Abstract
The antibacterial spectra and modes of action of synthetic peptides corresponding to mesenterocin 52B and leucocin B-TA33a greatly differ despite their high sequence homology. Circular dichroism experiments establish the capacity of each of these two peptides to partly fold into an amphiphilic helix that might be crucial for their adsorption at lipophilic-hydrophilic interfaces.
Among the various antimicrobial compounds produced by lactic acid bacteria, bacteriocins are bioactive single polypeptides or polypeptide complexes that are active against closely related bacterial species (14, 15, 22). These bacteriocins constitute a large family of metabolites, which can be subdivided into different classes based on their modes of action and their structures (14). In particular, class II bacteriocins are small, heat-stable, non-lanthionine-containing peptides, varying between 30 and 60 residues in length (<10 kDa) (8). Subgroups have been defined within class II, notably the class IIa bacteriocins, which contain a consensus YGNGV amino acid motif near the N terminus and are active against Listeria spp. Within the class IIa bacteriocins produced by some Leuconostoc strains (11, 12, 18, 20), the closely related leucocin A and mesentericin Y105 have been the subject of numerous studies, including conformational ones (9, 10). In low-polarity medium, mesentericin Y105 was found to be partially folded as an amphipathic helix spanning over residues 17 to 31 (9), and leucocin A is proposed to adopt an amphiphilic α-helical conformation in its C-terminal region, whereas the N-terminal part would fold into a three-stranded anti-parallel β-sheet conformation (10).
Within the Leuconostoc genus, some strains have been recently shown to produce more than one bacteriocin. In addition to mesenterocin 52A, which is identical to mesentericin Y105, Leuconostoc mesenteroides subsp. mesenteroides FR52 produces the 32-mer polypeptide mesenterocin 52B (20). The same bacteriocin has also been detected in culture extracts of L. mesenteroides Y105 (1). L. mesenteroides strain TA33a actually produces three different bacteriocins: class IIa leucocin A-TA33a, leucocin B-TA33a, and leucocin C-TA33a, a new anti-Listeria bacteriocin (18, 19). It is noteworthy that mesenterocin 52B and leucocin B-TA33a display narrow spectra of activity limited to the genera Leuconostoc and Weissella (18, 19, 20). Thus, they can be distinguished from other metabolites produced by Leuconostoc sp. strains (11, 12, 19, 20). The primary structures of the two bacteriocins are presented in Table 1. By shifting the mesenterocin 52B sequence forward by two positions, identical amino acids appear in 17 positions and lead to a 63% identity score between the two peptides. The similarity is most obvious in the central domain of the peptide, where the LTGPQQP motif is present in the two bacteriocins. As both mesenterocin 52B and leucocin B-TA33a are small heat-stable and non-lanthionine-containing peptides, their classification as bacteriocins from class II is obvious. Yet, in addition to the uncommon biological properties cited above, the sequences of both mesenterocin 52B and leucocin B-TA33a contain neither the YGNGV consensus motif shared by all other Leuconostoc bacteriocins nor the conserved disulfide bridge which is considered a characteristic feature of the IIa subgroup (8).
In this study, synthetic peptides representative of bacteriocins mesenterocin 52B and leucocin B-TA33a were characterized and compared with respect to their biological activities and secondary-structure propensities. To study the latter characteristic, both peptides were examined by circular dichroism (CD) under a variety of conditions including aqueous or micellar media.
MATERIALS AND METHODS
Synthesis of peptides.
Mesenterocin 52B and leucocin B-TA33a were synthesized according to the amino acid sequences reported earlier (19, 20). They were prepared by stepwise solidphase peptide synthesis, as described by Fleury et al. (9). Purity of the synthetic peptides was assessed by mass spectrum and solid-phase sequence analyses (9).
Microbial strains and bacteriocin activity.
