Abstract
Background
This study was conducted to assess the effect of electromechanical-assisted gait training intensity on walking ability in patients over 3-month post-stroke.
Methods
Data from two randomized controlled trials (RCTs) were collected under the same study design of assessment and intervention, excluding intervention time per session. After matching the inclusion criteria of two RCTs, the experimental groups of each RCT were defined as low-intensive (LI) and high-intensive (HI) group according to the intervention time per session. Primary outcome was the difference of the change in Functional Ambulatory Categories (FAC) between LI and HI gait training. Secondary outcomes were the difference of changes in mobility, walking speed, walking capacity, leg-muscle strength, balance and daily activity evaluated with Rivermead Mobility Index (RMI), 10 m walk test (10MWT), 6-min walk test (6MWT), Motricity Index (MI), Berg Balance Scale (BBS) and Modified Barthel Index (MBI) respectively.
Results
The FAC improved after gait training in both groups. The secondary outcomes also improved in both groups except RMI and MI in HI group. The change of all outcomes were not different between groups except RMI. The change of RMI in the LI group was greater than that in the HI group statistically, but it did not meet minimal clinically important difference.
Conclusions
The improvement of walking ability after LI or HI gait training was not different if providing the same total gait training time. By providing the electromechanical gait training intensively, we could shorten the gait training period to improve walking ability and customize the training program according to the patient training abilities.
Trial registration
Name of the registry: Clinical Research Information Service. Trial registration number: No. KCT0002195(RCT1), No. KCT0002552(RCT2). Date of registration: 10/04/2016(RCT1), 10/05/2017(RCT2). URL of the trial registry record: https://cris.nih.go.kr/cris/search
Keywords: Gait, Exoskeleton device, Rehabilitation, Stroke
Background
Electromechanical-assisted gait training for stroke patients has been rapidly developed in recent years, and is being used as a new method of rehabilitation [1]. It has been reported in many studies as a treatment option to replace or supplement conventional rehabilitation by means of focused, repetitive, and active motions for stroke patients [2–6]. For the clinical effects of electromechanical-assisted gait training, Mehrholz et al. [6] demonstrated that it could improve post-stroke independent walking recovery when combined with physical therapy in patients suffering from a stroke. However, the most effective frequency, duration, and timing of post-stroke robot-assisted gait training are still unresolved [1, 6].
Most traditional stroke rehabilitation programs lack exercise intensity and there is no standard evaluation tool [7]. The intensity of exercise refers to work rate or metabolic needs which could be quantified as heart rate, rating of perceived exertion, rate of oxygen consumption and walking speed [8–14]. However, it would be difficult for stroke survivors to increase their work rate or metabolic needs and walking speed because of the underlying disease and the risk of fall. The intensity of exercise for stroke survivors could be quantified as the number of gait repetitions although it is a crude measure of intensity [15]. If the electromechanical assisted gait provided the uniform repetitive leg motion during the intervention, the number of gait repetitions should be in direct proportion to intervention time and we could define the intervention time as exercise intensity for electromechanical assisted gait training.
Those electromechanical-assisted gait training is known to be effective for acute and sub-acute stroke patients [6–8], and meta-analysis suggests that the patients in the first three months after a stroke and those who are not able to walk should seem to benefit the most [16]. However, several studies have reported that electromechanically assisted gait training can improve gait function in patients with chronic stroke [4–6, 16].
Recently, electromechanical-assisted gait training by Exowalk® (Fig. 1) improved walking in chronic stroke patients although it was not superior to conventional therapy [17]. Exowalk® was developed for patients with gait difficulty to perform gait training by providing the normal gait pattern of a healthy person. It has a design as an exoskeleton and actualizes walking by the patient because most of the device’s components are located dorsally on the patient, including motorized wheels for the control of device speed and direction. This design provides a firm standing ability and obviates the need for an additional cane or walker. A questionnaire of previous studies revealed that stroke survivors should feel increased confidence in independent gait and showed high satisfaction rates [18].
Fig. 1.
