Abstract
Background
Risks for cardiovascular diseases, including myocardial infarction and stroke, are elevated in people with HIV infection (PWH). However, no trials of statin utilization with clinical cardiovascular disease (CVD) end points have been completed in PWH, and there are sparse real-world data regarding statin use and lipid-lowering effectiveness. We therefore used a unique cohort of PWH and uninfected controls to evaluate (1) differences in statin types used for PWH versus uninfected persons; (2) lipid lowering achieved by statin use for PWH versus uninfected persons; and (3) racial and ethnic disparities in appropriate statin use among PWH and uninfected persons.
Methods
We analyzed a cohort of 5,039 PWH and 10,011 uninfected demographically matched controls who received care at a large urban medical center between January 1, 2000, and May 17, 2017. Medication administration records, prescription data, and validated natural language processing algorithms were used to determine statin utilization. Statins were categorized by generic active ingredient name and intensity (high, moderate, or low). Lipid values collected in routine clinical care were available for analysis. The first set of analyses was restricted to PWH and uninfected matched controls taking statins and compared (1) differences in statin type and (2) difference in cholesterol levels after versus before statin initiation by HIV status. For the second set of analyses, we first used prevalent CVD risk factors to determine participants with statin indications and then determined how many of these participants were taking statins. We then compared statin utilization among persons with indications for statins by race/ethnic group for PWH and uninfected matched controls using multivariable-adjusted logistic regression.
Results
Among people prescribed statins, PWH were more likely than controls to have ever taken pravastatin (34.8% vs 12.3%, P < .001) or atorvastatin (72.2% vs 65.6%, P = .002) and less likely to have ever taken simvastatin (14.2% vs 39.5%, P < .001). Among PWH with indications for statin utilization, 55.7% of whites, 39.4% of blacks, and 45.8% of Hispanics were prescribed statins (P < .001). These differences in statin prescription by race/ethnicity remained significant after adjustment for demographics (including insurance status), cardiovascular risk factors, antiretroviral therapy use, HIV viremia, and CD4 count. These racial/ethnic disparities in statin utilization were less pronounced among uninfected persons.
Conclusions
Among PWH with statin indication(s), blacks and Hispanics were less likely than whites to have been prescribed a statin. These racial/ethnic disparities were less pronounced among uninfected persons. There were significant differences in type of statin used for PWH compared to uninfected matched controls. Future efforts addressing disparities in CVD prevention among PWH are warranted.
With widespread uptake of effective antiretroviral therapy (ART), morbidity and mortality from acquired immunodeficiency syndrome (ATDS)-defining illnesses have declined, whereas cardiovascular disease (CVD) has become more prominent among people with HIV infection (PWH).1–3 PWH have greater risks for atherosclerotic CVD (ASCVD) compared with uninfected controls.4 The elevated risk is due in part to the higher prevalence of traditional CVD risk factors such as smoking, diabetes, and dyslipidemia in PWH. However, after accounting for traditional risk factors, PWH still have a 1.5- to 2-fold greater risk of ASCVD than the general population.1,2,4,5
3-Hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors (“statins”), which substantially decrease vascular event rates across CVD risk levels, form the backbone of primary and secondary prevention of ASCVD in the general population.6,7 Whereas well-powered trials with adjudicated clinical end points have evaluated statins in the general population and are in evidence-based guidelines,8 similar data are unavailable among PWH, although the Randomized Trial to Prevent Vascular Events in HIV is ongoing (ClinicalTrials.gov Identifier: NCT02344290). Furthermore, some studies suggest that the cholesterol-lowering effectiveness of statins may be somewhat different for PWH compared with the general population, although it remains unclear whether these findings relate to different statin intensities, coadministration of different ART regimens, adherence, or other factors.9–12 Several studies have reported statin underutilization among PWH.13,14 Possible contributing factors include a care model that prioritizes ART and management of opportunistic infections with less emphasis on noninfectious chronic diseases, a dearth of statin safety and outcome data for PWH, and concerns over drug-drug interactions between ART and certain statins.13,15,16 With the transition of HIV to a chronic manageable disease state marked by chronic inflammation and immune activation, prevention and management of CVD and other chronic noninfectious comorbidities are expected to join center stage.
The studies that have evaluated statin utilization among PWH to date have generally lacked well-matched uninfected control populations and data granularity required for real-world assessment of statin types and intensity utilization. Furthermore, although differences by race and sex in statin utilization exist in the general population,17,18 the extent to which these differences exist among PWH is unknown. Given considerably lower rates of HIV care retention among black PWH versus white and Hispanic PWH,19 we anticipated that appropriate statin utilization would likewise be lowest among black PWH.
Therefore, in this study, we evaluated statin utilization, cholesterol-lowering effectiveness, and racial differences in statin use in PWH and demographically matched uninfected controls at a large urban medical system. Our hypotheses were that (1) significant differences in type and intensity of statin used exist for PWH versus uninfected controls, (2) lipid lowering after statin initiation in statin-naive PWH is lower than in statin-naive uninfected controls, and (3) black and Hispanic PWH are significantly less likely than white PWH to receive indicated statin therapy.
