Abstract
Introduction
Although adalimumab is the only approved biologic for the treatment of hidradenitis suppurativa (HS), the treatment response may not be satisfactory in all patients. Recently, many other biological agents, including interleukin 17 inhibitors such as ixekizumab, have shown promise.
Case Presentations
Five severe HS (Hurley stage III) patients resistant to conventional treatments and adalimumab for at least 3 months were recruited. Patients were prescribed ixekizumab with a scheme approved for psoriasis (160 mg once, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12.) The primary outcome measure was achieving the Hidradenitis Suppurativa Clinical Response (HiSCR) score following 12 weeks. Secondary outcome measures included the patient-reported Dermatology Life Quality Index (DLQI) and visual analog scale (VAS). Four of 5 patients (80%) achieved HiSCR. While improvement was observed in the VAS and DLQI scores of 4 patients, the decline was limited in 1 patient. No adverse event was recorded related to ixekizumab.
Conclusion
The result of our observation suggests that ixekizumab may be effective for HS, especially in challenging cases.
Keywords: Hidradenitis suppurativa, Biologic, Adalimumab, Ixekizumab, IL-17
Established Facts
Hidradenitis suppurativa is a chronic and relapsing inflammatory skin disease of the hair follicles, negatively impacting patients' quality of life.
Recent advances in disease pathogenesis have expanded our understanding of elevated levels of several pro-inflammatory cytokines, including serum levels of IL-17.
Recently, inhibition of pathogenic IL-17 blockade has shown promising results in isolated case reports.
Novel Insights
Ixekizumab may be effective for hidradenitis suppurativa, even in adalimumab-resistant cases.
Introduction/Literature Review
Hidradenitis suppurativa (HS) is a severe and debilitating skin disease characterized by painful subcutaneous nodules in intertriginous areas. The clinical course and disease severity are highly variable with substantial negative impacts on quality of life. Although numerous therapeutic approaches exist to treat HS, clinical management is still quite challenging, with existing therapies having limited efficacy [1].
Although the pathogenesis of HS has not been fully elucidated, aberrant expression of pro-inflammatory cytokines such as tumor necrosis factor, interleukin (IL)-1β, and IL-17 play a central role [2, 3]. Adalimumab is the only approved biologic for the treatment of HS; many other biological agents, including IL-17 inhibitors, appear promising [4, 5]. There are case reports of HS improved with ixekizumab [6, 7, 8]. We present the clinical characteristics and the course of 5 HS patients treated with 12 weeks of ixekizumab.
Case Presentations
Severe HS patients resistant to conventional treatments and adalimumab for at least 3 months were recruited. Off-label use of ixekizumab (Copellor®) was considered and discussed with the patients. Ixekizumab was used with a scheme approved by the US Food and Drug Administration for psoriasis (160 mg once, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12.) The primary outcome measure was achieving the Hidradenitis Suppurativa Clinical Response (HiSCR) score following 12 weeks. Secondary outcome measures included the patient-reported Dermatology Life Quality Index (DLQI) and visual analog scale (VAS).
All patients were Hurley III previously treated with adalimumab (Table 1). Four of 5 patients (80%) achieved HiSCR (2 patients at week 8 and the other 2 at week 12; Table 1; Fig. 1). One patient (patient 1) was unresponsive, and treatment was stopped at the 12th week due to lack of effectiveness and increased discharge. While improvement was observed in the VAS and DLQI scores of 4 patients, the decline was limited in patient 1 (Table 1). No serious adverse events such as new-onset inflammatory bowel disease or candidiasis were recorded due to the use of ixekizumab.
Table 1.
Clinical characteristics and medical history of the patients and their responses to ixekizumab
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Gender/age | M/53 | M/34 | M/29 | M/47 | M/56 |
| Hurley stage | III | III | III | III | III |
| Disease duration, years | 6 | 4 | 10 | 18 | 20 |
| Diagnostic delay, months | 24 | 36 | 84 | 120 | 180 |
| Smoking history, packet-years | 10 | 15 | 16 | 10 | 20 |
| Medical comorbidities | − | − | − | HT, DM | HT, DM |
| Previous treatments, weeks | |||||
| Adalimumab | 12 | 24 | 24 | 36 | 108 |
| Certolizumab pegol | − | − | − | − | 48 |
| Rifampicin-clindamycin | 12 | 10 | − | 10 | 12 |
| Antibiotics (≥3 cycle) | + | + | + | + | + |
| Achieved HiSCR, week | − | 12 | 8 | 12 | 8 |
| DLQI | |||||
| Baseline | 16 | 21 | 24 | 18 | 18 |
| Week 12 | 10 | 12 | 9 | 8 | 10 |
| VAS | |||||
| Baseline | 10 | 9 | 10 | 9 | 10 |
| Week 12 | 8 | 4 | 2 | 3 | 3 |
DM, diabetes mellitus; HT, hypertension.
