Abstract
Formation of neutrophil extracellular traps has been implicated in autoimmunity. However, the presence and clinical relevance of neutrophil extracellular traps in immune-complex-mediated cutaneous small and medium vessel vasculitides has not been investigated. This study retrospectively analysed 72 patients with histology-proven hypersensitivity vasculitis (n = 21), IgA vasculitis (n = 22), urticarial vasculitis (n = 22), erythema elevatum diutinum (n = 3) and polyarteritis nodosa (n = 4). Neutrophil extracellular traps were detected in hypersensitivity vasculitis, IgA vasculitis, urticarial vasculitis and erythema elevatum diutinum, but not in polyarteritis nodosa lesions. Neutrophil extracellular traps were found around inflamed vessels, and their formation was highest early after the onset of vasculitis and decreased progressively thereafter. Neutrophil extracellular traps were strongly correlated with the histological severity of vasculitis and the production of reactive oxygen species. Both hypersensitivity vasculitis and IgA vasculitis showed significantly more neutrophil extracellular traps than did urticarial vasculitis, independent of the histological severity and duration of vasculitis. These results provide evidence on the implication of neutrophil extracellular traps in the early phases of immune-complex-mediated small vessel vasculitis.
Key words: neutrophil extracellular traps, cutaneous small vessel vasculitis, reactive oxygen species, tissue damage
Vasculitis refers to a specific pattern of inflammation of the blood vessel walls. It can affect any organ system of the body, including the skin (1). Cutaneous vasculitis exclusively affects small vessels (in the superficial and mid-dermis) and medium-sized vessels (in the deep dermis or subcutis). Small vessel vasculitis (SVV) is broadly categorized based on its pathogenesis; whether it involves immune-complex (IC) deposition or antibody-mediated cytotoxicity (anti-neutrophil cytoplasmic antibody (ANCA) vasculitis). Leukocytoclastic vasculitis (LCV) is the histopathological term that refers to SVV, in which the inflammatory infiltrate is composed predominantly of neutrophils, which disintegrate releasing nuclear debris, a process named leukocytoclasia (1). LCV occurs due to the deposition of excess IC in post-capillary venules. The IC precipitates into the vessels, fixes complement, activates both complement pathways and leads to an intense immune reaction (2). Complement activation recruits neutrophils, which attempt to engulf the IC. Neutrophils degranulate and release free oxygen radicals, vasoactive amines and lysosomal proteolytic enzymes, which cause damage to the vessel wall (3, 4). Hypersensitivity vasculitis (HV) (2, 5), IgA vasculitis (6) and urticarial vasculitis (UV) (7–9) are acute SVV, and erythema elevatum diutinum (EED) is a chronic SVV (1, 10), which all share IC deposition as a central role in their pathogenesis, and LCV in their histology. Cutaneous polyarteritis nodosa (PAN) is predominantly a medium-sized vessel vasculitis. Its precise aetiology remains unknown; however, IC deposition plays a role in its pathogenesis (11). In contrast, in ANCA vasculitis, vessel damage is directly mediated by neutrophils rather than by IC deposition, and is therefore referred to as “pauci-immune” vasculitis (12).
SIGNIFICANCE
Neutrophils fight microbes by releasing neutrophil extracellular traps, which are DNA tracts studded with antimicrobial peptides. Neutrophil extracellular traps are implicated in autoimmunity. However, their presence and relevance in immune-complex-mediated cutaneous vasculitis is unknown. This study showed that neutrophil extracellular traps are found in the various immune-complex-mediated vasculitides: hypersensitivity vasculitis, IgA vasculitis, urticarial vasculitis, and erythema elevatum diutinum; and their level of production correlates with the severity of vasculitis and the amount of reactive oxygen species produced. Drugs targeting neutrophil extracellular traps are currently under development and might eventually be useful for treatment of difficult-to-treat chronic recurrent small vessel vasculitis, IgA vasculitis relapses, and the notoriously chronic and disfiguring erythema elevatum diutinum.
A major discovery in the last decade was the identification of neutrophil extracellular traps (NETs): a mechanism by which neutrophils externalize a fibrous network of web-like chromatin strands studded with antimicrobial peptides and histones (13). To release these NETs, activated neutrophils undergo NETosis, which is a form of cell death (14) dependent on the formation of reactive oxygen species (ROS) and on NADPH oxidase and myeloperoxidase (15–18). In recent years, NETs have been found to be implicated in immune defence, auto-inflammation and auto-immunity, such as systemic lupus erythematosus (SLE) (19, 20), rheumatoid arthritis (RA) (21), ANCA vasculitis (22) and Behçet’s disease (18). NETs cause tissue damage; with histones, major components of NETs, playing a predominant role in endothelium injury (18, 23, 24). In ANCA vasculitis, NETs provide a scaffold for alternative complement pathway activation, which, in turn, contributes to endothelial cell damage (25). On the other hand, IC trigger NETs in autoimmune diseases, such as RA (26). To date, NETs have not been described in IC-mediated cutaneous vasculitis. Therefore, the aim of this study was to investigate the presence and clinical relevance of NETs in cutaneous small and medium vessel vasculitides that share IC deposition in their pathogenesis.
