Abstract
Objective
The prevalence of phage 80/81 Staphylococcus aureus strains, the pandemic strains that were dominant in the 1950s, had declined in the 1960s and 1970s. However, these strains have reemerged in some countries in recent years. This study investigated the antibacterial resistance, virulence, and the genetic backgrounds of CC30-MSSA isolates obtained from patients in three tertiary hospitals.
Materials and Methods
Twenty-two CC30-MSSA isolates cultured from different clinical samples were investigated using antibiotic sensitivity testing, spa typing, multilocus sequence typing, and DNA microarray analysis.
Results
All 22 isolates were susceptible to vancomycin (MIC ≤2 μg/mL), teicoplanin (MIC ≤2 μg/mL), and cefoxitin but were resistant to penicillin G (n = 22; 100.0%), tetracycline (n = 12; 54.5%), ciprofloxacin (n = 15; 68.2%), cadmium acetate (n = 22; 100%), mercuric chloride (n = 13; 59.1%), and ethidium bromide (n = 3; 13.6%). The isolates belonged to sequence type, ST30, and five spa types: t012 (n = 12; 54.5%), t019 (n = 5; 22.7%), t017 (n = 2; 9.1%), t037 (n = 2; 9.1%), and t318 (n = 1; 4.5%). All 22 isolates were positive for agrIII, cap8, clfA, clfB, icaA, icaC, icaD, cna, and staphylococcal enterotoxin gene clusters (seg, sei, sem, sen, seo, seu). Eight isolates carried lukS-PV and lukF-PV that code for Panton-Valentine leukocidin.
Conclusion
The current CC30-MSSA isolates share phenotypic and genotypic characteristics with the pandemic phage 80/81 isolates that were common in the 1950s and 1960s. Continued surveillance is recommended to keep abreast of the changing epidemiology of S. aureus causing healthcare and community-associated infections.
Keywords: Methicillin-sensitive Staphylococcus aureus, Sequence type 30, spa typing, Panton-valentine leukocidin, DNA microarray
Highlights of the Study
Twenty-two clonal complex 30-methicillin-susceptible Staphylococcus aureus isolates were investigated using multilocus sequence typing, Staphylococcus protein A typing, and DNA microarray analysis.
The isolates belonged to sequence type 30 and five spa types: t012, t019, t017, t037, and t318 and were positive for enterotoxin genes (seg, sei, seu, seo, sem, sen).
Eight isolates (36.3%) were positive for genes that code for Panton-valentine leukocidin.
Introduction
Staphylococcus aureus remains a leading cause of human infections worldwide. S. aureus can cause skin and soft tissue infections (boils, carbuncles, and pustules), pneumonia, and infections of the bloodstream, bone and joint, and respiratory and gastrointestinal tracts [1]. Several phenotypic and genotypic typing methods have been applied to characterize S. aureus isolates for their virulence properties, genetic relatedness, and antibiotic resistance determinants. These methods include phage typing, pulsed-field gel electrophoresis, staphylococcal protein A typing (spa typing), and multilocus sequence typing (MLST) [2, 3].
Bacteriophage typing (phage typing) was the primary method for typing S. aureus for epidemiological purposes in the 1950s–1970s [4, 5]. Phage typing is based on the ability of a set of bacteriophages (typing phages) to lyse S. aureus strains and produce patterns known as bacteriophage types [4, 5]. Bacteriophage typing was used to identify strains of S. aureus designated as phage 80/81 based on their susceptibility to lysis by a set of specific bacteriophages known as phages 80, 81, and sometimes phage 83 [2, 4, 5].
The phage-type 80/81 strains that were isolated in the 1950s were resistant to penicillin by producing penicillinase [2, 4, 5]. Some of the strains also produced a leukocidin designated Panton-Valentine leukocidin (PVL), a pore-forming cytotoxin that causes destruction of leucocytes and tissue necrosis [6]. In S. aureus strains, PVL is encoded by two co-transcribed genes, lukS-PV and lukF-PV, which are carried by bacteriophages [6]. The phage 80/81 strains were responsible for outbreaks of infections including skin and soft tissue infections (boils, carbuncles, and pustules), sepsis, and pneumonia in Australia, Great Britain, Canada, and the USA in the 1950s [2, 4, 5].
