Abstract
Atopic dermatitis has a negative impact on quality of life in patients and their families. However, there have been very few studies of the impact of atopic dermatitis on adolescents and their relatives. The objective of this study was to evaluate the impact of atopic dermatitis in the daily lives of adolescents between 12 and 17 years of age in the French population and to assess the burden of the disease on their families. Quality of life was measured in 399 parents of adolescents with atopic dermatitis and in the adolescents themselves. Impairment of quality of life in the adolescents was associated with disease severity. Moreover, in children aged 12–14 years, quality of life was worse with increasing age, with decreasing disease duration, and when parents had atopic dermatitis. In children aged 15–17 years quality of life was worse when the parent who answered the questionnaire was male and when the parent was <45 years old. The burden of atopic dermatitis was higher in parents of older children, in parents with children with higher disease severity, with shorter disease duration, in male parents, and in parents aged < 45 years. The burden of atopic dermatitis in adolescents and their parents is considerable and should be taken into account in the management of atopic dermatitis.
Key words: adolescent, atopic dermatitis, Dermatology Life Quality Index, family burden, quality of life
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease. It is more frequent in children, but can occur at any age. AD affects 5–20% of children in Western countries (1, 2). In France, the prevalence of AD has recently been estimated as 4.6% in the general population over 15 years of age (3). The incidence of AD is increasing (4), in particular in industrialized countries. Therefore, AD is an important public health issue whose impact needs to be studied in depth. Great attention has been paid to AD in childhood, but fewer studies are available on the impact of this condition on young adults and adults. Due to its symptoms, mainly pruritus, and to its frequent occurrence in visible areas, AD may have a detrimental effect on patients’ lives, by negatively affecting different aspects of quality of life (QoL) (5, 6). In addition, AD may have a secondary impact on the patient’s family or caregiver. Basra & Finlay (7) proposed the concept of the “Greater Patient” to describe the group of people close to the patient who are thus affected in some way by his or her disease. The burden of AD in the family members of children has been well described (8–10). Family members are burdened with time-consuming treatment regimens, dietary and household changes, as well as a heavy financial impact. However, adult patients with AD may also involve their family members in the physical and psychosocial consequences of their condition (11, 12). Previous studies on AD have focused mainly on children and adults, neglecting the intermediate category, that of adolescents, who are a specific group of patients with their own experiences, preferences and beliefs (13). Trials are currently being conducted on adolescents with AD (14), taking into account that they may not necessarily respond to treatment in the same way as adults. Adolescence is, in fact, a transitional phase of growth in which biological, cognitive, social and emotional transformations take place. Adolescents face a series of challenges in developing their autonomy, identity and self-image. Body image plays a more important role at this age than it does in childhood and adulthood; thus, the impact of AD on QoL in adolescents is particularly high. Adolescents often have to deal with their disease by themselves, and they develop a personal attitude towards treatment (13), with the need for a rapid and persistent effect, and with generally low adherence. Thus, it is important specifically to study the burden AD places on adolescents and its consequences on their families.
SIGNIFICANCE
The burden of atopic dermatitis on adolescents and their parents was studied in a representative sample of adults with children aged 12–17 years with atopic dermatitis. Impairment of quality of life in the adolescents themselves was found to be associated, in particular, with disease severity. The burden of atopic dermatitis on parents was considerable. This aspect is particularly important in parents of children with atopic dermatitis, since they are involved in all aspects of care and in the problems the child encounters in their daily life. This has been studied in depth in parents of paediatric patients with atopic dermatitis, but not specifically in the adolescent group. Our results show that an effective treatment for AD should be aimed at improving adolescents’ QoL, and thus at reducing the burden of the disease on parents.
The objectives of this study were therefore to evaluate the impact of AD in the daily lives of adolescents between 12 and 17 years of age in the French population and to assess the burden of the disease on their families.
MATERIALS AND METHODS
Study design
This was an observational, cross-sectional study. The study was approved by the Institutional Review Board (NO3 - Nord-Ouest III, FRANCE, nr 19.04.11.96333) on 7 September 2019 and was conducted according to the principles of the Declaration of Helsinki.
Study population
A polling institute (HC Conseil Paris, France) conducted the survey between September and October 2019. A representative sample of the adult general population over 18 years of age was recruited using a stratified, proportional sampling with replacement design. Individuals who had children aged between 12 and 17 years with AD, diagnosed by a physician, were invited to participate in the study. Thus, the study population consisted of parents of adolescents aged 12–17 years who had AD. Inclusion criteria were: (i) being parents of a child aged 12–17 years with AD; (ii) able to understand the French language; and (iii) having given consent to participate after receiving written information about the study. Parents also gave consent for their child’s participation in the study.
Study procedures
Parents were asked to answer a questionnaire regarding sociodemographic and personal information, including age, professional level, absences from work due to their child’s disease, presence or history of AD in one or both parents, and number of days per year of absence at school of their children. Professional level was defined according to the socio-professional categories (CSP) set out by the French Institut National de la statistique et des etudes économiques (INSEE). Parents were further categorized into 2 categories: “plus” (+) and “minus” (–). The (+) category included individuals belonging to higher and intermediate managerial, administrative, professional, supervisory, clerical and junior managerial, administrative, and professional occupations; the (–) category included skilled, semi-skilled, unskilled manual and lowest grade occupations. Parents were also asked to answer a question about the attendance at therapeutic patient educational sessions: “did your child or one or both parents attend educational sessions for AD?”.
