Abstract
Objectives
Composite resins are the most preferred filling material because of their excellent aesthetic qualities. However, a filling material should also be biocompatible as well as aesthetic. The aim of this study was to determine the serum and saliva bisphenol-A (BPA) levels and to examine the effects of serum BPA on reproductive hormone levels after healthy men were treated with composite fillings.
Methods
Eighteen healthy males each received 2 composite restorations. Saliva and blood samples of subjects were collected before resin application and 1 day and 1, 3, and 5 weeks after the resin was applied. BPA amounts in samples were detected using high-performance liquid chromatography (HPLC). Serum gonadotropins, testosterone, sex hormone binding globulin, free androgen index, and oestrogen levels were measured with radioimmunological assay kits. Statistical analysis of data was made using Friedman, Wilcoxon signed ranks and Mann-Whitney U tests (α = 0.05).
Results
The amount of BPA released from composite resins over time was not significantly elevated in either saliva or serum (P > 0.5). In addition, serum BPA levels were significantly higher than saliva BPA levels for both composites (P < .05), but saliva and serum BPA levels were not statistically different when comparing the 2 composites (P > .05).
Conclusions
BPA from composite resins used in this study did not significantly alter serum hormone levels.
Key words: Composite resin, Oestrogen, Free androgen index, Gonadotropins, Testosterone, Sex hormone binding globulin
Introduction
Bisphenol A (BPA: 2,2-bis(-hydroxyphenyl) propane, CAS No: 80-05-7) is an industrial chemical that was synthesized in 1891 for the first time and was found in the 1930s to have oestrogenic effects. With an annual production of more than 2 million tons today, BPA is the main monomer used in the production of polycarbonate plastics and epoxy resins. Polycarbonate plastics are used in the manufacturing of dental resins, baby bottles, food storage containers, water bottles and bottle caps, glass lenses, CDs, DVDs, and electronic devices.1,2 Exposure to BPA is thought to have side effects on human health, particularly during infant development, which is a considerable public health problem. However, there are few basic studies on its possible effects on human health.1, 2, 3
Composite resins are the most preferred filling material today because of their excellent aesthetic qualities. The composite resins used in dentistry consist of resin matrix (organic phase) and fillers. Resin is the chemically active component of the composite. The most widely used monomers in resin matrix are urethane dimethacrylate (UDMA), bisphenol A glycidyl methacrylate (BISGMA), and triethylene glycol dimethacrylate (TEGDMA).4
Pure BPA is not found in dental products. BPA, BISGMA, and bisphenol A dimethacrylate (BISDMA) are the major resin monomers. If synthetic reactions cannot be completed cytokiometrically during the production of dental sealants containing BISGMA, BPA may be present in the product in an impure form. Composite resins are exposed to mechanical, bacterial, or thermal biodegradation in the oral cavity.5 BPA can also be found as a degradation product of BISDMA via salivary esterases that can hydrolyse the ester bond found in BISDMA monomers.6 As a result, BPA is absorbed into the blood from the gastrointestinal tract and redistributed to other tissues and conjugated in the liver in high doses to create BPA glucuronide, an important metabolite excreted in urine.7,8
BPA is thought to be an endocrine-disrupting chemical with toxic effects on reproduction.9 In animal studies conducted on rodents and in vitro studies, it has been shown that BPA has both oestrogenic and antiandrogenic effects.10, 11, 12 Studies have also shown that BPA exposure may have some side effects such as decreased epididymal or testicular sperm count, decreased sperm motility and speed, decreased epididymal weight, impaired insulin signal and glucose homeostasis, decreased steroidogenesis in the testis, and reduced serum follicle-stimulating hormone (FSH) and testosterone levels in the male reproductive system.13, 14, 15, 16, 17, 18 However, these observations are largely based on animal studies or in vitro experiments.
Because of exposure to environmental BPA and oestrogenic activity in vitro and in vivo, the potential negative effects of BPA exposure on human reproductive health have been a source of concern and have become a topic for further study.19 A number of epidemiological studies have been conducted on BPA levels measured in humans and its sources, and it has been concluded that dietary intake is the principal BPA source.20 Once BPA enters the body, it acts as an oestrogen agonist and androgen antagonist and may disrupt normal cell function and affect human health.11,21 However, only a few epidemiological studies have investigated the relationship between BPA exposure and health-related end points, and as a result, human studies on the possible health effects of BPA exposure are limited.22
The aim of this study was to compare the relationship between the levels of BPA released from composite fillings into the saliva and serum in healthy males, and serum FSH, luteinizing hormone (LH), testosterone (T), sex hormone binding globulin (SHBG), free androgen index (FAI), and oestrogen (E2).
The null hypotheses of the study are as follows:
-
1.
There is no difference between the amounts of BPA released from composite resins into saliva or serum over time.
-
2.
The amount of BPA released is similar between different composite types.
-
3.
The amount of BPA released into saliva and serum is similar for each composite resin.
-
4.
Serum hormone levels did not change over time compared to baseline levels.
