Abstract
Purpose of review
Barrett’s oesophagus is the only identifiable precursor lesion to oesophageal adenocarcinoma. The stepwise progression of Barrett’s oesophagus to dysplasia and invasive carcinoma provides the opportunity to intervene and reduce the morbidity and mortality associated with this lethal cancer. Several studies have demonstrated the efficacy and safety of endoscopic eradication therapy (EET) for the management of Barrett’s oesophagus related neoplasia. The primary goal of EET is to achieve complete eradication of intestinal metaplasia (CE-IM) followed by enrolment of patients in surveillance protocols to detect recurrence of Barrett’s oesophagus and Barrett’s oesophagus related neoplasia.
Recent findings
EET depends on early and accurate detection and diagnosis of Barrett’s oesophagus related neoplasia. All visible lesions should be resected followed by ablation of the remaining Barrett’s epithelium. After treatment, patients should be enrolled in endoscopic surveillance programmes. For nondysplastic Barrett’s oesophagus, surveillance alone is recommended. For low-grade dysplasia, both surveillance and ablation are reasonable options and should be decided on an individual basis according to patient risk factors and preferences. EET is preferred for high-grade dysplasia and intramucosal carcinoma. For T1b oesophageal adenocarcinoma, esophagectomy remains the standard of care, but endoscopic therapy can be considered in select cases.
Summary
EET is now standard of care and endorsed by societal guidelines for the treatment of Barrett’s oesophagus related neoplasia. Future studies should focus on risk stratification models using a combination of clinical data and biomarkers to identify ideal candidates for EET, and to predict recurrence. Optimal therapy for T1b cancer and surveillance strategy after CE-IM are topics that require further study.
Keywords: ablation, oesophageal cancer, quality in endoscopy, recurrence, resection, surveillance
INTRODUCTION
Oesophageal adenocarcinoma (EAC) is on the rise worldwide and the majority of patients present with late-stage disease associated with poor survival [1]. The only identifiable precursor lesion is Barrett’s oesophagus, which affects up to 5% of the general population [2]. Strategies to identify and screen high-risk individuals and enrol patients with Barrett’s oesophagus in endoscopic surveillance programmes are critical for cancer risk reduction [3]. Unfortunately, EAC miss rates in Barrett’s oesophagus are reported as high as 25% and we still have not impacted EAC outcomes at a population level [4,5].
Endoscopic Eradication Therapy (EET) is considered standard of care for management of Barrett’s oesophagus related neoplasia [high-grade dysplasia (HGD), intramucosal cancer (IMC) and select cases with low-grade dysplasia (LCD)], and endorsed by guidelines [6■,7■,8–11]. This paradigm shift has demonstrated survival outcomes comparable with esophagectomy, with far fewer adverse effects, and this strategy is cost-effective [12,13]. The basic principles of EET include resection of all visible lesions in Barrett’s metaplasia followed by ablation of the remaining Barrett’s epithelium with the goal of achieving complete eradication of intestinal metaplasia (CE-IM) and reduced progression to EAC. In this review, we highlight important data on outcomes related to EET, provide updates from recent guidelines and new technologies, and underscore the areas wherein future research is needed.
DYSPLASIA DETECTION AND DIAGNOSIS
Optimal outcomes for EET depend first on reliable detection of Barrett’s oesophagus related neoplasia. We suggest a 10-step approach for a high-quality examination (Table 1) that includes high definition white light endoscopy (HD-WLE) and the use of standardized reporting systems such as the Prague classification (circumferential and maximal extent of Barrett’s) and the Paris classification for superficial lesion [14–17].
Table 1.
Ten step approach to high-quality endoscopic examination of Barrett’s Esophagus using standardized reporting systems
| Approach | Rationale | |
|---|---|---|
| 1 | Consider use of a distal attachment cap | Facilitate visualization |
|
|
||
| 2 | Utilize CO2 insufflation and desufflation | Fine adjustments to luminal insufflation aid detection of subtle abnormalities |
|
|
||
| 3 | Clean mucosa well using water jet channel and carefully suction the fluid | Remove any distracting mucus or debris and minimize mucosal trauma |
|
|
||
| 4 | Identify esophageal landmarks, including the location of the diaphragmatic hiatus, gastroesophageal junction, and squamocolumnar junction | Critical for future exams |
|
|
||
| 5 | Examine the Barrett’s segment using high definition white light endoscopy | Standard of care |
|
|
||
| 6 | Examine the Barrett’s segment using chromoendoscopy (including virtual chromoendoscopy) | Enhances mucosa pattern and surface vasculature |
|
|
||
| 7 | Spend adequate time inspecting (consider 1 minute per cm) | Careful examination increases dysplasia detection |
|
|
||
| 8 | Use the Prague classification to describe the circumferential and maximal Barrett’s segment length | Standardized reporting system |
|
|
||
| 9 | Use the Paris Classification to describe superficial neoplasia | Standardized reporting system |
|
|
||
| 10 | Use the Seattle Protocol (in conjunction with advanced imaging modalities) to sample the Barrett’s segment | Increases dysplasia detection |
Sampling the Barrett’s oesophagus segment
The Seattle protocol remains the recommended strategy for sampling the Barrett’s oesophagus segment (four-quadrant biopsies at 1–2 cm intervals along the entire length) with additional targeted biopsies of areas suspicious for dysplasia (nodularity, erosions, luminal irregularities) [18]. This need for random biopsies continues to be the Achilles heel of Barrett’s oesophagus surveillance, as it only samples less than 5% of the Barrett’s oesophagus segment and is tedious and time-consuming, features that likely contribute to nonadherence with the protocol [19] that is associated with lower rates of dysplasia detection [odds ratio (OR) 0.53, 95% confidence interval (95% CI) 0.35–0.82] [20].
