Abstract
The association between rosacea and cardiovascular disease remains controversial. A systematic review and meta-analysis of the literature, from inception to 15 February 2020, was performed to compare cardiovascular risk and comorbidities in individuals with and without rosacea. Twelve studies, involving 40,752 patients with rosacea, were included. Compared with controls, patients with rosacea had higher systolic blood pressure (standardized mean difference (SMD) 0.293, 95% confidence interval (CI) 0.054–0.532), diastolic blood pressure (SMD 0.309, 95% CI 0.003–0.615), total cholesterol (SMD 1.147, 95% CI 0.309–1.984), low-density lipoprotein (SMD 0.792, 95% CI 0.174–1.409), C-reactive protein (SMD 0.26, 95% CI 0.099–0.421), greater epicardial fat thickness (SMD 1.945, 95% CI 1.595–2.296), and higher incidence of hypertension (odds ratio (OR) 1.204, 95% CI 1.097–1.332) and insulin resistance (OR 2.338, 95% CI 1.187–4.605). This study reveals that patients with rosacea are predisposed to increased subclinical cardiovascular risk.
Key words: cardiovascular disease, dyslipidaemia, hypertension, meta-analysis, risk factor, rosacea, systematic review
Rosacea is a chronic disease that occurs frequently in women and individuals with fair skin (1). The clinical features of rosacea include centrofacial erythema, flushing, telangiectasia, papules, pustules, and phymatous changes (1). Ocular involvement may also occur, characterized by burning, stinging sensation, conjunctival injection, and lid margin telangiectasia (1). The exact pathophysiology of rosacea remains unclear, but it is believed that chronic inflammation and vascular hyper-reactivity are the major contributing factors (2–4). Chronic inflammation also plays a pivotal role in the pathogenesis of atherosclerosis, which reflects the increased risk of cardiovascular (CV) diseases in various chronic inflammatory disorders, such as psoriasis (5–7). Since rosacea is also a chronic inflammatory disease, an important question is whether rosacea is a localized cutaneous disease or a disease with systemic ramifications. This question is important because, if systemic inflammation does occur in patients with rosacea, more aggressive monitoring and interventions for systemic comorbidities in patients with rosacea may be warranted.
SIGNIFICANCE
This study reveals that patients with rosacea are predisposed to increased subclinical cardiovascular risk, but there is insufficient evidence to demonstrate a higher incidence of overt cardiovascular comorbidities. Clinicians are advised to examine patients with rosacea for cardiovascular risk and comorbidities and to offer advice on lifestyle modifications.
Several observational studies have investigated the association between rosacea and various CV diseases, such as coronary artery disease (CAD), diabetes mellitus (DM), dyslipidaemia, and hypertension (HTN) (8, 9). However, to date, the results of these studies are inconclusive. The aim of this study was to examine the CV risk and comorbidities in patients with rosacea in an evidence-based manner, by conducting a systematic review and meta-analysis.
MATERIALS AND METHODS
The methodology of this study complied with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.
The study sought to examine CV risk in patients with rosacea in comparison with controls. The primary outcomes were risk factors for CV diseases and incidence of CV comorbidities in patients with rosacea compared with controls.
Data sources and search strategy
Databases (PubMed, Cochrane Library, and Embase) were searched from inception to 15 February 2020. The final date of searching was 10 March 2020. The search focused exclusively on clinical studies involving humans, and the results were reported without any language limit. The literature search was initially performed with more general terms. After that, based on the search results, more specific terms (e.g. epicardial fat thickness) were used to search the databases again. Keywords used in the literature searches were: “rosacea” combined with “cardiovascular disease”, “cardiovascular risk”, “coronary artery disease”, “myocardial infarction”, “heart failure”, “peripheral arterial occlusive disease”, “hypertension”, “diabetes mellitus”, “dyslipidemia”, “stroke”, “obesity”, “insulin resistance”, “metabolic syndrome”, “epicardial fat thickness” and “carotid intima media thickness”. Reference lists from the screened articles were reviewed in order to avoid missing any studies.
Eligibility criteria and study selection
Studies comparing (i) the risk factors for CV diseases and (ii) the incidence of CV comorbidities between patients with rosacea and controls were included in the analysis. Eligible case-control and cohort studies (both prospective and retrospective, population-based and institutionbased) were included. Review articles, case reports, case series, and conference abstracts were excluded. Case-control and cohort studies were included and case reports and case series excluded because we aimed to include studies with a higher level of evidence. Duplicated studies were excluded, but partially overlapping studies were included in the systematic review. Two investigators (TYT and YYC) independently screened the titles and the abstract of the articles. The full texts of articles reporting relevant data were assessed to determine eligibility. Any disagreement was resolved through discussion with a third investigator (YCH).
Quality assessment
The methodological quality of the included articles was evaluated based on an adapted version of the Newcastle–Ottawa Scale for cohort studies and the adapted version for cross-sectional studies, with a maximum score of 9 points for cohort studies and 7 points for cross-sectional studies. Quality assessment was performed independently (TYT and YYC) and any disagreement was resolved by the third investigator (YCH).
