Abstract
Objective
The intent was to analyse the association of periodontitis with the development of rheumatoid arthritis (RA) using a representative population-based cohort and longitudinal matched-cohort design.
Methods
Participants were 40 years of age or older and had not been diagnosed with RA between 2002 and 2006. Among the participants, those who were newly diagnosed with periodontitis between 2004 and 2006 (excluding cases that had already been diagnosed with periodontitis between 2002 and 2003) were allotted to the periodontitis group. Among the participants, those who had never been diagnosed with periodontitis between 2002 and 2006 formed the control group, matched by sex, age, and household income at a 1:1 ratio. From 2007 to 2018, the 2 groups (n = 691,506) were followed to monitor the development of RA. The t-test and χ2 test compared the general characteristics and health-related variables of both groups. The Kaplan-Meier method with a log-rank test was conducted to compare the incidence of RA in both groups. The hazard ratio (HR) and adjusted hazard ratio (aHR) were calculated using a Cox proportional hazard regression analysis to evaluate the risk of subsequent RA.
Results
Univariate analysis revealed that the periodontitis group was more likely to develop RA than the control group (hazard ratio 1.10), and multivariate analysis also revealed a higher incidence risk of RA (adjusted hazard ratio 1.09) in the periodontitis group.
Conclusions
Our findings demonstrate that periodontitis is associated with an increased risk of developing RA.
Key words: Epidemiology, Matched case-control studies, Periodontitis, Proportional hazard models, Rheumatoid arthritis
Introduction
Periodontitis is an inflammatory disease that destroys hard and soft tissues surrounding the teeth and is associated with bacterial colonies and biofilms.1 Several systemic diseases are also related to the action of inflammatory mediators, and periopathogenic bacteria may be the direct or indirect source of these mediators. In this regard, consistent evidence has suggested that periodontitis may affect systemic diseases.2,3 In a 2017 literature review of the association between periodontitis and systemic disease,4 various systemic diseases such as diabetes, obesity, metabolic syndrome, cognitive impairment, and rheumatoid arthritis (RA) were shown to be associated with periodontitis.
RA is reported to be found in 1% of the world's population,5,6 and chronic inflammation associated with RA causes cartilage damage and joint deformation, adversely affecting the quality of life.7 Its prevalence increases rapidly with age, affecting 2.2% of individuals age 30 and older, and 5.3% of individuals ages 65 and older. Leaving RA untreated may aggravate health and increase medical costs.8
It has been consistently reported that there is an epidemiological link between RA and periodontitis.3,9, 10, 11, 12 In a case-control study,9 a significant correlation between the prevalence of periodontitis and RA was verified, with consideration of confounders, and this correlation was more clearly seen in patients with more severe forms of periodontitis and RA. A study10 analysing previously published literature in relation to the 2 diseases reported that periodontal treatment helped improve the symptoms of patients with RA. However, a number of studies failed to find any correlation between the 2 diseases,13, 14, 15 and a previous study16 reported that tooth loss had a significant effect on RA, however, periodontitis had no significant effect on RA.
The authors conducted preliminary studies using the 6th (2013-2015) Korea National Health and Nutrition Examination Survey (KNHANES) and Korea's National Health Insurance Service–National Sample Cohort (NHIS–NSC) to investigate the epidemiological relationship between RA and periodontitis.11,12 Both studies were cross-sectional in nature and revealed an association between the prevalence of the 2 diseases. There have been a number of longitudinal studies3,17 using population-based databases in Taiwan and the United States, but such studies have not yet been performed in Korea. Therefore, we conducted a longitudinal matched-cohort study to assess the relationship between RA and periodontitis using the nationwide population-based database.