A newer approach to tissue collection uses an abrasive cytology brush for wide-area transepithelial sampling with computer-assisted three-dimension disease analysis (WATS3D; CDx Diagnostics, Suffern NY, USA). WATS is designed to capture tissue from a larger surface area than can be sampled by forceps biopsies, creating thick cytology specimens that can be analysed using computer constructs to recapitulate glandular structure. The 2019 ASGE guidelines provide a conditional recommendation for use of WATS based on their systematic review and metaanalysis of 6271 patients with Barrett’s oesophagus in whom WATS provided a 48% increase in relative and 10.6% increase in absolute dysplasia detection [6■]. The cost-effectiveness of WATS, implications of identifying additional cases of crypt dysplasia and the meaning of LGD on cytology compared with histology will need to be addressed in future studies.
Image-enhanced endoscopy to guide endoscopic eradication therapy
Advanced imaging modalities can improve detection and diagnosis of dysplasia in real-time and guide EET. Chromoendoscopy is the most commonly used adjunctive tool to demarcate lesions prior to resection and is recommended by ASGE guidelines based on data demonstrating a 9% absolute increase and 30% relative increase in dysplasia detection compared with HD-WLE [6■]. Although narrow band imaging (NBI; Olympus Center Valley PA, USA) is the most frequently studied platform, several other systems including iScan Optical Enhancement system (OE) and ELUXEO 7000 (Fujifulm, Japan) have demonstrated improved dysplasia detection in Barrett’s oesophagus [21,22].
Artificial intelligence
Computer-aided detection systems for detection and diagnosis of Barrett’s oesophagus have the potential to dramatically impact clinical practice. Several systems have been developed and tested including the ARGOS project in the Netherlands [23,24] and an artificial intelligence algorithm in the USA that can detect Barrett’s oesophagus neoplasia with 93.7% accuracy, 95.6% sensitivity and 91.8% specificity for an area under the curve (AUC) of 0.94 [25]. Preliminary data on the use of real-time artificial intelligence showed an accuracy of 89.9% in 14 cases with neoplastic Barrett’s oesophagus [26■■]. Machine learning is also being developed for volumetric laser endomicroscopy [27,28], and we anticipate continued advancements in the near future.
PATIENT SELECTION FOR ENDOSCOPIC ERADICATION THERAPY
At the present time, the histologic grade of dysplasia remains the best predictor for neoplastic progression of Barrett’s oesophagus and should direct EET. New data have substantially enriched the conversation on management for patients with NDBE and LGD. A population-based modelling study determined the optimal strategy as follows: for men with NDBE, surveillance every 3 years (five for women), and for LGD, EET is favoured over surveillance with the caveat that treatment should only be performed if LGD is confirmed on repeat endoscopy after 2 months of high-dose acid suppression (incremental cost-effectiveness ratio, $53044/quality- adjusted life year (QALY)) [29■]. A modelling study from the UK similarly demonstrated cost-effectiveness of EET for LGD and HGD [30].
Nondysplastic Barrett’s oesophagus: surveillance over no surveillance, surveillance over ablation
At the present time, there are insufficient data to inform which individuals with NDBE will progress and benefit from EET. Surveillance for NDBE is recommended by professional gastrointestinal societies every 3–5 years [6■]. Given the relatively low risk of progression, cost-benefit and impact depend on adherence to guideline-supported intervals for repeat endoscopy. Unfortunately, up to 30% of patients with NDBE are given a surveillance interval that is too soon (1–2 years) [31]. Risk stratification will be critical with future studies focused on a panel of biomarkers to predict progression and target these individuals for EET [32].
Low-grade dysplasia: endoscopic eradication therapy or surveillance
The management of LGD is challenging due to multiple factors, including variable reported rates of progression and significant interobserver variation among pathologists (including expert gastrointestinal pathologists) [33]. In an updated systematic review and meta-analysis of 619 patients with LGD, EET with radiofrequency ablation (RFA) had a lower rate of progression to HGD/EAC compared with surveillance (OR 0.07, 95% CI 0.02–0.22) [34]. The AGA Clinical Practice Committee state that both EET and continued surveillance are reasonable options for LGD [35■]. The risks and benefits of both strategies (EET and surveillance) should be discussed with the patient as well as the adverse events associated with ablation. Similarly, the most recent ASGE guideline statement emphasizes that a patient centred approach is critical for this patient population [6■].
