Abstract
Light hydrocarbons (LHs) separation is an important process in petrochemical industry. The current separation technology predominantly relies on cryogenic distillation, which results in considerable energy consumption. Adsorptive separation using porous solids has received widespread attention due to its lower energy footprint and higher efficiency. Thus, tremendous efforts have been devoted to the design and synthesis of high-performance porous solids. Among them, porous carbons display exceptional stability, tunable pore structure, and surface chemistry and thus represent a class of novel adsorbents upon achieving the matched pore structures for LHs separations. In this review, the modulation strategies toward advanced carbon-based adsorbents for LHs separation are firstly reviewed. Then, the relationships between separation performances and key structural parameters of carbon adsorbents are discussed by exemplifying specific separation cases. The research findings on the control of the pore structures as well as the quantification of the adsorption sites are highlighted. Finally, the challenges of carbonaceous adsorbents facing for LHs separation are given, which would motivate us to rationally design more efficient absorbents and separation processes in future.
1. Introduction
Light hydrocarbons (LHs, e.g., C1-C4 hydrocarbons) are basic feedstocks for fuels, plastics, and polymers [1–3]. The separation of LHs with similar physical and chemical properties is one of the most important but challenging petrochemical processes [4]. The dominated thermal-driven separation technology by the difference in boiling points (i.e., distillation) suffers from high energy consumption, which accounts for up to half of the total energy consumption in the chemical industry [1]. The adsorptive separation based on advanced adsorbents is a promising alternative for LHs separation because of the advantages in terms of low energy consumption, environmental friendliness, and easy regeneration [5–8].
Table 1 displays the physicochemical parameters of LHs of C1 to C4 and the background for their separation. Methane (CH4) is produced either from petrochemical sites or unconventional gas sources (e.g., coalbed methane and shale gas). The gas mixture contains a couple of impurities, mainly including N2, CO2, and a small fraction of C2+ hydrocarbons in some cases. The highly valuable C2-4 olefins are mainly produced by the steam cracking of feedstocks from gaseous resources, such as ethane (C2H6) and propane (C3H8), to liquid, heavier feedstocks, such as naphtha, gas oil, etc. The outlet stream is a gas mixture composed of mainly paraffin and olefin. Accordingly, the relevant separation includes CH4/N2, CH4/CO2, and CH4/CxHy separation for natural gas purification; CxH2x/CxH2x+2 for the production of high-purity C2-3 olefins; and saturated/unsaturated C4 isomer separation for producing the precursors for synthetic rubbers and additives for high-octane gasoline.
Table 1.
Physical properties of light hydrocarbons of C1 to C4 [4].
| Adsorbate | Boiling point (K) | Kinetic diameter (Å) | Dipole moment (×1018esu cm) | Polarizability (×10−25cm3) | Application background |
|---|---|---|---|---|---|
| CH4 | 111.6 | 3.8 | 0 | 25.9 | Natural gas purification |
| C2H6 | 184.6 | 4.4 | 0 | 44.3 | Producing high-purity ethylene |
| C2H4 | 169.4 | 4.2 | 0 | 42.5 | |
| C3H8 | 231.0 | 4.3-5.1 | 0.084 | 62.9 | Producing high-purity propylene |
| C3H6 | 225.5 | 4.7 | 0.366 | 62.9 | |
| n-C4H10 | 272.7 | 4.7 | 0.05 | 82.0 | Paraffin separation of normal paraffin from iso-paraffins |
| i-C4H10 | 261.3 | 5.3 | 0.132 | 81.4-82.9 | |
| n-C4H8 | 266.9 | 4.5 | 0.359-0.438 | 79.7-85.2 | Olefin separation |
| i-C4H8 | 266.3 | 4.8 | 0.501 | 78.9 | |
| C4H6 | 268.6 | 5.2 | 0 | 86.4 | Removal of dienes from olefins |
In the typical adsorptive separation process, the gas mixture flows into the adsorption column, and the weakly adsorbed adsorbate is preferentially drained out from the adsorption column. The adsorption separation process can be divided into separation and purification [9–11]. For the former, the separated gas components (i.e., the strongly adsorbed adsorbate) account for a large proportion of the mixture [12, 13]. For the latter, impurities (usually less than 2%) are more strongly adsorbed to obtain high-purity gas. For instance, the removal of acetylene (C2H2, about 1%) from the mixture of C2H4/C2H2 to upgrade the C2H4 stream is the so-called purification process [14, 15]. No matter which type of adsorption separation process, separation efficiency can be affected by the interactions between adsorbate and adsorbent and between different adsorbates, while the optimization of interactions mainly relies on the structural modification of the adsorbent.
With the advances in new porous solids, the adsorptive LHs separation is becoming more effective and playing a bigger role in molecular separation. Figure 1(a) shows the development of advanced adsorbents for LHs separation in the past two decades, which is exemplified by carbon materials and crystalline materials (e.g., MOFs and zeolites) [16]. Crystalline adsorbents have the advantages of well-defined pore structures and highly uniform micropore size. A typical example is zeolite material with 3D cage cavities. The charged skeletons or open metal sites provide tunable binding affinities towards unsaturated hydrocarbons and thus enable well control over a variety of host–guest interactions [4, 16]. However, the higher prices of raw materials and the stability of the crystalline adsorbents limit the industrial application of these materials. Only adsorbents with a good match between performance and cost are preferred for industrial production.
Figure 1.
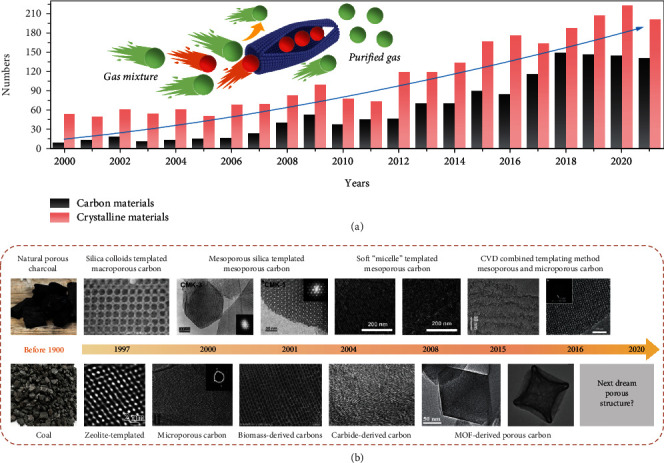
(a) The development of the field of “carbon materials” and “crystalline materials” on light hydrocarbons separation in the last two decades (web of science until March. 2022). (b) The research timeline on the development of porous carbons with defined pore structures.
Porous carbons hold promises as adsorbents for LHs separation because of their inherent advantages such as structural robustness, low cost, and high porosity [17–20]. Unlike crystalline materials, porous carbons hold promises as adsorbents for LHs separation because of their inherent advantages such as structural robustness, low cost, and high porosity. Structurally, porous carbons exhibit a unique slit-shape microporous structure, which is built from disorganized graphene-like planes, forming a tightly stacked structure with chemically stable and acid/base resistance [21, 22]. These disorganized, multimodal micropores usually limit the tunability of the pore structure at the sub-Ångstrom scale. In addition, the surface of most carbons is predominantly nonpolar, reflecting the highly hydrophobic property due to high-temperature treatment. After decades of development, researchers have been able to relatively well control the pore size and geometries (Figure 1(b)) for targeted separations. The common strategies include the judicious selection of polymer precursors, control of the carbonization temperature, and pre- or postfunctionalization [23–25]. Not only the tunable pore structure but also the rich controllability of surface chemistry also brings more possibilities to porous carbons [26, 27]. The diverse chemical sites of porous carbon show different binding affinities to adsorbate molecules and followingly different guest-host interactions [28, 29]. For instance, the oxygen-containing groups on porous carbons can improve the surface polarity and enhance the affinity for C2H6 [30]. Alternatively, the Ag(I) and Cu(I) species can interact strongly with olefins by π-π interactions [31, 32]. Apart from the above two points, porous carbons show high processability so that they can be engineered to a specific morphology or readily combine with other functional materials for advanced composite adsorbents [33]. By applying these strategies, porous carbons have proven to be powerful adsorbents for the separation of LHs.
Continuous progress has been made over porous carbon-based adsorbents in improving adsorption capacity, selectivity, mechanical stability, and so on. In order to promote the rapid development of carbonaceous adsorbents in the field of light hydrocarbon separation, it is necessary to summarize and sort out the structure-effect relationship of porous carbon. This motivates us to organize this review to bring potential readers a full picture of the adsorptive separation of LHs from synthesis protocols of porous carbons to their structural properties as well as performance assessments. In this review, we overviewed the separation mechanism and the preparation protocols and discussed the latest advances in LHs separation. Exemplified with typical LHs separation cases, we highlighted the correlations between the structural parameters of porous carbons and their separation performances. Furthermore, we emphasized the targeted design of carbonaceous adsorbents on the molecular level for ideal LHs separation performances. At last, we discussed the challenges and the research opportunities for the adsorptive separation of hydrocarbons in future.
2. Separation Mechanism
The separation of LHs is mainly achieved by distinguishing the diffusion rate and/or the adsorption affinities. Typically, the adsorption separation of LHs mechanism can be divided into three cases: thermodynamic effect, kinetic effect, and molecular sieving effect. Each mechanism was discussed as follows.
2.1. Thermodynamic Effect
Thermodynamically, the separation performance is based on different interaction forces between adsorbent and adsorbate [34, 35] (Figure 2(a)). The adsorbents usually contain gas-philic sites such as open metal active sites to selectively adsorb olefin by π-π interactions [36, 37]. Notably, the olefins have higher polarity than paraffin, which also leads to preferential enrichment of olefins molecules on the polar sites of porous carbon. More specifically, the surface functional groups or defective edge structures in porous carbons would show electric dipole or quadrupole interactions with guest LHs molecules [16].
Figure 2.
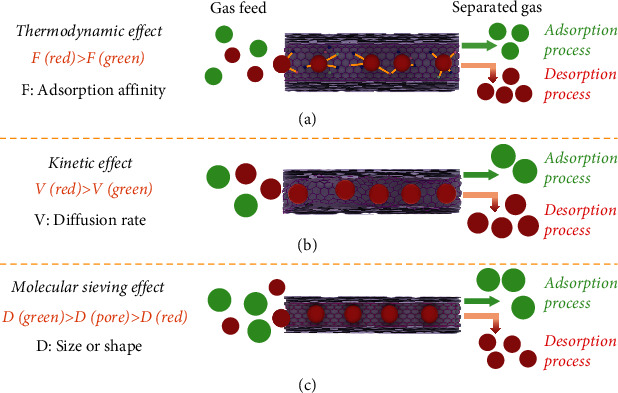
Proposed mechanisms for the adsorptive separation of LHs. (a) Thermodynamic effect. (b) Kinetic effect. (c) Molecular sieving effect.
2.2. Kinetic Effect
The gas separation by porous carbons is mostly dominated by the kinetic effect. The kinetic separation depends on different gas molecular diffusion rates of adsorbates traveling into the pores [38, 39] (Figure 2(b)). In most cases, the diffusion rates of olefins are faster than paraffins, because olefins have a lower molecular weight and boiling point than paraffins [40, 41]. Compared with the thermodynamic effect, the kinetic effect allows a low energy consumption for product recovery, because of the absence of affinity between LHs and adsorbents. As a result, the kinetic effect is more favorable for industrial processes. For clarity, the single-component adsorption isotherm is not suitable for determining a material whether achieves the LHs separation by kinetic effect or not. In general, time-resolved gas adsorption is typically used to verify the kinetic effect by comparing the diffusion constants of different gases. The diffusion time constants, which are obtained by fitting kinetic adsorption profiles, are the standard to evaluate the rate of diffusion.
2.3. Molecular Sieving Effect
The molecular sieving is an outcome of size/shape exclusion, which is the ideal case for gas separation [42] (Figure 2(c)). It should be mentioned that the molecular sieving effect can be considered a kinetic effect at the boundary conditions. In general, olefins have a smaller size than paraffin, when they have the same number of carbon atoms. Therefore, olefin can quickly enter the micropores, while paraffin is completely shielded from adsorption. However, if the micropore size is similar to the size of the adsorbate, it will cause a slow diffusion of the adsorbed component. Since the overall configuration of micropores is built from disorganized graphene-like planes in the porous carbons, the formation of uniform micropores is a highly challenging task. Although there are available quite a few kinds of carbon molecular sieves for the separation of CO2/N2 and H2/CO by the molecule sieving effect, it remains scarce for the time being to achieve the LHs separation based on the molecule sieving effect.
The gas adsorption separation is a complicated process, resulting in multiple separation mechanisms synergetic effect in most cases [43]. For instance, the micropore walls with size sieving properties are loaded with open active metal or doped heteroatoms. On the one hand, the size of the micropores can achieve LHs separation based on kinetics effect or molecular sieving effect; on the other hand, the adsorption sites on the pore walls can strengthen the target molecule interactions. Notably, practical separation processes are mainly controlled by synergistic thermodynamics and kinetic effects. The calculation of the combined selectivity is based on gas diffusivities and Henry's constants [44].
3. Strategies on Structural Modulations
Based on the three separation mechanisms above, the synthesis and modification of porous carbons can be controlled in a targeted manner. For thermodynamic effect, the adsorbent is required to create adsorption sites by doping heteroatoms and loading open metal active sites. In the case of molecular sieving effect and kinetic effect, it depends on the fitting pore size and pore geometry of the adsorbent. In addition, the adsorption capacity is closely related to the surface chemistry and the pore structure of the adsorbent. Thus, this section summarized the synthesis and modification strategies for high-performance carbonaceous adsorbents. Meanwhile, the relationship between pore structure, surface chemistry, and adsorbent capacity is discussed.
