Abstract
There is substantial evidence in support of an association between periodontitis and cardiovascular disease. The most important open question related to this association is causality. This article revisits the question of causality by reviewing intervention studies and systematic reviews and meta analyses published in the last 3 years. Where are we now in answering this question? Whilst systematic reviews and epidemiological studies continue to support an association between the diseases, intervention studies fall short in determining causality. There is a dearth of good-quality, blinded randomised control trials with cardiovascular disease outcomes. Most studies use surrogate markers/biomarkers for endpoints, and this is problematic as they may not be reflective of cardiovascular disease status. This review further highlights another issue with surrogate markers/biomarkers: the potential for collider bias. Ethical considerations surrounding nontreatment have led to calls for a well-annotated database containing in-depth dental health data. Finally, a relatively new and important risk factor for cardiovascular disease, clonal haematopoiesis of indeterminate potential, is discussed. Clonal haematopoiesis of indeterminate potential increases cardiovascular risk by more than 40%, and inflammation is a contributing factor. The impact of periodontal disease on this emerging risk factor has yet to be explored. Although the question of causality in the association between periodontal disease and cardiovascular disease remains unanswered, the importance of good oral health in maintaining good heart health is reiterated.
Key words: Periodontitis, Cardiovascular disease, Clonal haematopoiesis, Collider bias, Plaque
Background
Cardiovascular disease (CVD) is a catchall term to define disorders of the heart and blood vessels. An underlying cause of CVD is atherosclerosis. Atherosclerosis is a chronic, vascular inflammatory condition that results in the deposition of lipid in the artery wall (plaque).1, 2, 3 Plaque buildup and progression can reduce blood flow, leading to ischemia of downstream organs and tissues, and also promote clot formation.1, 2, 3 Major disorders of the heart associated with atherosclerosis include myocardial infarction (heart attack), heart failure, arrhythmia, heart valve disorders, and cardiomyopathy.2 Blood vessel disorders associated with atherosclerosis include coronary, carotid, and peripheral artery diseases; abdominal aortic aneurysm; and stroke.2 Worldwide, CVD is the leading cause of death, and this has been the case for the last 20 years, despite the availability of therapeutics and intensive interventional efforts.3
Periodontal disease (including gingivitis), resulting from a different kind of plaque, is estimated to affect 47.2% of adults in the United States aged 30 and older.4 This increases to ∼70% after age 65.4 In addition to oral care habits, factors contributing to periodontal disease include socioeconomic status, sex (men > women), education, diet, and smoking.4 Based on data from 2009 and 2010, severe periodontal disease was estimated to affect 11% of adults worldwide and was the sixth most prevalent disease (number of cases at a specific time point).5 Periodontitis and CVD incidence (the likelihood of new occurrences of disease) increase with age.3,5
Development of CVD is largely influenced by underlying risk factors. Some of these are immutable, including sex (male), age, and genetics (ethnic background, family history).6 In addition to these, there are modifiable risk factors that have been shown to be associated with atherosclerosis, including weight, glucose levels/insulin response, blood pressure, blood lipids (driven by diet), smoking, and physical inactivity.6 Because atherosclerosis is a chronic inflammatory illness, researchers sought to understand whether other inflammatory conditions could contribute to atherosclerosis and worsen disease.7 One of the inflammatory conditions studied was periodontitis. Multiple epidemiologic studies showed an association between periodontitis (excluding gingivitis) and CVD.8, 9, 10, 11, 12, 13, 14 In 2012, following more than 10 years of epidemiologic data supporting an association between periodontitis and CVD, the American Heart Association issued a scientific statement reiterating a link between the two diseases, but highlighting the lack of causal evidence.15
Associations/correlations uncovered by epidemiologic studies can significantly guide public health and therapeutic efforts. However, without evidence for causality, these efforts may be focused on an incorrect target. Thus, it could be that something else that is associated with the identified putative factor may actually account for disease. Correlative studies, whilst important, are considered to be at the lower end of the spectrum of scientific evidence.16 This is why, in the years since 2012, many studies have tried to address causality and to better understand the link between periodontitis and CVD. A range of studies, from cell culture to preclinical animal experiments to human clinical research and interventional trials, have been performed in order to evaluate whether there is a causal relationship between the two diseases.
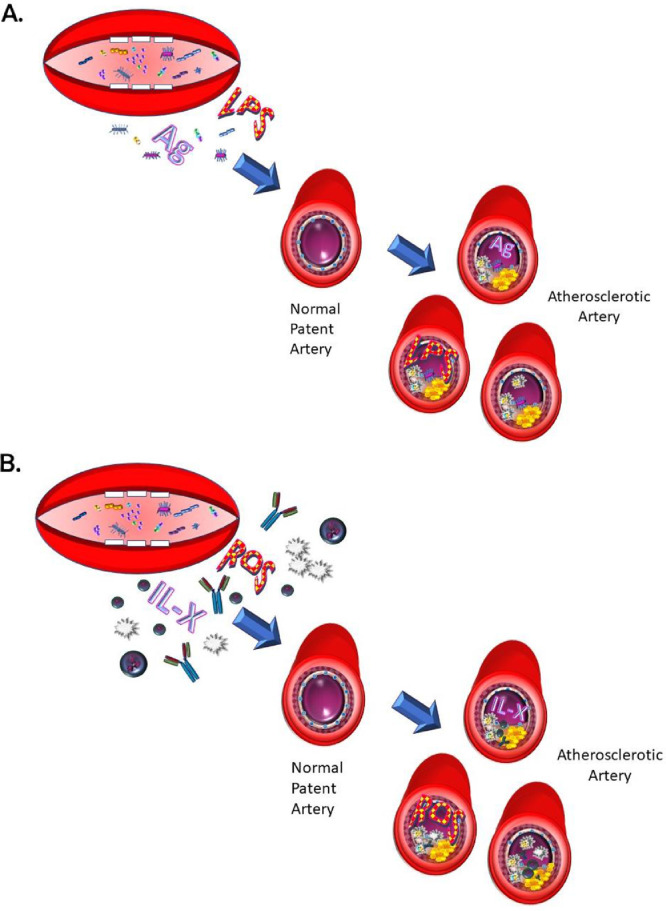
As a result of these studies, 2 main hypotheses have emerged to explain the link between the diseases. The first hypothesis suggests that oral bacteria involved in periodontal disease (or their by-products) can infect blood vessels or in some other way promote plaque formation and, thus, CVD.17,18 The second hypothesis postulates that inflammation as a result of periodontitis increases systemic inflammation and oxidative stress, and this contributes to and increases the already chronic inflammation present and in this way contributes to atherosclerosis and CVD.17,18 There is evidence to support both hypotheses, but it is sometimes conflicting and often indirect. An emerging hypothesis suggests that changes in the microbiome, either gut or oral, as a result of periodontal disease pathogens and/or inflammation, may impact inflammatory processes and chronic diseases, including periodontitis and atherosclerosis and, in this way, provide a mechanistic link.18 Studies in this area are too few to date for decisive conclusions, although it is clear that the microbiomes between healthy patients and those with chronic periodontitis have differences.18,19 Figure 1 summarises the prevailing hypotheses as to the connection between periodontal disease and CVD.
Fig. 1.
There are 2 main hypotheses to explain the link between periodontitis and cardiovascular disease. A, Bacteria or their by-products (primarily lipopolysaccharides or antigens) can disseminate from the oral cavity into blood vessels and, there, a host response can lead to damage and atherosclerotic plaque formation. B, Alternatively, localized inflammation from periodontitis enhances ongoing chronic inflammation due to atherosclerosis. Periodontal disease–derived reactive oxygen species contribute to systemic oxidative stress and inflammatory mediators, including various interleukins, chemokines, cytokines (IL-X), immunoglobulins, and inflammatory cells (lymphocytes, macrophages, neutrophils), contribute to a destructive immune response at areas in the vessel wall prone to atherosclerosis.
Since the American Heart Association statement in 2012, many studies, systematic reviews, and meta analyses have been published in this topic area. This concise clinical review aims to address the following questions: Is there a causal link between periodontitis and CVD? After more than 20 years of study, why is this such a difficult question to answer? Where are we today, and what can we expect from future studies?
Current findings and controversies
We conducted a PubMed search of the literature from the past 5 years, using the search terms “periodontal disease and cardiovascular disease and periodontitis and cardiovascular disease.” Nearly 400 articles were uncovered. We did an in-depth evaluation of interventional studies and systematic reviews with meta analyses from the last 3 years (2018-2020, including online ahead of print). Whilst not exhaustive, this article aims to highlight some of the more current findings and controversies as well as future directions.
Major findings
A recent consensus report was published as a result of a joint workshop organised by the European Federation of Periodontology and the World Health Federation.20 This report updated much of the current epidemiological evidence for significant association and mechanistic links between periodontitis and CVD. This provides an excellent in-depth and thorough discussion of the evidence underlying the association between periodontitis and CVD. This concise review is focused on the question of causality and does not seek to replicate the discussion of the evidence for association.