High-grade dysplasia, intramucosal cancer (or T1a oesophageal adenocarcinoma) and submucosal cancer (T1b oesophageal adenocarcinoma)
GI professional societies unanimously endorse EET over esophagectomy for Barrett’s oesophagus with HGD/IMC [7■,35■]. Endoscopic ultrasound should not be used for T-staging in this group, as it frequently results in overstaging [6■,36]. Surgery has always been the cornerstone of therapy for T1b EAC given the risk of lymph node metastasis; however, the management has recently become more nuanced. A risk stratified approach can be considered with endoscopic resection for patients with SM1 tumours (submucosal invasion limited to <500 μm) and low-risk features (well differentiated, small size <2 cm, no lymphovascular invasion). This has been endorsed by expert opinion as a reasonable alternative to esophagectomy, especially in patients who are poor surgical candidates [35■,37]. However, this decision requires input from a multidisciplinary tumour board and should account for patient preferences and overall health. Additional studies are needed to determine outcomes and predictors of recurrence as well as to define the role of adjuvant chemotherapy and radiation after endoscopic resection.
TECHNIQUES FOR ENDOSCOPIC ERADICATION THERAPY
Quality indicators and thresholds have been established for the preprocedure and intraprocedure aspects of EET with proposed thresholds (Table 2) [38]. All endoscopists performing EET should be familiar with established quality metrics and should evaluate their own performance on a regular basis. Our algorithm for management of Barrett’s oesophagus related neoplasia and implementation of EET where appropriate is provided (Fig. 1).
Table 2.
Quality indicators for endoscopic eradication therapy in Barrett’s esophagus
| Quality Metric | Threshold Benchmark | Type of measure |
|---|---|---|
| The rate at which the reading is made by a GI pathologist or confirmed by a second pathologist before EET is begun for patients in whom a diagnosis of dysplasia is made | 90% | Process |
| Centers in which EET is performed should have available HD-WLE and expertise in mucosal ablation and EMR techniques | -- | Process |
| The rate at which documentation of a discussion of the risks, benefits, and alternatives to EET is obtained from the patient prior to treatment | >98% | Process |
| The rate at which landmarks and length of BE is documented (eg, Prague grading system) in patients with BE before EET | 90% | Process |
| The rate at which the presence or absence of visible lesions is reported in patients with BE referred for EET | 90% | Process |
| The rate at which the BE segment is inspected by using HD-WLE | 95% | Process |
| The rate at which complete endoscopic resection (en bloc resection or piecemeal) is performed in patients with BE with visible lesions | 90% | Process |
| The rate at which a defined interval for subsequent EET is documented for patients undergoing EET who have not yet achieved CE-IM | 90% | Process |
| The rate at which CE-N and CE-IM is achieved by 18 months in patients with BE-related dysplasia or intramucosal cancer referred for EET | 80%, 70% | Outcome |
| The rate at which a recommendation is documented for endoscopic surveillance at a defined interval for patients who achieve CE-IM | 90% | Process |
| The rate at which biopsies of any visible mucosal abnormalities are performed during endoscopic surveillance after EET | 95% | Process |
| The rate at which an anti-reflux regimen is recommended for EET | 90% | Process |
| The rate at which adverse events are being tracked and documented in individuals after EET | 90% | Process |
Quality Indicators apply to pre-procedure (blue), intra-procedure (yellow), and post-procedure (green). GI, gastrointestinal; EET, endoscopic eradication therapy; HD-WLE, high-definition white light endoscopy; BE, Barrett’s esophagus; EMR, endoscopic mucosal resection; CE-IM, complete eradication of intestinal metaplasia; CE-N, complete eradication of neoplasia
Adapted from Wani et al (36)
FIGURE 1.
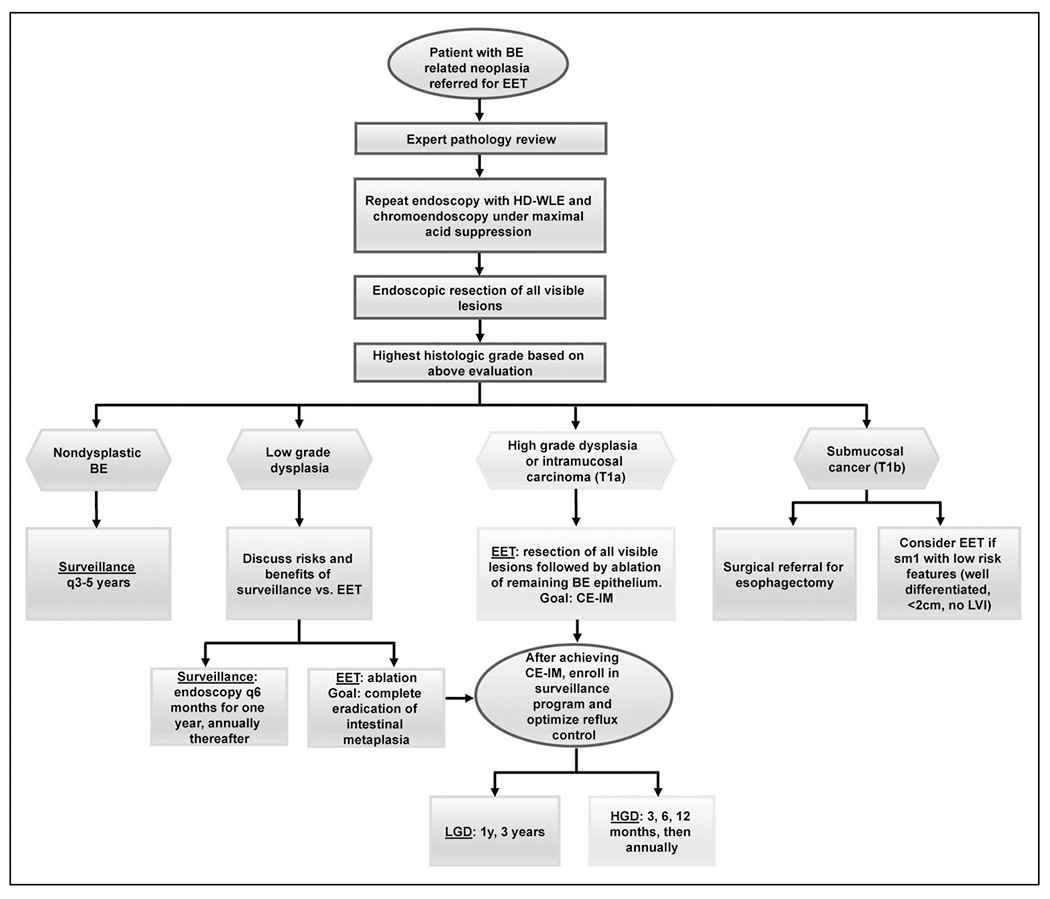