Methods
Participants
This study was conducted using the National Health Insurance Service-Customized Research Database (NHIS-CRDB) of Korea. In Korea, health insurance enrolment is mandated by law, and more than 99% of the population is enrolled in the NHIS. These individuals’ demographic and medical records are stored in the NHIS database. NHIS conducts a health checkup every year or 2, collects health-related behaviour information through a self-reported questionnaire, and acquires physical measurements (height, weight, blood pressure, etc.), blood tests, and urine tests. Information on test results is also included in the NHIS-CRDB, making it valuable research data on health-related topics. We conducted this study with approval from the institutional review board of Shinhan University (IRB No. SHIRB-201904-HR-092-02) and in full accordance with the World Medical Association Declaration of Helsinki. The requirement for written consent was waived by institutional review board because the study used the NHIS database, which contains only anonymised secondary data.
This study used a longitudinal matched-cohort design. Patients 40 years of age or older (as of 2006) who were newly diagnosed with periodontitis between 2004 and 2006 were selected as the periodontitis group, and those who had been diagnosed with periodontitis between 2002 and 2003 were excluded. Patients with periodontitis were defined as those having received periodontitis treatment (U1010; Subgingival curettage, U1051-1052; Periodontal flap surgery, U1060; Root conditioning, U1071-1072; Bone graft for alveolar bone defects, U1081-1083; Guided tissue regeneration) under the corresponding disease codes K052, K053, K054, or K056. For the control group, 1:1 matching with the periodontitis group was performed based on sex, age, and household income among the participants who had no history of periodontitis treatment between 2002 and 2006. For both the periodontitis group and the control group, those who were diagnosed with RA between 2002 and 2006, died before 2018, and did not receive any health examination between 2004 and 2006 were excluded.
Variables
For 12 years, from January 2007 to the last day of 2018, we observed whether RA (diagnostic code: M05, M06, M08) developed in the periodontitis and control groups. As possible confounders that can affect the 2 diseases, information such as sex, age, and household income was collected based on 2006 NHIS records. In addition, smoking status, alcohol consumption, body mass index (BMI), systolic blood pressure, fasting serum glucose level, and total cholesterol were collected from the most recent records of the health examination results between 2004 and 2006. Participants’ household income was classified into quartiles based on the health insurance premiums paid (low, medium-low, medium-high, high). Smoking was classified into ‘No,’ ‘former smoker,’ and ‘Yes.’ Current alcohol consumption status was classified into ‘Yes’ and ‘No,’ and age, systolic blood pressure, fasting serum glucose level, total cholesterol, and BMI were analysed as continuous variables.
Data analysis
A t-test and χ2 test were performed to compare the general characteristics and health-related variables of the periodontitis and control groups. The Kaplan-Meier method with a log-rank test was performed to estimate and compare the cumulative incidence of RA in the control and periodontitis groups. A Cox proportional hazard regression analysis was performed to determine the hazard ratio (HR) of RA based on the presence of periodontitis. Using a multivariate analysis incorporating all variables used in this study, the adjusted hazard ratio (aHR) was calculated. Subgroup analysis was conducted after stratifying the participants by sex. The proportional hazards assumption was evaluated graphically by analysis with log-log plots. All statistical analyses and plotting graphs were performed using the SAS statistical program (version 9.2, SAS Institute), and the statistical significance level was set to 0.05.
Results
The study included a total of 691,506 individuals, with 345,753 patients in the periodontitis group and 345,753 participants in the control groups. There was no difference in sex, age, or household income between the periodontitis and control groups, confirming that the 1:1 matching was appropriately performed (Table 1). The mean age of the study participants was 51.4 ± 8.2 years, and the proportion of males was 56.8%.
Table 1.