The emergence of penicillin resistance in S. aureus including the phage 80/81 strains led to the development and clinical use of methicillin, a penicillinase-resistant penicillin, to treat infections caused by penicillin-resistant phage 80/81 strains [1, 2]. The clinical use of methicillin subsequently resulted in the emergence of methicillin-resistant strains (MRSA), which has now become a global health problem [1]. The emergence of MRSA and their global expansion coincided with the decline in the prevalence of the phage 80/81 strains [2] and the replacement of phage typing as an epidemiological typing tool by molecular typing methods such as spa typing and MLST [2, 3]. The application of MLST on the phage 80/81 isolates obtained in the 1950s and early 1960s revealed that they belonged to S. aureus clonal complex 30 (CC30) and sequence type sequence type 30 (ST30) [2, 7]. It was subsequently revealed that contemporary S. aureus CC30 isolates include the community-associated MRSA lineage ST30-MRSA-IV, also known as the Southwest Pacific clone, and the healthcare-associated lineage ST36-MRSA-II, which is also known as the UK Epidemic MRSA-16 [2, 7]. Whereas the CC30-MRSA strains have spread globally in recent years [8], the pandemic phage-type 80/81 strains have been reported sporadically in the literature [2, 9, 10, 11].
While investigating the genetic backgrounds of S. aureus isolates obtained from clinical samples in tertiary hospitals in Jos, Plateau State of Nigeria using DNA microarray, we detected methicillin-sensitive S. aureus (MSSA) strains that belonged to the CC30 lineage (CC30-MSSA). Here we report further investigations conducted on the 22 CC30-MSSA isolates using a combination of molecular typing methods to establish their genetic relatedness to the pandemic phage 80/81 strains.
Materials and Methods
Sources and Identification of Bacterial Isolates
The S. aureus isolates were collected as part of routine diagnostic microbiology service. Strains were identified based on growth characteristics on blood agar plate (5% sheep blood), positive Gram's stain, catalase, and tube coagulase tests. In total, 214 S. aureus were isolated in 2017 and 2018 in the Jos University Teaching Hospital (JUTH; n = 8), Plateau State Specialist Hospital (PSSH; n = 12), and Bingham University Teaching Hospital (BUTH; n = 2). The isolates were cultured from wound swabs (n = 10), blood cultures (n = 9), ear swab (n = 1), urethral swab (n = 1), and urine specimen (n = 1) obtained from 13 male and 9 female patients and were preserved in semisolid agar medium (0.3% agar in brain heart infusion broth). Molecular characterization was performed at the Gram-Positive Bacteria Research Laboratory, Department of Microbiology, Faculty of Medicine, Kuwait University, Kuwait.
Susceptibility to Antibacterial Agents
The disk diffusion and minimum inhibitory concentration (MIC) methods were performed according to the guidelines of the Clinical Laboratory Standards Institute (CLSI) [12]. The following antimicrobial disks (Oxoid) were tested: benzyl penicillin (10 U), cefoxitin (30 μg), kanamycin (30 μg), mupirocin (200 μg and 5 μg), gentamicin (10 μg), erythromycin (15 μg), clindamycin (2 μg), chloramphenicol (30 μg), tetracycline (10 μg), trimethoprim (2.5 μg), fusidic acid (10 μg), rifampicin (5 μg), and ciprofloxacin (5 μg). The MIC for oxacillin, cefoxitin, mupirocin, vancomycin, and teicoplanin was determined with E-test strips (BioMerieux, Marcy l'Etoile, France). Susceptibility to fusidic acid was interpreted according to the British Society for Antimicrobial Chemotherapy [13]. S. aureus strains ATCC25923 and ATCC29213 were used as quality control strains for disc diffusion and MIC determination, respectively. The D test was used to test for inducible resistance to clindamycin. Susceptibility to three nonantibiotic compounds was tested with 6-mm discs impregnated with cadmium acetate (50 µg), mercuric chloride (109 µg), and ethidium bromide (50 µg) and S. aureus WBG248 was used as quality control strain [14].
Molecular Typing of S. aureus Isolates
DNA for staphylococcal protein A (spa typing) was isolated as described previously [15]. spa typing was performed on all isolates using protocol and primers published previously [16] and assigned to spa types using the spa typing website (http://www.spaserver.ridom.de). MLST was performed on all isolates as described by Enright et al. [17].
DNA Microarray Analysis
DNA microarray analysis was performed as described previously [18] to detect genes encoding species markers, SCCmec, virulence, and antibiotic resistance genes.