Strains used as indicator microorganisms are listed in Table 2 and were grown statically at 30°C in MRS broth (Biokar, Beauvais, France). The bacterial strains were maintained frozen at −24°C and were propagated twice in broth medium before use. Aqueous solutions of both bacteriocins were prepared by solubilizing 1.2 mg of the synthetic peptides per ml in a 5 mM phosphate buffer (pH 6.5). The solutions were then heated at 80°C during 20 min to prevent cell contamination. Bacteriocin activities were determined by the agar well diffusion method (20). MICs were determined by a critical dilution assay. Stationary-phase cells of Weissella paramesenteroides LMA 19 and Leuconostoc pseudomesenteroides CIP 103316 obtained after 16 h at 30°C in MRS broth were used to inoculate (10%, vol/vol) MRS broth. Bacteriocins were then added to a final concentration of 0.03 mg/ml. Samples were incubated at 30°C and aliquots were taken at appropriate intervals of time to determine the number of viable cells (in CFU per milliliter) by plate counts in MRS medium agar (12 g/liter) after incubation at 30°C for 48 h.
CD and amphipathic properties.
CD spectra were recorded by using a Jobin-Yvon CD6 dichrograph calibrated with epiandrosterone. Measurements were performed at 20°C using a 0.2-cm-path-length quartz cell, a 2-nm bandwidth, a scan speed of 20 nm/min, a time constant of 2 s, and at least three scan accumulations. A protein-free control spectrum was recorded for each condition and subtracted from the protein spectra. Peptides were dissolved in 20 mM phosphate buffer, pH 7, and most measurements were done at a peptide concentration of 24 μM. The results are reported as mean residual ellipticity in degrees per square centimeter per decimole. The contents in the α-helical structures of the peptides were calculated from the mean residual ellipticity at 222 nm (16). Hydrophobic moments were calculated according to the method of Eisenberg et al. (7), and the helical wheel representations were drawn as implemented in GCG, version 8.0.
RESULTS
Biological activities of mesenterocin 52B and leucocin B-TA33a.
The antibacterial spectra of activity of the two bacteriocins were assayed against 32 indicator strains belonging to the genera Leuconostoc and Weissella (Table 2), since no activity was detected against other gram-positive genera (18, 20). Mesenterocin 52B inhibited many more strains than did leucocin B-TA33a. At the bacteriocin concentration tested, some strains appeared to be resistant to both peptides. For the indicator strains sensitive to both bacteriocins, sensitivities to mesenterocin 52B and leucocin B-TA33a appeared to vary considerably from one strain to another, with over 100-fold differences between the two MICs for a given strain as a function of the tested bacteriocin, as well as over 100-fold differences between the MICs of a given bacteriocin as a function of the indicator strain used.
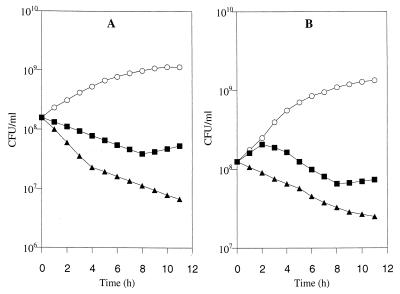
The modes of action of the two bacteriocins (0.03 mg/ml) were examined during the growth of W. paramesenteroides LMA 19 and L. pseudomesenteroides CIP 103316 in MRS broth at 30°C. Figure 1 shows bactericidal effects of the two peptides against the two sensitive strains, but there are marked differences. In both cases, leucocin B-TA33a displayed a more rapid rate of bactericidal action than mesenterocin 52B. With mesenterocin 52B, the bactericidal effect against W. paramesenteroides LMA 19 occurred immediately after bacteriocin addition, while it was detected only after 2 h of contact in the case of L. pseudomesenteroides CIP 103316. Furthermore, bactericidal action of mesenterocin 52B vanished after 8 h of contact, and survivor cells resumed growth.
FIG. 1.
Kinetics of death of W. paramesenteroides LMA 19 cells (A) and L. pseudomesenteroides CIP 103316 cells (B) in MRS broth at 30°C. ○, control; ■, mesenterocin 52B (0.03 mg/ml); ▴, leucocin B-TA33a (0.03 mg/ml).
CD experiments and helical propensities.
In the pH range of 4 to 8, the CD spectra of both peptides in aqueous solution are typical of an unordered conformation with an α-helical content below 2%. In 20 mM phosphate buffer, pH 7, the molar ellipticity does not depend on the peptide concentration (2 to 200 μM) and is characteristic of a random coil conformation. Within this concentration range and in aqueous media, both peptides thereby remain essentially disordered and monomeric. This is consistent with results provided by predictive methods related to the helical behavior of monomeric peptides (17), which lead to the conclusions that helix levels of both bacteriocins would be less than 3% under these CD conditions.