Methods
Cohort
The HIV Electronic Comprehensive Cohort of CVD Complications (HIVE-4CVD) is an electronic data repository of 5,039 PWH and 10,011 uninfected controls identified from the Northwestern Medicine Enterprise Data Warehouse (NMEDW). PWH were eligible for inclusion in the cohort if they were at least 18 years of age; received care at Northwestern Medicine (NM) between January 1, 2000, and May 17, 2017; and met at least 1 of the following criteria for HIV+ status: (1) positive HIV-1 antibody or serology, (2) positive (>0) HIV viral load, or (3) at least 3 separate dates on which HIV viral load and CD4 T-cell count were ordered concurrently. These criteria have been previously validated for identification of HIV-infected patients in electronic records.20 Uninfected controls were frequency-matched 2:1 with PWH based on age, sex, race/ethnicity, zip code of primary residence in the medical record, and clinic location within the NM system. The Northwestern University Institutional Review Board approved the HIVE-4CVD creation and research protocol with a waiver of consent applied.
Determination of statin type and dose
Each statin/dose combination was extracted from the NMEDW using text search algorithms across all inpatient and outpatient encounters, prescription documentation, and medication administration records for patients in HIVE-4CVD. Statin type and dose were determined using the RxNorm drug terminology (National Library of Medicine, Bethesda, MD). Generic statins were not differentiated from brand name statins. Start date was determined as the earliest date recorded at which any statin was recorded for each patient in HIVE-4CVD. The algorithm identified correctly spelled statins coupled with dosages in patient-reported home medication records, inpatient medication administration records, and prescription records. The natural language extraction algorithm was validated by manual review of randomly selected patient medical records, which revealed zero disagreements between the automatically extracted and manually reviewed statin/dose combinations. From the NMEDW, we were unable to directly assess patient adherence to statin medications prescriptions or documented history of administration.
Statin total cholesterol-lowering effectiveness
To determine changes in total cholesterol before and after statin initiation, prestatin cholesterol values for each patient were determined based on the final cholesterol panel prior to initiation of statin therapy. Poststatin lipid values were taken from each patient’s first cholesterol panel at least 60 days after the initiation of statin therapy; we did not include cholesterol panels from within the first 60 days after statin therapy initiation to ensure sufficient time for statin initiation and achieving of steady-state levels. Total cholesterol (TC) was the primary lipid variable analyzed. Low-density lipoprotein cholesterol (LDL-C) was also analyzed but as a secondary lipid variable because hypertriglyceridemia is particularly common in PWH and significantly elevated triglycerides invalidate LDL-C values calculated using the Friedewald equation (the source of the majority of LDL-C data in HIVE-4CVD). Analyses of lipid lowering following statin initiation were restricted to patients with lipid values prior to statin initiation (without any documented statin use prior to or on the day of the earliest cholesterol panel) who also had cholesterol panels checked at least 60 days after statin initiation.
Demographic and clinical covariates
Age, sex, insurance status, and race/ethnicity (white non-Hispanic, black non-Hispanic, Hispanic, other) were determined from administrative records from each patient’s first clinical encounter. Hypertension diagnoses were determined using International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes and use of antihypertensive medication. These methods were used instead of direct blood pressure measurements because of systematic differences in frequency and distribution of blood pressure measurements in different clinical settings (eg, inpatient vs outpatient). Diabetes mellitus (DM) diagnoses were determined using ICD-9 or ICD-10 codes and either a measured hemoglobin A1c >6.5% or use of diabetes medication. Coronary heart disease (CHD) diagnoses were established using administrative (ICD-9, ICD-10, or Current Procedural Terminology) codes for coronary revascularization, myocardial infarction, or a primary CHD diagnosis, lipid values and other laboratory values from January 1, 2000, to May 17, 2017, were extracted for all patients with any lipid levels checked in clinical care, regardless of fasting status. For PWH, nadir CD4+ T-cell counts (cells/mm3), peak HIV viral load, and use of ART were determined using methods we have published previously.21
Indications for statin therapy
Patients were categorized as having indications for statin therapy if they had a diagnosis of CHD or DM, as defined above, or if they ever had a TC ≥240 mg/dL.22 The 2013 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines and ASCVD risk scores were not used to determine indications for statin therapy because the majority of cohort calendar time in HIVE-4CVD predates these guidelines.
Statistical analyses
An overview of the cohort and analyses performed is included in Figure 1. The first set of analyses compared patterns of statin utilization and lipid lowering after statin initiation for PWH versus uninfected matched controls. We started by comparing demographic and clinical covariates for PWH versus uninfected controls overall and separately for PWH versus uninfected controls taking statins. We then restricted our analyses to PWH and uninfected controls taking statins and analyzed differences in use of individual statin type (“types”, eg, atorvastatin vs pravastatin) and intensity. Statin intensity was determined according to 2013 ACC/AHA guidelines, which classify “high”-intensity statins as those expected to achieve ≥50% LDL-c reduction, “moderate” a 30% to <50% LDL-c reduction, and “low” a <30% LDL-c reduction.6 Next, we compared intraindividual changes in TC before and after statin initiation for PWH versus uninfected controls. Multivariable-adjusted general regression models adjusted for age, sex, race/ethnicity, and baseline TC were used to evaluate the association of HIV status with poststatin changes in TC. Because of the possibility of statin intensity (and related lipid lowering) differing systematically for PWH versus uninfected persons, we performed sensitivity analyses of TC lowering by statin intensity (high, moderate, or low) for PWH versus uninfected controls.
Figure 1.