Results
DNA microarray analysis of 214 S. aureus obtained from patients in three different hospitals in Central Nigeria revealed that 22 of the isolates belonged to CC30-MSSA. The 22 CC30-MSSA isolates were characterized further using antibiogram, spa typing, and MLST.
All 22 isolates were susceptible to vancomycin (MIC ≤2 μg/mL), teicoplanin (MIC ≤2 μg/mL), cefoxitin, mupirocin, fusidic acid, erythromycin, clindamycin, gentamicin, kanamycin, trimethoprim, chloramphenicol, and rifampicin, but were resistant to penicillin G (n = 22; 100.0%), tetracycline (n = 12; 54.5%), and ciprofloxacin (n = 15; 68.2%). The isolates were also resistant to cadmium acetate (n = 22; 100%), ethidium bromide (n = 3; 13.6%), and mercuric chloride (n = 13; 59.1%).
The genetic determinants of the antibiotic resistance are summarized in Table 1. Penicillin resistance was mediated by blaZ, tetracycline resistance was mediated by tet(M), while resistance to mercuric chloride was mediated by merA and merB.
Table 1.
Antibiotic resistance determinants of CC30-MSSA isolates
| Resistance profile | MSSA genotypes |
|||||
|---|---|---|---|---|---|---|
| CC30/ST30-MSSA-t012 (N = 12) | CC30/ST30-MSSA-t017 (N = 2) | CC30/ST30-MSSA, (PVL+)-t019 (N = 5) | CC30/ST30-MSSA, (PVL+)-t037 (N = 2) | CC30/ST30-MSSA, (PVL+)-t318 (N = 1) | N | |
| Resistance phenotype | ||||||
| P | 12 | 2 | 5 | 2 | 1 | 22 |
| TE | 11 | 2 | − | − | − | 13 |
| CIP | 10 | 2 | − | 2 | − | 14 |
| Cd | 12 | 2 | 5 | 2 | 1 | 22 |
| Hg | 11 | 2 | − | − | − | 13 |
| Eb | − | 1 | − | 2 | − | 3 |
| Resistance genotypes | ||||||
| blaZ | 12 | 2 | 5 | 2 | 1 | 22 |
| tet(M) | 11 | 2 | − | − | − | 13 |
| merA | 11 | 2 | − | − | − | 13 |
| merB | 11 | 2 | − | − | − | 13 |
merA, merB, mercury resistance gene; blaZ, beta-lactamase gene; tet(M), tetracycline resistance gene; P, penicillin; TE, tetracycline; CIP, ciprofloxacin; Cd, cadmium acetate; Hg, mercuric chloride; Eb, ethidium bromide.
Virulence Determinants of the CC30-MSSA Isolates
All 22 isolates were positive for genes that code for accessory gene regulator type 3 (agr3) and capsular polysaccharide type 8 (cap8). The isolates were also positive for genes encoding hemolysins (hla, hlb, hld), biofilm synthesis proteins (icaA, icaC, icaD), serine protease E (spIE), clumping factors A/B (clfA, clfB), fibronectin-binding proteins A/B (fnbA, fnbB), collagen-binding adhesion (cna), enolase (eno), von Willebrand factor-binding protein (vwb), and immune evasion clusters including staphylokinase (sak), chemotaxis-inhibiting protein (chp), and staphylococcal complement inhibitor (scn).
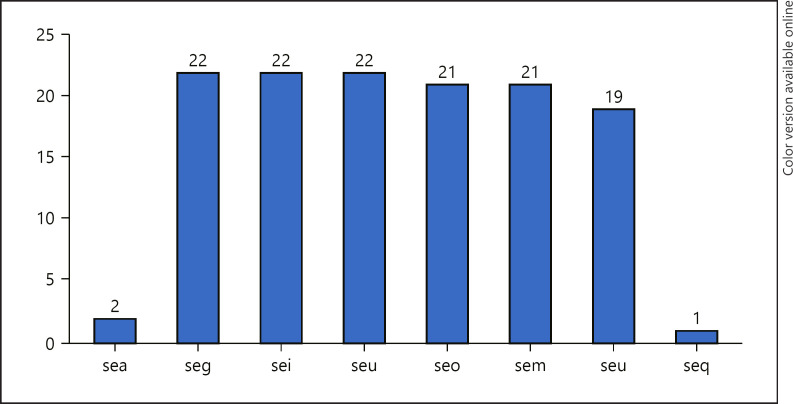
All isolates were positive for genes for enterotoxin gene cluster (egc), which consists of enterotoxins seg, sei, seu, seo, sem, and sen. Three isolates lacked seu, while one isolate lacked sem and seo. Two isolates were positive for sea. The distribution of the SE genes is presented in Figure 1. Eight isolates were positive for lukS-PV-lukF-PV that code for PVL. The gene for epidermal cell differentiation inhibitors (edinA) and (edinB) was detected in one isolate. All 22 isolates were negative for tst1 that codes for toxic shock syndrome toxin.