As a solvent suitable for spectroscopic experiments, trifluoroethanol (TFE) is known to induce and stabilize α-helical structures in peptides that possess an intrinsic tendency to adopt this kind of secondary structure. In the presence of TFE, both compounds give spectra typical of partly α-helical peptides, with a maximum α-helical content (39% for mesenterocin 52B and 43% for leucocin B-TA33a) obtained for a TFE concentration of 50% (results not shown). A further increase of the TFE concentration up to 75% does not result in any increase of the α-helical content.
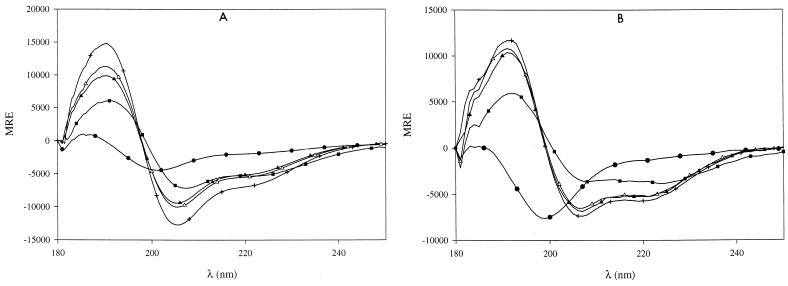
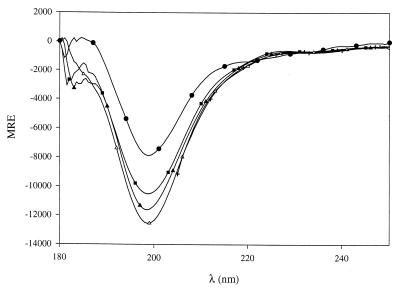
In order to examine the peptide behavior in membrane-mimicking media, CD spectra were then recorded in the presence of sodium dodecyl sulfate (SDS) at concentrations ranging from 1 μM to 20 mM. Surprisingly, concentrations of SDS as low as 2 μM induced a significant transition towards α helix formation (15% α-helicoidal content) for both peptides and a maximum of 36 to 38% of α helix content was obtained for SDS concentrations greater than or equal to 0.05 mM (Fig. 2). An isodichroic point is observed near 200 nm, indicating a local two-state (α-helical/random coil) population equilibrium (13) even at a high SDS concentration. Furthermore, since α-helical appearance is obtained for SDS concentrations much lower than the 8 mM critical micellar concentration (CMC), this implies that peptide structuralization does not depend upon micelle formation and that smaller aggregates of SDS are sufficient to induce the transition. In order to investigate whether the negative charge of SDS was essential for this interaction, similar experiments were carried out in the presence of tetradecyltrimethylammonium bromide (TTAB), a cationic counterpart of SDS with very similar physicochemical properties. The spectra obtained in TTAB (concentrations ranging from 1 μM to 10 mM, with a CMC of 3.6 mM [2]) are not significantly different from those obtained in aqueous solution and typically account for a random coil conformation (Fig. 3). Thus, given the nearly identical hydrophobic behaviors of the aliphatic parts of both SDS and TTAB, induction of α-helical formation within peptides appears to depend on the electrostatic properties of the polar head of the detergent used.
FIG. 2.
Effect of SDS on the CD spectra of leucocin B-TA33a and mesentorocin 52B in aqueous solution. The spectra of the two peptides (24 μM in 20 mM phosphate buffer, pH 7) were recorded in the absence or in the presence of various amounts of SDS. (A) Leucocin B-TA33a. Concentrations of SDS were as follows: none (●), 5 μM (■), 10 μM (▴), 30 μM (▵), and 10 mM (+). (B) Mesenterocin 52B. SDS concentrations were as follows: none (●), 20 μM (■), 50 μM (▴), 0.2 mM (▵), and 20 mM (+).
FIG. 3.