Materials and methods
Subjects and study design
This study was carried out with the ethical approval of Atatürk University Faculty of Dentistry Ethics Committee (20.11.2014/032) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all individual participants included in the study. This study was carried out on a total of 18 male subjects aged 19 to 24 years (21.11 ± 1.32) who were admitted to Atatürk University, Faculty of Dentistry, Restorative Dentistry Clinic.
G*Power 3.1.9.4 software (Heinrich-Heine Dusseldorf University, Dusseldorf, Germany) was used to determine the sample size based on using the following parameters: 80% power, 0.58 effect size, and α error at 0.05. A minimum sample size of 18 participants was assessed to be appropriate.
Inclusion criteria were as follows:
-
-
No systemic diseases.
-
-
Have not taken any medication in the last 3 months.
-
-
No smoking or alcohol use.
-
-
No bruxism or a habit of chewing gum frequently.
-
-
No filling materials in the mouth and no periodontal disease.
In the study, 2 composite materials (Charisma [Heraeus Kulzer GmbH] and Grandio [VOCO GmbH]) were applied according to the manufacturer's instructions. Clearfil S3 Bond (Kuraray) was used as a bonding agent. The information about the applied materials is given in Table 1.
Table 1.
Details of investigated materials.
| Materials | Content |
Manufacturer | Lot No | |
|---|---|---|---|---|
| Matrikx | Filler | |||
| Charisma | BISGMA, TEGDMA | 58 vol%, 78 wt%, Barium aluminum, fluoride glass Silicium dioxide | Heraeus Kulzer GmbH, Hanau, Germany | 010024 |
| Grandio | BISGMA, TEGDMA, UDMA | 71.4 vol%, 87 wt%, Glass-ceramic particles | VOCO GmbH Cuxhaven, Germany | 1525390 |
| Clearfil S3 Bond | BISGMA, HEMA | Colloidal silica | Kuraray, Okayama, Japan | 8R0053 |
BISGMA, bisphenol A-glycidyldimethacrylate; HEMA, 2-hydroxyethyl methacrylate; TEGDMA, triethylene glycol dimethacrylate; UDMA, urethane dimethacrylate.
The restorative materials were applied according to the manufacturer's instructions. The composite filling materials were given an anatomical form by placing into cavities not exceeding 2 mm in depth as 1 piece (bulk method) and polymerized for 40 seconds by using a light source (Elipar Freelight II, 3M-ESPE Dental Products). The wavelength of the light source was 430-480 nm and the light intensity was about 1200 mW/cm2. During the polymerization process, the tip of the light source was kept as close as possible to the restoration. The intensity of the light device was measured using a radiometer (Hilux Ultra Plus Curing Units; Benlioglu Dental). Finishing and polishing operations were completed using discs (Sof-Lex; 3M ESPE Dental Products). The amount of composite resin applied to each individual was calculated using the method that Gul et al23 used and the amount of applied composite resin was calculated in grams.
Individuals were asked not to eat on each test day and the fillings were applied between 9 am and 12 pm. Saliva and blood samples were taken prior to the application of the filling (baseline) and 1 day, 1 week, 3 weeks, and 5 weeks after the fillings were applied. Saliva samples were taken into sealed Eppendorf tubes, blood samples were taken into biochemical tubes and both samples were centrifuged at 3500 rpm for 10 minutes.23,24 The obtained samples were stored at −80 °C until analysis. The samples were sent to Atatürk University Medical Faculty, Biochemistry Laboratory for BPA and hormone analysis.
High-performance liquid chromatography analysis
HPLC conditions: The high-performance liquid chromatography (HPLC) device (Agilent Technologies 1100 Series) was used with a UV detector (Agilent Technologies 1100 Series) and chromatographic separation was performed on a BRISA LC2 C18 (250 × 3 mm, 5 µm; Technochroma). The mobile phase solvents were acetonitrile (A) and water (B) (70 A: 30 B, v/v), flow rate of 1.0 mL/min, and detection wavelength was set at 270 nm. The sample injection volume was 20 μL and the column temperature was room temperature.
Preparations of stock, serum calibration, and quality control solutions: The high purity BPA standard (CAS NO: 80-05-7) obtained from Sigma Aldrich was used for HPLC analysis. The stock solution of BPA (0.1 mg/mL) were prepared in acetonitrile. This solution was diluted to give the intermediate stock solution (1000 ng/mL). The calibration and quality control (QC) solutions for both saliva and serum were prepared by spiking into blank human serum and saliva with different standard BPA solutions to give final concentrations for BPA of 0.2-20 ng/mL for calibration and 0.5, 5, and 15 ng/mL for QC.
Extraction procedure of BPA from serum: This method was modified from the extraction method developed by Kardas et al25: 1 mL of human serum was placed in the Eppendorf tube and later, 200 µL of CH3COONH4 buffer (0.01 M, pH: 4.5) and 5 mL mixed solvent of n-hexane and diethyl ether were added. The mixture was briefly vortexed and 5 mL n-hexane and diethyl ether was added twice. The solution was vortexed for 5 minutes, centrifuged at 5000g for 5 minutes, and the supernatant layer was transferred and evaporated to dryness under nitrogen stream. The resulting samples were dissolved in 100 µL acetonitrile and injected (20 µL) into the HPLC system.
