Abstract
Genetic and biochemical evidence for a defective xylan degradation pathway was found linked to the xylose operon in three lactococcal strains, Lactococcus lactis 210, L. lactis IO-1, and L. lactis NRRL B-4449. Immediately downstream of the xylulose kinase gene (xylB) (K. A. Erlandson, J.-H. Park, W. El Khal, H.-H. Kao, P. Basaran, S. Brydges, and C. A. Batt, Appl. Environ. Microbiol. 66:3974–3980, 1999) are two open reading frames encoding a mutarotase (xylM) and a xyloside transporter (xynT) and a partial open reading frame encoding a β-xylosidase (xynB). These are functions previously unreported for lactococci or lactobacilli. The mutarotase activity of the putative xylM gene product was confirmed by overexpression of the L. lactis enzyme in Escherichia coli and purification of recombinant XylM. We hypothesize that the mutarotase links xylan degradation to xylose metabolism due to the anomeric preference of xylose isomerase. In addition, Northern hybridization experiments suggested that the xylM and xynTB genes are cotranscribed with the xylRAB genes, responsible for xylose metabolism. Although none of the three strains appeared to metabolize xylan or xylobiose, they exhibited xylosidase activity, and L. lactis IO-1 and L. lactis NRRL B-4449 had functional mutarotases.
Xylan, a polymer with a β-1,4-linked backbone of xylose, is the major carbohydrate in the hemicellulose portion of plant cell walls. One-third of all renewable carbon is xylan (24). Although xylan metabolism has not been previously reported for Lactobacillus and Lactococcus species, xylan degraders include a wide range of other microbes, including rumen organisms, plant pathogens, and soil microbes (4, 10, 24, 36). Before the xylose backbone of xylan is attacked, side chains such as acetyl and arabinofuranose are removed by a variety of enzymes (4). Extracellular endo-1,4-β-xylanases then degrade the xylan to xylo-oligomers, which are transported into the cell by permeases (5). Finally, intracellular β-xylosidases hydrolyze the xylo-oligomers to xylose.
Previously, we reported the discovery of the genes necessary for xylose metabolism (xylRAB) in three lactococcal strains, including a non-xylose-fermenting (Xyl−) dairy starter culture strain (13). We also discussed the relationship between habitat and functional xylose metabolism. The dairy fermentation environment, with its intense selection for rapid growth and acid production, is very different from the plant niche that Lactococcus lactis subsp. lactis strains originally inhabited. The ability to metabolize xylose is not essential for growth in dairy environments, and xylose-fermenting (Xyl+) strains of the exclusively dairy-associated L. lactis subsp. cremoris have not been reported. However, the necessary genes (xylR, xylA, and xylB) for the metabolism of the pentose sugar xylose are still present in Xyl− strains of lactococci, including L. lactis subsp. cremoris (3, 13).
In this work, we report the subsequent discovery of the genetic potential of both plant (Xyl+) and dairy (Xyl−) isolates of L. lactis to metabolize xylan, a function encoded by the xylM, xynT, and xynB genes immediately downstream of the xylRAB locus. The sequencing of xylM and xynTB from L. lactis NRRL B-4449, L. lactis IO-1, and L. lactis 210 and the measurement of mutarotase and xylanolytic activities are described here. We propose a new function linking xylose and xylan metabolism: the mutarotase encoded by the xylM gene.
MATERIALS AND METHODS
Bacterial strains, plasmids, and cultivation.
Strains and vectors are described in Table 1. All strains were routinely cultivated at 30°C in M17 medium (Difco, Detroit, Mich.) with 0.5% glucose or xylose. Escherichia coli strains were cultured with aeration at 37°C in Luria broth (LB; Sigma, St. Louis, Mo.) and with 100 μg of ampicillin (Sigma) per ml or 50 μg of carbenicillin (Sigma) per ml as appropriate.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genetic marker(s) | Source |
|---|---|---|
| Strains | ||
| Lactococcus lactis subsp. lactis 210 | Xyl− | J. Kondo (Marschall Products) |
| Lactococcus lactis subsp. lactis IO-1 | Xyl+ | P. Stansbury (University of Hertfordshire, Hertfordshire, United Kingdom) |
| Lactococcus lactis subsp. lactis NRRL B-4449 (formerly Lactobacillus xylosus) | Xyl+ | A. J. Sinskey (Massachusetts Institute of Technology) |
| Escherichia coli DH5α F′ | F′ endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacIZYA-argF) U169 deoR [φ80dlacΔ(lacZ)M15] | |
| Escherichia coli BL21 (DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3 [T7lac promoter]) | Novagen |
| Plasmids | ||
| pGEM-T | AprlacZ′ | Promega |
| pET19d | Apr | Novagen |
Cloning of xylM
The xylM gene was obtained previously from L. lactis 210 (13) via an inverse PCR. The FNDE-XM and RBAM-XM primers (Table 2) were designed to amplify xylM from L. lactis NRRL B-4449 and L. lactis IO-1 for cloning prior to sequencing. The PCR products were initially ligated to pGEM-T (Table 1) and then transformed into E. coli DH5α F′. The xylM gene was also subcloned in pET19d for use in the Novagen (Madison, Wis.) E. coli BL21 (DE3) overexpression system. Ligations were performed as described by Promega (Madison, Wis.) for pGEM-T or Sambrook et al. (28). Standard methods were used to prepare and transform competent cells (28).
TABLE 2.
