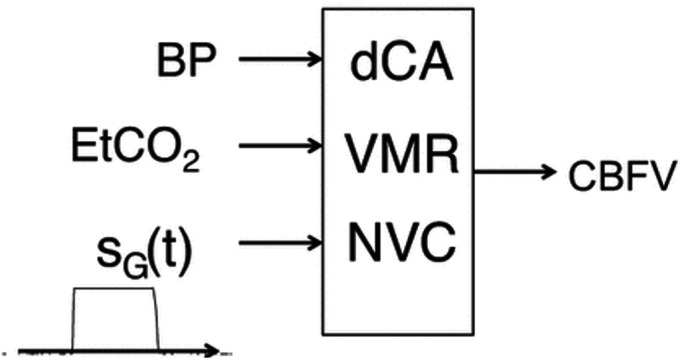
The effects of BP, EtCO2 and the passive elbow flexion manoeuvre were expressed in the time domain as an autoregressive moving average model (ARMA).40,45,46 At each time point, CBFV was modelled as the previous Nv CBFV values, plus the sum of the relative contributions of BP, EtCO2 and neural stimulation resulting from the manoeuvre (Figure 1 adapted with permission from Panerai et al. 42 ). The moving average terms, representing each of the three inputs, was comprised of Np (BP), Nc (EtCO2) and Ns (neural stimulation) samples, respectively. 42 [Nv,Np,Nc,Ns] represent the orders of the ARMA model. These were chosen as [2, 4, 1, 1] based on previous studies, and their suitability was assessed by the model prediction error, expressed as the correlation coefficient between measured and model predicted CBFV.40,45,46 The neural stimulation input was expressed by a gate function obtained by recording the electrical output of a metronome during the activation phase of the manoeuvre. 46 The relative contributions to the CBFV response by BP, CO2 and stimulation were quantified by their variance, notated as VARBP, VARCO2 and VARSTIM.
Figure 1.
Schematic model of the contribution of blood pressure (BP), dynamic cerebral autoregulation (dCA), end-tidal CO2 (EtCO2), vasomotor reactivity (VMR), gate function (SG(t)) and neurovascular coupling (NVC) to the cerebral blood flow velocity (CBFV) response to the passive elbow flexion paradigm. Adapted with permission from Panerai et al, 2019. 42
Once the ARMA model coefficients were estimated by means of least squares, CBFV step responses were obtained for each input, creating CBFV/BP, CBFV/CO2 and CBFV/STIM step responses. The CBFV/BP step response represents dCA, and allows for the estimation of the autoregulation index (ARI), by fitting of the Tiecks et al. model.9,46 The ARI ranges from zero to nine, with zero representing absence of autoregulation and nine the most efficient autoregulation that can be observed. The CBFV/CO2 step response represents the integrity of VMR, which was assessed by measuring the plateau of the CBFV/CO2 step response in cm.s−1.mmHg−1 of EtCO2. Finally, we quantified the effect of motor stimulation by the mean value of the CBFV/STIM step response for the same time interval of 30–40 s into the response.
Data were visually inspected, and participants with poor quality data were rejected. Step responses generated by the ARMA model from remaining participants were visually inspected, and those with temporal patterns that were not physiologically plausible were also rejected.
Statistical analysis
Demographic data
Physiological parameters at rest were inspected for normality using Q-Q plots. Values were compared between groups using independent t-tests for ordinal data, and the chi-squared test for nominal data.
Inputs into the CBFV response
A hierarchal approach was employed, whereby the Wilcoxon-signed rank test was utilised initially to compare the contribution of these inputs between hemispheres. In instances where significant hemispheric differences were noted, hemispheric data were analysed separately. Where no significant differences existed between hemispheres, bilateral data were subsequently averaged before testing for the effect of medication with Friedman ANOVA. In instances where there was no significant effect of medication, PD data were averaged between visits, prior to testing for the effect of disease state with the Mann-Whitney U test.
Step responses
Paired t-tests were used to compare bilateral hemispheric outputs for ARI, CBFV-EtCO2 and CBFV-STIM. In HC, data were compared between the dominant and non-dominant hemispheres, as assessed by the Edinburgh Handedness Inventory. 43 For PD participants, data were compared between the onset and non-onset hemispheres, as detailed by clinician assessment. Where no significant differences existed, these data were averaged.
Subsequently, the effect of medication was assessed using repeated measures ANOVA, with PD participants grouped by medication state (medicated vs. unmedicated). Where no significant differences existed, these data were averaged before independent t-tests were used to compare HC vs. PD. For parameters which demonstrated hemispheric differences, we employed the General Linear Model (GLM) to compare the dominant/non-dominant hemispheres (HC) against the onset/non-onset hemispheres (PD). Tukey post-hoc testing was utilised where significant differences were detected. Significance was set at p < 0.05.
Results
Recruitment and data quality
Fifty-five HC and forty-nine PD participants were recruited. Four participants within the HC group had their data rejected due to non-physiological model outputs, leaving 51 good quality, bilateral data sets. Of the forty-nine PD participants, three did not return for a repeat visit. Participants whose data were excluded, in either the medicated or unmedicated state, were entirely removed from the study, leaving 34 good quality, repeated, PD datasets. Supplementary Table S.1 details the rationale for exclusion and the numbers of excluded participants for each reason.