Characteristics of the study participants at baseline.
| Characteristic | Periodontitis | Control | P* | |
|---|---|---|---|---|
| Total | 345,753 (50.0) | 345,753 (50.0) | ||
| Sex, N (%) | Male | 196,341 (56.8) | 196,341 (56.8) | 1.000 |
| Female | 149,412 (43.2) | 149,412 (43.2) | ||
| Age, y, mean (SD) | 51.4 (8.2) | 51.4 (8.2) | 1.000 | |
| Household income, N (%) | Low | 52,532 (15.2) | 52,532 (15.2) | 1.000 |
| Medium-low | 53,424 (15.5) | 53,424 (15.5) | ||
| Medium-high | 84,465 (24.4) | 84,465 (24.4) | ||
| High | 155,332 (44.9) | 155,332 (44.9) | ||
| Smoking status, N (%) | No | 228,907 (66.2) | 234,588 (67.9) | <.001 |
| Former smoker | 39,788 (11.5) | 35,083 (10.2) | ||
| Yes | 77,058 (22.3) | 76,082 (22.0) | ||
| Alcohol consumption, N (%) | No | 246,028 (71.2) | 244,879 (70.8) | .002 |
| Yes | 99,725 (28.8) | 100,874 (29.2) | ||
| Body mass index, kg/m2, mean (SD) | 24.1 (3.0) | 24.0 (3.1) | <.001 | |
| Systolic blood pressure, mmHg, mean (SD) | 124.2 (16.3) | 124.8 (16.6) | <.001 | |
| Fasting serum glucose, mg/dL, mean (SD) | 97.7 (27.2) | 96.3 (24.6) | <.001 | |
| Total cholesterol, mg/dL, mean (SD) | 198.8 (44.0) | 198.5 (40.2) | .003 | |
SD = standard deviation.
P values were calculated by a χ2 test or independent t-test.
Examination of the characteristics of participants according to the development of RA (Table 2) revealed that women accounted for 60.6% of those who developed RA, and 39.4% of these were men (P < .001). The mean age of participants with RA was 52.8 ± 8.3 years, which was higher than that of participants without RA (51.1 ± 8.2 years) (P < .001).
Table 2.
Characteristics of the study participants according to occurrence of rheumatoid arthritis over the course of 12 years.
| Characteristic | Occurrence of rheumatoid arthritis |
P* | ||
|---|---|---|---|---|
| Yes | No | |||
| Total | 111,445 (16.1) | 580,061 (83.9) | ||
| Sex, N (%) | Male | 43,930 (39.4) | 348,752 (60.1) | <.001 |
| Female | 67,515 (60.6) | 231,309 (39.9) | ||
| Age, y, mean (SD) | 52.8 (8.3) | 51.1 (8.2) | <.001 | |
| Household income, N (%) | Low | 19,113 (17.2) | 85,951 (14.8) | <.001 |
| Medium-low | 18,406 (16.5) | 88,442 (15.3) | ||
| Medium-high | 27,131 (24.3) | 141,799 (24.5) | ||
| High | 46,795 (42.0) | 263,869 (45.5) | ||
| Smoking status, N (%) | No | 85,514 (76.7) | 377,981 (65.2) | <.001 |
| Former smoker | 9338 (8.4) | 65,533 (11.3) | ||
| Yes | 16,593 (14.9) | 136,547 (23.5) | ||
| Alcohol consumption, N (%) | No | 86,129 (77.3) | 404,778 (69.8) | <.001 |
| Yes | 25,316 (22.7) | 175,283 (30.2) | ||
| Body mass index, kg/m2, mean (SD) | 24.2 (2.9) | 24.0 (3.0) | <.001 | |
| Systolic blood pressure, mm Hg, mean (SD) | 123.8 (16.6) | 124.6 (16.4) | <.001 | |
| Fasting serum glucose, mg/dL, mean (SD) | 96.3 (24.7) | 97.1 (26.1) | <.001 | |
| Total cholesterol, mg/dL, mean (SD) | 199.3 (44.8) | 198.6 (41.6) | <.001 | |
SD = standard deviation.
P values were calculated by a χ2 test or independent t-test.
The 12-year follow-up results of RA development in each group are shown in Table 3. RA developed in 53,332 patients (15.4%) in the control group and 58,113 (16.8%) in the periodontitis group. The 12-year cumulative incidence of RA was calculated to be 1412 per 100,000 persons in the control group and 1546 in the periodontitis group. In other words, the RA incidence rate per 100,000 person-years was higher in the periodontitis group than in the control, corresponding to 128.8 and 117.6, respectively. In addition, the incidence of RA was higher in women than in men in both the control and periodontitis groups.