Fig. 1.
Distribution of genes for staphylococcal enterotoxins.
Molecular Characterization of CC30-MSSA Isolates
The CC30 isolates belonged to five spa types, t012 (n = 12; 54.5%), t019 (n = 5; 22.7%), t017 (n = 2; 9.1%), t037 (n = 2; 9.1%), and t318 (n = 1; 4.5%). MLST of the 22 isolates revealed that all belonged to the same sequence type, ST30. Twelve of the 22 ST30-MSSA isolates belonged to t012 (t012-/ST30-MSSA). The isolates were obtained from blood (n = 6), wound (n = 4), urethral swab (n = 1), and ear swab (n = 1). Ten of the t012 isolates were obtained from one hospital (PSSH), while each of the remaining two isolates was obtained from JUTH and BUTH. Eleven of the 12 isolates were resistant to tetracycline, ciprofloxacin, cadmium acetate, and mercuric chloride and harbored tet(M), merA, and merB (Table 1). Eleven of the t012 isolates were positive for genes for clumping factors, biofilm production, enterotoxin gene cluster (egc), and immune evasion clusters. None of t012 isolates were positive for lukS-PV-lukF-PV and tst1.
Two isolates belonging to t017-CC30/ST30-MSSA were isolated from urine and wound samples in two hospitals (JUTH and PSSH). Both isolates were resistant to penicillin G, tetracycline, ciprofloxacin, cadmium acetate, and mercuric chloride and carried blaZ tet(M) and merA and merB. One isolate was resistant to ethidium bromide. Both isolates were positive for sea and egc. One isolate lacked sem and seu.
Five isolates identified as t019-ST30-MSSA (PVL+) were obtained from blood (n = 2) and wound (n = 3) samples and belonged to spa type, t019. All five isolates were resistant to penicillin G mediated by blaZ. In addition, two of the isolates were resistant to cadmium acetate. All t019 isolates were positive for lukS-PV-lukF-PV (PVL) and egc.
Two isolates, identified as t037-ST30-MSSA (PVL+), were obtained from blood and wound samples of patients in the same hospital (JUTH). Both isolates were resistant to penicillin G, ciprofloxacin, cadmium acetate, and ethidium bromide. Both were positive for lukS-PV-lukF-PV (PVL). A single t318-ST30-MSSA (PVL+) isolate was isolated from a wound sample. It was resistant to penicillin G and cadmium acetate and was positive for blaZ. It was also positive for genes encoding epidermal cell differentiation inhibitors B and C (edinB and edinC), lukS-PV-lukF-PV, egc, and seq.
Discussion
This study investigated antibacterial resistance and virulence determinants in recent isolates of S. aureus belonging to CC30 (CC30-MSSA) obtained from three tertiary hospitals in North-Central Nigeria. S. aureus isolates belonging to CC30 have had significant impacts on human infections since it was first identified as phage-type 80/81 in the 1950s [2, 3, 4, 5]. The pandemic S. aureus phage 80/81 clone caused major outbreaks of infections in the 1950s and early 1960s in several countries including Australia, England, USA, and Canada [4, 5, 19]. The isolates of the phage 80/81 clone later acquired methicillin resistance (mecA) and evolved into the Southwest Pacific clone of community-associated MRSA (ST30-MRSA-IV [PVL+]) and the healthcare-associated MRSA clone (ST36-MRSA-II) [2, 7, 8]. Interestingly, the prevalence of the pandemic phage 80/81 strains declined with the increase in the prevalence of the CC30-MRSA clones [2]. However, there are recent reports of CC30-MSSA in some countries including Ecuador [9], Lebanon [10], Greece [11], Canada [19], USA [20, 21], and Germany [22]. The CC30-MSSA isolates in this study add to the growing list of recent reports of this lineage. The recent reports of the CC30-MSSA isolates may represent a reemergence of the pandemic phage 80/81 clone or the emergence of CC30-MRSA isolates that have lost mecA that codes for methicillin resistance. The reports may also show increasing awareness of CC30-MSSA as significant human pathogens [11, 19, 20, 21, 22, 23].