3.1. Tuning of Chemical Properties
The regulation of surface chemistry can achieve LHs separation by thermodynamic effect. In general, most porous carbons are prepared by carbonizing precursors in an inert atmosphere. The higher the carbonization temperature, the fewer polar functional groups remain on the carbon surface. Thus, carbon materials are inherently non-polar, and not suitable for LHs separation by thermodynamic effect. It is highly challenging to render a non-polar surface to be highly polar. Typically, the main methods of regulating surface chemistry for porous carbons include doping heteroatoms and loading open metal active sites such as Ag(I) and Cu(I). We will discuss the above methods of porous carbons preparation in more detail in the following.
3.1.1. Heteroatom Doping
Heteroatom doping is to replace some carbon atoms in the basal plane or edges of graphene layers with noncarbon atoms. It obviously changes the electron and charge properties of porous carbons; thus, it can tune their polarity for enhanced interaction between the carbon pore walls and the C=C/C≡C [45]. In principle, heteroatom doping can be carried out in two ways. One is the post-treatment, in which the pristine carbons are functionalized by surface reactions using dopant gases under optimized conditions. Dopant gases include NH3 for N-doping, H2S for S doping, and Cl2 for Cl doping [46–50]. Normally, the gas phase functionalization occurs at high temperatures (mostly 600-900 °C). Such harsh treatment sometimes leads to further shrinkage of the porous structure and reduction of the accessible pores. In some cases, the doping was found to be limited in the surface region, unlikely effective for bulk phase functionalization [51].
The other one is in-situ doping. In this strategy, the precursors, both amorphous and crystalline, are heteroatom-rich [52]. The amorphous precursors include phenolic resin, polyaniline, and polybenzoxazine [18]. The crystalline precursors show long-range order, which, in some cases, will be partly remained during carbonization. These would result in pores with a high-level heteroatom doping in both ways [53]. Besides, using various biomaterials as precursors is a common method for heteroatom doping [20]. It should be noted that the carbonization temperature will affect the degree of doping [18, 54]. Since the heteroatom is inherited from the precursor, the generated functional groups are more stable than the doping posttreatment. Overall, the in situ doping strategy is quite feasible and holds great potential for scaling up. Furthermore, doping with dual even multi-heteroatoms can be imparted, which would be fascinating for task-specific LHs separation.
3.1.2. Open Metal Active Sites
The decoration of Ag(I) or Cu(I) on the pore wall of adsorbents favors the trapping of olefin over paraffin due to π-π interactions between open metal active sites and π-bond of olefin. Generally, the metal species are adsorbed, reduced, and anchored to the sites of vacancies or defects of the porous carbons [55, 56]. In addition, the sulfurized porous carbons can improve the loading efficiency of Ag(I), because of the high affinity of the Ag(I) to the sulfur functionalities [31].
3.2. Tuning of the Porous Structure
According to IUPAC standard, the pores can be divided into macropores (>50 nm), mesopores (2-50 nm), and micropores (<2 nm) [57]. Among them, the micropore size is the dominant factor affecting the molecular sieving effect and kinetic effect. For most porous carbons, the overall configuration of micropores is built from disorganized graphene-like planes, normally composed of narrow slit-shaped apertures (<0.7 nm) and layer stacked nanovoids in the length scale of 0.7-2 nm [58]. Excellent selectivity will result from the size of the narrow micropore size distribution which falls right between the kinetic diameters of gas mixtures, enabling a sieving effect. A narrow micropore size distribution can also enhance the adsorption capacity in low pressure regions. However, the gas diffusion in the highly concentrated ultramicroporous channels is suppressed, leading to sluggish adsorption kinetics. The mesoporous structures are evidenced as an effective pathway for gas molecules diffusion [59]. Therefore, by adjusting pore sizes (e.g., the combination of mesopores and micropores), it is possible to achieve the fast transport of LHs and effective LHs separation performance. According to the literature, a close relationship between the formation of micropore structure and polymer precursors, pyrolysis conditions, and treatment methods has been figured out [60, 61]. We will discuss the pore structure control method of the porous carbons in more detail in the following.
3.2.1. Precursor Selection
The judicious choice of precursors is crucial for obtaining porous carbons with well-defined ultramicropores. Porous carbon derived from biomass, such as coal, wood, and fruit shells, can change pore size by changing carbonization conditions, but this strategy still does not precisely control ultramicropore size [62]. On the contrary, solution chemistry based on molecular level design is more effective, and a series of precise micropore regulation rules of porous carbon has been developed. It is different from the traditional process for the preparation of porous carbons by the solid-state method. Precisely distributed ultramicropores are created essentially by thermal transformation of cross-linked polymer networks, followed by the release of volatile fragments, dynamic aromatization, and alignment of the conjugated sp2-C-based microcrystalline domains [63]. Thus, the pore size distribution is also highly relevant to the carbon chain structure of the polymer precursors [64]. As a consequence, it is important to choose suitable crosslinked polymeric structures, which include but are not limited to polyacrylonitrile, polyvinylidene dichloroethylene, polyimide, phenolic resin, poly(ionic liquid)s, polythiophene, and polydopamine. For instance, Lu and co-workers reported that a novel type of ultramicroporous carbon sheets was obtained by using phenolic resin-based precursors with a hundred-nanometer-thickness, which showed narrow bimodal micropore distribution centered at 0.48 and 0.82 nm, respectively [64]. In addition, the influence of the aliphatic chain length of the primary amine moieties on the micropore structure of 2D polybenzoxazine-based porous carbons was also investigated by Lu and co-workers [65]. The result showed that the shorter the length of aliphatic amine moieties, the higher ordering of the stacking of graphene layers, and the higher the unimodality in micropore size achieved. Besides, the MOFs-related materials, which possess tunable pore size and surface chemistry, are sacrificial templates to produce micropores carbons [66, 67].
3.2.2. Treatment Method
Besides the selection of precursors, the pre-/post-treatment methods can effectively control the pore size of porous carbon. To date, a series of reliable treatments have been successfully developed, such as chemical activation, chemical vapor deposition (CVD), and water vapor activation [68]. As a typical pretreatment method, the chemical activation method can obtain ultramicroporous carbon by using alkali hydroxide or salts containing alkali ions [69]. For instance, Xiao and co-workers prepared a series of ultramicroporous starch-based carbon spheres (SC-M; M = Na, K, Rb), which could achieve sub-Ångstrom tunable ultramicropore apertures by using the alkali metal ion-exchange method. Remarkably, the ultramicropore sizes of SC-Na, SC-K, and SC-Rb are concentrated at 4.73, 4.83, and 4.93 Å, respectively. This indicates that the ultramicropore size of carbon can be finely tuned by activation using different sizes of metal ions. Among the materials, SC-K exhibits excellent C3H6/C3H8 separation performance by molecular sieving effect, and adsorption capacity ratios for C3H6/C3H8 is 5.36 [70] (Figures 3(a)–3(d)).
Figure 3.
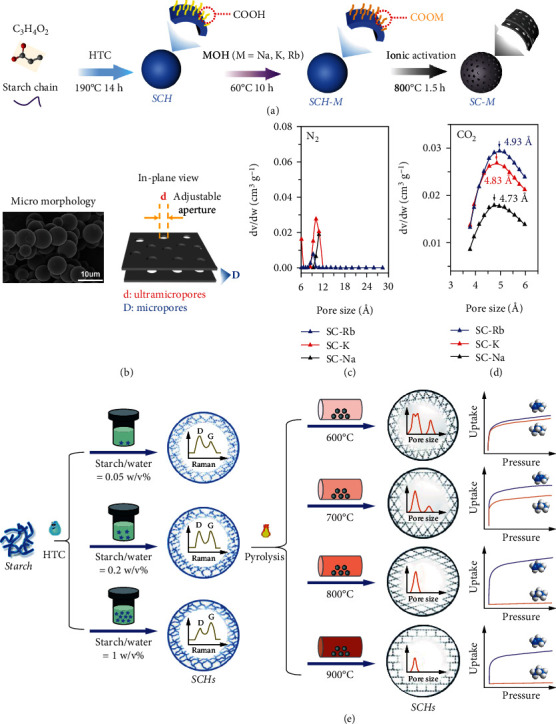
(a) The ultramicropore formation process for the starch-based carbon adsorbents (SC-M) by using the alkali metal ion-exchange method. (b) The schematic diagram of the microstructure. (c, d) Pore size distribution of SC-M [70]. (e) Synthesis schematic of starch-based carbon molecular sieve (SCMS) [71].
The experimental parameters, such as dosage, the ratio of raw materials, and activation temperature, are critical for micropore production in the pre-treatment. For instance, Xiao and co-workers controlled the concentration of starch hydrothermally to tune the size of micropores (SCH-X-Y, X = the starch/water ratio of 0.05, 0.2, and 1 w/v%, Y = different pyrolysis temperatures) [71]. Different starch hydrothermal concentrations can affect the graphitization and defect ratio of the carbon material, which was verified by Raman (Figure 3(e)). The ID/IG values of SCHs followed the order SCH-0.05 > SCH-0.2 > SCH-1. Among them, the low ID/IG value of the SCH-1 reflected relatively low lattice edges or defects on plane terminations of disordered graphite. The author analyzes that the higher starch concentration can produce more hydroxymethyl furfural and organic acids that would generate fewer defects of hydrochar in the polymerization and aromatization process, resulting in improvement in aromatic microdomains and microcrystallite size of SCHs. It is crucial for synthesizing carbon molecular sieves with uniform pore size. In addition, higher pyrolysis temperature rearranges the carbons toward graphitization and orderliness. Thus, the (002) crystal planes sharpen gradually with increasing pyrolysis temperature. Meanwhile, the micropore size reduces to about 4.78 Å which is between the kinetic diameter of C3H6 and C3H8. The CVD as a typical posttreatment method is an extraordinarily effective strategy to reduce the pore size of the porous carbons through the deposition of pyrolytic carbon from the hydrocarbon. However, it will reduce accessible micropore volume and inevitably decrease the adsorption capacity [72, 73]. Alternatively, the exploration of novel treatment methods and a flexible combination of different treatment methods will have effective ways to control the microporous size of porous carbons for efficient LHs separation in future.
3.3. Adsorption Capacity
The adsorption capacity is related to the surface chemistry and the pore structure of an adsorbent. Thermodynamically, the adsorbent has active sites that ensure moderate interactions with adsorbates, while it does not mean the more active sites, the higher the adsorption capacity. For instance, Deng et al. found that N content showed limited influence on the regression model by studying the regression models of pore volume under specific pore size and CO2 capacity [74]. At low pressure of 0.15 bar, the N-doping played a dominant role. A content of 5.1 wt% led to two folds higher CO2 capacity than that with N content of 1.5 wt%. However, at 1.0 bar, the CO2 capacity is dependent on the micropore volumes. Schwartz et al. conducted theoretical studies exemplifying CH4 as a gas probe [75]. In their case, the mean CH4 adsorption energies were found lowered due to the higher degree of oxidation over carbon adsorbents, which reduced the overall CH4 uptake as compared to the unoxidized surface.
The functional groups were also found beneficial to increase the adsorption capacity at high temperatures [62]. However, the strong binding sites will inevitably generate a large amount of adsorption heat during the adsorption process, resulting in a decrease in the adsorption capacity. In addition, for strong adsorption interactions, much lower pressures or higher temperatures are required for adsorbent regeneration in PSA and TSA processes. As a result, a balanced regulation of pore structure and surface chemistry can be used to achieve optimized performance for a specific separation process.
4. Separation Based on Thermodynamic Effect
As discussed above, huge attention has been paid to the development of novel porous carbons for LHs separation. However, as the opposite process of mixing, separation is generally not a spontaneous procedure, because it is a violation of the second law of thermodynamics. Consequently, gas separation often requires adsorbents with a considerable number of adsorption sites, strong guest-host interactions, and a large accessible surface area. In this section, we overviewed the porous carbon adsorbents applied in thermodynamic effect for LHs separation.
4.1. CH4
With a high heat of combustion (55.7 kJ/g), CH4 accounts for 22% of the global fuel consumption, and this value is expected to increase continuously in the future. Therefore, as a substitute for traditional fossil fuels, it is urgent to develop and utilize CH4 sources [76]. Natural gas is composed of CH4 and the predominant impurity of N2 and CO2, which must be separated. The CH4/N2 and CH4/CO2 separation performance of selected carbon-based adsorbents are summarized in Table 2. The porous carbon for CH4 purification is mainly divided into three types: biomass carbon, polymer-based carbon, and pitch-based carbon. Bio-based and polymer-based derived carbons can better achieve heteroatom doping, resulting in affinities for different gases. Among them, the polymer-derived carbon can achieve not only more controllable morphologies, such as carbon sheets and carbon spheres, but also more concentrated pore size distributions. For the capture of low concentration CH4, improving the selectivity and adsorption capacity of CH4 is the main task for the adsorbent. However, the adsorption capacity of CH4 is difficult to exceed 2 mmol g−1, because the CH4 has a low polarizability (2.6 × 10−24 cm3) and boiling point (-161 °C) as well as no quadrupole moment, so it is difficult to achieve effective adsorption of CH4 in ultramicropores at 25 °C. Although the high specific surface area of porous carbon can increase its adsorption capacity of CH4, the adsorption capacity of other gas will also increase, resulting in a decrease in selectivity. Probably, the preparation of high-density ultramicroporous channels conforming to the molecular dynamics-based model of CH4 is important for the construction of large-capacity and high-selectivity porous carbon adsorbents.
Table 2.