Extraction procedure of BPA from saliva: This method was modified from the extraction method developed by Demirkaya and Kadioglu26: 1 mL of sample was mixed with 0.5 mL of acetonitrile, vortexed for 1 minute and mixed in the shaker for 15 minutes at room temperature. Then, the solutions were centrifuged at 1200g for 10 minutes. The supernatant layer was separated and injected (20 µL) into the HPLC system.
Hormone analysis
E2, FSH, LH, testosterone, SHBG, and FAI levels in the obtained serum samples were measured according to the manufacturer's recommendations using commercial immunoassay kits (Beckman Coulter Inc.). E2 (Lot No: 671144) levels were determined in pg/mL, FSH (Lot No: 671161) and LH (Lot No: 771005) levels were in mIU/mL, testosterone (Lot No: 631414) levels were in ng/mL, and SHBG (Lot No: 630345) levels were determined in nmol/L. FAI levels were calculated as a % (testosterone × 100/SHBG).
Reference values were taken as 20-47 pg/mL for E2, 1.27-19.26 mIU/mL for FSH, 1.24-8.62 mIU/mL for LH, 1.75-7.81 ng/mL for testosterone, and 14.5-48.4 nmol/L for SHBG.
Statistical analysis
The data obtained were analysed using SPSS 18 statistical package software (IBM). First, the Kolmogorov-Smirnov test was used to determine whether the data showed normal distribution. The Friedman test was used to compare the amount of BPA levels released from both composites into saliva and serum over time and the changes in serum hormone levels during these time periods. The Wilcoxon signed rank test was used to determine the difference between groups in case of a difference between groups. The Mann-Whitney U test was used to compare the composite resins in terms of BPA amounts released. In addition, Wilcoxon signed ranks test was used to compare the amount of BPA released into saliva and serum for each composite resin. The Spearman correlation test was used to determine the relationship between weights of composite resins and the amount of BPA released into saliva and serum, as well as any relationship between serum BPA levels and hormone levels. Statistical significance was established as P < .05.
Results
HPLC diode-array detection (DAD) analysis, which is a powerful quantification method, was used in this study to determine the BPA level in serum and saliva. BPA showed the maximum peak at 270 nm and the retention time was 5.7 minutes according to the chromatograms. The chromatogram obtained from blank serum containing endogenous BPA and serum spiked with 1 ng/mL BPA are given in Figures 1A and B, respectively. The same way, the chromatogram obtained from blank saliva containing endogenous BPA and saliva spiked with 1 ng/mL BPA are shown in Figures 2A and B, respectively. There exists endogenous BPA in the pooled blank serum and saliva. So, standard addition method for the calibration curve was used in serum and saliva to determine endogenous levels of BPA. The different BPA concentrations were added into both blank serum and blank saliva. Calibration curves were obtained by plotting between peak areas and added BPA concentrations. The equations for calibration curves for both serum and saliva were obtained as y = 1.4571x + 0.6789 (R2 :0.9986, n:6) and y = 1.4952x + 0.6390 (R2: 0.9992, n:6) in the 0.2-20 ng/mL concentration range for determination of BPA and the endogenous BPA for both serum and saliva were calculated to be 1.12 ng/mL and 0.4 ng/mL, respectively.
Fig. 1.
HPLC-DAD chromatograms (A) Blank serum (1.12 ng/mL endogenous BPA) (B) Blank serum spiked BPA (1 ng/mL). BPA, bisphenol A; HPLC-DAD, high-performance liquid chromatography diode-array detection.
Fig. 2.
HPLC-DAD chromatograms (A) Blank saliva (0.4 ng/mL endogenous BPA) (B) Blank saliva spiked BPA (1 ng/mL). BPA, bisphenol A; HPLC-DAD, high-performance liquid chromatography diode-array detection.
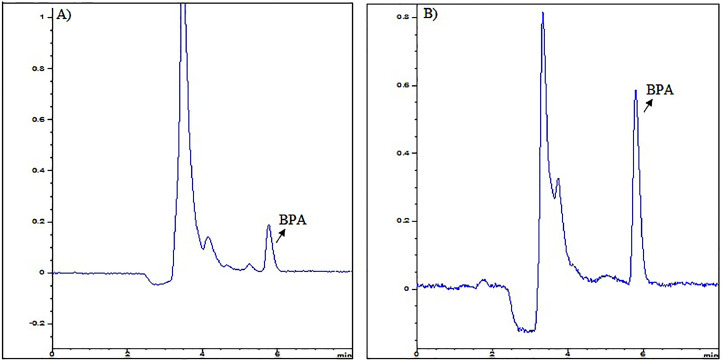
The method precision and accuracy were achieved by analyzing the intra-day (6 times per day) and inter-day (6 times once daily for 6 days) on QC samples (0.5, 5 and 15 ng/mL). The precision of the method for serum and saliva medium was given by the Relative Standard Deviation (% RSD) which calculated as 2.46% and 1.93%, respectively. The accuracy of the method was given by Percent Relative Error (RE%) and found as ±3.81% and ±2.45% for serum and saliva medium, respectively. The extraction recoveries of BPA in serum and saliva medium were determined to be 98.4 ± 4.21% and 99.5 ± 3.87% for all calibration concentration, respectively. According to the results, the method is accurate and precise for determination of BPA level in serum and saliva medium. The limit of quantification (LOQ) were calculated as 10 × σ (standard deviation of y-intercepts)/S (slope of the calibration curve) and found as 0.15 ng/mL for each medium. The developed and validated HPLC method was applied to quantify BPA in both serum and saliva samples of volunteers who exposure two different composite materials along 0 (baseline), 1 day, 1, 3 and 5 weeks. The obtained chromatograms from the subject after treatment for serum and saliva BPA were given the Figure 3.