The correlation coefficient between the recorded CBFV signal and the model predicted output was 0.99 ± 0.01 for HC, PD (medicated) and PD (unmedicated) inclusive.
Participant demographics and baseline physiological parameters
In those included, the mean age was 62.2 ± 10.3 years (31/51 male) for HC and 67.6 ± 9.4 years (22/34 male) for PD patients. Representative data from a single participant and mean population responses to stimulation are given in Figures 2 and 3, respectively. Resting CBFV was comparable between hemispheres in both study groups, and so these data were averaged for further analysis. The two groups were comparable with the exception of age. Disease state had no significant effect on resting parameters in either the medicated or unmedicated state (Table 1).
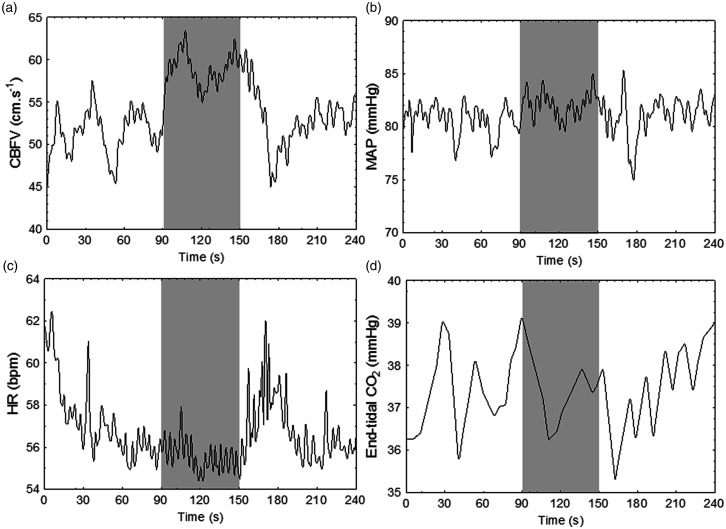
Figure 2.
Representative data from a 51 year old female participant, demonstrating the physiological response to the passive elbow flexion manoeuvre. Shaded area represents period of stimulation. (a) CBFV, averaged cerebral blood flow velocity across hemispheres; (b) MAP, mean arterial pressure; (c) HR, heart rate; (d) end-tidal CO2.
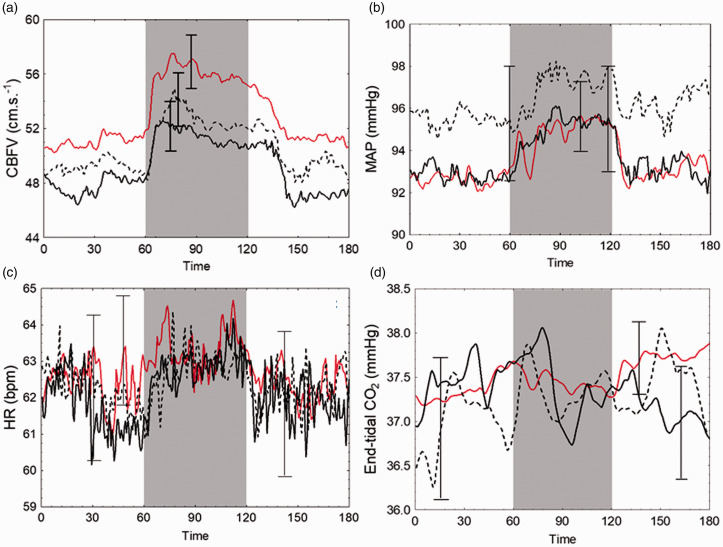
Figure 3.
Average data for physiological parameters in response to neural stimulation for healthy controls and PD subgroups. Shaded area represents period of stimulation. Solid red line, healthy controls; solid black line, PD medicated; black dotted line, PD unmedicated. Error bars are largest ± standard error at the point of occurrence. (a) CBFV, averaged cerebral blood flow velocity across both hemispheres. (b) MAP, mean arterial pressure. (c) HR, heart rate. (d) end-tidal CO2.
Table 1.
Participant physiological parameters at rest. Values are population mean ± SD.
| Parameter | HC (n = 51) | PD Medicated (n = 34) | PD Un-medicated (n = 34) | P-value - HC vs. medicated | P-value - HC vs. unmedicated |
|---|---|---|---|---|---|
| Mean CBFV (cm.s-1) | 49.7 ± 13.0 | 47.7 ± 9.6 | 48.6 ± 9.6 | 0.45 | 0.70 |
| MAP (mmHg) | 91.7 ± 10.0 | 90.9 ± 12.9 | 94.1 ± 13.1 | 0.73 | 0.35 |
| Heart rate (bpm) | 63.2 ± 9.1 | 61.8 ± 9.9 | 62.4 ± 9.4 | 0.48 | 0.68 |
| End-tidal CO2 (mmHg) | 38.2 ± 4.3 | 37.3 ± 3.9 | 37.7 ± 4.0a | 0.37 | 0.63 |
HC: healthy controls; PD-medicated: Parkinson’s disease patients with medication ON; PD-unmedicated: Parkinson’s patients with medication OFF; Mean CBFV: cerebral blood flow velocity averaged across hemispheres. MAP: mean arterial pressure.
