Abstract
Objective
This study aimed to compare the systemic and periodontal conditions between morbidly obese patients with and without hypertension who were candidates for bariatric surgery.
Methods
The study cohort had 111 morbidly obese patients stratified into two groups: patients with (G1 = 54) and without (G2 = 57) arterial hypertension. The following characteristics were compared between the two groups: (i) education level; (ii) anthropometric parameters [weight, height, body mass index (BMI), waist and hip circumferences and waist-to-hip ratio (WHR)]; (iii) risk of developing cardiovascular diseases (based on patients’ sex, age and WHR); (iv) behaviours regarding oral hygiene; and (v) periodontal status. The t-test, Mann–Whitney U-test, chi-square test and logistic regression were applied, with a significance level of 5%.
Results
Patients in G1 had a lower level of education (P = 0.002). There were no intergroup differences for weight (P = 0.211), height (P = 0.126), BMI (P = 0.551), waist circumference (P = 0.859) and WHR (P = 0.067); however, patients in G2 had a smaller hip circumference (P = 0.029), and 78% of patients in G1 had a high/very high risk of developing cardiovascular diseases. The prevalence of periodontitis was 72.2% (n = 39) in G1 and 38.6% (n = 22) in G2. On logistic regression analysis, age [adjusted odds ratio (OR) = 1.07; 95% CI = 1.01–1.13; P = 0.008) and the presence of arterial hypertension (OR = 2.77; 95% CI = 1.17–6.56; P = 0.019) were identified as the independent variables associated with periodontitis.
Conclusion
Morbid obesity and arterial hypertension are associated with a higher prevalence of cardiovascular diseases. Moreover, morbidly obese patients with hypertension have a higher prevalence of periodontitis and greater severity of periodontal disease than those without hypertension.
Key words: Periodontitis, Obesity, Hypertension, Social class
Introduction
Obesity is a chronic inflammatory disease that affects people in both developed and developing countries and may be associated with several comorbidities, such as dyslipidemia, cardiovascular disease, hypertension, type 2 diabetes mellitus, obstructive sleep apnoea syndrome and some types of cancer, and consequently negatively impacts an individual's quality of life.1, 2, 3, 4 Patients with obesity are in a chronic inflammatory state caused by the actions of inflammatory cytokines.5, 6, 7, 8, 9
The pathophysiological mechanism underlying arterial hypertension is similar to that for obesity. Hypertension is related to several factors, such as eating habits, glomerular filtration, and metabolic and neuroendocrine disorders. Scientific evidence shows a link between arterial hypertension with vascular inflammation and endothelial dysfunction.10,11 Vascular inflammation occurs through the action of inflammatory mediators, resulting in increased vascular permeability and changes in the cytoskeleton of endothelial cells, leading to an imbalance between vasodilation and vasoconstriction. Because of the active involvement of inflammatory mediators, vasoconstriction is more intense, triggering an increase in blood pressure.10,11 C-reactive protein plays an important role in this process.
Previous studies have highlighted the association between periodontal disease and obesity as the inflammatory markers released by the body fat of individuals with obesity influence the host immune response. Thus, patients with obesity present with exacerbated inflammation in the periodontium, even if they have a minimal amount of bacterial plaque.12, 13, 14, 15
In a systematic review with a meta-analysis, Martin-Cabezas et al.16 showed that periodontal disease appears to be associated with an increased risk of high blood pressure, although their findings were highly heterogeneous and there was no evidence of an associated cause for these conditions.16 Recently, another systematic review with meta-analysis, by Muñoz Aguilera et al.,17 confirmed a high prevalence of periodontitis in patients with hypertension. Their review included prospective cohorts to establish the temporal association between periodontitis and the incidence of arterial hypertension. However, there is still no evidence regarding the cause-and-effect relationship between these outcomes.
Considering that periodontitis and arterial hypertension share common risk factors, such as obesity, the confounding factors must be controlled when assessing the association of these outcomes. Given the lack of studies that have investigated the association between hypertension and periodontitis, with exclusion of the bias of obesity, this study aimed to compare the systemic and periodontal conditions between morbidly obese patients with and without arterial hypertension, who were candidates for bariatric surgery.
Materials and methods
This observational, cross-sectional, analytical study followed the reporting guidelines recommended by Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).18
Sample composition
Initially, 130 patients who were under medical follow-up for the treatment of morbid obesity were consecutively selected from July 2019 to February 2020 in a public multidisciplinary clinic. These patients had a body mass index (BMI) of ≥40 kg/m2 and were between 20 and 50 years of age. In addition, they were being monitored by endocrinologists, psychologists, nutritionists and surgeons, and their eating habits were being controlled by these professionals in order to guarantee weight loss or weight maintenance before bariatric surgery.