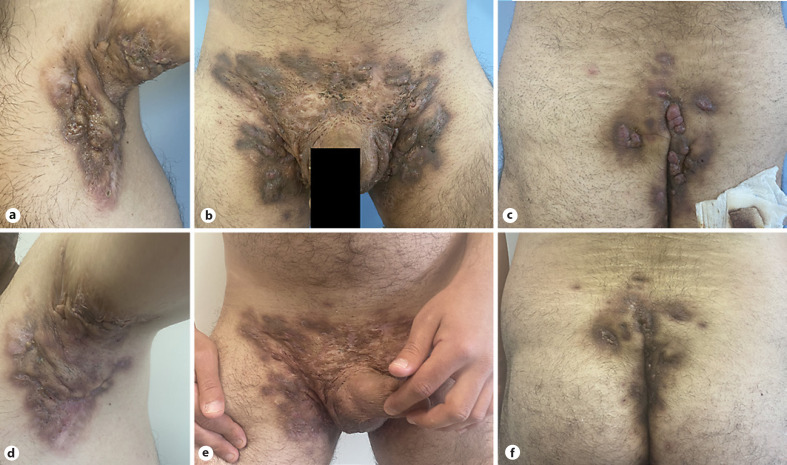
Fig. 1.
Clinical appearance of the affected area before and after treatment. A 29-year-old male patient had Hurley stage 3 HS. Before treatment, there were deep swollen inflamed nodules and abscesses, draining sinus tracts, and hypertrophic scar areas in the axilla, inguinal, and perianal regions (a–c). After 8 weeks of ixekizumab treatment, there was a reduction in the lesions' inflamed, plump, and discharged appearance (d–f). The patient achieved a HiSCR response at week 8.
Discussion
Although weekly adalimumab is effective for HS, approximately 40–60% of patients do not achieve benefit after 3 months of treatment, indicating primary treatment failure [9]. Considering the additional secondary failure in the following period, there is still a huge unmet need for HS treatments.
The IL-17 pathway has been implicated as a potential therapeutic target in HS, prompting clinical trials with various IL-17 inhibitors (secukinumab, brodalumab, and ixekizumab) [4]. While data are increasing regarding secukinumab and brodalumab, the literature on ixekizumab is limited [4, 6, 7, 8]. Ixekizumab, a humanized monoclonal immunoglobulin G 4 antibody, specifically binding to IL-17A, is very effective in treating moderate to severe psoriasis [4]. We found 3 case reports of ixekizumab for HS. Detailed information about one of them could not be accessed [6]; in one of the cases, severe HS lesions were unresponsive to adalimumab and improved with 12-week ixekizumab treatment [8]. In the other case, it was reported that simultaneous severe psoriasis and mild HS lesions improved with the standard psoriasis dose of ixekizumab [7]. Our findings also support the potential effectiveness of ixekizumab as a treatment for HS, especially in challenging cases that were not under-controlled with adalimumab.
Statement of Ethics
The local ethics committee provided ethical clearance and approval. Written informed consent was obtained from participants to publish the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have received no external funding.
Author Contributions
Pelin Esme and Ercan Caliskan: conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; and final approval of the version to be published. Aysenur Botsali: conception or design of the work and the acquisition, analysis, or interpretation of data for the work. Gulsen Akoglu: conception or design of the work; drafting the work or revising it critically for important intellectual content; and final approval of the version to be published.
Data Availability Statement
Data are available on request from the authors.
References
- 1.Alikhan A, Sayed C, Alavi A, Alhusayen R, Brassard A, Burkhart C, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II − topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81((1)):91–101. doi: 10.1016/j.jaad.2019.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marzano AV, Genovese G, Casazza G, Moltrasio C, Dapavo P, Micali G, et al. Evidence for a ‘window of opportunity’ in hidradenitis suppurativa treated with adalimumab: a retrospective, real-life multicentre cohort study. Br J Dermatol. 2021 Jan;184((1)):133–40. doi: 10.1111/bjd.18983. [DOI] [PubMed] [Google Scholar]
- 3.Matusiak Ł, Szczęch J, Bieniek A, Nowicka-Suszko D, Szepietowski JC. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: Implications for treatment with anti-IL-17 agents. J Am Acad Dermatol. 2017 Apr;76((4)):670–5. doi: 10.1016/j.jaad.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 4.Kashetsky N, Mufti A, Alabdulrazzaq S, Lytvyn Y, Sachdeva M, Rahat A, et al. Treatment outcomes of IL-17 inhibitors in hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2021 Aug 8;:12034754211035667. doi: 10.1177/12034754211035667. [DOI] [PubMed] [Google Scholar]
- 5.Esme P, Akoglu G, Caliskan E. Rapid response to certolizumab pegol in hidradenitis suppurativa: a case report. Skin Appendage Disord. 2021 Jan;7((1)):58–61. doi: 10.1159/000511284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odorici G, Pellacani G, Conti A. Ixekizumab in hidradenitis suppurativa in a psoriatic patient. G Ital Dermatol Venereol. 2020 Dec;155((6)):788–9. doi: 10.23736/S0392-0488.18.06135-7. [DOI] [PubMed] [Google Scholar]
- 7.Megna M, Ruggiero A, Di Guida A, Patrì A, Fabbrocini G, Marasca C. Ixekizumab: an efficacious treatment for both psoriasis and hidradenitis suppurativa. Dermatol Ther. 2020 Jul;33((4)):e13756. doi: 10.1111/dth.13756. [DOI] [PubMed] [Google Scholar]
- 8.Reardon K, Levin J, Levin C. Severe hidradenitis suppurativa with herpes simplex virus 1 superinfection and clinical responsiveness to ixekizumab. JAAD Case Rep. 2021 Jan 10;9:7–8. doi: 10.1016/j.jdcr.2020.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015 Apr;29((4)):619–44. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.