Abstract
The incidence pattern of lichen planus (LP) and LP-related mortality are unknown. The aim of this study was to assess these factors, based on Finnish nationwide registry data including 13,378 women with LP diagnosed during 1969 to 2012. The incidence rate for LP in 2003 to 2012 was 28 per 100,000 woman-years age-adjusted to the European Standard Population. Mortality was assessed using the standardized mortality ratio (SMR) with national mortality rates as the reference. All-cause mortality was increased (SMR 1.07, 95% confidence interval (95% CI) 1.02–1.11), with excess mortality from Hodgkin lymphoma (SMR 6.73, 95% CI 1.83–17.2), non-Hodgkin lymphoma (SMR 1.68, 95% CI 1.11–2.44), cancer of the oral cavity (SMR 10.5, 95% CI 5.99–17.0), cancer of the tongue (SMR 7.25, 95% CI 3.13–14.3), infections (SMR 1.78, 95% CI 1.14–2.64), respiratory diseases (SMR 1.31, 95% CI 1.07–1.57), and diseases of the digestive system (SMR 1.39, 95% CI 1.09–1.75). In conclusion, LP is a common disease and patients seem to have an impaired long-term prognosis.
Key words: lichen planus, epidemiology, incidence, mortality, cause of death
Lichen planus (LP) is a multifaceted disease of the skin and mucous membranes; it presents as many different subtypes and manifests in different parts of the body.
The most frequently affected site is the mucosa of the oral cavity (1, 2), and the second most common site is the skin (3). Oral LP affects women more often than men (4), whereas cutaneous LP shows no sex predilection (3). Infrequently affected sites of LP are the vulva, the vagina, the pharynx, the larynx, and the oesophagus. Despite the heterogeneity of the phenotypes of LP, all are caused by inflammation of the skin or mucous membranes. The aetiology of the inflammation, however, is unknown.
The exact incidence of LP and its different subtypes is unknown due to the care of patients with LP spreading across different medical specialties, in both primary and specialized healthcare. The crude incidence rate of LP in primary care in the UK between 1994 and 2003 was 38 per 100,000 woman-years (5). Cutaneous LP is typically diagnosed in women in their 40s or 50s, and oral LP 10 years later (2, 3).
SIGNIFICANCE
Lichen planus is a skin disease that may affect many different cutaneous or mucous parts of the body in individuals at all ages. Little is known of its incidence and long-term prognosis. This study analysed the incidence of lichen planus in women and the mortality of lichen planus patients, based on nationwide Finnish registry data. The incidence was 28 per 100,000 woman-years. The mortality of women with lichen planus was slightly increased because of excesses in mortality from several specific disease categories. These results confirm the importance of long-term and multidisciplinary follow-up of patients with lichen planus.
Treatment of LP is usually topical, with corticosteroid preparations as the gold standard (6). The erosive subtype may respond poorly to topical treatment, and systemic immunomodulatory agents are sometimes needed.
In the long run, cutaneous LP self-limits but often later recurs, and the mucosal variant is inevitably chronic (1–3). The quality of life of patients with LP is impaired (7). Long-term complications of erosive LP are scarring and malignant transformation (8, 9). Even though LP is known to cause significant morbidity, little is known of its effects on mortality.
The aim of this study was to assess the incidence of LP in women using nationwide hospital-based data from Finland. In addition, this study assessed the all-cause and cause-specific mortality of women diagnosed with LP.
MATERIALS AND METHODS
Data for this longitudinal descriptive study of all women in Finland were gathered from nationwide registries. All Finnish citizens are given a unique personal identification code at birth or immigration, which allows for identifying them in different registries.
Women diagnosed with LP between 1969 and 2012 were retrieved from the Hospital Discharge Registry (HDR) maintained by the Finnish Institute for Health and Welfare. This registry gathers both primary and secondary inpatient diagnoses starting from 1969, and all outpatient diagnoses from public hospitals from the beginning of 1998. Diagnoses within the HDR are recorded with the International Statistical Classification of Diseases and Related Health Problems (ICD) system: ICD-8 in 1969 to 1986, ICD-9 in 1987 to 1995, and ICD-10 since 1996. Before the 1980s, the reporting of all diagnosis codes to the HDR required manual input, and from the 1980s onwards, an automated electronic system has been adopted.