All 22 CC30-MMSA isolates in this study were resistant to penicillin G mediated by blaZ, with 54.5% of them resistant to tetracycline mediated by tet(M), which is comparable to the resistance characteristics of the phage group 80⁄81 isolates [2, 5, 24]. In addition, 68.2% of our isolates were resistant to ciprofloxacin, an antibiotic that was not available in the 1950s, indicating recent acquisition of ciprofloxacin resistance by these isolates. In contrast, a recent study conducted in Poland revealed that all S. aureus phage 80/81 strains obtained from patients with oral infection were sensitive to ciprofloxacin [25]. Resistance to tetracycline and ciprofloxacin has also been reported in ST30-MRSA isolates [15, 24, 25]. Although the isolates in this study were susceptible to erythromycin and clindamycin, 71% and 63% of CC30-MSSA causing ocular infections in patients in the USA were resistant to erythromycin and clindamycin, respectively [21]. The differences in the susceptibility patterns may reflect exposure to different antibiotics at the different clinical units or hospitals.
The 22 CC30-MSSA isolates were all resistant to cadmium acetate, while 59.1% of them were resistant to mercuric chloride. S. aureus strain, ATCC25923, a prepandemic phage 80/81 strain traditionally used as quality control strain for antibiotic susceptibility testing of S. aureus, has been reported to harbor a plasmid-mediated cadmium resistance [14], indicating that S. aureus acquired cadmium resistance prior to penicillin resistance.
The 22 isolates in this study belonged to sequence type 30 (ST30), accessory gene regulator type 3 (agrIII), and capsular polysaccharide type 8 but were heterogeneous in their spa types. The dominant spa type was t012 (12/22) followed by t019 (5/22), t017 (2/22), t037 (2/22), and t318 (1/22). Similarly, t012 was the dominant spa type among CC30-MSSA nasal colonizers in Germany [22] and among ST30-MSSA isolates in Lebanon [10]. The other spa types, t017 and t019, have also been associated with ST30-MSSA and ST30-MRSA isolates [15, 20, 22]. Interestingly, two of the isolates belonged to t037 that is usually associated with ST239-MRSA-III isolates [26]. ST239 isolates are considered to be mosaic strains formed by the recombination between ST8 and ST30 parents [7] but have spa types typical of ST30 strains [22], suggesting that the t037 isolates in this study are closer to ST239. This is supported by their multiresistance to ciprofloxacin, cadmium acetate, mercuric chloride, and ethidium bromide, which is a typical resistance pattern of ST239-MRSA isolates [26].
The isolates were positive for a variety of virulence-related genes. Eight of the 22 CC30-MSSA isolates comprising t019 (N = 5), t037 (N = 2), and t318 (N = 1) were positive for lukF-PV-lukS-PV that encodes PVL similar to the phage 80/81 that evolved into the Southwest Pacific MRSA clone [7, 8]. Similarly, Gomes et al. [27], who investigated S. aureus strains collected in Denmark between 1957 and 1973, found that two of three t012 isolates in their study were positive for PVL.
All 22 isolates were positive for agrIII, cap8, clfA, clfB, icaA, icaC, icaD, and cna as has also been reported in ST30-MRSA isolates [10, 15, 28]. The enterotoxin gene cluster (egc) was common among the ST30-MSSA isolates. However, sea was only detected in the t017 isolates, while seq was detected in a single isolate.
None of the isolates were positive for tst1 that encodes toxic shock syndrome toxin. In contrast, other studies conducted in different parts of the world have reported the carriage of tst1 in ST30 isolates [11, 15, 22, 29]. In addition, ST30-MRSA isolates recently emerged in Kuwait and Saudi Arabia that carried SCCmec type VI with fusidic acid resistance and tst1 [30], suggesting that tst1 has recently been acquired by ST30-MRSA in the Arabian Gulf.
Limitations of this study include the small number of isolates investigated and the absence of phage typing data on the isolates. Nevertheless, the data presented draw attention to the presence of CC30-MSSA isolates that possess phenotypic and molecular characteristics that are comparable to those of the phage 80/81 pandemic clone in an area where little is known about the existence of CC30-MRSA strains.