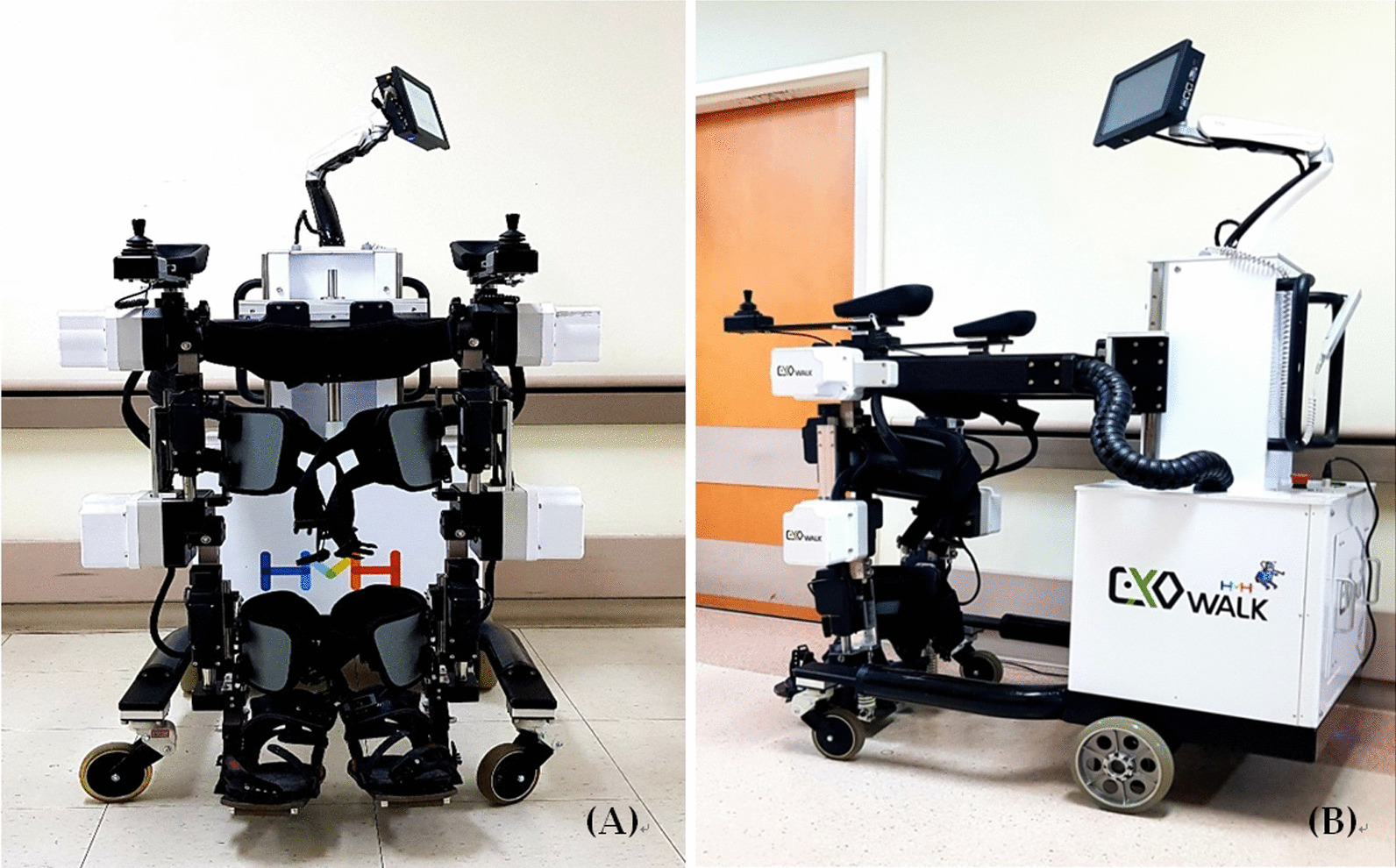
Exowalk.®, HMH Co., Ltd: a anterior view. b lateral view
There are two randomized controlled trials (RCTs) by Exowalk® which were conducted to investigate the effect of electromechanical-assisted gait training with the same kind of evaluation and the same type of gait training except for gait training intervention time per session [17, 18]. The intervention time per session was set as exercise intensity, and we hypothesized that high intensity of gait training could shorten the rehabilitation period for endurable stroke survivors. The purpose of this study was to assess the effect of electromechanical-assisted gait training intensity on the walking ability in patients over 3-month post-stroke.
Methods
Data acquisition
The data for each experimental group from two former clinical trials were selected [17, 18]. Both clinical trials were RCTs and conducted with the same kind of evaluation and the same type of gait training except for the intervention time per session.
The first clinical trial (RCT 1) was intended to assess the efficacy of electromechanical-assisted gait training on the walking ability of stroke patients based on ambulatory function, muscle strength, balance, gait speed and capacity. Forty patients with stroke who could stand alone were randomly assigned to the control and experimental groups. The experimental groups underwent gait training assisted by Exowalk® for 30 min per session, one session per day, 5 sessions per week, for a period of 4 weeks, and the total gait training time was 600 min [18]. The second clinical trial (RCT 2) was intended to assess the efficacy of electromechanical-assisted gait training on the walking ability of stroke patients who had a stroke over 3 months previously and could walk with or without another’s assistance. Forty patients were randomly assigned to the control and experimental groups. The experimental groups underwent gait training assisted by Exowalk® for 60 min per session, one session per day, 5 sessions a week, for a period of 2 weeks and the total gait training time was 600 min [17]. The study provided the additional gait training for a period of 2 weeks at the end of the intervention, but the outcome measures at the end of gait training of 2 weeks were adopted in this study in order to match the total gait training time of 600 min [17].