PATIENTS AND METHODS
Patients
After obtaining approval for the study from the Institutional Review Board of the American University of Beirut, a pathology database search was performed to identify cohort cases with SVV, including HV, IgA vasculitis (previously Henoch-Schonlein purpura (27)), UV, EED and cutaneous PAN diagnosed at the Department of Dermatology of the American University of Beirut Medical Center from January 1992 to June 2018. For HV and IgA, only cases which had had both haematoxylin and eosin stain (H&E) and direct immunofluorescence stain (DIF) performed were included in the study. Reviewed data included age at diagnosis, sex, clinical history of constitutional symptoms and systemic involvement, preceding infection and drug intake in the 4 weeks prior to diagnosis. Aetiology and systemic involvement were determined based on clinical, laboratory findings and physician notes.
Immunofluorescence and dihydroethidium staining
For DIF, skin specimens were soaked in 0.9% saline solution and processed immediately. The presence of IgG, IgA, IgM, C3 and/or fibrinogen deposits was evaluated on cryosections using anti-IgG (Dako F0202; Dako, DAKO/Agilent Technologies Inc., Santa Clara, CA, USA), anti-IgA (Dako F0204), anti-IgM (Dako F0203), anti-C3 (Dako F0201) and anti-Fibrinogen (Dako F0111), as described by the manufacturer. For elastase and citrullinated histone 3 (His-3-cit) single immunolabelling, formalin-fixed paraffin-embedded (FFPE) tissues were collected and 4-μm thick sections of tissues were mounted on glass slides. After deparaffinization in xylol, the slides were hydrated and then heat-mediated antigen retrieval was performed with sodium citrate buffer (for elastase) or Tris-EDTA buffer (for His-3-cit) for 15 min. Tissues were blocked with 3% normal goat serum (NGS, Nippon Chemi-Con, Japan) in phosphate-buffered saline (PBS) for 1 h. Incubation with anti-neutrophilic elastase (5 µg/ml, Abcam, Cambridge, UK, #ab68672) and anti-His-3-cit (10 µg/ml, Abcam, #ab5103) diluted in 1% NGS was performed overnight at 4°C. Slides were washed and incubated with anti-rabbit IgG-Alexa Fluor 488 secondary antibody (1 µg/ml, Invitrogen) for 1 h at room temperature. For elastase and His-3-cit double immunolabelling, FFPE tissues were co-stained with anti-neutrophilic elastase (10 µg/ml, sc-53388) and anti-His-3-cit (10 µg/ml, Abcam, #ab5103). After washes, tissues were incubated with anti-rabbit IgG-Alexa Fluor 488 (1 µg/ml, Invitrogen, Carlsbad, CA, USA) and anti-mouse IgG-Alexa Fluor 594 (1 µg/ml, Invitrogen) secondary antibodies. Nuclei were stained using Hoechst 33342 (0.5 mg/ml, Molecular probes, Eugene, Oregon, USA) for 10 min in the dark. Slides were washed and mounted using ProLong anti-fade (Thermo Fisher Scientific, Waltham, MA, USA) and observed under fluorescence microscopy (Zeiss, Oberkochen, Germany).
To assess the presence of ROS, FFPE specimens were cut into 4-μm sections and mounted on slides. After deparaffinization, tissues were incubated with dihydroethidium (DHE, 10 μM, Sigma, Saint Louis, Missouri, USA) for 30 min at 37ºC in a humidified chamber and protected from light. Images were captured using fluorescence microscopy.
Scoring and quantification
The histological sections of all cases were reviewed (CB) and the diagnoses were confirmed by a dermatopathologist (OA). Histological definition of LCV involved a predominant neutrophilic infiltrate, primarily affecting superficial post-capillary venules, with fibrinoid deposits in and around the vessel wall, endothelial swelling and extravasation of red blood cells (28). In order to assess the extent of inflammation, a score was given to each vasculitis case according to the density of the neutrophilic infiltrate and leukocytoclasia, as well as the extent of fibrinoid necrosis found on H&E-stained slides (1: mild, 2: moderate, 3: severe). This is referred to as the vasculitis score in the current paper. Semi-quantitative scores are frequently and routinely used for histopathology quantification. This score has been used extensively in research and in the clinical setting in all sorts of pathologies, including cutaneous ones (29–31). A distinct score for elastase, His-3-cit and DHE staining was given to each case according to the density of immunofluorescence (0: none; 1: low; 2: medium; 3: high). The investigators were blinded to the vasculitis score of each case while assessing the immunofluorescence.
