Abstract
Antibiotic-resistant Cutibacterium acnes has been reported worldwide, but data from Israeli patients with acne is currently lacking. This study evaluated the antibiotic susceptibility of C. acnes, isolated from 50 Israeli patients with acne to commonly prescribed antibiotics, using the Epsilometer test (E-test). Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) analysis, 16S rRNA sequencing and single locus sequence typing (SLST) molecular typing were used to identify and characterize C. acnes. Among 36 strains isolated, phylotype IA1 was most common. Resistance to at least one antibiotic was found in 30.6% of tested strains. Resistance rates were highest for erythromycin (25.0%), followed by doxycycline (19.4%), clindamycin (16.7%), minocycline (11.1%) and tetracycline (8.3%). Significant correlation was found between resistance to multiple antibiotics, with 5.6% of isolates resistant to all antibiotics tested. When reviewing resistances rate worldwide antibiotic resistance was found to be prevalent in Israel. Measures to limit the emergence of antibiotic-resistant strains of Cutibacterium acnes should be taken and alternative treatments should be sought.
Key words: acne, Cutibacterium acnes, antibiotic, resistance
Acne is a common chronic inflammatory disorder of the pilosebaceous unit, affecting approximately 80% of adolescents and young adults (1). It is characterized by comedones, papules, pustules, nodules and cysts over pilosebaceous rich areas, such as the face, chest, upper back and arms. As sequelae, it may cause pigmentary changes, permanent scarring, and severe psychological implications, such as depression, social isolation and suicidal ideation. The pathophysiology of acne involves abnormal follicular keratinization, excessive sebum production (under androgen control), modification of the distribution of Cutibacterium acnes clusters and an inflammatory response (1, 2). Recent research emphasizes the role of dysseborrhoea (alteration of the sebaceous lipid profile), and involvement of external factors, such as stress, irritation, cosmetics and potential dietary factors, in inducing inflammation (3, 4). The role of the skin microbiome remains to be fully elucidated, but it is possible that changes in the distribution of species/strains, a stable distribution with pathogenic alteration in response to internal or external stimuli, or a combination of these factors, are involved in the pathogenesis of acne (5). A disturbed skin barrier and alteration of the skin microbiome results in the proliferation of C. acnes (4).
SIGNIFICANCE
Cutibacterium acnes has a causative role in acne. Antibiotic resistance of Cutibacterium acnes has been reported worldwide, but there is no data regarding the antibiotic-resistance rates in Cutibacterium acnes in Israel. This study collected samples from 50 Israeli patients with acne and evaluated resistance rates for commonly prescribed antibiotics. Resistance to at least one antibiotic was found in 30.6% of isolated strains. Resistance rates were highest for erythromycin (25.0%), followed by clindamycin (16.7%) and doxycycline (19.4%), minocycline (11.1%) and tetracycline (8.3%). Antibiotic resistance was found to be prevalent in Israel. Measures to limit the emergence of antibiotic-resistant strains of Cutibacterium acnes should be taken and alternative treatments should be sought.
C. acnes, previously named Propionibacterium acnes, is a Gram-positive, preferential anaerobic rod, which is a dominant member of the microbiota of healthy human skin and an exclusive bacterial inhabitant of normal human facial sebaceous follicles (5). The anaerobic and lipid-rich conditions within the pilosebaceous unit provide an optimal microenvironment for the growth of C. acnes. Recent research has further elucidated the important role of C. acnes in the pathogenesis of acne. It has been shown to induce inflammatory responses in host skin parenchymal cells and immune cells through the activation of Toll-like receptors and the presence of host tissue-degrading enzymes (6) and is therefore considered an important target in the treatment of acne.
Developments in genomic research have shown that C. acnes consists of phylogenetically distinct cluster groups with different pathogenic traits. Some strains of C. acnes induce stronger cytokine/chemokine expression, as well as differentiation and proliferation of keratinocytes and sebocytes (7). These findings suggest that C. acnes strains may influence the severity of inflammatory acne. Exact identification of the pathogenic strains of C. acnes could lead to more selective and advanced therapeutics, aimed at these strains only.
Topical and oral antibiotics have been the mainstay of acne treatment for the past 40 years. Chronic course and prolonged therapy have contributed to the emergence of antibiotic-resistant C. acnes strains. Increases in antibioticresistant C. acnes have now been reported worldwide; however, data for different antibiotics and various regions or countries is incomplete. Many countries have reported that over 50% of strains of C. acnes are resistant, particularly to topical macrolides, and a correlation has been shown between the emergence of resistant C. acnes strains and antibiotic use. Collateral damage to the microbiome’s steady state and the emergence of antibiotic resistance in non-target bacteria is also a major concern (8). With progression into the “post-antibiotic era”, clinical guidelines for the treatment of acne, published by the international group “The Global Alliance to Improve Outcomes in Acne” in 2018, have excluded antibiotic agents as monotherapy (topically and systemically) in order to prevent further development of resistant strains (9). Development of new treatment modalities for acne is necessary and, regardless of acne, the need for novel antimicrobial agents is urgent.
To date, information regarding antibiotic resistance rates of C. acnes in Israeli patients with acne is lacking. The data provided here may contribute to raising the awareness of anti-microbial resistance and changing the way dermatologists use antibiotic agents to treat acne.
The aim of this study was to characterize the antibiotic susceptibility patterns of C. acnes isolated from Israeli patients with acne.