Data for all women with LP as a primary or secondary diagnosis code within the HDR between 1969 and 2012 were gathered for this study. The utilized ICD codes are specified in Table SI1.
Causes of death were retrieved from the Cause-of-Death Registry of Statistics Finland, where such data are collected since 1936. After death, the physician in charge usually issues the death certificate with the ICD code for the underlying cause of death. If an autopsy is required, the forensic pathologist defines the cause of death based on the findings of the autopsy. For comparing causes of death over the years, Statistics Finland has produced a 54-category short list for time series from 1971, which was used in this study (10).
For deaths of cancer patients, data from the Finnish Cancer Registry (FCR) were used. All cancer diagnoses have been reported to this registry since 1953. In addition, the registry is informed of deaths from Statistics Finland. In the case of a death of a cancer patient, the FCR reassesses whether the death was specifically due to the cancer.
The accuracy and completeness of data in the 3 aforementioned registries has been validated in several studies (11–13).
The dates for deaths and emigration were available from the national Population Register, kept by the Digital and Population Data Services Agency. Demographic data of the Finnish female population were taken from Statistics Finland.
The calculation of the incidence of LP was restricted to the last 10 years of the study period, i.e. 2003 to 2012, to avoid inclusion of prevalent cases whose first diagnosis was before the HDR started to collect data. For mortality, all women diagnosed during 1969 to 2012 were included. Follow-up for death began at the first diagnosis of LP or on January 1, 1971 (whichever was later). Follow-up ended in emigration, death, or on 31 December 2014, whichever came first. Women, who have both been diagnosed with LP and died between 1969 and 1971, should be excluded, but there were no such women in the study cohort.
The crude incidence rate of LP was calculated by dividing the number of new diagnoses by the size of the Finnish female population each year. The incidence rates were calculated by 5-year age groups and were age-adjusted both to the European Standard 2013 Population and to the World Standard Population.
Standardized mortality ratios (SMR) for all-cause and cause-specific deaths were calculated as ratios of the observed and the expected numbers of death. The observed deaths and person-years of follow-up were stratified by 5-year age groups, calendar periods, and follow-up periods (< 5 years, ≥ 5 years since the LP diagnosis). The expected numbers of deaths were calculated by multiplying the person-years of follow-up in each stratum with the respective mortality rate in the Finnish female population. These rates were obtained from Statistics Finland for non-cancer deaths, and from the FCR for cancer deaths. For the 95% confidence intervals (CI) a Poisson distribution of the observed deaths was assumed.
Permission for this study was obtained from the Finnish Institute for Health and Welfare (THL/1440/5.05.00/2013). Permission for use of the data on the causes of death was given by Statistics Finland (TK-53-1712-17). In Finland, no ethics committee approval is required for register-based studies.
RESULTS
The whole cohort of women with LP from 1969 to 2012 consisted of 13,378 women (Table I), of whom 84% had been diagnosed in outpatient settings and 16% in inpatient settings. LP was most often the primary reason for the hospital visit for the women in the cohort: LP was a primary diagnosis code for 84% of women diagnosed in inpatient settings and for 96% of women diagnosed in outpatient settings.
Table I.
Number of patients with lichen planus by age at the beginning of follow-up, and person-years of follow-up by dynamic age
| Age | Patients n (%) | Person-years n (%) |
|---|---|---|
| 0–9 years | 51 (0.4) | 182 (0.1) |
| 10–19 years | 164 (1.2) | 943 (0.7) |
| 20–29 years | 453 (3.4) | 3,051 (2.1) |
| 30–39 years | 982 (7.3) | 7,081 (5.0) |
| 40–49 years | 2,061 (15.4) | 15,846 (11.1) |
| 50–59 years | 3,835 (28.7) | 32,468 (22.7) |
| 60–69 years | 3,334 (24.9) | 39,598 (27.7) |
| 70–79 years | 1,927 (14.4) | 29,693 (20.8) |
| > 80 years | 571 (4.3) | 14,179 (9.9) |
| Total | 13,378 (100) | 143,040 (100) |
For incidence calculations between 2003 and 2012, 7,761 women were included. Of these women, 98% were diagnosed in outpatient settings and 2% in inpatient settings. LP was a primary diagnosis code in 95% of patients.