Statistical analysis
Statistical analyses were performed using SPSS (Chicago, IL, USA) Version 25.0 software package. Ordinal variables are presented as median and interquartile range. Age, the only continuous variable, is presented as mean ±standard deviation (SD). Data were considered statistically significant if p < 0.05. A χ2 test was used to assess the relationship between categorical variables. One-way ANOVA was used for age, with Tukey for post hoc analysis and Kruskal–Wallis 1-way ANOVA for ordinal variables with pairwise comparisons using the Dunn-Bonferroni for post hoc analysis. Ordinal logistic regression model was built to assess the relationship between the His-3-cit score and vasculitis type, after adjustment for the vasculitis score and duration of the vasculitis as possible confounding variables. Spearman’s rho was used for the correlation between ordinal variables.
RESULTS
Patients’ characteristics
A total of 43 cases of cutaneous SVV cases with both H&E and DIF performed were found, 21 of which had non-specific DIF and were referred to as HV, and 22 of which had IgA immunoreactivity and were referred to as IgA vasculitis. Sixty-two cases of UV were found, 22 of which were randomly selected for inclusion in the study. There were 3 cases of EED and 4 of PAN. For statistical analysis, we only included cases of acute SVV (HV, IgA vasculitis and UV); and only descriptive analysis was performed for EED and PAN cases due to their small sample size. Patient characteristics are described in Table I.
Table I.
Baseline characteristics of patients with vasculitis
| HV n = 21 | IgA vasculitis n = 22 | UV n = 22 | EED n = 3 | PAN n = 4 | p-value* | |
|---|---|---|---|---|---|---|
| Age, years, mean ± SD | 39.4 ± 22.4 | 27.9 ± 25.5 | 46.8 ± 16.1 | 23.3 ± 21.8 | 31 ± 9.0 | IgA vs. UV 0.018, LCV vs. IgA 0.226, LCV vs. UV 0.506 |
| Age, years, median | 43 | 16 | 45 | 19 | 31.5 | |
| Sex, n (%)a | ||||||
| Male | 13 (61.9) | 12 (54.5) | 2 (9.1) | 1 (33.3) | 1 (25) | UV vs. LCV 0.000 and UV vs. IgA 0.000 |
| Female | 8 (38.1) | 8 (36.4) | 20 (90.9) | 2 (66.7) | 3 (75) | UV vs. LCV 0.000 and UV vs. IgA 0.000 |
| Duration, n (%)a | ||||||
| < 1 week | 5 (26.3) | 6 (27.3) | 1 (4.5) | 0 (0) | 0 (0) | |
| 1–4 weeks | 7 (36.8) | 10 (45.5) | 3 (13.6) | 0 (0) | 1 (25) | |
| 1–6 months | 4 (21.1) | 2 (9.1) | 7 (31.8) | 0 (0) | 1 (25) | |
| > 6 months | 3 (15.8) | 2 (9.1) | 11 (50) | 3 (100) | 2 (50) | |
| Direct immunofluorescence, n (%) | ||||||
| C3 | 5 (23.8) | 7 (31.8) | – | – | – | 0.558 |
| IgG | 1 (4.8) | 3 (13.6) | – | – | – | 0.317 |
| IgM | 4 (19) | 5 (22.7) | – | – | – | 0.767 |
| IgA | 0 (0) | 22 (100) | – | – | – | 0.000 |
| Fibrinogen | 6 (28.6) | 2 (10.0) | – | – | – | 0.101 |
| 0 | 7 (33.3) | 0 (0) | – | – | – | 0.001 |
| Systemic involvement, n (%) | 7 (33.3) | 16 (80) | – | – | – | 0.003 |
| Joint involvement | 4 (19) | 9 (45) | – | – | – | 0.074 |
| Renal | ||||||
| Nephrotic range proteinuria | 2 (9.5) | 4 (20) | – | – | – | 0.343 |
| Haematuria | 3 (14.3) | 2 (10.0) | – | – | – | 0.675 |
| Gastrointestinal | ||||||
| Abdominal pain | 3 (14.3) | 8 (40) | – | – | – | 0.063 |
| Haematochezia | 2 (9.5) | 4 (20) | – | – | – | 0.343 |
| Aetiology, n (%) | ||||||
| Idiopathic | 6 (28.6) | 8 (40) | – | – | – | 0.440 |
| Infection | 6 (28.6) | 7 (35) | – | – | – | 0.658 |
| Drug | 5 (23.8) | 5 (20) | – | – | – | 0.768 |
| Malignancy | 1 (4.8) | 0 (0) | – | – | – | 0.323 |
| Autoimmune | 3 (14.3) | 1 (5) | – | – | – | 0.317 |
2 missing (9.1).