Summary of selected carbonaceous adsorbents for CH4 purification.
| Sample ID | Uptake (mmol g−1) | Qst (kJ mmol−1) | Selectivity | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| CH4 | N2 | CO2 | CH4 | CO2 | SCH4/N2 | SCO2/CH4 | ||
| PCNPsa | 1.17 | 0.28 | — | 22.3 | — | 10d | — | [77] |
| GOC-2a | 1.82 | 0.5 | — | 22.1 | — | 5.8e | — | [78] |
| SC-6a | 1.86 | 0.6 | — | 24.9 | — | 5.7e | — | [79] |
| PRC-850a | 1.12 | 0.38 | — | 23 | — | 5.4d | — | [80] |
| PSb | 1.38 | 0.45 | 3.63 | — | — | 3.1 | 15g | [81] |
| GLb | 0.45 | 0.17 | 1.65 | — | — | 2.7 | 54g | |
| OTS-1-550a | 1.12 | 0.3 | 2.8 | 25 | 37.3 | 5.9e | 7.4g | [82] |
| OTS-2-650a | 1.67 | 0.5 | 3.96 | 24.7 | 33.8 | 5.2e | 4.1g | |
| OTSS-3-350a | 0.9 | 0.26 | 2.83 | — | 39.2 | 5.6e | 9.5i | [83] |
| OTSS-2-450a | 0.93 | 0.3 | 2.94 | — | 47 | 4.9e | 12.8i | |
| CGUC-0.5-6a | 0.9 | 0.2 | 3.4 | — | 31 | 6.7e | 34.9h | [84] |
| CGUC-1-6a | 1.01 | 0.2 | 3.26 | — | 27.5 | 5.9e | 16.9h | |
| SNMC-1-600a | 1.45 | 0.5 | 3.98 | — | 27.5 | 5.1e | 6.9h | [74] |
| SNMC-2-600a | 1.57 | 0.5 | 4.24 | — | 26.4 | 4.2e | 4.3h | |
| SNMC-3-600a | 1.17 | 0.4 | 3.0 | — | 23.3 | 3.6e | 3.2h | |
| Co-NDPC-500a | 1.0 | 0.26 | 3.5 | 27 | 35 | 3.9f | 10.4e | [85] |
| Co-NDPC-600a | 1.2 | 0.27 | 5.3 | 28 | 53.8 | 4.4f | 11.4e | |
| CC-PNPc | 2.20 | — | 3.7 | — | — | — | 15.9i | [86] |
| P-CC-PNPc | 4.26 | — | 7.5 | — | — | — | 5.2i | |
| C30-CC-PNPc | 7.02 | — | 11.9 | — | — | — | 4.3i | |
| C85-CC-PNPc | 5.35 | — | 8.9 | — | — | — | 4.8i | |
| Chit_supCO2_P800a | 0.01 | — | 1.37 | — | — | — | 137j | [87] |
| Chit-PMO_P800a | 0.01 | — | 0.30 | — | — | — | 30j | |
| Chit-PMO_P800_crusheda | 0.14 | — | 1.01 | — | — | — | 7.2j | |
| C1000a | 1.75 | — | 3.7 | 17.4 | 20.7 | — | 28e | [88] |
| PHAa | 0.63 | — | 1.6 | 22.5 | 33.5 | — | 7.2e | [89] |
| NAHA-1a | 1.65 | — | 3.75 | 23 | 29.7 | — | 7e | |
| NAHA-2a | 1.5 | — | 4 | 23.5 | 29 | — | 6e | |
Gas uptake at a298 K and 100 kPa, b313 K and 100 kPa, and c298 K and 20 bar. IAST predicted selectivity for separation of CH4/N2 with mole ratios of d30/70 and e50/50. fUptake ratio of CH4/N2 as separation selectivity. IAST predicted selectivity for separation of CO2/CH4 with mole ratios of g50/50, h40/60, and i10/90. jUptake ratio of CO2/CH4 as separation selectivity.
4.1.1. CH4/CO2
In this section, we discussed the latest efforts from the materials aspect, i.e., the design of novel carbon adsorbents for CO2/CH4 separation. The presence of H2O and CO2 in natural gas will corrupt the transportation and storage system. To meet the specifications for fuel gas, CO2 must be removed. To date, many porous carbons showed excellent CO2 adsorption performance. However, due to the similar kinetic diameters of CH4 and CO2, it is still challenging for their efficient separation. In previous reports, both microporous structure and heteroatoms doping are beneficial for CO2 adsorption, specifically ultramicropores (<1 nm) and N doping. The ultramicropores not only provide storage space for CO2 but also confine CO2 by Van der Waals' forces. The N doping can increase the surface polarity and basicity, which achieves weak chemical interaction between CO2 and the pore wall. However, does the CO2 capture capacity increase with the increase of basic sites? To verify this issue, Deng and co-workers prepared three samples with almost identical pore structures and varied N content (6.78%, 4.81%, and 0.38%) from the oil-tea seed shell (OTSS) through direct activation with solid NaNH2, which acts as both porogen and nitrogen source [83]. The CO2 adsorption isotherms showed a nearly indistinguishable shape and adsorption performance at both 1 and 0.15 bar. In this case, the N-doped functional groups merely affected the CO2 adsorption behaviors. Besides, OTSS-2-550 with the largest micropore volume (0.63 cm3g−1) displayed the highest CO2 uptake of 5.65 mmol g−1 at 0 °C and 100 kPa. This revealed that the microporous structure is rather critical in contributing to the CO2 adsorption capacity. In addition, to further explore the effects between micropores and N-doped functionalities on CO2 adsorption. Deng and co-workers developed N-doped (ca. up to 5.11 wt%) microporous carbons (SNMC-X-Y, X = the mass ratio of KOH/SFRH =1/1, 2/1, and 3/1; Y = the activation temperature) by using an N-rich polymer as a precursor. The precursor was made from the polymerization of resorcinol and hexamethylenetetramine by using a one-pot melting-assisted and solvent-free method [74]. By studying the regression models of pore volume under specific pore size and CO2 capacity, the authors found that both high and low N content showed limited influence on the regression model. But the SNMC-1-600 with N content of 5.11 wt% showed about two folders higher CO2 capacity than that of SNMC-2-700 (1.5 wt%) at 0.15 bar. It implies that the CO2 capacity at 1 bar is dominated by micropores volumes rather than the amount of N-doped functional groups, while N content remarkably influences the CO2 capacity at 0.15 bar. Additionally, SNMC-3-800 with the highest SBET (3657.0 m2g−1) showed excellent CO2 capacity up to 22.06 mmol g−1 at 20 bar and 25 °C, due to the highest KOH/SFRH ratio and pyrolysis temperature. However, the authors only studied the storage of CO2 and did not study CO2/CH4 separation performance under high pressure.
For the pressure swing adsorption (PSA) process, it is essential to explore the gas separation performance under high pressure. Li and co-workers prepared the N-doped porous carbon spheres (Glc-C-4) with a high SBET of 3153 m2 g−1 via hydrothermal carbonization and then KOH activation [90]. The Glc-C-4 had a narrow pore size distribution and rich N-doped active sites, which could be used as an adsorbent for capturing CO2. The Glc-C-4 exhibited a high CO2 capacity (22.4 mmol g−1) and relatively low CH4 capacity (11 mmol g−1) under 298 K and 30 bar. With the increase of pressure, the heat of adsorption of CO2 increased gradually, while the heat of adsorption of both CH4 and N2 decreased, because of the energetical heterogeneity of adsorption sites for CH4 and N2. Thus, the separation selectivity was optimized, reaching 4.5 for CO2/CH4 (50 : 50 v:v) binary mixtures under 298 K and 30 bar.
4.1.2. CH4/N2
In this section, we will discuss several designed carbonaceous adsorbents for CH4/N2 separation. Although the chemical and physical activation methods are quite mature to enlarge the surface area and impart additional micropores of porous carbon, it still lacks effective control over the pore size at the microporous scale for the CH4/N2 separation performance. To solve this problem, it is essential to understand the separation mechanism of CH4 and N2 in porous carbons. Kumar and co-workers studied the effect of porous structure and pore geometry of carbonaceous adsorbents on the separation of dilute CH4 from CH4/N2 mixture [91]. Four kinds of nanostructured carbons were selected as model adsorbents, which include the theoretical carbon structures with ideally slit or tubular pores, the realistic porous carbons with disordered pore structures, and carbon foam structures. The Grand Canonical Monte Carlo (GCMC) simulation results showed that the pore structure could affect the CH4/N2 selectivity or the adsorption behavior. More specifically, they found that the nanotube-like pores with a confined pore size exhibited a CH4/N2 separation selectivity of 2.0 at the pressure range from 6 to 8 bar, while the microporous carbon with a slit-pore and carbon foam structures showed a slightly higher selectivity of ~3.0 at lower pressures. In summary, carbon adsorbents are hard to achieve high separation performances based on the thermodynamic effect.
In recent research, it is reported that some ultramicroporous carbons displayed excellent CH4/N2 separation performance [92]. However, the gas diffusion in the highly twists ultramicropores is restrained; thus, the adsorption capacity and adsorption kinetics need to be reinforced. To overcome the diffusion limitations, the conventional strategy is to build a hierarchical pore system by imparting meso/macropores. However, Lu and co-workers show a distinct and effective method by pillaring up the 2D ultramicroporous carbons nanoplates (PCNPs) through highly processible synthesis [77]. The pillared polymer nanoplates are based on a multicomponent sequential assembly, in which the protrusions were in situ grown on the soft 2D templates (self-assembly of triblock copolymers and stearic acid) by inducing sequential condensation of phloroglucinol, terephthalaldehyde, and p-phenylenediamine (p-PDA). The authors have demonstrated that PCNPs showed fast kinetics and high selectivity. In addition, not only the areal density but also the height of the protrusions can be regulated continuously by varying the concentration of p-PDA. At a low partial pressure of CH4, the PCNPs showed a high CH4/N2 selectivity up to 24, and the CH4 diffusion constant (1.18∗10−3s−1) was two orders of magnitude faster than the commercial carbon molecular sieves (CMS, Takeda 3kt, 1.19∗10−5s−1). The PCNPs with dense ultramicropores by nanoprotrusions greatly improved diffusion rate and resulted in selective separation of CH4/N2.
4.1.3. CH4/CxHy
In addition to CO2 and N2, the small fractions of LHs, such as C2H2, C2H4, C3H6, and C3H8, are also common impurities in natural gas. Thus, the separation of CH4/CxHy has also been explored extensively. In the aspect of material design, one way is to achieve a suitable pore structure; the other is a surface modification by heteroatom doping. For instance, Lu and co-workers devised an innovative kind of 2D flat carbon nanoplates (FCP), which was synthesized by stearic acid thermoregulated phase-transition approach [63]. The liquefied stearic acid (SA) at 80 °C was dispersed into F127 aqueous solution to form a uniform microemulsion, and then the temperature was lowered to 28 °C to change the SA from liquid to solid. After Ostwald ripening, the sheet SA template is obtained (Figures 4(a) and 4(b)). The FCP showed greatly well-organized and accessible ultramicropores preferentially orientated growth of more than 80% sp2-C and confined thickness. Remarkably, the pore sizes are uniformly concentrated at 0.53, 0.56, or 0.58 nm at different carbonization temperatures of 1000, 800, and 600 °C, respectively (Figure 4(c)). The author found that the carbon crystallites were stacked together into parallel multilayers at the regions close to the sheet boundary by using Brownian dynamics simulation and TEM. Thus, the 2D structure makes the parallel oriented growth and stack of carbon crystallites, achieving the single size of the micropores. For CH4 purification, the FCP exhibited excellent capacity of CO2 (5.2 mmol g−1), C2H6 (5.3 mmol g−1), and C3H8 (5.1 mmol g−1) (Figure 4(d)). The order of heat of adsorption is C3H8 > C2H6 > CO2 > CH4, exhibiting the same sequence as the molecular polarizabilities. It is indicating increased host-guest interactions originating from short-range attractive forces in uniform ultramicropores. The breakthrough experiment exhibited that FCP could efficiently distinguish natural gas mixture (x/CH4,10/90 v/v, x = CO2, C2H6, and C3H8) (Figure 4(e)). In addition, their tested breakthrough performance under wet conditions: C3H8/CH4/H2O (10%/87%/3%, v/v/v). The breakthrough results reveal that the separation performance of carbon nanoplate is not affected by the presence of moisture, indicating good moisture resistance. Thus, the construction of 2D structure porous carbon is a promising method to prepare high-efficiency carbonaceous adsorbents with narrow micropore size distribution.
Figure 4.
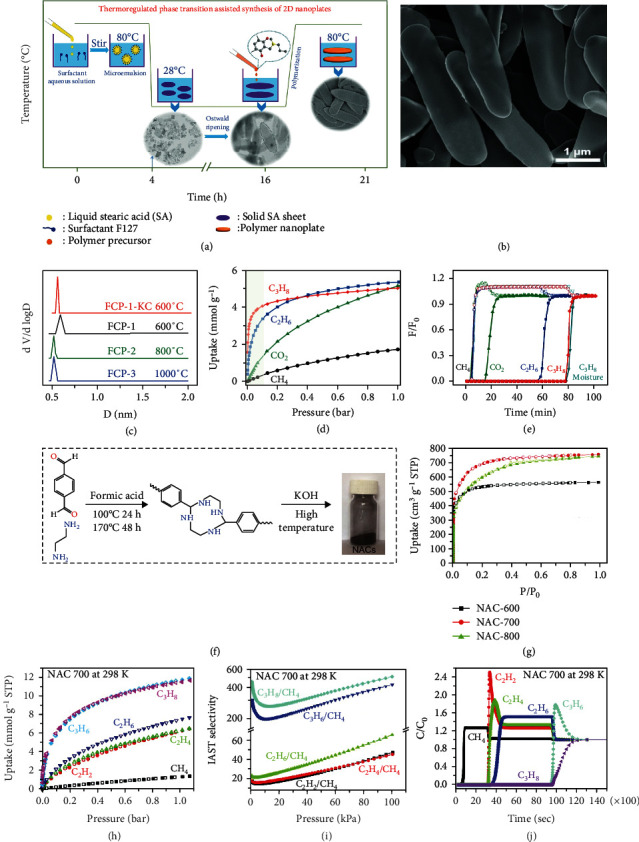
(a) Schematic illustration of the synthesis for the polymer nanoplates. (b) SEM image of FCP-1. (c) Pore size distribution. (d) Gas adsorption isotherms and (e) breakthrough profile of x/CH4 (10/90 v/v, X = CO2, C2H6, C3H8) of FCP-1-KC at 298 K and 1 bar [63]. (f) Schematic illustration of the synthesis process. (g) N2 adsorption isotherms. (h) LHs adsorption isotherms. (i) IAST selectivity and (j) breakthrough simulation profile in an equimolar 6-component CH4/C2H2/C2H4/C2H6/C3H6/C3H8 mixture [93].