Conclusion
This study has provided insights into the epidemiology of ST30-MSSA isolates recovered from healthcare facilities in Jos, Nigeria. The ST30-MSSA were isolated mostly from wound and blood infections, were resistant to penicillin G, and harbored blaZ and the enterotoxin genes cluster (egc), which were comparable to the characteristics of the pandemic phage-type 80/81 strain that were reported in the 1950s and early 1960s. Further surveillance studies are recommended to monitor the changing trends in epidemiology of S. aureus causing healthcare and community-associated infections for better patient management, prevention, and control of infection.
Statement of Ethics
This study was approved by the Ethical Committees of Jos University Teaching Hospital, Jos (approval No. JUTH/DCS/ADM/127/XXV/314), Plateau State Specialist Hospital, Jos (approval No. PSSH/ADM/ETH.CO/2017/006), and Bingham University Teaching Hospital, Jos, Plateau State (approval No. NHREC/21/05/2005/00495).
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Funding Sources
There was no external funding for this project.
Author Contributions
Conception and design of the study were contributed by Edet E. Udo. Methodology, acquisition, and analysis of data were carried by Samar S. Boswihi and Unyime C. Essien. Supervision of the study was contributed by Edet E. Udo and Nneka R. Agbakoba. Writing − original draft was carried by UCE, and writing − review and editing the manuscript were carried by Edet E. Udo, Unyime C. Essien, Samar S. Boswihi, and Nneka R. Agbakoba.
Data Availability Statement
All data generated during this study are included in this paper.
Acknowledgments
We are grateful to Mr. Nnamdi Uzoma and Mr. Ezra Dasun of the Department of Medical Laboratory Science University of Jos for technical assistance and Ms. Tina Verghese for assistance with spa typing (U.C.E.).
References
- 1.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–61. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Udo EE. Community-acquired methicillin-resistant Staphylococcus aureus: the new face of an old foe? Med Princ Pract. 2013;22((Suppl 1)):20–9. doi: 10.1159/000354201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson DA, Enright MC. Multilocus sequence typing and the evolution of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2004;10:92–7. doi: 10.1111/j.1469-0691.2004.00768.x. [DOI] [PubMed] [Google Scholar]
- 4.Roundtree P, Freeman V. Infections caused by a particular phage type of Staphylococcus aureus. Med J Aust. 1956;42:157–61. [PubMed] [Google Scholar]
- 5.Bynoe ET, Elder RH, Comtois RD. Phage-typing and antibiotic-resistance of staphylococci isolated in a general hospital. Can J Microbiol. 1956;2:346–58. doi: 10.1139/m56-041. [DOI] [PubMed] [Google Scholar]
- 6.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DA, Enright MC. Evolutionary models of the emergence of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:3926–34. doi: 10.1128/AAC.47.12.3926-3934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6:e17936. doi: 10.1371/journal.pone.0017936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zurita J, Barba P, Ortega-Paredes D, Mora M, Rivadeneira S. Local circulating clones of Staphylococcus aureus in Ecuador. Braz J Infect Dis. 2016;20:525–33. doi: 10.1016/j.bjid.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harastani HH, Araj GF, Tokajian ST. Molecular characteristics of Staphylococcus aureus isolated from a major hospital in Lebanon. Int J Infect Dis. 2014;19:33–8. doi: 10.1016/j.ijid.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Papadimitriou-Olivgeris M, Drougka E, Fligou F, Dodou V, Kolonitsiou F, Filos KS, et al. Spread of Tst-positive Staphylococcus aureus strains belonging to ST30 clone among patients and healthcare workers in two intensive care units. Toxins. 2017 Sep 4;9:270. doi: 10.3390/toxins9090270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing. Wayne, PA, USA: Twenty-second Information supplement M100-S25.CLSI; 2015. [Google Scholar]
- 13.British Society for Antimicrobial Chemotherapy . BSAC Disc diffusion method for Antimicrobial susceptibility testing. British Society for Antimicrobial Chemotherapy; 2005. [DOI] [PubMed] [Google Scholar]
- 14.Udo EE, Jacob LE, Mathew B. A cadmium resistance plasmid, pXU5, in Staphylococcus aureus, strain ATCC25923. FEMS Microbiol Lett. 2000;189:79–80. doi: 10.1111/j.1574-6968.2000.tb09209.x. [DOI] [PubMed] [Google Scholar]