p-value for difference between groups by x2 test for proportions, 1-way analysis of variance (ANOVA) for continuous variables and Kruskal–Wallis 1-way ANOVA for ordinal variables
SD: standard deviation; HV: hypersensitivity vasculitis; UV: urticarial vasculitis; EED: erythema elevatum diutinum; PAN: polyarteritis nodosa; LCV: leukocytoclastic vasculitis.
Hypersensitivity vasculitis and IgA vasculitis form more neutrophil extracellular traps than urticarial vasculitis
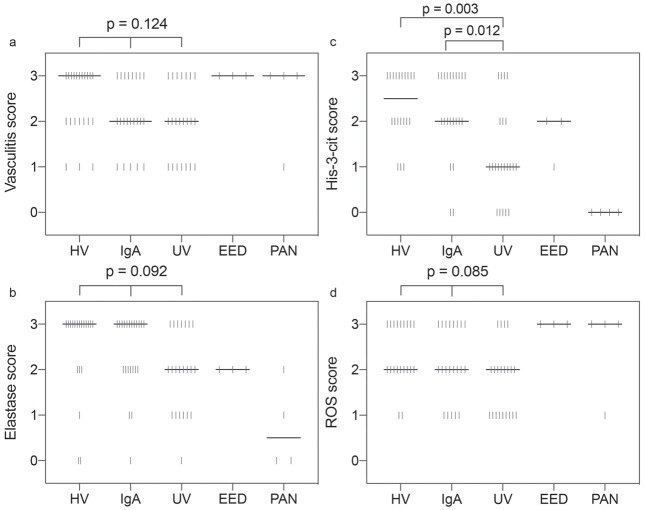
Lesions of HV had a median vasculitis score of 3 on H&E sections, whereas IgA vasculitis and UV lesions had a median vasculitis score of 2. This difference was not statistically significant (p = 0.124) (Figs 1, and 2a).
Fig. 1.
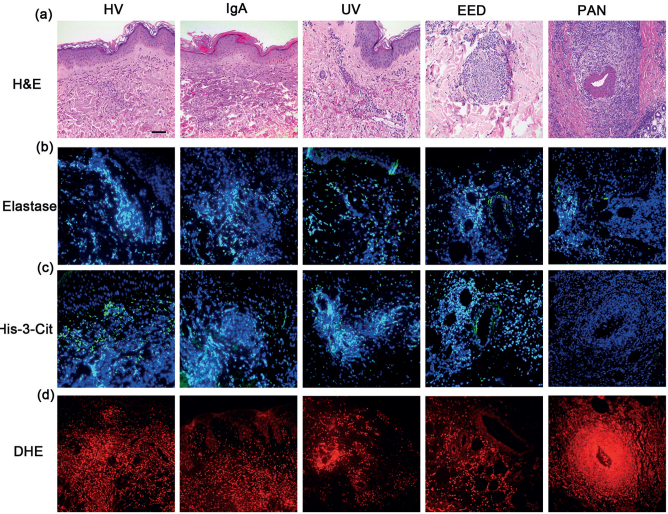
Histological characterization of cutaneous immune complex (IC)-mediated vasculitis, including hypersensitivity vasculitis (HV), IgA, urticarial vasculitis (UV), erythema elevatum diutinum (EED) and polyarteritis nodosa (PAN). (a) Haematoxylin and eosin staining; (b) immunostaining of elastase merged with Hoechst 33342; (c) immunostaining of His-3-cit merged with Hoechst 33342; (d) DHE staining. Scale bar: 25 µm.
Fig. 2.
Scatter dot plot – score distribution according to vasculitis type. Each vertical bar represents a single observation. The median is represented by the thick horizontal line. (a) Vasculitis score, (b) elastase score, (c) His-3-cit score, (d) reactive oxygen species (ROS) score. p-value for difference between groups obtained using Kruskal–Wallis 1-way analysis of variance (ANOVA) for ordinal variables with pairwise comparisons using the Dunn-Bonferroni for post hoc analysis. HV: hypersensitivity vasculitis; UV: urticarial vasculitis; EED: erythema elevatum diutinum; PAN: polyarteritis nodosa.