Algorithm for the management of Barrett’s oesophagus related neoplasia with endoscopic eradication therapy. BE, Barrett’s oesophagus; CE-IM, complete eradication of intestinal metaplasia; EET, endoscopic eradication therapy; HD-WLE, high definition white light endoscopy; HGD, high-grade dysplasia; LGD, low-grade dysplasia; LVI, lymphovascular invasion; sm1, submucosal invasion limited to <500 μm.
Ablation
RFA remains the preferred ablative modality for flat dysplastic Barrett’s oesophagus, though several studies have recently evaluated the efficacy of cryotherapy in this patient population. In a systematic review and meta-analysis of 386 patients treated with cryotherapy with liquid nitrogen, high rates of CE-IM (56.5%, 95% CI 48.5–64.2) and CE-D (83.5%, 95% CI 78.3–87.7) and CE-HGD (86.5%, 95% CI 64.4–95.8) were achieved with a low rate of adverse events at 4.7%. Cryotherapy has also demonstrated effectiveness as salvage therapy in patients who previously failed RFA (CE-IM 58.4%, 95% CI 47.2–68.8) [39]. Newer platforms such as the cryoballoon focal ablation that uses nitrous oxide demonstrated high rates of 1-year CE-IM (88%, median number of procedures 3) and CE-D (95%) in combination with EMR for 41 patients with treatment-naive or previously ablated Barrett’s oesophagus [40]. Other techniques for EET such as argon plasma coagulation or hot avulsion require further study before they can be incorporated into routine clinical practice [41,42].
Endoscopic mucosal resection versus endoscopic submucosal dissection
Endoscopic resection for visible neoplasia serves as a staging tool by providing a larger and deeper tissue specimen than biopsy samples to demonstrate depth of invasion. The limitation of endoscopic mucosal resection (EMR) is that piecemeal resection compromises the lateral margins and may lead to indefinite pathologic diagnoses such as ‘at least intramucosal carcinoma’. The main advantage of ESD is that en bloc resection offers more precise histology and higher rates of curative R0 resection (58.8 versus 11.7%, P = 0.01) [43] with lower rates of residual and local recurrence. The downside is that ESD is more technically challenging than EMR with a steep learning curve and higher adverse event rates.
ESD for Barrett’s oesophagus related neoplasia is gaining traction in the west. A systematic review and meta-analysis of ESD for 524 Barrett’s oesophagus related lesions (mean size 27 mm) showed high rates of en bloc resection (93%), complete R0 resection (75%) and curative resection rates (65%) with less than 2% rate of bleeding and/or perforation. Recurrence after curative resection was extremely low at 0.17% at 23 months [44■■]. Outcomes were similar in studies from Asia and the West. Trained experts in the USA advocate considering ESD over EMR in the following scenarios: lesions more than 15 mm that would necessitate piecemeal removal by EMR, morphology indicating high risk of submucosal invasion (Paris Is, Ip, IIc), advanced histology (‘at least IMC’) given the frequency with which final disease gets upgraded and incompletely resected/recurrent lesions wherein submucosal fibrosis and scarring can be expected [45■,46]. Future studies are required to validate these criteria for ESD over EMR.
GOAL OF ENDOSCOPIC ERADICATION THERAPY: COMPLETE ERADICATION OF INTESTINAL METAPLASIA
The aim of EET is CE-IM, defined as the absence of endoscopically visible Barrett’s oesophagus and histologic eradication of intestinal metaplasia for the entire pretreatment length of the Barrett’s oesophagus segment and the squamocolumnar junction. There is ample literature supporting the efficacy of EET in achieving this goal of CE-IM. An alternate endpoint of CE-D has been suggested, but it is associated with higher risk of dysplasia recurrence [relative risk (RR) 2.8, 95% CI 1.7–4.6] and specifically HGD/IMC (RR 3.6, 95% CI 1.45–9) [47]. One proposed explanation for this unacceptably high rate of recurrence in the CE-D group is patient related factors, wherein comorbidities and the overall clinical scenario dictated the stopping point for EET that subsequently impacted the risk of recurrence [48].