The crude and age-adjusted incidence rates (European Standard Population) were similar and remained stabile between 2003 and 2012, at 26–31 per 100,000 woman-years (Fig. 1). The respective rate adjusted for age according to the World Standard Population fluctuated between 17 and 20 per 100,000 woman-years.
Fig. 1.
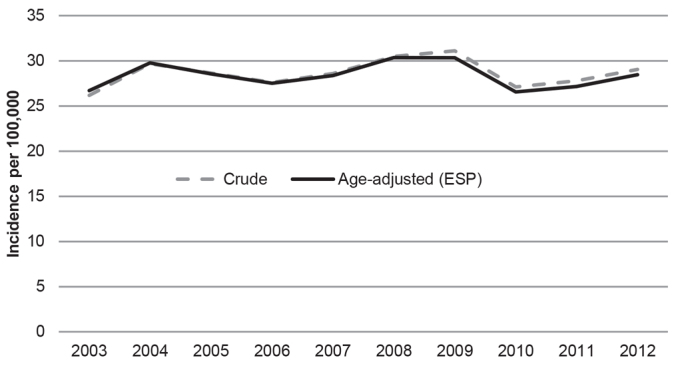
Crude and age-adjusted (European Standard Population (ESP) 2013) incidence rate of lichen planus in Finland 2003 to 2012, per 100,000 person-years.
The age-specific incidence rate was low in girls and increased slowly through the reproductive years (Fig. 2). The incidence was highest in women in the perimenopausal and postmenopausal age groups and reached its maximum (64 per 100,000 woman-years) in women aged 65 to 69 years.
Fig. 2.
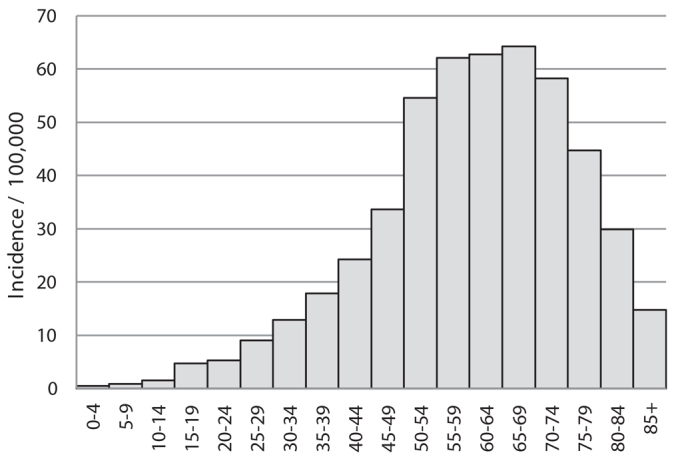
Age-specific incidence rates of lichen planus in Finland 2003 to 2012, per 100,000 person-years.
For mortality calculations the whole cohort of 13,378 patients with LP contributed altogether 143,040 person-years and a mean of 10.7 years of follow-up (Table I). Most deaths in the cohort were due to the common causes of death of women in general; circulatory diseases, malignancies, dementia and Alzheimer’s disease (Table II).
Table II.