Review of intervention studies from the last 3 years
Nine intervention studies published in the last 3 years (2018-2020) have addressed the topic of causality between periodontitis and CVD.21, 22, 23, 24, 25, 26, 27, 28, 29 The major findings of these studies are summarised in Table 1. Four of these studies used CVD end points, whilst the remainder used surrogate markers for CVD. Of the 4 that used CVD end points, the largest was an investigation using the Korean National Health Insurance System-National Health Screening Cohort, by Park et al.25 This cohort had ∼250,000 patients, with a mean follow-up of 9.5 years, and recorded nearly 15,000 deaths related to CVD.25 The authors found that CVD risk was higher in participants with more tooth loss, caries, or periodontitis (assessed by a dental professional).25 Oral hygiene behaviours (tooth brushing frequency, dental checkups) were associated with decreased CVD risk.25 A limitation in this study, however, was that the latter were self-reported.
Table 1.
Interventional studies.
| Reference | Major findings | Limitations |
|---|---|---|
| Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J 2019;40:3459–70. doi:10.1093/eurheartj/ehz646 | In this interventional study, 101 patients with moderate to severe periodontitis who were also diagnosed with high blood pressure were randomised into 1 of 2 treatment arms: intensive periodontal treatment which included sub- and supragingival scaling plus chlorhexidine or control periodontal treatment, which included supragingival scaling only. The primary outcome measure was mean ambulatory 24-hour systolic blood pressure. At 2 months, there was a significant difference between the groups, with periodontal disease status correlating with improvement in systolic blood pressure. Secondary outcome measures, including diastolic blood pressure, endothelial function, certain cytokines, and immune cells implicated in hypertension were also improved. The authors conclude “A causal relationship between periodontitis and [blood pressure] was observed”… | Blood pressure is a significant risk factor for CVD but is an indirect marker for CVD. |
| Lobo MG, Schmidt MM, Lopes RD, et al. Treating periodontal disease in patients with myocardial infarction: a randomized clinical trial. Eur J Intern Med 2020;71:76–80. doi:10.1016/j.ejim.2019.08.012 | This randomised control trial enrolled patients who had myocardial infarction and were diagnosed with severe periodontal disease (in hospital) between August 2012 and January 2015 into control or periodontal treatment groups (n = 24/group). The primary outcome measure was brachial artery flow-mediated vasodilation and the follow-up period was 6 months. CVD events was a secondary outcome measure. There was a significant difference in primary outcome but not CVD events. | Use of a surrogate marker (brachial artery flow-mediated vasodilation) for CVD outcome. Small group size; heavily male-skewed. |
| Montenegro MM, Ribeiro IWJ, Kampits C, et al. Randomized controlled trial of the effect of periodontal treatment on cardiovascular risk biomarkers in patients with stable coronary artery disease: preliminary findings of 3 months. J Clin Periodontol 2019;46:321–31. doi:10.1111/jcpe.13085 | Single-blind randomised controlled trial of patients with stable coronary artery disease and severe chronic periodontal disease. The test group received supragingival plaque control via calculus removal and “personalized oral hygiene instructions.” They next received “… up to four sessions of subgingival scaling and root planing (SRP) per quadrant, under local anaesthesia, over a maximum period of 14 days. Patients were followed by means of individual periodontal maintenance or recall visits (professional plaque removal and reinforcement of oral hygiene instructions) once monthly during the 3 months of follow‐up” (n = 39). The control group received plaque and calculus removal and “standard oral hygiene instruction” (n = 43). The outcome measure was levels of cardiovascular-related biomarkers. After 3 months, there were significant improvements in periodontal disease parameters but no differences in biomarkers. | 3-month follow-up only. Heavily skewed towards females (74%). |
| Nishi H, Takahashi S, Ohta K, et al. Effects of perioperative oral care on postoperative inflammation following heart valve surgery. Oral Dis 2021;27(6):1542–50. doi:10.1111/odi.13682 | In this study, patients with single valve heart surgery were divided into 2 groups: 111 patients received a cleaning or scaling up to 3 days before and at least twice a week after, and 112 patients received no oral care. The outcome measures were white blood cell count, white blood cell/neutrophil ratio, C-reactive protein levels, and temperature. All parameters decreased after day 1 in both groups, but by a slightly larger degree in the oral care group. The length of hospital stay was unaffected. | Not clear whether the differences are clinically significant. |
| Park SY, Kim SH, Kang SH, et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur Heart J 2019;40:1138–45. doi:10.1093/eurheartj/ehy836 | This study involved 247,696 healthy adults, aged 40 years or older, with a median follow-up of 9.5 years, from the Korean National Health Insurance System-National Health Screening Cohort. Based on medical records, there were 14,893 CVD deaths (myocardial infarction, cardiac death, stroke, and heart failure). CVD risk was higher in participants with more tooth loss, caries, or periodontal disease (assessed by a dental professional). Oral hygiene behaviours (tooth brushing frequency, dental checkups) were associated with decreased CVD risk. | Oral care was self-reported. |
| Pedroso JF, Lotfollahi Z, Albattarni G, et al. Influence of periodontal disease on cardiovascular markers in diabetes mellitus patients. Sci Rep 2019;9:16138. doi:10.1038/s41598-019-52498-7 | Diabetic patients (older than 35 years with type 2 diabetes for more than 5 years) with gingivitis or periodontitis (stage III/IV grade B/C) were enrolled (24/group). The gingivitis group received supragingival scaling and prophylaxis, whilst the periodontitis group received scaling and root planning; both groups received maintenance therapy every 3 months. Periodontal parameters and outcome measures (glycemia, hemoglobin A1c, levels of total cholesterol, HDL and LDL cholesterol, triglycerides, high-sensitivity-C-reactive protein, oxidised LDL) were obtained at baseline and 6 and 12 months posttreatment. High-sensitivity C-reactive protein was found to have improved significantly in the periodontitis group after 12 months. Although there was no change in oxidised LDL, the authors suggest that there was improvement in the quality of the LDL particles. | Biomarkers of CVD are used as outcome measures. |
| Saffi MAL, Rabelo-Silva ER, Polanczyk CA, et al. Periodontal therapy and endothelial function in coronary artery disease: a randomized controlled trial. Oral Dis 2018;24:1349–57. doi:10.1111/odi.12909 | In this trial, 69 patients with stable coronary disease and severe periodontitis were divided into 2 groups. One group received nonsurgical periodontal therapy (oral hygiene instructions, supragingival calculus removal, subgingival scaling, and root planning [up to 4 sessions/quadrant] and monthly maintenance, n = 31), whilst the control group received supragingival plaque and calculus removal and oral hygiene instruction at the start of the study (n = 38). The outcome measures were endothelial function (brachial artery flow-mediated dilation) and concentrations of endothelial inflammatory proteins in serum at baseline and 3 months after therapy. There was no significant improvement in any of the parameters in the group receiving therapy when compared to baseline. CVD outcome measures worsened in the control group, leading to significant difference between the groups. | Biomarkers of CVD are used as outcome measures. 3 month only follow-up. Almost identical to Montenegro et al. |
| Santos-Paul MA, Neves RS, Gowdak LHW, et al. Cardiovascular risk reduction with periodontal treatment in patients on the waiting list for renal transplantation. Clin Transplant 2019;33:e13658. doi:10.1111/ctr.13658 | In this study, 206 patients undergoing dialysis and on the waiting list for kidney transplant received periodontal disease treatment (74% had moderate to severe periodontal disease) and were compared with 203 historical controls who did not receive periodontal disease treatment. Patients were followed for 24 months or until death or transplant. The authors found that severity of periodontal disease was correlated with coronary artery disease severity. Cardiovascular events, coronary events, and cardiovascular death were significantly reduced in the treatment group. | Periodontal disease status of the controls is not known. |
| Seinost G, Horina A, Arefnia B, et al. Periodontal treatment and vascular inflammation in patients with advanced peripheral arterial disease: a randomized controlled trial. Atherosclerosis 2020;313:60–9. doi:10.1016/j.atherosclerosis.2020.09.019 | In this randomised controlled study, patients with peripheral arterial disease and severe periodontitis were placed in one of 3 groups (n = 30/group): control (no treatment), nonsurgical periodontal therapy plus antibiotics, or nonsurgical periodontal therapy without antibiotics. The primary CVD outcome measure was vascular inflammation; secondary CVD outcome measures were changes in vascular biomarkers and cardiovascular events. Although there were significant improvements in periodontal disease parameters in the treated groups, there were no significant differences in primary or secondary CVD outcomes. | 76% of study participants received statin therapy. |
CVD, cardiovascular disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
A study with a particularly vulnerable cohort, patients receiving dialysis and on a kidney transplant waiting list, showed that periodontal disease treatment significantly reduced cardiovascular events, coronary events, and cardiovascular death (24-month follow-up; 206 patients).28 In this otherwise compelling study, a limitation was that the comparison was to historical controls (thus, their periodontal disease status is unknown).28 In the third study that used CVD end points, patients had peripheral arterial disease and severe periodontitis and were treated with scaling and root planing, with or without antibiotic treatment (n = 30/group).29 Compared to the control group, there were no changes in CVD outcomes 3 months posttherapy despite significant improvements in oral health parameters.29
Finally, in the study by Lobo et al., patients hospitalised for myocardial infarction were assessed for periodontal disease.22 Those with severe periodontitis (4 mm or greater clinical attachment loss, probing depth 6 mm or greater in 5 or more teeth, gingival bleeding in 8 teeth or more) were allocated into control or treatment groups.22 Treatment was intensive: teeth were restored, restorations were repaired if necessary, and root tips removed. Supragingival calculus was removed, followed by up to 4 sessions of scaling and root planing. There were follow-up sessions at 3 and 6 months. At 6 months, there was also assessment of primary and secondary outcome measures. There was a significant difference in brachial artery flow-mediated dilation, the primary outcome measure and an indicator of endothelial cell dysfunction, as a result of treatment, but there was no difference in CVD events (myocardial infarction, stent thrombosis, urgent revascularisation, death).22 A limitation to this study was the small group size (n = 24/group); the calculated group size necessary for 80% power was 44 per group, but the investigators could not reach that level. Based on the smaller group size, the calculated power is 67%.22 The P value was lowered to .25 for significance as a “penalty.”22
In the 5 studies using surrogate markers of CVD as outcome measures, one of the best was a study analysing blood pressure. Czesnikiewicz-Guzik et al. enrolled 101 patients with high blood pressure and moderate to severe periodontitis and divided them into a treatment group (sub- and supragingival scaling plus chlorhexidine) and control group (supragingival scaling only).21 At 2 months, there was a significant difference in 24-hour ambulatory systolic blood pressure (primary outcome measure) in the patients in the treatment group.21 Secondary outcome measures, including diastolic blood pressure, endothelial function, certain cytokines, and immune cells implicated in hypertension were also improved.21 Hypertension is a key risk factor for CVD.