MATERIALS AND METHODS
Patients
Patients, over 10 years of age, with acne vulgaris attending the Dermatology Clinic at Hadassah Hebrew University Medical Center, were invited to participate in the study. Written informed consent was obtained from the participants and/or their parents after providing a detailed explanation of the study. Clinical information, including age, sex, age of onset, previous or current anti-acne treatments, acne severity (according to the Physician Global Assessment) (10) and acne distribution, were obtained from medical records. The study was approved by the local ethics review board (HMO-0073-19).
Specimen collection and processing
The skin over acne lesions was first cleaned with 70% isopropyl alcohol wipes. Comedonal or pustular content was squeezed manually or punctured with a sterile needle. The content was collected on a sterile swab (Copan ESwab®, Murrieta, CA, USA) and inoculated into the surface of culture plates containing Wilkins-Chalgren agar (Oxoid, Hampshire, England) supplemented with furazolidone to inhibit the growth of staphylococci. The plates were incubated under anaerobic conditions (anaerobic chamber or anaerobic bags) between 48 h to one week, and examined daily for the appearance of typical colonies. If the culture was negative after one week, another culture was prepared from the original bacterial swab that was kept refrigerated. Isolation streaks were performed to obtain single colonies. Each isolated strain was viewed microscopically. To confirm that these bacteria were C. acnes, matrix-assisted laser desorption ionization-time of flight (MALDITOF) analysis was performed (11). For strains unrecognized by MALDI-TOF, 16S PCR and sequencing were performed using Ilumina’s universal 16S primers (https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomiclibraryprepguide15044223b.pdf) (12). Sequences were compared with a database using BLAST software (https://blast.ncbi.nlm.nih.gov/Blast.cgi). All confirmed C. acnes strains had undergone a PCR reaction with the single locus sequence typing (SLST) method primers, described herein, and were recognized by these specific primers. Furthermore, when the PCR product was sequenced and compared to the BLAST database, it matched C. acnes in 99% similarity.
Single locus sequence typing
Molecular characterization of C. acnes was performed using SLST, as described by Scholz et al. (13). This rapid method uses a single locus in the C. acnes genome to classify the strain and to distinguish between the defined C. acnes phylotypes. The following primers were used for SLST: forward primer 5’-CAGCGGCGCTGCTAAGAACTT-3’; reverse primer 5’-CCGGCTGGCAAATGAGGCAT-3’. The PCR product was sequenced and compared with different known SLST types (http://medbac.dk/slst). Discrimination between the different phylogenetic clusters of C. acnes, and adaptation to the phylotypes described in the traditional typing method, was performed using the scheme provided by Scholz et al. (13).
Antibiotic susceptibility
Following bacterial identification, C. acnes isolates were subcultured in Wilkins broth (Oxoid, Basingstoke, UK) and suspended at a density of 1.0 McFarland. Bacterial lawns were prepared on anaerobic blood plates (Novamed, Jerusalem, Israel) and allowed to dry. Antibiotic susceptibility was assessed by determining a minimal inhibitory concentration (MIC) using an Epsilometer test (E-test) (ETEST® bioMérieux, St. Louis, MO, USA). The MIC was determined at 48 h following incubation under anaerobic conditions as the point on the scale at which the ellipse of growth inhibition intercepts the plastic strip. The antibiotics used were: erythromycin, clindamycin, tetracycline, doxycycline and minocycline. The breakpoints used to define susceptibility or resistance to clindamycin and tetracycline followed the recommendations set out by the Clinical and Laboratory Standards Institute (CLSI) (14); resistance to clindamycin was defined at a MIC above 2 µg/ml and tetracycline at a MIC above 4 µg/ml. Since no standards exist for breakpoints of erythromycin, doxycycline and minocycline, those with a MIC of ≥ 0.5 µg/ml (for erythromycin) and ≥ 1 µg/ml (for doxycycline and minocycline) were defined as resistant, according to definitions used in previous studies (15).
Antibiotic susceptibility patterns in relation to characteristics of patients and acne
The antibiotic susceptibility patterns of C. acnes to the different antibiotics were compared between the patients; sub-grouped according to sex, age (> 20 years and 20 years or less), acne duration (< 2 years and 2 years or more), acne severity (mild, moderate and severe, according to Physician’s Global Assessment) (10) and acne distribution (facial only and face plus trunk). The patients were also sub-grouped according to negative or positive history of previous therapy and previous antibiotic therapy for acne. The resistance patterns to multiple antibiotics were examined as well as the correlation with different phylotypes.
Statistical analysis
Data were analysed using IBM SPSS Statistics V22.0 (https://www.ibm.com/analytics/spss-statistics-software). Descriptive statistics were used to display the research results. Sequential variances (age, acne duration) were compared using Student’s t-test. Categorical variances were compared by χ2 test. A p-value of ≤ 0.05 was considered statistically significant.
RESULTS
Patients’ characteristics
Patients’ demographic and clinical characteristics are shown in Table I. Only the 36 patients in whom C. acnes was isolated are presented. Only a minority of the patients had severe acne. Among treatments for acne, antibiotic treatment was administered in 18 patients and included topical clindamycin, erythromycin, neomycin, oral minocycline and doxycycline. Other therapies for acne included topical benzoyl peroxide, topical retinoids, isotretinoin and oral contraceptive pills.
Table I.