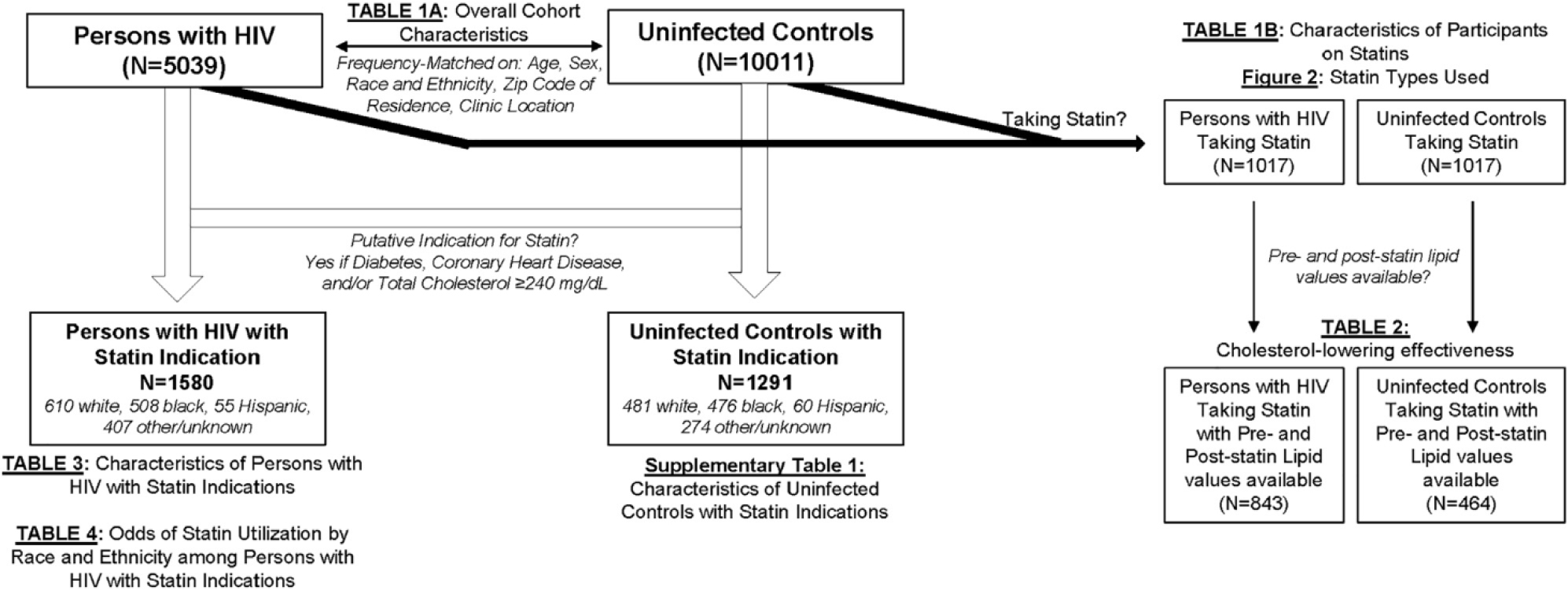
Overview of cohort and analyses.
The second set of analyses evaluated disparities by race/ethnicity (non-Hispanic white, non-Hispanic black, or Hispanic; other races/ethnicities were excluded because of insufficient numbers) in statin utilization among PWH with putative indications for statin use. Multivariable logistic regression models adjusted for age, sex, insurance status, DM, hypertension, CHD, HIV-specific variables (nadir CD4+ T-cell count, peak HIV viral load, any antiretroviral use, and any protease inhibitor use), and baseline LDL-C were used to analyze racial/ethnic disparities in statin use among PWH for whom statin use was indicated. We chose to restrict analyses to persons with statin indications and then perform multivariable adjustment (rather than propensity matching-based analyses) because we judged this approach to be most clinically relevant and reproducible in other cohorts. We included protease inhibitors as a separate variable from any ART use because of the potential for drug interactions between boosted protease inhibitors and statins,23 which may have led to safety concerns for statin administration by HIV and cardiology providers. A sensitivity analysis examining racial/ethnic differences in statin use among uninfected controls for whom statins were indicated was also performed. An additional analysis of antihypertensive medication use among persons with hypertension was performed to determine if patterns of indicated medication use across race/ethnic groups were similar or different than with statins.
All analyses were performed using Stata version 14 (StataCorp 15, College Station, TX). A P < .05 was considered statistically significant.
Funding source
The research and creation of this paper were supported by funding from the American Heart Association (Fellow-to-Faculty Transition Award FTF 31200010; Feinstein M. J., PI). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Results
Statin type, dose, and lipid lowering for PWH and uninfected matched controls
Demographic characteristics and relevant clinical covariates of PWH and uninfected controls in HIVE-4CVD overall (n=15,050) and persons in HIVE-4CVD with any documented statin use (n=1830) are summarized in Tables I and II, respectively. Our PWH and control cohorts were well matched by age, sex, and race. In the overall cohort (Table I), PWH were sicker than uninfected persons (more likely to have diabetes and hypertension and to be taking statins). In the nested cohort of PWH and uninfected persons taking statins (Table II), the composition of age, sex, and race/ethnicity was similar for PWH and uninfected persons, and uninfected persons were marginally sicker (slightly more likely to have diabetes and hypertension). Regarding specific statin used (Figure 2), PWH who ever used statins were significantly more likely than uninfected controls to have been prescribed pravastatin (34.8% vs 12.3%; P < .001), atorvastatin (72.2% vs 65.7%; P = .002), and rosuvastatin (19.4% vs 14.3%; P = .004) but less likely to have taken simvastatin (14.2% vs 39.5%; P < .001). There was no significant difference in the proportion of patients who took high-intensity statins (atorvastatin ≥40 mg daily or rosuvastatin ≥20 mg daily; P = .75). Analyses of cholesterol lowering after statin initiation revealed no statistically significant difference in TC or LDL-C lowering after statin initiation for PWH compared to uninfected controls, although there was a nonsignificant pattern of somewhat less TC and LDL-C lowering among PWH (Table III).