Summary of primers
| Primer | Sequence (5′–3′) | Location (nt)a | Comments |
|---|---|---|---|
| r-xylM | ACCAATGTCCAAAAATCGCGG | 5040–5060 | xynT and xynB inverse PCR |
| Ip1225 | CTCAACCGTTAAGCGAATCAT | 3949–3929 | xynT and xynB inverse PCR |
| FNDE-XM | GGAATTCCATATGACATTTACCATTTCC | 4176–4194 | xylM cloning, Northern primer |
| RBAM-XM | CGGGATCCTTAATTCGTTGTAAATAG | 5164–5181 | xylM cloning, Northern primer |
| F210xb | CGCATTTGCACTTGAACAT | 2512–2530 | xylB Northern primer |
| R210xb | GCTTTAGTGACCGCTTCTA | 4029–4011 | xylB Northern primer |
| M13–40 | GTTTTCCCAGTCACGAC | NA | Vector sequencing primer |
| M13rev (ht) | GCTTTAGTGACCGCTTCTA | NA | Vector sequencing primer |
Numbering corresponds to that for the L. lactis IO-1 xyl-xyn operon (including upstream sequences) (GenBank accession number AF092041). NA, not available.
Inverse PCR.
To obtain the L. lactis 210 xynT and xynB genes downstream of xylM, an inverse PCR with the r-xylM and Ip1225 primers (Table 2) was performed as described previously (13). Briefly, ligation reaction mixtures contained Csp6I (New England Biolabs, Beverly, Mass.)-digested chromosomal DNA at concentrations of 25 to 750 ng. The resulting self-ligated DNA was used at a final concentration of 5 ng/μl in the PCR.
PCR primers were designed from the L. lactis 210 sequence to obtain the xynT and xynB genes from L. lactis IO-1 and L. lactis NRRL B-4449.
PCR amplification.
PCRs were carried out with PCR buffer (20 mM Tris-HCl [pH 8.3], 50 mM KCl) in a final volume of 50 μl. Reaction mixtures contained 1 μl of self-ligated chromosomal DNA or crude cell lysate prepared as described by Czajka and Batt (11), 50 pmol of each primer, 100 mM (each) deoxynucleoside triphosphate, 1 U of AmpliTaq DNA polymerase (Perkin-Elmer, Foster City, Calif.), and 1.5 mM MgCl2. A Perkin-Elmer 2400 thermocycler was programmed with a 4-min hold at 94°C; 30 cycles of 1 min at 94°C, 1 min at 55 to 65°C (depending on the primer melting temperature), and 1 min at 72°C; and a 10-min hold at 72°C. For inverse PCR, the extension time at 72°C was increased to 2 min.
Sequencing and sequence analysis.
Genes were sequenced at the Cornell University BioResource Center using an ABI Prism 373A Stretch automated sequencer. Sequences were analyzed using several programs from the Lasergene software suite (DNASTAR, Inc., Madison, Wis.): EditSeq version 3.12, SeqMan version 3.53, and MegAlign version 3.05. We also performed BLAST X searches (1) of the GenBank database with our nucleotide sequences to identify homologous genes.
Overexpression and purification of XylM.
A 200-ml culture of E. coli BL21(pLbx10XM) was grown in LB supplemented with 50 μg of carbenicillin/ml at 37°C with aeration until it reached an optical density at 600 nm (OD600) of 0.65. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.2 mM, and the culture was incubated for an additional 2.5 h at 30°C with aeration. The cell pellet was harvested by centrifugation and frozen at −70°C until purification of the enzyme was performed. Mutarotase labeled with a histidine tag was extracted and batch purified under native conditions using Ni-nitrilotriacetic acid (NTA) Superflow resin according to the instructions of the manufacturer (Qiagen, Chatsworth, Calif.). The protein was further concentrated with a Centricon-10 spin column (Amicon, Beverly, Mass.) and resuspended in 10 mM Tris-HCl (pH 7.5). Purified XylM was run on a sodium dodecyl sulfate (SDS)-polyacrylamide gel to estimate its molecular weight (28).
Xylan-degrading activity.
Cell pellets and medium supernatants were harvested from 100-ml cultures of the three lactococcal strains grown in M17 medium-xylose to an OD600 of 0.4 to 1.0. The supernatants were concentrated approximately 20-fold by ultrafiltration (Millipore; 10,000-molecular-weight cutoff). The cell pellets were resuspended in 3 ml of 50 mM potassium phosphate buffer (pH 6), French pressed at 10,000 lb/in2, and centrifuged at 5,000 × g for 10 min to separate the cytoplasmic extracts from the membrane fractions. The membrane fractions were resuspended in 50 mM potassium phosphate buffer (pH 6).
The xylanase reaction mixtures consisted of 50 to 200 μl of sample and 100 μl of birch wood xylan (Sigma; 1% [wt/vol] in potassium phosphate buffer) brought to a final volume of 400 μl with 50 mM potassium phosphate buffer (pH 6). After overnight incubation at 37°C, the reaction mixtures were mixed with 1 ml of dinitrosalicylic acid reagent (22) and boiled for 15 min. The amount of reducing sugar released was measured at an absorbance of 600 nm using an LKB Biochrom Ultrospec II and compared against a standard curve prepared using various concentrations of xylose. One unit of enzyme activity was defined as the amount of enzyme required to release 1 μmol of reducing sugar per min.
Xylosidase activity was determined by measuring the hydrolysis of p-nitrophenyl-β-d-xylopyranoside. The xylosidase reactions comprised 50- or 150-μl samples and 100 μl of p-nitrophenyl-β-d-xylopyranoside (Sigma; 20 mM) brought to a final volume of 500 μl with 50 mM potassium phosphate buffer (pH 6). The reaction mixtures were incubated at 37°C, and the reactions were terminated with 500 μl of a stop reagent (1 M Na2CO3 [pH 9.0]). The amount of p-nitrophenol released was determined spectrophotometrically at 420 nm. An enzyme activity unit was defined as the amount of enzyme that produced 1 μmol of p-nitrophenol per min.
Mutarotase assay.
The α and β anomers of xylose can be easily discriminated via 13C nuclear magnetic resonance (NMR) spectroscopy, so this technique was performed to compare the rate of xylose mutarotation in cell extracts and the rate of spontaneous (nonenzymatic) mutarotation. Samples were prepared as follows. Cultures (100 ml) were grown to an OD600 of 0.4 to 1.0 in LB or M17 medium containing 0.5% glucose or xylose. The cell pellets were washed, resuspended in 3 ml of 10 mM Tris-HCl (pH 7.5), and then sonicated. Assay samples were prepared immediately before measurement by resuspending 16 mg of xylose (99% α anomer; Sigma) in NMR buffer (10 mM Tris-HCl [pH 7.5], 15% D2O [Sigma]) and adding 5 to 400 μl of cell extract to a final volume of 800 μl. Spontaneous mutarotation was evaluated with 16 mg of xylose dissolved in 800 μl of NMR buffer.