Participant
To minimize the selection bias, we matched the stroke duration and initial functional status of stroke patients in RCT 1 and RCT 2. The inclusion criteria of this study were revised in terms of duration since stroke onset and initial ambulatory function by FAC. The revised inclusion criteria were as follows: (1) ischemic or hemorrhagic stroke confirmed by a brain imaging study; (2) age above 19 years; (3) hemiplegia or hemiparesis confirmed by physical examination, with the ability to walk with help (FAC 2–5); (4) patients with sufficient cognitive function to control walking speed and direction; (5) stroke with onset more than 3 months previously. The exclusion criteria were the same as in RCT 1 and RCT 2 and were as follows: (1) poor cognition (unable to obey a command or a Mini-Mental Status Exam (MMSE) of less than 10); (2) trunk ataxia, inability to stand; (3) severe spasticity (Modified Ashworth Scale (MAS) Grade 3 and 4); (4) severe leg osteoarthritis, inability to walk, and (5) inability to undergo gait training.
The number of subjects in the experimental groups in RCT 1 and RCT 2 was 18 each. Two patients in RCT 1 were excluded because they had a stroke less than 3 months previously and their FAC was level 1. Four patients in RCT 2 were excluded because their FAC was level 6 and they could walk independently without help. Thus, the number of subjects included in this study was 30 persons.
Interventions
The experimental group of RCT 1 was allocated to the low-intensity (LI) group with the electromechanical-assisted gait training for 30 min per session. The experimental group of RCT 2 was allocated to the high-intensity (HI) group and performed the same intervention for 60 min per session. Intervention and evaluation were performed by different physiotherapists with 5 years or more of experience, in order to increase reliability by minimizing the measurement error. This was a single-blind clinical trial in that outcome assessors were blind. At enrollment, patients were instructed not to reveal their allocation arm to the outcome assessor. The researcher who performed the randomization and data analyses was not involved in assessment and training.
Measurements and analyses
Both LI and HI groups had the same outcome measures. The primary outcome was the change of FAC [19]. Secondary outcome were the changes of Rivermead Mobility Index (RMI) [20], 10-m walk test (10MWT) [21], 6-min walk test (6MWT) [22], Motricity Index (MI) [23], Berg Balance Scale (BBS) [24], and Modified Barthel Index (MBI) [25]. All assessments were conducted within 1 week before and after gait training.
FAC represented the walking ability on a 6-point scale. Walking ability was assessed by the need for walking assistance [19]. RMI was a test to assess mobility based on 15 items ranging from turning over in bed to running based on a question format of Yes or No [20]. The 10MWT was used to measure the walking ability of the subjects which was used to measure walking velocity based on average speed (meter/s) after three times of 10-m walking [21]. The 6MWT was used to measure walking capacity, which was recorded as the distance calculated by the number of repetitions in a 30-m cycle for 6 min [22]. MI was calculated only for the lower legs to represent muscle strength. The total MI score was recorded in a range of 0 to 100 points, with the higher numbers representing good muscle strength [23]. BBS testing is a clinical measurement method used to measure the risk of falls in stroke patients with the higher the score, the better the balance ability [24]. MBI is the ADL outcome measures, and consisted of 10 items: feeding, personal hygiene (grooming), bathing, dressing, toilet transfer, bladder control, bowel control, chair/bed transfers, stair climbing, and ambulation [25]. All assessments were conducted within a week pre-gait training and post-gait training, and the patients used the same walking assistance for the assessment of 10MWT and 6MWT during pre and post-gait training. All values are presented as mean and standard deviation (mean ± SD).
Continuous data were compared using the t-test, binary data using a χ2 test, to compare the data between the LI and HI groups. The significance of changes between pre-gait training and post-gait training in each group was assessed by using a paired Wilcoxon signed-rank test. Age was different between the two groups (Table 1) and we analyzed the data between groups with covariance of age (Table 3). Mixed model ANCOVA adjusted by the effect of age between groups (LI versus HI group) and within group (pre versus post-gait training time) showed the same result (Table 4). All statistical analyses were done using SPSS Version 22.0 (SPSS, Chicago, IL, USA). Statistical significance level was set at p < 0.05.
Table 1.
The baseline characteristics of the low-intensity and high-intensity groups
| LI group (n = 16) 30 min (4 weeks) |
HI group (n = 14) 60 min (2 weeks) |
p valuea | |
|---|---|---|---|
| Age (years) | 46.94 ± 15.61 | 61.86 ± 11.33 | 0.006 |
| Sex | 0.282 | ||
| Male, n (%) | 10 (62.5%) | 6 (42.9%) | |
| Female, n (%) | 6 (37.5%) | 8 (57.1%) | |
| Post-stroke duration, days | 475.31 ± 411.42 | 511.07 ± 292.91 | 0.789 |
| Stroke type | 0.431 | ||
| Ischemic, n (%) | 8 (50%) | 9 (64.3%) | |
| Hemorrhagic, n (%) | 8 (50%) | 5 (35.7%) |
SD, standard deviation; LI, low intensity; HI, high intensity
aT-test and χ² test SD
Table 3.