P-value; unpaired t-tests.
aEtCO2 data from two participants excluded due to unreliable capnography trace.
Variance contributions to the CBFV response
The contributions of BP, EtCO2 and neural stimulation to ΔCBFV (VARBP, VARCO2, VARSTIM, respectively) did not differ significantly between hemispheres across all groups; therefore, these data were averaged. Differences in medication state did not significantly affect VARBP, VARCO2 or VARSTIM, and therefore PD data were averaged across the two visits prior to comparisons against HC. VARBP, VARCO2 and VARSTIM were statistically similar between the HC and PD populations (Table 2). VARSTIM was the dominant input in both PD patients (p < 0.01) and HC (p < 0.001).
Table 2.
Fraction of the CBFV response variance (mean ± SD) explained by each of the three inputs (BP, EtCO2, stim) stratified by study group. PD data averaged between visits.
| Parameter | HC (n = 51) | PD (n = 34) | P-value |
|---|---|---|---|
| VARBP | 0.27 ± 0.19 | 0.31 ± 0.20 | 0.25 |
| VARCO2 | 0.25 ± 0.18 | 0.25 ± 0.19 | 0.74 |
| VARSTIM | 0.48 ± 0.16 | 0.43 ± 0.20 | 0.25 |
| P-value | <0.001* | <0.01* | – |
HC: healthy controls; PD: Parkinson’s disease patients; VARBP: variance contribution of blood pressure; VARCO2: variance contribution of end-tidal CO2; VARSTIM: variance contribution of stimulation by manoeuvre.
P-value comparing HC vs. PD, Mann-Whitney U test. *P-value comparing separate variance contributions, Friedman ANOVA.
Step responses
CBFV step responses to the influences of BP, EtCO2 and neural stimulation did not show significant differences between hemispheres in the PD population. However, the step response to neural stimulation was significantly greater in the dominant hemisphere in HC as compared to the non-dominant hemisphere (p < 0.001). Therefore, hemispheric data for the step response to neural stimulation were analysed separately for HC.
Estimates of ARI were significantly depressed in PD compared to HC (6.2 ± 2.3 vs 7.5 ± 1.5, p < 0.001, Figure 4(a)). The step response to changes in EtCO2, used to quantify VMR, was significantly dampened in the PD population (p < 0.01, Table 3, Figure 4(b)). On GLM, significant differences existed in the CBFV/STIM step response, which on post-hoc testing was identified as being between the dominant hemisphere in HC and the onset hemisphere in the PD population (4.5 ± 0.6 vs 3.2 ± 0.5, p = 0.04, Table 3, Figure 4(c)). However, no other significant differences were detected between hemispheres and study groups.
Figure 4.
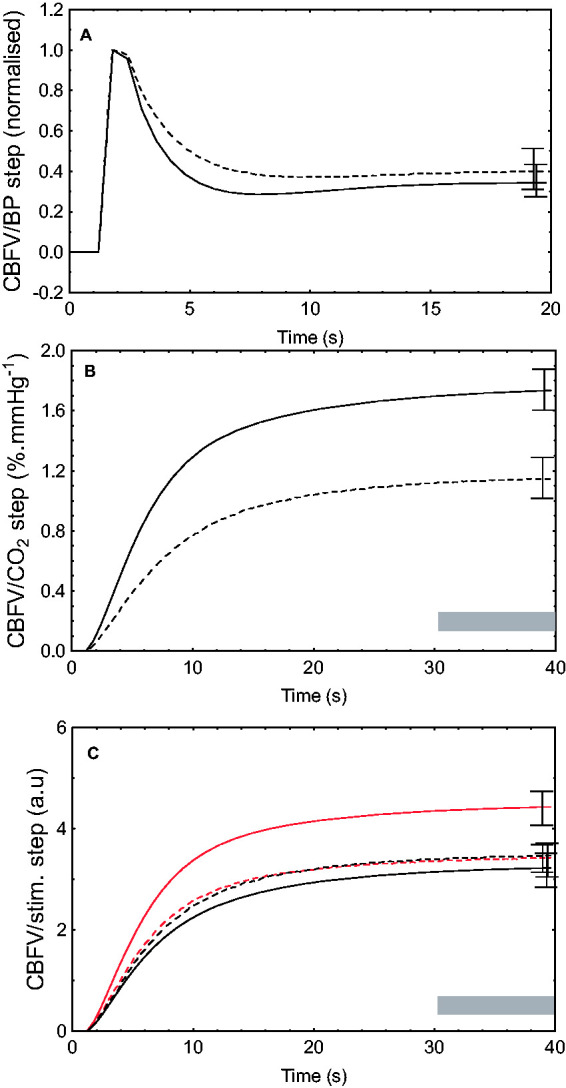
Mean CBFV step responses to fluctuations in blood pressure (BP, a), end-tidal CO2 (b) and neural stimulation (c). In (a) and (b), solid line represents healthy controls (HC); dashed line, subjects with Parkinson’s disease. In (c), solid red line represents healthy controls dominant hemisphere; small dashed red line, HC non-dominant hemisphere; dotted black line, Parkinson’s patients non-onset hemisphere, solid black line, Parkinson’s patients onset hemisphere. CBFV, cerebral blood flow velocity. Error bars represent standard error of the mean at point of maximal occurrence. Grey shading represents time interval where mean value of the step response was calculated (Table 3).