Patient’s demographic and clinical characteristics
| Characteristic | Patients with acne (n = 36) |
|---|---|
| n (%) | |
| Age, median (range) | 19 (11–30) |
| ≤ 20 years | 14 (38.9) |
| > 20 years | 22 (61.1) |
| Sex | |
| Male | 9 (25.0) |
| Female | 27 (75.0) |
| Acne severity* | |
| Mild | 16 (45.7) |
| Moderate | 15 (42.9) |
| Severe | 4 (11.4) |
| Acne duration** | |
| ≥ 2 years | 28 (82.4) |
| < 2 years | 6 (17.6) |
| Acne distribution | |
| Only face | 20 (55.6) |
| Face plus either back, chest, arms or neck | 16 (44.4) |
| Prior and current treatments*** | |
| No treatment | 6 (18.8) |
| Treatment (antibiotic or other) | 26 (81.2) |
| Antibiotic treatment (topical and oral) | 18 (56.3) |
Only the 36 patients in whom C. acnes was isolated are shown.
Information missing for:
1 patient;
2 patients;
4 patients.
Identification of Cutibacterium acnes strains
Throughout the 6-month study period from December 2017 to May 2018, comedonal or pustular content samples were obtained from 50 patients with acne vulgaris (Table SI1). Bacteria were isolated from 44 samples on plates and in liquid culture. Twenty-six of the isolates were identified as C. acnes using MALDI-TOF (Table SI1). The 10 isolates that were not identified were sent to 16S rRNA sequencing (Table SI1). These analyses showed that C. acnes strains were present in 36 patients (isolation rate 72%). The other isolates were Cutibacterium avidum (7 strains; 6 isolated from facial skin and 1 isolated from the back) and Cutibacterium granulosum (1 strain isolated from facial skin) (Table SI1).
Phylotype identification
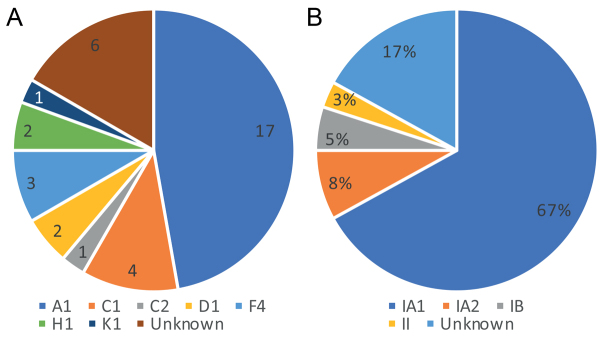
The SLST typing of the strains was tested further using the medbac website (http://medbac.dk/slst/pacnes). The data are presented in Fig. 1. Seventeen strains were of the A1 SLST type, and the remainder were C1 (4 strains), C2 (1 strain), D1 (2 strains), F4 (3 strains), H1 (2 strains) and K1 (1 strain). Six strains were recognized by the SLST method, but the sequence did not match any currently recognized strain. Since they seem to represent new SLST types the sequencing process was repeated for a second confirmation, and the data were sent to the medbac website to be added to the database. Their sequences were confirmed as C. acnes in the BLAST database, in 97.54% similarity (Appendix S11), with another independent C. acnes identification described herein (Table SI1). Conversion of the SLST types to the phylotypes according to traditional typing is shown in Fig. 1 (13).
Fig. 1.
Cutibacterium acnes molecular typing in the 36 clinical isolates. (A) Single locus sequence typing. (B) Phylotypes (traditional typing).
Antibiotic susceptibility assay in Israeli patients with acne
The isolates and their resistance profiles, with the number of strains inhibited at various MICs, are shown in Table II. The MIC ranges in the current study were 0.016–256 µg/ml for erythromycin, 0.016–256 µg/ml for clindamycin, 0.047–256 µg/ml for tetracycline, 0.047–64 µg/ml for doxycycline and 0.023–32 µg/ml for minocycline. MIC50, the MIC required to inhibit the growth of 50% of bacteria, of the 36 strains were all below the resistance breakpoint of all antibiotics tested. MIC50 was lowest for erythromycin (≤ 0.016 µg/ml) and highest for tetracycline and doxycycline (0.190 µg/ml). MIC90, the MIC required to inhibit the growth of 90% of bacteria, were all above the resistance breakpoint of the antibiotics tested and showed the highest value for erythromycin (≥ 256 µg/ml) and lowest value for minocycline (1 µg/ml).
Table II.
The isolates examined and their resistance profiles, with the number of strains inhibited at various minimum inhibitory concentrations (MICs)
| Antibiotics | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resistance breakpoint (μg/ml) | Resistant strains n (%) | |||||||||||||||||||||||
| ≤ 0.016 | 0.023 | 0.032 | 0.047 | 0.064 | 0.094 | 0.125 | 0.19 | 0.25 | 0.38 | 0.5 | 0.75 | 1 | 1.5 | 2 | 3 | 4 | 8 | 32 | 48 | 64 | ≥ 256 | MIC50 | MIC90 | |
| Erythromycin | 18 | 6 | 2 | 1 | 9 | ≤ 0.016 | ≥ 256 | |||||||||||||||||
| Clindamycin | 2 | 5 | 7 | 3 | 4 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 2 | 1 | 3 | 0.064 | 4 | ||||||
| Tetracycline | 2 | 4 | 1 | 7 | 10 | 3 | 1 | 4 | 1 | 2 | 1 | 0.19 | 4 | |||||||||||
| Doxycycline | 1 | 2 | 2 | 12 | 2 | 7 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 0.19 | 4 | |||||||
| Minocycline | 1 | 7 | 12 | 5 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 0.047 | 1 |
All resistant isolates (with resistance to at least one of the antibiotics tested) are shown in Table III. Overall, resistance to at least one antibiotic was found in 30.6% of the isolated strains. The antibiotic that showed the highest percentage of resistant strains was erythromycin, with 25.0% resistant strains, followed by doxycycline (19.4%), clindamycin (16.7%), minocycline (11.1%) and tetracycline (8.3%) (Fig. 2).