Table 3.
Incidence rate of rheumatoid arthritis.
| RA cases N (%) | Total person-years | RA incidence rate per 100,000 person-years |
|||
|---|---|---|---|---|---|
| 12-year cumulative incidence | Incidence rate | 95% CI | |||
| Control | |||||
| Total (n = 345,753) | 53,332 (15.4) | 3777,896 | 1412 | 117.6 | 1399.8-1423.6 |
| Male (n = 196,341) | 20,608 (10.5) | 2203,129 | 935 | 77.9 | 922.8-948.2 |
| Female (n = 149,412) | 32,724 (21.9) | 1574,767 | 2078 | 173.2 | 2055.9-2100.4 |
| Periodontitis | |||||
| Total (n = 345,753) | 58,113 (16.8) | 3759,561 | 1546 | 128.8 | 1533.3-1558.3 |
| Male (n = 196,341) | 23,322 (11.9) | 2197,920 | 1061 | 88.4 | 1047.6-1074.7 |
| Female (n = 149,412) | 34,791 (23.3) | 1561,641 | 2228 | 185.7 | 2204.8-2251.1 |
CI = confidence interval; RA = rheumatoid arthritis.
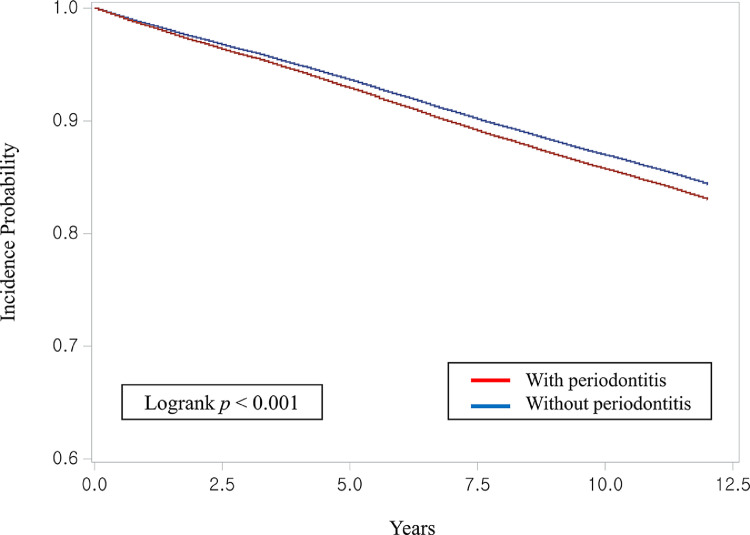
The Cox proportional hazard regression analysis results are shown in Table 4 and the Figure. In the univariate analysis, the periodontitis group was shown to be more likely to develop RA than the control group (HR, 1.10; P < .001), and the multivariate analysis incorporating all variables used in this study also showed that the periodontitis group was at a higher risk of developing RA (aHR, 1.09; P < .001). In the multivariate analysis, women were found to be at a higher risk of developing RA (aHR, 2.09; P < .001), and older individuals were more likely to develop RA (aHR, 1.02; P < .001). The risk of RA was lowest when household income was ‘high’ (aHR, 0.94; P < .001), and the incidence of RA was highest in the case of former smokers (aHR, 1.06; p<0.001). In addition, the risk of developing RA was higher in those that drank alcohol than in nondrinkers (aHR, 1.06; P < .001), and the higher the BMI, the higher the risk of RA (aHR, 1.01; P <.001). Systolic blood pressure, fasting serum glucose level, and total cholesterol levels revealed an aHR close to 1.00. In the subgroup multivariate analysis (Table 5), the incidence of RA was higher in the periodontitis group than in the control group in both men and women, and the aHR value was higher for men than for women (1.13 and 1.07, respectively; P < .001).