The NMR experiments were conducted at Cornell University (Chemistry Department NMR Facility) using a Varian XL-400 spectrometer set at 25°C. Carbon spectra were measured at 1- or 2-min intervals, and 48 transients were collected at each time point. Continuous Waltz-16 modulated broadband proton decoupling was used with a pulse sequence of 24.7°, a sweep width of 25,000 Hz, and an acquisition time of 1.199 s. Conditions were not optimized for precise quantitation. Dioxane (66.66 ppm) was used as a reference to set the scale of chemical shifts. Under these conditions, the C1 carbon of 13C-xylose had an α-anomer peak at 95 ppm and a β-anomer peak at 91 ppm. Mutarotation rates were calculated based on the rate of change of the β-anomer peak amplitude relative to the sum of the amplitudes for the two anomers. The enzymatic mutarotation was defined as the sample mutarotation rate minus the spontaneous mutarotation rate. Data were normalized based on protein concentrations.
Northern hybridizations.
RNA was isolated from xylose-grown and glucose-grown L. lactis using the hot phenol method of van der Vossen et al. (37). The RNA was precipitated with LiCl as described by Zantinge and Wessels (38). The formamide-denatured RNA was electrophoresed through a 1% agarose gel containing 2.2 M formaldehyde and then transferred by capillary action to a nylon membrane using 20× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) as described by Sambrook et al. (28). After the RNA was fixed by baking the membrane at 80°C for 2 h, the membrane was prehybridized for 3 h at 68°C in modified Church buffer (33). Hybridization was carried out overnight at 68°C with digoxigenin-labeled DNA probes in modified Church buffer. The xylB and xylM probes were prepared by PCR incorporation of digoxigenin-dUTP using the F210xb-R210xb and FNDE-XM–RBAM-XM primer pairs, respectively. A Boehringer Mannheim Biochemicals (Indianapolis, Ind.) Genius kit was used for detection.
Nucleotide sequence accession numbers.
The xylM and xynTB gene sequences for L. lactis NRRL B-4449, L. lactis IO-1, and L. lactis 210 can be found in GenBank under accession numbers AF092042, AF092041, and AF092040, respectively.
RESULTS
Two xylan metabolism genes (xynTB) and one novel mutarotase gene (xylM) were sequenced from three lactic acid bacteria, including one Xyl− strain. A combination of PCR products generated with traditional and inverse primers was assembled to give 3.3, 3.0, and 3.3 kb of sequence downstream of xylB from L. lactis NRRL B-4449, L. lactis IO-1, and L. lactis 210, respectively (Fig. 1).
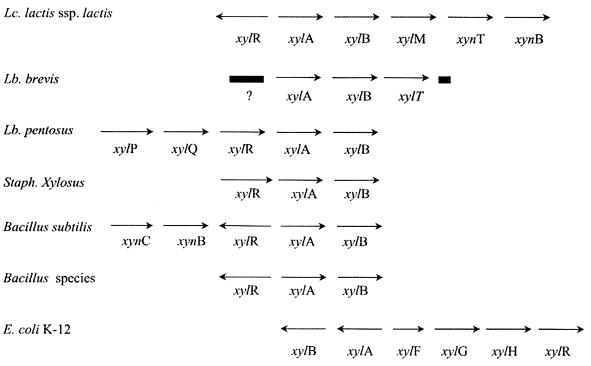
FIG. 1.
Organization of the xyl and xyn loci in various bacteria. The xylR gene encodes a regulatory protein; xylA encodes a xylose isomerase; xylB encodes a xylulose kinase; xylM encodes a mutarotase; xynT encodes a putative xylo-oligomer transporter; xynB encodes a β-1,4-xylosidase; xylT encodes a xylose symporter; xylP encodes an isoprimeverose symporter; xylQ encodes an α-xylosidase; xynC encodes a membrane-bound protein (xylo-oligomer permease?); xylF encodes a xylose-binding protein; xylG encodes an ATP-binding protein; and xylH encodes a membrane transporter (ABC-type xylose transport). The question mark represents a sequenced ORF of unknown function. Data are from the following sources (in parentheses): L. lactis subsp. lactis (13; this work), L. brevis (6, 8), Lactobacillus pentosus (9, 20, 21), Staphylococcus xylosus (34), B. subtilis (16), Bacillus species (30, 31), and E. coli (12, 19, 27, 35).
Mutarotase.