Table III.
Antibiotic resistance pattern of resistant isolates (with resistance to at least one of the antibiotics examined)
| Sample (#) | SLST type | Phylotype (traditional typing) | Erythromycin | Clindamycin | Tetracycline | Doxycycline | Minocycline |
|---|---|---|---|---|---|---|---|
| 1 | A1 | IA1 | ≥ 256 | ≥ 256 | ≥ 256 | 64 | 32 |
| 2 | H1 | IB | ≥ 256 | 4 | 8 | 8 | 1 |
| 3 | A1 | IA1 | ≥ 256 | 3 | 8 | 32 | 0.032 |
| 4 | A1 | IA1 | ≥ 256 | ≥ 256 | 1 | 1 | 0.5 |
| 5 | A1 | IA1 | ≥ 256 | ≥ 256 | 1 | 3 | 0.032 |
| 6 | A1 | IA1 | ≥ 256 | 3 | 4 | 4 | 0.75 |
| 7 | F4 | IA2 | ≥ 256 | 1.5 | 1 | 1 | 0.5 |
| 8 | A1 | IA1 | ≥ 256 | 2 | 1 | 0.75 | 0.064 |
| 9 | A1 | IA1 | ≥ 256 | 2 | 0.19 | 0.25 | 0.094 |
| 10 | C1 | IA1 | 0.016 | 0.032 | 0.125 | 0.125 | 1.5 |
| 11 | A1 | IA1 | 0.016 | 0.064 | 0.25 | 0.25 | 1 |
Red box: above resistance breakpoint.
SLST: single locus sequence typing.
Fig. 2.
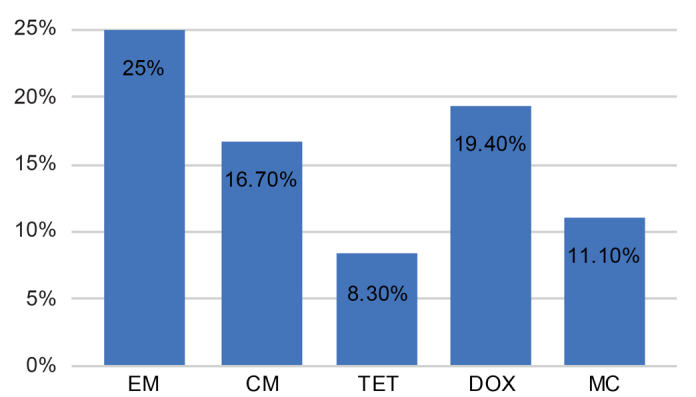
Percentage of Cutibacterium acnes resistant strains among the 36 clinical isolates. Resistant breakpoints: clindamycin > 2 μg/ml; erythromycin ≥ 0.5 μg/ml, doxycycline and minocycline ≥ 1 μg/ml; tetracycline > 4 μg/ml. Antibiotics: EM; erythromycin; CM: clindamycin; TET: tetracycline; DOX: doxycycline; MC: minocycline.
Minimal inhibitory concentration differences regarding patients’ characteristics
No statistically significant differences were found between MIC and age, sex, acne characteristics (severity, distribution and duration), previous or current treatments, or between phylotypes.
Antibiotic susceptibility patterns
The antibiotic resistance profiles of the 11 resistant strains are shown in Table III. In most cases, resistance to one or more antibiotics showed a trend towards a higher MIC for the remaining antibiotic/s even though the MIC did not exceed the resistance breakpoint. Six (16.7%) strains had crossresistance among MLS (macrolides– lincosamides–streptogramins), namely, clindamycin and erythromycin. Seven (19.4%) had crossresistance between the MLS and cycline antibiotics, and 3 (8.3%) strains had crossresistance among cycline antibiotics. Interestingly, different susceptibility patterns were seen within the same family of tetracycline. Statistically significant correlations were found between resistance to all pairs of antibiotics tested, except for minocycline and doxycycline (p = 0.302); erythromycin and minocycline (p = 0.01431); clindamycin and erythromycin, doxycycline or tetracycline (p = 0.00001), clindamycin and minocycline (p = 0.028), erythromycin and doxycycline (p = 0.00001), erythromycin and tetracycline ((p = 0.0027), minocycline and tetracycline (p = 0.0001) and doxycycline and tetracycline (p = 0.014).
DISCUSSION
Antibiotics have been a mainstay of acne treatment for many years. The antibiotic treatment is administered for its antibacterial effect, addressing C. acnes colonization, but also for its anti-inflammatory and immunomodulatory properties (16). Widely used topical antibiotics for acne in Israel include clindamycin 1% gel and erythromycin 2% solution or emulsion. Systemic antibiotics, such as minocycline and doxycycline, are indicated for moderate-to-severe inflammatory acne or after a failure of topical therapy. A typical treatment course may last several months and the selective pressure from long-term use is considerable. As previously mentioned, antibioticresistance of C. acnes has been reported worldwide (15, 17-34), but antibiotic susceptibility patterns of C. acnes in Israeli patients with acne, to the best of our knowledge, have not been determined. This is the first report to address C. acnes antibiotic resistance rates in Israel.