The change of outcome measures before and after gait training and the difference of values between the low-intensity and high-intensity group
| LI group (n = 16) 30min (4 weeks) |
HI group (n = 14) 60min (2 weeks) |
p value | |||
|---|---|---|---|---|---|
| Mean ± SD | LS mean ± SE | Mean ± SD | LS mean ± SE | ||
| FAC | 0.63 ± 0.61 | 0.68 ± 0.15 | 0.43 ± 0.51 | 0.36 ± 0.16 | 0.200 |
| RMI | 1.50 ± 1.41 | 1.54 ± 0.30 | 0.36 ± 0.63 | 0.30 ± 0.32 | 0.015 |
| 10MWT | 0.24 ± 0.73 | 0.27 ± 0.14 | 0.56 ± 0.51 | 0.02 ± 0.15 | 0.284 |
| 6MWT | 20.96 ± 27.31 | 18.28 ± 6.26 | 21.35 ± 18.39 | 24.41 ± 6.75 | 0.537 |
| MI | 6.81 ± 6.55 | 6.70 ± 1.63 | 2.07 ± 5.32 | 2.19 ± 1.76 | 0.090 |
| BBS | 6.50 ± 4.41 | 6.39 ± 1.06 | 3.43 ± 3.20 | 3.55 ± 1.14 | 0.099 |
| MBI | 6.38 ± 4.89 | 11.36 ± 18.73 | 8.52 ± 3.42 | 8.89 ± 3.69 | 0.946 |
P-value by the analysis of covariance of age between LI and HI groups
Numbers are mean ± standard deviation
LS mean, least square mean; SE, standard error; FAC, Functional Ambulation Categories; RMI, Rivermead Mobility Index; 10MWT, 10-meter walk test; 6MWT, 6-minute walk test; MI, Motricity Index; BBS, Berg Balance Scale; MBI, Modified Barthel Index
Table 4.
Mixed model analysis of covariance (ANCOVA) between time (pre versus post-gait training) and group (low versus high-intensity)
| Sum of Squares | df | Mean square | F | p | |
|---|---|---|---|---|---|
| FAC | |||||
| Time | 0.010 | 1 | 0.010 | 0.059 | 0.811 |
| Group | 0.292 | 1 | 0.292 | 0.242 | 0.627 |
| Time*Group | 0.006 | 1 | 0.006 | 0.036 | 0.851 |
| RMI | |||||
| Time | 0.374 | 1 | 0.374 | 0.528 | 0.474 |
| Group | 38.731 | 1 | 38.731 | 4.486 | 0.044 |
| Time*Group | 5.100 | 1 | 5.100 | 7.192 | 0.012 |
| 10MWT | |||||
| Time | 0.007 | 1 | 0.007 | 0.043 | 0.837 |
| Group | 0.353 | 1 | 0.353 | 0.427 | 0.519 |
| Time*Group | 0.185 | 1 | 0.185 | 1.221 | 0.279 |
| 6MWT | |||||
| Time | 1594.689 | 1 | 1594.689 | 4.710 | 0.039 |
| Group | 6727.920 | 6727.920 | 1.037 | 0.318 | |
| Time*Group | 185.851 | 1 | 185.851 | 0.549 | 0.465 |
| MI | |||||
| Time | 4.058 | 1 | 4.058 | 0.219 | 0.644 |
| Group | 91.715 | 1 | 91.715 | 0.349 | 0.560 |
| Time*Group | 42.347 | 1 | 42.347 | 2.280 | 0.143 |
| BBS | |||||
| Time | 7.429 | 1 | 7.429 | 0.995 | 0.328 |
| Group | 343.204 | 1 | 343.204 | 1.441 | 0.241 |
| Time*Group | 27.710 | 1 | 27.710 | 3.711 | 0.065 |
| MBI | |||||
| Time | 53.713 | 1 | 53.713 | 0.657 | 0.425 |
| Group | 828.643 | 1 | 828.643 | 3.216 | 0.084 |
| Time*Group | 0.387 | 1 | 0.387 | 0.005 | 0.946 |
2 × 2 Mixed model ANCOVA adjusted by the effect of age
Type III Sum of Squares; Df, Degrees of freedom; MS, Mean Square; FAC, Functional Ambulation Categories; RMI, Rivermead Mobility Index; 10MWT, 10-meter walk test; 6MWT, 6-minute walk test; MI, Motricity Index; BBS, Berg Balance Scale; MBI, Modified Barthel Index
Results
Sixteen patients were included in LI group, and 14 patients were included in HI group in this study. Age in HI group was significantly greater than in LI group (Table 1). Therefore, the data was adjusted by the effects of age using the analysis of covariance (ANCOVA) and least-square mean (LS mean) represents the average covariate value (Table 3).