Histone citrullination, which plays a key role in chromatin decondensation and NETs formation, was used as a marker of NETs (32). Double immunolabelling showed that, when present, Hist-3-cit co-localizes with elastase and extracellular DNA, which is a hallmark of NETs (Fig. S11).
HV and IgA vasculitis lesions had a median elastase staining score of 3, whereas UV lesions had a median score of 2. This difference was not statistically significant (p = 0.092) (Figs 1, 2b).
Interestingly, HV displayed the highest His-3-cit score, with a median of 2.5 compared with a median of 2 for IgA vasculitis and 1 for UV. Both HV and IgA vasculitis were found to have a significantly higher His-3-cit score than UV (p =0.003 and p =0.012, respectively); however, there was no statistically significant difference between scores for HV and IgA vasculitis (p = 1.000) (Figs 1, 2c).
Neutrophil extracellular traps are formed mainly around the inflamed vessels
In addition to the differences in His-3-cit scores among all vasculitis groups, there were also differences in the distribution pattern of this protein. In HV and IgA vasculitis the His-3-cit distribution was both superficial and deep dermal perivascular, whereas the distribution in UV was predominantly superficial perivascular (Fig. 1, Table SI1). This distribution follows that of the vasculitic changes in the corresponding vasculitides. The distribution pattern of His-3-cit was co-localized to the elastase staining (Fig. 1, Table SI1). These results highlight the role of NETs in endothelial damage in IC-mediated vasculitis.
Reactive oxygen species are produced around the inflamed vessels in cutaneous vasculitides
Production of ROS is essential for activation of neutrophils and subsequent release of NETs and tissue damage (18). Using DHE staining, we investigated the presence of ROS in IC-mediated cutaneous SVV to correlate it with the extent of vasculitis and NETs formation. Both HV and IgA vasculitis had a median score of 2, higher than the UV median score of 1, although this difference was not statistically significant (p = 0.085) (Figs 1, 2d). The pattern of distribution of ROS was mainly perivascular and mimicked that of His-3-cit and elastase (Fig. 1, Table SI1).
Formation of neutrophil extracellular traps is strongly correlated with vasculitis score and production of elastase and reactive oxygen species
The His-3-cit score was positively and strongly correlated with vasculitis score (R =0.618, p =0.000), elastase score (R = 0.726, p =0.000) and ROS score (R =0.767, p = 0.000). The last 2 correlations remained strong even after adjusting for the vasculitis score (adjusted elastase score (R =0.627, p =0.000) and ROS score (R =0.627, p = 0.000)).
Formation of neutrophil extracellular traps is highest early after the onset of vasculitis and decreased progressively thereafter
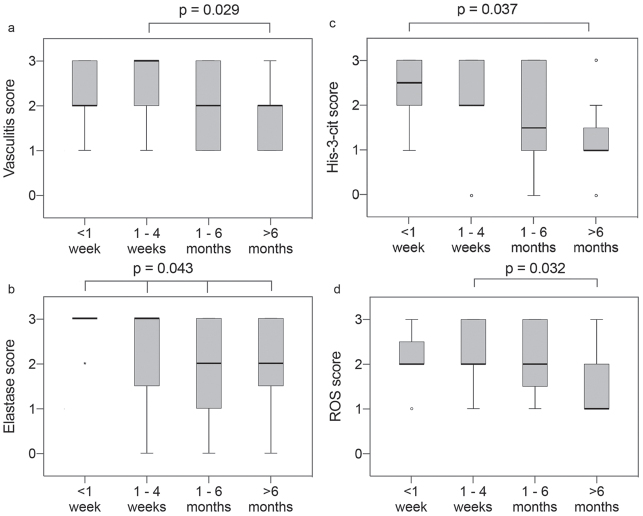
Formation of NETs was dependent on the biopsy timing; the earlier the biopsy was taken after onset of vasculitis, the more NETs were detected. Cases from vasculitides that had started less than one week before the biopsy had a median His-3-cit score of 2.5, compared with 1 for cases biopsied > 6 months after onset of rash (p = 0.037) (Fig. 3c). A similar trend was observed for the vasculitis, elastase, and ROS scores (Figs. 3a, b, d).
Fig. 3.
Boxplots – score distribution according to duration since onset of vasculitis for hypersensitivity vasculitis (HV), IgA and urticarial vasculitis (UV), < 1 week (n = 12), 1–4 weeks (n = 20), 1–6 months (n = 12), > 6 months (n = 16). The median is represented by the thick line across the box. The top and bottom box lines show the first and third quartiles. The whiskers show the maximum and minimum values, with the exceptions of outliers, which are represented by circles. (a) Vasculitis score, (b) elastase score, (c) His-3-cit score, (d) reactive oxygen species (ROS) score. p-value for difference between groups obtained using Kruskal–Wallis 1-way analysis of variance (ANOVA) for ordinal variables with pairwise comparisons using the Dunn-Bonferroni for post hoc analysis.