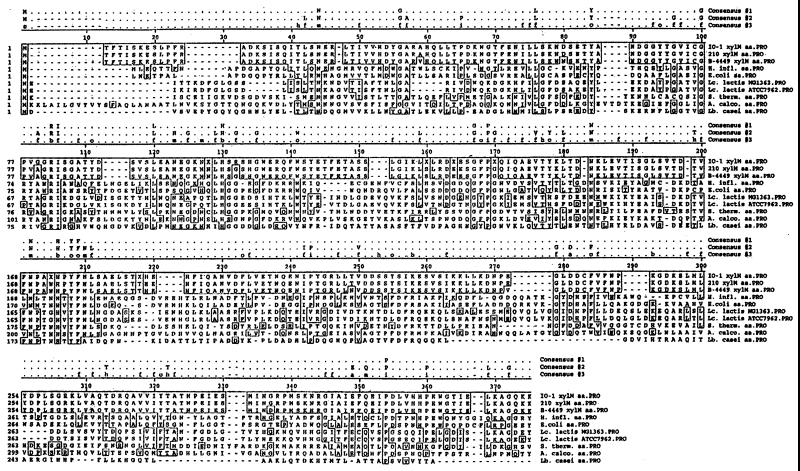
A gap of only 2 nucleotides (nt) exists between the stop codon of xylB and the start codon of another 1,005-bp open reading frame (ORF). The translated ORF is homologous (averages of 30% identical and 46% similar) over its entire length to galactose mutarotases from E. coli, Haemophilus influenzae, Acinetobacter calcoaceticus, and Streptococcus thermophilus. Mutarotases catalyze the interconversion of the β and α anomers of carbohydrates. Figure 2 shows an alignment of the L. lactis 210, IO-1, and NRRL B-4449 XylM sequences to the sequences of seven other mutarotases ranging in size from 335 to 381 amino acids. Approximately 5% (26) of the amino acids are identical for all 10 strains. Thirty-one additional residues (10%) are conserved or similar among the mutarotases. The L. lactis 210, IO-1, and NRRL B-4449 XylM sequences differ at only eight positions. Our XylM sequences are most similar (40%) to S. thermophilus GalM. Although promoter prediction by a neural network (26; M. G. Reese, N. L. Harris, and F. H. Eeckman, Electronic Proc. 1996. Pacific Symp. Biocomput., abstr., 1996) suggests two possible transcription start sites for our xylM, located 34 or 45 nt upstream of the start codon (data not shown), we also have evidence linking xylM transcription to xylAB transcription (see Northern hybridization results below).
FIG. 2.
Alignment of the L. lactis IO-1, L. lactis NRRL B-4449, and L. lactis 210 XylM sequences with seven galactose mutarotases. GenBank accession numbers for the GalM sequences are as follows: A. calcoaceticus, X03893; S. thermophilus, M38175; E. coli, U13636; H. influenzae, X65934; L. lactis ATCC 7962, U60828; L. lactis MG1363, AJ011653; and Lactobacillus casei, AF005933. Three consensus sequences are indicated above the alignment. Consensus sequence 1 includes residues that are conserved in all 10 mutarotases. Consensus sequence 2 includes residues that are conserved in 8 of 10 mutarotases. Consensus sequence 3 includes residues that are similar in 8 of 10 mutarotases. Residue groupings of consensus sequence 3 are as follows: a, acidic residues (DE); b, basic (HKR); f, aliphatic (AGILV); m, amide (NQ); o, aromatic (FWY); h, hydroxyl (ST); i, imino (P); and s, sulfur (CM). Residues identical to those in IO-1 XylM are boxed.
Mutarotase activity.
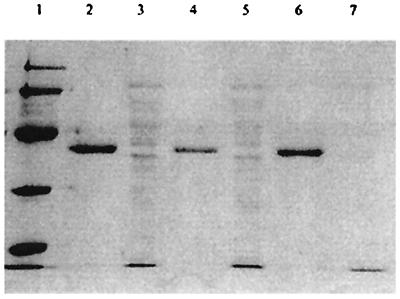
Concentrated crude cell extracts of xylose-grown L. lactis NRRL B-4449, IO-1, and 210 were tested for mutarotase activity using 13C NMR. The results are shown in Table 3. The specific mutarotase activities of L. lactis IO-1 and L. lactis NRRL B-4449 extracts were 2.9 and 0.9 U/mg of protein, respectively. L. lactis 210 did not have mutarotase activity above the base (spontaneous) rate. Growth on glucose decreased the L. lactis NRRL B-4449 specific mutarotase activity 29-fold, to almost undetectable levels. The L. lactis NRRL B-4449 mutarotase gene was overexpressed in E. coli BL21 under the control of the inducible T7lac promoter. Concentrated cell extracts prepared from induced cells exhibited high levels of mutarotase activity (38.7 U/mg of protein) compared to those in control BL21 cells transformed with pET19d. Histidine-tagged mutarotase was purified as described in Materials and Methods. The purified mutarotase (including 10 histidine residues) had an apparent molecular mass of approximately 40 kDa, as estimated by SDS-polyacrylamide gel electrophoresis (PAGE) (Fig. 3). Molecular masses of 37 to 40 kDa have been reported for galactose mutarotases (15, 23). The purified protein had a specific mutarotase activity of 67 U/mg (Table 3).
TABLE 3.
Mutarotase activities in L. lactis IO-1, L. lactis 210, and L. lactis NRRL B-4449 and of overexpressed L. lactis 210 XylM purified from recombinant E. coli BL21
| Sample | Mutarotation ratea(R2) | Enzymatic mutarotation (U)b | Specific mutarotase activity (U/mg of protein) |
|---|---|---|---|
| L. lactis 210 lysate | 5.12 (0.66) | 0 | 0 |
| Xylose standard (spontaneous) | 5.13 (0.92) | ||
| L. lactis IO-1 lysate | 9.08 (0.87) | 2.34 | 2.9 |
| Xylose standard | 6.74 (0.93) | ||
| L. lactis NRRL B-4449 lysate | 5.93 (0.93) | 1.03 | 0.9 |
| Xylose standard | 4.90 (0.98) | ||
| L. lactis NRRL B-4449 glucose-grown lysate | 4.81 (0.95) | 0.13 | 0.1 |
| Xylose standard | 4.67 (0.97) | ||
| Overexpressed crude XylM | 7.59 (0.93) | 1.16 | 38.7 |
| B21 control | 6.43 (0.94) | ||
| Purified XylM | 9.66 (0.94) | 2.68 | 67.0 |
| Xylose standard | 6.98 (0.98) |
Mutarotation rate is the change in the relative beta peak height per minute.
Enzymatic mutarotation is the sample rate minus the spontaneous rate.
FIG. 3.
SDS-PAGE analysis of L. lactis recombinant mutarotase-His tag fusion protein purified from E. coli BL21 using nickel column chromatography. Lane 1, molecular weight markers (low series; Bio-Rad); lane 2, nickel column eluate from recombinant E. coli BL21 carrying L. lactis 210 xylM; lane 3, flowthrough of nickel column eluate from recombinant E. coli BL21 carrying L. lactis 210 xylM; lane 4, nickel column eluate from recombinant E. coli BL21 carrying L. lactis NRRL B-4449 xylM; lane 5, flowthrough of nickel column eluate from recombinant E. coli BL21 carrying L. lactis NRRL B-4449 xylM; lane 6, nickel column eluate from recombinant E. coli BL21 carrying L. lactis IO-1 xylM; lane 7, flowthrough of nickel column eluate from recombinant E. coli BL21 carrying L. lactis IO-1 xylM.
