Abstract
Slowly depolarizing currents applied for one minute have been shown to activate C-nociceptors and provoke increasing pain in patients with neuropathy. This study examined the effect of transcutaneous slowly depolarizing currents on pruritus in patients with atopic dermatitis. C-nociceptor-specific electrical stimulation was applied to areas of eczema-affected and non-affected skin in 26 patients with atopic dermatitis. Single half-sine wave pulses (500 ms, 0.2–1 mA) induced itch in 9 patients in eczema-affected areas of the skin (numerical rating scale 5 ± 1), but pain in control skin (numerical rating scale 6±1).Sinusoidal stimuli (4 Hz, 10 pulses, 0.025–0.4 mA) evoked itch in only 3 patients in eczema-affected areas of the skin but on delivering pulses for one minute (0.05–0.2 mA) 48% of the patients (n = 12) reported itch with numerical rating scale 4 ± 1 in areas of eczema-affected skin. The number of patients reporting itch in eczema-affected areas of the skin increased with longer stimulation (p < 0.005). These results demonstrate a reduced adaptation of peripheral C-fibres conveying itch in patients with atopic dermatitis. Sensitized spinal itch processing had been suggested before in atopic dermatitis patients, and this could be present also in our patients who therefore might benefit from centrally acting antipruritic therapy.
Key words: itch, C-nociceptor, transcutaneous electrical stimulation
Transcutaneously delivered rectangular-shaped electrical stimuli of high frequency (up to 200 Hz) has been shown to activate primary afferent skin nerve fibres and, when administered to the wrist and ankle, can evoke itch in healthy control subjects and patients with atopic dermatitis (AD) (1–4). The long pulse duration (2–8 ms) and the long delay between stimulation and sensation suggest that unmyelinated C-fibres are critically involved (1), but rectangular electrical pulses preferentially activate thick myelinated axons. We recently developed electrical stimulation paradigms that preferentially activate either mechano-sensitive (5) or both mechanosensitive and -insensitive (“silent”) C-nociceptors (6) in hairy human skin (7). Chemical activation of these 2 C-nociceptor classes in the skin has been shown to drive itch (8, 9), but spinal circuits involved in chemical (and also mechanical) itch processing have to be considered (10). In particular, gastrin-releasing peptide (GRP) and GRP-receptor (GRPR) positive neurones (11, 12) as well as natriuretic polypeptide b (Nppb) receptor expressing neurones in the dorsal spinal cord (13, 14) have been identified as major components of spinal itch circuits. Intriguingly, only repetitive burst activation of presynaptic GRP positive neurones was sufficient to depolarize post-synaptic GRP-receptor positive neurones and thereby relay pruritoceptive information (11).
SIGNIFICANCE
This study showed that electrical stimulation, which is known specifically to activate unmyelinated nociceptors, evoked itch in approximately 50% of 26 patients with atopic dermatitis when one minute slowly depolarizing pulses were delivered transcutaneously to eczema affected skin. The number of patients perceiving itch increased with longer stimulation (p < 0.005) and adaptation of peripheral C-nociceptors was less pronounced. Sensitized itch-conveying C-fibres and facilitated central processing may explain the persistence of itch in patients with atopic dermatitis. Patients identified by the electrical stimulation protocol, used in this study, as having sensitized spinal processing for itch, might benefit from centrally acting antipruritic therapy.
In the current study patients with AD were stimulated with slowly depolarizing electrical stimuli that specifically activate unmyelinated C-fibres. In order to assess peripheral nociceptor accommodation and the potential “opening of the spinal gate for itch” (11), sinusoidal pulses were delivered continuously for 1 min to the patients’ eczematous and control skin. This particular stimulation paradigm of ongoing sinusoidal stimulation was perceived as increasingly painful in patients with painful neuropathy (6) and it was hypothesized that it might evoke progressively increasing pruritus in patients with chronic itch in a similar fashion.
MATERIALS AND METHODS
The study procedure was approved by the local ethics committee of the University of Heidelberg and the study protocol was in accordance with the principles of the Declaration of Helsinki. All patients had AD, as diagnosed by an experienced dermatologist (EW), and were recruited at the Department of Occupational Dermatology (University of Heidelberg). A total of 26 patients (10 female, 16 male, mean age 48 ± 21 years) signed written informed consent and participated in the study. None of the patients were told not to scratch the areas that had been most itchy over the last few days. All patients were using non-medical skin care products or moisturizing ointments for eczema treatment at the time of investigation. One patient was on long-term cyclosporine treatment for AD. Two patients took oral non-sedative antihistamines prior to the investigation because of allergic rhinoconjunctivitis. None of the patients were told not to use steroid creams before the investigation.