Table 4.
Covariates associated with rheumatoid arthritis.
| Characteristic | Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P* | aHR | 95% CI | P* | ||
| Periodontitis, control = 1 | Yes | 1.10 | 1.08-1.11 | <.001 | 1.09 | 1.08-1.11 | <.001 |
| Sex, male = 1 | Female | 2.16 | 2.13-2.19 | <.001 | 2.09 | 2.05-2.12 | <.001 |
| Age, y | 1.02 | 1.02-1.02 | <.001 | 1.02 | 1.02-1.02 | <.001 | |
| Household income, low = 1 | Medium-low | 0.94 | 0.92-0.96 | <.001 | 1.01 | 0.98-1.03 | .655 |
| Medium-high | 0.87 | 0.86-0.89 | <.001 | 0.97 | 0.96-0.99 | .005 | |
| High | 0.81 | 0.80-0.83 | <.001 | 0.94 | 0.92-0.95 | <.001 | |
| Smoking status, no = 1 | Former | 0.65 | 0.64-0.67 | <.001 | 1.06 | 1.03-1.08 | <.001 |
| Yes | 0.56 | 0.55-0.57 | <.001 | 0.93 | 0.91-0.95 | <.001 | |
| Alcohol consumption, no = 1 | Yes | 0.70 | 0.69-0.71 | <.001 | 1.06 | 1.04-1.08 | <.001 |
| Body mass index, kg/m2 | 1.01 | 1.01-1.01 | <.001 | 1.01 | 1.01-1.01 | <.001 | |
| Systolic blood pressure, mm Hg | 1.00 | 1.00-1.00 | <.001 | 1.00 | 1.00-1.00 | <.001 | |
| Fasting serum glucose, mg/dL | 1.00 | 1.00-1.00 | <.001 | 1.00 | 1.00-1.00 | .005 | |
| Total cholesterol, mg/dL | 1.00 | 1.00-1.00 | <.001 | 1.00 | 1.00-1.00 | .012 | |
aHR = adjusted hazard ratio; CI = confidence interval; HR = hazard ratio.
P values were calculated by a Cox proportional hazard regression analysis.
Figure.
Kaplan-Meier curve showing the cumulative incidence of rheumatoid arthritis with and without periodontitis.
Table 5.
HRs (95% CIs) for rheumatoid arthritis by sex.
| Characteristic | Male (n = 392,682) |
Female (n = 298,824) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||
| HR (95% CI) | P* | aHR (95% CI) | P* | HR (95% CI) | P* | aHR (95% CI) | P* | ||
| Periodontitis, control = 1 | Yes | 1.13 (1.11-1.16) | <.001 | 1.13 (1.11-1.15) | <.001 | 1.07 (1.06-1.09) | <.001 | 1.07 (1.06-1.09) | <.001 |
| Age, y | 1.03 (1.03-1.03) | <.001 | 1.03 (1.03-1.03) | <.001 | 1.01 (1.01-1.01) | <.001 | 1.01 (1.01-1.01) | <.001 | |
| Household income, low = 1 | Medium-low | 0.99 (0.96-1.03) | .732 | 1.04 (1.01-1.08) | .023 | 1.01 (0.98-1.03) | .495 | 1.00 (0.98-1.03) | .976 |
| Medium-high | 0.93 (0.90-0.96) | <.001 | 1.01 (0.98-1.04) | .577 | 1.00 (0.97-1.02) | .787 | 0.98 (0.96-1.00) | .095 | |
| High | 0.90 (0.88-0.93) | <.001 | 0.97 (0.94-1.00) | .022 | 0.96 (0.94-0.98) | <.001 | 0.95 (0.93-0.97) | <.001 | |
| Smoking status, no = 1 | Former | 1.04 (1.01-1.07) | .003 | 1.07 (1.05-1.10) | <.001 | 0.98 (0.91-1.06) | .627 | 0.98 (0.91-1.06) | .616 |
| Yes | 0.87 (0.85-0.89) | <.001 | 0.94 (0.92-0.96) | <.001 | 1.12 (1.07-1.18) | <.001 | 1.09 (1.04-1.15) | <.001 | |
| Alcohol consumption, no = 1 | Yes | 1.00 (0.98-1.01) | .595 | 1.04 (1.02-1.06) | <.001 | 1.05 (1.03-1.08) | <.001 | 1.08 (1.05-1.11) | <.001 |
| Body mass index, kg/m2 | 1.01 (1.01-1.01) | <.001 | 1.01 (1.01-1.01) | <.001 | 1.01 (1.01-1.01) | <.001 | 1.01 (1.01-1.01) | <.001 | |
| Systolic blood pressure, mm Hg | 1.00 (1.00-1.00) | .002 | 1.00 (1.00-1.00) | <.001 | 1.00 (1.00-1.00) | .043 | 1.00 (1.00-1.00) | <.001 | |
| Fasting serum glucose, mg/dL | 1.00 (1.00-1.00) | .041 | 1.00 (1.00-1.00) | .123 | 1.00 (1.00-1.00) | .350 | 1.00 (1.00-1.00) | .026 | |
| Total cholesterol, mg/dL | 1.00 (1.00-1.00) | .483 | 1.00 (1.00-1.00) | .896 | 1.00 (1.00-1.00) | <.001 | 1.00 (1.00-1.00) | .864 | |
aHR =adjusted hazard ratio; CI = confidence interval; HR = hazard ratio.
P values were calculated by a Cox proportional hazard regression analysis.
Discussion