Recurrence after endoscopic eradication therapy/complete eradication of intestinal metaplasia
Tremendous progress in endoscopic therapy for Barrett’s oesophagus related neoplasia through expansion of tools and technologies has led to significantly more individuals undergoing EET, which raises important questions about their disease course following CE-IM. Recurrence is defined as histologic evidence of intestinal metaplasia or dysplasia on biopsies or resection specimens taken from the oesophagus or squamocolumnar junction after CE-IM or CE-D is achieved, with or without endo scopically visible Barrett’s oesophagus. Recurrence of intestinal metaplasia occurs in 8–10% of patients yearly [49]. Predictors of recurrence include older age, increased Barrett’s oesophagus length, hiatal hernia, gastroesophageal reflux disease, baseline grade of dysplasia and number of sessions required to achieve CE-IM, whereas treatment at high volume centres is associated with a lower risk of recurrence [50,51■,52]. The histology of recurrence is usually the same or lower than the index histology. The majority of recurrences can be managed using the same EET techniques with resection of visible lesions and ablation of the remaining Barrett’s oesophagus segment.
Time to recurrence and implications for surveillance
The long-term durability of EET and the need for continued surveillance poses a challenge to endoscopists. Just as baseline histology is the best predictor of progression, it is the most useful marker for neoplastic recurrence post CE-IM. The timing of recurrence was examined in a systematic review and meta-analysis of 1973 patients, which showed the highest incidence rate of intestinal metaplasia recurrence in the first year (12%) compared with the second (7%) and third year (3%) (RR 1.8, 95% CI 1.29–2.49). Dysplasia detection and HGD/EAC detection was also higher in the first year than the years after (RR 1.92, 95% CI 1.32–2.8 and RR 1.58, 95% CI 0.94–2.65) [53■■]. The authors hypothesize that high rates of disease in the first year may be due to incompletely treated disease rather than recurrence, warranting more intensive surveillance in the first year following CE-IM. Recurrence tends to occur within 2 cm of the squamocolumnar junction and is often nonvisible, thus a modified biopsy protocol that focuses random sampling in the distal oesophagus has been suggested [54].
A modelling study based on data from USA and UK RFA registries determined optimal surveillance intervals post CE-IM as follows: LGD: at 1 and 3 years, and HGD/IMC: at 3 then 6 months, 1 year, then annually [55■■]. These intervals were recently supported by expert opinion and endorsed by an AGA Clinical Practice Update document [35■]. Our own data from a multicentre prospective study of 807 patients showed overall low rates of recurrent intestinal metaplasia (15%, incidence rate 5.2/100 PY) and recurrent dysplasia (4.5%, incidence rate 1.6/100 PY), with recurrence peaking at 1.6 years after achieving CE-IM. These results suggest that aggressive surveillance may not be needed in the first year [51■]. Another multicentre study of 594 patients demonstrated that risk of CE-IM remained constant over time with a 19% cumulative risk of Barrett’s oesophagus recurrence within 2 years and additional 49% risk over the next almost 9 years [56]. Future studies should focus on risk stratification tools for recurrence and validation of proposed surveillance intervals.
Finally, reflux control is essential post EET and there may be an expanding role for antireflux surgery or endoscopy with transoral incisionless fundoplication. Data are needed to determine the optimal timing of these interventions and their potential impact on surveillance intervals.
CONCLUSION
At the present time, it is recommended that EET be performed at academic medical centres that have experienced gastrointestinal pathologists and skilled interventional endoscopists. As the number of patients at risk for Barrett’s oesophagus increase, and the technologies and techniques related to EET expand, innovative educational platforms will need to adapt to reach and teach new trainees and practicing endoscopists [57]. Novel research and development of biomarkers will help us to develop risk prediction models to determine which patients are at highest risk for progression or recurrence [58,59]. In the meantime, performance of excellent endoscopic examinations according to published quality indicators, adherence to professional society guideline recommendations for surveillance intervals and aggressive EET with a multimodal approach always focusing on the goal of CE-IM remain our best chance to halt the progression of Barrett’s oesophagus and decrease the incidence and mortality of EAC.
KEY POINTS.
Endoscopic eradication therapy is now standard of care for Barrett’s oesophagus related neoplasia based on studies demonstrating its efficacy and safety.
The basic principles of endoscopic eradication therapy for Barrett’s oesophagus related neoplasia include resection of all visible lesions followed by ablation of the remaining Barrett’s epithelium.
Surveillance is recommended for nondysplastic Barrett’s oesophagus.
Both surveillance and ablation are reasonable options for low-grade dysplasia and should be considered on an individual basis according to patient risk factors and preferences.
Gastroenterology professional societies unanimously endorse endoscopic eradication therapy over esophagectomy for Barrett’s oesophagus with high-grade dysplasia or intramucosal carcinoma.