Saffi et al. used brachial artery flow-mediated dilation as an outcome measure in a study of 69 patients with stable coronary artery disease and severe periodontitis (patients with 10 or more teeth, clinal attachment loss of 6 mm or more and probe depth of 5 mm or more in 2 or more nonadjacent teeth).27 The 38 patients in the control group received supragingival plaque and calculus removal and oral hygiene instruction at the start of the study, whilst the 31 patients in the treatment group received oral hygiene instructions, supragingival calculus removal, subgingival scaling and root planing (up to 4 sessions/quadrant), and monthly maintenance.27 There were no significant improvements in any of the primary or secondary outcome measures in the treatment group, but because outcomes worsened in the control group, there were differences between the groups.27 Using an identical study protocol, Montenegro et al. divided patients with stable coronary artery disease and severe periodontitis into 2 groups as outlined above (n = 43 and n = 39 for control and treatment groups, respectively).23 Outcome measures were biomarkers of CVD (C-reactive protein, glycated haemoglobin, and plasma levels of lipids and various cytokines).23 Simiar to what was reported by Saffi et al., there were no improvements in any of the biomarkers as a result of treatment.23
In a study by Nishi et al., 223 patients undergoing heart valve surgery were placed into an oral care or control group.24 The oral care group received a cleaning or scaling up to 3 days before surgery.24 The outcome parameters measured were white blood cell count, white blood cell/neutrophil ratio, C-reactive protein levels, and temperature.24 All outcomes decreased in both groups after postoperative day 1, and there were small but significant differences between the groups for outcomes on most days. However, this did not alter length of hospitalisation stay; thus, the clinical relevance of these differences is not clear.24
Pedroso et al., enrolled patients with type 2 diabetes (older than 35 years and diagnosed for at least 5 years) with gingivitis or periodontitis (stage III/IV grade B/C) into treatment groups (n = 24/group).26 The gingivitis group received supragingival scaling and prophylaxis, whilst the periodontitis group received scaling and root planing; both groups received maintenance therapy every 3 months.26 Outcome measures (glycaemia; haemoglobin A1c; total, high-density lipoprotein [HDL], and low-density lipoprotein [LDL] cholesterol; triglycerides; high-sensitivity C-reactive protein [hsCRP], oxidised LDL) were assessed at baseline and 6 and 12 months.26 At 12 months, hsCRP was found to have improved significantly in the periodontitis group.26 Although there was no change in levels of oxidised LDL, the authors showed there was improvement in the quality of the LDL particles.26
As is clear from this discussion, there is great variability in the types of intervention studies undertaken (patient pool, periodontal treatment) and the outcomes measured, which makes it difficult to formulate broad conclusions. Intensive periodontal treatment seems to reduce CVD event outcomes, or surrogates, in certain patient pools, but why in others there is no improvement remains unknown. One would expect a number of parameters to improve in concert if there is a change in CVD risk, but in many studies, this is not the case. Thus, the clinical relevance of changes in CVD surrogates to actual CVD outcomes remains unresolved.
Challenges for establishing causality
There is general agreement that, to date, there have been few high-quality studies with CVD end points; most studies use surrogate markers or biomarkers that are assumed to reflect CVD end points. The main obstacle to high-quality, double-blind, randomised controlled trials with CVD end points is ethical; nontreatment is not an option. A conclusion of many researchers who have conducted systematic reviews is that the establishment of a large-scale, well-annotated longitudinal database that considers, in detail, oral health parameters would provide a mechanism around this and be a major step forwards towards understanding whether the relationship between periodontitis and CVD is causal.30
The use of surrogate markers or biomarkers of CVD end points
The Heart & Stroke Foundation estimates that there are about 35,000 heart attacks and 62,000 strokes each year in Canada.31 This is approximately 3 CVD events/1000 people. Given this relative rarity of CVD events, the reason most clinical trials use surrogate markers for CVD outcomes is that to measure actual CVD events, the length of time of the study must be quite long (unless it is a particularly vulnerable population) and the population quite large to achieve appropriate power for statistical analyses.
There is a wide variety of surrogates/biomarkers used in the place of CVD end points, as demonstrated by the description of the intervention trials from the last 3 years. These are sometimes indicators of cardiac damage (troponin I or T, brain natriuretic peptide, and N-terminal pro brain natriuretic peptide), blood clots (fibrin D-dimers, tissue plasminogen activator antigen), and endothelial dysfunction (brachial artery flow-mediated dilation, von Willebrand factor). Others are associated with inflammation (C-reactive protein or hsCRP, various cytokines). Some biomarkers are risk factors for CVD (blood pressure; lipid profiles, including total, very-low-density lipoprotein, LDL, and HDL cholesterol; triglyceride levels; and fasting glucose and haemoglobin A1c levels for diabetes). Oxidised or modified forms of LDL, thiobarbituric acid reactive substances, and malondialdehyde are also used as biomarkers because these are indicators of oxidative stress.
The validity of a surrogate to predict CVD end points is extremely important, as is the degree by which a change in a surrogate reflects therapeutic efficacy on CVD end points. It is generally thought that the most predictive surrogates are directly involved in causality.32 There are multiple potential issues with the use of surrogates to determine causality in the case of periodontitis and CVD, with the greatest issue being the predictive value of a given surrogate.32 Thus, although an intervention may “improve” a biomarker associated with CVD, for many of these, the level of improvement, if any, that is clinically relevant to a CVD outcome is unknown. A second issue is the time frame of improvement: for many intervention trials, the follow-up period is quite short. Considering that atherosclerosis takes years to develop, long-term follow-up is essential to understand clinical relevance. A third issue is relative importance. Some studies have multiple surrogate end points; one would think that if there is improvement in CVD risk, there would be similar improvement in all or most surrogates that are considered to be in the same pathway. When only 1 or a subset changes, it is difficult to understand the impact on CVD outcomes. Bikdeli et al. conducted a review of cardiovascular intervention trials that took place from 1990 to 2011 and listed primary surrogate end points.33 They uncovered 59 trials that reported subsequent clinical CVD end points.33 Of these, only 24 validated the surrogate end point.33 Some of these surrogates that did not correlate with CVD end points, such as plasma LDL and cholesterol levels, markers of oxidation and coagulation, blood pressure, and carotid intima-media thickness, are familiar surrogates to those that read the periodontitis-CVD literature.
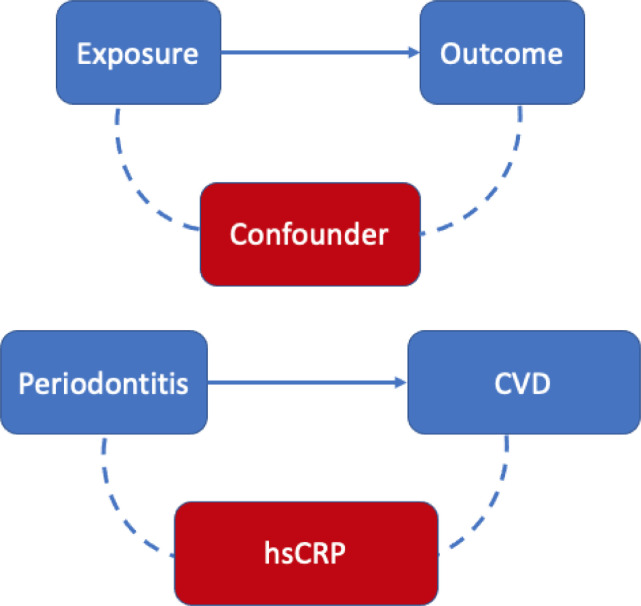
Surrogate markers or biomarkers may also be problematic because they reflect a bias in selection of the pool of patients studied, leading to what is known as collider bias. An excellent analysis by Leite et al. discusses the pitfalls of collider bias and how this may lead to an association between variables, even when one does not exist.34 This cross-sectional human study assessed for the presence of a collider bias in the association between periodontitis and CVD. Collider bias is an unmeasured confounder that may explain the association between the two variables. Figure 2 depicts how a collider bias may influence the association between exposure and outcome. The confounder studied was the inflammatory biomarker, hsCRP. High levels of circulating hsCRP are associated with atherosclerotic plaque formation.35,36 Inflammatory processes and a known genetic condition (APOE-CI-CII gene) can result in high hsCRP levels.37 Data analysis stratified the population by hsCRP levels: individuals with lower hsCRP levels (<3 mg/L) and individuals with higher hsCRP levels (≥3 mg/L). For individuals with lower hsCRP levels, no association was found between periodontitis and CVD (odds ratio [OR], 1.3; 95% confidence interval [CI], 0.8-2.0).34 In contrast, individuals with higher hsCRP levels had a strong association between periodontitis and CVD (OR, 2.2; 95% CI, 1.1-4.2).34 This study suggests that hsCRP distorts the association between periodontitis and CVD. As increased hsCRP may be due to inflammation, genetic conditions, or both, using this surrogate biomarker may be confounding the association of periodontitis and CVD.