Data extraction
Two reviewers independently extracted and collected the data in a tabular form. The extracted data included: country, study type, inclusion criteria, sample size, study results, and quality scores (Table I). The age, sex, and laboratory data for patients with rosacea and the controls were also extracted. Detailed data for the studies regarding the association with CV comorbidities are shown in Table II, which included the crude and adjusted odds ratio (OR)/incidence rate ratio (IRR) and the adjusted variables.
Table I.
Summary of included studies
| Study | Study design | Country | Inclusion period and criteria | Sample size, n |
Concomitant systemic treatment | Outcomes measurementa | Study result | Scoreb | |
|---|---|---|---|---|---|---|---|---|---|
| Rosacea | Control | ||||||||
| Population-based study | |||||||||
| Hua et al. (8), 2015 | Cross-sectional case-control | Taiwan | 1997–2010 Without diagnoses of acne, seborrhoeic dermatitis, and cutaneous lupus erythematosus |
33,553 | 67,106 | No limitation; Not report | HTN, DM, Dyslipidaemia, CAD, PAOD, Cerebral infarction | Patients with rosacea are more likely to have dyslipidaemia and HTN. They are also at increased risk of CAD | 7 |
| Marshall et al. (10), 2016 | Retrospective cohort | USA | 2005–2007 30–64 years old At least 1 year of follow-up data and 6 months of baseline data |
2,105 | 4,263 | No limitation; Not report | CVD (ischaemic heart disease, transient cerebral ischaemia, heart failure, and occlusion of cerebral arteries) | Patients with rosacea did not have an increased 1-year risk of CVD | 8 |
| Egeberg et al. (11), 2016 | Retrospective cohort | Denmark | 1997–2012 Without a history of MI or stroke before study start |
4,948 | 23,823 | No limitation; Not report | MACE (MI, ischaemic stroke, haemorrhagic stroke, and CV death) | Patients with rosacea were not associated with increased risk of adverse CV outcomes or death | 9 |
| Sinikumpu et al. (33), 2019 | Cross-sectional case-control | Finland | 46-year-old females from NFBC1966 | 146 | 278 | No limitation; Not report | A–D, G | Females with rosacea had increased CIMT | 7 |
| Institution-based study | |||||||||
| Duman et al. (9), 2014 | Cross-sectional case-control | Turkey | Without chronic inflammatory disorder | 60 | 50 | Tetracycline (13.3%) Isotretinoin (10%) |
B, C, E | Rosacea patients may have a high risk of CVD | 6 |
| Tsiskarishvili et al. (34), 2015 | Cross-sectional case-control | Georgia | Rosacea patients | 50 | 50 | No limitation; Not report | B | Rosacea is associated with 6 higher cholesterol, LDL, and TG and a lower HDL | 6 |
| Rainer et al. (35), 2015 | Cross-sectional case-control | USA | November 2012–August 2013 >18 years | 65 | 65 | No limitation; Not report | Metabolic disease (DM, HTN, dyslipidaemia, and obesity) | Rosacea is associated with 6 metabolic disease and in a skin severity-dependent manner | 6 |
| Belli et al. (36), 2016 | Cross-sectional case-control | Turkey | January to June 2015 Without disease associated with glucose metabolism, CAD, any other chronic inflammatory disease, and a history of drug use that may affect carbohydrate metabolism |
47 | 50 | Tetracycline (23.4%) Isotretinoin (4.3%) |
A–F | Association between rosacea and IR and some parameters of CV risk factors | 6 |
| Belli et al. (37), 2017a | Cross-sectional case-control | Turkey | January 2015–January 2016 Without known DM, CAD, hyperlipidaemia, and chronic inflammatory disease |
61 | 60 | No limitation; Not report | A–F | Association between rosacea and IR and some parameters of CV risk factors | 6 |
| Belli & Altun (38), 2017b | Cross-sectional case-control | Turkey | January 2015–November 2016 Without CVD peripheral vascular disease, DM, and any other inflammatory disease |
85 | 90 | No limitation; Not report | A–F | Rosacea patients did not have an increased risk of CVD | 6 |
| Belli et al. (39), 2017c | Cross-sectional case-control | Turkey | January to October 2016 Without CVD peripheral vascular disease, DM, and chronic inflammatory disease, and pregnancy |
40 | 40 | No limitation; Not report | A–G | Rosacea patients had significantly higher and EFT and CIMT | 6 |
| Gurel & Turan (40), 2019 | Cross-sectional case-control | Turkey | January to December 2017 Without CVD peripheral vascular disease, DM, COPD, PAOD, stroke, chronic inflammatory disease, and pregnancy |
52 | 52 | No limitation; Not report | A–C, G | Rosacea patients had significantly higher and EFT and CIMT | 6 |
A: blood pressure; B: lipid profile, C: C-reactive protein; D: glucose; E: insulin resistance, F: metabolic syndrome, G: carotid intima-media thickness/epicardial fat thickness.
The methodological quality of the studies were rated using an adapted version of the Newcastle–Ottawa Scale (NOS) for cohort studies with a maximum score of 9 points and for crosssectional studies with a maximum score of 7 points.