Xyloside transport.
A 1,485-bp ORF, located 53 to 54 nt downstream of the xylM stop codon, encompasses a putative xyloside transport gene, xynT. XynT appears to be a hydrophobic protein (232 hydrophobic residues out of 494) with at least 11 transmembrane domains, as predicted by a Kyte-Doolittle hydrophobicity plot (18a) (data not shown). XynT is homologous to a variety of di- and trisaccharide transporters, with 40 to 46% amino acid similarity to E. coli glucuronide permease (UidB), lactose permease of E. coli and S. thermophilus (LacY), Pediococcus raffinose carrier protein (RafF), and melibiose carrier protein of Salmonella enterica serovar Typhimurium (MelB). Our prediction that the product of xynT could be involved in xyloside transport is also based upon its location upstream of xylan metabolism genes (see below). XynT is also 44% similar to a putative Lactobacillus pentosus proton symporter (XylP) that has recently been demonstrated to transport α-xylosides (9). The xynT gene is not homologous to the xylT (Lactobacillus brevis) or xylE (E. coli) xylose transporter genes.
Xylosidase.
The L. lactis 210 downstream xylM inverse PCR product contained one additional 806-bp ORF, beginning 24 nt downstream of xynT. We have sequenced 457 and 770 nt of this ORF from L. lactis IO-1 and L. lactis NRRL B-4449, respectively. The product of the ORF has strong similarity to the first 264 amino acids of β-1,4-xylosidases from Bacillus subtilis (GenBank accession number G69735), Bacillus pumilus (P07129), and E. coli (P77713). It is also quite similar to broad-specificity Selenomonas ruminatum xylosidase-arabinosidase Xsa (GenBank accession number AAB97967). Xylosidases are typically intracellular enzymes that further break down xylosides to xylose. The product of our ORF, which we have named xynB, is 57% identical and 66% similar to Selemonas Xsa and 52% identical and 64% similar to B. subtilis XynB (data not shown). To a lesser degree, our XynB is also homologous to endoarabinases from B. subtilis and Pseudomonas fluorescens.
Xylan-degrading activity.
L. lactis IO-1 and L. lactis NRRL B-4449 did not show significant increased growth in M17 medium (a carbon-limited complex medium) plus xylan versus in M17 medium alone (data not shown). Also, IO-1 did not grow better in M17medium–0.25% xylobiose than in M17 medium alone (data not shown). To determine if these strains possess any xylan-degrading activity, xylanase and xylosidase assays were performed with culture supernatants, cytoplasmic extracts, and membrane fractions. Although no xylanase activity was detected in the supernatants, the cytoplasmic extracts of L. lactis IO-1 and L. lactis NRRL B-4449 produced enzymatic activities of 1 × 10−3 and 6 × 10−4 U mg of protein−1, respectively. Xylosidase activity was detected in the cytoplasmic extracts and the membrane fractions of all three strains. The specific activities of β-xylosidases in the cytoplasmic extracts were 0.36, 0.003, and 0.01 U mg−1 for L. lactis IO-1, L. lactis 210, and L. lactis NRRL B-4449, respectively.
Transcription analysis.
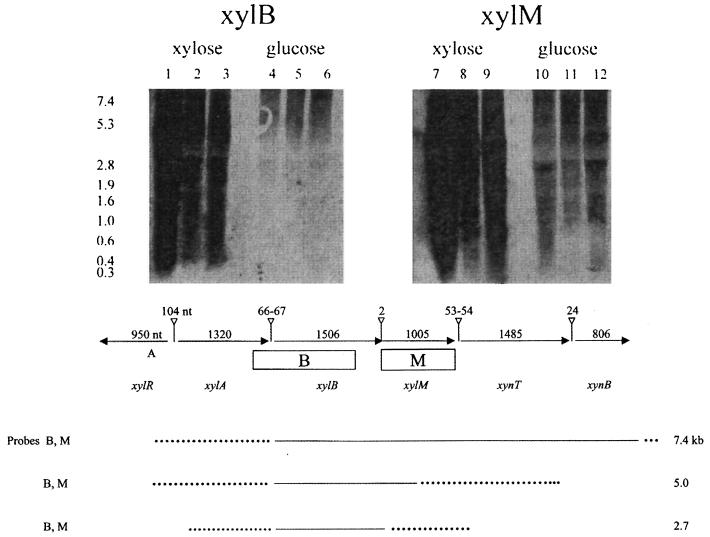
In order to determine the transcriptional relationships of our xylose and xylan genes, we performed Northern hybridizations with xylB and xylM as probes. Figure 4 shows the autoradiograph (top panel) and also schematically depicts our results (bottom panel) with RNA extracted from xylose-grown cultures of L. lactis IO-1, 210, and NRRL B-4449. The xylB probe overlaps the 3′ end of xylA, while the xylM probe contains the complete ORF and does not overlap any other genes. Three different xyl and xyn transcripts were produced in xylose-grown L. lactis 210, IO-1, and NRRL B-4449. Both probes hybridized to 7.4-, 5.0-, and 2.7-kb transcripts. The 7.4-kb transcript is long enough to encompass xylABM and xynTB. The 5.0-kb transcript could include either xylABM or xylBMxynT, due to the overlapping probes. Similarly, the 2.7-kb transcript could include xylAB or xylBM. These transcripts were present at much higher levels in L. lactis 210 than in L. lactis IO-1 or NRRL B-4449 (Fig. 4, top panel), perhaps accumulating because the inducing signal (xylose) could not be metabolized. In contrast, the three transcripts were not detectable in glucose-grown cells even after very long exposure times (Fig. 4, top panel). Although neural network analysis (26; Reese et al., Electronic Proc. 1996 Pacific Symp. Biocomput.) suggests the existence of potential promoters upstream of each of the xyl and xyn genes (data not shown), it appears from the Northern hybridization data that the xyl genes are cotranscribed and may also be cotranscribed with the xyn genes.