Adolescents were asked to complete different questionnaires on their QoL. Parents were also asked to complete a questionnaire on the burden of their child’s disease, to evaluate their personal health status, and to evaluate the clinical severity of their child’s AD. All the questionnaires were anonymous. The adolescents’ and their parents’ questionnaires were linked by a code to form a dyad.
The questionnaires are described below.
Clinical severity: the Patient-Oriented Eczema Measure. Clinical severity was assessed by the parent using a proxy version of the Patient-Oriented Eczema Measure (POEM) (15). POEM is a measurement tool for monitoring disease activity in children and adults with atopic eczema, and it is completed by the patient or the caregiver when the patient is a child. The questionnaire asks about the frequency of occurrence of 7 symptoms during the previous week: itching, sleep, bleeding, weeping, cracking, flakiness, dryness. Possible answers are given on a 5-point scale: 0 = no days;1 = 1– 2 days; 2 = 3–4 days; 3 = 5–6 days; 4 = every day. The total score is obtained by adding up the scores of the single items (possible scores from 0 to 28). The proposed bandings for POEM scores (16) are: 0–2 (clear/almost clear); 3–7 (mild); 8–16 (moderate); 17–24 (severe); and 25–28 (very severe). Three groups were created: mild (0–7), moderate (8–16), and severe (17–28).
Quality of life measures: EQ-5D. The EQ-5D (17) allows the current general health-related QoL to be measured across all medical fields and across the general population. EQ-5D has been used to assess patients with skin conditions (18), showing a good overall validity. EQ-5D consists of 2 parts. Here, we report only the results of the first part, which is a visual analogue scale (VAS) recording respondents’ self-rated health from 0 to 10 (worst to best imaginable health state). EQ-5D was completed both by the child and the parent, each with regards to their own health status. The child-friendly version of the EQ-5D (EQ-5D-Y) (19) was used for the adolescents.
Dermatology Life Quality Index (DLQI). The DLQI (20) is a simple questionnaire that evaluates the impact of a skin disease on patient’s QoL. The DLQI consists of 10 items covering symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment. Each item is scored on a 4-point scale, from 0 to 3, with higher scores indicating greater impairment of QoL. The following score band descriptors have been validated (21): total score 0–1 = no effect at all on the patient’s life; 2–5 = small effect on the patient’s life; 6–10 = moderate effect on the patient’s life; 11–20 = very large effect on the patient’s life; 21–30 = extremely large effect on the patient’s life. In this study, the DLQI was used in children aged 15–17 years.
Children’s Dermatology Life Quality Index (CDLQI). The CDLQI (22) is a QoL instrument that aims to evaluate the impact of a skin disease and its treatment on the lives of children under 16. As in the DLQI, there are 10 questions, with possible answers scores from 0 to 3: “not at all” to “very much”. Higher scores indicate a worse QoL. In this study, the CDLQI was used in children aged 12–14 years.
Burden of disease in the family: Atopic dermatitis Burden Scale-Family (ABS-F). The ABS-F questionnaire (23) is a validated tool that assesses the burden of AD in families of children with AD. This questionnaire consists of 14 items that can be summarized into 4 dimensions. For each item, possible answers were scored 0–3: “no without hesitation” (0), “I don’t know” (1), “maybe” (2), and “yes without hesitation” (3).
Statistical methods
Since the study was descriptive, no sample calculation was necessary. A sample of 400 parents was considered adequate for the purposes of the study. Categorical values are described as numbers and percentages, and continuous variables as means and standard deviations (SD). The mean scores of the different instruments were compared for different levels on different variables using t-tests. Correlations between instrument scores were calculated using the Pearson correlation coefficient. Three linear regression models were performed, with the CDLQI, DLQI, and ABS-F scores as dependent variables. The independent variables were: sex and age of the child, disease severity as evaluated by the parent (POEM questionnaire) in 3 categories (mild, moderate, and severe), duration of the disease (< 5 years and ≥ 5), sex and age of the parent, and presence of AD in parents.
RESULTS
Study population
A total of 400 parents met the inclusion criteria, agreed to participate in the study and completed the questionnaires. One respondent (0.25%) was excluded because of inconsistent responses. Therefore, a total of 399 parents were included in the final analysis. Fig. 1 summarizes the number of parents and adolescents included in the analysis and the administered questionnaires. Table I describes the socio-demographic and personal characteristics of the parents. The mean ± SD age of the parents was 41.4 ± 8.2 years, and 67.4% of the respondents were women. Twelve of the 13 major French regions were represented. AD was present in 46.9% of parents, and 54.6% of them reported a history of AD. Fifty-eight parents (14.5%) reported that one or both of parents or their child had attended therapeutic patient educational sessions.
Fig. 1.