CAD: coronary artery disease; CIMT: carotid intima media thickness; COPD: chronic obstructive pulmonary disease; CV: cardiovascular; CVD: cardiovascular disease; DM: diabetes mellitus; EFT: epicardial fat thickness; HDL: high-density lipoprotein; HTN: hypertension; IR: insulin resistance; LDL: low-density lipoprotein; MI: myocardial infarction; NFBC: Northern Finland Birth Cohort; PAOD: peripheral arterial occlusive disease; TG: triglyceride.
Table II.
Detailed data and results of studies with incidence of cardiovascular comorbidities
| Study | Data sources | Rosacea definition | CV risk and disease definition | Risk with (95% CI) | Adjusted variable | Follow-up period | |
|---|---|---|---|---|---|---|---|
| Hua et al. (8), 2015 | National Health | ICD-9: 695.3 | HTN | ICD-9: 401–402 | Adjusted OR: 1.17 (1.12–1.21) | Age, sex | Cross-section |
| Insurance Research | DM | ICD-9: 250 | Adjusted OR: 1.01 (0.97–1.06) | ||||
| Database in Taiwan | Dyslipidaemia | ICD-9: 272 | Adjusted OR: 1.41 (1.36–1.46) | ||||
| CAD | ICD-9: 411–414 | Adjusted OR: 1.20 (1.14–1.26) | Age, sex, HTN, DM, | ||||
| PAOD | ICD-9: 440.2, 444.2 | Adjusted OR: 1.02 (0.93–1.11) | dyslipidaemia. | ||||
| Cerebral infarction | ICD-9: 433–434, 436–437 | Adjusted OR: 0.98 (0.91–1.06) | |||||
| Rainer et al. (35), 2015 | Johns Hopkins Hospital | National Rosacea | HTN | Hospital diagnosis | Crude OR: 2.8 (1.1–7.2) | N/A | Cross-section |
| Society Criteria | Metabolic disease | DM,HTN, dyslipidaemia | Crude OR: 2.4 (1.04–5.4) | ||||
| Marshall et al. (10), 2016 | MarketScan™ Commercial Claims and Encounters database | ICD-9: 695.3 | Ischaemic heart disease | ICD-9: 410–414 | Crude OR: 0.833 (0.691–1.004) Adjusted OR: 0.894 (0.7321.091) | Age, sex, DM, Charlson comorbidity index | One year |
| Transient cerebral ischaemia | ICD-9: 435 | ||||||
| Heart failure | ICD-9: 428 | ||||||
| Occlusion of cerebral arteries | ICD-9: 433, 434 | ||||||
| Egeberg et al. (11), 2016 | Danish civil personal register | ICD-10: I.71 | HTN | Use of 2 antihypertensive drugs | Crude OR: 1.234 (1.115–1.366)* | Age, sex, comorbidities, medication (anticholesterol and anti-migraine), and socioeconomic status | Until migration, death from any cause, or the occurrence of an end point. |
| DM | Either a hospital diagnosis or use of glucose-lowering drugs | Crude OR: 1.203 (1.012–1.431)* | |||||
| MI | ICD-10 codes I21–I22 | Crude IRR: 0.86 (0.65–1.13) | |||||
| Adjusted IRR: 0.75 (0.57–1.00) | |||||||
| Ischaemic stroke | ICD-10 codes I63–I64 | Crude IRR: 1.19 (0.96–1.49) | |||||
| Adjusted IRR: 1.08 (0.86–1.35) | |||||||
| Hemorrhagic | ICD-10 codes I60–I61 | Crude IRR: 1.11 (0.67–1.82) | |||||
| stroke | Adjusted IRR: 1.01 (0.61–1.67) | ||||||
| CV death | ICD-10 codes I00–I99 | Crude IRR: (1.26 1.02–1.57) | |||||
| Adjusted IRR: 0.99 (0.80–1.24) | |||||||
| MACE | Included all above | Crude IRR: 1.14 (0.99–1.32) | |||||
| Adjusted IRR: 0.99 (0.86–1.15) | |||||||
Odds ratio (OR) and confidence interval (CI) were calculated from original data.
CAD: coronary artery disease; CIMT: carotid intima media thickness; COPD: chronic obstructive pulmonary disease; CV: cardiovascular; CVD: cardiovascular disease; DM: diabetes mellitus; EFT: epicardial fat thickness; HDL: high-density lipoprotein; HTN: hypertension; ICD: International Classification of Diseases; IRR: incidence rate ratio; MACE: major adverse cardiac event; MI: myocardial infarction; N/A: not available; PAOD: peripheral arterial occlusive disease.