FIG. 4.
(Top) Autoradiograph of RNA isolated from xylose-grown and glucose-grown L. lactis IO-1, L. lactis 210, and L. lactis NRRL B-4449 probed with a region including xylB and xylM. Lanes 1, 4, 7, and 10, L. lactis 210; lanes 2, 5, 8, and 11, L. lactis NRRL B-4449; lanes 3, 6, 9, and 12, L. lactis IO-1. (Bottom) Transcription analysis of the xyl and xyn loci of xylose-grown L. lactis IO-1, L. lactis 210, and L. lactis NRRL B-4449. The top portion of panel B shows the organization and sizes of the genes (indicated by horizontal arrows) and intergenic regions (vertical lines ending in open triangles) and the locations of the Northern probes (rectangles). The bottom portion of panel B shows the predicted transcript boundaries (solid lines or, where there is uncertainty, broken lines). The transcripts (sizes indicated on the right) which hybridized to the probes (listed on the left) could lie anywhere within the depicted boundaries.
DISCUSSION
Many lactic acid bacteria are found in plant environments, but xylan metabolism has not been described for these bacteria. Xylose metabolism has been described for and characterized in lactic acid bacteria but is rare in L. lactis. We have discovered a metabolic pathway with the potential to metabolize xylan to xylose and to xylulose-5-phosphate in L. lactis. This xylan pathway is encoded by six genes clustered together at a single locus: xylRABM-xynTB.
Although L. lactis IO-1, L. lactis 210, and L. lactis NRRL B-4449 were not able to grow on xylan or xylobiose, we found ORFs encoding a putative xyloside transporter (xynT) and part of a xylosidase (xynB). Our XynB is highly homologous to characterized β-1,4-xylosidases from B. subtilis, B. pumilus, and Butyrivibrio fibrisolvens, as well as a xylosidase-arabinosidase from the rumen bacterium Selenomonas. Our partial ORF encodes over one-half (268 amino acids) of a typical xylosidase (517 to 535 amino acids). We identified the remainder of the xynB sequence in the low-redundancy genomic sequence database of L. lactis IL-1403 (Institut National de la Recherche Agronomique [INRA], Jouy en Josas, France), and its similarity to other xylosidase genes was confirmed by a BLASTX search.
L. lactis IO-1 exhibited a significant level of intracellular xylosidase activity compared to other known xylosidase producers. Specific xylosidase activities of 1.2 U mg−1, 0.21 U mg−1, and 0.09 U mg−1 have been previously reported for Aspergillus nidulans (18), Bacillus sp. strain K-1 (25), and Clostridium cellulolyticum (29). Low xylosidase activities were also detected in the cytoplasmic extracts of L. lactis 210 and L. lactis NRRL B-4449.
We further tested the xylanolytic activity with a general xylanase assay measuring the release of reducing sugars from xylan. The initial breakdown of xylan involves extracellular xylanase activity, but this activity was not detected in the culture supernatants of the three lactococcal strains tested. Instead, we detected activities in the cytoplasmic extracts of L. lactis IO-1 and L. lactis NRRL B-4449, but they were not significant compared to those of other known xylanases. Xylanase specific activities as high as 9.3 U mg−1 and 4.8 U mg−1 have been reported for Aspergillus ochraceus 42 (2) and Bacillus sp. strain K-1 (25), respectively. We suspected that the xylanase activity was in fact a xylosidase activity encoded by xynB.
We have not identified a putative xylanase gene in L. lactis, but the genomic sequence of L. lactis IL-1403 revealed the presence of a gene highly homologous to the endo-1,4-β-xylanase D gene from a different species. This gene (nt 281456 f to 282572 f) is located far from the xylose-xyloside operon (nt 1543238 r to 1547484 r) that we identified. However, even if Lactococcus has the genetic potential to effectively degrade xylan, our three strains, including the plant environmental isolates L. lactis NRRL B-4449 and L. lactis IO-1, seem to have lost that function. It is possible that L. lactis has evolved to exploit the xylanase secretion of other bacteria in the environment, transporting the resulting xylo-oligosaccharides for further metabolism.
We hypothesize that the ORF downstream of xynB encodes a xyloside transporter, XynT. Previous characterization of oligoxyloside transport has implicated an ATP-binding cassette (ABC) transport system. When an ATP-binding component of an ABC transport system, msiK, is inactivated in Streptomyces lividans, the transport of cellobiose and xylobiose is abolished (17). Genes encoding two potential membrane components of an ABC xyloside transporter, xynB and xynC, have been sequenced from Thermoanaerobacterium thermosulfurigenes (GenBank accession number U50952). Although our XynT is not homologous to these ABC proteins, several pieces of evidence support its putative function. First, XynT is homologous to known di- and trisaccharide transporters as well as an α-xyloside transporter (encoded by the xylP gene) recently described by Chaillou et al. (9). Second, xynT is located between the xylose mutarotase and xylosidase genes, downstream of the xylose metabolic locus. B. subtilis has a similar organizational relationship for the xyl and xyn loci (xynC-xynB-xylR-xylA-xylB0) (16). Third, almost half of the XynT amino acid residues (232 of 494) are hydrophobic, indicative of a membrane-associated protein. Another gene located further downstream of xynB and showing high homology to d-xylose, hexose, or monosaccharide transporter genes is included in the lactococcal genome database (INRA). This finding further supports our hypothesis that XynT is involved in xyloside rather than xylose transport.