As for carbon matrix modification by heteroatom doping, N-doping is an effective strategy for improving gas adsorption capacity and separation selectivity. For instance, Deng and co-workers reported one kind of N-doped (ca. 3.6 wt%) activated carbons by using an N-rich polymer as a precursor, which was made from the polymerization of terephthalaldehyde and ethylenediamine [93] (Figures 4(f) and 4(g)). The NACs showed a high C2 and C3 capacity of 7.59 and 11.77 mmol g−1, respectively. This material exhibited a high separation selectivity of 66 for C2/C1 and 502 for C3/C1 under mild conditions (Figures 4(h) and 4(i)). As expected, the breakthrough experiments in the 6-component mixture (CH4/C2H2/C2H4/C2H6/C3H6/C3H8) demonstrate that the separation can be realized (Figure 4(j)). The authors believed that N functionalization played a key role in enhancing the adsorption capacity and selectivity. On the one hand, the N-doping led to stronger interactions with those easily polarized hydrocarbons; on the other hand, the pore size was optimized simultaneously.
4.2. C2H4/C2H6
The exploration of high-performance carbonaceous adsorbents is underway for C2H4/C2H6 separation. Table 3 compared the selective adsorption properties of the carbonaceous adsorbents, in which the capacity, the adsorption heat, and the IAST selectivity were compared. Notably, the adsorbents without open metal sites all exhibit the property of preferentially adsorbing C2H6 in the C2H4/C2H6 separation. For this unique phenomenon, researchers have made various explanations from the perspective of pore size and chemisorption site, which will be reviewed next. However, why are there no carbonaceous adsorbents without adsorption sites on the preferential adsorption of C2H4? This topic is an opportunity for carbon scientists.
Table 3.
Summary of the selected carbonaceous adsorbents for C2H4/C2H6 separation.
| Sample ID | Uptake (mmol g−1) | Qst (kJ mmol−1) | Selectivity SC2H4/C2H6 | Ref. | ||
|---|---|---|---|---|---|---|
| C2H4 | C2H6 | C2H4 | C2H6 | |||
| CMK-3a | 2.9 | 3.1 | 20 | 19 | 0.94c | [32] |
| 4CuCl/CMK-3a | 3.2 | 1.7 | 53 | 15 | 1.88c | |
| 8CuCl/CMK-3a | 3.6 | 1.3 | 66 | 13 | 2.77c | |
| MC-S-Ag-3b | 3.4 | 2.6 | — | — | 2.4d | [94] |
| CuCl(6.0)/ACa | 2.7 | 0.8 | — | — | 52d | [95] |
| CuCl(8.0)/ACa | 2.6 | 0.7 | — | — | 69.4d | |
| beta-ZTC-O 2.2a | 4.9 | 7.3 | 17 | 24 | 1.5e | [96] |
| EMT-ZTCa | 4.1 | 5.9 | 24 | 25 | 1.6e | |
| FAU-ZTCa | 4.0 | 5.0 | 28 | 25 | 1.7e | |
| 25CPDA@A-ACsb | 5.9 | 7.2 | — | — | 2.75f | [30] |
| 50CPDA@A-ACsb | 6.3 | 7.1 | 32 | 35 | 3f | |
| 75CPDA@A-ACsb | 5.9 | 7.1 | — | — | 2.79f | |
| NAC-800b | 4.5 | 5.8 | 22 | 24 | 0.77e | [90] |
| ANPC-1-800b | 5.7 | 6.8 | 30 | 34 | 1.54e | [97] |
| MGA-700-4b | 6.0 | 7.3 | 24 | 29 | 6.76f | [98] |
| MGA-750-3b | 5.7 | 7.0 | 22 | 28 | 6.44f | |
| C-PDA-3b | 5.1 | 6.6 | 22 | 22 | 1.83e | [99] |
Gas uptake at a303 K and 100 kPa and b298 K and 100 kPa. cUptake ratio of C2H4/C2H6 as separation selectivity; IAST predicted selectivity for separation of C2H4/C2H6 with mole ratios of d50/50; IAST predicted reverse selectivity for separation of C2H4/C2H6 with mole ratios of e50/50 and f15/1.
In the thermodynamic effect, the strong host-guest interactions between open metal active sites and C2H4 result in a distinct difference of porous carbon in adsorption affinity for C2H6 and C2H4. For instance, Jiang and co-workers synthesized ordered mesoporous carbon and then incorporated the CuCl species by solid-state grinding [32]. After thermal treatment, the CuCl nanoparticles were high dispersed, with no metallic Cu nanoparticles observed. The adsorbent can selectively adsorb C2H4 via π-π interactions, and the selectivity increases with the increasing loading of CuCl. Similarly, Gao and co-workers prepared the Cu(I) decorated activated carbon (AC) [95]. They first introduced the CuCl2 that was reduced to CuCl by thermal treatment under N2 atmosphere. The obtained CuCl/AC adsorbents achieved a C2H4 uptake of 2.57 mmol g−1 at 30 °C and 1 bar. The C2H4/C2H6 separation selectivity reached 69. Notably, it is necessary to construct chemical sites for anchoring open active metals in subsequent studies, preventing open active metal sites from agglomeration and oxidation.
The C2H6-selective adsorption technology is considered a favorable strategy for energy saving in C2H6/C2H4 separation, which can directly afford high-purity C2H4. However, C2H6-selective adsorbents often have poor selectivity because of the insufficiency of adsorption sites. Although facing many challenges, the research on C2H6-philic carbonaceous adsorbents has been made great development in the past few years. For instance, to introduce adsorption sites with C2H6 affinity, Li and co-workers reported a novel composite porous carbon (CPDA@A-ACs) prepared by growing polydopamine (CPDA) on asphalt-based activated carbons (A-ACs) as a precursor [30]. With the increasing amount of CPDA loading, the O contents increased from 13.25 to 17.79%, and N contents increased from 3.07 to 4.06%. The bands of C=O, N-H and C-N can be observed in the CPDA@A-ACs composites rather than in the A-AC. This shows that the O/N concentrations increased by the CPDA loading. In addition, with the loading increases of CPDA, the pore size distributions of PDA@A-ACs are reduced and shift toward micropores. The 50PDA@A-ACs achieved the C2H6 uptake of 4.71 mmol g−1 and the C2H4 uptake of 3.81 mmol g−1 at 30 kPa and 298 K. The 50CPDA@A-ACs showed IAST selectivity of 3 for the C2H6/C2H4 binary mixtures (1/15, v/v). The Forcite module in Materials Studio calculations had confirmed that C2H6/C2H4 adsorption selectivity originated from the strong binding sites of C2H6, which are derived from C-H···π, CH···N/O (Figures 5(a)–5(c)). The binding energies followed the order of O sites > C-sp2 sites > N sites, so the O functional groups were proved to play an important role in enhancing C2H6/C2H4 separation selectivity by comparing the binding energies. Similarly, the Li group reported another series of carbon >materials, which can selectively adsorb C2H6 efficiently [98, 99]. The underlying mechanism was interpreted by Molecular simulation. The result shows that C2H6 has sp3 configuration, which could interact with O and N functional groups on the pore wall through H-bonding more efficiently (Figures 5(d) and 5(e)).
Figure 5.
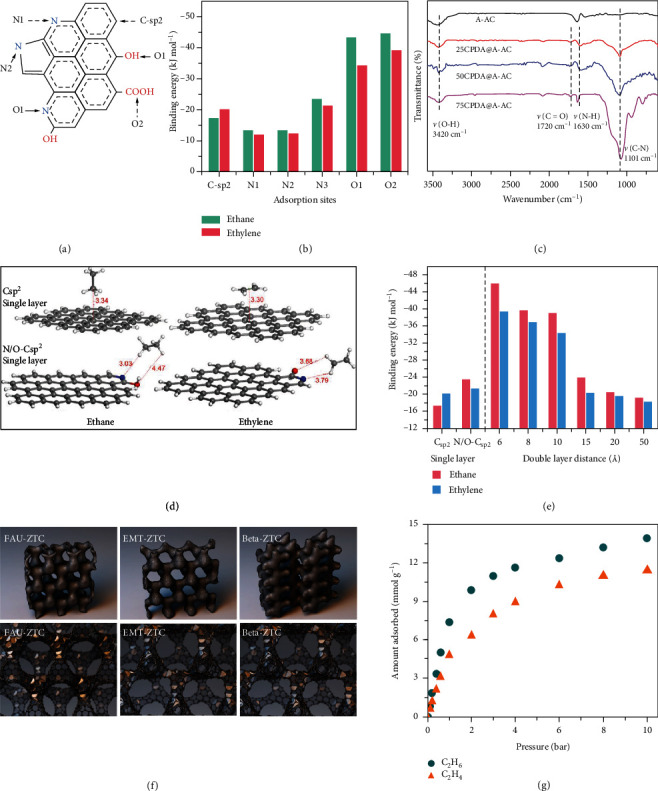
(a) Schematic illustration of O and N interaction sites in porous carbon and (b) corresponding binding energy of C2H4/C2H6 with different interaction sites. (c) FTIR spectra of samples [30]. (d) Interaction between C2H4/C2H6 and the Csp2 and N/O-Csp2 single layers. (e) The interaction energy between C2H4/C2H6 and different pore sizes of Csp2 and N/O-Csp2 double carbon layer is referred to as that of the Csp2, N/O-Csp2 single carbon layers [98]. (f) Schematic structures and Schwarzite models of samples. (g) Adsorption isotherms of beta-ZTC at 303 K [96].
Previous reports showed that carbonaceous adsorbents can selectively adsorb C2H6 at low pressures (1 bar or less than 1 bar). From the perspective of engineering, it is important to enhance the C2H6 selective adsorption capacity of the adsorbent from the C2H6/C2H4 binary mixture at high pressures (10 bar or higher). Recently, Chang and co-workers introduced zeolites (FAU, EMT, and beta) as the templates for the synthesis of microporous three-dimensional (3D) graphene-like porous carbons with C2H6 selective [96]. The FAU-, EMT-, and beta-zeolite templated carbons (ZTC) have different pore sizes of 1.2, 1.1, and 0.9 nm, respectively (Figure 5(f)). Among them, the beta-ZTC is composed of numerous polygon graphene rings that provide different degrees of strain, which was indicated by 13C NMR, Raman, and XPS. Thus, the concentrations of other types of defects and a diverse range of polygons in beta-ZTC were proven to be higher than those in FAU- and EMT-ZTCs. Interestingly, the beta-ZTC with the most curved graphene structure showed preferential adsorption of C2H6 (14 mmol g−1) over C2H4 (11.2 mmol g−1) at 10 bar and 303 K (Figure 5(g)). Conversely, a graphene sheet without curvature does not achieve C2H6/C2H4 separation. Thus, the author thought that the negatively curved graphene structure could affect the C2H6/C2H4 separation performance. Not only that, unlike C2H6-selective carbon materials reviewed above, the C2H6-selective adsorption performance of beta-ZTC was not affected by controlling the oxygen content [30, 97, 98]. Breakthrough experiments showed that the directly obtained C2H4 purity is 99.9% at 5 bar.
4.3. C3H6/C3H8
Similar to the thermodynamic separation of other olefin/paraffin, C3H6 generally engages in stronger interactions with polar surfaces than C3H8 [100, 101]. Depending on the difference in pore microenvironment, porous carbons can effectively achieve the separation of C3H6 and C3H8. As mentioned before, the thermodynamic effect has been an effective factor in the selective separation of C3H6/C3H8. The majority of research has been dedicated to the preferential adsorption of C3H6 by π-π interactions between open metal active sites and olefin. For instance, Comroe and co-workers reported that Ag(I)-doped microporous carbons were synthesized by using furfuryl alcohol as a precursor [94]. The SBET of the microporous carbons with Ag(I)-doping content of 0.7-2.5 at.% can achieve 915-1193 m2/g. The IAST selectivity of C3H6/C3H8 is in the range of 2.4~5. It suggested that the porous carbons with the Ag(I) content of 2.5 at.% more affinity towards C3H6. If Ag(I) was not enough, it will not capture enough C3H6 by π-π complexation. From the theoretical point of view, selective adsorption of C3H6 from C3H6/C3H8 mixtures was largely investigated in the framework of the Density Functional Theory (DFT). The DFT proved reliable results in reproducing binding energies and equilibrium geometries in good agreement with the experimental data since it allows for obtaining accurate information on the electron transfer during the adsorption process. For instance, Chakraborty and co-workers performed a combined experimental and theoretical study, explaining how Ag(I)-doped porous carbon can effectively separates C3H6/C3H8 briary mixture [31]. The frame of molecular orbital theory and DFT results indicated that the d-π complexation between the S-Ag(I) open active metal sites and olefin (C2H4 or C3H6) existed in pores. This interaction makes the adsorption energy between the olefins and paraffin a substantial difference. When the narrow pores contain paraffin, Ag(I) could interact with the sp2-C on the other side of the graphene plane, rather than paraffin. However, in presence of olefin molecules, π-π bond interaction occurs between Ag(I) and olefins, rather than sp2-C interaction in the graphene plane.
For the purification of feeds containing low concentration C3H8, porous carbon with an affinity for C3H8 will be an ideal adsorbent, which can directly obtain high-purity C3H6. For instance, Mendes and co-workers constructed novel carbonaceous adsorbents for C3H8/C3H6 separation, which had an excellent affinity for C3H8 over C3H6 [102]. The carbonaceous adsorbents were synthesized by using phosphoric acid-treated phenolic resin as a precursor. Next, the adsorbents were placed in the C3H6 atmosphere for 12 days. The phosphoric acid and C3H6 treatments led to changes in the surface chemistry properties of carbonaceous adsorbents. The adsorbents surface pretreated with phosphoric acid exists C=O and P-O-C functional groups, whereas C3H6 can be used as a cleaner of these O functional groups and produce C=C and P=O functional groups in porous carbon inner surface. Meanwhile, the C3H6 treatment can open the constriction pore to more straight interpore connections, promoting the kinetic diffusion of C3H8. Thus, the sample displays a higher uptake for C3H8 (2.9 mmol g−1) over C3H6 (1.4 mmol g−1) at 100 kPa and 25 °C.