Fig. 2.
The unmeasured confounder (eg, high-sensitivity C-reactive protein) may account for the association that may or may not exist between periodontitis and cardiovascular disease. This is known as collider bias.
In summary, care has to be taken in the use and interpretation of surrogate markers. Surrogate biomarkers may not accurately describe changes in CVD outcomes or the impact of periodontitis and therapeutic interventions; reliance on these markers does not bring us closer to determination of causality.
Studies that use CVD outcomes
Even when CVD outcomes are utilised, there are significant issues in interpreting results. Aldossri et al. evaluated publications from 85 studies (1993-2018) “to systematically map clinical heterogeneity and methodological gaps in assessing the relationship between poor oral health and CVD outcomes.”38 No randomised control trials met their inclusion criteria. They found that there were no consistent standards for the definition of oral health parameters or CVD outcomes.38 For example, no studies used the same definition for nonfatal cardiovascular events.38 Similarly, oral health parameters to define periodontal disease differed between studies and included such variables as gingival inflammation, tooth mobility, pocket depth, and bone loss.38 Methodological gaps were found in most studies. The authors concluded, “The identified clinical heterogeneity and methodological gaps interfere with summarising existing evidence and understanding their practical implications.”38
Liu et al. updated a previous systematic review entitled “Periodontal Therapy for Primary or Secondary Prevention of Cardiovascular Disease in People With Periodontitis.”39 The inclusion criteria for CVD outcomes in this review were death and/or cardiovascular events, with a follow-up period of at least 1 year.39 There were 2 studies included since the last update in 2017 (the original review was in 2014). The first had the goal of primary prevention of CVD in patients with both periodontitis and metabolic syndrome, comparing scaling, root planning, and antibiotics to supragingival scaling.40 In this study, there were 4 CVD events (1 with mortality) in the 164 participants, all in the treatment group.40 One patient in the treatment group and 2 in the control group showed progression in their periodontitis.40 The authors used GRADE Working Group grades of evidence and found that there was very low‐certainty evidence of an effect. In the secondary prevention study reviewed, 303 participants were either treated with scaling and root planing or given an explanation of their oral examination with instructions to see a dentist (“community care”).41 One-year follow-up included only 37 patients; thus, the authors concluded that there was high risk of attrition bias and that the data collected were not useful for analysis.41 Overall, Liu et al. found no reliable evidence regarding primary or secondary prevention of CVD in people with diagnosed chronic periodontitis.39
Recent progress in determining causality
Table 2 summarises the 18 systematic reviews and meta-analyses uncovered in our search. There have been 2 systematic reviews and meta-analyses focused on tooth loss (as a surrogate marker for periodontitis) and CVD outcomes, and they are of note for their lack of agreement. Peng et al. investigated the association between tooth loss and all-cause mortality, including a dose-response analysis from studies published between 2003 and 2018 (15 prospective studies, 19,577 cases/306,807 total participants), and included subgroup analyses for CVD (7 cohort studies, 1526 cases/125,716 total participants) and coronary heart disease (CHD) (5 cohort studies, 1899 cases/46,130 total participants).42 Although they found an association and linearity in dose-response for every 10 lost teeth for all-cause mortality, subgroup analyses failed to show significance for CVD or CHD.42 Very high heterogeneity was noted. Romandini et al. investigated periodontitis, edentulism, and all-cause mortality in a review and meta-analysis that included 57 studies (48 cohorts) involving >5.7 million total participants from studies published between 1993 and 2019.43 They found a significant association between periodontitis or edentulism with all-cause mortality, CVD, CHD, and cerebrovascular diseases.43 Interestingly, the association was not influenced by severity of periodontitis, but by age, with the association more evident in patients younger than 65.43
Table 2.
Systematic reviews.
| Reference | Main findings | Limitations |
|---|---|---|
| Almeida A, Fagundes NCF, Maia LC, et al. Is there an association between periodontitis and atherosclerosis in adults? A systematic review. Curr Vasc Pharmacol 2018;16:569–82. doi:10.2174/1570161115666170830141852 | A systematic review of 7 literature databases (to January 2017) uncovered 2138 studies; 4 were included in the review.The authors concluded that an association exists and is due to an increase in inflammatory mediators, specifically C-reactive protein and interleukin 6. |
Based on few studies. Surrogate markers for end points. |
| Cheng F, Zhang M, Wang Q, et al. Tooth loss and risk of cardiovascular disease and stroke: a dose-response meta-analysis of prospective cohort studies. PLoS One 2018;13:e0194563. doi:10.1371/journal.pone.0194563 | Meta-analysis included 17 cohort studies (through March 2017) with 879,084 participants and 43,750 cases. 28 reported on tooth loss and CVD; 8 reported on tooth loss and stroke. Tooth loss was associated with a significant risk of CVD and stroke. For every 2 teeth lost, coronary heart disease risk increased by 3% and stroke risk increased by 3%. |
Tooth loss is an indirect surrogate marker for periodontitis. Included studies differed with respect to CVD outcomes: heart failure, myocardial infarction, coronary heart disease, ischemic heart disease. Outcomes were self-reported. Different conclusion compared to Peng et al. |
| D'Isidoro O, Perrotti V, Hui WL, et al. The impact of non-surgical therapy of periodontal disease on surrogate markers for cardiovascular disease: A literature review. Am J Dent 2019;32:191-200. | 28 articles reviewed (through December 2018). The authors conclude: “The initial phase of periodontal therapy has a positive impact on the short-term reduction of a series of systemic markers that are considered as surrogate markers AVD” (atherosclerotic vascular disease). | Surrogate markers of CVD are used as outcome measures. Meta-analysis could not be done due to heterogeneity of the studies. Great variability in follow-up period (none-18 years). |
| Fiorillo L, Cervino G, Laino L, et al. Porphyromonas gingivalis, periodontal and systemic implications: a systematic Review. Dent J 2019;7. doi:10.3390/dj7040114 | 21 studies included from 2009-2019. Cardiology outcome measures: lipoprotein concentration, endothelial permeability, lipoprotein binding in the intima, CVD, CHD, chronic infection of the heart, atherosclerosis and atherosclerosis risk factors, cytokines. The authors conclude that “P. gingivalis has implications in the onset of different systemic pathologies, including rheumatoid arthritis, cardiovascular pathologies, and neurodegenerative pathologies,” after synthesis of the articles. |
Porphyromonas gingivalis is used as a biomarker of periodontal disease. Of the included studies, 14 had moderate and 4 had high risk of bias. Cardiology end points were very variable. |
| Froum SJ, Hengjeerajaras P, Liu KY, et al. The link between periodontitis/peri-implantitis and cardiovascular disease: a systematic literature review. Int J Periodontics Restorative Dent 2020;40:e229-e33. doi:10.11607/prd.4591 | 51 articles from 1990 to 2020 included. The authors conclude that infection and inflammatory pathways can explain a link between periodontal disease and CVD, but because periodontal disease interventional studies have shown conflicting results on CVD, cause and effect cannot be ascribed. Too few studies on peri-implantitis to draw conclusions. |
Outcome measurements concerning CVD and characterisation of periodontitis were very varied in the different studies. |
| Joshi C, Bapat R, Anderson W, et al. Detection of periodontal microorganisms in coronary atheromatous plaque specimens of myocardial infarction patients: A systematic review and meta-analysis. Trends Cardiovasc Med 2021;31:69-82. doi:10.1016/j.tcm.2019.12.005 | 14 studies investigating the presence of periodontal disease pathogens in plaque; 12/14 reported the presence of bacteria. Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans were the most frequently reported. | Mostly small studies and male-skewed. Few controls and high level of heterogeneity. DNA was also found in areas of the vasculature that are resistant to atherosclerosis, raising the question of causality. |
| Kaschwich M, Behrendt CA, Heydecke G, et al. The association of periodontitis and peripheral arterial occlusive disease-a systematic review. Int J Mol Sci 2019;20:2936. doi:10.3390/ijms20122936 | 17 studies included (through 12/2018). Both longitudinal studies and case-controlled studies reviewed. All studies identified an association between periodontal disease and peripheral arterial occlusive disease. 3 causative mechanisms leading to vessel damage highlighted: (1) periodontal pathogen colonisation of the vessel, (2) systemic inflammation, and (3) autoimmunity vs host protein(s) as a result of infection with the oral periodontal disease pathogen, which may combine with systemic inflammation (2). |
Some studies used tooth loss as a biomarker of periodontal disease. Assessment for CVD varied and in some cases was self-reported. |
| Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J. Risk of incident cardiovascular disease in people with periodontal disease: a systematic review and meta-analysis. Clin Exp Dent Res 2021;7:109-22. doi:10.1002/cre2.336 | Review of study results published between 1996 and October 2019. Included 32 longitudinal studies; 30 eligible for meta-analyses. Found a small but consistent increased risk of CVD associated with periodontal disease. Highest evidence was for stroke, followed by coronary heart disease. Evidence for myocardial infarction was not significant. Men and those with severe periodontal disease were at the highest risk for increased risk of CVD. |