Observed and expected numbers of deaths and standardized mortality ratios (SMR) with 95% confidence intervals (95% CI) for selected causes of death in women with lichen planus within a follow-up of more than 5 years
| Cause of death | Observed | Expected | SMR | 95% CI |
|---|---|---|---|---|
| All causes | 1,813 | 1,701 | 1.07 | 1.02–1.11 |
| All diseases | 1,742 | 1,636 | 1.06 | 1.02–1.11 |
| Infections | 24 | 13 | 1.78 | 1.14–2.64 |
| Cancer | 417 | 366 | 1.14 | 1.03–1.25 |
| Lip | 1 | 0.2 | 6.85 | 0.17–38.2 |
| Tongue | 8 | 1 | 7.25 | 3.13–14.3 |
| Oral cavity | 16 | 1.53 | 10.5 | 5.99–17.0 |
| Pharynx | 3 | 1 | 3.08 | 0.64–9.00 |
| Oesophagus | 9 | 6 | 1.61 | 0.74–3.05 |
| Larynx | 2 | 0.3 | 5.89 | 0.71–21.3 |
| Lung, trachea | 50 | 41 | 1.22 | 0.90–1.60 |
| Breast | 52 | 53 | 0.98 | 0.73–1.28 |
| Genitals | 56 | 43 | 1.29 | 0.97–1.67 |
| Cervix uteri | 2 | 4 | 0.53 | 0.06–1.89 |
| Corpus uteri | 14 | 12 | 1.18 | 0.65–1.98 |
| Ovary | 28 | 21 | 1.31 | 0.87–1.89 |
| Vulva | 3 | 2 | 1.34 | 0.28–3.91 |
| Vagina | 2 | 0.7 | 2.75 | 0.33–9.92 |
| Skin, non-melanoma | 2 | 1 | 1.61 | 0.19–5.80 |
| Non-Hodgkin lymphoma | 27 | 16 | 1.68 | 1.11–2.44 |
| Hodgkin lymphoma | 4 | 0.6 | 6.73 | 1.83–17.2 |
| Endocrine, nutritional and metabolic diseases | 27 | 22 | 1.25 | 0.82–1.81 |
| Diabetes mellitus | 22 | 18 | 1.22 | 0.76–1.83 |
| Dementia, Alzheimer’s disease | 223 | 232 | 0.96 | 0.84–1.09 |
| Diseases of circulatory system | 766 | 761 | 1.01 | 0.94–1.07 |
| Ischaemic heart diseases | 429 | 403 | 1.07 | 0.97–1.16 |
| Other heart diseases | 76 | 73 | 1.04 | 0.82–1.29 |
| Cerebrovascular diseases | 185 | 198 | 0.94 | 0.81–1.07 |
| Diseases of respiratory system | 105 | 80 | 1.31 | 1.07–1.57 |
| Bronchitis, emphysema | 35 | 22 | 1.57 | 1.09–2.17 |
| Diseases of digestive system | 73 | 52 | 1.39 | 1.09–1.75 |
| Diseases of genitourinary system | 28 | 19 | 1.44 | 0.96–2.08 |
| Alcohol related diseases and accidental poisoning by alcohol | 11 | 19 | 0.59 | 0.30–1.06 |
| Accidents and violence | 68 | 62 | 1.09 | 0.85–1.38 |
| Suicide | 9 | 9 | 1.00 | 0.46–1.90 |
The SMRs were invariably lower within the first 5 years of follow-up than later on (Table SII1). This indicates a healthy patient effect, and therefore, only the mortality figures of follow-up of more than 5 years are presented in Table II.
The all-cause mortality of women with LP was slightly increased (SMR 1.07, 95% CI 1.02–1.11) (Table II). The excess mortality arose from infections (SMR 1.78, 95% CI 1.14–2.64), respiratory diseases (SMR 1.31, 95% CI 1.07–1.57), bronchitis and emphysema (SMR 1.57, 95% CI 1.09–2.17), diseases of the digestive system (SMR 1.39, 95% CI 1.09–1.75), and cancers (SMR 1.14, 95% CI 1.03–1.25) (Table II). There was excess mortality from cancers of the oral cavity (SMR 10.5, 95% CI 5.99–17.0) and tongue (SMR 7.25, 95% CI 3.13–14.3), and from non-Hodgkin (SMR 1.68, 95% CI 1.11–2.44) and Hodgkin lymphoma (SMR 6.73, 95% CI 1.83–17.2).