Study protocol
Patients were informed about the aim of the study and familiarized with the transcutaneous electrode being used for transcutaneous electrical stimulation. A pair of rounded bipolar platinum electrodes (diameter 0.4 mm, distance 2 mm, Nørresundby, Denmark) were mounted in an applicator printed with a 3D-printer and attached to the subject’s skin (Fig. 1). A training session was run to familiarize the patients with the slowly depolarizing electrical stimulation protocol and the use of the numerical rating scale (NRS) for stimulus-evoked itch or pain estimation. For electrical stimulation, sine wave and half sine wave pulses were generated by a constant current stimulator (Digitimer DS5, Welwyn Garden City, UK) connected to a Digital-Analogue Converter (DAQ NI USB-6221, National Instruments, Austin, TX, USA) controlled by Dapsys 8 software (www.dapsys.net). A single half sine wave pulse of 500-ms duration was applied to non-affected (normal) skin of the patient and with increasing intensities of 0.2–0.4–0.8 mA. After each stimulus, the patient was requested to rate the intensity of itch or pain on the NRS with the endpoints 0 (no sensation felt) and 10 (maximum sensation that can be imagined). Subsequently, sine wave pulses of 4 Hz and 2.5-s duration (=10 sinusoidal cycles) were delivered to the same skin site, at increasing intensities of 0.05–0.1–0.2 mA, and maximum itch or pain were rated by the patient on the NRS. In addition, patients were instructed to report when the stimulation was no longer felt.
Fig. 1.
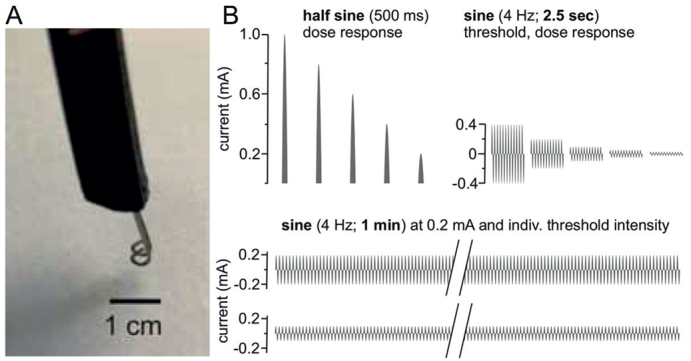
(A) A rounded bipolar electrode used for transcutaneous administration of slowly depolarizing electrical currents (scale bar: 1 cm to identify the dimension of the stimulation electrode). The platinum electrodes were mounted in a non-conducting plastic applicator. (B) Electrical profiles delivered to eczematous and non-eczematous (control) skin of patients with atopic dermatitis (AD). Half sine wave stimuli of randomized current intensities (0.2–1 mA) were applied in 10-s intervals (top left). Perception and pain sensation thresholds to 4-Hz sinusoidal pulses were assessed and an intensity-dose-response curve to 10 sinusoidal pulses (2.5-s duration) delivered randomized with 0.025–0.4 mA evaluated (top right). Finally, sine wave pulses were administered continuously for 60 s (bottom) at current intensities of perception threshold (individual threshold intensity) and 0.2 mA, respectively, and sensation recorded in 10-s intervals (5-min interval between skin site stimulation).
After the training session (data not included in the analyses) the eczema site for electrical stimulation was selected. With this aim, the patients pointed to areas that had been most itchy over the last few days. Only intact skin sites on eczema-affected were selected as test areas. The investigated eczema areas were located on the lower (n = 8) and upper (n = 3) arm, the elbow region (n = 6) and the wrist (n =2), as well as on the neck (n =5) and lower leg (n = 2). If possible, a contra-lateral and non-affected site was chosen for stimulating non-affected skin. Should that not be applicable, a site without lesion, and preferably on the forearm, was selected as control.
The electrical stimulation protocols outlined below were applied to the control (no eczema) and eczematous skin site involving one repetition at each site. Mean values of the perceived intensities (NRS) were calculated for each stimulus (Fig. 1) at each site for analysis.
Half sine wave stimulation
Starting at the healthy control skin site, single half sine wave pulses of 500-ms duration were administered with a current intensity of 0.2–0.4–0.6–0–8–1 mA in randomized order. Between each stimulus, an interval of 10 s was maintained, allowing the patient to scale the perceived intensity of sensation (NRS 0–10) and to indicate whether itch or pain was felt. After a pause of 2 min, the half sine wave stimulation protocol was administered to the eczema and the NRS value as well as the quality of sensation (itch or pain) recorded. The stimulation cycle (control/eczema skin) was repeated once.