20 studies only investigated one sex (14 male, 6 female). In some studies, periodontal disease/CVD was self-reported. In 1 study, the outcome measure was hypertension. 21 studies were judged critical and 11 serious in terms of risk of bias as per ROBINS-I (evaluates the risk of bias in results reported for 2 or more interventions in nonrandomised studies). "Critical" suggests that the study is too problematic to draw any conclusions about the intervention. |
| Lavigne SE, Forrest JL. An umbrella review of systematic reviews examining the relationship between type 2 diabetes and periodontitis: position paper from the Canadian Dental Hygienists Association. Can J Dent Hyg 2021;55:57-67. | Asked the question: “For adults in good general health who are diagnosed with periodontal disease, will receiving non-surgical periodontal therapy (NSPT), as compared to not receiving NSPT, lower their risk for cardiovascular diseases?” Included published studies between 2007 and 2019. 7 studies reviewed, 6 with meta-analyses. The 1 study using cardiovascular events as an outcome measure was rated very low-quality due to high risk of bias as a result of deviations from the study protocol and lack of follow-up. The study provided “insufficient evidence to either support or refute whether NSPT (nonsurgical periodontal therapy) could prevent the recurrence of [cardiovascular] event.” “Biological plausibility” of an association has been shown by demonstration of increased levels of systemic cytokines and mechanistic studies based on this. Overall conclusion of this umbrella review was that no causal relationship between periodontal disease and CVD can be confirmed. |
Only 1 study used cardiovascular events as an outcome measure; 6/7 studies used surrogate markers. 2 studies were of low quality. |
| Liu W, Cao Y, Dong L, et al. Periodontal therapy for primary or secondary prevention of cardiovascular disease in people with periodontitis. Cochrane Database Syst Rev 2019;12:CD009197. doi:10.1002/14651858.CD009197.pub4 | 2014 review updated in 2017 and now 2019. 2 new randomised control trials included. Inclusion criteria for CVD outcome were death and/or cardiovascular events, with a follow-up of at least 1 year. Overall conclusion: “For primary prevention of cardiovascular disease (CVD) in people diagnosed with periodontitis and metabolic syndrome, very low‐certainty evidence was inconclusive about the effects of scaling and root planning plus antibiotics compared to supragingival scaling. There is no reliable evidence available regarding secondary prevention of CVD in people diagnosed with chronic periodontitis and CVD.” |
The primary prevention study showed low-certainty evidence that was inconclusive; only 1 death was recorded in the follow-up period. The secondary prevention study had too few patients with sufficient follow-up and did not report deaths. |
| Muñoz Aguilera E, Suvan J, Buti J, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res 2020;116:28-39. doi:10.1093/cvr/cvz201 | Reviewed 81 studies published up to December 2018; 40 were included in a meta-analysis. Moderate-severe and severe periodontal disease were associated with hypertension, and prospective studies showed that periodontitis increased the likelihood of a hypertension diagnosis later. Periodontitis was associated with increases in both systolic and diastolic blood pressure. 3 studies researched potential mechanisms but only 1 showed differences with the hypertensive group. In that study, serum neutrophil enzymes (elastase and matrix metalloproteinases 8 & 9) were increased in periodontitis patients with hypertension, compared to hypertensive patients and healthy patients. |
Interventional studies were inconclusive. (5 showed a decrease in blood pressure following periodontal therapy, whilst 6 showed no change. 1 study showed an increase in blood pressure 1 day after periodontal therapy.) |
| Natto ZS, Hameedaldain A. methodological quality assessment of meta-analyses and systematic reviews of the relationship between periodontal and systemic diseases. J Evid Based Dent Pract 2019;19:131-9. doi:10.1016/j.jebdp.2018.12.003 | Assessment of 42 systematic reviews using 2 quality tools: Overview Quality Assessment Questionnaire and A Measurement Tool to Assess Systematic Reviews. | Outcome measures included a mix of conditions: diabetes, obesity, and CVD. |
| Peng J, Song J, Han J, et al. The relationship between tooth loss and mortality from all causes, cardiovascular diseases, and coronary heart disease in the general population: systematic review and dose-response meta-analysis of prospective cohort studies. Biosci Rep 2019;39:BSR20181773. doi:10.1042/BSR20181773 | 18 prospective studies from 1966-2018. Found a significant association between tooth loss and all-cause mortality, but not CVD/CHD. Dose-response analysis on 15 studies: 19,577 cases out of 306,807 participants. However, subgroup and sensitivity analyses showed inconsistencies. |
Tooth loss is an indirect surrogate marker for periodontitis. Included studies differed with respect to CVD outcomes: heart failure, myocardial infarction, coronary heart disease, ischemic heart disease. Outcomes were self-reported. Different conclusion from Cheng et al. |
| Roca-Millan E, González-Navarro B, Sabater-Recolons MM, et al. Periodontal treatment on patients with cardiovascular disease: systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal 2018;23:e681-90. doi:10.4317/medoral.22725 | Review and meta-analysis of 10 clinical trials up to 2017. The authors found a statistically significant decrease in C-reactive protein and white blood cell levels in patients who had nonsurgical periodontal treatment in comparison to controls receiving no treatment. | Most outcomes were biomarkers of CVD. Some studies had no follow-up period or periods as short as 1 month. Longest follow-up period was 12 months. Small number of participants in many studies. Heterogeneity in diagnosis of periodontal disease and CVD. 2 studies included patients with underlying systemic disease. In one study, antibiotics were used. |
| Romandini M, Baima G, Antonoglou G, et al. Periodontitis, edentulism, and risk of mortality: a systematic review with meta-analyses. J Dent Res 2021;100:37-49. doi:10.1177/0022034520952401 | Review of 57 studies including 5.7 million people. 10 had follow-up periods of more than 10 years. No included study had a high risk of bias. The meta-analyses showed that periodontitis/edentulism was associated with all-cause mortality and mortality from CVD, cancer, coronary heart disease, and cerebrovascular disease. Edentulism was also associated with mortality from pneumonia. Sensitivity analyses utilising only the highest-quality studies showed increased relative-risk levels. Analysis showed that periodontitis increased risk of all-cause and CVD mortality. The association was not affected by severity of periodontitis, but by age: the association was more evident in patients younger than 65. |
In 12 studies, edentulism was self-reported. In 30 studies, the control group for edentulism was the presence of at least 1 tooth. Cause of tooth loss may not be periodontal disease. Relevant potential effect modifiers were not analysed. |
| Seitz MW, Listl S, Bartols A, et al. Current knowledge on correlations between highly prevalent dental conditions and chronic diseases: an umbrella review. Prev Chronic Dis 2019;16:E132. doi:10.5888/pcd16.180641 | Umbrella systematic review focused on 10 chronic diseases with the highest burden in Germany and 3 most prevalent oral conditions. Of 32 studies included between 1995 and 2017, only 4 were rated as high-quality. Periodontitis was most often correlated with 1 chronic disease, whilst type 2 diabetes was the chronic disease most often correlated with a dental condition. Periodontitis and CVD was the most next frequently reported association. |
2 included studies were low-quality. The strength of evidence was limited. The evidence to assess causality of these disease correlations remains unclear. |
| Taylor HL, Rahurkar S, Treat TJ, et al. Does nonsurgical periodontal treatment improve systemic health? J Dent Res 100:253-60. doi:10.1177/0022034520965958 | A review of 52 systematic reviews on the effect of nonsurgical periodontal disease treatment on disease outcomes: diabetes (21 studies), adverse birth outcomes (15 studies), cardiovascular disease (8 studies), obesity (3 studies), rheumatoid arthritis (3 studies), and chronic kidney disease (2 studies). Most studies were found to use surrogate end points or biomarkers instead of disease end points. Using A MeaSurement Tool to Assess systematic Reviews (AMSTAR)-2, 92% of studies were rated low or critically low in terms of confidence levels due to nonregistration of the study protocol, lack of justification for study exclusion, risk of bias as a result of study inclusion, and inappropriate meta-analysis techniques. The authors concluded: “There is a dearth of robust evidence on whether nonsurgical periodontal treatment improves systemic disease outcomes.” |
Included studies showed moderate heterogeneity; 2 were of low quality. |
| Voinescu I, Petre A, Burlibasa M, et al. Evidence of connections between periodontitis and ischemic cardiac disease-an updated systematic review. Maedica 2019;14:384-90. doi:10.26574/maedica.2019.14.4.384 | This systematic review included 17 studies in English and French from 2014 to 2019. The authors note that “a significant relationship between the periodontal and cardiovascular pathology in 17 selected studies was observed,” but do not critically assess the studies in detail. |
A “huge methodological heterogeneity” amongst the studies is noted by the authors. No randomised control studies were included. Only 40% of included studies had a control group. Cardiac diagnosis was heterogenous. In some studies, periodontal disease was self-reported. Sex and age data were not always stated in all studies. Only 2 studies gave race-related information. Diseases included varied widely amongst reviewed studies. |
CHD, coronary heart disease; CVD, cardiovascular disease.