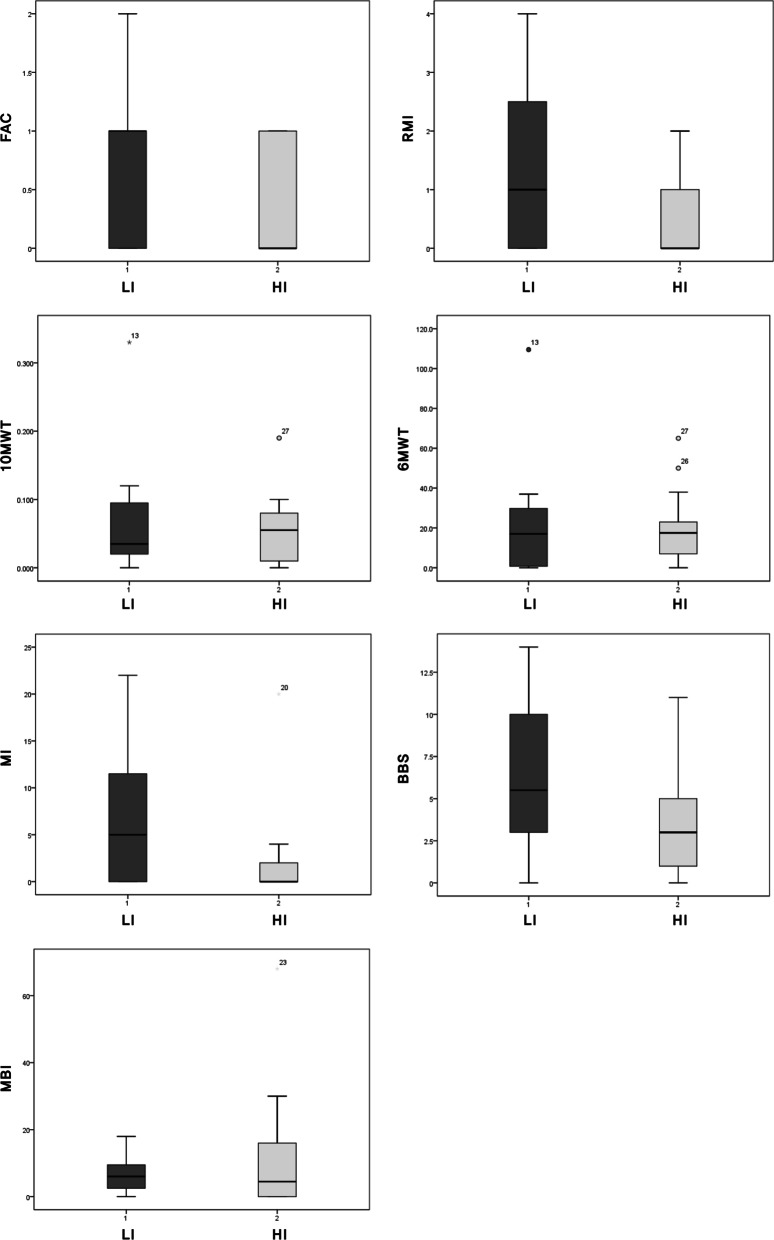
All outcome measures pre-gait training were not different statistically between LI and HI group (p > 0.5). In the LI group, the FAC was 3.19 ± 1.04 pre-gait training and 3.81 ± 1.22 post-gait training, and the FAC improved significantly post-gait training (p = 0.004). In the HI group, the FAC was 3.43 ± 1.09 pre-gait training and 3.86 ± 1.17 post-gait training, and the FAC also improved significantly post-gait training (p = 0.014). Most secondary outcomes in the LI and HI group improved significantly post-gait training (Table 2, Fig. 2). Whereas 10MWT, 6MWT, BBS, and MBI in HI group were improved significantly post-gait training, RMI and MI were not improved significantly (Table 2). The change of FAC after gait training was 0.63 ± 0.61in the LI group and 0.43 ± 0.51in the HI group. The changes of FAC were not different between the LI and HI groups (p = 0.200). The change of RMI in the LI group was greater than that in the HI group statistically (p < 0.015), but it was not reach the level of minimal clinically important difference (Table 3). Mixed model ANCOVA adjusted by the effect of age between groups (LI versus HI group) and within each group (pre versus post-gait training time) showed the same result (Table 4).
Table 2.
The outcome measures before and after gait training
| LI group (n = 16) 30 min (4 weeks) |
HI group (n = 14) 60 min (2 weeks) |
|||||
|---|---|---|---|---|---|---|
| Pre-gait training | Post-gait training | P value | Pre- gait training | Post-gait training | p value | |
| FAC | 3.19 ± 1.04 | 3.8 ± 1.22 | 0.004 | 3.43 ± 1.09 | 3.86 ± 1.17 | 0.014 |
| RMI | 5.38 ± 2.39 | 6.87 ± 2.60 | 0.003 | 7.71 ± 3.73 | 7.93 ± 3.47 | 0.317 |
| 10MWT | 0.47 ± 0.83 | 0.72 ± 1.56 | 0.002 | 0.38 ± 0.28 | 0.43 ± 0.29 | 0.010 |
| 6MWT | 89.87 ± 68.64 | 108.34 ± 74.10 | 0.017 | 105.07 ± 90.65 | 125.00 ± 94.68 | 0.006 |
| MI | 44.44 ± 14.27 | 51.25 ± 12.94 | 0.003 | 54.29 ± 18.97 | 56.36 ± 17.57 | 0.068 |
| BBS | 28.38 ± 13.52 | 34.8 ± 13.85 | 0.001 | 31.79 ± 18.81 | 35.21 ± 18.25 | 0.002 |
| MBI | 58.19 ± 16.98 | 64.56 ± 17.34 | 0.001 | 63.86 ± 20.59 | 75.21 ± 14.55 | 0.012 |
P-value by Wilcoxon signed-rank test between pre-gait training and post-gait training
Numbers are mean ± standard deviation
FAC, Functional Ambulation Categories; RMI, Rivermead Mobility Index; 10MWT, 10-meter walk test; 6MWT, 6-minute walk test; MI, Motricity Index; BBS, Berg Balance Scale; MBI, Modified Barthel Index
Fig. 2.