Table 3.
CBFV step response parameters to changes in blood pressure, end-tidal CO2 and neural stimulation.
| Step response input | HC (n = 51) | PD (n = 34) | P-value | ||
|---|---|---|---|---|---|
| BP (ARI) | 7.5 ± 1.5 | 6.2 ± 2.3 | <0.01 | ||
| CO2 step (cm.s−1.mmHg−1) | 1.8 ± 1.5 | 0.93 ± 0.7 | <0.01 | ||
|
Dominant |
Non-dominant |
Onset |
Non-onset |
||
| Stimulus (a.u.) | 4.5 ± 2.3 | 3.5 ± 2.5 | 3.2 ± 2.0 | 3.4 ± 1.6 | *0.02 |
Note: ARI describes the temporal pattern of the CBFV step response to the BP input. For the CO2 and stimulus inputs, the CBFV step response is represented by the mean value of the plateau (Figure 4) from 30 to 40 s into the response. Values are means ± SD. Hemispheric data were averaged for ARI and the CO2 step response, but significant differences between hemispheres in HC led to the response to stimulation being analysed separately.
HC: healthy controls; PD: Parkinson’s disease patients; BP: blood pressure; ARI: autoregulation index.
P-values, unpaired t-test.
*p-value from the General Linear Model. Post-hoc Tukey testing identified the difference between HC (dominant) and PD (onset), p = 0.04.
Discussion
Key findings
To our knowledge, this is the first study to assess cerebral haemodynamics in a PD population through a multi-variate approach. We have demonstrated that the relative explanatory contribution of the inputs into the cerebrovascular response, namely BP, EtCO2 and passive motor stimulation, do not vary significantly between hemispheres nor according to medication state or disease state as compared to HC. The passive elbow flexion manoeuvre was the dominant input in both HC and PD patients, with a variance contribution notably greater than that of BP or EtCO2.
Additionally, our approach has shown that the autoregulatory response to fluctuations in BP is depressed in the PD population compared to HC, although whether this is a true impairment remains unclear and will be discussed further. VMR is also impaired, and we found that there was a statistically significant difference in the hemispheric response to passive motor stimulation between the dominant hemisphere in HC, and the onset hemisphere in the PD population.
Together, these results suggest that the relative contributory factors to CBF changes following neural stimulation are similar in both HC and PD patients, yet the disease process causes alterations in cerebrovascular regulatory functions that manifest as differences in dCA, VMR and NVC in those affected by the disease. This finding appears to be independent of the presence of anti-parkinsonian medication.
dCA in PD
Previous attempts to assess dCA in this population have led to conflicting results, most likely due to the substantial heterogeneity in these studies. Studies have used different techniques to induce changes in CBFV including head-up tilt (HUT)27,33,47 and the cold-pressor test.28,48 Importantly, a variety of modelling techniques have been employed. Several studies assessed CA by comparing CBFV between HC and PD patients before and after stimulation,28,47,49 while others used both the pulsatility index and cerebrovascular reactivity.27,48 No studies involving PD patients have employed ARI as their metric to assess CA, and only one study employed transfer function analysis (TFA), 33 which is a widely accepted and verified technique for the reliable assessment of dCA.35,36 In their study, Haubrich et al. 33 used the tilt-table test, and demonstrated that PD patients with orthostatic hypotension had BP instability induced by tilting, but the dCA response was preserved as quantified by measures of phase and gain.
In the present study, we generated estimates of ARMA-ARI in response to BP fluctuations induced by a passive motor paradigm. Tiecks’ classical paper describes a population mean ARI of 5 ± 1, derived from the thigh-cuff manoeuvre. 9 In the present study we report results of 7.5 ± 1.5 vs 6.2 ± 2.3 for HC and PD patients, respectively, which are higher than Tiecks’ reference point. One interpretation of these data, at face value, may be to conclude that our populations had unusually effective autoregulation. However, existing literature demonstrates that estimates of ARMA-ARI are known to be elevated compared to Tiecks’ reference values 50 and therefore direct comparison is inappropriate.
While we have demonstrated a significant difference in the dCA response between HC and PD subjects, we cannot reliably conclude that dCA is impaired in those with PD. Instead, we conclude that dCA appears to be depressed in PD patients relative to our population of HC, but whether this represents true inhibition is uncertain. Further work using a paradigm that induces large BP oscillations such as the squat-stand manoeuvre, or the thigh cuff manoeuvre, would provide a more challenging assessment of dCA and may clarify this uncertainty. 51
VMR in PD
In the present study, we have demonstrated a significant difference in VMR between HC and PD patients. As with dCA, the existing literature on VMR in PD is conflicting. Previous studies have found VMR to be impaired 29 and intact,37–39 and again there was considerable heterogeneity in terms of study size and measurement technique. The single study that found VMR to be impaired utilised a breath-holding paradigm in a population of fifteen PD patients, in both their medicated and unmedicated states. 29 As in our study, they found an impairment in VMR, relative to HC, which was independent of medication state.