4.4. Separations of C4 Isomer
To date, different types of adsorbents have been reported for the separation of C4 isomers, including MOFs, zeolites, and porous carbons [103–105]. Among them, the adsorption separation of C4 isomers is still in its infancy by using porous carbons, and a few publications had been reported to date. For instance, Xiao and co-workers synthesized a series of starch-based ultramicroporous carbon spheres by using the alkali metal ion-exchange method to control micropore size (SC-M; M = Na, K, Rb). The ultramicropore size of SC-M was tuned on the sub-Ångstrom level [70]. The potassium derivative SC-K can achieve high uptake of C4H6 at 100 kPa and 298 K (2.36 mmol g−1). The C4H6/n-C4H8 and C4H6/i-C4H8 adsorption capacity ratios of SC-K reached 3.75 and 4.72, respectively. The excellent selectivity of SC-K originates from extremely narrow micropores of 4.83 Å. In addition, the bicomponent breakthrough experiment results showed that the SC-K had an outstanding performance in the dynamic separation of C4 alkadiene/alkenes. On top of that, the adsorbent can be regenerated easily under He flow at 353 K.
Although research on the adsorption separation of C4 isomers by porous carbons is limited, there are some reports about carbon molecular sieving membranes (CMMSs) for butane isomer separation. For instance, Yang and co-workers introduced ultramicropores CMMSs, which were synthesized by pyrolysis of P84 polymer [106]. The slit-like ultramicropores of CMSMs were tailored on the Ångstrom level by fine-tuning the pyrolysis temperature. The best average separation factor of n-butane/iso-butane is 74 which emerged in CMMS obtained by pyrolyzing at 600 °C (CMMS-600). The CMMS-600 has ultramicropores centered at 6.0 Å which lies between the kinetic diameters of n-butane and iso-butane. Thus, it is a feasible design method for synthesizing the effective carbonaceous adsorbents by using the above CMMSs membranes precursors.
5. Separation Based on Kinetic Effect
Unlike equilibrium separation, the kinetic separation is reached by the differences in diffusion rates of gas mixtures. The size of the pore entrance plays a critical role in restricting the diffusion of certain adsorbates, leading to partial size exclusion [107, 108]. In this section, we highlighted the main research progress of LHs kinetic separation using porous carbon. We summarized and compared several best-performing porous carbons in kinetic separation.
5.1. CH4/N2
Taking advantage of the difference in molecule kinetic diameters of N2 (0.36 nm) and CH4 (0.38 nm) molecules can achieve separation. The purification can be effectively achieved through precise control of the pore size/shape to make CH4 diffuse in porous carbons, but it significantly restricts the diffusion of the N2 molecules [109–112]. For instance, Zhang and coworkers prepared modified CMS materials with a chemical vapor deposition method to decrease the micropore size of activated carbon [113]. By precisely controlling the deposition temperature, time, and flow rate of benzene, the CMS pore size decreased to form an aperture ideally sized to accommodate N2 but not CH4. As a result, the CMS-G showed a high CH4/N2 kinetic selectivity (35.26) and a good performance in the PSA experiment (enrichment of 30.20% for CH4). Besides, for the CMS, it is necessary to consider the barrier resistance at the entrance of the micropore and the pore diffusion resistance for N2/CH4 separation, as proposed by Yang and co-workers [114]. The authors used the dual resistance model to determine the adsorption kinetics of binary gas mixtures by batch uptake experiments. It indicated that the adsorption rate of binary gas mixtures can be controlled by the surface barrier resistance at the entrance of the micropore and diffusion in the micropore interior. The transport of CH4 (6.04∗107 s−1) is much slower than that of N2 (1.21∗1010 s−1). Accordingly, although CH4 breaks through together with N2 in a breakthrough experiment, the CH4 was hardly adsorbed because of the slower diffusion transport than N2.
5.2. CH4/CO2
As for CH4/CO2 separation, CO2 is easier to diffuse into the pores because of its smaller kinetic size (0.33 nm) than that of CH4. The adsorption performances of CO2 and CH4 on a commercial CMS were reported by Rocha and co-workers [115]. The CMS can effectively trap CO2 and impede the diffusion of CH4, to achieve kinetic and thermodynamic combined selectivity. The diffusion of gases in the adsorbent clearly showed that CO2 diffused through the micropores much faster than CH4. It indicated that the constriction at the entrance of the micropore has an important contribution to the overall diffusion resistance. The equilibrium for CO2 was reached after approximately 800 s, while the equilibrium for CH4 was reached over 25 h. The CO2 and CH4 diffusion constant were calculated to be 0.01 and 0.0001 s−1, respectively. Kapoor and co-workers evaluated the CH4/CO2 kinetic separation performance by using a carbon molecular sieve [116]. It is shown that the CO2 with stronger adsorption has a quicker diffusion rate and the diffusion time constant is 9.7∗10−4s−1. On the contrary, the CH4 has a slower diffusion rate, and the diffusion time constants were calculated to be 5∗10−6s−1. The CO2/CH4 diffusivity rate ratio was 180 at 298 K. This is beneficial for CH4/CO2 kinetic separation, particularly when CH4 was the desired product.
5.3. C3H6/C3H8
Analogous to other LHs separation, the pore aperture size/shape of carbon materials play a key role in achieving the kinetic separation of C3H6/C3H8. For instance, Liu and co-workers prepared a separation of C3H6/C3H8 novel CMS, which was synthesized through pyrolysis a gel-type strong acid cation exchange resin [117] (Figures 6(a) and 6(b)). With the pyrolysis temperature enhanced from 700 to 850 °C, the adsorption half-time ratio (t0.5(C3H8)/t0.5(C3H6)) increased from 1 to more than 100, and the diffusion rate of the C3H8 was noticeably slower. The C3H6/C3H8 kinetic selectivity (90) is similar to that of zeolite 4A, while the C3H6 diffusion rate is 30 times faster than that of zeolite 4A. However, the diffusion ratio of C3H6 is slowed down for carbon materials pyrolysis at 1000 °C, which is not conducive to the kinetic separation of C3H6/C3H8. By comparing the pressure change (ΔP) from start to finish of adsorption in a closed environment, the authors further explored the effect of pyrolysis temperatures on the adsorption capacity of C3H6 and C3H8. Since the pore size of carbon materials gets narrowed as pyrolysis temperature increases, the pore volume close to the size of C3H8 with a larger molecular diameter is reduced, but close to that of the C3H6 changes little (Figures 6(c) and 6(d)). Recently, Liu and coworkers further systematically studied the separation performance of C3H6/C3H8 on the polyvinylidene chloride copolymer (PVDC) derived porous carbons [118]. This work can precisely control the micropore size of PVDC-derived CMS by choosing polymer precursor and pyrolysis temperature. With the pyrolysis temperature increased from 700 to 1500 °C, the effective micropore size can decrease to 6.2-4.2 Å. The CMS-18 (1500°C pyrolysis) showed an obvious difference in the diffusivity rate of C3H6/C3H8, and the diffusivity of C3H6 (D/r2 =1.6∗10−5s−1) was 123 times faster than C3H8 (D/r2 =1.3∗10−7s−1), because the pore aperture is 4.2 Å, which can block C3H8 adsorption. The C3H8 did not reach equilibrium even after 20 days.
Figure 6.
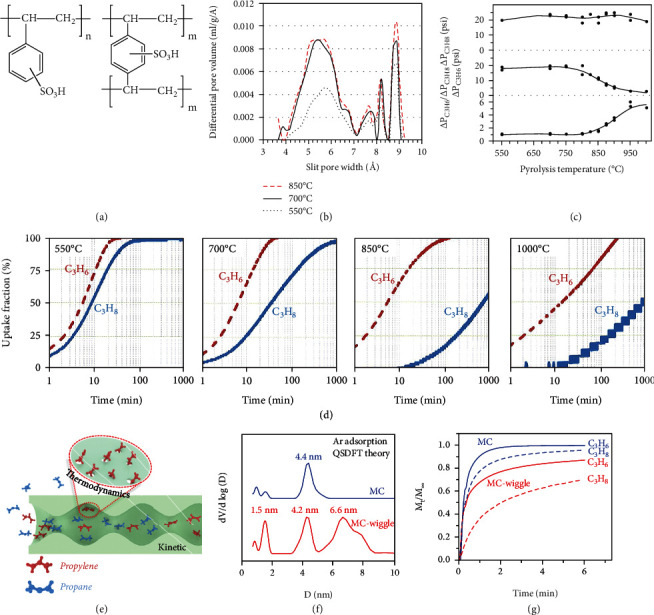
(a) Simplified chemical structure of cation exchange resin: monosulfonated polystyrene. (b) Pore size distribution. (c) The pressure drops of C3H6 (top) and C3H8 (middle) and the ratio of the two (bottom) during adsorption at different carbonization temperatures. (d) Time-resolved adsorption curve for CMS samples with different carbonization temperatures [117]. (e) Illustration of the proposed mechanism for C3H6/C3H8 separation in MC-wiggle. (f) Pore size distributions. (g) Time-resolved absorption curves for C3H6/C3H8 at 298 K [119].
Our group recently demonstrated a new strategy to create wiggling mesopores in monolithic carbon (MC-wiggle) through a perturbed self-assembly method [119]. By such nanoscale mesopore geometry engineering, we achieved a C3H6/C3H8 separation selectivity of around 39.0 and a C3H6 uptake of 2.6 mmol g−1, which outperform not only the reported carbonaceous adsorbents but also the top-performing crystalline adsorbents like MOFs and zeolites. Fundamentally, this is the first wiggling transport behavior observed on mesoporous carbon materials, which featured a unique two-step desorption process for all the gas probes in terms of Ar, N2, H2O, and toluene. Besides the gas adsorption techniques, the state-of-the-art electron tomography was applied to reconstruct the 3D wiggling mesoporous channels. Based on this, we proposed that the bigger C3H8 molecules were easily colliding with and bouncing back the geometrical bulges in the pore channel, resulting in a significantly lower diffusion rate of C3H8 (2.1∗10−4s−1) in the MC-wiggle, while the smaller C3H6 (4.2∗10−3s−1) were less affected, thus amplifying the kinetic selectivity. It is different from the common thermodynamic effect that relies on strong binding sites. This is the first wiggling transport-induced kinetic enhanced C3H6/C3H8 separation (Figures 6(e)–6(g)).
6. Structured Carbon Adsorbents
Carbon adsorbents are often produced as powders that need post-shaping by pressurizing or using binders before their practical applications [120, 121]. In comparison to powders, pellet adsorbents exhibit greater operational flexibility. Microscopically, the 3D continuous hierarchical structures warrant multiple advantages such as fast heat and mass transfer, low pressure drop, and high contacting efficiency [122, 123]. The shaping methods in terms of extrusion, colloidal processing, coatings of honeycombs, etc. are widely explored, where normally require organic or inorganic binders. Binder-free synthesis represents a research frontier. For instance, our group developed a series of carbon pellets derived from functional polymer precursors by a one-step sol-gel method [59, 124, 125]. Although these carbon adsorbents showed a hierarchically porous structure, the connectivity of the mass transport channels can be further improved.
In addition, the development of complex-shaped carbon adsorbents is another research direction. For instance, 3D printing technique enables more options for the geometric design of adsorbent pellets, which can be directly used in adsorption processes. Thus, this technique has been employed as a viable option for the production of shaped carbon adsorbents [126]. Recently, Garnica and co-workers reported that sponge-like carbons with tailored channel architecture and porosity were prepared by combined sol-gel polymerization and 3D printing technology [127]. Mendes and co-workers prepared composite adsorbents composed of porous carbon and 13X zeolite, which were then 3D printed using bentonite as a binder [128]. However, the use of 3D printed porous carbon adsorbents for LHs separation is rarely reported, which could be a developing trend in future.