Data analysis
A pooled estimate of the laboratory and image examinations regarding the risk of CV diseases was performed for patients with rosacea and compared with that of controls. In addition, a pooled estimation was performed, comparing the incidence of CV comorbidities between patients with rosacea and controls. Pooled analyses were only performed for at least 2 studies, reporting the results in a similar form. Analyses of continuous data were performed using standardized mean difference (SMD) with 95% confidence interval (CI), while those of dichotomous data were conducted using OR with 95% CI. Adjusted estimates were chosen instead of raw ones, if they were provided in included studies. Heterogeneity testing was conducted by using the I square test. A random effects model was used for all analyses because of potential heterogeneity. Funnel plots and tests for publication bias (Egger’s test and Begg and Mazumdar test) were performed. The software used for statistical analyses was Comprehensive Meta-Analysis Version 3 software (Biostat Inc., Englewood, NJ, USA).
RESULTS
Search results and trial characteristics
Of the 232 studies screened, 12 studies involving 40,752 patients with rosacea met the inclusion criteria (Fig. 1). Two of the 12 studies were cohort studies (both population-based studies) and the remaining 10 studies were cross-sectional case-control studies (2 population-based studies and 8 institution-based studies). Four of the 12 studies reported the incidence of CV comorbidities in patients with rosacea, and the other 8 studies described the laboratory and image data related to CV risk factors in patients with rosacea. Four cross-sectional studies were performed at the same medical centre and the study periods showed an overlap. Only 2 of the 4 studies were considered for the meta-analysis. Table I summarizes the characteristics of the studies, with quality scores ranging from 6 to 9. Most cross-sectional studies lost one point of score from the selection part owing to lack of description for the non-respondents.
Fig. 1.

Flow diagram for study identification.
Risk factors for cardiovascular diseases
The common risk factors for CV diseases measured in the included studies were blood pressure, lipid profile, fasting blood glucose levels, C-reactive protein (CRP) levels, carotid intima media thickness (CIMT), and epicardial fat thickness (EFT). Dyslipidaemia, HTN, insulin resistance (IR), metabolic syndrome, and DM were also defined as risk factors for CV diseases. All these data were summarized in Table III and aggregated to compare the risk factors for CV diseases in patients with rosacea with those in controls.
Table III.
Detailed data and results of studies with outcomes of physical examination and laboratory data
| Study | Group | Age, years Mean±SD |
Sex,M:F n |
SBP, mmHg Mean±SD |
DBP, mmHg Mean ± SD |
Total cholesterol, mg/dl Mean±SD |
LDL, mg/dl Mean ±SD |
HDL, mg/dl Mean±SD |
TG, mg/dl Mean±SD |
CRP, mg/dl Mean±SD |
FBG, mg/dl Mean±SD |
CIMT, mm Mean±SD |
EFT, mm Mean ±SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duman et al (9), 2014 | Rosacea | 44.70± 12.90 | 20:40 | 199.19 ±36.60 | 121.02±31.54 | 53.80 ± 14.06 | 119.05 ±53.93 | 0.429 | |||||
| Control | 42.30± 12.30 | 17:33 | 162.83 ±30.05 | 101.48±23.47 | 50.17 ±8.43 | 121.94±64.57 | 0.423 | ||||||
| Tsiskarishvili et al. (34), 2015 | Rosacea | 35–65a | 20:30 | 280.25 ±24.33 | 180.00± 19.75 | 24.00 ±5.62 | 234.50 ±27.45 | ||||||
| Control | 35–65a | 20:30 | 190.60 ±10.40 | 135.00± 12.40 | 45.70 ±8.90 | 170.50 ±11.5 | |||||||
| Belli et al. (36), 2016 | Rosacea | 50.80 | 12:35 | 127.45 ±17.35 | 81.49± 8.34 | 218.53 ±33.37 | 132.96±31.21 | 58.96 ±15.75 | 131.49 ±69.6 | 4.86 ±12.86 | 96.57 ±13.05 | ||
| Control | 50.90 | 11:39 | 118.60 ±15.25 | 76.00± 8.81 | 201.80 ±36.48 | 119.84± 27.46 | 59.20 ±17.22 | 103.26 ±50.25 | 2.78 ±4.93 | 92.48 ±6.89 | |||
| Belli et al. (37), 2017a | Rosacea | 50.50 ±8.60 | 16:45 | 126.80 ±16.71 | 82.29± 9.20 | 214±35.47 | 129.87 ±31.46 | 57.19 ± 14.51 | 83.09 ± 8.79 | 3.33 ±3.11 | 96.42 ± 14.53 | ||
| Control | 49.80 ±9.70 | 13:47 | 118.50 ± 14.12 | 75.83± 9.26 | 199.76 ±34.89 | 119.00±26.39 | 57.62 ±16.69 | 76.04± 9.39 | 2.26 ±2.22 | 91.83 ±7.36 | |||