However, the majority of studies report no evidence of impairment. Krainik et al. used a hypercapnic stimulus and BOLD-MRI to assess VMR in a population of ten PD patients and eight HC, and found no significant differences between the two study groups both in the presence and absence of dopaminergic medication. 38 Al-Bachari et al. also used a hypercapnic challenge in the context of an fMRI study and found no significant difference in cerebrovascular reactivity. 39 Interestingly, Hanby et al. used induced hyperventilation on the same study cohort as reported here, and found no evidence of impairment in VMR. 37
The contrast between the present findings and those of Hanby is particularly interesting given the data were taken from the same study cohort, albeit performing a different manoeuvre as part of a series of recordings. On one hand, we might expect the induced hyperventilation used by Hanby to be more sensitive to differences in VMR, given the larger changes in pCO2 as compared to our passive motor paradigm. 37 However, the analysis approach utilised did not account for the concomitant changes in BP and neural stimulation induced by the manoeuvre. Additionally, the analysis technique used was relatively simplistic; only a ratio between changes in CBFV and changes in EtCO2 was calculated. This fails to consider the dynamic response of CBFV to changes in pCO2, which are automatically accounted for in the ARMA model and expressed by the CBFV/EtCO2 step response. 42
Future work utilising a hypercapnic manoeuvre in the context of ARMA modelling might be more sensitive, given evidence that haemodynamic parameters and pCO2 demonstrate a non-linear relationship. 52 With small fluctuations, as in the present study (Figure 3), EtCO2 is within the region of greater sensitivity of the logistic curve. 52 At this point on the curve, there is a comparatively linear relationship, and small changes in EtCO2 induce noticeable changes in CBFV. In contrast, with a large change in PaCO2, as induced by hyperventilation, parameters enter the region of the logistic curve where sensitivity is significantly reduced and changes in EtCO2 lead to a relatively small change in CBFV.
NVC in PD
To our knowledge, the existing TCD literature contains four studies that assess NVC in the PD population, none of which have demonstrated statistically significant evidence of impairment.31,32,34,53 The only study that utilised a motor paradigm was that of Troisi et al., who used a thumb-finger opposition task to study twelve PD patients in their medicated state, seven of whom returned for a repeat visit after abstaining from medication. 31 The other studies used visual stimulation32,34 or cognitive tasks 53 to assess NVC.
Troisi et al. found no significant effect of disease or medication state on NVC, but their analysis technique did not account for the temporal pattern of the CBFV response. Instead, they reported a single peak %change in CBFV following stimulation. By reporting a single value, the temporal relationship between both the initiation and cessation of stimulation, and the CBFV response cannot be interpreted. In contrast, Gutteridge et al. reported NVC data from 21 PD patients, and measured the latency of the CBFV response. 53 They described a non-significant trend towards an attenuated and delayed response to cognitive stimulation in the PD population.
In the present study, GLM demonstrated a significant difference in the magnitude of the CBFV/STIM step response between the dominant hemisphere in HC and the onset hemisphere in PD patients. We hypothesise that the ARMA approach has allowed us to detect this difference, which may have been present in other studies but was not statistically demonstrable. By accounting for the co-variates of BP and EtCO2, we have been able to isolate the cerebrovascular response to neural stimulation with greater sensitivity and thus have identified a significant difference.
Clinical perspectives
Cerebral autoregulation is known to be impaired in a variety of disease states, including acute ischaemic stroke, traumatic brain injury and sepsis.56–58 Our data suggest that PD may be added to this list of conditions.
In the case of acute stroke, there is a growing movement towards individualised care, whereby BP is titrated to ensure an adequate but not excessive cerebral perfusion pressure in the acute setting. This also applies to the operating table, whereby recent recommendations suggest that BP should be rigidly controlled peri- and intra-operatively in the stroke patient to reduce the risk of further cerebral infarction or secondary haemorrhage as a result of impaired autoregulation. 59
As yet there has been no such consensus on the integrity of cerebral autoregulation in PD, and so the clinical implications of our findings should be carefully considered in individual cases where patients exhibit signs and symptoms in keeping with autoregulatory dysfunction, for example in those with orthostatic hypotension. Additionally, our data suggest that measures should be taken peri- and intra-operatively to closely regulate BP in PD patients requiring general anaesthesia, thus ensuring adequate but not excessive cerebral perfusion. Further validation of our findings would be required before any definitive recommendations can be made for clinical practice.
Limitations
The key limitation associated with the use of TCD is the assumption that CBFV is a reliable reflection of CBF. We assume that the diameter of the insonated vessel does not vary significantly throughout the paradigm, despite fluctuations in EtCO2. In the present study, variation in EtCO2 was small throughout the paradigm (Figure 3), making significant changes in arterial diameter unlikely.26,54
Another limitation of this study is the proportion of participants whose data were rejected. The majority of exclusions occurred during and after the ARMA modelling process (Suppl. Table S.1). We acknowledge that the reduction in our sample size may have increased the likelihood of type II error. However, given that our findings demonstrate depression in dCA, VMR and NVC, if there is statistical error, it is likely that the error is an underestimation of differences as opposed to failing to demonstrate them.