Fig. 3.
HPLC-DAD chromatograms of the obtained from the subject after treatment for (A) serum (1.68 ng/mL BPA) (B) saliva (0.47 ng/mL BPA). BPA, bisphenol A; HPLC-DAD, high-performance liquid chromatography diode-array detection.
Table 2 shows the BPA levels released from 2 different composite resins into saliva and serum over time, the serum hormone levels measured in these time periods, and the results of the statistical comparison. As a result of the Kolmogorov Smirnov test on the distribution of the obtained data, it was determined that the data were not normally distributed (P < .05).
Table 2.
BPA levels released from 2 different composite resins into saliva and serum over time, the serum hormone levels measured in these time periods, and the results of the statistical comparison.
| Materials | Parameters | Baseline | 1 day | 1 week | 3 weeks | 5 weeks |
|---|---|---|---|---|---|---|
| Grandio | BPA saliva (ng/mL) | 0.29 ± 0.19A | 0.46 ± 0.23A | 0.52 ± 0.54A | 0.49 ± 0.29A | 0.34 ± 0.24A |
| BPA serum (ng/mL) | 1.42 ± 0.31A | 1.17 ± 0.34AB | 1.21 ± 0.31AB | 1.01 ± 0.16B | 1.49 ± 1.04AB | |
| E2 (pg/mL) | 36.00 ± 13.26A | 30.10 ± 18.81A | 40.70 ± 15.18A | 33.60 ± 16.24A | 33.70 ± 19.48A | |
| FSH (mIU/mL) | 4.38 ± 2.95A | 4.23 ± 3.56AB | 3.88 ± 2.78ABC | 3.70 ± 2.78BC | 3.55 ± 2.68C | |
| LH (mIU/mL) | 4.66 ± 2.44AB | 4.22 ± 2.36AB | 4.40 ± 1.91AB | 3.80 ± 1.29B | 2.86 ± 1.9C | |
| Testosterone (ng/mL) | 3.66 ± 0.82A | 3.94 ± 0.64A | 4.04 ± 0.80A | 4.67 ± 0.98B | 3.76±1.42A | |
| SHBG (nmol/L) | 21.27 ± 15.36A | 22.61 ± 15.77A | 21.54 ± 14.41A | 21.89 ± 14.15A | 24.63 ± 12.27A | |
| FAI | 0.71 ± 0.27AB | 0.70 ± 0.22AC | 0.78 ± 0.28AB | 0.85 ± 0.31B | 0.58 ± 0.26C | |
| Charisma | BPA saliva (ng/mL) | 0.20 ± 0.15A | 0.31 ± 0.27A | 0.33 ± 0.22A | 0.31 ± 0.23A | 0.28 ± 0.14A |
| BPA serum (ng/mL) | 1.27 ± 0.24A | 0.98 ± 0.08B | 1.39 ± 0.59AB | 1.06 ± 0.14AB | 1.66 ± 0.78A | |
| E2 (pg/mL) | 33.75 ± 17.74A | 34.25 ± 12.77A | 31.38 ± 21.39A | 31.75 ± 11.30A | 37.00 ± 17.27A | |
| FSH (mIU/mL) | 5.24 ± 2.98A | 4.95 ± 2.49A | 5.01 ± 2.86A | 4.96 ± 2.74A | 4.91 ± 2.70A | |
| LH (mIU/mL) | 4.07 ± 1.80AB | 3.99 ± 1.35A | 3.54 ± 1.35A | 4.96 ± 0.89B | 3.82 ± 1.47AB | |
| Testosterone (ng/mL) | 3.66 ± 0.60AC | 3.51 ± 0.79AB | 3.15 ± 0.66B | 3.69 ± 0.91ABC | 3.99 ± 0.75C | |
| SHBG (nmol/L) | 16.71 ± 4.24A | 15.69 ± 4.61B | 17.10 ± 5.15A | 18.49 ± 7.01AC | 19.65 ± 6.92C | |
| FAI | 0.80 ± 0.16A | 0.86 ± 0.24A | 0.66 ± 0.20B | 0.78 ± 0.29AB | 0.76 ± 0.19A |
BPA, bisphenol A; E2, oestrogen; FAI, free androgen index; FSH, follicle-stimulating hormone; LH, luteinizing hormone; SHBG, sex hormone binding globulin.
Data shown are mean ± SD. Different uppercase letters indicate a significant difference for values within that row.