Table I.
Characteristics of PWH and uninfected controls in HIVE-4CVD
| HIV+ (n = 5039) | Uninfected controls (n = 10,011) | P value | |
|---|---|---|---|
|
| |||
| Average age, y | 49.0 | 48.6 | .06 |
| Age 40–75 y, n (%) | 3839 (76.2%) | 7578 (75.7%) | .51 |
| Sex, n male (%) | 4166 (82.7%) | 8262 (82.5%) | .824 |
| Race | <.001 | ||
| Black, n (%) | 1572 (32.4%) | 2771 (27.7%) | |
| White, n (%) | 1680 (34.6%) | 3008 (30.1%) | |
| Hispanic, n (%) | 172 (3.5%) | 461 (4.6%) | |
| Other/unknown, n (%) | 1615 (32.1%) | 3771 (37.7%) | |
| HTN diagnosis, n (%) | 1674 (33.2%) | 1775 (17.7%) | <.001 |
| DM diagnosis, n (%) | 463 (9.2%) | 541 (5.4%) | <.001 |
| TC, baseline, mg/dL | 171.89 | 182.5 | <.001 |
| HDL, baseline, mg/dL | 39.74 | 45.80 | <.001 |
| TG, baseline, mg/dL | 161.13 | 126.98 | <.001 |
| Statin therapy, n (%) | 1017 (20.2%) | 813 (8.1%) | <.001 |
| Nadir CD4, cells/mm3 | 276.41 | n/a | |
| ART, n (%) | 4251 (84.7%) | n/a | |
| Protease inhibitor, n (%) | 2363 (46.9%) | n/a | |
HTN, hypertension.
Table II.
Characteristics of PWH and uninfected controls taking statins in HIVE-4CVD
| Patients ever taking statins |
|||
|---|---|---|---|
| PWH (n = 1017) | Uninfected controls (n = 813) | P value | |
|
| |||
| Age, y | 56.7 | 57.1 | .19 |
| Age 40–75 y, n (%) | 946 (93.0%) | 750 (92.2%) | .49 |
| Sex, n male (%) | 887 (87.2%) | 718 (88.4%) | .43 |
| Race | .11 | ||
| Black, n (%) | 260 (25.7%) | 248 (30.7%) | |
| White, n (%) | 480 (45.2%) | 365 (47.4%) | |
| Hispanic, n (%) | 72 (7.1%) | 48 (5.8%) | |
| Other/unknown, n (%) | 205 (20.2%) | 152 (18.7%) | |
| HTN diagnosis, n (%) | 739 (72.7%) | 638 (78.5%) | .004 |
| DM diagnosis, n (%) | 249 (24.5%) | 267 (32.8%) | <.001 |
| TC, baseline, mg/dL | 189.69 | 192.14 | .18 |
| HDL, baseline, mg/dL | 38.12 | 43.00 | <.001 |
| TG, baseline, mg/dL | 198.54 | 151.87 | <.001 |
| Statin therapy, n (%) | NA | NA | |
| Nadir CD4, cells/mm3 | 286.75 | NA | |
| ART use, n (%) | 988 (97.15%) | NA | |
| Protease inhibitor–based ART, n (%) | 606 (59.59%) | NA | |
Figure 2.