We sought to assess the relationship between periodontitis and RA using NHIS-CRDB data of Korean patients between 2002 and 2018. Monitoring RA incidence for 12 years revealed that the incidence of RA was 16.8% in the periodontitis group compared with 15.4% in the control group. In the Cox proportional hazards model, the periodontitis group showed a higher probability of RA development compared to the control group (HR, 1.10), and even when controlling for all the confounders used in this study, the periodontitis group was shown to have a higher risk of developing RA (aHR, 1.09). This was consistent with a previously published cross-sectional study12 in which patients with periodontitis were more likely to have RA (odds ratio 1.07) as compared to those without periodontitis. A longitudinal study3 using the Taiwanese National Health Insurance Research Database on periodontitis and RA development also reported a higher risk of RA in the periodontitis group compared with the group without periodontitis (HR, 1.91). In a study conducted in the United States,17 female patients with a history of periodontitis were more likely to develop RA than those without (relative risk 1.18), but the results were not statistically significant. In contrast, our subgroup analysis revealed that the risk of developing RA was significantly higher in female patients with periodontitis. However, this previous study is different from the present study in terms of its methods. Indeed, the previous study only enrolled women participants, and periodontitis history was determined using periodontal surgery and tooth loss as proxies, which may have led to the discrepancies between the previous study and ours.
As shown, periodontitis can be a risk factor for RA because of the similar aetiology between the 2 diseases.6 RA is regarded as an autoimmune disease, and periodontitis is classified as an inflammatory disease. Although the pathogenesis is not exactly the same, they have a similar aetiology on the cellular and molecular levels including: (i) increased infiltration of immune and inflammatory cells, such as monocytes, T lymphocytes, B lymphocytes, and neutrophils;18 (ii) an imbalance between pro-inflammatory and anti-inflammatory cytokines release;19 and (iii) shared genetic risk markers, such as polymorphic HLA-DRB1 locus or single nuclear polymorphisms in cytokine genes, including the interleukin-6 gene.20 A study21 reported that the subgingival microbiota of patients with recently developed RA is similar to that of patients who have had RA for a long time and healthy participants with relatively severe periodontitis. Because periodontitis and RA have similar aetiology on the cellular and molecular levels, the treatment of 1 disease can affect the prognosis of the other.22
In this study, RA was more likely to develop in women and in older individuals, consistent with the results of a previous study.12 In contrast, in the case of former smokers, alcohol consumption was more likely to be associated with RA development, which is different from the results of a previous study12,23 that showed a higher incidence of RA in nonsmokers and nondrinkers, but similar to the findings of Li et al.24 In addition, the higher the BMI, the higher the risk of developing RA, consistent with the findings of Kim et al.25 However, the risk of RA was lowest when household income was ‘high,’ which was different from the results of previous studies.12,25 Another report noted that patients with recently developed RA have a high incidence of periodontitis, regardless of their age and smoking history.21 As can be seen from these studies, health-related behaviours, such as alcohol consumption and smoking, vary across studies, and follow-up research is warranted to clarify the relationship.