Effect of TTAB on the CD spectra of mesenterocin 52B in aqueous solution (24 μM peptide, 10 mM phosphate, pH 7). The spectra were recorded in the wavelength range of 180 to 250 nm except for the highest concentrations of TTAB (205 to 252 nm) because of the strong absorption of this compound at short wavelengths. TTAB concentrations were as follows: none (●), 1 mM (■), 2 mM (▴), 4 mM (▵), and 10 mM (+).
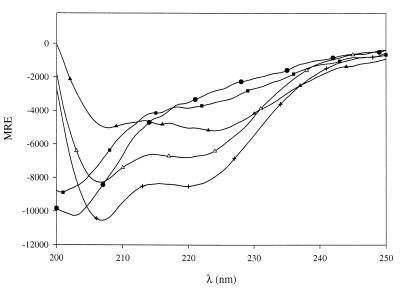
Finally, CD spectra were recorded in the presence of dodecyllysophosphatidylcholine (LPC) (concentration range of 55 μM to 10 mM, with a CMC value of 1.1 mM [21]). α-Helical structure was induced in leucocin B-TA33a and mesenterocin 52B only once the LPC concentration exceeded the CMC and maximal α-helical content (42 and 40%, respectively) was reached for 3.3 mM LPC (Fig. 4). Similar experiments carried out at different peptide concentrations in order to change the lipid/peptide ratio for the same LPC concentrations led essentially to the same conclusions: a micellar environment is required to induce peptide structuralization. The zwitterionic nature of the LPC polar head proceeds from the simultaneous presence of a negatively charged phosphodiester group and a positively charged quaternary ammonium group. If the negative charge of SDS is essential to the formation of bacteriocin secondary structures, especially at submicellar concentrations (see above), isolated LPC molecules may be unable to tightly interact with the peptides because of the contradictory effects resulting from their positive and negative neighboring charges. In contrast, a micellar environment would allow a local concentration of negative charges by appropriately orienting the LPC molecules close to the bacteriocin molecules and thus would provide a suitable environment for the initial adhesion of both peptides to the micellar interface.
FIG. 4.
CD spectra of mesenterocin 52B in aqueous solution (24 μM peptide, 10 mM phosphate, pH 7) at the following LPC concentrations: none (●), 0.6 mM (■), 1 mM (▴), 2mM (▵), and 3.3 mM (+).
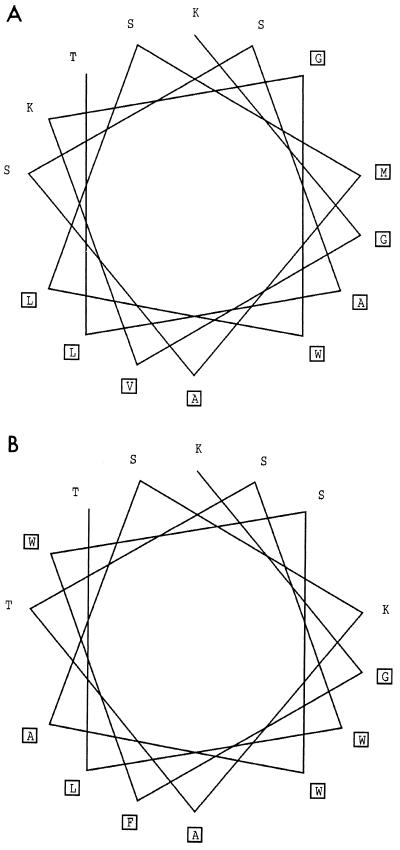
In order to account for the amphipathic character likely associated with the occurrence of peptide-surfactant attractive interactions, we looked for the hydrophobic moment profile along the sequences of the two bacteriocins folded into an α-helical structure. The highest magnitude of putative amphipathy was observed within a fragment ranging from 10 to 15 residues in length and centered at positions 8 and 10 in the mesenterocin 52B and leucocin B-TA33a sequences, respectively. The α-helical wheel representation (Fig. 5) clearly illustrates the amphiphilic character of this fragment for the two bacteriocins.
FIG. 5.
Edmunsen α-helical wheel representation of the amphiphilic region of leucocin B-T33a (A) and mesenterocin 52B (B). The amphiphilic region starts with residue 3 and ends with residue 17 in the leucocin B-T33a sequence, whereas it starts with residue 1 and ends with residue 15 for mesenterocin B52. The boxes denote hydrophobic residues.