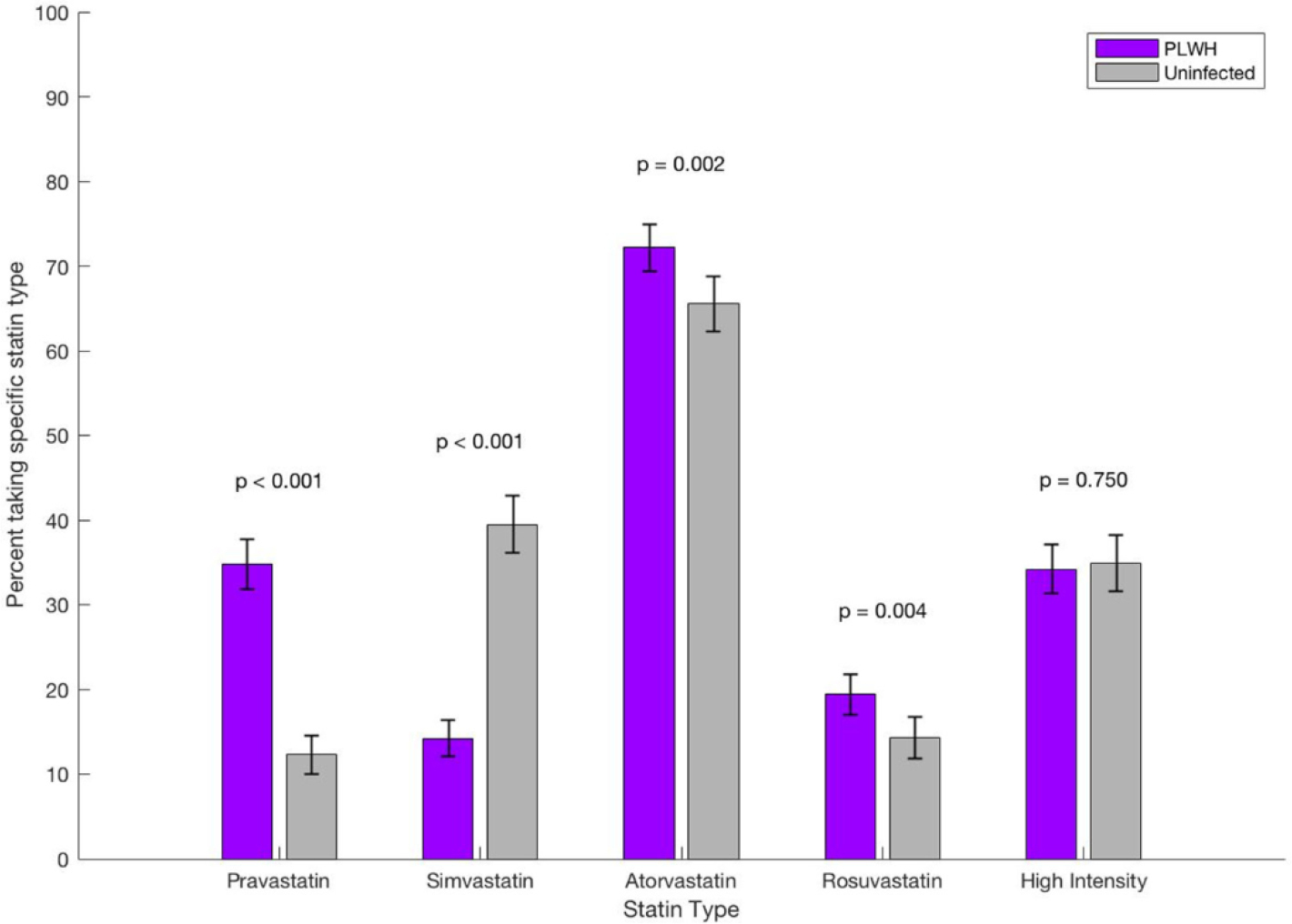
Statin types used among persons in HIVE-4CVD ever taking statins.
Table III.
Total cholesterol change with statin use for PWH and uninfected controls
| PWH (n = 843) | Uninfected controls (n = 464) | P value | |
|---|---|---|---|
|
| |||
| Prestatin TC, mean (mg/dL) | 203.3 (199.7, 206.9) | 202.2 (197.5, 207.0) | .72 |
| Poststatin TC, mean (mg/dL) | 182.6 (179.0, 186.2) | 173.7 (169.7, 177.6) | .002 |
| Change in TC, mean (mg/dL) | −22.9 (−19.6, −26.2) | −28.5 (−23.7, −33.3) | .06 |
| Difference in TC lowering, adjusted for age, sex, and race | 5.4 (11.5, −0.7) | Referent | .08 |
| Difference in TC lowering, adjusted for age, sex, race, and prestatin TC | 4.0 (10.1, −2.1) | Referent | .20 |
| Difference in TC lowering, adjusted for age, sex, race, prestatin TC, and whether or not statin intensity was ever reduced | 3.8 (9.9, −2.3) | Referent | .22 |
| Prestatin LDL-C, mean | 127.7 (124.5, 131.0) | 130.0 (125.7, 134.4) | .41 |
| Poststatin LDL-C, mean | 104.8 (102.0, 107.6) | 103.0 (99.6, 106.5) | .44 |
| Change in LDL-C, mean | −23.3 (−20.4, −26.3) | −27.0 (−22.6, −31.3) | .16 |
| Difference in LDL-C lowering, adjusted for age, sex, and race | 3.80 (9.11, −1.61) | Referent | .17 |
| Difference in LDL-C lowering, adjusted for age, sex, race, and prestatin LDL-C | 2.57 (7.12, −1.97) | Referent | .27 |
| Participants on high-intensity statins (n = 256 for PWH, n = 129 for controls) | |||
| Change in TC, mean (mg/dL) | −22.1 (−28.7, −15.5) | −27.7 (−36.3, −19.2) | .32 |
| Participants on moderate-intensity statins (n = 605 for PWH, n = 296 for controls) | |||
| Change in TC, mean (mg/dL) | −23.1 (−27.0, −19.2) | −26.5 (−32.0, −21.0) | .32 |
| Participants on low-intensity statins (n = 73 for PWH, n = 197 for controls) | |||
| Change in TC, mean (mg/dL) | −17.7 (−24.6, −10.9) | −17.8 (−27.9, −7.7) | .99 |
Statin intensity was determined according to 2013 ACC/AHA guidelines, which classify “high”-intensity statins as those expected to achieve ≥50% LDL-c reduction, “moderate” a 30% to <50% LDL-c reduction, and “low” a <30% LDL-c reduction.6
Racial and ethnic differences in statin utilization PWH
When we evaluated statin utilization among PWH with indications for statins, significant differences by race and ethnicity were apparent (Table IV). Whereas 340 of 610 white PWH (55.7%) with indications for statin were prescribed a statin, only 200 of 508 black (39.4%) and 66 of 143 Hispanic (45.8%) PWH with statin indications received a statin prescription (P < .001). After adjustment for age, sex, insurance status, hypertension, DM, CHD, nadir CD4+ T-cell count, peak HIV viral load, antiretroviral therapy, and baseline LDL-c (Table V), black PWH with statin indications were significantly less likely than white PWH with statin indications to be prescribed statins (odds ratio 0.59, 95% CI 0.42–0.85, P = .004). The pattern was similar but did not reach statistical significance for Hispanic versus white PWH with indications for statins (odds ratio 0.60, 95% CI 0.36–1.02; P = .06).