Although the amount of BPA released from the composite resins was less than that observed in saliva, it was increased compared to baseline levels; however, this difference was not statistically significant (P > .05). In terms of the amount of BPA released into serum, it was found that the amounts of BPA released over time in both composite resins did not show a significant increase (P > .05). The Wilcoxon signed rank test performed to compare the levels of BPA in serum and saliva showed that the amount of BPA released in both composite resins was statistically significantly higher in serum compared to the amount released in the saliva (P < .05). Based on the results of the Mann-Whitney U test performed to compare composite resins in terms of amounts of BPA released into saliva and serum, no statistically significant difference was found between the 2 composite resins (P < .05).
When serum hormone levels were analysed over time after composite resins were applied, no significant difference was found in both composite resins in terms of E2 in all time periods (P > .05). When the measured amounts of FAI, FSH, and LH after application of both composite resins were compared, the levels of all 3 hormones significantly decreased compared to baseline for Grandio (P < .05), but this decrease was not statistically significant for Charisma (P > .05). Although there was an increase in testosterone, this was not significant compared to baseline (P > .05). SHBG increased in comparison with baseline, but this increase was not statistically significant (P > .05). Decreases and increases in the hormone levels did not go beyond the normal reference intervals in all time periods.
The mean filling weights were 0.05 ± 0.03 g and 0.10 ± 0.09 g for Grandio and Charisma, respectively. There was no statistically significant correlation between the filling weights and the BPA amounts released into serum and saliva for both composite resins (P > .05). In addition, there was no statistically significant correlation between BPA levels released into serum from both composite resins and hormones levels (P > .05).
Discussion
Composite resins are the most preferred filling material because of their excellent aesthetic qualities. However, a filling material should also be biocompatible as well as aesthetic. Biocompatibility is the ability of a material to be compatible with the biological functions of the tissue without showing toxic and harmful effects. Ideally, the dental materials used in the mouth should be harmless for oral tissues. Moreover, they should not cause systemic or local toxicity, mutagenicity, or carcinogenic effects.4,27
In this study, saliva and serum BPA levels were measured after 2 different composite resins were applied to healthy male subjects, and the effect of BPA on reproductive hormones was investigated. The HPLC method was used to determine the amount of BPA in saliva and serum samples. The HPLC technique is the most suitable method for eluting the nonpolar compounds forming the composite resin monomer and also has the advantage of separating the components according to their hydrophobic order.28 Also, because the monomers can be dissolved in the mobile phase in the HPLC method, the separation process is carried out at a more controlled level. The molecules consisting of high-molecular-weight monomers such as BISGMA can be decomposed in the gas chromatography technique, and only decomposition products can be detected. Therefore, the HPLC method is more suitable for determining the type and amount of monomers released from composite resins. For these reasons, the amount of monomer released from the composite resins in present study was measured using the HPLC method.29
In recent years, following the application of fissure sealant and composites, the results of studies evaluating the levels of BPA, BISGMA and BISDMA in saliva have been compared.2,30,31 BPA derivatives have been detected in some studies conducted in vitro and in vivo.30,31 In this regard, Lewis et al32 measured BPA release from 28 commercially available dental products (20 composites, 8 sealants) using HPLC and indicated that the amount of released BPA was too small to be measured. As a result, researchers concluded that dental resins could not be a source of BPA. Fleisch et al2 stated that BPA can be detected in saliva for 3 hours after the resin is placed. In their study, Sasaki et al33 applied 9 different composite resins (Z100, Progress, Palfique, Metafile Flo, UNIFIL S, Beautifil, Xeno CFII, Prodigy, Clearfil ST) to 21 individuals and found that the BPA amounts released into saliva varied between 15 and 100 ng/mL. They also found that gargling for 30 seconds after polymerization significantly reduced the rate of BPA release. In another study, Kingman et al34 measured the amount of BPA released into saliva before and after composite resin treatment. Researchers have argued that BPA detected in saliva may be a good indicator of BPA released from resin because it is possibly the highest amount that can be measured because saliva is in direct contact with the resin. Researchers have stated that the amount of BPA measured before resin application is basal BPA and that the amount of BPA measured after resin application may be the amount of BPA directly associated with resin. In their study, Kingman et al34 found the level of BPA measured before resin application was 0.43 ng/mL, 0.64 ng/mL after 1 hour, and 0.41 ng/mL after 9 to 30 hours. The BPA rate returned to baseline after 30 hours.
Because the aim of this study was to measure the amounts of BPA released after resin application, we aimed to measure BPA levels in saliva, which is the first point of contact with the resin, and BPA levels in serum were also measured to examine the effect on the hormonal system. We found higher serum BPA levels than saliva BPA levels may be attributed to the continuous ingestion of saliva and the cumulative effect of basal BPA levels. The fact that this condition continues during the follow-up period supports our claim.
In many studies where serum BPA levels were measured using different analytical techniques, human serum BPA levels were found to vary between 0.2 and 20 ng/mL.35 However, the data recorded in biological monitoring studies show that the estimated nonmetabolized BPA in human blood or serum samples is in a steady state in the range of 0.5-3 ng/mL (2-13 nM).35,36 When we look at studies on the cytotoxic doses of monomers released from composite resins, this dose was found to be 0.01 mmol/L (10000 nM) for BPA.37 Studies have reported that BPA released from dental materials is minimal and not at a level that can pose a health risk.38,39
After the composite resin was applied, BPA released from composite resins into saliva and serum were measured at certain intervals up to 5 weeks, and the maximum value measured in saliva was 0.52 ng/mL, whereas the maximum value measured in serum was 1.66 ng/mL. Looking at the results obtained, it was found that even the highest BPA levels released into saliva and serum among all time intervals were below the levels indicated in the literature. In this respect, the data obtained in the present study are consistent with the mentioned literature.