Sensory electrical thresholds for sine wave stimuli
Next, the perception and pain thresholds of the patients’ control and eczema skin to 4 Hz sine wave stimuli were evaluated. Sinusoidal pulses were administered for 2.5 s (10 sinusoidal cycles) with increasing current intensities of 0.005–0.01–0.025–0.05–0.1–0.2–0.4 mA, and the patients were requested to indicate when they first perceived the stimulus (perception threshold) and when it was felt unpleasant (painful or itchy). A time interval of 5 s was applied before the current was increased.
Dose-response to sine wave stimuli
A dose response curve with 4-Hz sine wave stimulation was recorded (Fig. 1). Ten sinusoidal pulses (2.5 s) were applied with current intensities of 0.025–0.05–0.1–0.2–0.4 mA in randomized order (10-s time interval in between) and patients were asked to indicate the perceived intensity on the NRS (0–10), as well as to report whether the sensation was itchy or painful. The stimulation protocol started at the control skin site, followed by the eczema Stimulation at each site was performed twice.
Continuous sine wave stimulation
In order to record a potential accommodation of C-nociceptors, sinusoidal 4 Hz pulses were delivered continuously for 1 min (Fig. 1). The patients’ sensation (NRS 0–10) was recorded at 5 and 10 s after stimulus onset and thereafter in 10 s intervals until the end of stimulation. First, the current intensity for continuous stimulation was set at the individually identified value at which 10 pulses were perceived as unpleasant (see above). Stimuli were delivered to control skin and patients were asked to estimate magnitude of perception (NRS) and whether the sensation became itchy during the 1-min stimulation period. In addition, patients were instructed to indicate as soon as the stimulation was no longer felt. After a resting period of 5 min the stimulation protocol was repeated on the eczema-affected skin. Secondly, the current intensity was set to 0.2 mA and the 4 Hz sine wave pulses delivered for 1 min, again starting on the control skin followed by the eczema site 5 min later. Similar to the measures described above, patients were instructed to rate their sensation (NRS) in regular time-intervals, indicate when perception became itchy, and when stimulation was no longer felt.
Statistical analysis
Data were analysed by analysis of variance (ANOVA) and Bonferroni post hoc tests, using Statistica 7.1 (StatSoft Inc., Tulsa, OK, USA) with p < 0.05 to identify significant differences between the factorial groups “skin site” – “current intensity” – “time of stimulation”. Mann-Whitney U test was used as non-parametric comparison of 2 independent groups (“patient perceived itch” vs “patient perceived pain” on electrical stimulation). All values are depicted as mean ± standard error of mean (SEM).
RESULTS
All patients were diagnosed “atopic dermatitis” and had a history of the disease for more than 8 years. At the time of investigation, no patient had acute itch. Electrical stimuli were delivered and corresponding NRS recordings obtained from unaffected (control) and eczematous skin sites. Both skin sites were tested twice in alternating order. Offline analysis revealed no significant (n.s) difference for test repetition (ANOVA, n.s.) and thus mean NRS-values were calculated from each site for statistical analysis.
Sensory thresholds to sine wave stimulation
Perception thresholds for stimulation with 4 Hz sinusoidal pulses were 0.05 ± 0.02 mA and not significantly different between control and eczema (ANOVA, n.s.). Current thresholds for inducing an unpleasant sensation of pain or itch were virtually identical in both sites (0.1 ± 0.08 mA; ANOVA, n.s.).
Half sine wave stimulation
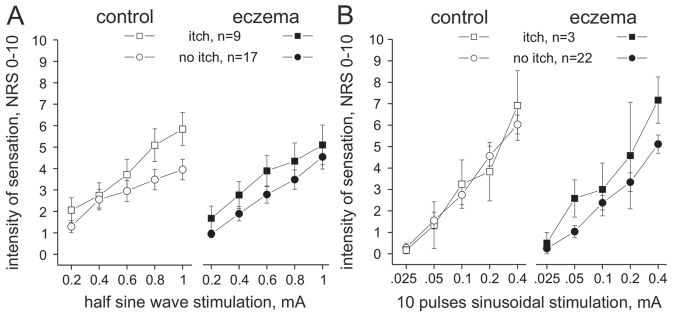
The perceived intensity of sensation after single half sine wave pulse stimulation was stronger with increasing current intensity (ANOVA, p < 0.0001), but did not differ significantly between control and eczema (ANOVA, p > 0.4, Fig. 2A). Intriguingly, half sine wave pulses evoked an itch sensation in 9 patients, whereas 17 reported pain. Significant interaction was identified between the factorial groups “evoked itch”, “current intensity”, and “skin site” (ANOVA, p < 0.04), revealing that stronger half sine wave stimuli caused increasing itch. Maximum sensation on application of a 1-mA half sine pulse was, on mean NRS 4 ± 0.5 in control skin of patients without itch and NRS 5.8 ± 0.8 in patients perceiving itch (Mann-Whitney U test, p > 0.06, Fig. 2A). In eczema-affected skin, the mean intensity of sensation was NRS 4.5 ± 0.6 (no itch), and NRS 5.1 ± 0.9 in patients responding with itch (Mann-Whitney U test, p > 0.5). No significant sex differences were identified (ANOVA, p > 0.4).