Table IV.
Characteristics of PWH with likely indications for statin use, by race/ethnicity
| Black (n = 508) | Hispanic (n = 143) | White (n = 610) | P | |
|---|---|---|---|---|
|
| ||||
| Age, y (mean) | 54.0 (53.2–54.8) | 52.2 (50.7–53.8) | 55.9 (55.2–56.7) | |
| Sex, n male (%) | 385 (75.8%) | 113 (79.0%) | 561 (92.0%) | <.001 |
| HTN diagnosis, n (%) | 412 (81.1%) | 96 (67.1%) | 404 (66.2%) | <.001 |
| DM diagnosis, n (%) | 185 (36.4%) | 58 (40.6%) | 137 (22.5%) | <.001 |
| CHD diagnosis, n (%) | 306 (60.2%) | 65 (45.5%) | 237 (38.9%) | <.001 |
| Nadir CD4 (95% CI), cells/mm3 | 199.9 (182.9–216.8) | 261.5 (226.2–296.8) | 272.0 (255.9–288.1) | <.001 |
| Log10 of peak HIV viral load | 9.9 (9.6–10.2) | 9.1 (8.5–9.7) | 8.6 (8.3–8.9) | <.001 |
| ART use, n (%) | 444 (87.4%) | 131 (91.6%) | 583 (95.6%) | <.001 |
| Protease inhibitor–based ART, n (%) | 298 (58.7%) | 86 (60.14%) | 364 (59.7%) | .92 |
| Taking statin, n (%) | 200 (39.4%) | 66 (45.8%) | 340 (55.7%) | <.001 |
Table V.
Multivariable-adjusted odds of statin utilization by race and ethnicity among persons with HIV with likely indications for statins
| Model 1 | P value | Model 2 | P value | Model 3 | P value | Model 4 | P value | Model 5 | P value | Model 6 | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Black (vs white) | 0.52 (0.41, 0.66) | <.01 | 0.56 (0.44, 0.73) | <.01 | 0.57 (0.44, 0.74) | <.01 | 0.51 (0.38, 0.66) | <.01 | 0.54 (0.39, 0.74) | <.01 | 0.59 (0.42, 0.85 | .004 |
| Hispanic (vs white) | 0.57 (0.40, 0.83) | <.01 | 0.70 (0.48, 1.03) | .07 | 0.73 (0.49, 1.09) | .12 | 0.67 (0.45, 1.01) | .06 | 0.60 (0.37, 0.97) | .04 | 0.60 (0.36, 1.02) | .06 |
| Age | 1.06 (1.05, 1.08) | <.01 | 1.06 (1.05, 1.08) | <.01 | 1.05 (1.04, 1.07) | <.01 | 1.06 (1.04, 1.08) | <.01 | 1.06 (1.04, 1.08) | <.001 | ||
| Female sex | 0.85 (0.62, 1.19) | .35 | 0.85 (0.60, 1.12) | .35 | 0.84 (0.60, 1.19) | .33 | 0.94 (0.63, 1.40) | .90 | 0.91 (0.59, 1.42) | .69 | ||
| Private insurance | 1.02 (0.36, 2.86) | .97 | 0.98 (0.35, 2.77) | .97 | 1.15 (0.36, 3.72) | .81 | 1.90 (0.44, 8.17) | .39 | ||||
| Medicare | 0.96 (0.54, 1.69) | .88 | 0.87 (0.49, 1.55) | .64 | 0.97 (0.49, 1.91) | .92 | 0.79 (0.38, 1.64) | .53 | ||||
| Medicaid or other public aid | 0.93 (0.54, 1.59) | .78 | 0.93 (0.54, 1.60) | .79 | 0.97 (0.51, 1.84) | .92 | 0.83 (0.42, 1.64) | .59 | ||||
| HTN | 1.63 (1.21, 2.20) | <.01 | 1.51 (1.06, 2.15) | .02 | 1.44 (0.97, 2.13) | .07 | ||||||
| DM | 1.35 (1.02, 1.79) | <.01 | 1.53 (1.10, 2.13) | .01 | 1.99 (1.36, 2.91) | <.001 | ||||||
| CHD | 0.99 (0.76, 1.29) | .94 | 1.36 (0.99, 1.87) | .06 | 1.87 (1.30, 2.69) | .001 | ||||||
| Nadir CD4 (per 100 cells/mm3 higher) | 1.20 (1.12, 1.29) | <.01 | 1.14 (1.04, 1.24) | .003 | ||||||||
| Log10 of peak viral load | 0.99 (0.94, 1.04) | .56 | 0.98 (0.93, 1.04) | .61 | ||||||||
| ART use | 8.84 (3.53, 22.1) | <.01 | 8.56 (2.72, 26.9) | <.01 | ||||||||
| Protease inhibitor–based ART | 1.36 (0.98, 1.86) | .06 | 1.31 (0.92, 1.86) | .13 | ||||||||
| Baseline LDL (per 10 mg/dL higher) | 1.11 (1.06, 1.15) | <.001 | ||||||||||
Sensitivity analyses revealed that these racial differences were less pronounced among uninfected persons in HIVE-4CVD, although they were statistically significant after adjustment for demographics and cardiovascular risk factors (Supplementary Table I). In a separate analysis, there were no consistent or significant differences in antihypertensive medication use for black versus white versus Hispanic PWH or uninfected persons with hypertension (data not shown).