Although BPA values in saliva and serum increased initially, this increase was not significant. Moreover, the amounts of released BPA were similar for both composite resins (P > .05). Conversely, Joskow et al30 concluded that BPA exposure after Delton LC sealant placement was significantly higher than exposure after placement of Helioseal F. Patients treated with Delton LC had significantly higher doses of BPA (110 µg) than did those treated with Helioseal F (5.5 µg) (P < .0001). As a result, the first and second hypotheses of the present study were accepted, but the third hypothesis was rejected because the amount of BPA released into the serum from composite resins was significantly higher than BPA released into saliva (P < .05).
In the literature, researchers also used urine to quantify BPA exposure in humans. Urinary BPA (uBPA) concentrations increased 24 hours after dental treatment. The 2 studies with the largest sample sizes34,40 found statistically significant increases >40% in uBPA concentrations at 24 hours posttreatment (both P values < .01). The 1 study that examined uBPA concentrations beyond 1 month posttreatment found that concentrations returned to baseline by 14 days after treatment and remained at baseline 6 month after treatment. 40
It is known that gonadal hormones affect human reproductive activities. When considered in terms of mechanisms of action, FSH induces spermatogenesis through receptors in Sertoli cells found in the seminiferous tubules of the testis.41 Testosterone is secreted by the adult Leydig cells in men and is mainly regulated by the LH. The majority of serum testosterone is bound to SHBG. SHBG is a glycoprotein responsible for the transport of testosterone and oestradiol in the bloodstream.42,43 SHBG concentration increases with oestrogen and decreases with androgen. For this reason, SHBG production is stimulated by oestradiol and is blocked by testosterone. It is clear that exposure.44 To endocrine disruptors that affect gonadal hormone levels may cause changes in the reproductive performance of the individual. For this reason, the relationship between the BPA released from composite resins and reproductive hormones was also examined in the present study. When considered in terms of the role of hormones on the human reproductive system, studying the rate of release of a chemical that can affect the endocrine system, and its relationship with the hormones contributes to the importance of our study.
Studies in the literature are usually based on the examination of the effect of BPA on the human endocrine system resulting from environmental sources. Based on our literature survey, our study is the first to examine the effect of BPA, which is released from composite resins into saliva and serum, on reproductive hormones. When we look at the studies investigating the relationship between BPA and reproductive hormones in humans, Hanaoka et al45 reported that uBPA levels of 42 workers exposed to BPA were significantly higher than the controls and that there was also an inverse relationship between uBPA levels and serum FSH levels. In their study, Meeker et al46 found a positive correlation between BPA levels and serum FSH and a negative correlation between BPA and LH, FAI, and E2 in urine specimens taken from 167 male subjects. Mendiola et al47 investigated the relationship between uBPA levels and serum hormones in fertile men and found a negative relationship between BPA and FAI and a positive relationship between BPA and SHBG. Takeuchi et al48 found that there was a positive relationship between serum BPA levels and serum total and free testosterone levels in 11 male subjects. Zhou et al49 found that serum levels of BPA were negatively correlated with serum free testosterone and FAI levels, whereas there was a positive correlation between BPA and serum SHBG levels. Cha et al50 found a positive relationship between BPA and LH but a negative relationship between BPA and FSH in individuals with work-related BPA exposure. Lassen et al51 conducted a study on 308 young male subjects and found a positive relationship between BPA and LH but a negative relationship between BPA and FSH. As can be seen in the literature, the results differ between studies. This disparity can be explained by the differences in the populations in which the studies were carried out, the number of individuals, and the measurement methods of the samples.
With respect to the endocrine system, we found that BPA does not activate the endocrine system, and the minimal changes that occur in hormone levels do not go beyond the reference values. In this respect, the fourth hypothesis has been partially accepted. In addition, there was no statistically significant correlation between the released BPA levels and hormone levels (P > .05). Given the results of our study, the amounts of BPA released from the resins used are well below the toxic doses. This may explain why the endocrine system is not activated.
The limitation of this study is the small number of individuals in the study sample because of the difficulty of finding individuals who fit the inclusion criteria. In addition, diets of the participants were not changed. However, the fact that the samples were repeatedly taken at specific time intervals instead of a single sample makes our study valuable in terms of monitoring BPA levels. However, there is a need for studies with larger samples to confirm the results.
Conclusions
BPA was found in both serum and saliva released from composite resins. However, it was determined that the released BPA levels were below the toxic doses and did not alter hormonal balance. Additionally, there is a need for studies involving different materials and a larger number of individuals.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Neslihan Celik, Fatma Betul Ozgeris, and Fatma Demirkaya-Miloglu. The first draft of the manuscript was written by Pinar Gul and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None disclosed.
Footnotes
This study was presented at the Restorative Dentistry Association's 21st International Scientific Congress, Eskişehir, Turkey, December 1-3, 2017.