Fig. 2.
(A) Intensity of sensation (numerical rating scale (NRS) 0–10) recorded upon single half sine wave pulses of 500-ms duration and up to 1-mA intensity delivered to non-affected control skin (left panel, open symbols) and in the eczema-affected skin (right panel, solid symbols). Itch was recorded in 9 patients (squares) and pain (no itch) in 17 patients (circles). The intensity of sensation increased current intensity dependently, but did not differ significantly between patient groups (itch vs no itch) or investigated skin sites (control vs eczema). Error bars indicate standard error of the mean (SEM). (B) Intensity of sensation (NRS 0–10) recorded upon 2.5-s 4-Hz sinusoidal stimuli (10 pulses) delivered at intensities of 0.025–0.4 mA to non-affected control skin (left panel, open symbols) and in the eczema-affected skin (right panel, solid symbols). Three patients responded with itch from in the eczema-affected skin (squares) and n = 22 patients reported pain (no itch, circles). The intensity of sensation increased current intensity dependently, but was not significantly different between the patient groups (itch vs no itch) or the investigated skin site (control vs eczema). Error bars indicate SEM.
Sine wave dose-response
Sinusoidal 4 Hz stimuli evoked a current intensity-dependent increase of pain (ANOVA, p < 0.0001). No significant difference was recorded between control and eczema sites (ANOVA, p > 0.8), revealing an mean NRS of 6.2 ± 0.4 (control) and 5.4 ± 0.4 (eczema) upon 2.5 s stimuli at 0.4 mA (Fig. 2B). Only 3 patients reported an itch during the 10 sine wave pulses, but no significant difference in the NRS values was calculated between the patient groups (ANOVA, p > 0.4). No significant sex differences were identified (ANOVA, p > 0.4).
Continuous sine wave stimulation for 1 min
In order to identify whether ongoing sine wave stimulation induces increasing itch in AD the current study delivered sinusoidal pulses at intensities of 0.05, 0.1 and 0.2 mA for 1 min. The choice of whether a current intensity of 0.05 or 0.1 mA was delivered was dependent on the patients’ individual sensory threshold of stimulus-perceived unpleasantness, which had been measured previously in control and eczematous skin. Accordingly, continuous sine wave stimuli of 0.05 mA were delivered to 23 and a current of 0.1 mA to 19 patients with AD. In addition, all but one patient (who withdrew due to stimulus unpleasantness) received stimuli of intensity 0.2 mA.
Continuous sine wave stimulation of 0.05 mA (n = 23) induced itch in 9 patients and pain (no itch) in 14 patients. Of the 9 patients, 2 reported itch in control skin and 8 reported itch in the eczema-affected skin (88%; Fig. 3A). The intensity of the sensation was significantly different between the patients’ groups (itch vs non-itch, ANOVA, p < 0.02). In the eczema, a maximum NRS of approximately 3 ± 0.7 was recorded in itch patients compared with NRS 1 ± 0.3 in non-itch patients (Mann-Whitney U test, p < 0.04) at 40–60 s of stimulation (Fig. 3A). No significant sex differences were identified between patients (ANOVA, p > 0.1).
Fig. 3.