The change of outcome measures after intervention with low-intensity (LI) and high-intensity (HI) gait training
Discussion
This study was conducted to assess the effect of electromechanical-assisted gait training intensity on walking ability in stroke patients. Generally intensity of exercise was defined as percent of heart rate maximum and high-intensity exercise could be more potent stimulus in enhancing walking competency in stroke survivors [12–14]. However, stroke is the second most common cause of death after ischemic heart disease and a strong cause of difficulties in walking [31, 32]. During gait training for the stroke patients who had walking difficulties, it was impractical to apply high-intensity gait training of over 70–95% heart rate maximum [12–14]. In this study, the intensity of gait training was defined as the intervention time per session because electromechanical-assisted gait training could provide repetitive leg motion during the intervention and the number of repetitions could be determined according to intervention time and gait speed. The speed of electromechanical-assisted gait training was set by 10MWT pre-gait training and adjusted by the clinician during the period of the clinical trial. Because there were no differences of 10MWT between LI and HI group in both pre and post-gait training, the number of repetitive leg motions should not be different within the same intervention time between groups, either. The intervention time of gait training per session was 60 min in HI group. Thus, the number of repetitions per session in HI group was twice those in LI group. Because the improvement of walking ability in LI and HI group was not different after matching the inclusion criteria and total intervention time, we suggested that intensive gait training could shorten the gait training period and the clinician could customize the training program according to the patient training abilities.
Mehrholz et al. [16] investigated 36 trials of electromechanical-assisted gait training involving 1472 participants and concluded that electromechanical-assisted gait training in combination with physiotherapy increased the odds of participants becoming independent in walking, but did not significantly increase their walking velocity or walking capacity. However, they interpreted the results with caution, because some trials investigated people who were independent in walking at the start of the study, and they found differences between the trials in terms of the duration of intervention and frequency. It is still uncertain what is the most effective frequency and intervention time of electromechanical-assisted gait training.
Some studies applied the intervention time for around 30 min per day or session [26–28] because it is a tolerable exercise time for stroke patients. However, a few studies tried the intervention time for 60 min per session [29, 30] and it was tolerable for chronic stroke patients who could walk with or without help. Bang and Shin [29] reported that chronic stroke patients who had robot-assisted gait training for 60 min a day, 5 days a week, for 4 weeks, showed better walking abilities and balance than those who had treadmill gait training. However, Stein et al. [30] reported that robotic therapy for ambulatory stroke patients with chronic hemiparesis using a robotic knee brace resulted in only modest functional benefits that were comparable to those from a group exercise intervention, although they did the robot therapy for 60 min a day, 3 days a week, for 6 weeks. We also tried intensive 60-min gait training to get a better result by increasing the intervention time per session and found that the 60-min electromechanical-assisted gait training improved ambulatory function as much as the physical therapist-assisted gait training although the improvements did not meet the minimal clinically important difference (MCID) [17]. The change of RMI in HI group in RCT 2 was 0.17 ± 0.70 and it was not included in the result because MCID of the change of RMI was 3.0 [33]. In this study, the change of RMI was 1.54 ± 0.30 in LI group and 0.30 ± 0.32 in HI group, and both did not meet MCID although the difference between groups were significant (Table 3).
Stroke patients who could walk with another’s assistance (FAC 2, 3) or requiring help (FAC 4, 5) were included in this study. Chronic stroke patients who could walk with help participated actively in RCT 2 because they wanted walk well. And they could tolerate 60-min gait training and eagerly wanted to obviate the need for a cane or another’s assistance. This study had new inclusion criteria of the chronic patients who had stroke duration over 3 months and those who could walk help. The patients of FAC 6 in RTC 2 who could walk independently were excluded, because they could walk without help and did not expect further improvement of FAC. We intended to find out whether we could shorten the gait training period if providing the electromechanical-assisted gait training intensively, because Exowalk® could provide unlimited repetition and most accurate motion. This study found the same improvement of walking ability after 2 or 4 weeks of gait training if providing the same total intervention time. Electromechanical-assisted device in this study provide repetitive training with symmetric gait motion for stroke patients. Improvement of gait symmetry was achieved after symmetrical walking training and it related to the balance [34]. We needed to investigate the quantitative gait analysis.
We tried to compared the data of LI and HI group to control group in each LI and HI group after applying the revised inclusion criteria. In the previous RCT 1 study of LI group [18], we analyzed the data with covariance of age and stroke duration which were different between control and experimental group before gait training. After adjusting the data with covariance of age and duration, the change of all outcomes was not different between groups. In the previous RTC 2 study of HI group [17], the change of both primary and secondary outcomes were not different between control and experimental group although they improved significantly after gait training within each groups. In this study by the revised inclusion criteria, the change of all outcomes after gait training was not different, either. However, the number of patients by the revised inclusion was small and we need statistical adjustment. This study was conducted by single electromechanical assisted gait trainer of Exowalk® and we need to review the articles of LI and HI gait training because the meta-analysis by many electromechanical assisted gait trainers would be more informative.