References
- 1.Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59:296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleisch AF, Sheffield PE, Chinn C, Edelstein BL, Landrigan PJ. Bisphenol A and related compounds in dental materials. Pediatrics. 2010;126:760–768. doi: 10.1542/peds.2009-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caserta D, Mantovani A, Marci R, et al. Environment and women's reproductive health. Hum Reprod Update. 2011;17:418–433. doi: 10.1093/humupd/dmq061. [DOI] [PubMed] [Google Scholar]
- 4.Rogalewicz R, Batko K, Voelkel A. Identification of organic extractables from commercial resin-modified glass-ionomers using HPLC-MS. J Environ Monitor. 2006;8:750–758. doi: 10.1039/b604149c. [DOI] [PubMed] [Google Scholar]
- 5.Kang YG, Kim JY, Kim J, Won PJ, Nam JH. Release of bisphenol A from resin composite used to bond orthodontic lingual retainers. Am J Orthod Dentofacial Orthop. 2011;140(6):779–789. doi: 10.1016/j.ajodo.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Soderholm KJ, Mariotti A. BIS-GMA-based resins in dentistry: are they safe? J Am Dent Assoc. 1999;130:201–209. doi: 10.14219/jada.archive.1999.0169. [DOI] [PubMed] [Google Scholar]
- 7.Fisher JW, Twaddle NC, Vanlandingham M, Doerge DR. Pharmacokinetic modeling: prediction and evaluation of route dependent dosimetry of bisphenol A in monkeys with extrapolation to humans. Toxicol Appl Pharmacol. 2011;257:122–136. doi: 10.1016/j.taap.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM. The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci. 2000;54:3–18. doi: 10.1093/toxsci/54.1.3. [DOI] [PubMed] [Google Scholar]
- 9.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Magdalena P, Ropero AB, Soriano S, et al. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012;355:201–207. doi: 10.1016/j.mce.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75:40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- 12.Melzer D, Harries L, Cipelli R, et al. Bisphenol A exposure is associated with in vivo estrogenic gene expression in adults. Environ Health Perspect. 2011;119:1788–1793. doi: 10.1289/ehp.1103809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloom MS, Kim D, vom Saal FS, et al. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril. 2011;96:672–677. doi: 10.1016/j.fertnstert.2011.06.063. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Cruz SC, Jubendradass R, Jayakanthan M, Rani SJA, Mathur PP. Bisphenol A impairs insulin signaling and glucose homeostasis and decreases steroidogenesis in rat testis: An in vivo and in silico study. Food Chem Toxicol. 2012;50:1124–1133. doi: 10.1016/j.fct.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Goodman J, Witorsch R, McConnell E, et al. Weight-of-evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit Rev Toxicol. 2009;39:1–75. doi: 10.3109/10408440903279946. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko M, Okada R, Yamamoto K, et al. Bisphenol A acts differently from and independently of thyroid hormone in suppressing thyrotropin release from the bullfrog pituitary. Gen Comp Endocr. 2008;155:574–580. doi: 10.1016/j.ygcen.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Nanjappa MK, Simon L, Akingbemi BT. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol Reprod. 2012;86:135. doi: 10.1095/biolreprod.111.095349. 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salian S, Doshi T, Vanage G. Neonatal exposure of male rats to Bisphenol A impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology. 2009;265:56–67. doi: 10.1016/j.tox.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect. 2001;109:55–60. doi: 10.1289/ehp.0110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meeker JD, Ehrlich S, Toth TL, et al. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol. 2010;30:532–539. doi: 10.1016/j.reprotox.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–439. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhuang W, Wu K, Wang Y, et al. Association of serum bisphenol-A concentration and male reproductive function among exposed workers. Arch Environ Contam Toxicol. 2015;68:38–45. doi: 10.1007/s00244-014-0078-7. [DOI] [PubMed] [Google Scholar]
- 23.Gul P, Akgul N, Alp HH, Kiziltunc A. Effects of composite restorations on oxidative stress in saliva: An in vivo study. J Dent Sci. 2015;10:394–400. [Google Scholar]
- 24.Yıldız MAH, Gul P, Bakan N, Özcan M. Lipid peroxidation and DNA oxidation caused by dental filling materials. J Dent Sci. 2017;12:233–240. doi: 10.1016/j.jds.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kardas F, Bayram AK, Demirci E, et al. Increased serum phthalates (MEHP, DEHP) and bisphenol A concentrations in children with autism spectrum disorder: the role of endocrine disruptors in autism etiopathogenesis. J Child Neurol. 2016;31:629–635. doi: 10.1177/0883073815609150. [DOI] [PubMed] [Google Scholar]
- 26.Demirkaya F, Kadioglu Y. Development and validation of a reversed phase-HPLC-DAD method for determination of bisphenol-A in artificial saliva. Asian J Chem. 2010;22:153–158. [Google Scholar]