DISCUSSION
Some lactic acid bacterial strains produce more than one type of bacteriocin (6, 18, 20). So, erroneous conclusions regarding the relationship between structure and activity of a single bacteriocin could be drawn from activity measurements assessed only with culture supernatant (6). A convenient way to avoid this problem is to use the synthetic counterparts of natural bacteriocins (9), readily obtained by peptide synthesis when posttranslational modifications do not occur. In the case of synthetic mesenterocin 52B and leucocin B-TA33a, their activities were previously shown to be similar to those obtained with equivalent peptides isolated from natural sources (18, 20).
The antibacterial activity of each bacteriocin appears to be strongly dependent on the indicator strain used. Furthermore, mesenterocin 52B and leucocin B-TA33a display different antibacterial spectra and activities despite their notable sequence homology. No global rule can, then, be deduced from the comparative analysis of the two sets of inhibitory data. The sequence alignment of the two peptides (Table 1) reveals a homogeneous rate of homology along the whole sequences and does not enable one to simply relate their activity profile to specific fingerprints at the primary structure level.
Structure and activity investigations were then conducted by CD experiments in order to determine whether or not mesenterocin 52B and leucocin B-TA33a adopt the same conformation in different aqueous lipophilic micellar systems. The structural studies presented here clearly establish that both bacteriocins are able to adopt a partially helical structure at amphiphilic interfaces, provided that the required conditions (hydrophobic environment together with the presence of negative charges) are fulfilled. Therefore, despite the great differences in activity discussed above, the two peptides exhibit exactly the same behavior from a structural point of view, and predictions regarding the positions of helical elements along their sequences (Fig. 5) obviously support the hypothesis of identical three-dimensional properties. This similarity in the behaviors of the two peptides when placed in a membrane-mimicking environment correlates with the observation that the two bacteriocins are able to spontaneously adsorb on the same strains, even if these strains are not sensitive to one or both of the compounds (results not shown). This probably occurs in a first general step of interaction between a bacteriocin molecule and the target cell, during which a contact could be established between the peptide and the bacterium, but which would not preclude an efficient bactericidal effect. Apart from the likely promoting role played by negatively charged phospholipid heads (4) that was confirmed in the present study by the comparison between the results obtained in the presence of SDS and TTAB, the initial adsorption of a bacteriocin thus appears to depend only on its capacity to adopt an amphipathic conformation in an interfacial environment. After the achievement of an efficient adsorption process, it is still not clear in which step different behaviors between a sensitive and a nonsensitive strain take place. In that context, the putative requirement for a specific receptor of proteinaceous nature remains an open question at present (3, 5), especially if one considers the bacteriocin oligomerization step that would undoubtedly be required for the pore formation process.
As underlined in the introduction, numerous studies have been carried out on class IIa bacteriocins with the aim of identifying the regions involved in cell recognition and bactericidal action. Altogether, these studies have established that the pore formation process occurs through several recognition steps involving different structural features of the bacteriocins (for a complete review, see reference 8). However, it is not possible to take advantage of these former studies to find clues concerning the mechanisms of mesenterocin 52B and leucocin B-TA33a. Indeed, it appears that these two bacteriocins are rather atypical and differ clearly in sequence and in activity spectra from class IIa bacteriocins as well as from bacteriocins of any other subgroup. This raises the issue of the reliability of the classification scheme used for bacteriocins, which has already been addressed by Jack et al. (14) and Ennahar et al. (8) with class IIa. In order to rationally refine the criteria used for classification, a deeper knowledge of the mechanism of action is required. In particular it would be of considerable interest to make further enquiries in the field of the membrane properties which certainly differentiate one bacterial species from another, or even one strain from another, regarding their sensitivities to bacteriocins.
TABLE 1.
Amino acida sequence alignment of mesenterocin 52B and leucocin B-TA33a
Identical amino acids are in bold type.
TABLE 2.