The isolation rate of C. acnes from 50 patients with acne in this study was only 72%. Isolation rate of C. acnes in previous studies was shown to be between 57% (32) and 96% (31), but mostly around 80% (15, 17). If other Cutibacterium are included, the isolation rate in the current study increases to 88%. Accurate methods to identify C. acnes, as used in this study, may contribute to this relatively low isolation rate.
C. acnes recognition was performed initially with MALDI-TOF. The bacteria that were not recognized by this method were then sent to 16S rRNA sequencing, which recognized all the bacteria as either C. acnes or as a different Cutibacterium species. All C. acnes isolates were then phylotyped using the SLST method.
Previous studies have shown that different C. acnes phylogroup strains have a different effect on the innate immune response and pathogenic potential in inducing acne lesions (7). Phylotype 1A1 is considered an acne-associated type. Although specimens were not collected from healthy controls or healthy skin to analyse the skin microbiome in comparison with acne lesions in the current study, the phylotype distribution, consisting mainly of the 1A1 phylotype, is in correlation with previous studies (7). Other phylotypes found in our study (IA2, IB, II) have also been recovered from acneic skin, but their rates of recovery are low, again, in accordance with the current findings. The focus of future in vitro work should be on the 1A1 phylotype, as it appears to be the major pathogen related to acne.
This study used the E-test to assess the antibiotic susceptibility of 5 antibiotics used widely to treat acne in Israel. Methods used to assess the antibiotic resistance of C. acnes in previous reports, included the conventional agar dilution method (27, 30, 31, 35–38), E-test (15, 19, 24, 33, 34, 39) and disk diffusion assay (17). When using the E-test method to assess antibiotic susceptibility, there are no definite standards in breakpoints of antibiotic resistance. The same MIC interpretation criteria used for the agar dilution method were used in the current study, as previous reports have shown a high degree of agreement between these 2 methods (17).
Table II shows the different phenotypes, with various MIC values indicating low or high levels of resistance. It is possible that the different phenotypes correlate with different mutations and resistance mechanism. Molecular analysis of the resistance mechanisms is needed for further investigation.
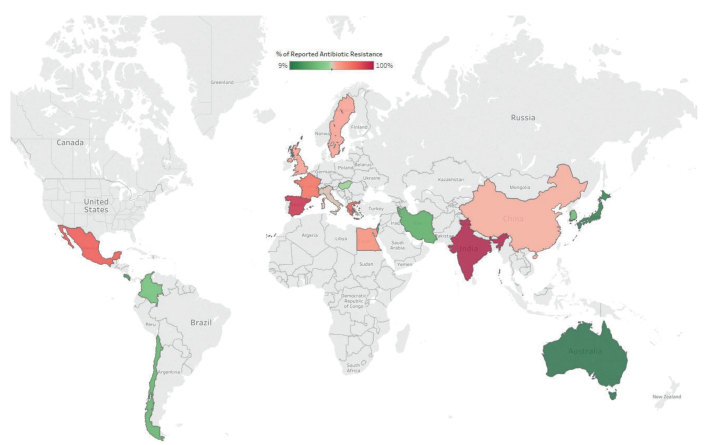
Data on the percentages of resistant C. acnes from different countries worldwide show a wide variation, as seen in Table IV and Fig. 3. The data shown here are taken from the latest publication from each country. Regional differences are remarkable and dynamic changes are also observed over time. The prevalence of carriage of resistant isolates was reported to be as high as 94% in Spain and the UK (30, 40), and 98% in India (41). In other countries, such as Korea, Japan, Chile and Australia, the reported incidence of resistance was much lower, but still demonstrated a trend towards higher MIC values over time (15, 37, 39, 42). A study from the UK showed an increase in antibiotic-resistant C. acnes strains, from 34% in 1991 to 64% in 1997 (40). A recent review describes a gradual increase in C. acnes antibiotic resistance; from 20–25% in the 1970s–1980, to 50–60% in the 1990s, reaching a peak at 75% in the early 2000 and decreasing to 30–40% within the past decade (43).
Table IV.