Larvin et al. conducted a systematic review of randomised controlled trials and longitudinal cohort studies. Thirty-two studies (30 with meta-analyses) were included that spanned publication years 1996 to 2019.44 They uncovered a small but consistent increased risk of CVD.44 The highest subcategory risk was for stroke, followed by CHD.44 Men with severe periodontitis showed the highest risk of CVD.44
Plausible mechanisms in terms of causality
In the discussion of causality, the mechanisms with the most evidentiary support are increased inflammation, increased oxidative stress, and bacteremia, resulting in dissemination of oral bacteria to the aorta and other sites.4,5 Joshi et al. published a systematic review and meta-analysis investigating the detection of periodontal microorganisms in atherosclerosis plaque from patients following myocardial infarction.45 In 12 of 14 studies included, the investigators reported the presence of bacterial DNA in plaque samples.45 An interesting note, however, is that they also reported recovery of bacterial DNA in areas of the vasculature that are resistant to atherosclerosis, furthering the controversy over causality.45 In a recent study by Gode et al., fewer than 5% of patients who underwent surgery for atherosclerotic vascular disease (96 in total) had DNA from periodontitis pathogens in isolated tissue; this was not significantly different from tissue from patients who underwent surgery for rheumatic heart disease (85 in total).46
Munoz Aguilera et al. conducted a systematic review and meta-analysis of 40 studies investigating periodontitis and hypertension.47 Hypertension is a major risk factor for CVD and could be an important causal link. Increased systemic or local vascular inflammation (via bacteremia) and/or interaction with the sympathetic nervous system have been proposed as mechanistic connections between the 2 diseases.47 Increased reactive oxygen species generation (oxidative stress), leading to vascular dysfunction, would precipitate hypertension in the context of “classical pro-hypertensive mechanisms” including a salt-rich diet, obesity, and stress.47 Their analysis showed that moderate and severe periodontitis were both associated significantly with hypertension.47 They also noted that in 5 of 12 studies, blood pressure declined after periodontal disease therapy.47
Two umbrella systematic reviews approached the question of causality from a different perspective, by investigating the effect of nonsurgical periodontal disease treatment on health, including heart health. Taylor et al. found that most studies did not use disease end points, but rather surrogates.30 Using A Measurement Tool to Assess Systematic Reviews (version 2), they found that systematic reviews and meta analyses in the field did not meet inclusion criteria.48 They concluded that “there is a dearth of robust evidence on whether nonsurgical periodontal treatment improves systemic disease outcomes.”30 Similarly, Lavigne et al., in a position paper from the Canadian Dental Hygienists Association, asked the question: “For adults in good general health who are diagnosed with periodontal disease (Population), will receiving non-surgical periodontal therapy (NSPT) (Intervention), as compared to not receiving NSPT (Comparison group), lower their risk for cardiovascular diseases? (Outcome).”49 Based on their analysis, they failed to find a causal relationship between periodontal disease treatment and risk for CVD.49
Emerging topics and future directions
Additional new areas of research in establishing causality are being driven by emerging technology. For example, the impact of the oral and systemic microbiome, and indeed the establishment of such microbiomes and the critical factors involved, are all exciting frontiers. Understanding not only which microorganisms are present but also how they interact and synergise with one another and to processes in our body, to perhaps gain different characteristics that may contribute to pathology, are creating new paradigms and points of therapeutic intervention in CVD and periodontal disease.50, 51, 52, 53, 54 With the application of single-cell RNA-sequencing to interrogate the transcriptome, the impact of the immune system to periodontal disease pathogens and the mystery of susceptibility may be unraveled, resulting in a greater understanding of the intersecting pathologies.53, 54, 55, 56, 57 This can result in tailored immunotherapies or other precision medicine–type approaches.58
At present, the assumption of many intervention trials is that current periodontal disease therapies could lower or reverse risk of CVD. This may be much more difficult than presumed. An emerging topic that we will discuss in detail poses the following dilemma: What if the mechanism of disease alters properties of stem cells, which persist even after improvements in oral health?
Perhaps a significant but overlooked development in this area is the discovery of a new risk factor for cardiovascular disease: clonal haematopoiesis (CH, also known as age-related CH).59,60 First recognised in 2014 for the link to CVD, a subset of CH known as clonal haematopoiesis of indeterminate potential (CHIP) has been studied extensively for its association with mutations that may ultimately lead to haematological cancer in the context of other factors.61
Clonal haematopoiesis
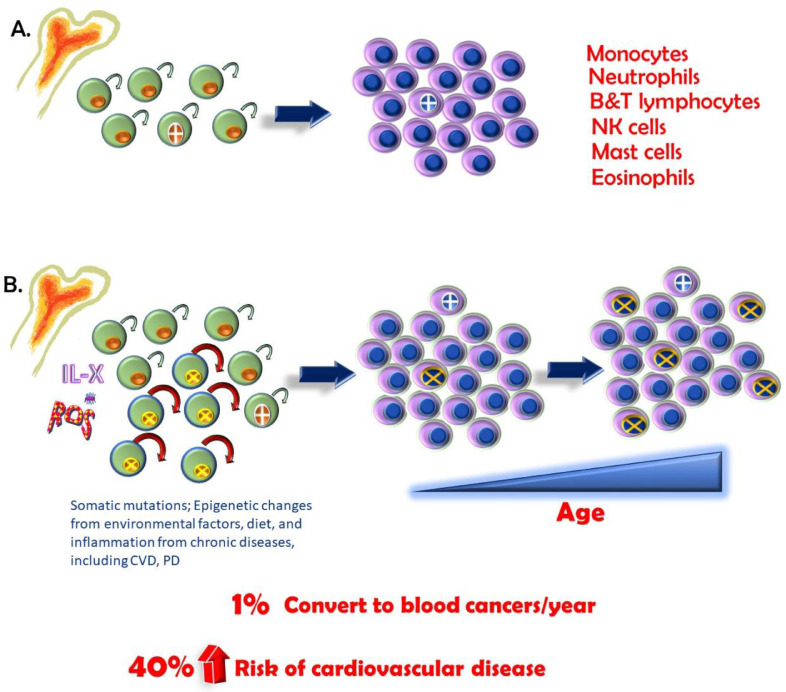
As we age, we acquire somatic (non-germ cell) mutations. High-throughput next-generation sequencing has become increasingly less costly, and “personalised” medicine based on genomics is more attainable. As a result, multiple studies, many related to cancers, reported genomic and exomic (exons; portions of genes that are not translated into protein) sequences of thousands of patients and controls. These revealed that a percentage of blood cells carried mutations that could indicate risk of haematological cancer.59 Because blood cells originate from the bone marrow, this suggested (and it was later confirmed) that a haematopoietic stem cell harbored the mutation and, as a result, led to the clonal expansion of this mutation (see Figure 3).60,61 The initial mutation likely occurred randomly. CH has been recognised to occur with aging since the 1990s. When there is a clonal population of blood cells with a frequency ≥2%, this is defined as CHIP, because of the association with clinical outcomes.62
Fig. 3.
A, Haematopoietic stem cells (HSCs) reside in the bone marrow. They have proliferative and self-renewing properties (arrow). These HSCs give rise to all our differentiated blood cells, including those of the immune system (listed). During our lifetime, mutations arise (cell with cross), but most have no consequences, although rare oncogenic mutations can lead to blood cancers. Their offspring make up a small percentage of total blood cells. B, As we age, we accumulate mutations in our HSCs. These may be due to somatic mutations; epigenetic changes from environmental factors, including diet; and inflammation from chronic diseases, such as cardiovascular disease (CVD) and periodontal disease. Most, again, are inconsequential. However, a mutation that leads to a selective advantage of an HSC may arise (cells with X). This advantage may be increased renewal capacity or increased proliferative capacity. As a result, there is a skewing in the differentiated cell population such that they carry that mutation, and as we age, this becomes more pronounced. Less than 1% per year may result in a blood cancer. More commonly, however, this clonal expansion of differentiated cells carrying the mutation is associated with a 40% increase in risk of CVD.