| Belli et al. (38), 2017b | Rosacea | 50.60 ±8.50 | 20:65 | 212.71 ±36.27 | 129.56±31.58 | 55.91 ±13.79 | 135.39 ± 70.31 | 3.35 ±3.31 | 96.71 ± 14.27 | ||||
| Control | 50.8o±9.00 | 23:67 | 204.14±33.53 | 121.91 ± 25.43 | 57.59 ± 16.47 | 117.23 ± 54.49 | 2.33 ±2.13 | 93.56 ±9.05 | |||||
| Belli et al. (39), 2017c | Rosacea | 50.40 ± 7.60 | 9:31 | 123.14± 16.94 | 80.00± 10.57 | 215.54±40.78 | 133.74± 33.39 | 53.89 ±12.31 | 141.45 ± 75.06 | 3.16 ±3.16 | 95.97 ± 14.96 | 0.72± 0.19 | 4.46 ±0.65 |
| Control | 50.50 ± 8.00 | 10:30 | 114.36 ± 14.11 | 74.61 ± 6.82 | 206.31 ± 30.45 | 121.76±24.68 | 59.87 ± 16.98 | 123.24±59.11 | 2.26 ±1.99 | 94.49 ±11.46 | 0.61 ± 0.12 | 3.28 ±0.59 | |
| Sinikumpu et al. (33), 2019 | Rosacea | 46 | 0:146 | 121.61 ±15.47 | 83.78± 9.96 | 200.77 ± 30.89 | 124.71 ± 30.12 | 64.86± 15.44 | 97.35±52.21 | 1.9 ±3.5 | 93.69 ± 14.41 | 0.61 ± 0.074 | |
| Control | 46 | 0:278 | 118.62 ±15.07 | 82.09± 10.19 | 198.84±31.27 | 123.55±29.73 | 64.48± 15.06 | 94.69 ±50.44 | 1.4±2.3 | 92.79 ±9.08 | 0.59± 0.067 | ||
| Gurel et al. (40), 2019 | Rosacea | 50.70 ±7.90 | 23:29 | 121.54± 18.51 | 76.15± 11.90 | 211.61 ±43.82 | 126.21 ± 36.58 | 54.4± 11.52 | 154.71 ±90.46 | 3.06 ±2.66 | 0.692 ±0.068 | 5.61 ±0.68 | |
| Control | 50.30 ±8.40 | 23:29 | 121.54± 15.13 | 76.92± 8.75 | 188.93 ±32.88 | 106.83±30.13 | 54.9± 12.14 | 132.88 ±58.87 | 1.97 ±1.34 | 0.56± 0.048 | 4.34±0.6 |
These data are presented as ranges.
SD: standard deviation; CIMT: carotid intima media thickness; CRP: C-reactive protein; DBP: diastolic blood pressure; EFT: epicardial fat thickness; FBG: fast blood glucose; HDL: high-density lipoprotein; LDL: low-density lipoprotein; SBP: systolic blood pressure; TG: triglyceride.
Cardiovascular comorbidities
The CV comorbidities included CAD, heart failure, stroke, peripheral arterial occlusive disease, and CV death (Table II). One cohort study showed that the one-year CV risk in patients with rosacea was similar to that in the controls after adjustment for age, sex, and comorbidities (DM and Charlson Comorbidity Index) (OR 0.894, 95% CI 0.732–1.091) (10). Another cohort study with a long-term follow-up also showed no difference in the incidence of major adverse CV events after adjustment for comorbidities, medication, and socioeconomic status (IRR 0.99, 95% CI 0.86–1.15) (11). However, one cross-sectional study showed that the risk of CAD in patients with rosacea was significantly higher than that in controls, even after adjustment for DM, HTN, and dyslipidaemia (OR 1.20, 95% CI 1.14–1.26) (8). No pooled analysis was performed, owing to differences in study designs and the heterogeneous definition of CV outcomes.
Statistical analysis
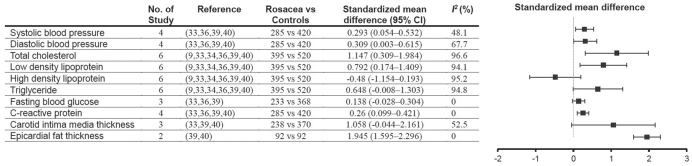
The results of the metaanalyses are shown in Figs 2 and 3. Patients with rosacea had higher systolic blood pressure (SMD 0.293, 95% CI 0.054–0.532), diastolic blood pressure (SMD 0.309, 95% CI 0.003–0.615), total cholesterol levels (SMD 1.147, 95% CI 0.309–1.984), low-density lipoprotein levels (SMD 0.792, 95% CI 0.174–1.409), and CRP levels (SMD 0.26, 95% CI 0.099–0.421) in comparison with controls. The CIMT was similar between patients with rosacea and controls (SMD 1.058, 95% CI −0.044–2.161), but the EFT in patients with rosacea was higher than that in controls (SMD 1.945, 95% CI 1.595–2.296). Pooled analyses showed that the incidence of HTN (OR 1.204, 95% CI 1.097–1.332) and IR (OR 2.338, 95% CI 1.187–4.605) in patients with rosacea was significantly higher than that in controls, but the incidence of metabolic syndrome and DM was similar in the 2 groups. Tests for publication bias were not performed because only a limited number of studies were included in the meta-analysis.
Fig. 2.
Forest plots. The forest plots showed the pooled estimates of cardiovascular risk factors in patients with rosacea in comparison with controls. Compared with controls, patients with rosacea had higher systolic blood pressure, diastolic blood pressure, total cholesterol, low-density lipoprotein, and C-reactive protein. Carotid intima media thickness was similar between patients with rosacea and controls, but epicardial fat thickness was greater in rosacea patients than in controls. CI: confidence interval.