We discovered a new function related to xylan metabolism and encoded immediately downstream of xylB by xylM. This mutarotase function could link together xylose metabolism and xylan metabolism. The first enzyme in the xylose utilization pathway, xylose isomerase, has a marked preference for α-d-xylose over β-d-xylose (14, 32). However, the end product of xylan breakdown is β-d-xylose. Although xylose mutarotation occurs spontaneously in solution, the actual rates in vivo are not known. The spontaneous rate of galactose mutarotation was shown to be insufficient for E. coli growth on lactose, because a deletion of the mutarotase gene (galM) resulted in very slow growth (7). If a bacterium transported and metabolized only free xylose, a mutarotase would not be necessary. If a bacterium metabolized xylan or xylo-oligosides, a xylose mutarotase could give an additional competitive advantage because of the relatively high initial concentration of β-xylose. The xylM gene could be part of the multicistronic xylose operon because of its close proximity to the stop codon of xylB. In addition, xylA and xylM probes both hybridized to a 7-kb mRNA transcript (among other transcripts), large enough to encompass xylA, xylB, and xylM.
Recently, other mutarotase sequences from L. lactis subsp. lactis ATCC 7962 and L. lactis subsp. cremoris MG1363 have been deposited in GenBank (accession numbers U60828 and AJ011653, respectively). These mutarotases are reportedly involved in lactose and galactose metabolism via the Leloir pathway. Our XylM sequence is approximately 28% identical and 46% similar to these GalM sequences. The dramatically increased xylose mutarotase activity that we observed upon overexpression and purification of L. lactis NRRL B-4449 XylM confirmed the existence of a mutarotase separate from galactose mutarotases.
In conclusion, we have discovered the genetic potential in L. lactis for two related metabolic capacities, xylose utilization and xylan utilization, encoded by the xylRABM and xynTB genes. Based on transcription analysis, these genes are organized in what could be a single xylABM-xynTB operon, potentially regulated by xylR. The Xyl− dairy starter culture strain L. lactis 210 has experienced a loss of function in an environment no longer selective for xylose metabolism. Plant environmental isolates such as L. lactis NRRL B-4449 and L. lactis IO-1 retain the ability to metabolize xylose. Despite the functioning xylose metabolism and xylosidase capability of L. lactis IO-1 and L. lactis NRRL B-4449, these two strains could not grow on xylan or xylobiose. Further studies are necessary to elucidate the metabolic defect. Finally, we propose a new enzyme associated with xylan metabolism, a mutarotase encoded by xylM, that may speed the further metabolism of xylose released from xylan.
ACKNOWLEDGMENTS
We thank David Wilson and Diana Irwin for help with the xylosidase and xylanase assays. We thank Alexei Sorokin (INRA, Jouy en Josas, France) for providing us with information about the genomic sequence of L. lactis IL-1403.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Meyers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Amitabha D, Geeta N. Production of xylanolytic enzymes during growth on pulverized grass by Aspergillus ochraceus-42. Lett Appl Microbiol. 1995;20:141–144. [Google Scholar]
- 3.Basaran P. M.S. thesis. Ithaca, N.Y: Cornell University; 1996. [Google Scholar]
- 4.Bastawade K B. Xylan structure, microbial xylanases and their mode of action. World J Microbiol Biotechnol. 1992;8:353–368. doi: 10.1007/BF01198746. [DOI] [PubMed] [Google Scholar]
- 5.Biely P. Microbial xylanolytic systems. Trends Biotechnol. 1985;3:286–290. [Google Scholar]
- 6.Bor Y-C, Moraes S-P, Lee S-P, Crosby W L, Sinskey A J, Batt C A. Cloning and sequencing the Lactobacillus brevisgene encoding xylose isomerase. Gene. 1992;114:127–131. doi: 10.1016/0378-1119(92)90718-5. [DOI] [PubMed] [Google Scholar]
- 7.Bouffard G G, Rudd K E, Adhya S L. Dependence of lactose metabolism upon mutarotase encoded in the gal operon in Escherichia coli. J Mol Biol. 1994;244:269–278. doi: 10.1006/jmbi.1994.1728. [DOI] [PubMed] [Google Scholar]
- 8.Chaillou S, Bor Y-C, Batt C A, Postma P W, Pouwels P H. Molecular cloning and functional expression in Lactobacillus plantarum 80 of xylT, encoding the d-xylose-H+ symporter of Lactobacillus brevis. Appl Environ Microbiol. 1998;64:4720–4728. doi: 10.1128/aem.64.12.4720-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaillou S, Lokman B C, Leer R J, Posthuma C, Postma P W, Pouwels P H. Cloning, sequence analysis, and characterization of the genes involved in isoprimeverose metabolism in Lactobacillus pentosus. J Bacteriol. 1998;180:2312–2320. doi: 10.1128/jb.180.9.2312-2320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford D L, Crawford R L. Microbial degradation of lignocellulose: the lignin component. Appl Environ Microbiol. 1976;31:374–380. doi: 10.1128/aem.31.5.714-717.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czajka J, Batt C A. Verification of causal relationships between Listeria monocytogenesisolates implicated in food-borne outbreaks of listeriosis by randomly amplified polymorphic DNA patterns. J Clin Microbiol. 1994;32:1280–1287. doi: 10.1128/jcm.32.5.1280-1287.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis E O, Henderson P J F. The cloning and DNA sequence of the gene xylE for xylose-proton symport in Escherichia coliK-12. J Biol Chem. 1987;192:583–587. [PubMed] [Google Scholar]