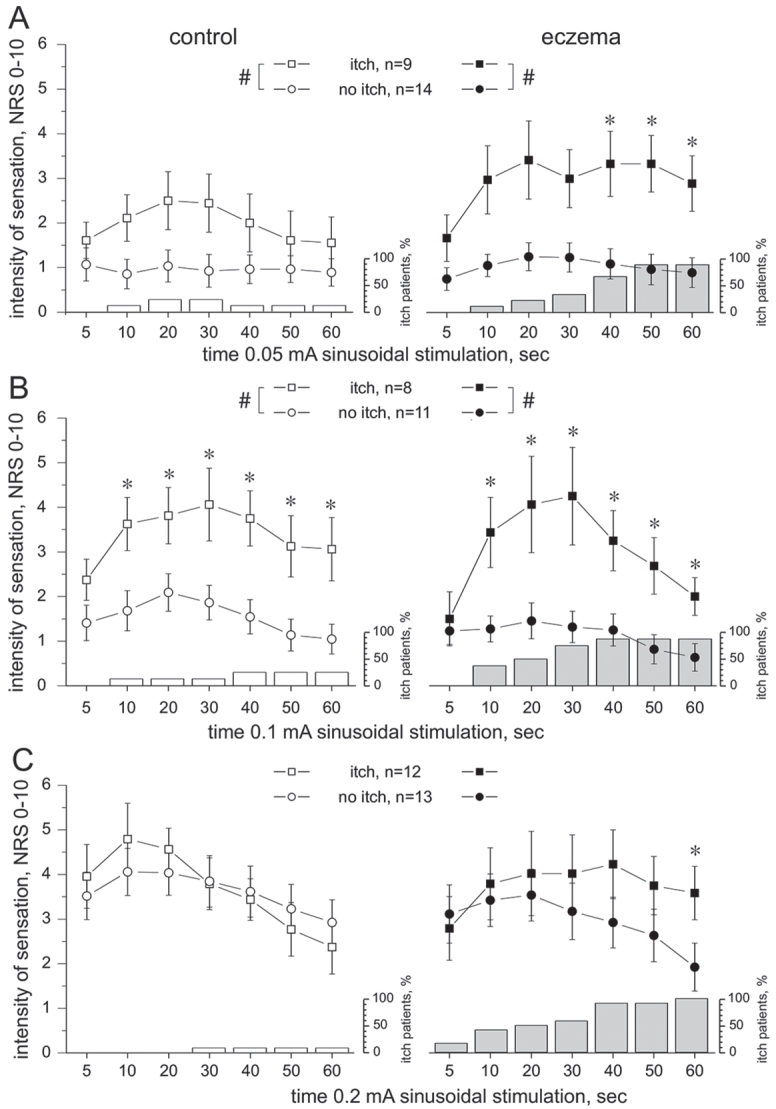
Time course of the intensity of sensation (numerical rating scale; NRS) recorded during 60 s lasting 4 Hz sine wave stimulation delivered to nonaffected control skin (left panel, open symbols) or eczema (right panel, solid symbols). Note that the number of patients perceiving itch in the eczema-affected skin increases the longer the sine wave stimuli were delivered (grey columns, depicted in % of patients reporting itch). Three patients felt itch when stimulating control skin (open columns). Error bars indicate standard error of the mean (SEM). (A) Sinusoidal currents of 0.05 mA evoked itch in 9 patients (squares), but pain in 14 patients (circle). The intensity of sensation was significantly different between the patient groups (itch vs non-itch, analysis of variance (ANOVA), #p < 0.02,), particularly in the eczema-affected skin during 40–60 s of stimulation (Mann-Whitney U test *p < 0.04). Also, 2 patients reported itch when stimulating control skin. (B) Sinusoidal currents of 0.1 mA evoked itch in 8 (squares) and pain in 11 (circles) patients with atopic dermatitis (AD). The intensity of sensation was significantly different between the groups (ANOVA, #p < 0.005) at 10–60 s of stimulation (Mann–Whitney U test, *p < 0.05) in both control skin (left panel) and eczema (right panel). (C) Continuous sine wave stimulation of 0.2 mA evoked in approximately 50% of patients itch (n = 12) and in 50% pain (n = 13). The intensity of sensation was not significantly different between the patient groups (itch vs no-itch, ANOVA, p > 0.05) or the skin sites (control vs eczema, ANOVA, p > 0.3), but pain declined continuously (solid circles), whereas itch remained significantly elevated in the eczema-affected skin (Mann–Whitney U test, *p < 0.04).
When delivering current intensities of 0.1 mA (n = 19), 8 patients reported itch and 11 patients reported pain (Fig. 3B). No significant difference of intensity was recorded between control and eczema (ANOVA, p > 0.1). At both skin sites, mean maximum intensities of NRS 4 ± 1.1 were recorded from patients with itch. In contrast, significantly lower NRS of 1.5 ± 0.3 were assessed in the non-itch group (ANOVA, p < 0.005). In particular, significant NRS differences were calculated during 10–60 s of stimulation (Mann-Whitney U test p < 0.05, Fig. 3B). No significant sex differences were identified (ANOVA, p > 0.3).