Fig. 3.
Forest plots. Pooled analyses showed the incidence of hypertension and insulin resistance was significantly higher in patients with rosacea than in controls, but the incidence of metabolic syndrome and diabetes mellitus was similar in the 2 groups. CI: confidence interval.
DISCUSSION
This meta-analysis reveals that patients with rosacea have significant risk factors for CV diseases, including higher systolic blood pressure, diastolic blood pressure, total cholesterol levels, low-density lipoprotein levels, CRP levels, and EFT. Moreover, this study showed that the incidence of IR in patients with rosacea was significantly higher than in controls, but the incidence of DM and metabolic syndrome was not increased in patients with rosacea compared with that in controls, thereby suggesting a subclinical derangement in patients with rosacea.
No meta-analysis of CV comorbidities was performed, due to substantial study heterogeneity and a limited number of eligible studies. The population-based cross-sectional study conducted by Hua et al. (8) showed that patients with rosacea had a higher risk of CAD than controls; in contrast, the cohort study conducted by Egeberg et al. (11) revealed that patients with rosacea had a similar risk of myocardial infarction as controls. However, in the cross-sectional study, the definition of CAD did not include the International Classification of Disease-9 (ICD-9) code 410 (acute myocardial infarction), which is the most severe form of CAD (8). This might explain the different results between the 2 studies. Both studies concluded that patients with rosacea did not have a higher risk of ischaemic stroke than controls (8, 11). The current evidence is not sufficient to demonstrate that patients with rosacea have higher incidence of CV comorbidities, such as myocardial infarction and stroke.
The mechanisms underlying the susceptibility of patients with rosacea to increased CV risk may be attributable to the common pathological pathways in these diseases. Patients with rosacea have an increased level of cathelicidin, an antimicrobial peptide, in their skin (2, 3). Besides the levels of cathelicidin, the forms of cathelicidin in patients with rosacea are different from those in healthy individuals (2). Cathelicidin enhances angiogenesis, leukocyte chemotaxis, and the expression of extracellular matrix components (2). Cathelicidin is processed by serine protease kallikrein 5, which is expressed extensively in the epidermis of patients with rosacea (3, 12). Recent studies have shown increased levels of cathelicidin in atherosclerotic plaques and correlations between the genetic expression of cathelicidin and CV risk factors (13–15). Cathelicidin has also been reported to promote IR in obese individuals (16). Furthermore, serine proteases are also involved in the pathogenesis of atherosclerosis (17). In addition, patients with rosacea are reported to have a decreased activity of paraoxone-1 (PON1), an antioxidant enzyme, and increased oxidative stress (18). Decreased activity of PON1 has also been shown in patients with dyslipidaemia, HTN, and DM (19, 20). Increased oxidative stress is widely believed to be a pivotal mechanism in atherosclerosis (21)
Tetracyclines, which are commonly used for treating rosacea, have not only antimicrobial effects, but also anti-inflammatory properties, and they could potentially be used to treat both cutaneous and systemic inflammation (22, 23). Tetracyclines inhibit matrix metalloproteinases (MMPs), which are important enzymes in the vascular pathophysiology of both rosacea and atherosclerosis (22, 24). A large cohort study showed that patients with rosacea receiving tetracyclines (with variable dosage and duration) had a decreased risk of developing vascular diseases (25). Axisa et al. (26) showed, in a randomized controlled trial (RCT), that doxycycline (200 mg daily for 2–8 weeks) decreased the expression of MMP-1 in atherosclerotic carotid plaques In multiple RCTs, sub-antimicrobial doses of doxycycline therapy (20 mg twice daily for 3 months in Koppikar et al. (27), 6 months in Brown et al. (28) and 2 years in Payne et al. (29)) have also been shown to reduce the levels of CRP and MMP-9. The beneficial effects of treatment with tetra-cyclines on both cutaneous and systemic inflammation corroborate the hypothesis that rosacea and CV diseases share common pathophysiological pathways, and these effects may serve to explain the relatively subclinical CV derangements in patients with rosacea, as shown in the current study.