- 27.Celik N, Askin S, Gul MA, Seven N. The effect of restorative materials on cytokines in gingival crevicular fluid. Arch Oral Biol. 2017;84:139–144. doi: 10.1016/j.archoralbio.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Alshali RZ, Salim NA, Sung R, Satterthwaite JD, Silikas N. Qualitative and quantitative characterization of monomers of uncured bulk-fill and conventional resin-composites using liquid chromatography/mass spectrometry. Dent Mater. 2015;31:711–720. doi: 10.1016/j.dental.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Cebe MA, Cebe F, Cengiz MF, Cetin AR, Arpag OF, Ozturk B. Elution of monomer from different bulk fill dental composite resins. Dent Mater. 2015;31:E141–E149. doi: 10.1016/j.dental.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Joskow R, Barr DB, Barr JR, Calafat AM, Needham LL, Rubin C. Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants. J Am Dent Assoc. 2006;137:353–362. doi: 10.14219/jada.archive.2006.0185. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman-Downs JM, Shuman D, Stull SC, Ratzlaff RE. Bisphenol A blood and saliva levels prior to and after dental sealant placement in adults. J Dent Hyg. 2010;84:145–150. [PubMed] [Google Scholar]
- 32.Lewis JB, Rueggeberg FA, Lapp CA, Ergle JW, Schuster GS. Identification and characterization of estrogen-like components in commercial resin-based dental restorative materials. Clin Oral Investig. 1999;3:107–113. doi: 10.1007/s007840050087. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki N, Okuda K, Kato T, et al. Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. J Mater Sci Mater Med. 2005;16:297–300. doi: 10.1007/s10856-005-0627-8. [DOI] [PubMed] [Google Scholar]
- 34.Kingman A, Hyman J, Masten SA, et al. Bisphenol A and other compounds in human saliva and urine associated with the placement of composite restorations. J Am Dent Assoc. 2012;143:1292–1302. doi: 10.14219/jada.archive.2012.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cienc Saude Coletiva. 2012;17:407–434. doi: 10.1590/s1413-81232012000200015. [DOI] [PubMed] [Google Scholar]
- 37.Kita K, Jin YH, Sun Z, et al. Increase in the levels of chaperone proteins by exposure to beta-estradiol, bisphenol A and 4-methoxyphenol in human cells transfected with estrogen receptor alpha cDNA. Toxicol In Vitro. 2009;23:728–735. doi: 10.1016/j.tiv.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Johnson R, Anderson D, Bakko D. Bisphenol-A exposure from dental sealants is minimal and does not cause increased morbidity or mortality (UT CAT# 2313) Tex Dent J. 2013;130:214. [PubMed] [Google Scholar]
- 39.Watanabe M, Hase T, Imai Y. Change in the bisphenol A content in a polycarbonate orthodontic bracket and its leaching characteristics in water. Dent Mater J. 2001;20:353–358. doi: 10.4012/dmj.20.353. [DOI] [PubMed] [Google Scholar]
- 40.Maserejian NN, Trachtenberg FL, Wheaton OB, et al. Changes in urinary bisphenol A concentrations associated with placement of dental composite restorations in children and adolescents. J Am Dent Assoc. 2016;147(8):620–630. doi: 10.1016/j.adaj.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyes-Fuentes A, Veldhuis JD. Neuroendocrine physiology of the normal male gonadal axis. Endocrinol Metab Clin North Am. 1993;22:93–124. [PubMed] [Google Scholar]
- 42.Munell F, Suarez-Quian CA, Selva DM, Tirado OM, Reventos J. Androgen-binding protein and reproduction: Where do we stand? J Androl. 2002;23:598–609. [PubMed] [Google Scholar]
- 43.Selby C. Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem. 1990;27(Pt 6):532–541. doi: 10.1177/000456329002700603. [DOI] [PubMed] [Google Scholar]
- 44.Elmlinger MW, Kuhnel W, Wormstall H, Doller PC. Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin Lab. 2005;51:625–632. [PubMed] [Google Scholar]
- 45.Hanaoka T, Kawamura N, Hara K, Tsugane S. Urinary bisphenol A and plasma hormone concentrations in male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents. Occup Environ Med. 2002;59:625–628. doi: 10.1136/oem.59.9.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol. 2010;44:1458–1463. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendiola J, Jorgensen N, Andersson AM, et al. Are environmental levels of bisphenol A associated with reproductive function in fertile men? Environ Health Perspect. 2010;118:1286–1291. doi: 10.1289/ehp.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeuchi T, Tsutsumi O. Serum bisphenol A concentrations showed gender differences, possibly linked to androgen levels. Biochem Bioph Res Co. 2002;291:76–78. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Q, Miao M, Ran M, et al. Serum bisphenol-A concentration and sex hormone levels in men. Fertil Steril. 2013;100:478–482. doi: 10.1016/j.fertnstert.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Cha BS, Koh SB, Park JH, Eom A, Lee KM, Choi HS. Influence of occupational exposure to bisphenol A on the sex hormones of male epoxy resin painters. Mol Cell Toxicol. 2008;4:230–234. [Google Scholar]
- 51.Lassen TH, Frederiksen H, Jensen TK, et al. Urinary bisphenol A levels in young men: association with reproductive hormones and semen quality. Environ Health Perspect. 2014;122:478–484. doi: 10.1289/ehp.1307309. [DOI] [PMC free article] [PubMed] [Google Scholar]