Formation of neutrophil extracellular traps is not correlated with systemic involvement or aetiology
For HV and IgA vasculitis specifically, there was no statistically significant difference in the vasculitis severity score nor in the formation of NETs between cases with and without systemic involvement (median score 2 and 2.5, respectively; p = 0.723). Similarly, there was no significant difference in the vasculitis severity score nor in the formation of NETs among the different aetiologies (p = 0.619).
Hypersensitivity vasculitis and IgA vasculitis produce significantly more neutrophil extracellular traps than urticarial vasculitis, independent of the vasculitis score and duration of vasculitis
Results of the ordinal regression, whereby the His-3-cit score was regressed against the acute cutaneous SVV, show that for HV compared with UV, the odds of having a higher level of His-3-cit score compared with a lower level is 8 (95% confidence interval (95% CI) 2.37–26.98, p = 0.001). For IgA vasculitis compared with UV, the odds of having a higher level of His-3-cit score compared with a lower level is 6.30 (95% CI 1.97–20.23, p = 0.002).
We then adjusted for vasculitis score and duration of vasculitis. For HV compared with UV, the adjusted odds of having a higher level of His-3-cit score compared with a lower level becomes 12.38 (95% CI 2.71–56.66, p = 0.001). For IgA vasculitis compared with UV, the adjusted odds of having a higher level of His-3-cit score compared with a lower level becomes 8.65 (95% CI 1.90–39.37, p = 0.005). These results show that, in HV and IgA vasculitis, there is significantly more NETs formation compared with UV, independent of the vasculitis score and duration.
Erythema elevatum diutinum and polyarteritis nodosa
Among the three EED lesions, two had a His-3-cit score of 2 and one had a score of 1. Elastase and His-3-cit staining were found around deeper vessels, intertwined within the concentric storiform fibrosis and areas of fibrinoid necrosis (Fig. 1). This highlights the role of NETs in endothelial damage in a chronic form of IC-mediated cutaneous SVV.
No NETs were detected in the 4 examined lesions of PAN, even in lesions with a high vasculitis score. The elastase staining, when present, was found mainly within and around the inflamed medium-sized vessel (Fig. 1).
DISCUSSION
These results demonstrate the presence of NETs in the various IC-mediated cutaneous SVV. NETs were found specifically around inflamed vessels at areas of high neutrophilic infiltration. The density of NETs was strongly correlated with the severity of vessel inflammation, the amount of ROS production. Neutrophil extracellular traps were found around inflamed vessels, and their formation was highest early after the onset of vasculitis. These results indicate a role of NETs in inducing vascular damage in IC-mediated vasculitis. This is in line with various examples in the literature, whereby NETs were shown to induce vascular damage in different types of vasculitis. In ANCA vasculitis, NETs provide a scaffold for alternative complement pathway activation, which, in turn, contributes to endothelial damage (25). In SLE, matrix metalloproteinase-9 contained in NETs induce activation of endothelial matrix metalloproteinase-2 contributing to endothelium damage (33). Furthermore, in SLE a subset of neutrophils termed “low-density granulocytes” were shown to mediate extensive endothelial cell death through their enhanced capacity to form NETs (34). We have previously demonstrated that, in Behçet’s disease, NETs cause endothelial cell death through a preG0/G1 cell cycle arrest (18). Moreover, NETs play important roles in the formation of thrombosis, atherosclerosis, and other vascular complications (34, 35).
IC deposition plays a central role in the pathogenesis of HV (2), IgA vasculitis (6), UV (7) and EED (1, 10) through complement activation, neutrophilic infiltration, and the release of destructive enzymes leading to vessel damage. Immobilized ICs have been reported recently to induce the release of NETs from human neutrophils in vitro (36). IC deposition in vessel walls occurs early and precedes cellular infiltration and inflammation (2). This is corroborated by our findings showing that vasculitides biopsied earlier in time since the onset of vasculitis demonstrated stronger NETs formation. In addition, we have demonstrated the presence of ROS and its strong correlation with NETs production in those specific vasculitides. Formation of NETs requires the production of ROS by NADPH oxidase and myeloperoxidase (17). This process is mediated by FcgRIIIb in association with macrophage-1 Ag, and the intracellular signalling pathways involved in IC-induced NETosis is the tyrosine kinase Src/Syk pathway, which downstream regulates the PI3K/Akt, p38 MAPK, and ERK1/2 pathways (36). Moreover, IgAIC in plasma and synovial fluid of patients with RA has also been shown to activate neutrophils and induce NETs via FcaRI (26).