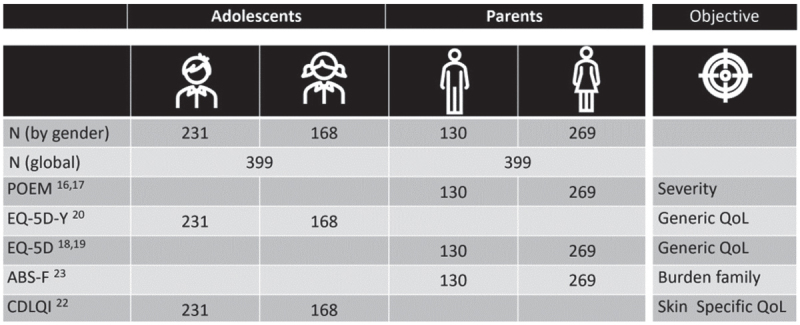
Number of parents and adolescents with atopic dermatitis (AD) who answered to the different questionnaires. ABS-F: Atopic dermatitis Burden Scale-Family; CDLQI: Children’s Dermatology Life Quality Index; DLQI: Dermatology Life Quality Index; POEM: Patient-Oriented Eczema Measure.
Table I.
Description of the study population: parents
| Variable | n (%) |
|---|---|
| Parent | |
| Mother | 269 (67.4) |
| Father | 130 (32.6) |
| Age | |
| < 45 years | 193 (54.1) |
| ≥ 45 years | 164 (45.9) |
| Professional level* | |
| Socio-professional category + | 198 (49.6) |
| Socio-professional category - | 148 (37.1) |
| Unemployed | 53 (13.3) |
| Absence at work** during one year | |
| At least one absence | 85 (26.2) |
| None | 239 (73.8) |
| Presence of atopic dermatitis | |
| One of the parents | 160 (40.1) |
| Both parents | 27 (6.8) |
| None | 212 (53.1) |
| History of atopic dermatitis | |
| One of the parents | 186 (46.6) |
| Both parents | 32 (8.0) |
| None | 181 (45.4) |
| Educational sessions on atopic dermatitis (child or parent) | |
| Yes | 58 (14.5) |
| No | 341 (85.5) |
The (+) category included individuals belonging to higher and intermediate managerial, administrative, professional, supervisory, clerical and junior managerial, administrative, and professional occupations; the (-) category included skilled, semi-skilled, unskilled manual and lowest grade occupations.
Due to their child’s atopic dermatitis.
Among adolescents (Table II), 57.9% were boys, and half of them were 15 years old or more, according to the selection criteria. The duration of the disease was 5 years or more in 49.1% of cases. Severity, as evaluated by the POEM, was mild in 57.6%, moderate in 32.8% and severe in 9.5% of patients.
Table II.
Description of the study population: adolescents
| Variable | |
|---|---|
| Sex, n (%) | |
| Boys | 231 (57.9) |
| Girls | 168 (42.1) |
| Age, n (%) | |
| 12–14.9 years | 200 (50.0) |
| 15–17.9 years | 199 (50.0) |
| Disease severity: POEM, n (%) | |
| Mild | 230 (57.6) |
| Moderate | 131 (32.8) |
| Severe | 38 (9.5) |
| Duration of the disease, n (%) | |
| < 5 years | 203 (50.9) |
| ≥ 5 years | 196 (49.1) |
| Days of absence from school during one year, mean ± SD | 6.8 ±5.9 |
POEM: Patient-Oriented Eczema Measure; SD: standard deviation.
Quality of life in children and their parents according to socio-demographic and clinical variables
Mean ± SD CDLQI score was 8.7 ± 7.1, indicating a moderate effect on QoL, and mean ± SD DLQI score was 12.8 ± 11.1, indicating a very large effect on QoL (Table III). Low patient’s and parent’s QoL were associated with a higher clinical severity (p = 0.02 for moderate vs mild condition in EQ-5D VAS of parents, and p < 0.001 for all the other comparisons). In adolescents QoL was worse for a disease duration of < 5 years (p = 0.003 for 12–14 years old, p = 0.01 for 15–17 years old). QoL in parents and in children aged 15–17 years was lower when AD was present in parents (p = 0.02 in parents, and 0.006 and 0.02 in the two adolescents’ groups). Children health status and QoL were worse when the father participated in the study (p = 0.02 for CDLQI, p < 0.001 for the other QoL variables). Having attended therapeutic patient educational sessions was strongly associated with low QoL (p < 0.001 for all groups, except parent EQ-5D with p = 0.007).
Table III.