Discussion
In this study, we evaluated real-world statin utilization for PWH and demographically matched uninfected controls in clinical care at a large university-based health care system. We found that black and Hispanic PWH with indications for statins (CHD, DM, and/or hypercholesterolemia with TC ≥240 mg/dL) were significantly less likely than white PWH to be prescribed statins even after adjustment for demographic and clinical factors; these difference were not as pronounced among uninfected persons and may represent a racial disparity in cardiovascular disease prevention for PWH. Regarding statin types, PWH were approximately 3 times more likely to take pravastatin than uninfected controls and approximately one-third as likely to take simvastatin. Lipid lowering after statin initiation was not significantly different for PWH versus uninfected controls.
Our finding of dramatic racial differences in statin utilization by race among PWH but not uninfected controls is concerning and may reflect a “double jeopardy” phenomenon, whereby racial/ethnic minorities with HIV may be particularly vulnerable. Black and Hispanic PWH with DM, CHD, and/or significant hypercholesterolemia consistently had 0.5–0.6 times the odds of white PWH to be taking a statin after considerable multivariable adjustment, including for demographics, clinical characteristics, HIV viremia and immunosuppression, and baseline LDL-C levels. Although we expected some racial/ethnic differences in statin utilization given previous studies in uninfected patients demonstrating lower statin utilization rates among racial/ethnic minorities compared with whites, 24–27 the extent to which this potential racial disparity was present among PWH (and more pronounced than for uninfected persons) was surprising. One potential reason for this would be if racial/ethnic disparities in access to care—particularly subspecialty and CVD care—are greater among PWH. Another possible related explanation is that HIV transmission factors most associated with socioeconomic vulnerability and fragmented care—such as intravenous drug use—are more common among black and Hispanic PWH than white PWH. The design of our study precludes us from determining why participants were or were not prescribed statins; we sought to account for obvious socioeconomic and access-related causes by adjusting for insurance status, although the possibility of residual confounding certainly exists. Furthermore, HIVE-4CVD does not have reliable substance use data, so we were not able to include these data in our analyses. Nevertheless, our findings may reflect a public health need for improved CVD screening and prevention programs among certain racial and ethnic groups of PWH. In the short term, further studies in other diverse cohorts of PWH in clinical care are needed to confirm our findings.
We also found highly significant differences in the statin types and doses prescribed for PWH versus uninfected persons in HIVE-4CVD. These differences were expected, particularly the relatively low rates of simvastatin utilization among PWH, for whom simvastatin is relatively contraindicated.15 Meanwhile, pravastatin was 3 times as common among PWH on statins versus uninfected controls on statins, suggesting that this was a common alternative for PWH who may have otherwise been prescribed simvastatin. Certainly, much of the relative excess in pravastatin among PWH may be driven by concerns regarding statin interactions with ART. These concerns are based primarily on pharmacokinetic data which have, appropriately, led to simvastatin being contraindicated among PWH.15,23,28 It remains unclear whether the benefits of higher-intensity statins (particularly atorvastatin 40 mg daily or greater and rosuvastatin 20 mg daily or greater) outweigh their risks for PWH given the potential for adverse effects but also the substantial potential CVD risk-reducing benefit of intensified statin therapy.
Similar to previous studies, we found somewhat (though not statistically significant) less lipid lowering for PWH versus controls taking statins, with percent LDL-C reduction similar to those in a previous study of Kaiser Permanente data that did not distinguish statin type and dose.9–11 Whether this reflects differences in statin adherence, statin doses being decreased more commonly among PWH (with resulting reduced lipid lowering), or other mechanisms is unclear. Our finding that achieved LDL-C levels on statins were similar for PWH and uninfected persons corroborates the findings of a recent study in the Multicenter AIDS Cohort Study.29
A key strength of this study is that the cohort represents a racially and ethnically diverse cohort receiving inpatient and/or outpatient care in an urban university-based health care system. Our findings may therefore be generalizable to similar routine care settings, which is a limitation of cohorts that require regular and frequent participant follow-up. There were several limitations to this study. As with any study of nonrandomized medication data, confounding by indication limits potential comparison between groups. We sought to address this (particularly for the race/ethnic differences in statin use among PWH) by restricting our analyses of statin utilization to patients with likely indications for statins based on diagnoses of DM, CHD, and/or significant hypercholesterolemia. We judged this to be more clinically relevant alternative to matching on propensity scores for statin use, which would have been limited by heterogeneous collection of demographic and clinical variables in this cohort. Nevertheless, residual confounding by indication is certainly possible, and we were unable to control for other factors that may have influenced provider decision making regarding statin prescription. We sought to limit the extent to which confounders may have influenced our analyses through multivariable adjustment, after which the effect size for statin use by race/ethnicity among PWH remained consistent. Based on the nature of this cohort, there was no standardized procedure for measurement of baseline and follow-up lipid panels, and statin prescription data may have been incomplete. Finally, although most guidelines and previous studies use LDL-C to guide and evaluate statin therapy, we focused primarily on TC in our analyses because very high rates of hypertriglyceridemia in our population (particularly among PWH) complicated LDL-C calculation when the Freidewald formula was used.