DISCUSSION
In this nationwide study, the incidence of LP remained constant during 2003 to 2012: 28 per 100,000 woman-years. LP diagnoses were rare in children, and the highest incidence was observed within postmenopausal age groups. The mortality of women with LP was somewhat higher than in the general female population due to excess mortality from respiratory diseases, lymphomas, cancers of the oral cavity and tongue, infections, and diseases of the digestive system.
A strength of this study is its data. Finnish registries have gathered data from the whole population for a long period of time. The registries are continuously evaluated for data accuracy and completeness (11–13). The current study gained access to a large number of patients with LP and enabled data for the women to be merged between registries.
However, registry data also entail limitations. The diagnosis codes used for LP do not specify the subtype or location of the disease. LP is a heterogeneous disease group with different phenotypes, and mortality may vary between them.
Furthermore, some of the diagnoses of LP may be incorrect. Diagnosing LP poses a challenge: many different diseases may mimic LP clinically. Moreover, the histological picture may be unspecific or highly variable. To overcome this problem, several diagnostic criteria for LP have been presented over the years. Some allow a clinical-only diagnosis, but recommend a biopsy in uncertain cases (14). Others require both typical clinical picture and confirmatory histology for diagnosis (6, 15–17). Within registry data, the diagnostic procedure is not recorded. In the current cohort, there may be patients who have been clinically misdiagnosed or miscoded as having LP. Even so, we assume them to be few, because the LP diagnoses in the data have been made by specialists in secondary or tertiary care.
The study cohort includes patients hospitalized for LP from 1969 and patients treated in outpatient settings in hospitals from 1998. Therefore, the study cohort does not represent all women diagnosed in Finland with LP. The mildest cases of LP diagnosed and treated only in the primary healthcare are lacking in this study. The manual extraction and input of data into the HDR in the beginning of the study period may have been incomplete, leading to more patients missing. Therefore, the incidence rates do not include all cases of LP, and the SMR results may not be generalizable to all patients with LP.
The data available for this study do not include information on several factors known to affect mortality, such as smoking, alcohol consumption, body mass index, physical inactivity, diet, socioeconomic status, and parity. It is difficult to estimate whether the prevalence of those factors differs among patients with LP and the general population and therefore could cause bias in the SMRs.
We do not know how patients in our cohort were treated. Systemic treatment, such as corticosteroids or methotrexate, could affect mortality. In psoriasis and psoriatic arthritis, methotrexate has been suggested to lower the risk of cardiovascular disease (18). In contrast, an increased risk of cardiovascular events was observed among users of oral corticosteroids in a population-based case-control study (19). Evidence in LP is lacking.
Previous research on the incidence of LP is scarce and done with small populations. In the UK, the crude incidence of any LP between 1994 and 2003 was 38 per 100,000 woman-years, with data collected from a pool of patients observed by sentinel general practitioners (5). This is not comparable to our incidence rate because our cohort is based on patients diagnosed in specialized healthcare. The incidence of oral LP has varied greatly in 3 studies: it was 14 per 100,000 woman-years calculated from health registers of Olmsted county, Minnesota in the USA, 188 per 100,000 in a screening programme in a Japanese city, and 250 per 100,000 among Indian villagers (20–22). None of these rates are comparable to ours.
In previous literature, the mean age at the onset of symptoms or at diagnosis of LP has been 57–61 years for oral LP and 46 years for cutaneous LP (2, 3, 23). The current cohort includes patients with any LP and the mean age at diagnosis (57 years) is thus comparable to the previous estimates.
The lack of research on the mortality of patients with LP is pronounced. An older study compared the percentages of causes of death among 50 patients with LP to several reference populations (24). Numbers of deaths in specific cause-of-death categories were small, and no systematic differences in mortality patterns of patients with LP and referents were found.
The cause for the elevated mortality in the LP cohort from diseases of many organ systems (infections, respiratory diseases, digestive diseases) and from different cancers (cancer of the oral cavity and tongue, and lymphomas) remains ambiguous.