- 15.Boswihi SS, Udo EE, Monecke S, Mathew B, Noronha B, Verghese T, et al. Emerging variants of methicillin-resistant Staphylococcus aureus genotypes in Kuwait Hospitals. PLoS One. 2018;13:e0195933. doi: 10.1371/journal.pone.0195933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmsen D, Claus H, Witte W, Rothgänger J, Claus H, Turnwald D, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41:5442–8. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–15. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monecke S, Jatzwauk L, Weber S, Slickers P, Ehricht R. DNA microarray-based genotyping of methicillin-resistant Staphylococcus aureus strains from Eastern Saxony. Clin Microbiol Infect. 2008;14:534–45. doi: 10.1111/j.1469-0691.2008.01986.x. [DOI] [PubMed] [Google Scholar]
- 19.McGavin MJ, Arsic B, Nickerson NN. Evolutionary blueprint for host- and niche-adaptation in Staphylococcus aureus clonal complex CC30. Front Cell Infect Microbiol. 2012;2:48. doi: 10.3389/fcimb.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park KH, Greenwood-Quaintance KE, Uhl JR, Cunningham SA, Chia N, Jeraldo PR, et al. Molecular epidemiology of Staphylococcus aureus bacteremia in a single large Minnesota medical center in 2015 as assessed using MLST, core genome MLST and spa typing. PLoS One. 2017;12:e0179003. doi: 10.1371/journal.pone.0179003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wurster JI, Bispo PJM, Tyne DV, Cadorette JJ, Boody R, Gilmore MS. Staphylococcus aureus from ocular and otolaryngology infections are frequently resistant to clinically important antibiotics and are associated with lineages of community and hospital origins. PLoS One. 2018;13:e0208518. doi: 10.1371/journal.pone.0208518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtfreter S, Grumann D, Balau V, Barwich A, Kolata J, Goehler A, et al. Molecular epidemiology of Staphylococcus aureus in the general population in Northeast Germany: results of the study of health in Pomerania (SHIP-TREND-0) J Clin Microbiol. 2016;54:2774–85. doi: 10.1128/JCM.00312-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen G, Monecke S, Brus O, Ehricht R, Söderquist B. Long term molecular epidemiology of methicillin-susceptible Staphylococcus aureus bacteremia isolates in Sweden. PLoS One. 2014;9:e114276. doi: 10.1371/journal.pone.0114276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae E, Kim CK, Jang JH, Sung H, Choi Y, Kim MN. Impact of community-onset methicillin-resistant Staphylococcus aureus on Staphylococcus aureus bacteremia in a Central Korea Veterans Health Service Hospital. Ann Lab Med. 2019;39:158–66. doi: 10.3343/alm.2019.39.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garbacz K, Kwapisz E, Piechowicz L, Wierzbowska M. Staphylococcus aureus isolated from the oral cavity: phage susceptibility in relation to antibiotic resistance. Antibiotics. 2021;10((11)):1329. doi: 10.3390/antibiotics10111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boswihi SS, Udo EE, Al-Sweih N. Shifts in the clonal distribution of methicillin-resistant Staphylococcus aureus in Kuwait Hospitals: 1992–2010. PLoS One. 2016;1511:e0162744. doi: 10.1371/journal.pone.0162744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes AR, Westh H, de Lencastre H. Origins and evolution of methicillin-resistant Staphylococcus aureus clonal lineages. Antimicrob Agents Chemother. 2006;50:3237–44. doi: 10.1128/AAC.00521-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolo J, Miragaia M, Turlej-Rogacka A, Empel J, Bouchami O, Faria NA, et al. High genetic diversity among community-associated Staphylococcus aureus in Europe: results from a multicenter study. PLoS One. 2012;7:e34768. doi: 10.1371/journal.pone.0034768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Udo EE, Al-Lawati BA, Al-Muharmi Z, Thukral SS. Genotyping of methicillin-resistant Staphylococcus aureus in the Sultan Qaboos University Hospital, Oman reveals the dominance of Panton-Valentine leucocidin-negative ST6-IV/t304 clone. New Microbes New Infect. 2014;2:100–5. doi: 10.1002/nmi2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senok A, Slickers P, Hotzel H, Boswihi S, Braun SD, Gawlik D, et al. Characterization of a novel SCCmec VI element harboring fusC in an emerging Staphylococcus aureus strain from the Arabian Gulf region. PLoS One. 2019;14:e0223985. doi: 10.1371/journal.pone.0223985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this study are included in this paper.