The primary goal of endoscopic eradication therapy is to achieve complete eradication of intestinal metaplasia (CE-IM) followed by enrolment of these patients in surveillance protocols to detect recurrence of Barrett’s oesophagus and related neoplasia.
Financial support and sponsorship
JK received funding from National Institutes of Health (NIH) T32-DK007038
SW is supported by the University of Colorado Department of Medicine Outstanding Early Scholars Program.
Footnotes
Conflicts of interest
SW is a consultant for Medtronic, Boston Scientific and Interpace.
This paper has not been published in its current form or a substantially similar form (in any format), it has not been accepted for publication elsewhere and it is not under consideration by another publication.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Coleman HG, Xie SH, Lagergren J. The epidemiology of esophageal adenocarcinoma. Gastroenterology 2018; 154:390–405. [DOI] [PubMed] [Google Scholar]
- 2.Hayeck TJ, Kong CY, Spechler SJ, et al. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus 2010; 23:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammad TA, Thrift AP, El-Serag HB, Husain NS. Missed opportunities for screening and surveillance of Barrett’s esophagus in veterans with esophageal adenocarcinoma. Dig Dis Sci 2019; 64:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visrodia K, Singh S, Krishnamoorthi R, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: a systematic review and meta-analysis. Gastroenterology 2016; 150:599–607e7; quiz e14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thrift AP. Barrett’s esophagus and esophageal adenocarcinoma: how common are they really? Dig Dis Sci 2018; 63:1988–1996. [DOI] [PubMed] [Google Scholar]
- 6.Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc 2019; 90:335–359e2. [DOI] [PubMed] [Google Scholar]; ■ Updated ASGE guidelines using a robust systematic review and meta-analysis with GRADE methodology provide key recommendations.
- 7.Standards of Practice C, Wani S, Qumseya B, et al. Endoscopic eradication therapy for patients with Barrett’s esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018; 87:907–931e9. [DOI] [PubMed] [Google Scholar]; ■ ASGE Standards of Practice Committee provides seven summary statements for EET in Barrett’s esophagus using the GRADE methodology.
- 8.Beg S, Ragunath K, Wyman A, et al. Quality standards in upper gastrointestinal endoscopy: a position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut 2017; 66:1886–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017; 49:191–198. [DOI] [PubMed] [Google Scholar]
- 10.Fock KM, Talley N, Goh KL, et al. Asia-Pacific consensus on the management of gastro-oesophageal reflux disease: an update focusing on refractory reflux disease and Barrett’s oesophagus. Gut 2016; 65:1402–1415. [DOI] [PubMed] [Google Scholar]
- 11.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. American College of G. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111:30–50quiz 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wani S, Drahos J, Cook MB, et al. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: a population based study. Gastrointest Endosc 2014; 79:224–232e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inadomi JM, Saxena N. Screening and surveillance for Barrett’s esophagus: is it cost-effective? Dig Dis Sci 2018; 63:2094–2104. [DOI] [PubMed] [Google Scholar]
- 14.Kolb J, Wani S. Barrett’s esophagus: current standards in advanced imaging. Transl Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006; 131:1392–1399. [DOI] [PubMed] [Google Scholar]
- 16.Paris Workshop on Columnar Metaplasia in the Esophagus and the Esophagogastric Junction, Paris, France, December 11-12 2004. Endoscopy; 2005; 37:879–920. [DOI] [PubMed] [Google Scholar]
- 17.Gorrepati VS, Sharma P. How should we report endoscopic results in patient’s with Barrett’s esophagus? Dig Dis Sci 2018; 63:2115–2121. [DOI] [PubMed] [Google Scholar]
- 18.Wani S, Gaddam S. Editorial: best practices in surveillance of Barrett’s esophagus. Am J Gastroenterol 2017; 112:1056–1060. [DOI] [PubMed] [Google Scholar]
- 19.Wani S, Williams JL, Komanduri S, et al. Time trends in adherence to surveillance intervals and biopsy protocol among patients with Barrett’s esophagus. Gastroenterology 2020; 158:770–772.e2. [DOI] [PubMed] [Google Scholar]
- 20.Wani S, Williams JL, Komanduri S, et al. Endoscopists systematically undersample patients with long-segment Barrett’s esophagus: an analysis of biopsy sampling practices from a quality improvement registry. Gastrointest Endosc 2019; 90:732–741.e3. [DOI] [PubMed] [Google Scholar]
- 21.Everson MA, Lovat LB, Graham DG, et al. Virtual chromoendoscopy by using optical enhancement improves the detection of Barrett’s esophagus-associated neoplasia. Gastrointest Endosc 2019; 89:247–256e4. [DOI] [PubMed] [Google Scholar]
- 22.de Groof AJ, Swager AF, Pouw RE, et al. Blue-light imaging has an additional value to white-light endoscopy in visualization of early Barrett’s neoplasia: an international multicenter cohort study. Gastrointest Endosc 2019; 89:749–758. [DOI] [PubMed] [Google Scholar]
- 23.de Groof J, van der Sommen F, van der Putten J, et al. The Argos project: the development of a computer-aided detection system to improve detection of Barrett’s neoplasia on white light endoscopy. United European Gastroenterol J 2019; 7:538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Groof AJ, Struyvenberg MR, van der Putten J, et al. Deep-learning system detects neoplasia in patients with Barrett’s esophagus with higher accuracy than endoscopists in a multi-step training and validation study with benchmarking. Gastroenterology 2020; 158:915e4–929e4. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto R, Requa J, Tyler D, et al. Artificial intelligence using convolutional neural networks for real-time detection of early esophageal neoplasia in Barrett’s esophagus (with video). Gastrointest Endosc 2020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Ebigbo A, Mendel R, Probst A, et al. Real-time use of artificial intelligence in the evaluation of cancer in Barrett’s oesophagus. Gut 2020; 69:615–616. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ The first study on use of real-time artificial intelligence for Barrett’s oesophagus related neoplasia. Artificial intelligence has enormous potential to dramatically impact clinial practice through improved detection and diagnosis to guide endoscopic eradication therapy.