Quality of life and burden of disease scores in different levels of sociodemographic and clinical variables in 399 adolescents with atopic dermatitis (AD) and their parents
| Variable | EQ-5D VAS Parents Mean ± SD |
p-value | EQ-5D VAS Adolescents Mean ± SD |
p-value | CDLQI Children 12–14 years Mean ± SD |
p-value | DLQI Children 15–17 years Mean ± SD |
p-value | ABS-F Parents Mean ± SD |
p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 73.8 ± 21.3 | 72.7 ± 22.2 | 8.7 ± 7.1 | 12.8 ± 11.1 | 10.0 ± 8.0 | |||||
| Severity (POEM) | ||||||||||
| Mild | 77.0 ± 19.8 | 78.2 ± 19.4 | 5.6 ± 5.6 | 6.7 ± 6.8 | 8.6 ± 9.2 | |||||
| Moderate | 72.3 ± 20.8 | 0.02 * | 68.8 ± 21.5 | < 0.001 * | 12.7 ± 7.0 | < 0.001 * | 12.4 ± 7.0 | < 0.001 * | 16.9 ± 10.4 | < 0.001 * |
| Severe | 59.1 ± 25.0 | < 0.001 ** | 51.7 ± 25.4 | < 0.001 ** | 15.9 ± 4.1 | 0.07** | 18.2 ± 6.8 | < 0.001 ** | 24.3 ± 10.2 | < 0.001 ** |
| Sex (child) | ||||||||||
| Boys | 74.0 ± 20.9 | 71.7 ± 22.8 | 9.0 ± 7.4 | 10.5 ± 8.0 | 13.9 ± 11.4 | |||||
| Girls | 73.4 ± 21.8 | 0.77 | 73.9 ± 21.4 | 0.33 | 8.4 ± 6.8 | 0.58 | 9.2 ± 7.8 | 0.27 | 11.4 ± 10.4 | 0.03 |
| Age (child) | ||||||||||
| 12–14.9 years | 75.0 ± 19.1 | 74.6 ± 21.2 | 8.6 ± 7.0 | 11.7 ± 10.2 | ||||||
| 15–17.9 years | 72.6 ± 23.3 | 0.27 | 70.6 ± 23.1 | 0.07 | 10.0 ± 7.9 | 14.1 ± 11.8 | 0.03 | |||
| Duration of the disease | ||||||||||
| < 5 years | 73.4 ± 20.2 | 70.7± 22.4 | 10.0 ± 7.2 | 11.6 ± 8.4 | 14.7 ± 11.4 | |||||
| ≥ 5 years | 74.2 ± 22.5 | 0.72 | 74.6± 21.9 | 0.08 | 7.0 ± 6.7 | 0.003 | 8.7 ± 7.3 | 0.01 | 10.9 ± 10.4 | 0.001 |
| Presence of AD in parents | ||||||||||
| One or both of them | 71.2 ± 21.4 | 70.5± 23.0 | 10.2 ± 7.4 | 11.4 ± 8.4 | 14.5 ± 11.3 | |||||
| None | 76.0± 21.0 | 0.02 | 74.4± 21.4 | 0.08 | 7.4 ± 6.7 | 0.006 | 8.8± 7.4 | 0.02 | 11.4 ± 10.7 | 0.005 |
| Parent | ||||||||||
| Father | 72.9± 21.0 | 66.4± 23.4 | 10.6 ± 6.9 | 13.2 ± 7.9 | 16.8 ± 11.7 | |||||
| Mother | 74.2± 21.5 | 0.58 | 75.6 ± 21.0 | < 0.001 | 8.0 ± 7.1 | 0.02 | 8.1± 7.4 | < 0.001 | 11.0 ± 10.3 | < 0.001 |
| Age (parent) | ||||||||||
| < 45 years | 76.4± 19.3 | 73.8 ± 20.4 | 8.3 ± 6.9 | 10.6 ± 8.0 | 12.6 ± 11.0 | |||||
| ≥ 45 years | 73.1 ± 22.2 | 0.14 | 74.9 ± 23.4 | 0.62 | 8.2 ± 7.4 | 0.90 | 7.9 ± 6.5 | 0.02 | 11.0 ± 10.0 | 0.16 |
| Professional level (parent)*** | ||||||||||
| CSP+ | 74.7 ± 20.7 | 70.9 ± 21.7 | 10.3 ± 7.7 | 10.4 ± 7.8 | 14.0 ± 11.2 | |||||
| CSP− | 73.8 ± 21.9 | 0.68 | 73.9 ± 23.6 | 0.20 | 7.5 ± 6.6 | 0.01 | 9.1 ± 7.7 | 0.24 | 11.8 ± 11.1 | 0.06 |
| Educational sessions on AD (child or parent) | ||||||||||
| Yes | 66.8 ± 24.3 | 59.3 ± 23.6 | 18.0 ± 5.5 | 20.0 ± 7.0 | 25.7 ± 9.1 | |||||
| No | 75.0 ± 20.5 | 0.007 | 74.8 ± 20.5 | < 0.001 | 7.2 ± 6.1 | < 0.001 | 8.2 ± 6.7 | < 0.001 | 10.7 ± 9.8 | < 0.001 |
Moderate vs mild;
Severe vs moderate;
The (+) category included individuals belonging to higher and intermediate managerial, administrative, professional, supervisory, clerical and junior managerial, administrative, and professional occupations; the (−) category included skilled, semi-skilled, unskilled manual and lowest grade occupations.
CSP; socio-professional category; DLQI: Dermatology Life Quality Index; CDLQI: Children’s Dermatology Life Quality Index; ABS-F: Atopic dermatitis Burden Scale-Family; VAS: visual analogue scale. Figures in bold indicate statistical significance at p < 0.05.