Finally, a sine wave current intensity of 0.2 mA was delivered for 60 s (n =25), which evoked itch in 12 patients and burning pain (no itch) in 13 patients. Itch or pain intensity did not differ significantly between patient groups (itch vs no itch, ANOVA, p > 0.5) or the investigated skin sites (control vs eczema, ANOVA, p > 0.3). A significant interaction was identified between the factorial groups “itch patients”, “skin site” and “duration of stimulation” (ANOVA, p < 0.005), revealing that pain sensation continuously declined in the eczema-affected skin, whereas itch remained significantly elevated until 60 s of stimulation (Mann-Whitney U test p < 0.04, Fig. 3C). No significant sex differences were identified between patients (ANOVA, p > 0.3)
Note that the number of patients reporting itch increased progressively with increasing length of stimuli (depicted in columns, Fig. 3). Eventually, at the end of the stimulation period, the maximum numbers of itch-responders was recorded. Three individuals reported itch in non-affected control skin upon sine wave stimulation, i.e. 2 patients at 0.05 and 0.1 mA, and one patient at 0.2 mA. Also, the patients’ sensation stopped almost immediately within 2 s after termination of the 60-s sine wave stimulation (not shown).
Stimulus duration dependent itch development
Increasingly more patients developed an itch sensation the longer the sine wave stimulation was delivered to the eczema (Fig. 4). Within 10 s of sinusoidal stimulation 5 patients (19%) reported itch, at 30 s 9 patients (35%) reported itch, and at the end of the stimulation period (60 s) itch was reported by 14 of the overall 26 patients (54%, Fig. 4A). Delivering a single half sine wave pulse of 500-ms duration evoked itch in 8 patients (30%) in the eczema and in one patient in control skin (Fig. 4B).
Fig. 4.
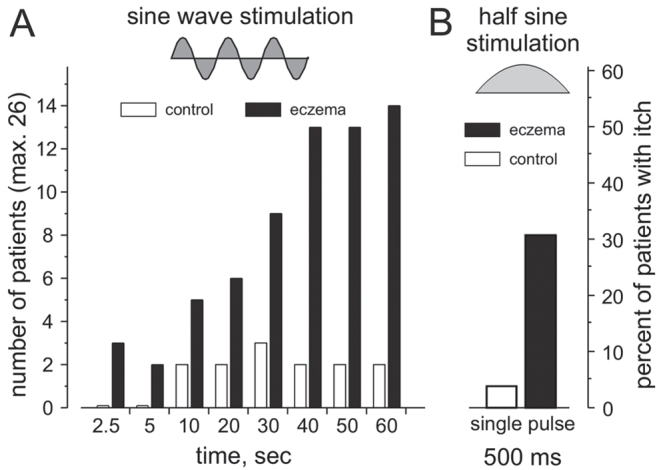
Number (left ordinate) and percentage (right ordinate) of patients reporting itch upon: (A) sine wave stimulation of increasing duration; (B) single half sine wave pulse delivered to non-affected control skin (open columns) and in the eczema-affected skin (black columns). Inlet depicts the 2 different electrical stimulation wave forms and current intensities, set at 0.025–0.2 mA for sine wave and 0.2–1 mA for half sine wave stimuli. Note that with increasing duration of sinusoidal stimulation the number of patients with atopic dermatitis (AD) reporting itch increases up to 14 (54%) out of 26 patients. A single half sine wave pulse of 500-ms duration caused itch in 8 patients (approximately 30%) in the eczema-affected skin and in one patient in non-affected control skin.
DISCUSSION
This study investigated somatosensory responses in patients with AD to slowly depolarizing currents, delivered transcutaneously, with 500-ms half sine wave pulses, and 4-Hz sine wave stimuli, both delivered to eczematous and non-affected (control) skin. Half sine wave pulses induced itch in the eczema of approximately one-third of patients. Sine wave pulses delivered continuously for 1 min evoked itch in approximately 50% of the patients (all of them also perceived half sine wave itch). Intriguingly, the number of patients reporting itch upon sinusoidal stimulation increased progressively with increasing (ongoing) sinusoidal stimulation time. Employing this novel electrical stimulation protocol we confirm that activation of polymodal nociceptors (half sine wave pulses (5)) as well as additional recruitment of silent nociceptors (sine wave pulses (6)) induces itch in affected skin in a subgroup of patients with AD. The progressively increasing occurrence of itch upon ongoing sinusoidal stimulation indicates that sustained peripheral input from unmyelinated primary afferent neurones may facilitate spinal itch transmission; for instance by activating GRPR neurones, as shown recently (11).