7. Conclusions and Perspectives
Porous carbon materials have attracted tremendous research interest for LHs separation in recent years, and the increasing number of publications on this topic signifies its importance. As compared with other types of adsorbent materials, carbon-based materials could play a bigger role in LHs separation, particularly in the aspect of practical applications. It shows a great variety in structural modulation which includes its pore structure (size, geometry), morphology (sphere, sheet, fiber), and chemical properties. In addition, doping heteroatoms into carbon matrix can also well modify their separation factor due to the enhanced interaction between the decorated adsorption sites and the specific gas. The full combination of open active metal sites (such as Cu+ and Ag+) and porous carbon provides a good choice to further promote the carbonaceous adsorbent of olefin selectivity. Furthermore, scientists should pay more attention to the gas diffusion behaviors in slit-type micropores and consider the separation performance from a kinetic point of view. Despite the available progress, there is still a huge demand for the development of advanced carbonaceous adsorbents for LHs separation in the chemicals industry. We listed the main issues and/or developing trends in this field as follows:
Judiciously integrating porous carbons with other functional materials (e.g., zeolites, MOFs, COF, ZIF, and POPs) would mitigate the drawbacks of the individual components and provide synergistic effects to enhance LHs separation performance. The unique carbon composites can better achieve separate those multiple and complex components mixtures, such as C1-C4 mixed gas. It has been reported that loading functional materials in porous carbon or physically mixing can quickly construct functional carbonaceous adsorbents. However, if the functional materials are introduced during the precursor synthesis stage, more precise control and design will be achieved, which is more challenging. Because the high-temperature carbonization process will destroy the structure of functional materials, thus the use of functional material with high thermal stability may be a good choice. In conclusion, scientists need to propose more novel and efficient strategies to construct a composite structure to improve the comprehensive performance of carbonaceous adsorbents
To date, the control of pore shapes of carbonaceous adsorbents has not been paid enough attention. It is precisely the subtleties in the local geometries that have a profound influence on the gas behavior properties. However, porous carbon with unusual silt microporous morphology is built from disorganized graphene-like planes, which is difficult to achieve the same flexible design as crystalline materials at the molecular scale. Thus, the key to the slit pore size regulation lies in the stacking parameters between the graphite crystallites, which include the angle, the degree of bending, and the continuity. The current strategy is still difficult to precise control for units of single-layer graphite, including chemical activation, chemical deposition, and control of the carbonization process. Carbon scientists should focus more on the gradient control of the molecular structure in the chemical synthesis of carbon precursors, such as the number of benzene rings, carbon-chain lengths, and group positions. It may be a very potent strategy to precisely control the stacking parameters between graphite layers through differences in molecular structure, which can achieve narrow ultramicropores in carbon materials with sub-Ångstrom precision
Researchers have made efforts trying to quantify the role of heteroatom doping for carbon adsorbents. The selective adsorption of LHs molecules over carbons with less functional groups mainly depends on matched pore structure, such as pore size, geometry, and connectivity. In this case, it is a surface coverage and micropore filling process. The gas adsorption isotherm usually has a lower adsorption capacity at low pressure than carbon materials with preferential adsorption sites, due to the lack of strong interaction. However, for the thermodynamic mechanism, further research is needed to unveil the contribution of nanopores and surface functional groups to the selective adsorption of targeted hydrocarbons
Olefin and CH4 are the desired product; the reversed paraffin/impurity gas selective (“reverse” selective) materials would be more favorable. The “reverse” selective carbonaceous adsorbents will greatly simplify the separation process and reduce the energy consumption for adsorbent regeneration as well as enhance gas purity. Thus, the further development of the “reverse” selectivity of carbonaceous adsorbents is also an area that requires deeper research and insight into the separations of LHs. For instance, the generality of the “reverse” selective of C2H6/C2H4 needs to be further clarified. Why do carbonaceous adsorbents without open metal sites generally have higher adsorption capacity for C2H6 than for C2H4? Can the separation of C2H4 and C2H6 be achieved by kinetics or size sieving effect?
Comprehensive and systematic characterizations will be of great help for observing gas adsorption behaviors and the evaluation of adsorbents practical performance. The adsorption of LHs/impurity gases in micropores can be obtained by gravimetric or volumetric methods, thereby revealing the overall state of the adsorbed LHs/impurity gases. However, the status of LHs/impurity gas in confined slit pores and their interaction with specific adsorption sites on the pore walls is still elusive. Thus, it highly requires advanced characterizations, particularly in situ techniques (e.g., in situ NMR and inelastic neutron scattering) with molecular level even atomic resolution to probe LHs/impurity gas adsorption behaviors in the subnanometer pores
For industrial applications, many factors must be considered, such as stability, scale-up, cost, and multicycle renewability. Under stringent criteria of industry, we have to point out that the reported most adsorbents are in the basic level to demonstrate the laboratory-scale viability, without considering practical applications. Therefore, future exploration must address the “performance parameters spectrum” relevant to commercial applications, rather than blindly pursuing LHs separation performance
All in all, porous carbons can exhibit unparalleled LHs separation and purification performances because of their intrinsic structural advantages and hold great promise for practical applications. Of course, these goals require interdisciplinary know-how input, by profiting knowledge from other disciplines, including organic chemistry, material science, and solid-state chemistry. We have a reason to believe that foreseeable improvements will be made in the LHs separation community in near future.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (No. 21975037); the Fundamental Research Funds for the Central Universities (Nos. DUT20GJ215 and DUT18RC(3)075); and the Liao Ning Revitalization Talents Program (No. XLYC1807205).
Contributor Information
Guang-Ping Hao, Email: guangpinghao@dlut.edu.cn.
An-Hui Lu, Email: anhuilu@dlut.edu.cn.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sholl D. S., Lively R. P. Seven chemical separations to change the world. Nature . 2016;532(7600):435–437. doi: 10.1038/532435a. [DOI] [PubMed] [Google Scholar]
- 2.Bereciartua P. J., Cantín Á., Corma A., et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science . 2017;358(6366):1068–1071. doi: 10.1126/science.aao0092. [DOI] [PubMed] [Google Scholar]
- 3.Cadiau A., Adil K., Bhatt P. M., Belmabkhout Y., Eddaoudi M. A metal-organic framework–based splitter for separating propylene from propane. Science . 2016;353(6295):137–140. doi: 10.1126/science.aaf6323. [DOI] [PubMed] [Google Scholar]
- 4.Li J.-R., Kuppler R. J., Zhou H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chemical Society Reviews . 2009;38(5):1477–1504. doi: 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- 5.Wang H., Liu Y., Li J. Designer metal-organic frameworks for size-exclusion-based hydrocarbon separations: progress and challenges. Advanced Materials . 2020;32, article 2002603(44) doi: 10.1002/adma.202002603. [DOI] [PubMed] [Google Scholar]
- 6.Chen K.-J., Madden D. G., Mukherjee S., et al. Synergistic sorbent separation for one-step ethylene purification from a four-component mixture. Science . 2019;366(6462):241–246. doi: 10.1126/science.aax8666. [DOI] [PubMed] [Google Scholar]
- 7.Chai Y., Han X., Li W., et al. Control of zeolite pore interior for chemoselective alkyne/olefin separations. Science . 2020;368(6494):1002–1006. doi: 10.1126/science.aay8447. [DOI] [PubMed] [Google Scholar]
- 8.Cui X., Chen K., Xing H., et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science . 2016;353(6295):141–144. doi: 10.1126/science.aaf2458. [DOI] [PubMed] [Google Scholar]
- 9.Bloch E. D., Queen W. L., Krishna R., Zadrozny J. M., Brown C. M., Long J. R. Hydrocarbon separations in a metal-organic framework with open iron (II) coordination sites. Science . 2012;335(6076):1606–1610. doi: 10.1126/science.1217544. [DOI] [PubMed] [Google Scholar]
- 10.Suo X., Cui X., Yang L., et al. Synthesis of ionic ultramicroporous polymers for selective separation of acetylene from ethylene. Advanced Materials . 2020;32(29, article 1907601) doi: 10.1002/adma.201907601. [DOI] [PubMed] [Google Scholar]
- 11.Suo X., Yu Y., Qian S., Zhou L., Cui X., Xing H. Tailoring the pore size and chemistry of ionic ultramicroporous polymers for trace sulfur dioxide capture with high capacity and selectivity. Angewandte Chemie-International Edition . 2021;60(13):6986–6991. doi: 10.1002/anie.202013448. [DOI] [PubMed] [Google Scholar]
- 12.Liu J., Goss J., Calverley T., et al. Carbon molecular sieve fiber with 3.4-4. 9 Angstrom effective micropores for propylene/propane and other gas separations. Microporous and Mesoporous Materials . 2020;205, article 110341 doi: 10.1016/j.micromeso.2020.110341. [DOI] [Google Scholar]
- 13.Kim A.-R., Yoon T.-U., Kim E.-J., et al. Facile loading of Cu(I) in MIL-100(Fe) through redox-active Fe(II) sites and remarkable propylene/propane separation performance. Chemical Engineering Journal . 2018;331:777–784. doi: 10.1016/j.cej.2017.09.016. [DOI] [Google Scholar]
- 14.Zhang Y., Hu J., Krishna R., et al. Rational design of microporous MOFs with anionic boron cluster functionality and cooperative dihydrogen binding sites for highly selective capture of acetylene. Angewandte Chemie-International Edition . 2020;132(40):17817–17822. doi: 10.1002/ange.202007681. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z., Xiong X., Xiong J., et al. A robust Th-azole framework for highly efficient purification of C2H4 from a C2H4/C2H2/C2H6 mixture. Nature Communications . 2020;11(1) doi: 10.1038/s41467-020-16960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Peh S. B., Zhao D. Alternatives to cryogenic distillation: advanced porous materials in adsorptive light olefin/paraffin separations. Small . 2019;15(25, article 1900058) doi: 10.1002/smll.201900058. [DOI] [PubMed] [Google Scholar]
- 17.Liu R.-S., Shi X.-D., Wang C.-T., et al. The advances in post-combustion CO2 capture by physical adsorption: from materials innovation to separation practice. ChemSusChem . 2021;14(6):1428–1471. doi: 10.1002/cssc.202002677. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Shao Y., Mei S., et al. Polymer-derived heteroatom-doped porous carbon materials. Chemical Reviews . 2020;120(17):9363–9419. doi: 10.1021/acs.chemrev.0c00080. [DOI] [PubMed] [Google Scholar]
- 19.Peng L., Hung C.-T., Wang S., et al. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. Journal of the American Chemical . 2019;141(17):7073–7080. doi: 10.1021/jacs.9b02091. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z., Yao S., Hu X., et al. Sacrificial synthesis of supported Ru single atoms and clusters on N-doped carbon derived from covalent triazine frameworks: a charge modulation approach. Advanced Science . 2021;8(3, article 2001493) doi: 10.1002/advs.202001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao G.-P., Li W.-C., Qian D., Lu A.-H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Advanced Materials . 2010;22(7):853–857. doi: 10.1002/adma.200903765. [DOI] [PubMed] [Google Scholar]
- 22.Guo L.-P., Li W.-C., Qiu B., Ren Z.-X., Du J., Lu A.-H. Interfacial assembled preparation of porous carbon composites for selective CO2 capture at elevated temperatures. Journal of Materials Chemistry A . 2019;7(10):5402–5408. doi: 10.1039/C8TA12122B. [DOI] [Google Scholar]
- 23.Liang C., Li Z., Dai S. Mesoporous carbon materials: synthesis and modification. Angewandte Chemie-International Edition . 2008;47(20):3696–3717. doi: 10.1002/anie.200702046. [DOI] [PubMed] [Google Scholar]
- 24.Salleh W. N. W., Ismail A. F., Matsuura T., Abdullah M. S. Precursor selection and process conditions in the preparation of carbon membrane for gas separation: a review. Separation & Purification Reviews . 2011;40(4):261–311. doi: 10.1080/15422119.2011.555648. [DOI] [Google Scholar]
- 25.Hao G.-P., Sun Q., Zhang X.-Q., Li W.-C., Lu A.-H. Chemical synthesis of carbon materials with intriguing nanostructure and morphology. Macromolecular Chemistry and Physics . 2012;2013(10-11):1107–1131. [Google Scholar]