- 27.Trindade AJ, McKinley MJ, Fan C, et al. Endoscopic surveillance of Barrett’s esophagus using volumetric laser endomicroscopy with artificial intelligence image enhancement. Gastroenterology 2019; 157:303–305. [DOI] [PubMed] [Google Scholar]
- 28.Struyvenberg MR, van der Sommen F, Swager AF, et al. Improved Barrett’s neoplasia detection using computer-assisted multiframe analysis of volumetric laser endomicroscopy. Dis Esophagus 2019; 33:pii: doz065. [DOI] [PubMed] [Google Scholar]
- 29.Omidvari AH, Ali A, Hazelton WD, et al. Optimizing management of patients with Barrett’s esophagus and low-grade or no dysplasia based on comparative modeling: optimizing Barrett’s esophagus management. Clin Gastroenterol Hepatol 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ A population-based modelling study provides optimal strategy for NDBE and LGD along with important cost-effectiveness analysis.
- 30.Pollit V, Graham D, Leonard C, et al. A cost-effectiveness analysis of endoscopic eradication therapy for management of dysplasia arising in patients with Barrett’s oesophagus in the United Kingdom. Curr Med Res Opin 2019; 35:805–815. [DOI] [PubMed] [Google Scholar]
- 31.Wani S, Williams JL, Komanduri S, et al. Over-utilization of repeat upper endoscopy in patients with nondysplastic Barrett’s esophagus: a quality registry study. Am J Gastroenterol 2019; 114:1256–1264. [DOI] [PubMed] [Google Scholar]
- 32.Duits LC, Klaver E, Bureo Gonzalez A, et al. The Amsterdam ReBus progressor cohort: identification of 165 Barrett’s surveillance patients who progressed to early neoplasia and 723 nonprogressor patients. Dis Esophagus 2019; 32:pii: doy037. [DOI] [PubMed] [Google Scholar]
- 33.Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and management of low-grade dysplasia in barrett’s esophagus: expert review from the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology 2016; 151:822–835. [DOI] [PubMed] [Google Scholar]
- 34.Pandey G, Mulla M, Lewis WG, et al. Systematic review and meta-analysis of the effectiveness of radiofrequency ablation in low grade dysplastic Barrett’s esophagus. Endoscopy 2018; 50:953–960. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P, Shaheen NJ, Katzka D, Bergman JJGHM. Clinical practice update: endoscopic treatment of Barrett’s esophagus with dysplasia and/or early cancer. Gastroenterology 2020; 158:760–769. [DOI] [PubMed] [Google Scholar]; ■ Updated recommendations by the AGA Clinical Practice Committee on endoscopic therapy for Barrett’s esophagus related neoplasia.
- 36.Qumseya BJ, Bartel MJ, Gendy S, et al. High rate of over-staging of Barrett’s neoplasia with endoscopic ultrasound: systemic review and meta-analysis. Dig Liver Dis 2018; 50:438–445. [DOI] [PubMed] [Google Scholar]
- 37.Othman MO, Lee JH, Wang K. Clinical practice update on the utility of endoscopic submucosal dissection in T1b esophageal cancer: expert review. Clin Gastroenterol Hepatol 2019; 17:2161–2166. [DOI] [PubMed] [Google Scholar]
- 38.Wani S, Muthusamy VR, Shaheen NJ, et al. Development of quality indicators for endoscopic eradication therapies in Barrett’s esophagus: the TREAT-BE (Treatment with Resection and Endoscopic Ablation Techniques for Barrett’s Esophagus) Consortium. Gastrointest Endosc 2017; 86:1–17e3. [DOI] [PubMed] [Google Scholar]
- 39.Mohan BP, Krishnamoorthi R, Ponnada S, et al. Liquid nitrogen spray cryotherapy in treatment of Barrett’s esophagus, where do we stand? A systematic review and meta-analysis. Dis Esophagus 2019; 32: pii: doy130. [DOI] [PubMed] [Google Scholar]
- 40.Canto MI, Shaheen NJ, Almario JA, et al. Multifocal nitrous oxide cryoballoon ablation with or without EMR for treatment of neoplastic Barrett’s esophagus (with video). Gastrointest Endosc 2018; 88:438–446e2. [DOI] [PubMed] [Google Scholar]
- 41.Aranda-Hernandez J, Shimamura Y, Grin A, et al. Hot avulsion may be effective as salvage treatment for focal Barrett’s esophagus remaining after endoscopic therapy for dysplasia or early cancer: a preliminary study. Endoscopy 2018; 50:8–13. [DOI] [PubMed] [Google Scholar]
- 42.Peerally MF, Bhandari P, Ragunath K, et al. Radiofrequency ablation compared with argon plasma coagulation after endoscopic resection of high-grade dysplasia or stage T1 adenocarcinoma in Barrett’s esophagus: a randomized pilot study (BRIDE). Gastrointest Endosc 2019; 89:680–689. [DOI] [PubMed] [Google Scholar]
- 43.Terheggen G, Horn EM, Vieth M, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut 2017; 66:783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang D, Zou F, Xiong S, et al. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018; 87:1383–1393. [DOI] [PubMed] [Google Scholar]; ■■ A comprehensive analysis demonstrates the efficacy and safety of endoscopic submucosal dissection for Barrett’s esophagus related neoplasia. Results were similar in studies from Asia and from the West, which is important, as this technque becomes more common in the USA.