Study limitations
A major limitation of the current study is that only a few eligible studies were included. However, some analyses included population-based studies, which had large sample sizes of study subjects, thereby compensating more or less the paucity of existing eligible studies. Of note, many of the included studies were conducted in Turkey, which may lead to bias when the research findings are generalized to a broader population. Ethnicity and genetics may play critical roles in the risk of rosacea and cardiovascular diseases. Rosacea is found to be more prevalent in people of Celtic and Northern European origin and is less commonly seen in people with darker skin, possibly due to masking of symptoms by darker skin pigmentation or genetic differences (30). In addition, ethnic differences in cardiovascular risk could lead to potential bias. For example, Chaturvedi revealed that South Asians have higher risk of insulin resistance and heart diseases compared with Europeans. (31). Onat (32) reported that mortality from coronary heart disease in Turkey was among the highest in selected European countries. Therefore, inclusion of a high proportion of Turkish studies could potentially be a major source of bias. However, the sample sizes of these Turkish studies are relatively small compared with the large sample sizes of other population-based studies conducted in other regions of the world. Another limitation is that the use of systemic medications, such as tetracycline and isotretinoin, could not be adjusted in our analysis, because most included studies did not provide such details (Table I). The use of tetracycline may lead to underestimation, while the use of isotretinoin may lead to overestimation, of some parameters in the current analysis. However, the duration of tetracycline treatment for rosacea is often short, and isotretinoin is infrequently used to treat rosacea. Therefore, the potential biases caused by the use of these medications might not be marked. Future studies are encouraged to take this factor into consideration. Lastly, some of the analyses were limited by substantial heterogeneity, which might result from differences in age, ethnicity, types and severity of rosacea among included studies. Unfortunately, subgroup and meta-regression analysis could not be performed because of insufficient data reported in the included studies.
Conclusion
The current study revealed that patients with rosacea are predisposed to increased CV risk, such as HTN, dyslipidaemia, IR, and high EFT. However, patients with rosacea do not have a higher incidence of overt CV comorbidities, such as myocardial infarction and stroke, although this incidence may have been underestimated due to the common use of tetracyclines. Clinicians are advised to examine patients with rosacea for CV risk and comorbidities. Patients should also be given advice regarding lifestyle modifications. Future studies should investigate the link between cutaneous and internal inflammation and examine the potential benefits of sub-antimicrobial doses of tetracyclines as primary prevention for CV diseases in patients with rosacea.
ACKNOWLEDGEMENTS
The authors thank Taipei Medical University and Wan Fang Hospital, Taipei Medical University for financial support under grant number 108TMU-WFH-02, which made this study possible.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Gallo RL, Granstein RD, Kang S, Mannis M, Steinhoff M, Tan J, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol 2018; 78: 148–155. [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci 2009; 55: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med 2007; 13: 975–980. [DOI] [PubMed] [Google Scholar]
- 4.Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol 2013; 69: S15–S26. [DOI] [PubMed] [Google Scholar]
- 5.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- 6.Lockshin B, Balagula Y, Merola JF. Interleukin 17, inflammation, and cardiovascular risk in patients with psoriasis. J Am Acad Dermatol 2018; 79: 345–352. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135–1143. [DOI] [PubMed] [Google Scholar]
- 8.Hua TC, Chung PI, Chen YJ, Wu LC, Chen YD, Hwang CY, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol 2015; 73: 249–254. [DOI] [PubMed] [Google Scholar]
- 9.Duman N, Ersoy Evans S, Atakan N. Rosacea and cardiovascular risk factors: a case control study. J Eur Acad Dermatol Venereol 2014; 28: 1165–1169. [DOI] [PubMed] [Google Scholar]
- 10.Marshall VD, Moustafa F, Hawkins SD, Balkrishnan R, Feldman SR. Cardiovascular disease outcomes associated with three major inflammatory dermatologic diseases: a propensity-matched case control study. Dermatol Ther 2016; 6: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egeberg A, Hansen PR, Gislason GH, Thyssen JP. Assessment of the risk of cardiovascular disease in patients with rosacea. J Am Acad Dermatol 2016; 75: 336–339. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J 2006; 20: 2068–2080. [DOI] [PubMed] [Google Scholar]
- 13.Edfeldt K, Agerberth B, Rottenberg ME, Gudmundsson GH, Wang XB, Mandal K, et al. Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arterioscler Thromb Vasc Biol 2006; 26: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 14.Ciornei CD, Tapper H, Bjartell A, Sternby NH, Bodelsson M. Human antimicrobial peptide LL-37 is present in atherosclerotic plaques and induces death of vascular smooth muscle cells: a laboratory study. BMC Cardiovasc Disord 2006; 6: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benachour H, Zaiou M, Samara A, Herbeth B, Pfister M, Lambert D, et al. Association of human cathelicidin (hCAP18/LL-37) gene expression with cardiovascular disease risk factors. Nutr Metab Cardiovasc Dis 2009; 19: 720–728. [DOI] [PubMed] [Google Scholar]