- 26.Heidarinejad Z., Dehghani M. H., Heidari M., Javedan G., Ali I., Sillanpää M. Methods for preparation and activation of activated carbon: a review. Environmental Chemistry Letters . 2020;18(2):393–415. doi: 10.1007/s10311-019-00955-0. [DOI] [Google Scholar]
- 27.Yan P., Zhang B., Wu K.-H., Su D., Qi W. Surface chemistry of nanocarbon: characterization strategies from the viewpoint of catalysis and energy conversion. Carbon . 2019;143:915–936. doi: 10.1016/j.carbon.2018.11.085. [DOI] [Google Scholar]
- 28.Hao G.-P., Mondin G., Zheng Z., et al. Unusual ultra-hydrophilic, porous carbon cuboids for atmospheric-water capture. Angewandte Chemie-International Edition . 2015;54(6):1941–1945. doi: 10.1002/anie.201409439. [DOI] [PubMed] [Google Scholar]
- 29.Cai R., You B., Chen M., Wu L. Metal-free core-shell structured N-doped carbon/carbon heterojunction for efficient CO2 capture. Carbon . 2019;150:43–51. doi: 10.1016/j.carbon.2019.05.001. [DOI] [Google Scholar]
- 30.Liang W., Wu Y., Xiao H., Xiao J., Li Y., Li Z. Ethane-selective carbon composites CPDA@A-ACs with high uptake and its enhanced ethane/ethylene adsorption selectivity. AICHE Journal . 2018;64(9):3390–3399. doi: 10.1002/aic.16182. [DOI] [Google Scholar]
- 31.Luca G. D., Saha D., Chakraborty S. Why Ag(I) grafted porous carbon matrix prefers alkene over alkane? An inside view from ab-initio study. Microporous and Mesoporous Materials . 2021;316, article 110940 doi: 10.1016/j.micromeso.2021.110940. [DOI] [Google Scholar]
- 32.Jiang W.-J., Sun L.-B., Yin Y., Song X.-L., Liu X.-Q. Ordered mesoporous carbon CMK-3 modified with Cu(I) for selective ethylene/ethane adsorption. Separation Science and Technology . 2013;48(6):968–976. doi: 10.1080/01496395.2012.712600. [DOI] [Google Scholar]
- 33.Qian D., Lei C., Hao G.-P., Li W.-C., Lu A.-H. Synthesis of hierarchical porous carbon monoliths with incorporated metal−organic frameworks for enhancing volumetric based CO2 capture capability. ACS Applied Materials & Interfaces . 2012;4(11):6125–6132. doi: 10.1021/am301772k. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., Huang N.-Y., Zhang X.-W., et al. Selective aerobic oxidation of a metal-organic framework boosts thermodynamic and kinetic propylene/propane selectivity. Angewandte Chemie-International Edition . 2019;58(23):7692–7696. doi: 10.1002/anie.201902209. [DOI] [PubMed] [Google Scholar]
- 35.Chang Z., Lin R.-B., Ye Y., Duan C., Chen B. Construction of a thiourea-based metal organic framework with open Ag+ sites for the separation of propene/propane mixtures. Journal of Materials Chemistry A . 2019;7(44):25567–25572. doi: 10.1039/C9TA08614E. [DOI] [Google Scholar]
- 36.Iucolano F., Aprea P., Caputo D., Colella C., Eić M., Huang Q. Adsorption and diffusion of propane and propylene in Ag+-impregnated MCM-41. Adsorption . 2008;14(2-3):241–246. doi: 10.1007/s10450-008-9106-0. [DOI] [Google Scholar]
- 37.Grande C. A., Araujo J. D. P., Cavenati S., Firpo N., Basaldella E., Rodrigue A. E. New π-complexation adsorbents for propane-propylene separation. Langmuir . 2004;20(13):5291–5297. doi: 10.1021/la036400s. [DOI] [PubMed] [Google Scholar]
- 38.Li L., Lin R.-B., Wang X., et al. Kinetic separation of propylene over propane in a microporous metal-organic framework. Chemical Engineering Journal . 2018;354:977–982. doi: 10.1016/j.cej.2018.08.108. [DOI] [Google Scholar]
- 39.Olson D. H., Camblor M. A., Villaescusa L. A., Kuehl G. H. Light hydrocarbon sorption properties of pure silica Si-CHA and ITQ-3 and high silica ZSM-58. Microporous and Mesoporous Materials . 2004;67(1):27–33. doi: 10.1016/j.micromeso.2003.09.025. [DOI] [Google Scholar]
- 40.Xue D.-X., Cadiau A., Weseliński L. J., et al. Topology meets MOF chemistry for pore-aperture fine tuning: ftw-MOF platform for energy-efficient separations via adsorption kinetics or molecular sieving. Chemical Communications . 2018;54(49):6404–6407. doi: 10.1039/C8CC03841D. [DOI] [PubMed] [Google Scholar]
- 41.Zürner A., Kirstein J., Döblinger M., Bräuchle C., Bein T. Visualizing single-molecule diffusion in mesoporous materials. Nature . 2007;450(7170):705–708. doi: 10.1038/nature06398. [DOI] [PubMed] [Google Scholar]
- 42.Wang H., Dong X., Colombo V., et al. Tailor-made microporous metal-organic frameworks for the full separation of propane from propylene through selective size exclusion. Advanced Materials . 2018;30(49, article 1805088) doi: 10.1002/adma.201805088. [DOI] [PubMed] [Google Scholar]
- 43.Saha D., Bao Z., Jia F., Deng S. Adsorption of CO2, CH4, N2O, and N2on MOF-5, MOF-177, and zeolite 5A. Environmental Science & Technology . 2010;44(5):1820–1826. doi: 10.1021/es9032309. [DOI] [PubMed] [Google Scholar]
- 44.Ding Q., Zhang Z., Yu C., et al. Exploiting equilibrium-kinetic synergetic effect for separation of ethylene and ethane in a microporous metal-organic framework. Science Advances . 2020;6(15) doi: 10.1126/sciadv.aaz4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benzigar M. R., Talapaneni S. N., Joseph S., et al. Recent advances in functionalized micro and mesoporous carbon materials: synthesis and applications. Chemical Society Reviews . 2018;47(8):2680–2721. doi: 10.1039/C7CS00787F. [DOI] [PubMed] [Google Scholar]
- 46.Ling Z., Wang Z., Zhang M., et al. Sustainable synthesis and assembly of biomass-derived B/N co-doped carbon nanosheets with ultrahigh aspect ratio for high-performance supercapacitors. Advanced Functional Materials . 2016;26(1):111–119. doi: 10.1002/adfm.201504004. [DOI] [Google Scholar]
- 47.Ullah S., Shi Q., Zhou J., et al. Advances and trends in chemically doped graphene. Advanced Materials Interfaces . 2020;24(7, article 2000999) doi: 10.1002/admi.202000999. [DOI] [Google Scholar]
- 48.Zhu T., Zhou J., Li Z., Li S., Si W., Zhuo S. Hierarchical porous and N-doped carbon nanotubes derived from polyaniline for electrode materials in supercapacitors. Journal of Materials Chemistry A . 2014;2(31):12545–12551. doi: 10.1039/C4TA01465K. [DOI] [Google Scholar]
- 49.Shao Y., Sui J., Yin G., Gao Y. Nitrogen-doped carbon nanostructures and their composites as catalytic materials for proton exchange membrane fuel cell. Applied Catalysis B: Environmental . 2008;79(1):89–99. doi: 10.1016/j.apcatb.2007.09.047. [DOI] [Google Scholar]
- 50.Cao Y., Yu H., Tan J., et al. Nitrogen-, phosphorous-and boron-doped carbon nanotubes as catalysts for the aerobic oxidation of cyclohexane. Carbon . 2013;57:433–442. doi: 10.1016/j.carbon.2013.02.016. [DOI] [Google Scholar]
- 51.Hu L., Hou J., Ma Y., Li H., Zhai T. Multi-heteroatom self-doped porous carbon derived from swim bladders for large capacitance supercapacitors. Journal of Materials Chemistry A . 2016;4(39):15006–15014. doi: 10.1039/C6TA06337C. [DOI] [Google Scholar]
- 52.Jurcakova D. H., Puziy A. M., Poddubnaya O. I., García F. S., Tascón J. M. D., Lu G. Q. Highly stable performance of supercapacitors from phosphorus-enriched carbons. Journal of the American Chemical . 2009;131(14):5026–5027. doi: 10.1021/ja809265m. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y., Zhou H., Zhu X., et al. Simultaneous oxidative and reductive reactions in one system by atomic design. Nature Catalysis . 2021;4(2):134–143. doi: 10.1038/s41929-020-00563-0. [DOI] [Google Scholar]
- 54.Hao G.-P., Tang C., Zhang E., et al. Thermal exfoliation of layered metal-organic frameworks into ultrahydrophilic graphene stacks and their applications in Li-S batteries. Advanced Materials . 2017;29, article 1702829(37) doi: 10.1002/adma.201702829. [DOI] [PubMed] [Google Scholar]
- 55.Li Z., Wang D., Wu Y., Li Y. Recent advances in the precise control of isolated single-site catalysts by chemical methods. National Science Review . 2018;5(5):673–689. doi: 10.1093/nsr/nwy056. [DOI] [Google Scholar]
- 56.Yang X.-F., Wang A., Qiao B., Li J., Liu J., Zhang T. Single-atom catalysts: a new frontier in heterogeneous catalysis. Accounts of Chemical Research . 2013;46(8):1740–1748. doi: 10.1021/ar300361m. [DOI] [PubMed] [Google Scholar]
- 57.Stein A., Wang Z., Fierke M. A. Functionalization of porous carbon materials with designed pore architecture. Advanced Materials . 2009;21(3):265–293. doi: 10.1002/adma.200801492. [DOI] [Google Scholar]
- 58.Hao G.-P., Jin Z.-Y., Sun Q., Zhang X.-Q., Zhang J.-T., Lu A.-H. Porous carbon nanosheets with precisely tunable thickness and selective CO2 adsorption properties. Energy & Environmental Science . 2013;6(12):3740–3747. doi: 10.1039/c3ee41906a. [DOI] [Google Scholar]
- 59.Hao G.-P., Li W.-C., Qian D., et al. Structurally designed synthesis of mechanically stable poly(benzoxazine-co-resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents. Journal of the American Chemical Society . 2011;133(29):11378–11388. doi: 10.1021/ja203857g. [DOI] [PubMed] [Google Scholar]
- 60.Lei L. F., Bai L., Lindbråthen A., Pan F. J., Zhang X. P., He X. Z. Carbon membranes for CO2 removal: status and perspectives from materials to processes. Chemical Engineering Journal . 2020;401, article 126084 doi: 10.1016/j.cej.2020.126084. [DOI] [Google Scholar]
- 61.Xia J.-L., Yan D., Guo L.-P., Dong X.-L., Li W.-C., Lu A.-H. Hard carbon nanosheets with uniform ultramicropores and accessible functional groups showing high realistic capacity and superior rate performance for sodium-ion storage. Advanced Materials . 2020;32(21, article 2000447) doi: 10.1002/adma.202000447. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X., Zheng H., Li G., et al. Ammoniated and activated microporous biochar for enhancement of SO2 adsorption. Journal of Analytical and Applied Pyrolysis . 2021;156, article 105119 doi: 10.1016/j.jaap.2021.105119. [DOI] [Google Scholar]
- 63.Zhang L.-H., Li W.-C., Liu H., et al. Thermoregulated phase transition synthesis of two-dimensional carbon nanoplates rich in sp2 carbon and unimodal ultramicropores for kinetic gas separations. Angewandte Chemie-International Edition . 2018;57(6):1632–1635. doi: 10.1002/anie.201712913. [DOI] [PubMed] [Google Scholar]
- 64.Lee H.-M., Heo Y.-J., An K.-H., et al. A study on optimal pore range for high pressure hydrogen storage behaviors by porous hard carbon materials prepared from a polymeric precursor. International Journal of Hydrogen Energy . 2018;43(11):5894–5902. doi: 10.1016/j.ijhydene.2017.09.085. [DOI] [Google Scholar]
- 65.Zhang L.-H., Li W.-C., Tang L., et al. Primary amine modulated synthesis of two-dimensional porous nanocarbons with tunable ultramicropores. Journal of Materials Chemistry A . 2018;6(47):24285–24290. doi: 10.1039/C8TA09545K. [DOI] [Google Scholar]
- 66.Wang C., Kim J., Tang J., et al. New strategies for novel MOF-derived carbon materials based on nanoarchitectures. Chem . 2020;6(1):19–40. doi: 10.1016/j.chempr.2019.09.005. [DOI] [Google Scholar]
- 67.Li W., Hu S., Luo X., et al. Confined amorphous red phosphorus in MOF-derived N-doped microporous carbon as a superior anode for sodium-ion battery. Advanced Materials . 2017;29(16, article 1605820) doi: 10.1002/adma.201605820. [DOI] [PubMed] [Google Scholar]
- 68.Kyotani T. Control of pore structure in carbon. Carbon . 2000;38(2):269–286. doi: 10.1016/S0008-6223(99)00142-6. [DOI] [Google Scholar]
- 69.Li S., Han K., Li J., Li M., Lu C. Preparation and characterization of super activated carbon produced from gulfweed by KOH activation. Microporous and Mesoporous Materials . 2017;243:291–300. doi: 10.1016/j.micromeso.2017.02.052. [DOI] [Google Scholar]
- 70.Du S., Wang X., Huang J., et al. Ultramicroporous carbons featuring sub-Ångstrom tunable apertures for the selective separation of light hydrocarbon. AICHE Journal . 2021;67(9, article e17285) doi: 10.1002/aic.17285. [DOI] [Google Scholar]
- 71.Du S., Huang J., Anjum A. W., Xiao J., Li Z. A novel mechanism of controlling ultramicropore size in carbons at sub-angstrom level for molecular sieving of propylene/propane mixtures. Journal of Materials Chemistry A . 2021;9(42):23873–23881. doi: 10.1039/D1TA07261G. [DOI] [Google Scholar]
- 72.Yoshimune M., Haraya K. Simple control of the pore structures and gas separation performances of carbon hollow fiber membranes by chemical vapor deposition of propylene. Separation and Purification Technology . 2019;223:162–167. doi: 10.1016/j.seppur.2019.04.065. [DOI] [Google Scholar]
- 73.Khodabakhshi S., Kiani S., Niu Y., et al. Facile and environmentally friendly synthesis of ultramicroporous carbon spheres: a significant improvement in CVD method. Carbon . 2021;171:426–436. doi: 10.1016/j.carbon.2020.08.056. [DOI] [Google Scholar]
- 74.Zhang P., Zhong Y., Ding J., et al. A new choice of polymer precursor for solvent-free method: preparation of N-enriched porous carbons for highly selective CO2 capture. Chemical Engineering Journal . 2019;355:963–973. doi: 10.1016/j.cej.2018.08.219. [DOI] [Google Scholar]
- 75.Jagiełło J., Sanghani P., Bandosz T. J., Schwarz J. A. Thermodynamic study of high-pressure adsorption of methane on activated carbons: the effect of oxidation on pore structure and adsorption energy heterogeneity. Carbon . 1992;30(3):507–512. doi: 10.1016/0008-6223(92)90050-7. [DOI] [Google Scholar]
- 76.Kumar K. V., Preuss K., Titirici M. M., Reinoso F. R. Nanoporous materials for the onboard storage of natural gas. Chemical Reviews . 2017;117(3):1796–1825. doi: 10.1021/acs.chemrev.6b00505. [DOI] [PubMed] [Google Scholar]