Additionally, we acknowledge that we assessed VMR using spontaneous fluctuations in EtCO2 as part of a passive motor paradigm. Using a hyperventilation manoeuvre, as employed by Hanby et al., 37 would generate larger fluctuations in EtCO2 and may provide a more robust challenge to the cerebrovasculature. However, we note previous studies have used spontaneous fluctuations in EtCO2 as part of a multivariate approach and have generated physiologically relevant and plausible results.40–42,46 Further multivariate work employing induced fluctuations in EtCO2 would help clarify if the choice of paradigm has an impact our findings.
Finally, we note that our HC and PD populations were slightly mismatched in terms of age, with a mean difference of 5.4 years between the two groups (p = 0.01). Given the small magnitude of this difference, and the predominant view that there is no significant effect of ageing on autoregulatory processes, 55 it is highly unlikely that this has confounded the findings of our study.
Conclusions
In summary, our study is the first to use a multi-variate approach to assess cerebral haemodynamic regulation in a PD population. We have shown that the relative explanatory contributions of BP, EtCO2 and neural stimulation into the CBFV response are comparable between HC and PD patients. Medication state in PD patients had no significant effect, and in both groups, neural stimulation was the dominant input.
Despite comparatively similar contributions of BP, EtCO2 and neural stimulation to the CBFV response, we demonstrated differences in dCA, VMR and NVC in the PD population. Therefore, we hypothesise that the disease process of PD causes alterations in cerebrovascular regulatory functions that manifest as reductions in the efficiency of dCA, VMR and NVC. Further work using different stimuli may shed light on whether this finding is isolated to the passive elbow flexion manoeuvre.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211065204 for Cerebrovascular responses to somatomotor stimulation in Parkinson’s disease: A multivariate analysis by Sam C Barnes, Ronney B Panerai, Lucy Beishon, Martha Hanby, Thompson G Robinson and Victoria J Haunton in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: LB is a research training fellow funded by the Dunhill Medical Trust (RTF1806\27).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: VJH, TGR and RBP designed research. VJH and MH performed experiments. SB, LB and RBP analysed data. SB and RBP prepared figures. SB and RBP drafted the manuscript. All authors approved the final version of the manuscript.
ORCID iDs: Sam C Barnes https://orcid.org/0000-0001-6009-406X
Ronney B Panerai https://orcid.org/0000-0001-6983-8707
Supplemental material: Supplemental material for this article is available online.
References
- 1.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers 2017; 3: 1–21. [DOI] [PubMed] [Google Scholar]
- 2.Bartels AL, Leenders KL. Parkinson's disease: the syndrome, the pathogenesis and pathophysiology. Cortex 2009; 45: 915–921. [DOI] [PubMed] [Google Scholar]
- 3.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008; 79: 368–376. [DOI] [PubMed] [Google Scholar]
- 4.Saredakis D, Collins-Praino LE, Gutteridge DS, et al. Conversion to MCI and dementia in Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 2019; 65: 20–31. [DOI] [PubMed] [Google Scholar]
- 5.Monastero R, Cicero CE, Baschi R, et al. Mild cognitive impairment in Parkinson’s disease: the Parkinson’s disease cognitive study (PACOS). J Neurol 2018; 265: 1050–1058. [DOI] [PubMed] [Google Scholar]
- 6.Berganzo K, Tijero B, Gonzalez-Eizaguirre A, et al. Motor and non-motor symptoms of Parkinson's disease and their impact on quality of life and on different clinical subgroups. Neurología 2016; 31: 585–591. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi A. Autonomic nervous system disorders in Parkinson’s disease. Eur Neurol 1991; 31: 41–47. [DOI] [PubMed] [Google Scholar]
- 8.Aaslid R, Lindegaard KF, Sorteberg W, et al. Cerebral autoregulation dynamics in humans. Stroke 1989; 20: 45–52. [DOI] [PubMed] [Google Scholar]
- 9.Tiecks FP, Lam AM, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 1995; 26: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 10.Markwalder T, Grolimund P, Seiler RW, et al. Dependency of blood flow velocity in the middle cerebral artery on end-tidal carbon dioxide partial pressure – a transcranial ultrasound doppler study. J Cereb Blood Flow Metab 1984; 4: 368–372. [DOI] [PubMed] [Google Scholar]
- 11.Panerai RB, Deverson ST, Mahony P, et al. Effect of CO2 on dynamic cerebral autoregulation measurement. Physiol Meas 1999; 20: 265–275. [DOI] [PubMed] [Google Scholar]
- 12.Caldwell HG, Howe CA, Chalifoux CJ, et al. Arterial carbon dioxide and bicarbonate rather than pH regulate cerebral blood flow in the setting of acute experimental metabolic alkalosis. J Physiol 2021; 599: 1439–1457. [DOI] [PubMed] [Google Scholar]