Family burden according to socio-demographic and clinical variables
As shown in the final column of Table III, family burden was strongly positively associated with clinical severity (p < 0.001). A higher family burden was reported by parents of boys compared with girls (p = 0.03), of children of 15 years or more compared with the younger group (p = 0.03), and of patients with a disease duration of < 5 years (p = 0.001). Also, the family burden was higher when AD was present in parents (p = 0.005), when the father participated in the study (p < 0.001), and when the child or the parent attended therapeutic patient educational sessions (p < 0.001).
Quality of life and family burden: multivariate analysis
The regression models confirmed most of the results obtained in the univariate analysis. In children aged 12–14 years (Fig. 2) QoL decreased with increasing age (p < 0.001), with increasing disease severity (p < 0.001), with decreasing disease duration (p = 0.005), and when parents had AD (p = 0.031). In children aged 15–17 years higher DLQI scores (Fig. 3) were associated with higher disease severity (p < 0.001), sex of the parent (male) (p = 0.001), and younger age of the parent (p < 0.001). Family burden, as measured by the ABS-F (Fig. 4), was higher in parents of older children (p = 0.01), with higher disease severity (p < 0.001), shorter disease duration (p = 0.013), in male parents (p = 0.004), and in younger parents (p = 0.006). Pearson correlation coefficients were 0.79 between the CDLQI and ABS-F and 0.83 between the DLQI and ABS-F.
Fig. 2.
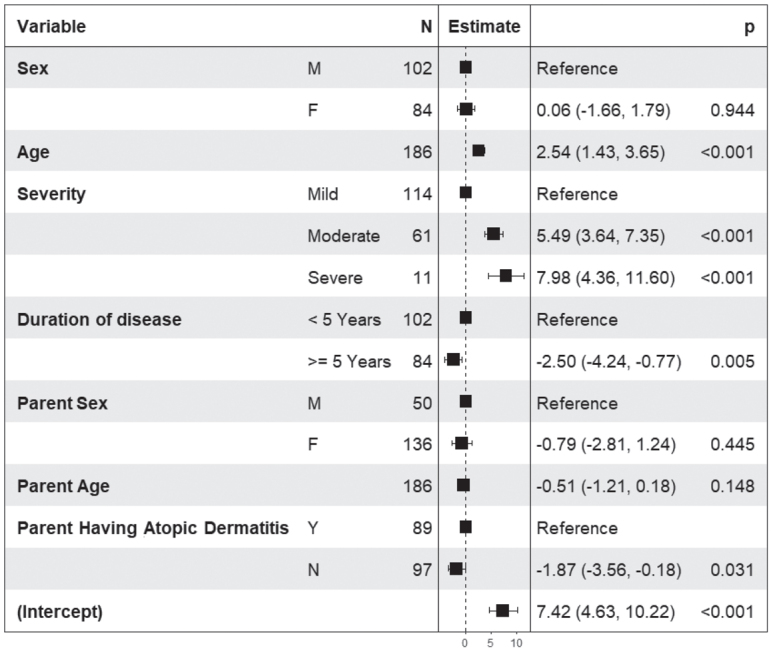
Results of the linear regression model in 200 adolescents (12–14 years old) with atopic dermatitis (AD). Children’s Dermatology Life Quality Index (CDLQI) score is the dependent variable, and the independent variables are sex and age of the child, disease severity as evaluated by the patient in 3 categories (mild, moderate, severe), duration of the disease (< 5 and ≥ 5 years), sex and age of the parents, and presence of AD in parents.
Fig. 3.
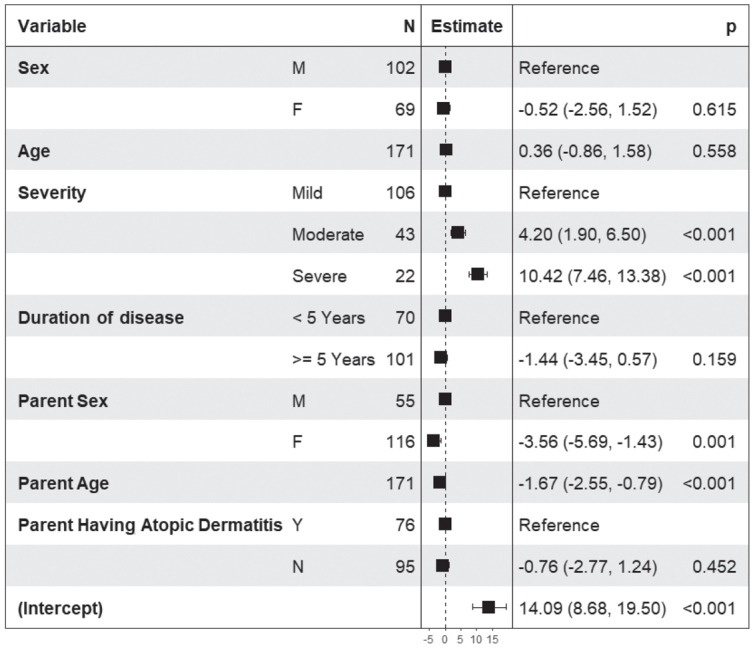
Results of the linear regression model in 199 adolescents (15–17 years old) with atopic dermatitis (AD). Dermatology Life Quality Index (DLQI) score is the dependent variable, and the independent variables are sex and age of the child, disease severity as evaluated by the patient in 3 categories (mild, moderate, severe), duration of the disease (< 5 and ≥ 5 years), sex and age of the parents, and presence of AD in parents.