- 77.Xu S., Li W.-C., Wang C.-T., Tang L., Hao G.-P., Lu A.-H. Self-pillared ultramicroporous carbon nanoplates for selective separation of CH4/N2. Angewandte Chemie-International Edition . 2021;60(12):6339–6343. doi: 10.1002/ange.202014231. [DOI] [PubMed] [Google Scholar]
- 78.Dong Z., Li B., Shang H., et al. Ultramicroporous carbon granules with narrow pore size distribution for efficient CH4 separation from coal-bed gases. AICHE Journal . 2021;67(9, article e17281) doi: 10.1002/aic.17281. [DOI] [Google Scholar]
- 79.Du S., Wu Y., Wang X., et al. Facile synthesis of ultramicroporous carbon adsorbents with ultra-high CH4 uptake by in situ ionic activation. AICHE Journal . 2020;66(7, article e16231) doi: 10.1002/aic.16231. [DOI] [Google Scholar]
- 80.Tang R., Dai Q., Liang W., et al. Synthesis of novel particle rice-based carbon materials and its excellent CH4/N2 adsorption selectivity for methane enrichment from low-rank natural gas. Chemical Engineering Journal . 2020;384, article 123388 doi: 10.1016/j.cej.2019.123388. [DOI] [Google Scholar]
- 81.Park J., Attia N. F., Jung M., et al. Sustainable nanoporous carbon for CO2, CH4, N2, H2 adsorption and CO2/CH4 and CO2/N2 separation. Energy . 2018;158:9–16. doi: 10.1016/j.energy.2018.06.010. [DOI] [Google Scholar]
- 82.Zhang Y., Zhang P., Yu W., et al. Facile and controllable preparation of ultramicroporous biomass-derived carbons and application on selective adsorption of gas-mixtures. Industrial & Engineering Chemistry Research . 2018;57(42):14191–14201. doi: 10.1021/acs.iecr.8b02139. [DOI] [Google Scholar]
- 83.Zhang Y., Liu L., Zhang P., et al. Ultra-high surface area and nitrogen-rich porous carbons prepared by a low-temperature activation method with superior gas selective adsorption and outstanding supercapacitance performance. Chemical Engineering Journal . 2019;355:309–319. doi: 10.1016/j.cej.2018.08.169. [DOI] [Google Scholar]
- 84.Zhang P., Wang J., Fan W., et al. Ultramicroporous carbons with extremely narrow pore size distribution via in-situ ionic activation for efficient gas-mixture separation. Chemical Engineering Journal . 2019;375, article 121931 doi: 10.1016/j.cej.2019.121931. [DOI] [Google Scholar]
- 85.Fu N., Yu J., Zhao J., et al. In-situ preparation of nitrogen-doped unimodal ultramicropore carbon nanosheets with ultrahigh gas selectivity. Carbon . 2019;149:538–545. doi: 10.1016/j.carbon.2019.04.076. [DOI] [Google Scholar]
- 86.Attia N. F., Jung M., Park J., Jang H., Lee K., Oh H. Flexible nanoporous activated carbon cloth for achieving high H2, CH4, and CO2 storage capacities and selective CO2/CH4 separation. Chemical Engineering Journal . 2020;379, article 122367 doi: 10.1016/j.cej.2019.122367. [DOI] [Google Scholar]
- 87.Lourenco M. A. O., Nunes C., Gomes J. R. B., Pires J., Pinto M. L., Ferreira P. Pyrolyzed chitosan-based materials for CO2/CH4 separation. Chemical Engineering Journal . 2019;362:364–374. doi: 10.1016/j.cej.2018.12.180. [DOI] [Google Scholar]
- 88.Wang W., Yuan D. Mesoporous carbon originated from non-permanent porous MOFs for gas storage and CO2/CH4 separation. Scientific Reports . 2015;4(1):1–7. doi: 10.1038/srep05711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yuan B., Wang J., Chen Y., Wu X., Luo H., Deng S. Unprecedented performance of N-doped activated hydrothermal carbon towards C2H6/CH4, CO2/CH4, and CO2/H2 separation. Journal of Materials Chemistry A . 2016;4(6):2263–2276. doi: 10.1039/C5TA08436A. [DOI] [Google Scholar]
- 90.Wang X., Yuan B., Zhou X., et al. Novel glucose-based adsorbents (Glc-Cs) with high CO2 capacity and excellent CO2/CH4/N2 adsorption selectivity. Chemical Engineering Journal . 2017;327:51–59. doi: 10.1016/j.cej.2017.06.074. [DOI] [Google Scholar]
- 91.Kumar K. V., Reinoso F. R. Co-adsorption of N2 in the presence of CH4 within carbon nanospaces: evidence from molecular simulations. Nanotechnology . 2013;24(3, article 035401) doi: 10.1088/0957-4484/24/3/035401. [DOI] [PubMed] [Google Scholar]
- 92.Ning P., Li F., Yi H., et al. Adsorption equilibrium of methane and carbon dioxide on microwave-activated carbon. Separation and Purification Technology . 2012;98:321–326. doi: 10.1016/j.seppur.2012.07.001. [DOI] [Google Scholar]
- 93.Wang J., Krishna R., Yang T., Deng S. Nitrogen-rich microporous carbons for highly selective separation of light hydrocarbons. Journal of Materials Chemistry A . 2016;4(36):13957–13966. doi: 10.1039/C6TA04939G. [DOI] [Google Scholar]
- 94.Saha D., Toof B., Krishn R., et al. Separation of ethane-ethylene and propane-propylene by Ag(I) doped and sulfurized microporous carbon. Microporous and Mesoporous Materials . 2020;299, article 110099 doi: 10.1016/j.micromeso.2020.110099. [DOI] [Google Scholar]
- 95.Gao F., Wang Y., Wang X., Wang S. Ethylene/ethane separation by CuCl/AC adsorbent prepared using CuCl2 as a precursor. Adsorption . 2016;22(7):1013–1022. doi: 10.1007/s10450-016-9810-0. [DOI] [Google Scholar]
- 96.Lee S.-K., Park H., Yoon J. W., et al. Microporous 3D graphene-like zeolite-templated carbons for preferential adsorption of ethane. ACS Applied Materials & Interfaces . 2020;12(25):28484–28495. doi: 10.1021/acsami.0c04228. [DOI] [PubMed] [Google Scholar]
- 97.Zhang P., Wen X., Wang L., et al. Algae-derived N-doped porous carbons with ultrahigh specific surface area for highly selective separation of light hydrocarbons. Chemical Engineering Journal . 2020;381, article 122731 doi: 10.1016/j.cej.2019.122731. [DOI] [Google Scholar]
- 98.Wang X., Wu Y., Peng J., et al. Novel glucosamine-based carbon adsorbents with high capacity and its enhanced mechanism of preferential adsorption of C2H6 over C2H4. Chemical Engineering Journal . 2019;358:1114–1125. doi: 10.1016/j.cej.2018.10.109. [DOI] [Google Scholar]
- 99.Wang X., Wu Y., Zhou X., et al. Novel C-PDA adsorbents with high uptake and preferential adsorption of ethane over ethylene. Chemical Engineering Science . 2016;155:338–347. doi: 10.1016/j.ces.2016.08.026. [DOI] [Google Scholar]
- 100.Lin J. Y. S. Molecular sieves for gas separation. Science . 2016;353(6295):121–122. doi: 10.1126/science.aag2267. [DOI] [PubMed] [Google Scholar]
- 101.Christopher C. C. E., Dutta A., Orcid S. F., Karimi I. A. Process synthesis and optimization of propylene/propane separation using vapor recompression and self-heat recuperation. Industrial & Engineering Chemistry Research . 2017;56(49):14557–14564. doi: 10.1021/acs.iecr.7b03432. [DOI] [Google Scholar]
- 102.Andradea M., Parnell A. J., Bernardo G., Mendes A. Propane selective carbon adsorbents from phenolic resin precursor. Microporous and Mesoporous Materials . 2021;320, article 111071 doi: 10.1016/j.micromeso.2021.111071. [DOI] [Google Scholar]
- 103.Bender M. An overview of industrial processes for the production of olefins-C4 hydrocarbons. ChemBioEng Reviews . 2014;1(4):136–147. doi: 10.1002/cben.201400016. [DOI] [Google Scholar]
- 104.Gehre M., Guo Z., Rothenberg G., Tanase S. Sustainable separations of C4-hydrocarbons by using microporous materials. ChemSusChem . 2017;10(20):3947–3963. doi: 10.1002/cssc.201700657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cui J., Zhang Z., Hu J., et al. Geometry control of adsorption sites in sulfonate-pillared hybrid ultramicroporous materials for efficient C4 olefin separations. Chemical Engineering Journal . 2021;425, article 130580 doi: 10.1016/j.cej.2021.130580. [DOI] [Google Scholar]
- 106.Zhou Y., Wang Y., Ban Y., et al. Carbon molecular sieving membranes for butane isomer Separation. AICHE Journal . 2019;65(11, article e16749) doi: 10.1002/aic.16749. [DOI] [Google Scholar]
- 107.Lee C. Y., Bae Y.-S., Jeong N. C., et al. Kinetic separation of propene and propane in metal-organic frameworks: controlling diffusion rates in plate-shaped crystals via tuning of pore apertures and crystallite aspect ratios. Journal of the American Chemical . 2011;133(14):5228–5231. doi: 10.1021/ja200553m. [DOI] [PubMed] [Google Scholar]
- 108.Jaroniec M., Tóth J. Adsorption of gas mixtures on heterogeneous solid surfaces: I. extension of T6th isotherm on adsorption from gas mixtures. Journal of the American Chemical . 1976;254(7):643–649. doi: 10.1007/BF01753693. [DOI] [Google Scholar]
- 109.Zhong D.-L., Daraboina N., Englezos P. Recovery of CH4 from coal mine model gas mixture (CH4/N2) by hydrate crystallization in the presence of cyclopentane. Fuel . 2013;106:425–430. doi: 10.1016/j.fuel.2013.01.029. [DOI] [Google Scholar]
- 110.Yang J., Bai H., Shang H., Wang J., Li J., Deng S. Experimental and simulation study on efficient CH4/N2 separation by pressure swing adsorption on silicalite-1 pellets. Chemical Engineering Journal . 2020;388, article 124222 doi: 10.1016/j.cej.2020.124222. [DOI] [Google Scholar]
- 111.Liu J., Xie L., Elsworth D., Gan Q. CO2/CH4Competitive adsorption in shale: implications for enhancement in gas production and reduction in carbon emissions. Environmental Science & Technology . 2019;53(15):9328–9336. doi: 10.1021/acs.est.9b02432. [DOI] [PubMed] [Google Scholar]
- 112.Hou Q., Wu Y., Zhou S., Wei Y., Caro J., Wang H. Ultra-tuning of the aperture size in stiffened ZIF-8_Cm frameworks with mixed-linker strategy for enhanced CO2/CH4 separation. Angewandte Chemie-International Edition . 2019;58(1):327–331. doi: 10.1002/anie.201811638. [DOI] [PubMed] [Google Scholar]
- 113.Zhang J., Qu S., Li L., et al. Preparation of carbon molecular sieves used for CH4/N2 separation. Journal of Chemical & Engineering Data . 2018;63(5):1737–1744. doi: 10.1021/acs.jced.8b00048. [DOI] [Google Scholar]
- 114.Yang Y., Ribeiro A. M., Li P., Yu J.-G., Rodrigues A. E. Adsorption equilibrium and kinetics of methane and nitrogen on carbon molecular sieve. Industrial & Engineering Chemistry Research . 2014;53(43):16840–16850. doi: 10.1021/ie502928y. [DOI] [Google Scholar]
- 115.Rocha L. A. M., Andreassen K. A., Grande C. A. Separation of CO2/CH4 using carbon molecular sieve (CMS) at low and high pressure. Chemical Engineering Science . 2017;164:148–157. doi: 10.1016/j.ces.2017.01.071. [DOI] [Google Scholar]
- 116.Kapoor A., Yang R. T. Kinetic separation of methane-carbon dioxide mixture by adsorption on molecular sieve carbon. Chemical Engineering Science . 1989;44(8):1723–1733. doi: 10.1016/0009-2509(89)80014-4. [DOI] [Google Scholar]
- 117.Liu J., Liu Y., Talay D., Calverley E., Brayden M., Martinez M. A new carbon molecular sieve for propylene/propane separations. Carbon . 2015;85:201–211. doi: 10.1016/j.carbon.2014.12.089. [DOI] [Google Scholar]
- 118.Liu J., Calverley E. M., McAdon M. H., et al. New carbon molecular sieves for propylene/propane separation with high working capacity and separation factor. Carbon . 2017;123:273–282. doi: 10.1016/j.carbon.2017.07.068. [DOI] [Google Scholar]
- 119.Yuan Y.-F., Wang Y.-S., Zhang X.-L., et al. Wiggling mesopores kinetically amplified adsorptive separation of propylene/propane. Angewandte Chemie-International Edition . 2021;60(35):19063–19067. doi: 10.1002/anie.202106523. [DOI] [PubMed] [Google Scholar]
- 120.Liu L., Liu Z., Yang J., Huang Z., Liu Z. Effect of preparation conditions on the properties of a coal-derived activated carbon honeycomb monolith. Carbon . 2007;45(14):2836–2842. doi: 10.1016/j.carbon.2007.08.006. [DOI] [Google Scholar]
- 121.Arami-Niya A., Rufford T. E., Zhu Z. Activated carbon monoliths with hierarchical pore structure from tarpitch and coal powder for the adsorption of CO2, CH4 and N2. Carbon . 2016;103:115–124. doi: 10.1016/j.carbon.2016.02.098. [DOI] [Google Scholar]
- 122.Wang M., Li Y., Pan M., et al. Shape-customizable macro-/microporous carbon monoliths for structure-to-functionality CO2 adsorption and novel electrical regeneration. Advanced Materials Technologies . 2017;2(10) doi: 10.1002/admt.201700088. [DOI] [Google Scholar]
- 123.Sui S., Zhu S., Su L., et al. Assembly multifunctional three-dimensional carbon networks by controlling intermolecular forces. ACS Applied Materials & Interfaces . 2018;10(42):36284–36289. doi: 10.1021/acsami.8b12978. [DOI] [PubMed] [Google Scholar]
- 124.Du J., Li W.-C., Ren Z.-X., Guo L.-P., Lu A.-H. Synthesis of mechanically robust porous carbon monoliths for CO2 adsorption and separation. Journal of Energy Chemistry . 2020;42:56–61. doi: 10.1016/j.jechem.2019.06.006. [DOI] [Google Scholar]
- 125.Guo L.-P., Hu Q.-T., Zhang P., Li W.-C., Lu A.-H. Polyacrylonitrile derived sponge-like micro/macroporous carbons for selective CO2 separation. Chemistry–European Journal . 2018;24(33):8369–8374. doi: 10.1002/chem.201800631. [DOI] [PubMed] [Google Scholar]
- 126.Lawson S., Li X., Thakkar H., Rownaghi A. A., Rezaei F. Recent advances in 3D printing of structured materials for adsorption and catalysis applications. Chemical Reviews . 2021;121(10):6246–6291. doi: 10.1021/acs.chemrev.1c00060. [DOI] [PubMed] [Google Scholar]
- 127.Chaparro-Garnica C. Y., Bailón-García E., Davó-Quiñonero A., Lozano-Castello D., Bueno-López A. Sponge-like carbon monoliths: porosity control of 3D-printed carbon supports and its influence on the catalytic performance. Chemical Engineering Journal . 2022;432(15, article 134218) doi: 10.1016/j.cej.2021.134218. [DOI] [Google Scholar]
- 128.Mendes D. N., Gaspar A., Ferreira I., Mota J. P., Ribeiro R. P. 3D-printed hybrid zeolitic/carbonaceous electrically conductive adsorbent structures. Chemical Engineering Research and Design . 2021;174:442–453. doi: 10.1016/j.cherd.2021.08.020. [DOI] [Google Scholar]