Reported resistance rates of Cutibacterium acnes from different countries worldwide
| Percentages of antibiotic resistant C. acnes strains isolated from patients with acne | ||||||||
|---|---|---|---|---|---|---|---|---|
| Location | Study | Year | EM | CM | TC | DC | MC | Any antibiotic |
| Israel | Our study | 2019 | 25 | 17 | 9 | 19 | 11 | 31 |
| China | Zhang et al. (34) | 2019 | 49 | 29 | 0 | 0 | ≥ 49 | |
| Singapore | Yang et al. (33) | 2018 | 31 | 33 | 22 | 22 | 0 | ≥ 33 |
| Japan | Nakase et al. (27) | 2017 | 55 | 50 | – | 5 | 0 | ≥ 55 |
| India | Biswal et al. (19) | 2016 | 8 | 11 | 9 | – | 0 | ≥ 11 |
| Greece | Giannopoulos et al. (23) | 2015 | 32 | 29 | – | 1 | 0 | ≥ 32 |
| Chile | Schafer et al. (31) | 2013 | 12 | 7 | 0 | 0 | – | 34 |
| Colombia | Mendoza et al. (25) | 2013 | 35 | 15 | 8 | 9 | 1 | 40 |
| Egypt | Abdel Fattah et al. (17) | 2013 | 48 | 65 | – | 6 | – | ≥ 66 |
| Australia | Toyne et al. (15) | 2012 | 6 | – | – | – | – | 9 |
| Korea | Moon et al. (26) | 2012 | 27 | 30 | 3 | 7 | 10 | 37 |
| France | Dumont-Wallon et al. (20) | 2010 | 75 | – | 9 | 9 | – | 75 |
| Iran | Soodabeh et al. (32) | 2011 | 15 | 43 | 7 | 2 | – | 31 |
| Mexico | Gonzalez et al. (24) | 2010 | 46 | 36 | 14 | 20 | 0 | ≥ 82 |
| Italy | Bettoli et al. (18) | 2006 | 50 | 35 | 2 | – | – | 56 |
| Turkey | Ergin et al. (21) | 2006 | 12 | 10 | 2 | 0 | – | ≥ 12 |
| Costa Rica | Rodriguez-Cavallini et al. (29) | 2004 | 19 | 23 | 19 | – | – | ≥ 23 |
| Europe | Oprica & Nord (28) | 2004 | 17 | 15 | – | – | 3 | 29 |
| Hungary | Ross et al. (30) | 2003 | 45 | 40 | 0 | – | – | 51 |
| Spain | Ross et al. (30) | 2003 | 90 | 90 | 5 | – | – | 94 |
| Sweden | Ross et al. (30) | 2003 | 45 | 45 | – | – | 15 | 60 |
| UK | Ross et al. (30) | 2003 | 50 | 50 | – | – | 25 | 60 |
| Germany | Fluhr et al. (22) | 1999 | 11 | 7 | – | 0 | 0 | ≥ 11 |
Antibiotics: EM: erythromycin; CM: clindamycin; TET: tetracycline; DOX: doxycycline; MC: minocycline.
Fig. 3.
Resistance rates of Cutibacterium acnes reported from different countries worldwide.
The reason for the difference in the in vitro antibiotic susceptibility patterns of C. acnes among different countries is not clear. It may be attributed to the different antibiotic prescribing habits, different drug availability, but also different patients’ ethnicities and different C. acnes phylogenetic strains. The lack of implementation of international treatment guidelines probably contributes to the difference in antibiotic susceptibility patterns. Also, when evaluating and comparing resistance rates, many variables should be taken into consideration, including study setting, demographic background, sampling site, sampling technique, type and severity of acne, pretreatment and definition of antibiotic resistance (43).
Resistance to erythromycin in the patients in the current study showed an “all or none” pattern (Fig. 4) with susceptible bacterial strains being highly sensitive (MIC ≤0.0.64 µg/ml) or highly resistant (MIC ≥ 256 µg/ml). This finding is in accordance with previous studies and is probably explained by the resistance mechanism determined by mutations in the 23S RNA gene or acquired ermX transposon (28). This suggests that erythromycin might still be effective in the group of patients with the non-mutated C. acnes and that does not possess the erm(X) gene.
Fig. 4.
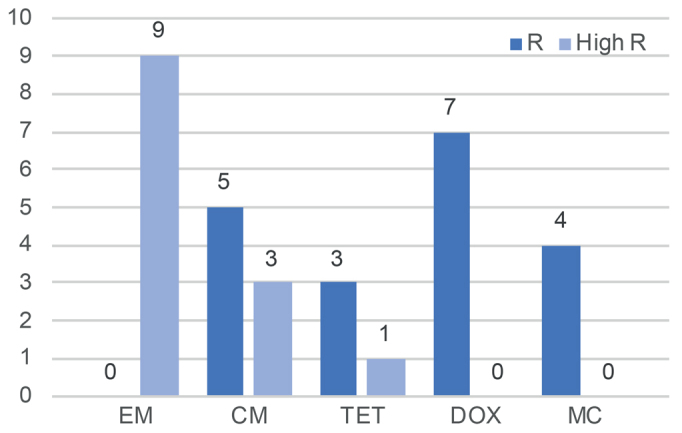
Resistant vs highly resistant isolates. R high: minimal inhibitory concentration (MIC) ≥ 256 µg/ml. R: breakpoint≤ MIC< 256 µg/ml. Antibiotics: EM: erythromycin; CM: clindamycin; TET: tetracycline; DOX: doxycycline; MC: minocycline.
The lack of statistically significant differences in MIC in regard to the characteristics of patients or acne in our study, is probably due to the small sample size. Other studies have shown a correlation between the emergence of resistant C. acnes strains and antibiotic use (15, 18, 20–23), acne severity (20–21, 24), sex (24), age (25), disease duration (25), clinical unresponsiveness and acne relapses (26).
The high degree of cross-resistance between erythromycin and clindamycin found in the current study is in agreement with previous studies (18, 27–28). An association between resistance to tetracycline, doxycycline and minocycline was also reported (29). As in the current study, cross-resistance between the MLS and cycline antibiotics has been described in a study from Hong Kong (25). Clinical implications may suggest lack of response of acne to other antibiotic treatments in a patient who did not respond to one antibiotic treatment.
The main limitation of the current study was the small sample size. In addition, the patients attending the dermatology outpatient clinic in our centre may not represent the general population, as most patients with acne are managed in community-based dermatology clinics. Another important issue is the lack of standardization in resistance cut-off points for C. acnes. Altering the cut-off points may have a significant impact on our results. Discrepancies between resistance breakpoints and methods to assess antibiotic susceptibility indicate the need for a standardized susceptibility test method for C. acnes. Taking all these limitations into consideration, we have still shown that antibiotic resistance of C. acnes in Israel is prevalent and should be taken into consideration when prescribing antibiotic treatment for acne. Other limitation is that there is no control healthy group.