Fig. 4.
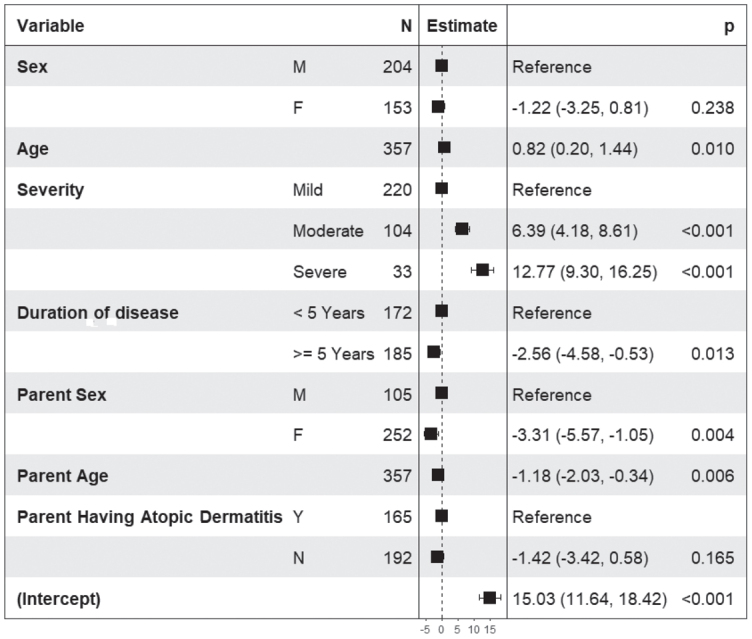
Results of the linear regression model in 399 parents of adolescents (12–17 years old) with atopic dermatitis (AD). Atopic Dermatitis Burden Scale-Family (ABSF) score is the dependent variable, and the independent variables are sex and age of the child, disease severity as evaluated by the patient in 3 categories (mild, moderate, severe), duration of the disease (< 5 and ≥ 5 years), sex and age of the parents, and presence of AD in parents.
Presence of atopic dermatitis in parents and quality of life and family burden
In the univariate analysis (Table III), when AD was present in one or both parents, QoL in children (measured with CDLQI and DLQI) and in parents (measured with EQ-5D) was significantly worse than when they did not have AD (p = 0.02, < 0.001, < 0.001, respectively). Also, family burden (ABS-F) was significantly higher when the parent/s did have AD (p = 0.031). The association was significant also in the linear regression model with the CDLQI as dependent variable (Fig. 2).
DISCUSSION
This study observed a moderate effect of AD on dermatology-related QoL in the younger group of patients (12–14 years old), while the impact on adolescents 15 years or older was very large. This finding may suggest that the, so-called, group of adolescents is not a homogeneous category, but should be further divided into at least 2 sub-groups with specific characteristics. Differences were observed even though the age range was restricted from 12 to less than 18 years, while the World Health Organization (WHO) defines adolescents as individuals aged 10–19 years old. Among the sparse specific studies evaluating QoL in adolescents with AD, a study (24) on Asian adolescents aged between 11 and 16 years reported an association between CDLQI scores and disease severity, as in the current study. Another study (25), which more specifically investigated depression and anxiety in adolescents with AD, found an association of those aspects with low QoL and sleep loss.
In the current study, QoL of adolescents was lower when the disease had started less than 5 years previously. It is possible that, over time, patients learn to deal with the disease and to use coping strategies. A low QoL was also reported in adolescents with a parent who had AD at the time of the study or in the past. In fact, it has been noted that the way in which an individual reacts to an illness has a lot to do with how his/ her parents reacted to the same illness when he/she was a child. Parents who had experienced AD are probably more worried about their child’s disease; hence the burden of the disease may be higher. Moreover, they may pay particular attention to the child, taking him/ her to the doctor frequently and letting him/her stay at home from school. These behaviours may thus pass on parental concern to their children, increasing the burden of the disease.
In the present study, the strongest association was observed between adolescents’ QoL and the severity of the disease. Such a finding is in line with other studies (26, 27). Nonetheless, in those studies, while QoL was also measured by the DLQI or the CDLQI, the clinical severity score used was the SCORAD, a physician-assessed severity score (28). In contrast, the current study used a patient/caregiver-reported severity outcome, the POEM, which may explain the higher correlation found between the DLQI and disease severity, i.e. a correlation coefficient of approximately 0.5, while they found a correlation of approximately 0.3–0.4 between QoL measures and the SCORAD. In fact, clinical severity and QoL reflect different aspects of disease burden, the first being based on objective clinical signs and symptoms and the other on the psychosocial impact of the disease. It is likely that the evaluation of clinical severity by the patient him/herself or the caregiver is a thorough evaluation of the disease, including their subjective experience of the disease in daily life.