Limitations
This study was conducted by combining two separate RCTs to evaluate the effect of gait training intensity on walking ability instead of designing a prospective study because it was difficult to assign the different walking capacity patients to different intervention time randomly. Because we had two types of gait training regimen, we compared the result in advance and tried to find the effect of intensive gait training. Although two RCTs had the same protocol of intervention and evaluation, they had different inclusion criteria and were vulnerable to selection bias.
No power calculations were performed because sample data for this study from two previous trials. we set intervention time as exercise intensity because it was difficult to increase work demand by walking in the patients with walking difficulties. We need to investigate the carry over compare the effect by evaluating outcome measures at 4 weeks in the HI group, because we shortened the intervention period by intensive gait training and expected the improvement to be lasted.
Conclusions
We could expect the same improvement of walking ability after LI or HI gait training, if we provided the same total gait training time. We could shorten the gait training period by providing the gait training intensively and we could customize the training program according to the patient training abilities.
Abbreviations
- MMSE
Mini-Mental State Examination
- FAC
Functional Ambulation Categories
- RMI
Rivermead Mobility Index
- 10MWT
10Meter walk test
- 6MWT
6Minute walk test
- MI
Motricity Index
- BBS
Berg Balance Scale
- MBI
Modified Barthel Index
Author contributions
All authors contributed to the study and preparation of the manuscript. BSK have made substantial contributions to conception and design. CSY drafting and revising the manuscript, acquisition of data. YGN participated in the statistical analysis and interpretation of data. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Availability of data and materials
The data presented in this study are available on reasonable request from the corresponding author.
Declarations
Ethics approval and consent to participate
RCT 1 and RCT 2 were approved by ethics committee at the Dongguk University Ilsan Hospital’s Institutional Review Board (Approve No. 2016-64, 2017-18) and registered at the clinical research information service (CRIS Registration No. KCT0002195, KCT0002552). All participant signed informed consent forms after the detail explanation of each study.
Consent for publication
Not applicable.
Competing interests
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morone G, Paolucci S, Cherubini A, Angelis DD, Venturiero V, Coiro P, et al. Robot-assisted gait training for stroke patients: current state of the art and perspectives of robotics. Neuropsychiatr Dis Treat. 2017;13:1303–1311. doi: 10.2147/NDT.S114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362(19):1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrholz J, Hädrich A, Platz T, Kugler J, Pohl M. Electromechanical and robot-assisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. 2012;(6):CD006876. [DOI] [PubMed]
- 4.Swinnen E, Beckwée D, Meeusen R, Baeyens JP, Kerckhofs E. Does robot-assisted gait rehabilitation improve balance in stroke patients? A systematic review. Top Stroke Rehabil. 2014;21(2):87–100. doi: 10.1310/tsr2102-87. [DOI] [PubMed] [Google Scholar]
- 5.Belas Dos Santos M, Barros de Oliveira C, Dos Santos A, Garabello Pires C, Dylewski V, MarioArida R. A Comparative Study of Conventional Physiotherapy versus Robot-Assisted Gait Training Associated to Physiotherapy in Individuals with Ataxia after Stroke. Behav Neurol. 2018;2018:2892065. doi: 10.1155/2018/2892065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrholz J, Elsner B, Werner C, Kugler J, Pohl M. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2013;2013(7):CD006185. doi: 10.1002/14651858.CD006185.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–2553. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 8.Cornelissen VA, Arnout J, Holvoet P, Fagard RH. Influence of exercise at lower and higher intensity on blood pressure and cardiovascular risk factors at older age. J Hypertens. 2009;27(4):753–762. doi: 10.1097/HJH.0b013e328322cf60. [DOI] [PubMed] [Google Scholar]
- 9.Cornelissen VA, Verheyden B, Aubert AE, Fagard RH. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J Hum Hypertens. 2010;24(3):175–182. doi: 10.1038/jhh.2009.51. [DOI] [PubMed] [Google Scholar]
- 10.Draganidis D, Chatzinikolaou A, Jamurtas AZ, Barbero JC, Tsoukas D, Theodorou AS, et al. The time-frame of acute resistance exercise effects on football skill performance: the impact of exercise intensity. J Sports Sci. 2013;31(7):714–722. doi: 10.1080/02640414.2012.746725. [DOI] [PubMed] [Google Scholar]
- 11.Helgerud J, Rodas G, Kemi OJ, Hoff J. Strength and endurance in elite football players. Int J Sports Med. 2011;32(9):677–682. doi: 10.1055/s-0031-1275742. [DOI] [PubMed] [Google Scholar]