Despite these limitations, our findings indicate potential race/ethnicity disparities in statin use among PWH that require further study and suggest a need for improved CVD prevention efforts among racial and ethnic minority PWH. Future studies should confirm these findings in other cohorts and focus on implementation efforts to improve CVD prevention among PWH.
Supplementary Material
Acknowledgments
Funding acknowledgements
The authors acknowledge the American Heart Association (Fellow-to-Faculty Transition Award FTF 31200010; Feinstein M. J., PI) for its funding support. The funding source had no role in study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication.
Footnotes
Disclosures
The authors report no relevant conflicts of interest to disclose.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahj.2018.11.012.
References
- 1.Palella FJ Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34. [DOI] [PubMed] [Google Scholar]
- 2.Islam FM, Wu J, Jansson J, et al. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012;13:453–68. [DOI] [PubMed] [Google Scholar]
- 3.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol 2016;117:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paisible AL, Chang CC, So-Armah KA, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr 2015;68:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012;59:1891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 7.Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
- 9.Silverberg MJ, Leyden W, Hurley L, et al. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med 2009;150:301–13. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed MH, Al-Atta A, Hamad MA. The safety and effectiveness of statins as treatment for HIV-dyslipidemia: the evidence so far and the future challenges. Expert Opin Pharmacother 2012;13:1901–9. [DOI] [PubMed] [Google Scholar]
- 11.Johns KW, Bennett MT, Bondy GP. Are HIV positive patients resistant to statin therapy? Lipids Health Dis 2007;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh S, Willig JH, Mugavero MJ, et al. Comparative effectiveness and toxicity of statins among HIV-infected patients. Clin Infect Dis 2011;52:387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement ME, Park LP, Navar AM, et al. Statin utilization and recommendations among HIV- and HCV-infected veterans: a cohort study. Clin Infect Dis 2016;63:407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Todd JV, Cole SR, Wohl DA, et al. Underutilization of statins when indicated in HIV-seropositive and seronegative women. AIDS Patient Care STDs 2017;31:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinstein MJ, Achenbach CJ, Stone NJ, et al. A systematic review of the usefulness of statin therapy in HIV-infected patients. Am J Cardiol 2015;115:1760–6. [DOI] [PubMed] [Google Scholar]
- 16.Kelly SG, Krueger KM, Grant JL, et al. Statin prescribing practices in the comprehensive care for HIV-infected patients. J Acquir Immune Defic Syndr 2017;76:e26–9. [DOI] [PubMed] [Google Scholar]
- 17.Gu A, Kamat S, Argulian E. Trends and disparities in statin use and low-density lipoprotein cholesterol levels among US patients with diabetes, 1999–2014. Diabetes Res Clin Pract 2018;139:1–10. [DOI] [PubMed] [Google Scholar]
- 18.Schroff P, Gamboa CM, Durant RW, et al. Vulnerabilities to health disparities and statin use in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. J Am Heart Assoc 2017;6, e005449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasgupta S, Oster AM, Li J, et al. Disparities in consistent retention in HIV care—11 states and the District of Columbia, 2011–2013. MMWR Morb Mortal Wkly Rep 2016;65:77–82. [DOI] [PubMed] [Google Scholar]
- 20.Felsen UR, Beilin EY, Cunningham CO, et al. Development of an electronic medical record-based algorithm to identify patients with unknown HIV status. AIDS Care 2014;26:1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steverson AB, Pawlowski AE, Schneider D, et al. Clinical characteristics of HIV-infected patients with adjudicated heart failure. Eur J Prev Cardiol 2017;24:1746–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- 23.Chauvin B, Drouot S, Barrail-Tran A, et al. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet 2013;52:815–31. [DOI] [PubMed] [Google Scholar]
- 24.Tran HV, Waring ME, McManus DD, et al. Underuse of effective cardiac medications among women, middle-aged adults, and racial/ethnic minorities with coronary artery disease (from the National Health and Nutrition Examination Survey 2005 to 2014). Am J Cardiol 2017;120:1223–9. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Sehgal NL, Ayanian JZ, et al. National trends in statin use by coronary heart disease risk category. PLoS Med 2005;2, e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann D, Reynolds K, Smith D, et al. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann Pharmacother 2008;42:1208–15. [DOI] [PubMed] [Google Scholar]
- 27.Qato DM, Lindau ST, Conti RM, et al. Racial and ethnic disparities in cardiovascular medication use among older adults in the United States. Pharmacoepidemiol Drug Saf 2010;19:834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fichtenbaum G, Gerber JG, Rosenkranz SL, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS 2002;16:569–77. [DOI] [PubMed] [Google Scholar]
- 29.Monroe AK, Fu W, Zikusoka MN, et al. Low-density lipoprotein cholesterol levels and statin treatment by HIV status among multicenter AIDS cohort study men. AIDS Res Hum Retrovir 2015;31:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