In conclusion, antibiotic resistance of C. acnes represents a major concern in Israel, as it does worldwide. Further studies are recommended to assess the in vitro antibiotic resistance and variables affecting the resistance rate in a larger group of patients with acne. Measures to limit the emergence of resistant strains of C. acnes, such as limiting the use of antibiotics, should be taken, and new alternative treatments should be sought.
ACKNOWLEDGEMENTS
Funding sources. Gishur Fund of Hadassah Medical Center, George and Linda Hiltzick’s donation to Hadassah Medical Center.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol 2013; 168: 474–485. [DOI] [PubMed] [Google Scholar]
- 2.Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol 2013; 133: 2152–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dréno B, Bettoli V, Araviiskaia E, Sanchez Viera M, Bouloc A. The influence of exposome on acne. J Eur Acad Dermatol Venereol 2018; 32: 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dréno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol 2017; 31: 8–12. [DOI] [PubMed] [Google Scholar]
- 5.Ramasamy S, Barnard E, Dawson TL Jr, Li H. The role of the skin microbiota in acne pathophysiology. Br J Dermatol 2019; 181: 691–699. [DOI] [PubMed] [Google Scholar]
- 6.Lheure C, Grange PA, Ollagnier G, Morand P, Désiré N, Sayon S, et al. TLR-2 Recognizes Propionibacterium acnes CAMP Factor 1 from highly inflammatory strains. PLoS One 2016; 11: e0167237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin J, Watterson S, Layton AM, Bjourson AJ, Barnard E, McDowell A. Propionibacterium acnes and acne vulgaris: new insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms 2019; 7: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis 2016; 16: e23–33. [DOI] [PubMed] [Google Scholar]
- 9.Thiboutot DM, Dréno B, Abanmi A, Alexis AF, Araviiskaia E, Barona Cabal MI, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol 2018; 78: S1–S23.e1. [DOI] [PubMed] [Google Scholar]
- 10.Pascoe VL, Enamandram M, Corey KC, Cheng CE, Javorsky EJ, Sung SM, et al. Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol 2015; 151: 375–381. [DOI] [PubMed] [Google Scholar]
- 11.Lay JO Jr. MALDI-TOF mass spectrometry of bacteria*. Mass Spectrom Rev 2001; 20: 172–194. [DOI] [PubMed] [Google Scholar]
- 12.Patel JB. 16S rRNA gene sequencing for bacterial pathogen identification in the clinical laboratory. J Mol Diagn 2001; 6: 313–321. [DOI] [PubMed] [Google Scholar]
- 13.Scholz CF, Jensen A, Lomholt HB, Bruggemann H, Kilian M. A novel high-resolution single locus sequence typing scheme for mixed populations of propionibacterium acnes in vivo. PLoS One 2014; 9: e104199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wayne P. Performance Standards for antimicrobial susceptibility testing: twenty-seventh informational supplement M100-S27. Wayne, PA: CLSI; 2017. [Google Scholar]
- 15.Toyne H, Webber C, Collignon P, Dwan K, Kljakovic M. Propionibacterium acnes (P. acnes) resistance and antibiotic use in patients attending Australian general practice. Australas J Dermatol 2012; 53: 106–111. [DOI] [PubMed] [Google Scholar]
- 16.Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant? implications of resistance for acne patients and prescribers. Am J Clin Dermatol 2003; 4: 813–831. [DOI] [PubMed] [Google Scholar]
- 17.Abdel Fattah N, Darwish Y. In vitro antibiotic susceptibility patterns of propionibacterium acnes isolated from acne patients: an Egyptian university hospital-based study. J Eur Acad Dermatol Venereol 2013; 27: 1546–1551. [DOI] [PubMed] [Google Scholar]
- 18.Bettoli V, Borghi A, Rossi R, Ferroni M, Rigolin F, Virgili A. Antibiotic resistance of propionibacteria. Dermatology 2006; 212: 206. [DOI] [PubMed] [Google Scholar]
- 19.Biswal I, Gaind R, Kumar N, Mohanty S, Manchanda V, Khunger N, et al. In vitro antimicrobial susceptibility patterns of Propionibacterium acnes isolated from patients with acne vulgaris. J Infect Dev Ctries 2016; 10: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 20.Dumont-Wallon G, Moyse D, Blouin E, Dréno B. Bacterial resistance in French acne patients. Int J Dermatol 2010; 49: 283–288. [DOI] [PubMed] [Google Scholar]
- 21.Ergin Ç, Ergin Ş, Kaleli İ, Kaçar N, Şengül M, Erdoğan BŞ. Nasal antibiotic-resistant propionibacterium acnes carriage in acne vulgaris patients in Turkey. J Dermatol 2006; 33: 899–901. [DOI] [PubMed] [Google Scholar]
- 22.Fluhr J, Gloor M, Dietz P, Höffler U. In vitro activity of 6 antimicrobials against propionibacteria isolates from untreated acne papulopustulosa. Zentralbl Bakteriol 1999; 289: 53–61. [DOI] [PubMed] [Google Scholar]