Antimicrobial activitiesa of synthetic mesenterocin 52B and leucocin B-TA33a (1.2 mg/ml) against 28 bacterial indicator strains in a 5 mM phosphate buffer (pH 6.5)
| Indicator organism | Strain designation(s)b | Diam of zone of inhibition (mm) for bacteriocin:
|
|
|---|---|---|---|
| 52B | B-TA33a | ||
| Leuconostoc mesenteroides subsp. mesenteroides | CIP 5417, LMA 7, LMA 33 | 0 | 0 |
| LMA 100 | 10 | 0 | |
| DSM 20240 | 17 | 0 | |
| DSM 20243 | 19 | 0 | |
| LMA 38 | 14 | 8 | |
| Leuconostoc mesenteroides subsp. dextranicum | LMA 15, LMA 131 | 13 | 0 |
| LMA 24 | 0 | 0 | |
| DSM 20484 | 17 | 6 | |
| Leuconostoc mesenteroides subsp. cremoris | DSM 20346 | 16 | 0 |
| INRA 361 | 17 | 7 | |
| DSM 20200 | 17 | 5 | |
| Leuconostoc lactis | LMA 28, LMA 63, DSM 20202 | 0 | 0 |
| Leuconostoc pseudomesenteroides | CIP 103316 | 18 | 17 |
| Leuconostoc carnosum | CIP 103319 | 0 | 8 |
| Leuconostoc citreum | CIP 103405 | 0 | 0 |
| Weissella paramesenteroides | LMA 19 | 18 | 13 |
| LMA 39 | 14 | 5 | |
| LMA 133 | 12 | 0 | |
| LMA 134 | 0 | 0 | |
| DSM 20288 | 5 | 8 | |
| Weissella minor | CIP 102978 | 0 | 0 |
| Weissella confusa | CIP 103172 | 15 | 9 |
| Weissella viridescens | CIP 102810 | 10 | 9 |
| Weissella halotolerans | CIP 103005 | 10 | 6 |
Results were obtained using the agar well diffusion method, the well diameter (5 mm) being subtracted from the total zone diameter.
INRA, Institut National de la Recherche Agronomique, Jouy-en-Josas, France; CIP, Collection de l'Institut Pasteur, Paris, France; DSM, Deutsche Sammlung von Mikroorganismen, Göttingen, Germany; LMA, Laboratoire de Microbiologie Alimentaire, ENSAIA-INPL, Nancy, France.
ACKNOWLEDGMENTS
We thank A. Delfour and P. Nicolas (Institut Jacques Monod, Université Paris VII, Paris, France) for providing the synthetic peptides. CD experiments were carried out in the Service Commun de Biophysicochimie des interactions moléculaires of the Université Henri Poincaré, Nancy I, France.
REFERENCES
- 1.Biet F, Berjeaud J M, Worobo R W, Cenatiempo Y, Frémaux C. Heterologous expression of the bacteriocin mesentericin Y105 using the dedicated transport system and the general secretion pathway. Microbiology. 1998;144:2845–2854. doi: 10.1099/00221287-144-10-2845. [DOI] [PubMed] [Google Scholar]
- 2.Castedo A, Del Castillo J L, Suarez-Filloy M J, Rodriguez J R. Effect of temperature on the mixed micellar tetradecyltrimethylammonium bromide-butanol system. J Colloid Interface Sci. 1997;196:148–156. doi: 10.1006/jcis.1997.5201. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Shapira R, Eisenstein M, Montville T J. Functional characterization of pediocin PA-1 binding to liposomes in the absence of a protein receptor and its relationship to a predicted tertiary structure. Appl Environ Microbiol. 1997;63:524–531. doi: 10.1128/aem.63.2.524-531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Ludescher R D, Montville T J. Influence of lipid composition on pediocin PA-1 binding to phospholipid vesicles. Appl Environ Microbiol. 1998;64:3530–3532. doi: 10.1128/aem.64.9.3530-3532.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chikindas M L, Garcia-Garcera M J, Driessen A J M, Ledeboer A M, Nissen-Meyer J, Nes I F, Abee T, Konings W N, Venema G. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl Environ Microbiol. 1993;59:3577–3584. doi: 10.1128/aem.59.11.3577-3584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eijsink V G H, Skeie M, Middelhoven P H, Brurberg M B, Nes I. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl Environ Microbiol. 1998;64:3275–3281. doi: 10.1128/aem.64.9.3275-3281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenberg D, Weiss R M, Terwilliger T C. The helical hydrophobic moment: a measure of the amphiphilicity of a helix. Nature. 1982;299:371–374. doi: 10.1038/299371a0. [DOI] [PubMed] [Google Scholar]