The increased mortality from infections could arise from the use of systemic immunosuppressing medications for treatment, whereas the increased mortality from respiratory diseases, mainly form bronchitis and emphysema, most likely relates to patients with LP smoking more than the general population. The relationship of LP and smoking is controversial, but because mortality from lung cancer and respiratory diseases was increased in this study, we hypothesize that patients with LP smoke more than the general population.
Chronic inflammation may promote development of cancer, and several inflammatory diseases are known to increase the risk of cancer in organs where inflammation is present (25). We demonstrated this in LP in a previous study: the risks of cancers of the mouth, larynx, oesophagus, and vulva were increased (9). Therefore, the increased mortality from cancers of the oral cavity and tongue in this study was expected.
Some inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus, have been linked to increased risk of haematological malignancies due to systemic dysregulation of the immune system (25, 26). In this study, the mortality from non-Hodgkin and Hodgkin lymphomas was increased in women with LP. An increased risk of lymphomas has been observed in patients with psoriasis, another skin condition characterized with local inflammation (27). In psoriasis, inflammation may span from a local to a systemic level (28), and therefore, it is tempting to speculate, that, in addition to local inflammation, LP also causes systemic alterations in the immune system. Another explanation for the link between LP and lymphomas could be shared predisposing factors.
Increased mortality from cancers of the oral cavity and tongue in the LP cohort could, in part, be explained by excess consumption of alcohol and smoking, well-known risk factors for these cancers. Alcohol consumption among the patients with LP is probably not high, since mortality from alcohol-related causes was reduced. However, as stated above, smoking may be more common among the women in this cohort.
This nationwide study of women diagnosed with LP in hospital settings shows a stabile incidence of LP, with a majority of diagnoses made among postmenopausal age groups. Excess mortality from diseases of some organ systems and cancers among these women is suggested. Therefore, the need for multidisciplinary treatment and follow-up is further emphasized.
ACKNOWLEDGEMENTS
Conflicts of interest. Oskari Heikinheimo has received personal fees from Bayer Health Care AG, Gedeon-Richter, Sandoz AG, HRA-Pharma, and from Vifor Pharma, outside the submitted work. The other authors have no conflicts of interest to declare.
Footnotes
REFERENCES
- 1.Silverman S, Jr, Gorsky M, Lozada-Nur F. A prospective follow-up study of 570 patients with oral lichen planus: persistence, remission, and malignant association. Oral Surg Oral Med Oral Pathol 1985; 60: 30–34. [DOI] [PubMed] [Google Scholar]
- 2.Carbone M, Arduino PG, Carrozzo M, Gandolfo S, Argiolas MR, Bertolusso G, et al. Course of oral lichen planus: a retrospective study of 808 northern Italian patients. Oral Dis 2009; 15: 235–243. [DOI] [PubMed] [Google Scholar]
- 3.Irvine C, Irvine F, Champion RH. Long-term follow-up of lichen planus. Acta Derm Venereol 1991; 71: 242–244. [PubMed] [Google Scholar]
- 4.Axell T, Rundquist L. Oral lichen planus – a demographic study. Community Dent Oral Epidemiol 1987; 15: 52–56. [DOI] [PubMed] [Google Scholar]
- 5.Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect 2005; 133: 985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ioannides D, Vakirlis E, Kemeny L, Marinovic B, Massone C, Murphy R, et al. European S1 guidelines on the management of lichen planus: a cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol 2020; 34: 1403–1414. [DOI] [PubMed] [Google Scholar]