- 23.Giannopoulos L, Papaparaskevas J, Refene E, Daikos G, Stavrianeas N, Tsakris A. MLST typing of antimicrobial-resistant propionibacterium acnes isolates from patients with moderate to severe acne vulgaris. Anaerobe 2015; 31: 50–54. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez R, Welsh O, Ocampo J, Hinojosa-Robles RM, Vera-Cabrera L, Delaney ML, et al. In vitro antimicrobial susceptibility of propionibacterium acnes isolated from acne patients in northern Mexico. Int J Dermatol 2010; 49: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 25.Mendoza N, Hernandez PO, Tyring SK, Haitz KA, Motta A. Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int J Dermatol 2013; 52: 688–692. [DOI] [PubMed] [Google Scholar]
- 26.Moon SH, Roh HS, Kim YH, Kim JE, Ko JY, Ro YS. Antibiotic resistance of microbial strains isolated from Korean acne patients. J Dermatol 2012; 39: 833–837. [DOI] [PubMed] [Google Scholar]
- 27.Nakase K, Hayashi N, Akiyama Y, Aoki S, Noguchi N. Antimicrobial susceptibility and phylogenetic analysis of Propionibacterium acnes isolated from acne patients in Japan between 2013 and 2015. J Dermatol 2017; 44: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 28.Oprica C, Nord CE, Bacteria ESGoARiA. European surveillance study on the antibiotic susceptibility of Propionibacterium acnes. Clin Microbiol Infect 2005; 11: 204–213. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Cavallini E, Vargas-Dengo P. Bacterial etiology and antibiotic susceptibility in patients with acne. Revista Biomédica 2004; 15: 101–106. [Google Scholar]
- 30.Ross J, Snelling A, Carnegie E, Coates P, Cunliffe W, Bettoli V, et al. Antibiotic-resistant acne: lessons from Europe. Br J Dermatol 2003; 148: 467–478. [DOI] [PubMed] [Google Scholar]
- 31.Schafer F, Fich F, Lam M, Gárate C, Wozniak A, Garcia P. Antimicrobial susceptibility and genetic characteristics of Propionibacterium acnes isolated from patients with acne. Int J Dermatol 2013; 52: 418–425. [DOI] [PubMed] [Google Scholar]
- 32.Soodabeh Z, Behrouz V, Hamid A. Determination of microbial agents of acne vulgaris and Propionibacterium acnes antibiotic resistance in patients referred to dermatology clinics in Kerman, Iran. Jundishapur J Microbiol 2011. [Google Scholar]
- 33.Yang S, Long V, Liau M, Lee S, Toh M, Teo J, et al. A profile of propionibacterium acnes resistance and sensitivity at a tertiary dermatological centre in Singapore. Br J Dermatol 2018; 179: 200–201. [DOI] [PubMed] [Google Scholar]
- 34.Zhang N, Yuan R, Xin KZ, Lu Z, Ma Y. Antimicrobial susceptibility, biotypes and phylotypes of clinical cutibacterium (formerly propionibacterium) acnes strains isolated from acne patients: an observational study. Dermatol Ther (Heidelb) 2019; 9: 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oprica C, Emtestam L, Lapins J, Borglund E, Nyberg F, Stenlund K, et al. Antibiotic-resistant propionibacterium acnes on the skin of patients with moderate to severe acne in Stockholm. Anaerobe 2004; 10: 155–164. [DOI] [PubMed] [Google Scholar]
- 36.Lomholt HB, Kilian M. Clonality and anatomic distribution on the skin of antibiotic resistant and sensitive Propionibacterium acnes. Acta Derm Venereol 2014; 94: 534–538. [DOI] [PubMed] [Google Scholar]
- 37.Kurokawa I, Nishijima S, Kawabata S. Antimicrobial susceptibility of propionibacterium acnes isolated from acne vulgaris. Eur J Dermatol 1999; 9: 25–28. [PubMed] [Google Scholar]
- 38.Fan Y, Hao F, Wang W, Lu Y, He L, Wang G, et al. Multicenter cross-sectional observational study of antibiotic resistance and the genotypes of propionibacterium acnes isolated from Chinese patients with acne vulgaris. J Dermatol 2016; 43: 406–413. [DOI] [PubMed] [Google Scholar]
- 39.Song M, Seo SH, Ko HC, Oh CK, Kwon KS, Chang CL, et al. Antibiotic susceptibility of propionibacterium acnes isolated from acne vulgaris in Korea. J Dermatol 2011; 38: 667–673. [DOI] [PubMed] [Google Scholar]
- 40.Coates P, Vyakrnam S, Eady EA, Jones CE, Cove JH, Cunliffe WJ. Prevalence of antibiotic-resistant propionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribution study. Br J Dermatol 2002; 146: 840–848. [DOI] [PubMed] [Google Scholar]
- 41.Sardana K, Gupta T, Kumar B, Gautam HK, Garg VK. Cross-sectional pilot study of antibiotic resistance in propionibacterium acnes strains in Indian acne patients using 16S-RNA polymerase chain reaction: a comparison among treatment modalities including antibiotics, benzoyl peroxide, and isotretinoin. Indian J Dermatol 2016; 61: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishida N, Nakaminami H, Noguchi N, Kurokawa I, Nishijima S, Sasatsu M. Antimicrobial susceptibilities of propionibacterium acnes isolated from patients with acne vulgaris. Microbiol Immunol 2008; 52: 621–624. [DOI] [PubMed] [Google Scholar]
- 43.Karadag AS, Aslan Kayıran M, Wu CY, Chen W, Parish LC. Antibiotic resistance in acne: changes, consequences and concerns. J Eur Acad Dermatol Venereol 2020. May 31. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]