Itch upon electrical stimulation
Traditionally, itch is induced experimentally by the application of chemicals, for instance histamine (endogenously released from, for example, mast cells) or mucunain (cowhage spicules), which leads to consecutive activation of C-nociceptor subclasses characterized as mechano-insensitive (responding to, for example, histamine) or mechano-responsive (responding to, for example, cowhage spicules) (9). Indirect neuronal activation in the skin using itch-provoking chemical stimuli suggested a differential contribution of C-fibre classes in atopic itch (15, 16). Such chemically induced nociceptor activation involves a receptor-mediated transduction mechanism. For direct identification of particular neuronal subclasses involved in pathological itch, axonal electrical stimulation protocols of primary afferent neurons would be needed in order to circumvent the aforementioned chemical signal transduction mechanisms. It was demonstrated decades ago that rectangular electrical pulses of high frequency (25–200 Hz) and up to 5-ms pulse duration can elicit itch in the wrist and ankle in humans (1–4). Recently, slowly depolarizing electrical stimulation profiles that specifically activate mechano-responsive and mechano-insensitive C-fibres have been determined (5, 6). The current study found that, in eczematous skin of AD, this electrical stimulation paradigm caused itch in approximately 50% of patients, and thereby confirmed that both subclasses of C-nociceptors can provoke itch. It is notable that the electrically induced itch sensation disappeared immediately after termination of the stimulus. It is therefore assumed that the recorded itch is not a chemical response; for instance, caused by the release of histamine from skin mast cells, in which case the itch sensation would have lasted for several minutes after electrical stimulus offset.
It may be considered that a reduced descending inhibitory control is present in some (i.e. those patients responding with itch), but not all, of our investigated patients with AD. The electrical stimulation paradigm caused intense burning pain in the skin of healthy subjects (5, 6). Similarly, the majority of patients in the current study reported pain on electrical stimulation of non-affected skin. Given that itch can be suppressed by painful stimuli (17–20) pain is expected to be the dominant sensation rather than itch. However, some patients with AD perceived itch in the eczema, and this observation perhaps might be due to an altered itch inhibitory control mechanism comparable to the recently reported decreased conditioned pain modulation observed in subjects with chronic pruritus (21). Admittedly, a reduced descending inhibition of itch is difficult to control. Sine wave stimuli delivered with threshold intensity (0.05–0.1 mA) caused itch in fewer patients than it did at supra-threshold (0.2 mA) electrical stimulation. This result appears rather contradictory, as lower intensities of pulses would cause less painful counter stimuli, and thus should be more likely be perceived as itch. On the other hand, threshold sine wave stimulation might be too low to evoke a substantial spinal synaptic input sufficient to drive central pruriceptive neurones.
One intriguing observation was the long-lasting and progressively increasing itch sensation during the 1-min electrical sine wave stimulation period. In patients with chronic pain a similar dynamic of (in this case) pain perception was observed previously upon continuous sinusoidal stimulation, particularly at neuropathically painful skin sites, but also in non-painful areas (6). In AD, the addressed “itch”-fibres (mechanically responsive and mechano-insensitive C-nociceptors) apparently reveal a comparable lack of adaptation, both in the affected and non-affected (control) skin from patients who reported itch upon slowly depolarizing stimulation. In these patients an axonal sensitization of peripheral pruritoceptors may be considered, but central (spinal or supra-spinal) mechanisms of itch sensitization, as discussed below (11), could also be involved.
Triggering spinal itch?
Approximately 30% of patients in the current study responded with pruritus to half sine wave stimulation in eczema-affected areas, and the occurrence of itch increased with higher current intensities (0.6–1 mA). Notably, stronger half sine wave currents enhance action potential discharges of polymodal nociceptors (5). The longer the C-nociceptors were stimulated by electrical sine wave stimulation (6) the more patients with AD felt this stimulation as an itch. The progressively increasing development of itch with electrical stimulation might be due to an increased spinal synaptic input that is required to trigger itch, as shown recently (11). The authors demonstrated that repetitive bursts of presynaptic GRP neurones induce progressive depolarization of postsynaptic GRP-sensing neurones sufficient to relay spinal pruriceptive information (11). It may thus be hypothesized that the supra-threshold half sine wave, as well as the ongoing sine wave stimulation in the eczema-affected areas in the current study provides the peripheral input to trigger sufficient spinal GRP release entailed to provoke itch in a subgroup of patients with AD. The electrical stimulation profile in the current study thus provides a simple and fast experimental tool to assess axonal peripheral sensitization or facilitated central itch processing in patients with chronic itch. Patients identified as likely to have facilitated spinal processing of itch might benefit from centrally acting antipruritic therapy.