In our study, when looking at acute cutaneous SVV altogether, the amount of NETs production was strongly correlated with the severity of vessel inflammation. Nevertheless, for the same degree of inflammation there was no difference in the densities of NETs between HV and IgA vasculitis, which are histologically indistinguishable; whereas UV demonstrated less NETs. Interestingly, fibrinoid deposits, which represent direct signs of vessel damage, are always present in HV and IgA vasculitis, whereas most skin biopsies of UV demonstrate perivascular nuclear debris without fibrin deposits (37). This suggests that, in IC-mediated cutaneous SVV, NETs are associated with more fibrinoid damage. Moreover, in all 3 cases of EED, NETs were located mainly around deeper vessels, intertwined within areas of fibrinoid necrosis. It is noteworthy that all of our cases of UV were normocomplementemic, which have less fibrinoid deposits than hypocomplementemic UV (38).
None of the 4 cases of cutaneous PAN demonstrated any formation of NETs despite the heavy neutrophilic infiltrate. To date, the pathogenesis of cutaneous PAN is unknown, and although IC have been described in vessel walls of cutaneous PAN, they have been found in the deep muscular medium-sized vessels in only 50% of cases (11). Furthermore, IC are known to be deposited in the vessels for only a short period of time, and our 4 cases of cutaneous PAN might correspond to later stages where IC might have already been degraded (5, 39). Nevertheless, it is also possible that NETs are implicated only in specific inflammatory processes and in particular types of vasculitides, and that they may not be involved in the pathogenesis of PAN.
In conclusion, the current study demonstrates, for the first time, the presence of NETs and ROS in the different IC-mediated cutaneous SVV. We were able to demonstrate that the relationship between IC and NETs, previously described in isolated neutrophils from healthy adult volunteers (36), holds true for the pathogenic neutrophils found in the skin biopsies of the different cutaneous IC-mediated vasculitides. As new therapeutics targeting the formation of NETs are emerging (40), the findings of the current study might be particularly helpful for the difficult-to-treat cutaneous SVV, such as chronic recurrent SVV, IgA vasculitis relapses and the notoriously chronic and disfiguring EED.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Carlson JA, Chen KR. Cutaneous vasculitis update: small vessel neutrophilic vasculitis syndromes. Am J Dermatopathol 2006; 28: 486–506. [DOI] [PubMed] [Google Scholar]
- 2.Gower RG, Sams WM, Jr., Thorne EG, Kohler PF, Claman HN. Leukocytoclastic vasculitis: sequential appearance of immunoreactants and cellular changes in serial biopsies. J Invest Dermatol 1977; 69: 477–484. [DOI] [PubMed] [Google Scholar]
- 3.Wasi S, Murray RK, Macmorine DR, Movat HZ. The role of PMN-leucocyte lysosomes in tissue injury, inflammation and hypersensitivity. II. Studies on the proteolytic activity of PMN-leucocyte lysosomes of the rabbit. Br J Exp Pathol 1966; 47: 411–423. [PMC free article] [PubMed] [Google Scholar]
- 4.Tosca N, Stratigos JD. Possible pathogenetic mechanisms in allergic cutaneous vasculitis. Int J Dermatol 1988; 27: 291–296. [DOI] [PubMed] [Google Scholar]
- 5.Braverman IM, Yen A. Demonstration of immune complexes in spontaneous and histamine-induced lesions and in normal skin of patients with leukocytoclastic angitis. J Invest Dermatol 1975; 64: 105–112. [DOI] [PubMed] [Google Scholar]
- 6.Heineke MH, Ballering AV, Jamin A, Ben Mkaddem S, Monteiro RC, Van Egmond M. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schonlein purpura). Autoimmun Rev 2017; 16: 1246–1253. [DOI] [PubMed] [Google Scholar]
- 7.Black AK. Urticarial vasculitis. Clin Dermatol 1999; 17: 565–569. [DOI] [PubMed] [Google Scholar]
- 8.Mehregan DR, Gibson LE. Pathophysiology of urticarial vasculitis. Arch Dermatol 1998; 134: 88–89. [DOI] [PubMed] [Google Scholar]
- 9.Mehregan DR, Hall MJ, Gibson LE. Urticarial vasculitis: a histopathologic and clinical review of 72 cases. J Am Acad Dermatol 1992; 26: 441–448. [DOI] [PubMed] [Google Scholar]
- 10.Laymon CW. Erythema elevatum diutinum. A type of allergic vasulitis. Arch Dermatol 1962; 85: 22–28. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Perez JL, Schroeter AL, Winkelmann RK. Cutaneous periarteritis nodosa: immunofluorescence studies. Arch Dermatol 1980; 116: 56–58. [PubMed] [Google Scholar]