To obtain information on items such as smoking status, alcohol consumption, blood pressure, blood glucose level, total cholesterol, and BMI, which are potential confounding factors between the 2 diseases, the participants in this study were limited to individuals who had received a health examination, despite the possibility of incurring a selection bias. In addition, because the data used in this study consist of the medical records from medical institutions, this study is also limited in that those who did not visit the medical institution could not participate in the study. Although RA and periodontitis are both influenced by genetic factors, it is difficult to include genetic factors as confounders in this study because the NHIS-CRDB data used are limited to the variables investigated. Therefore, it is necessary to use caution when generalising the results of this study. Because we used claims data, information on the clinical characteristics of periodontitis, such as onset, severity, and distribution, were not available. Despite these limitations, however, this study is significant in that the relationship between periodontitis and the development of RA was deduced by conducting a longitudinal cohort analysis of NHIS-CRDB data, a data set representative of the Korean population, based on accurate specialist-delivered diagnoses. In addition, age, sex, and household income are known to have a strong effect on RA and periodontitis incidence, and there is a clear limitation when controlling for the role of these confounders by a statistical model alone. This being said, the use of the matching method to establish the control group in this study enhances the credibility of the findings.
Conclusion
In conclusion, this study analysed 17 years of medical records from 691,506 patients, thereby confirming that periodontitis could be a risk factor for RA. Further efforts to identify mediators shared by the 2 diseases are warranted to further clarify the relationship between these 2 diseases.
Funding
None disclosed.
Conflict of interest
None disclosed.
Acknowledgements
This study was conducted using data from National Health Insurance Service (Study Control Number: NHIS-2019-1-533).
References
- 1.Mercado F, Marshall RI, Klestov AC, et al. Is there a relationship between rheumatoid arthritis and periodontal disease? J Clin Periodontol. 2000;27:267–272. doi: 10.1034/j.1600-051x.2000.027004267.x. [DOI] [PubMed] [Google Scholar]
- 2.Grasso MA, Comer AC, DiRenzo DD, et al. Using big data to evaluate the association between periodontal disease and rheumatoid arthritis. AMIA Annu Symp Proc. 2015;2015:589–593. [PMC free article] [PubMed] [Google Scholar]
- 3.Chou YY, Lai KL, Chen DY, et al. Rheumatoid arthritis risk associated with periodontitis exposure: a nationwide, population-based cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon SY. Chonnam National University; Gwangju: 2017. A literature review of association between periodontal disease and systemic disease. [thesis] [Google Scholar]
- 5.Dev YP, Khuller N, Basavaraj P, et al. Rheumatoid arthritis among periodontitis patients in baddi industrial estate of Himachal Pradesh, India: a cross sectional study. J Clin Diagn Res. 2013;7:2334–2337. doi: 10.7860/JCDR/2013/6237.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persson GR. Rheumatoid arthritis and periodontitis - inflammatory and infectious connections. Review of the literature. J Oral Microbiol. 2012;4:11829. doi: 10.3402/jom.v4i0.11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho SY, Kang SY, Lee HJ, Lee WI. A comparative evaluation of the diagnostic value of anti-cyclic citrullinated peptide and rheumatoid factor in rheumatoid arthritis. Korean J Lab Med. 2008;28:39–45. doi: 10.3343/kjlm.2008.28.1.39. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO)/Republic of Korea. WHO MINDbank: more inclusiveness needed in disability and development. Korea: National Health Plan 2020. Available from: http://www.mindbank.info/item/4070. Accessed 2 February 2020.