ACKNOWLEDGEMENTS
The authors thank Frau Ilona Roßbach for careful proofreading of the manuscript.
The study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft DFG), project grant PruSearch FOR 2690.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Ikoma A, Handwerker H, Miyachi Y, Schmelz M. Electrically evoked itch in humans. Pain 2005; 113: 148–154. [DOI] [PubMed] [Google Scholar]
- 2.Shelley WB, Arthur RP. The neurohistology and neurophysiology of the itch sensation in man. AMA Arch Derm 1957; 76: 296–323. [DOI] [PubMed] [Google Scholar]
- 3.Tuckett RP. Itch evoked by electrical stimulation of the skin. J Invest Dermatol 1982; 79: 368–373. [DOI] [PubMed] [Google Scholar]
- 4.Edwards AE, Shellow WV, Wright ET, Dignam TF. Pruritic skin diseases, psychological stress, and the itch sensation. A reliable method for the induction of experimental pruritus. Arch Dermatol 1976; 112: 339–343. [PubMed] [Google Scholar]
- 5.Rukwied R, Thomas C, Obreja O, Werland F, Kleggetveit IP, Jorum E, et al. Slow depolarizing stimuli differentially activate mechanosensitive and silent C-nociceptors in human and pig skin. Pain 2020; 161: 2119–2128. [DOI] [PubMed] [Google Scholar]
- 6.Jonas R, Namer B, Stockinger L, Chisholm K, Schnakenberg M, Landmann G, et al. Tuning in C-nociceptors to reveal mechanisms in chronic neuropathic pain. Ann Neurol 2018; 83: 945–957. [DOI] [PubMed] [Google Scholar]
- 7.Jonas R, Namer B, Schnakenberg M, Soares S, Pakalniskis J, Carr R, et al. Sympathetic efferent neurons are less sensitive than nociceptors to 4 Hz sinusoidal stimulation. Eur J Pain 2020; 24: 122–133. [DOI] [PubMed] [Google Scholar]
- 8.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci 2008; 28: 7659–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol 2008; 100: 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen XJ, Sun YG. Central circuit mechanisms of itch. Nat Commun 2020; 11: 3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pagani M, Albisetti G.W, Sivakumar N, Wildner H, Santello M, Johannssen HC, et al. How gastrin-releasing peptide opens the spinal gate for itch. Neuron 2019; 103: 102–117.e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry DM, Liu XT, Liu B, Liu XY, Gao F, Zeng X, et al. Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors. Nat Commun 2020; 11: 1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science 2013; 340: 968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Polgar E, Solinski HJ, Mishra SK, Tseng PY, Iwagaki N, et al. Circuit dissection of the role of somatostatin in itch and pain. Nat Neurosci 2018; 21: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen HH, Elberling J, Solvsten H, Yosipovitch G, Arendt-Nielsen L. Nonhistaminergic and mechanical itch sensitization in atopic dermatitis. Pain 2017; 158: 1780–1791. [DOI] [PubMed] [Google Scholar]
- 16.Papoiu AD, Tey HL, Coghill RC, Wang H, Yosipovitch G. Cowhage-induced itch as an experimental model for pruritus. A comparative study with histamine-induced itch. PLoS One 2011; 6: e17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson HJ, Psouni E, Carstam R, Schouenborg J. Profound inhibition of chronic itch induced by stimulation of thin cutaneous nerve fibres. J Eur Acad Dermatol Venereol 2004; 18: 37–43. [DOI] [PubMed] [Google Scholar]
- 18.Brull SJ, Atanassoff PG, Silverman DG, Zhang JM, LaMotte RH. Attenuation of experimental pruritus and mechanically evoked dysesthesiae in an area of cutaneous allodynia. Somatosens Mot Res 1999; 16: 299–303. [DOI] [PubMed] [Google Scholar]
- 19.Ward L, Wright E, McMahon SB. A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain 1996; 64: 129–138. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One 2011; 6: e22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pogatzki-Zahn EM, Pereira MP, Cremer A, Zeidler C, Dreyer T, Riepe C, et al. Peripheral sensitization and loss of descending inhibition is a hallmark of chronic pruritus. J Invest Dermatol 2020; 140: 203–211.e204. [DOI] [PubMed] [Google Scholar]