- 13.Peebles KC, Ball OG, MacRae BA, et al. Sympathetic regulation of the human cerebrovascular response to carbon dioxide. J Appl Physiol 2012; 113: 700–706. [DOI] [PubMed] [Google Scholar]
- 14.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006; 100: 328–335. [DOI] [PubMed] [Google Scholar]
- 15.Peterson EC, Wang Z, Britz G. Regulation of cerebral blood flow. Int J Vasc Med 2011; 2011: 823525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang T, Sun Y, Lu Z, et al. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev 2017; 34: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips AA, Chan FH, Zheng MMZ, et al. Neurovascular coupling in humans: physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab 2016; 36: 647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beishon LC, Williams CA, Panerai RB, et al. The assessment of neurovascular coupling with the Addenbrooke’s cognitive examination: a functional transcranial doppler ultrasonographic study. J Neurophysiol 2018; 119: 1084–1094. [DOI] [PubMed] [Google Scholar]
- 19.Sorond FA, Schnyer DM, Serrador J, et al. Cerebral blood flow regulation during cognitive tasks: effects of healthy aging. Cortex 2008; 44: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moody M, Panerai RB, Eames PJ, et al. Cerebral and systemic hemodynamic changes during cognitive and motor activation paradigms. Am J Physiol Regul Integr Comp Physiol 2005; 288: R1581–R1588. [DOI] [PubMed] [Google Scholar]
- 21.Gröschel K, Terborg C, Schnaudigel S, et al. Effects of physiological aging and cerebrovascular risk factors on the hemodynamic response to brain activation: a functional transcranial doppler study. Eur J Neurol 2007; 14: 125–131. [DOI] [PubMed] [Google Scholar]
- 22.Salinet ASM, Silva NCC, Caldas J, et al. Impaired cerebral autoregulation and neurovascular coupling in middle cerebral artery stroke: Influence of severity? J Cereb Blood Flow Metab 2019; 39: 2277–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wintermark M, Fischbein NJ, Smith WS, et al. Accuracy of dynamic perfusion CT with deconvolution in detecting acute hemispheric stroke. Am J Neuroradiol 2005; 26: 104–112. [PMC free article] [PubMed] [Google Scholar]
- 24.Syme PD. The use of transcranial doppler ultrasonography as a cerebral stethoscope 'for the assessment and treatment of acute stroke. J Royal Col Phys Edinburgh 2006; 36: 17. [Google Scholar]
- 25.Evans DH. Physical and technical principles. Front Neurol Neurosci 2006; 21: 1–18. [DOI] [PubMed] [Google Scholar]
- 26.Willie CK, Colino FL, Bailey DM, et al. Utility of transcranial doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 2011; 196: 221–237. [DOI] [PubMed] [Google Scholar]
- 27.Angeli S, Marchese R, Abbruzzese G, et al. Tilt-table test during transcranial doppler monitoring in Parkinson's disease. Parkinsonism Relat Disord 2003; 10: 41–46. [DOI] [PubMed] [Google Scholar]
- 28.Rätsep T, Asser T. Subthalamic stimulation improves the cerebral hemodynamic response to the cold pressure test in patients with Parkinson's disease. J Clin Ultrasound 2012; 40: 547–553. [DOI] [PubMed] [Google Scholar]
- 29.Hamdy MM, Sadallah HM, Elsalamawy DH. The study of vasoreactivity of the cerebral vessels in patients with Parkinson disease. Egypt J Neurol. Psych and Neurosurg 2012; 49: 353–357. [Google Scholar]
- 30.Gurevich T, Gur AY, Bornstein NM, et al. Cerebral vasomotor reactivity in Parkinson's disease, multiple system atrophy and pure autonomic failure. J Neurol Sci 2006; 243: 57–60. [DOI] [PubMed] [Google Scholar]
- 31.Troisi E, Peppe A, Pierantozzi M, et al. Emotional processing in Parkinson's disease. J Neurol 2002; 249: 993–1000. [DOI] [PubMed] [Google Scholar]
- 32.Azevedo E, Santos R, Freitas J, et al. Deep brain stimulation does not change neurovascular coupling in non-motor visual cortex: an autonomic and visual evoked blood flow velocity response study. Parkinsonism Relat Disord 2010; 16: 600–603. [DOI] [PubMed] [Google Scholar]
- 33.Haubrich C, Pies K, Dafotakis M, et al. Transcranial doppler monitoring in Parkinson’s disease: cerebrovascular compensation of orthostatic hypotension. Ultrasound Med Biol 2010; 36: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 34.Rosengarten B, Dannhardt V, Burr O, et al. Neurovascular coupling in Parkinson's disease patients: effects of dementia and acetylcholinesterase inhibitor treatment. J Alzheimers Dis 2010; 22: 415–421. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R, Zuckerman JH, Giller CA, et al. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol 1998. Jan; 274: 233. [DOI] [PubMed] [Google Scholar]
- 36.Claassen JAHR, Meel-van den Abeelen ASS, Simpson DM, et al.; International Cerebral Autoregulation Research Network (CARNet). Transfer function analysis of dynamic cerebral autoregulation: a white paper from the international cerebral autoregulation research network. J Cereb Blood Flow Metab 2016; 36: 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanby MF, Panerai RB, Robinson TG, et al. Is cerebral vasomotor reactivity impaired in Parkinson disease? Clin Auton Res 2017; 27: 107–111. [DOI] [PubMed] [Google Scholar]