Interestingly and more recently, bioinformatic interrogation of these data sets showed not only that these mutations were associated with increased risk of haematological cancers but that all-cause death was also increased.63 What was especially striking was the increase in myocardial infarction and stroke (hazard ratio, 2.0 and 2.6, respectively).63 In adults younger than 40, the incidence of CHIP is less than 1%, and this rises to 10% in those older than 65 and to 30% in those older than 70.62,64 However, the incidence of CHIP is much higher in adults with a history of myocardial infarction before age 50 (2%).61 Other studies show that CHIP may also be a disease modifier in valve disease and heart failure.65, 66, 67, 68, 69, 70, 71
Specific genes have been identified, and it is believed that they may increase self-renewal capacity of haematopoietic stem cells or in some other way lead to a growth/survival advantage. The most-often mutated genes are related to epigenetic mechanisms (DNA [cytosine-5]-methyltransferase 3A, Ten-Eleven Translocation methylcytosine dioxygenase 2 [TET2], and ASXL1).65, 66, 67, 68,72 Epigenetics relates to processes that maintain DNA in a conformational state that either promotes or inhibits expression. Although it was expected that CHIP would associate with haematologic cancers, the surprise was the degree of risk associated with CVD and stroke, similar in magnitude to hypertension.73
More recent studies have shown that the association between CHIP and CVD may be causal. Whilst increased blood cell proliferation fits well with the pathogenesis of blood cancers, a different mechanism probably underscores increased CVD. The CHIP mutation in the TET2 gene would render the allele null. Transplant of TET2 heterozygous or null bone marrow into a mouse model of atherosclerosis (the LDL receptor knockout mouse) increased atherosclerosis plaque burden dose-dependently.74 Loss of TET2 increased inflammation in macrophages, a critical cell type in the development of atherosclerosis.74, 75, 76, 77, 78 This increase in inflammation is considered to lead to recruitment of monocytes and other immune cells and drive the increase in lesion burden. In humans, Tet2 gene mutations increase circulating levels of interleukin-6 and tumor necrosis factor (TNF)-alpha.77
Relevant to periodontitis, some studies suggest that inflammation drives CH. Studies in mice show that selection for TET2 mutant haematopoietic stem cells is enhanced by inflammation.30,79,80 Infections may select for haematopoietic stem cells with self-renewal/proliferation advantages, as inflammatory cells are depleted in the periphery as a result of infections.81 In experimental systems, TET2 mutant myeloid cell expansion was driven by microbial infection.79,82 TET2 mutant cells were resistant to death upon lipopolysaccharide exposure and more efficiently repopulated the bone marrow following severe infection. TET2 mutant haematopoietic stem cells were also more proliferative following TNF-alpha exposure.78 Certain myeloproliferative blood cancers have been shown to be driven by inflammation, further suggesting a role.83 If this role is proven, inflammation/infection could provide a potential to-date-unexplored mechanistic link between periodontitis in CH associated CVD. Are strategic therapies for TET2 mutants and CH farfetched? Recent publications suggest not.80,84
Conclusions
Studies continue to support a link between oral health and CVD, but causality remains unproven. Multiple studies, however, show improvements in cardiovascular risk factors following periodontal treatments, albeit, the follow-up periods are mostly short. Reasonable evidence supports the contribution of good oral health to overall and heart health. The safe bet? Continue to advise patients that a healthy mouth supports a healthy heart.
Funding
Funding was provided by the Department of Dentistry, University of Alberta, Fund for Dentistry (MF).
Conflict of interest
None disclosed.
REFERENCES
- 1.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 3.Head T, Daunert S, Goldschmidt-Clermont PJ. The aging risk and atherosclerosis: a fresh look at arterial homeostasis. Front Genet. 2017;8:216. doi: 10.3389/fgene.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eke PI, Thornton-Evans GO, Wei L, et al. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. 2018;149:576–588. doi: 10.1016/j.adaj.2018.04.023. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonetti MS, Jepsen S, Jin L, et al. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. 2017;44:456–462. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- 6.World Heart Federation. What is cardiovascular disease? Available from: https://world-heart-federation.org/what-is-cvd/#:~:text=Cardiovascular%20disease%20(CVD)%20is%20a,vessels%20(veins%20and%20arteries. Accessed 18 September 2021.
- 7.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction. BMJ. 1989;298:779–781. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeStefano F, Anda RF, Kahn HS, et al. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattila KJ, Valtonen VV, Nieminen M, et al. Dental infection and the risk of new coronary events: prospective study of patients with documented coronary artery disease. Clin Infect Dis. 1995;20:588–592. doi: 10.1093/clinids/20.3.588. [DOI] [PubMed] [Google Scholar]
- 11.Beck J, Garcia R, Heiss G, et al. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 12.Joshipura KJ, Rimm EB, Douglass CW, et al. Poor oral health and coronary heart disease. J Dent Res. 1996;75:1631–1636. doi: 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]
- 13.Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. 1999;6:7–11. doi: 10.1177/204748739900600102. [DOI] [PubMed] [Google Scholar]
- 14.Wu T, Trevisan M, Genco RJ, et al. Periodontal disease and risk of cerebrovascular disease: the First National Health and Nutrition Examination Survey and its follow-up study. Arch Intern Med. 2000;160:2749–2755. doi: 10.1001/archinte.160.18.2749. [DOI] [PubMed] [Google Scholar]
- 15.Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 2012;125:2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 16.Hung M, Bounsanga J, Voss MW. Interpretation of correlations in clinical research. Postgrad Med. 2017;129:902–906. doi: 10.1080/00325481.2017.1383820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demmer RT, Desvarieux M. Periodontal infections and cardiovascular disease: the heart of the matter. J Am Dent Assoc. 2006;137(Suppl):14S–20S. doi: 10.14219/jada.archive.2006.0402. [DOI] [PubMed] [Google Scholar]
- 18.Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21:426–440. doi: 10.1038/s41577-020-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Hemme C, Beleno J, et al. Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. ISME J. 2018;12:1210–1224. doi: 10.1038/s41396-017-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanz M, Marco Del Castillo A, Jepsen S, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. 2020;47:268–288. doi: 10.1111/jcpe.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. 2019;40:3459–3470. doi: 10.1093/eurheartj/ehz646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobo MG, Schmidt MM, Lopes RD, et al. Treating periodontal disease in patients with myocardial infarction: A randomized clinical trial. Eur J Intern Med. 2020;71:76–80. doi: 10.1016/j.ejim.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Montenegro MM, Ribeiro IWJ, Kampits C, et al. Randomized controlled trial of the effect of periodontal treatment on cardiovascular risk biomarkers in patients with stable coronary artery disease: preliminary findings of 3 months. J Clin Periodontol. 2019;46:321–331. doi: 10.1111/jcpe.13085. [DOI] [PubMed] [Google Scholar]
- 24.Nishi H, Takahashi S, Ohta K, et al. Effects of perioperative oral care on postoperative inflammation following heart valve surgery. Oral Dis. 2021;27(6):1542–1550. doi: 10.1111/odi.13682. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Kim SH, Kang SH, et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur Heart J. 2019;40:1138–1145. doi: 10.1093/eurheartj/ehy836. [DOI] [PubMed] [Google Scholar]
- 26.Pedroso JF, Lotfollahi Z, Albattarni G, et al. Influence of periodontal disease on cardiovascular markers in diabetes mellitus patients. Sci Rep. 2019;9:16138. doi: 10.1038/s41598-019-52498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saffi MAL, Rabelo-Silva ER, Polanczyk CA, et al. Periodontal therapy and endothelial function in coronary artery disease: a randomized controlled trial. Oral Dis. 2018;24:1349–1357. doi: 10.1111/odi.12909. [DOI] [PubMed] [Google Scholar]
- 28.Santos-Paul MA, Neves RS, Gowdak LHW, et al. Cardiovascular risk reduction with periodontal treatment in patients on the waiting list for renal transplantation. Clin Transplant. 2019;33:e13658. doi: 10.1111/ctr.13658. [DOI] [PubMed] [Google Scholar]
- 29.Seinost G, Horina A, Arefnia B, et al. Periodontal treatment and vascular inflammation in patients with advanced peripheral arterial disease: a randomized controlled trial. Atherosclerosis. 2020;313:60–69. doi: 10.1016/j.atherosclerosis.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Taylor HL, Rahurkar S, Treat TJ, et al. Does nonsurgical periodontal treatment improve systemic health? J Dent Res. 2021;100(3):253–260. doi: 10.1177/0022034520965958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heart and Stroke Foundation of Canada. Saving lives. Available from: https://www.heartandstroke.ca/what-we-do/our-impact/saving-lives#:~:text=Each%20year%2C%20an%20estimated%2035%2C000%20cardiac%20arrests%20occur%20in%20Canada. Accessed 18 September 2021.