- 12.McKinney EF, Willcocks LC, Broecker V, Smith KG. The immunopathology of ANCA-associated vasculitis. Semin Immunopathol 2014; 36: 461–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018; 18: 134–147. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoiber W, Obermayer A, Steinbacher P, Krautgartner WD. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules 2015; 5: 702–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirchner T, Moller S, Klinger M, Solbach W, Laskay T, Behnen M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators Inflamm 2012; 2012: 849136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol 2012; 92: 841–849. [DOI] [PubMed] [Google Scholar]
- 18.Safi R, Kallas R, Bardawil T, Mehanna CJ, Abbas O, Hamam R, et al. Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in Behcet’s disease. J Dermatol Sci 2018; 92: 143–150. [DOI] [PubMed] [Google Scholar]
- 19.Van Avondt K, Fritsch-Stork R, Derksen RH, Meyaard L. Ligation of signal inhibitory receptor on leukocytes-1 suppresses the release of neutrophil extracellular traps in systemic lupus erythematosus. PLoS One 2013; 8: e78459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 2016; 22: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013; 5: 178ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009; 15: 623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med 2009; 15: 1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 2012; 7: e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiber A, Rousselle A, Becker JU, von Massenhausen A, Linkermann A, Kettritz R. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci U S A 2017; 114: E9618–E9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aleyd E, Al M, Tuk CW, van der Laken CJ, van Egmond M. IgA Complexes in plasma and synovial fluid of patients with rheumatoid arthritis induce neutrophil extracellular traps via FcalphaRI. J Immunol 2016; 197: 4552–4559. [DOI] [PubMed] [Google Scholar]
- 27.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65: 1–11. [DOI] [PubMed] [Google Scholar]
- 28.Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol 2006; 45: 3–13. [DOI] [PubMed] [Google Scholar]
- 29.Cribier B, Couilliet D, Meyer P, Grosshans E. The severity of histopathological changes of leukocytoclastic vasculitis is not predictive of extracutaneous involvement. Am J Dermatopathol 1999; 21: 532–536. [DOI] [PubMed] [Google Scholar]
- 30.Lee JJ, Vilain RE, Granter SR, Hu NR, Bresler SC, Xu S, et al. 5-Hydroxymethylcytosine is a nuclear biomarker to assess biological potential in histologically ambiguous heavily pigmented melanocytic neoplasms. J Cutan Pathol 2017; 44: 249–255. [DOI] [PubMed] [Google Scholar]
- 31.Henriksen KL, Rasmussen BB, Lykkesfeldt AE, Moller S, Ejlertsen B, Mouridsen HT. Semi-quantitative scoring of potentially predictive markers for endocrine treatment of breast cancer: a comparison between whole sections and tissue microarrays. J Clin Pathol 2007; 60: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009; 184: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 2015; 74: 1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011; 187: 538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becatti M, Emmi G, Silvestri E, Bruschi G, Ciucciarelli L, Squatrito D, et al. Neutrophil activation promotes fibrinogen oxidation and thrombus formation in Behcet disease. Circulation 2016; 133: 302–311. [DOI] [PubMed] [Google Scholar]
- 36.Behnen M, Leschczyk C, Moller S, Batel T, Klinger M, Solbach W, et al. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcgammaRIIIB and Mac-1. J Immunol 2014; 193: 1954–1965. [DOI] [PubMed] [Google Scholar]
- 37.Davis MD, Brewer JD. Urticarial vasculitis and hypocomplementemic urticarial vasculitis syndrome. Immunol Allergy Clin North Am 2004; 24: 183–213, vi. [DOI] [PubMed] [Google Scholar]
- 38.Davis MD, Daoud MS, Kirby B, Gibson LE, Rogers RS, 3rd. Clinicopathologic correlation of hypocomplementemic and normocomplementemic urticarial vasculitis. J Am Acad Dermatol 1998; 38: 899–905. [PubMed] [Google Scholar]
- 39.Cream JJ, Bryceson AD, Ryder G. Disappearance of immunoglobulin and complement from the Arthus reaction and its relevance to studies of vasculitis in man. Br J Dermatol 1971; 84: 106–109. [DOI] [PubMed] [Google Scholar]
- 40.Kimura H, Matsuyama Y, Araki S, Koizumi A, Kariya Y, Takasuga S, et al. The effect and possible clinical efficacy of in vivo inhibition of neutrophil extracellular traps by blockade of PI3K-gamma on the pathogenesis of microscopic polyangiitis. Mod Rheumatol 2018; 28: 530–541. [DOI] [PubMed] [Google Scholar]