Another important aspect is the burden that AD has on the family. In an editorial, Finlay (29) defined 3 dimensions of skin disease burden: “now”, “long term” and “family”, the first 2 concerning the patient and the third dimension involving family members or caregivers. When the patient is a child or an adolescent, parents are involved in all aspects of care and management, as well as in the problems the child encounters in their daily life. This aspect has been studied in depth in parents of paediatric patients with AD (30–33), and specific instruments have been created (34). However, most of the previous studies on the family burden of AD included either very young children, or children and adolescents grouped together. The current study specifically examined the burden of AD on the family when the patient was aged between 12 and 17 years.
As in studies concerning children, the strongest association was observed between family burden and disease severity. Moreover, when the duration of AD was less than 5 years, the burden on the family was higher. The hypothesis is the same as that concerning the lower QoL in patients with short disease duration, i.e. the ability to implement coping strategies during that time. On this matter, the results are discordant (30); however, different age ranges and instruments have been used in previous studies. The current study found that fathers of children with AD were more impacted than mothers by their child’s AD. This finding does not align with other studies that found a higher burden in mothers (32).
There are limitations of a questionnaire-based recruitment, which may lead to bias concerning the diagnosis, although only children whom parents declared that the diagnosis of AD was confirmed by a dermatologist were included in the study. On the other hand, the fact that the recruitment did not take place in a hospital or in dermatological practice may allow us to detect patients who were diagnosed with AD, but who do not go regularly to the hospital or the dermatologist, since, for example, they have a mild condition. Moreover, the current study used the POEM, a patient-centred questionnaire, as a measure of clinical severity, and thus, it is more comparable to QoL outcomes.
In order to decrease the burden of AD in parents, educational interventions may be implemented. Previous studies have shown that educational and psychological programmes for parents of paediatric patients with AD are effective in improving patients’ QoL and in reducing severity of disease (35–37). Moreover, Staab et al. (36) also showed that adolescents who attended educational sessions reported reduced severity of eczema and improved QoL compared with the control group. The present study observed that only 14.5% of children’ parents or adolescents had attended therapeutic patient educational sessions for AD. However, due to the cross-sectional design of this study, it was not possible to evaluate the effectiveness of these sessions. Paradoxically, QoL was worse, and the burden of the disease higher, when parents or adolescents had participated in patient education. This may be due to selection bias. In fact, patients and parents of patients with higher severity of AD may be those seeking more educational sessions than those with mild severity. Unfortunately, we lack data related to the severity of AD of participants prior to attending the educational programme.
In conclusion, the burden of AD in adolescents and their parents is considerable and should be taken into account in the management of the disease. It is evident that clinical severity and QoL measures are complementary, and both are needed to gain a thorough picture of AD. In clinical practice, it is important to ask specifically about impairment of QoL by either using a specific instrument or asking pertinent questions. Effective treatment for AD is essential for adolescents’ QoL improvement and reduced burden of the disease on parents. However, in the long-term management of the disease, educational interventions appear to be more effective than conventional treatment, and should be implemented both for patients and their parents.
ACKNOWLEDGEMENTS
Funding sources: We received support from Sanofi-Genzyme, which had no role in the design, the analysis and the interpretation of the study.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Mathiesen SM, Thomsen SF. The prevalence of atopic dermatitis in adults: systematic review of population studies. Dermatol Online J 2019; 25: pii: 13030/qt6nj0x5k0. [PubMed] [Google Scholar]
- 2.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 national survey of children’s health. J Invest Dermatol 2011; 131: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richard M-A, Corgibet F, Beylot-Barry M, Barbaud A, Bodemer C, Chaussade V, et al. Sex- and age-adjusted prevalence estimates of five chronic inflammatory skin diseases in France: results of the “OBJECTIFS PEAU” study. J Eur Acad Dermatol Venereol 2018; 32: 1967–1971. [DOI] [PubMed] [Google Scholar]
- 4.Mohn CH, Blix HS, Halvorsen JA, Nafstad P, Valberg M, Lagerløv P. Incidence trends of atopic dermatitis in infancy and early childhood in a nationwide prescription registry study in Norway. JAMA Netw Open 2018; 1: e184145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol 2018; 121: 340–347. [DOI] [PubMed] [Google Scholar]
- 6.Drucker AM. Atopic dermatitis: burden of illness, quality of life, and associated complications. Allergy Asthma Proc 2017; 38: 3–8. [DOI] [PubMed] [Google Scholar]