- 8.Ennahar S, Sashihara T, Sonomoto K, Ishizaki A. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev. 2000;24:85–106. doi: 10.1111/j.1574-6976.2000.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 9.Fleury Y, Abdel Dayem M, Montagnes J J, Chaboisseau E, Le Caer J P, Nicolas P, Delfour A. Covalent structure, synthesis, and structure-function studies of mesentericin Y10537, a defensive peptide from gram-positive bacteria Leuconostoc mesenteroides. J Biol Chem. 1995;271:14421–14429. doi: 10.1074/jbc.271.24.14421. [DOI] [PubMed] [Google Scholar]
- 10.Fregeau-Gallagher N L, Sailer M, Niemczura W P, Nakashima T T, Stiles M E, Vederas J C. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry. 1997;36:15062–15072. doi: 10.1021/bi971263h. [DOI] [PubMed] [Google Scholar]
- 11.Hastings J W, Sailer M, Johnson K, Roy K L, Vederas J C, Stiles M E. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J Bacteriol. 1991;173:7491–7500. doi: 10.1128/jb.173.23.7491-7500.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hechard Y, Derijard B, Letellier F, Cenatiempo Y. Characterization and purification of mesentericin Y105, an anti-Listeria bacteriocin from Leuconostoc mesenteroides. J Gen Microbiol. 1992;138:2725–2731. doi: 10.1099/00221287-138-12-2725. [DOI] [PubMed] [Google Scholar]
- 13.Holtzer M E, Holtzer A. Alpha-helix to random coil transitions: determination of peptide concentration from the isodichroic point. Biopolymers. 1992;12:1675–1677. doi: 10.1002/bip.360321209. [DOI] [PubMed] [Google Scholar]
- 14.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 16.Kortemme T, Creighton T E. Ionisation of cysteine residues at the termini of model alpha-helical peptides. Relevance to unusual thiol pKa values in proteins of the thioredoxin family. J Mol Biol. 1995;253:799–812. doi: 10.1006/jmbi.1995.0592. [DOI] [PubMed] [Google Scholar]
- 17.Munoz V, Serrano L. Development of the multiple sequence approximation within the AGADIR model of α-helix formation: comparison with Zimm-Bragg and Lifson-Roig formalisms. Biopolymers. 1997;41:495–509. doi: 10.1002/(SICI)1097-0282(19970415)41:5<495::AID-BIP2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Papathanasopoulos M A, Krier F, Revol-Junelles A-M, Lefebvre G, Le Caer J P, von Holy A, Hastings J W. Multiple bacteriocin production by Leuconostoc mesenteroides TA33a and other Leuconostoc/Weissella strains. Curr Microbiol. 1997;35:331–335. doi: 10.1007/s002849900264. [DOI] [PubMed] [Google Scholar]
- 19.Papathanasopoulos M A, Dykes G A, Revol-Junelles A-M, Delfour A, von Holy A, Hastings J W. Sequence and structural relationships of leucocins A-, B- and C-TA33a from Leuconostoc mesenteroides TA33a. Microbiology. 1998;144:1343–1348. doi: 10.1099/00221287-144-5-1343. [DOI] [PubMed] [Google Scholar]
- 20.Revol-Junelles A-M, Mathis R, Krier F, Delfour A, Lefebvre G. Leuconostoc mesenteroides subsp. mesenteroides FR52 synthetize two distinct bacteriocins. Lett Appl Microbiol. 1996;23:120–124. doi: 10.1111/j.1472-765x.1996.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 21.Stafford R E, Fanni T, Dennis E A. Interfacial properties and critical micelle concentration of lysophospholipids. Biochemistry. 1989;13:5113–5120. doi: 10.1021/bi00438a031. [DOI] [PubMed] [Google Scholar]
- 22.Tagg J R, Dajani A S, Wannamaker L W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976;40:722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]