- 45.Yang D, Othman M, Draganov PV. Endoscopic mucosal resection vs endoscopic submucosal dissection for Barrett’s esophagus and colorectal neoplasia. Clin Gastroenterol Hepatol 2019; 17:1019–1028. [DOI] [PubMed] [Google Scholar]; ■ This study suggested scenarios wherein endoscopic submucosal dissection might be considered over endoscopic mucosal resection for Barrett’s esophagus related neoplasia. Further studies are required to validade these criteria.
- 46.Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol 2019; 17:16–25e1. [DOI] [PubMed] [Google Scholar]
- 47.Sawas T, Alsawas M, Bazerbachi F, et al. Persistent intestinal metaplasia after endoscopic eradication therapy of neoplastic Barrett’s esophagus increases the risk of dysplasia recurrence: meta-analysis. Gastrointest Endosc 2019; 89:913–925e6. [DOI] [PubMed] [Google Scholar]
- 48.Shaheen NJ. Where is the finish line for endoscopic eradication therapy in Barrett’s esophagus? Gastrointest Endosc 2019; 89:926–928. [DOI] [PubMed] [Google Scholar]
- 49.Kahn A, Shaheen NJ, Iyer PG. Approach to the post-ablation Barrett’s esophagus patient. Am J Gastroenterol 2020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Soroush A, Poneros JM, Lightdale CJ, Abrams JA. Shorter time to achieve endoscopic eradication is not associated with improved long-term outcomes in Barrett’s esophagus. Dis Esophagus 2019; 32:pii: doz026. [DOI] [PubMed] [Google Scholar]
- 51.Wani S Recurrence is rare and peaks at 18 months following complete eradication of intestinal metaplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol 2020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]; ■ A multicenter analysis provides data on the rate of recurrence following CE-IM and timing of recurrence, which has implications for surveillance intervals and will inform future guidelines.
- 52.Tan MC, Kanthasamy KA, Yeh AG, et al. Factors associated with recurrence of Barrett’s esophagus after radiofrequency ablation. Clin Gastroenterol Hepatol 2019; 17:65–72e5. [DOI] [PubMed] [Google Scholar]
- 53.Sawas T, Iyer PG, Alsawas M, et al. Higher rate of Barrett’s detection in the first year after successful endoscopic therapy: meta-analysis. Am J Gastroenterol 2018; 113:959–971. [DOI] [PubMed] [Google Scholar]; ■■ A comprehensive review and meta-analysis provides data on the timing of recurrence after CE-IM is achieved. High rates of recurrence in the first year suggest this might be more a result of incompletely treated disease rather than recurrence.
- 54.Omar M, Thaker AM, Wani S, et al. Anatomic location of Barrett’s esophagus recurrence after endoscopic eradication therapy: development of a simplified surveillance biopsy strategy. Gastrointest Endosc 2019; 90:395–403. [DOI] [PubMed] [Google Scholar]
- 55.Cotton CC, Haidry R, Thrift AP, et al. Development of evidence-based surveillance intervals after radiofrequency ablation of Barrett’s esophagus. Gastroenterology 2018; 155:316–326e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ A modeling study based on USA and UK data informed updated surveillance intervals that are now widely used.
- 56.Sami SS, Ravindran A, Kahn A, et al. Timeline and location of recurrence following successful ablation in Barrett’s oesophagus: an international multicentre study. Gut 2019; 68:1379–1385. [DOI] [PubMed] [Google Scholar]
- 57.Soetikno R, Kolb JM, Nguyen-Vu T, et al. Evolving endoscopy teaching in the era of the millennial trainee. Gastrointest Endosc 2019; 89:1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konda VJA, Souza RF. Biomarkers of Barrett’s esophagus: from the laboratory to clinical practice. Dig Dis Sci 2018; 63:2070–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duits LC, Lao-Sirieix P, Wolf WA, et al. A biomarker panel predicts progression of Barrett’s esophagus to esophageal adenocarcinoma. Dis Esophagus 2019; 32:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