- 38.Krainik A, Maillet A, Fleury V, et al. Levodopa does not change cerebral vasoreactivity in Parkinson's disease. Mov Disord 2013; 28: 469–475. [DOI] [PubMed] [Google Scholar]
- 39.Al-Bachari S, Parkes LM, Vidyasagar R, et al. Arterial spin labelling reveals prolonged arterial arrival time in idiopathic Parkinson's disease. Neuroimage Clin 2014; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panerai RB, Salinet AS, Robinson TG. Contribution of arterial blood pressure and PaCO2 to the cerebrovascular responses to motor stimulation. Am J Physiol Heart Circ Physiol 2012; 302: H459–66. [DOI] [PubMed] [Google Scholar]
- 41.Panerai RB, Eyre M, Potter JF. Multivariate modeling of cognitive-motor stimulation on neurovascular coupling: transcranial doppler used to characterize myogenic and metabolic influences. Am J of Physiol Heart Circ Physiol 2012; 303: R395–407. [DOI] [PubMed] [Google Scholar]
- 42.Panerai RB, Hanby MF, Robinson TG, et al. Alternative representation of neural activation in multivariate models of neurovascular coupling in humans. J Neurophysiol 2019; 122: 833–843. [DOI] [PubMed] [Google Scholar]
- 43.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 44.Salinet AS, Robinson TG, Panerai RB. Reproducibility of cerebral and peripheral haemodynamic responses to active, passive and motor imagery paradigms in older healthy volunteers: a fTCD study. J Neurosci Methods 2012; 206: 143–150. [DOI] [PubMed] [Google Scholar]
- 45.Maggio P, Salinet AS, Robinson TG, et al. Influence of CO2 on neurovascular coupling: interaction with dynamic cerebral autoregulation and cerebrovascular reactivity. Physiol Rep 2014; 2: e00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salinet AS, Robinson TG, Panerai RB. Effects of cerebral ischemia on human neurovascular coupling, CO2 reactivity, and dynamic cerebral autoregulation. J Appl Physiol 2015; 118: 170–177. [DOI] [PubMed] [Google Scholar]
- 47.Mihci E, Dora B, Balkan S. Transcranial doppler ultrasonographic evaluation of cerebral circulation during passive tilting in patients with Parkinson's disease. J Clin Ultrasound 2007; 35: 138–143. [DOI] [PubMed] [Google Scholar]
- 48.Tsai S, Chen S, Leu T, et al. Impairment of cerebral hemodynamic response to the cold pressor test in patients with Parkinson's disease. Parkinsonism Relat Disord 2009; 15: 94–100. [DOI] [PubMed] [Google Scholar]
- 49.Niehaus L, Böckeler GC, Kupsch A, et al. Normal cerebral hemodynamic response to orthostasis in Parkinson's disease. Parkinsonism Relat Disord 2002; 8: 255–259. [DOI] [PubMed] [Google Scholar]
- 50.Panerai RB, Eames PJ, Potter JF. Variability of time-domain indices of dynamic cerebral autoregulation. Physiol Meas 2003; 24: 367–381. [DOI] [PubMed] [Google Scholar]
- 51.Barnes SC, Ball N, Haunton VJ, et al. The cerebro-cardiovascular response to periodic squat-stand maneuvers in healthy subjects: a time-domain analysis. Am J Physiol Heart Circ Physiol 2017; 313: H1240–H1248. [DOI] [PubMed] [Google Scholar]
- 52.Minhas JS, Panerai RB, Robinson TG. Modelling the cerebral haemodynamic response in the physiological range of PaCO2. Physiol Meas 2018; 39: 065001. [DOI] [PubMed] [Google Scholar]
- 53.Gutteridge DS, Saredakis D, Badcock NA, et al. Cerebrovascular function during cognition in Parkinson's disease: a functional transcranial doppler sonography study. J Neurol Sci 2020; 408: 116578. [DOI] [PubMed] [Google Scholar]
- 54.Verbree J, Bronzwaer AS, Ghariq E, et al. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 2014; 117: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 55.Beishon L, Clough RH, Kadicheeni M, et al. Vascular and haemodynamic issues of brain ageing. Pflügers Archiv-Eur J Physiol 2021; 473: 735–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis 2003; 16: 69–75. [DOI] [PubMed] [Google Scholar]
- 57.Rangel-Castilla L, Gasco J, Nauta HJ, et al. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus 2008; 25: E7. [DOI] [PubMed] [Google Scholar]
- 58.Schramm P, Klein KU, Falkenberg L, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care 2012; 16: R181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minhas JS, Rook W, Panerai RB, et al. Pathophysiological and clinical considerations in the perioperative care of patients with a previous ischaemic stroke: a multidisciplinary narrative review. Br J Anaesth 2020; 124: 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211065204 for Cerebrovascular responses to somatomotor stimulation in Parkinson’s disease: A multivariate analysis by Sam C Barnes, Ronney B Panerai, Lucy Beishon, Martha Hanby, Thompson G Robinson and Victoria J Haunton in Journal of Cerebral Blood Flow & Metabolism