- 13.Erlandson K A, Park J-H, El Khal W, Kao H-H, Basaran P, Brydges S, Batt C A. Dissolution of xylose metabolism in Lactococcus lactis. Appl Environ Microbiol. 1999;66:3974–3980. doi: 10.1128/aem.66.9.3974-3980.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feather S M, Deshpande V, Lybyer M J. Anomeric specificity during some isomerase reactions. Biochem Biophys Res Commun. 1970;38:859–863. doi: 10.1016/0006-291x(70)90799-0. [DOI] [PubMed] [Google Scholar]
- 15.Gatz C, Altshmied J, Hillen W. Cloning and expression of the Acinetobacter calcoaceticus mutarotase gene in Escherichia coli. J Bacteriol. 1986;168:31–39. doi: 10.1128/jb.168.1.31-39.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastrup S. Analysis of the Bacillus subtilis xylose regulon. In: Ganesan A T, Hoch J A, editors. Genetics and biotechnology of bacilli. Vol. 2. New York, N.Y: Academic Press, Inc.; 1988. pp. 79–83. [Google Scholar]
- 17.Hurtubise Y, Shareck F, Kluepfel D, Morosoli R. A cellulase/xylanase-negative mutant of Streptomyces lividans1326 defective in cellobiose and xylobiose uptake is mutated in a gene encoding a protein homologous to ATP-binding proteins. Mol Microbiol. 1995;17:367–377. doi: 10.1111/j.1365-2958.1995.mmi_17020367.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Ramon D. Purification and regulation of the synthesis of a β-xylosidase from Aspergillus nidulans. FEMS Microbiol Lett. 1996;135:287–293. [Google Scholar]
- 18a.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 19.Lawlis V B, Dennis M S, Chen E Y, Smith D H, Henner D J. Cloning and sequencing of the xylose isomerase and xylulokinase genes of Escherichia coli. Appl Environ Microbiol. 1984;47:15–21. doi: 10.1128/aem.47.1.15-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lokman B C, Van Santen P, Verdoes J C, Kruse J, Leer R J, Posno M, Pouwels P H. Organization and characterization of three genes involved in d-xylose catabolism in Lactobacillus pentosus. Mol Gen Genet. 1991;230:161–169. doi: 10.1007/BF00290664. [DOI] [PubMed] [Google Scholar]
- 21.Lokman B C, Leer R J, van Sorge R. Promoter analysis and transcriptional regulation of Lactobacillus pentosusgenes involved in xylose catabolism. Mol Gen Genet. 1994;245:117–125. doi: 10.1007/BF00279757. [DOI] [PubMed] [Google Scholar]
- 22.Miller G L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem. 1959;31:426–428. [Google Scholar]
- 23.Poolman B, Royer T, Mainzer S E, Schmidt B F. Carbohydrate utilization in Streptococcus thermophilus: characterization of the genes for aldose 1-epimerase (mutarotase) and UDP glucose 4-epimerase. J Bacteriol. 1990;172:4037–4047. doi: 10.1128/jb.172.7.4037-4047.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prade R A. Xylanases: from biology to biotechnology. Biotechnol Genet Eng Rev. 1995;13:101–131. doi: 10.1080/02648725.1996.10647925. [DOI] [PubMed] [Google Scholar]
- 25.Ratanakhanokchai K, Kyu K L, Tanticharoen M. Purification and properties of a xylan-binding endoxylanase from alkaliphilic Bacillussp. strain K-1. Appl Environ Microbiol. 1999;65:694–697. doi: 10.1128/aem.65.2.694-697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reese M G. Diploma thesis. Heidelberg, Germany: German Cancer Research Center; 1994. [Google Scholar]
- 27.Rosenfeld S A, Stevis P E, Ho W Y. Cloning and characterization of the xyl genes from Escherichia coli. Mol Gen Genet. 1984;194:410–415. doi: 10.1007/BF00425552. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 2. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Saxena S, Fierobe H-P, Gaudin C, Guerlesquin F, Belaich J-P. Biochemical properties of a β-xylosidase from Clostridium cellulolyticum. App Environ Microbiol. 1995;61:3509–3512. doi: 10.1128/aem.61.9.3509-3512.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheler A, Rygus T, Allmansberger R, Hillen W. Molecular cloning, structure, promoters and regulatory elements for transcription of the Bacillus licheniformis-encoded regulon for xylose utilization. Arch Microbiol. 1991;155:526–534. doi: 10.1007/BF00245345. [DOI] [PubMed] [Google Scholar]
- 31.Schmiedel D, Kintrup M, Kuster E, Hillen W. Regulation of expression, genetic organization and substrate specificity of xylose uptake in Bacillus megaterium. Mol Microbiol. 1997;23:1053–1062. doi: 10.1046/j.1365-2958.1997.2881654.x. [DOI] [PubMed] [Google Scholar]
- 32.Schray K J, Rose I A. Anomeric specificity and mechanism of two pentose isomerases. Biochemistry. 1971;10:1058–1062. doi: 10.1021/bi00782a019. [DOI] [PubMed] [Google Scholar]
- 33.Shifman I M, Stein D G. A reliable and sensitive method for non-radioactive Northern blot analysis of nerve growth factor mRNA from brain tissues. J Neurosci Methods. 1995;59:205–208. doi: 10.1016/0165-0270(94)00184-i. [DOI] [PubMed] [Google Scholar]
- 34.Sizemore C, Buchnere E, Rygus T, Witke C, Goetz F, Hillen W. Organization, promoter analysis and transcriptional regulation of the Staphylococcus xylosusxylose utilization operon. Mol Gen Genet. 1991;227:377–384. doi: 10.1007/BF00273926. [DOI] [PubMed] [Google Scholar]
- 35.Song S, Park C. Organization and regulation of the d-xylose operons in Escherichia coliK-12: XylR acts as a transcriptional activator. J Bacteriol. 1997;179:7025–7032. doi: 10.1128/jb.179.22.7025-7032.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson J A. Molecular biology of xylan degradation. FEMS Microbiol Rev. 1993;104:65–82. doi: 10.1111/j.1574-6968.1993.tb05864.x. [DOI] [PubMed] [Google Scholar]
- 37.van der Vossen J, van der Lelie D, Venema G. Isolation and characterization of Streptococcus cremorisWg2-specific promoters. Appl Environ Microbiol. 1987;53:2452–2457. doi: 10.1128/aem.53.10.2452-2457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zantinge H D, Wessels J G H. Comparison of poly(A)-containing RNAs in different cell types of the lower eukaryote Schizophyllum commune. J Biochem. 1979;101:251–260. doi: 10.1111/j.1432-1033.1979.tb04238.x. [DOI] [PubMed] [Google Scholar]