- 32.Weintraub WS, Luscher TF, Pocock S. The perils of surrogate endpoints. Eur Heart J. 2015;36:2212–2218. doi: 10.1093/eurheartj/ehv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bikdeli B, Punnanithinont N, Akram Y, et al. Two decades of cardiovascular trials with primary surrogate endpoints: 1990-2011. J Am Heart Assoc. 2017;6:e005285. doi: 10.1161/JAHA.116.005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leite FRM, Nascimento GG, Peres KG, et al. Collider bias in the association of periodontitis and carotid intima-media thickness. Community Dent Oral Epidemiol. 2020;48:264–270. doi: 10.1111/cdoe.12525. [DOI] [PubMed] [Google Scholar]
- 35.Quispe R, Michos ED, Martin SS, et al. High-sensitivity C-reactive protein discordance with atherogenic lipid measures and incidence of atherosclerotic cardiovascular disease in primary prevention: the ARIC Study. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker PM, MacFadyen JG, Glynn RJ, et al. Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: secondary analyses from the Cardiovascular Inflammation Reduction Trial. Eur Heart J. 2020;41:2952–2961. doi: 10.1093/eurheartj/ehaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott P, Chambers JC, Zhang W, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aldossri M, Farmer J, Saarela O, et al. Oral health and cardiovascular disease: mapping clinical heterogeneity and methodological gaps. JDR Clin Trans Res. 2020 doi: 10.1177/2380084420953121. [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Cao Y, Dong L, et al. Periodontal therapy for primary or secondary prevention of cardiovascular disease in people with periodontitis. Cochrane Database Syst Rev. 2019;12 doi: 10.1002/14651858.CD009197.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez NJ, Quintero A, Casanova PA, et al. Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: a controlled clinical trial. J Periodontol. 2012;83:267–278. doi: 10.1902/jop.2011.110227. [DOI] [PubMed] [Google Scholar]
- 41.Offenbacher S, Beck JD, Moss K, et al. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. 2009;80:190–201. doi: 10.1902/jop.2009.080007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng CH, Yang YS, Chan KC, et al. Periodontal treatment and the risks of cardiovascular disease in patients with type 2 diabetes: a retrospective cohort study. Intern Med. 2017;56:1015–1021. doi: 10.2169/internalmedicine.56.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romandini M, Baima G, Antonoglou G, et al. Periodontitis, edentulism, and risk of mortality: a systematic review with meta-analyses. J Dent Res. 2021;100:37–49. doi: 10.1177/0022034520952401. [DOI] [PubMed] [Google Scholar]
- 44.Larvin H, Kang J, Aggarwal VR, et al. Risk of incident cardiovascular disease in people with periodontal disease: a systematic review and meta-analysis. Clin Exp Dent Res. 2021;7:109–122. doi: 10.1002/cre2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joshi C, Bapat R, Anderson W, et al. Detection of periodontal microorganisms in coronary atheromatous plaque specimens of myocardial infarction patients: a systematic review and meta-analysis. Trends Cardiovasc Med. 2021;31:69–82. doi: 10.1016/j.tcm.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Gode S, Sarp TZ, Saribas S, et al. The prevalence of periodontal pathogenic bacteria in atherosclerotic cardiovascular disease. Clin Lab. 2020;66 doi: 10.7754/Clin.Lab.2020.191146. [DOI] [PubMed] [Google Scholar]
- 47.Munoz Aguilera E, Suvan J, Buti J, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. 2020;116:28–39. doi: 10.1093/cvr/cvz201. [DOI] [PubMed] [Google Scholar]
- 48.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavigne SE, Forrest JL. An umbrella review of systematic reviews of the evidence of a causal relationship between periodontal disease and cardiovascular diseases: position paper from the Canadian Dental Hygienists Association. Can J Dent Hyg. 2020;54:32–41. [PMC free article] [PubMed] [Google Scholar]
- 50.Tang WH, Hazen SL. The gut microbiome and its role in cardiovascular diseases. Circulation. 2017;135:1008–1010. doi: 10.1161/CIRCULATIONAHA.116.024251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gencer B, Li XS, Gurmu Y, et al. Gut microbiota-dependent trimethylamine N-oxide and cardiovascular outcomes in patients with prior myocardial infarction: a nested case control study from the PEGASUS-TIMI 54 Trial. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 53.Maekawa S, Onizuka S, Katagiri S, et al. RNA sequencing for ligature induced periodontitis in mice revealed important role of S100A8 and S100A9 for periodontal destruction. Sci Rep. 2019;9:14663. doi: 10.1038/s41598-019-50959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen H, Ye F, Guo G. Revolutionizing immunology with single-cell RNA sequencing. Cell Mol Immunol. 2019;16:242–249. doi: 10.1038/s41423-019-0214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Papalexi E, Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol. 2018;18:35–45. doi: 10.1038/nri.2017.76. [DOI] [PubMed] [Google Scholar]
- 57.Belkina AC, Azer M, Lee JJ, et al. Single-cell analysis of the periodontal immune niche in type 2 diabetes. J Dent Res. 2020;99:855–862. doi: 10.1177/0022034520912188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pei J, Li F, Xie Y, et al. Microbial and metabolomic analysis of gingival crevicular fluid in general chronic periodontitis patients: lessons for a predictive, preventive, and personalized medical approach. EPMA J. 2020;11:197–215. doi: 10.1007/s13167-020-00202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Genovese G, Jaiswal S, Ebert BL, et al. Clonal hematopoiesis and blood-cancer risk. N Engl J Med. 2015;372:1071–1072. doi: 10.1056/NEJMc1500684. [DOI] [PubMed] [Google Scholar]
- 60.Shlush LI. Age-related clonal hematopoiesis. Blood. 2018;131:496–504. doi: 10.1182/blood-2017-07-746453. [DOI] [PubMed] [Google Scholar]
- 61.Burns SS, Kapur R. Putative mechanisms underlying cardiovascular disease associated with clonal hematopoiesis of indeterminate potential. Stem Cell Reports. 2020;15:292–306. doi: 10.1016/j.stemcr.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steensma DP. Clinical implications of clonal hematopoiesis. Mayo Clin Proc. 2018;93:1122–1130. doi: 10.1016/j.mayocp.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calvillo-Arguelles O, Jaiswal S, Shlush LI, et al. Connections between clonal hematopoiesis, cardiovascular disease, and cancer: a review. JAMA Cardiol. 2019;4:380–387. doi: 10.1001/jamacardio.2019.0302. [DOI] [PubMed] [Google Scholar]
- 65.Abplanalp WT, Cremer S, John D, et al. Clonal hematopoiesis-driver DNMT3A mutations alter immune cells in heart failure. Circ Res. 2021;128:216–228. doi: 10.1161/CIRCRESAHA.120.317104. [DOI] [PubMed] [Google Scholar]
- 66.Abplanalp WT, Mas-Peiro S, Cremer S, et al. Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with severe degenerative aortic valve stenosis or chronic post ischemic heart failure. JAMA Cardiol. 2020;5:1–6. doi: 10.1001/jamacardio.2020.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bazeley P, Morales R, Tang WHW. Evidence of clonal hematopoiesis and risk of heart failure. Curr Heart Fail Rep. 2020;17:271–276. doi: 10.1007/s11897-020-00476-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dorsheimer L, Assmus B, Rasper T, et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 2019;;4:25–33. doi: 10.1001/jamacardio.2018.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evans MA, Walsh K. A single-cell analysis of DNMT3A-mediated clonal hematopoiesis in heart failure. Circ Res. 2021;128:229–231. doi: 10.1161/CIRCRESAHA.120.318575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sano S, Wang Y, Yura Y, et al. JAK2 (V617F)-mediated clonal hematopoiesis accelerates pathological remodeling in murine heart failure. JACC Basic Transl Sci. 2019;4:684–697. doi: 10.1016/j.jacbts.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yura Y, Sano S, Walsh K. Clonal hematopoiesis: a new step linking inflammation to heart failure. JACC Basic Transl Sci. 2020;5:196–207. doi: 10.1016/j.jacbts.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zink F, Stacey SN, Norddahl GL, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130:742–752. doi: 10.1182/blood-2017-02-769869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibson CJ, Steensma DP. New insights from studies of clonal hematopoiesis. Clin Cancer Res. 2018;24:4633–4642. doi: 10.1158/1078-0432.CCR-17-3044. [DOI] [PubMed] [Google Scholar]
- 74.Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Sano S, Yura Y, et al. Tet2-mediated clonal hematopoiesis in nonconditioned mice accelerates age-associated cardiac dysfunction. JCI Insight. 2020;5 doi: 10.1172/jci.insight.135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sano S, Oshima K, Wang Y, et al. CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res. 2018;123:335–341. doi: 10.1161/CIRCRESAHA.118.313225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cull AH, Snetsinger B, Buckstein R, et al. Tet2 restrains inflammatory gene expression in macrophages. Exp Hematol. 2017;55:56–70. doi: 10.1016/j.exphem.2017.08.001. e13. [DOI] [PubMed] [Google Scholar]
- 78.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abegunde SO, Buckstein R, Wells RA, et al. An inflammatory environment containing TNFalpha favors Tet2-mutant clonal hematopoiesis. Exp Hematol. 2018;59:60–65. doi: 10.1016/j.exphem.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 80.Cai Z, Kotzin JJ, Ramdas B, et al. Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells mitigates stress-induced abnormalities and clonal hematopoiesis. Cell Stem Cell. 2018;23:833–849. doi: 10.1016/j.stem.2018.10.013. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitroulis I, Kalafati L, Bornhauser M, et al. Regulation of the bone marrow niche by inflammation. Front Immunol. 2020;11:1540. doi: 10.3389/fimmu.2020.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meisel M, Hinterleitner R, Pacis A, et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature. 2018;557:580–584. doi: 10.1038/s41586-018-0125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cook EK, Luo M, Rauh MJ. Clonal hematopoiesis and inflammation: partners in leukemogenesis and comorbidity. Exp Hematol. 2020;83:85–94. doi: 10.1016/j.exphem.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 84.Guan Y, Hasipek M, Tiwari AD, et al. TET-dioxygenase deficiency in oncogenesis and its targeting for tumor-selective therapeutics. Semin Hematol. 2021;58:27–34. doi: 10.1053/j.seminhematol.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]