- 7.Basra MKA, Finlay AY. The family impact of skin diseases: the greater patient concept. Br J Dermatol 2007; 156: 929–937. [DOI] [PubMed] [Google Scholar]
- 8.Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol 2005; 22: 192–199. [DOI] [PubMed] [Google Scholar]
- 9.Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. J Clin Pract 2006; 60: 984–992. [DOI] [PubMed] [Google Scholar]
- 10.Misery L, Finlay AY, Martin N, Boussetta S, Nguyen C, Myon E, et al. Atopic dermatitis: impact on the quality of life of patients and their partners. Dermatology 2007; 215: 123–129. [DOI] [PubMed] [Google Scholar]
- 11.Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. The burden of atopic dermatitis in US adults: health care resource utilization data from the 2013 National Health and Wellness Survey. J Am Acad Dermatol 2018; 78: 54–61.e1. [DOI] [PubMed] [Google Scholar]
- 12.Arima K, Gupta S, Gadkari A, Hiragun T, Kono T, Katayama I, et al. Burden of atopic dermatitis in Japanese adults: analysis of data from the 2013 National Health and Wellness Survey. J Dermatol 2018; 45: 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosse RC, Bouvy ML, Daanen M, De Vries TW, Koster ES. Adolescents’ perspectives on atopic dermatitis treatmentexperiences, preferences, and beliefs. JAMA Dermatology 2018; 154: 824–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatology 2019; 156: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: Development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004; 140: 1513–1519. [DOI] [PubMed] [Google Scholar]
- 16.Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol 2013; 169: 1326–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.EuroQoL Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy (New York) 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Brazier J, Longworth L. EQ-5D in skin conditions: an assessment of validity and responsiveness. Eur J Health Econ 2015; 16: 927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wille, N., Badia, X., Bonsel, G.et al. Development of the EQ-5D-Y: a childfriendly version of the EQ-5D. Qual Life Res 2010; 19: 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 21.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol 2005; 125: 659–664. [DOI] [PubMed] [Google Scholar]
- 22.Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 2010; 132: 942–949. [DOI] [PubMed] [Google Scholar]
- 23.Méni C, Bodemer C, Toulon A, Merhand S, Perez-Cullell N, Branchoux S, et al. Atopic dermatitis burden scale: creation of a specific burden questionnaire for families. J Eur Acad Dermatol Venereol 2013; 27: 1426–1432. [DOI] [PubMed] [Google Scholar]
- 24.Ng MSY, Tan S, Chan NHQ, Foong AYW, Koh MJA. Effect of atopic dermatitis on quality of life and its psychosocial impact in Asian adolescents. Australas J Dermatol 2018; 59: e114–117. [DOI] [PubMed] [Google Scholar]
- 25.Slattery MJ, Essex MJ, Paletz EM, Vanness ER, Infante M, Rogers GM, et al. Depression, anxiety, and dermatologic quality of life in adolescents with atopic dermatitis. J Allergy Clin Immunol 2011; 128: 668–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DH, Li K, Seo SJ, Jo SJ, Yim HW, Kim CM, et al. Quality of life and disease severity are correlated in patients with atopic dermatitis. J Korean Med Sci 2012; 27: 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holm JG, Agner T, Clausen ML, Thomsen SF. Quality of life and disease severity in patients with atopic dermatitis. J Eur Acad Dermatology Venereol 2016; 30: 1760–1767. [DOI] [PubMed] [Google Scholar]
- 28.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993; 186: 23–31. [DOI] [PubMed] [Google Scholar]
- 29.Finlay AY. The three dimensions of skin disease burden: “now”, “long term” and “family”. Br J Dermatol 2013; 169: 963–964. [DOI] [PubMed] [Google Scholar]
- 30.Chernyshov P V, Ho RC, Monti F, Jirakova A, Velitchko SS, Hercogova J, et al. An international multi-center study on self-assessed and family quality of life in children with atopic dermatitis. Acta Dermatovenerol Croat 2015; 23: 247–253. [PubMed] [Google Scholar]
- 31.Jang HJ, Hwang S, Ahn Y, Lim DH, Sohn M, Kim JH. Family quality of life among families of children with atopic dermatitis. Asia Pac Allergy 2016; 6: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marciniak J, Reich A, Szepietowski JC. Quality of life of parents of children with atopic dermatitis. Acta Derm Venereol 2017; 97: 711–714. [DOI] [PubMed] [Google Scholar]
- 33.Yang EJ, Beck KM, Sekhon S, Bhutani T, Koo J. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol 2019; 36: 66–71. [DOI] [PubMed] [Google Scholar]
- 34.Sampogna F, Finlay AY, Salek SS, Chernyshov P, Dalgard FJ, Evers AWM, et al. Measuring the impact of dermatological conditions on family and caregivers: a review of dermatology-specific instruments. J Eur Acad Dermatol Venereol 2017; 31: 1429–1439. [DOI] [PubMed] [Google Scholar]
- 35.Zhao M, Liang Y, Shen C, Wang Y, Ma L, Ma X. Patient education programs in pediatric atopic dermatitis: a systematic review of randomized controlled trials and meta-analysis. Dermatol Ther 2020; 10: 449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staab D, Diepgen TL, Fartasch M, Kupfer J, Lob-Corzilius T, Ring J, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ 2006; 332: 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ersser SJ, Cowdell F, Latter S, Gardiner E, Flohr C, Thompson AR, et al. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev 2007. Jul 18; CD004054. [DOI] [PubMed] [Google Scholar]