- 16.Braster Q, Silvestre-Roig C, Hartwig H, Kusters P, Aarts S, den Toom M, et al. Cathelicidin regulates myeloid cell accumulation in adipose tissue and promotes insulin resistance during obesity. Thromb Haemost 2016; 115: 1237–1239. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Touchard A, Henry TD, Sangiorgi G, Spagnoli LG, Mauriello A, Conover C, et al. Extracellular proteases in atherosclerosis and restenosis. Arterioscler Thromb Vasc Biol 2005; 25: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 18.Takci Z, Bilgili SG, Karadag AS, Kucukoglu ME, Selek S, Aslan M. Decreased serum paraoxonase and arylesterase activities in patients with rosacea. J Eur Acad Dermatol Venereol 2015; 29: 367–370. [DOI] [PubMed] [Google Scholar]
- 19.Kota SK, Meher LK, Kota SK, Jammula S, Krishna SVS, Modi KD. Implications of serum paraoxonase activity in obesity, diabetes mellitus, and dyslipidemia. Indian J Endocrinol Metab 2013; 17: 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yildiz A, Gur M, Demirbag R, Yilmaz R, Akyol S, Aslan M, et al. Paraoxonase and arylesterase activities in untreated dipper and non-dipper hypertensive patients. Clin Biochem 2008; 41: 779–784. [DOI] [PubMed] [Google Scholar]
- 21.Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep 2017; 19: 42. [DOI] [PubMed] [Google Scholar]
- 22.Dosal J, Keri J. Rosacea and cardiovascular disease: is there an association? J Am Acad Dermatol 2015; 73: 308–309. [DOI] [PubMed] [Google Scholar]
- 23.Alikhan A, Kurek L, Feldman SR. The role of tetracyclines in rosacea. Am J Clin Dermatol 2010; 11: 79–87. [DOI] [PubMed] [Google Scholar]
- 24.Castro MM, Kandasamy AD, Youssef N, Schulz R. Matrix metalloproteinase inhibitor properties of tetracyclines: therapeutic potential in cardiovascular diseases. Pharmacol Res 2011; 64: 551–560. [DOI] [PubMed] [Google Scholar]
- 25.Dosal JR, Rodriguez GL, Pezon CF, Li H, Keri JE. Effect of tetracyclines on the development of vascular disease in veterans with acne or rosacea: a retrospective cohort study. J Invest Dermatol 2014; 134: 2267–2269. [DOI] [PubMed] [Google Scholar]
- 26.Axisa B, Loftus IM, Naylor AR, Goodall S, Jones L, Bell PR, et al. Prospective, randomized, double-blind trial investigating the effect of doxycycline on matrix metalloproteinase expression within atherosclerotic carotid plaques. Stroke 2002; 33: 2858–2863. [DOI] [PubMed] [Google Scholar]
- 27.Koppikar RS, Agrawal SV. The effect of sub-antimicrobial dose-doxycycline periodontal therapy on serum inflammatory biomarker C-reactive protein levels in post-menopausal women: a 2-year, double-blinded, randomized clinical trial. Contemp Clin Dent 2013; 4: 71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol 2004; 24: 733–738. [DOI] [PubMed] [Google Scholar]
- 29.Payne JB, Golub LM, Stoner JA, Lee H-m, Reinhardt RA, Sorsa T, et al. The effect of subantimicrobial-dose–doxycycline periodontal therapy on serum biomarkers of systemic inflammation: a randomized, double-masked, placebo-controlled clinical trial. J Am Dent Assoc 2011; 142: 262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexis AF, Callender VD, Baldwin HE, Desai SR, Rendon MI, Taylor SC. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol 2019; 80: 1722–1729. e1727. [DOI] [PubMed] [Google Scholar]
- 31.Chaturvedi N. Ethnic differences in cardiovascular disease. Heart 2003; 89: 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onat A. Risk factors and cardiovascular disease in Turkey. Atherosclerosis 2001; 156: 1–10. [DOI] [PubMed] [Google Scholar]
- 33.Sinikumpu SP, Jokelainen J, Auvinen J, Puukka K, Kaikkonen K, Tasanen K, et al. Increased risk of cardiovascular diseases in female rosacea patients: a nested case-control study. Acta Derm Venereol 2019; 99: 705–706. [DOI] [PubMed] [Google Scholar]
- 34.Tsiskarishvili NV, Katsitadze A, Tsiskarishvili T, Tsiskarishvili NI. [Capillary fragility and some hemostatic parameters in patients with rosacea]. Georgian Med News 2015: 33–36 (in Russian). [PubMed] [Google Scholar]
- 35.Rainer BM, Fischer AH, Luz Felipe da Silva D, Kang S, Chien AL. Rosacea is associated with chronic systemic diseases in a skin severity-dependent manner: results of a case-control study. J Am Acad Dermatol 2015; 73: 604–608. [DOI] [PubMed] [Google Scholar]
- 36.Belli AA, Ozbas Gok S, Akbaba G, Etgu F, Dogan G. The relationship between rosacea and insulin resistance and metabolic syndrome. Eur J Dermatol 2016; 26: 260–264. [DOI] [PubMed] [Google Scholar]
- 37.Belli AA, Kara A, Ozbas Gok S. Can hematologic parameters be an indicator of metabolic disorders accompanying rosacea? Acta Dermatovenerol Croat 2017; 25: 145–150. [PubMed] [Google Scholar]
- 38.Belli AA, Altun I. Assessment of Framingham risk score and systemic coronary risk evaluation in rosacea patients. Dermatologica Sinica 2017; 35: 127–130. [Google Scholar]
- 39.Belli AA, Altun I, Altun I. Thickness of carotid intima and epicardial fat in rosacea: a cross-sectional study. An Bras Dermatol 2017; 92: 820–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gürel G, Turan Y. Noninvasive assessment of subclinical atherosclerosis in patients with rosacea. G Ital Dermatol Venereol 2019. Feb 4 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]