- 12.Gjellesvik TI, Becker F, Tjønna AE, Indredavik B, Indredavik B, Lundgaard E, et al. Effects of high-intensity interval training after stroke (the HIIT stroke study) on physical and cognitive function: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2021;102(9):1683–1691. doi: 10.1016/j.apmr.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Luo L, Zhu S, Shi L, Wang P, Li M, Yuan S. High intensity exercise for walking competency in individuals with stroke: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2019;28(12):104414. doi: 10.1016/j.jstrokecerebrovasdis.2019.104414. [DOI] [PubMed] [Google Scholar]
- 14.Holleran CL, Rodriguez KS, Echauz A, Leech KA, Hornby TG. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. J Neurol Phys Ther. 2015;39(2):95–102. doi: 10.1097/NPT.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 15.Cho JE, Yoo JS, Kim KE, Cho ST, Jang WS, Cho KH, et al. Systematic review of appropriate robotic intervention for gait function in subacute stroke patients. Biomed Res Int. 2018;2018:4085298. doi: 10.1155/2018/4085298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehrholz J, Thomas S, Werner C, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2017;5(5):CD006185. [DOI] [PMC free article] [PubMed]
- 17.Nam YG, Park JW, Lee HJ, Nam KY, Choi MR, Yu CS, et al. Effects of electromechanically assisted gait trainer (Exowalk®) in patients with chronic stroke: A randomized controlled trial. J Rehabil Med. 2020;52(9):jrm00097. doi: 10.2340/16501977-2723. [DOI] [PubMed] [Google Scholar]
- 18.Nam YG, Lee JW, Park JW, Lee HJ, Nam KY, Park JH, et al. Effects of electromechanical exoskeleton-assisted gait training on walking ability of stroke patients: a randomized controlled trial. Arch Phys Med Rehabil. 2019;100(1):26–31. doi: 10.1016/j.apmr.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired. Reliab Meaningfulness Phys Ther. 1984;64(1):35–40. doi: 10.1093/ptj/64.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Collen FM, Wade DT, Robb GF, Bradshaw CM. The rivermead mobility index: a further development of the rivermead motor assessment. Int Disabil Stud. 1991;13(2):50–54. doi: 10.3109/03790799109166684. [DOI] [PubMed] [Google Scholar]
- 21.van Loo MA, Moseley AM, Bosman JM, de Bie RA, Hassett L. Inter-rater reliability and concurrent validity of walking speed measurement after traumatic brain injury. Clin Rehabil. 2003;17(7):775–779. doi: 10.1191/0269215503cr677oa. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron D, Bohannon RW. Criterion validity of lower extremity Motricity Index scores. Clin Rehabil. 2000;14(2):208–211. doi: 10.1191/026921500675786655. [DOI] [PubMed] [Google Scholar]
- 24.Berg K, Wood-Dauphinée S, Williams JI, Gayton D. Measuring balance in elderly: preliminary development of an instrument. Physiother Can. 1989;41(6):304–311. doi: 10.3138/ptc.41.6.304. [DOI] [Google Scholar]
- 25.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 26.Erdoğan Uçar D, Paker N, Buğdaycı D. Lokomat: a therapeutic chance for patients with chronic hemiplegia. NeuroRehabilitation. 2014;34(3):447–453. doi: 10.3233/NRE-141054. [DOI] [PubMed] [Google Scholar]
- 27.Cho DY, Park SW, Lee MJ, Park DS, Kim EJ. Effects of robot-assisted gait training on the balance and gait of chronic stroke patients: focus on dependent ambulators. J Phys Ther Sci. 2015;27(10):3053–3057. doi: 10.1589/jpts.27.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han EY, Im SH, Kim BR, Seo MJ, Kim MO. Robot-assisted gait training improves brachial-ankle pulse wave velocity and peak aerobic capacity in subacute stroke patients with totally dependent ambulation: Randomized controlled trial. Medicine (Baltimore) 2016;95(41):e5078. doi: 10.1097/MD.0000000000005078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bang DH, Shin WS. Effects of robot-assisted gait training on spatiotemporal gait parameters and balance in patients with chronic stroke: a randomized controlled pilot trial. NeuroRehabilitation. 2016;38(4):343–349. doi: 10.3233/NRE-161325. [DOI] [PubMed] [Google Scholar]
- 30.Stein J, Bishop L, Stein DJ, Wong CK. Gait training with a robotic leg brace after stroke: a randomized controlled pilot study. Am J Phys Med Rehabil. 2014;93(11):987–994. doi: 10.1097/PHM.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76(1):27–32. doi: 10.1016/S0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 33.Geroin C, Mazzoleni S, Smania N, Gandolfi M, Bonaiuti D, Gasperini G, et al. Systematic review of outcome measures of walking training using electromechanical and robotic devices in patients with stroke. J Rehabil Med. 2013;45(10):987–996. doi: 10.2340/16501977-1234. [DOI] [PubMed] [Google Scholar]
- 34.Bovonsunthonchai S, Aung N, Hiengkaew V, Tretriluxana J. A randomized controlled trial of motor imagery combined with structured progressive circuit class therapy on gait in stroke survivors. Sci Rep. 2020;10(1):6945. doi: 10.1038/s41598-020-63914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.