- 7.Van Cranenburgh OD, Nijland SB, De Korte J, Lindeboom R, De Rie MA, Ter Stege JA, et al. Satisfaction with treatment and health-related quality of life among patients with lichen planus: a web-based survey. Eur J Dermatol 2016; 26: 113–116. [DOI] [PubMed] [Google Scholar]
- 8.Setterfield JF, Neill S, Shirlaw PJ, Theron J, Vaughan R, Escudier M, et al. The vulvovaginal gingival syndrome: a severe subgroup of lichen planus with characteristic clinical features and a novel association with the class II HLA DQB1*0201 allele. J Am Acad Dermatol 2006; 55: 98–113. [DOI] [PubMed] [Google Scholar]
- 9.Halonen P, Jakobsson M, Heikinheimo O, Riska A, Gissler M, Pukkala E. Cancer risk of lichen planus: a cohort study of 13,100 women in Finland. Int J Cancer 2018; 142: 18–22. [DOI] [PubMed] [Google Scholar]
- 10.Official Statistics of Finland (OSF): Causes of death. ISSN=1799-5078. Helsinki: Statistics Finland. [accessed 2020 April 14].Available from: http://www.stat.fi/til/ksyyt/ksyyt_2018-11-12_luo_001_en.html. [Google Scholar]
- 11.Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health 2012; 40: 505–515. [DOI] [PubMed] [Google Scholar]
- 12.Lahti RA, Penttila A. The validity of death certificates: routine validation of death certification and its effects on mortality statistics. Forensic Sci Int 2001; 115: 15–32. [DOI] [PubMed] [Google Scholar]
- 13.Pukkala E, Engholm G, Hojsgaard Schmidt LK, Storm H, Khan S, Lambe M, et al. Nordic Cancer Registries – an overview of their procedures and data comparability. Acta Oncol 2018; 57: 440–455. [DOI] [PubMed] [Google Scholar]
- 14.Simpson RC, Thomas KS, Leighton P, Murphy R. Diagnostic criteria for erosive lichen planus affecting the vulva: an international electronic-Delphi consensus exercise. Br J Dermatol 2013; 169: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer IR, Lucas RB, Pindborg JJ, Sobin LH. Definition of leukoplakia and related lesions: an aid to studies on oral pre-cancer. Oral Surg Oral Med Oral Pathol 1978; 46: 518–539. [PubMed] [Google Scholar]
- 16.van der Meij EH, van der Waal I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med 2003; 32: 507–512. [DOI] [PubMed] [Google Scholar]
- 17.Cheng YS, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 122: 332–354. [DOI] [PubMed] [Google Scholar]
- 18.Horreau C, Pouplard C, Brenaut E, Barnetche T, Misery L, Cribier B, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol 2013; 27: 12–29. [DOI] [PubMed] [Google Scholar]
- 19.Souverein PC, Berard A, Van Staa TP, Cooper C, Egberts ACG, Leufkens HGM, et al. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart 2004; 90: 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, Ma JE, Mara KC, Weaver AL, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019; 58: 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagao T, Ikeda N, Fukano H, Hashimoto S, Shimozato K, Warnakulasuriya S. Incidence rates for oral leukoplakia and lichen planus in a Japanese population. J Oral Pathol Med 2005; 34: 532–539. [DOI] [PubMed] [Google Scholar]
- 22.Bhonsle RB, Pindborg JJ, Gupta PC, Murti PR, Mehta FS. Incidence rate of oral lichen planus among Indian villagers. Acta Derm Venereol 1979; 59: 255–257. [PubMed] [Google Scholar]
- 23.Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol 2002; 46: 207–214. [DOI] [PubMed] [Google Scholar]
- 24.Anonide A, Rebora A. What lichen planus patients die of. A retrospective study. Int J Dermatol 1989; 28: 524–526. [DOI] [PubMed] [Google Scholar]
- 25.Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 2012; 32: 1119–1136. [PMC free article] [PubMed] [Google Scholar]
- 26.Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev 2017; 16: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 27.Pouplard C, Brenaut E, Horreau C, Barnetche T, Misery L, Richard MA, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol 2013; 27: 36–46. [DOI] [PubMed] [Google Scholar]
- 28.Reich K. The concept of psoriasis as a systemic inflammation: implications for disease management. J Eur Acad Dermatol Venereol 2012; 26: 3–11. [DOI] [PubMed] [Google Scholar]