- 9.Rodríguez-Lozano B, González-Febles J, Garnier-Rodríguez JL, et al. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: a case-control study. Arthritis Res Ther. 2019;21:27. doi: 10.1186/s13075-019-1808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calderaro DC, Corrêa JD, Ferreira GA, et al. Influence of periodontal treatment on rheumatoid arthritis: a systematic review and meta-analysis. Rev Bras Reumatol Engl Ed. 2017;57:238–244. doi: 10.1016/j.rbre.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Jung ES, Choi YY, Lee KH. Relationship between rheumatoid arthritis and periodontal disease in Korean adults: data from the sixth Korea National Health and Nutrition Examination Survey, 2013 to 2015. J Periodontol. 2019;90:350–357. doi: 10.1002/JPER.18-0290. [DOI] [PubMed] [Google Scholar]
- 12.Lee KH, Choi YY. Rheumatoid arthritis and periodontitis in adults: using the Korean National Health Insurance Service – National Sample Cohort. J Periodontol. 2020;91:1186–1193. doi: 10.1002/JPER.19-0311. [DOI] [PubMed] [Google Scholar]
- 13.Ceccarelli F, Saccucci M, Di Carlo G, et al. Periodontitis and rheumatoid arthritis: the same inflammatory mediators? Mediators Inflamm. 2019;2019 doi: 10.1155/2019/6034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khantisopon N, Louthrenoo W, Kasitanon N, et al. Periodontal disease in Thai patients with rheumatoid arthritis. Int J Rheum Dis. 2014;17:511–518. doi: 10.1111/1756-185X.12315. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz P, Bissada NF, Palomo L, et al. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol. 2009;80:535–540. doi: 10.1902/jop.2009.080447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi ES, Cho HA. Association between oral health status and rheumatoid arthritis. J Dent Hyg Sci. 2015;15:612–619. [Google Scholar]
- 17.Arkema EV, Karlson EW, Costenbader KH. A prospective study of periodontal disease and risk of rheumatoid arthritis. J Rheumatol. 2010;37:1800–1804. doi: 10.3899/jrheum.091398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkataraman A, Almas K. Rheumatoid arthritis and periodontal disease. An Update. N Y State Dent J. 2015;81:30–36. [PubMed] [Google Scholar]
- 19.Javed F, Ahmed HB, Mikami T, et al. Cytokine profile in the gingival crevicular fluid of rheumatoid arthritis patients with chronic periodontitis. J Investig Clin Dent. 2014;5:1–8. doi: 10.1111/jicd.12066. [DOI] [PubMed] [Google Scholar]
- 20.Perricone C, Ceccarelli F, Valesini G. An overview on the genetic of rheumatoid arthritis: a never-ending story. Autoimmun Rev. 2011;10:599–608. doi: 10.1016/j.autrev.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Scher JU, Ubeda C, Equinda M, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64:3083–3094. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz P, Bissada NF, Palomo L, et al. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol. 2009;80:535–540. doi: 10.1902/jop.2009.080447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KM. Korea University; Seoul: 2018. Factors influencing self-rated health in people with osteoarthritis and rheumatoid arthritis. [thesis] [Google Scholar]
- 24.Li R, Tian C, Postlethwaite A, et al. Rheumatoid arthritis and periodontal disease: what are the similarities and differences? Int J Rheum Dis. 2017;20:1887–1901. doi: 10.1111/1756-185X.13240. [DOI] [PubMed] [Google Scholar]
- 25.Kim SY, Nam HS, Kang C. Prevalence of arthritis and related factors among Korean adults. J Korea Acad Industr Coop Soc. 2012;13:4073–4081. [Google Scholar]