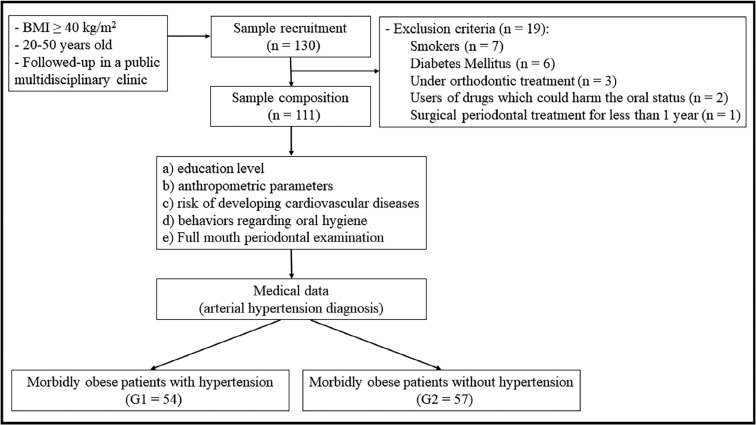
The following exclusion criteria were adopted: neuromotor weaknesses; previous consumption of alcoholic beverages more than three times a week; use of illicit drugs; previous history of surgical treatment for periodontitis (self-reported information); and loss of more than two teeth per hemiarch. Nineteen individuals were excluded for the following reasons: smoking (n = 7); presence of diabetes mellitus (n = 6); under orthodontic treatment (n = 3); use of drugs which could harm the oral status (e.g., anti-hypertensive, immunosuppressive, anticonvulsant or calcium channel-blocking drugs, such as cyclosporine, phenytoin or nifedipine) (n = 2); undergoing surgical periodontal treatment for <1 year (n = 1) (Figure 1).
Fig. 1.
Flowchart showing participant recruitment and composition of the study sample. BMI, body mass index.
Thus, the final cohort consisted of 111 patients who were stratified into two groups: morbidly obese (BMI ≥ 40.00 kg/m2) with arterial hypertension (G1; n = 54); and morbidly obese (BMI ≥ 40.00 kg/m2) without arterial hypertension (G2; n = 57). The classification of nutritional status was based on the protocol adopted by the World Health Organization according to the patients’ BMI, as shown in Table 1. Information regarding the diagnosis of arterial hypertension (systolic blood pressure of >140 mmHg and/or diastolic blood pressure of >90 mmHg) was obtained from the medical records of each patient.17
Table 1.
Classification of nutritional status based on body mass index (BMI)
| Classification | BMI (kg/m2) |
|---|---|
| Underweight | <18.50 |
| Normal weight | 18.50–24.99 |
| Pre-obese | 25.00–29.99 |
| Obesity class I | 30.00–34.99 |
| Obesity class II | 35.00–39.99 |
| Obesity class III | ≥40.00 |
Standardization of the examiner
Data collection for this study was conducted only by one blinded dentist. To ensure standardization of the collected data, the examiner was calibrated under the guidance of a researcher (SHCSP) who was experienced in epidemiological surveys. For the calibration process, 15 patients were selected and followed up in three stages. In the first stage, the examiner performed a full-mouth periodontal analysis. At the second stage, the same measurements were performed by the same examiner, 15 days after the initial first stage, to evaluate the intraexaminer coefficient of agreement. At the third stage, 15 days after the second examination, a full-mouth periodontal analysis was performed in the same patients by the supervisor, who was an expert in epidemiological studies, to evaluate the coefficient of agreement between the examiners. Intervals of 15 days were used because of the possibility of periodontal alteration after the first examination (rupture of the periodontal fibres).19 Inter- and intra-examiner kappa values of reliability, of 0.92 and 0.95, respectively, were obtained. The patients included in the examiner calibration process were not included in the sample of the present study.
Education level
The level of education for each patient was categorized as follows: 0, illiterate; 1, incomplete elementary school; 2, complete elementary school; 3, incomplete high school; 4, complete high school; 5, incomplete higher education; 6, complete higher education; 7, specialization; 8, master's degree; 9, doctorate. The highest qualification obtained by patients at the time of the interview was recorded.
Anthropometric assessment
The weight and height for each patient were obtained and used to calculate their BMI. A standardized stadiometer (Wood 2.20; WCS Ind., Curitiba, Paraná, Brazil) was used to measure patients’ height, and weight was measured using a calibrated automatic scale (MIC model 300PP; Micheletti Ind., São Paulo, São Paulo, Brazil).
Waist, hip and waist-to-hip ratio (WHR) measurements were performed to assess abdominal obesity, which served as an indicator of the risk of cardiovascular disease.20 The risk of developing cardiovascular diseases was evaluated according to patients’ sex, age and WHR and was categorized as low, moderate, high or very high risk,21 and allocated scores of 1, 2, 3 and 4, respectively, in order to ensure an ordinal qualitative statistical analysis (Table 2).
Table 2.
Classification of the risk of developing cardiovascular diseases, based on gender, age and waist-to-hip ratio
| Risk of developing cardiovascular diseases |
||||
|---|---|---|---|---|
| Age (years) | Low | Moderate | High | Very high |
| Women | ||||
| 20–29 | <0.71 | 0.71–0.77 | 0.78–0.82 | >0.82 |
| 30–39 | <0.72 | 0.72–0.78 | 0.79–0.84 | >0.84 |
| 40–49 | <0.73 | 0.73–0.79 | 0.80–0.87 | >0.87 |
| 50–59 | <0.74 | 0.74–0.81 | 0.82–0.88 | >0.88 |
| Men | ||||
| 20–29 | <0.83 | 0.83–0.88 | 0.89–0.94 | >0.94 |
| 30–39 | <0.84 | 0.84–0.91 | 0.92–0.96 | >0.96 |
| 40–49 | <0.88 | 0.88–0.95 | 0.96–1.00 | >1.00 |
| 50–59 | <0.90 | 0.90–0.96 | 0.97–1.02 | >1.02 |
Periodontal disease
Full-mouth periodontal analysis was performed for all patients. All teeth present (excluding the third molars) were evaluated. Probing pocket depth (PPD) and gingival recession or gingival overgrowth (if present) were assessed and used to calculate clinical attachment loss (CAL). PPD was measured from the gingival margin to the bottom of the periodontal pocket, and CAL was measured from the cemento–enamel junction to the bottom of the periodontal pocket.22 These parameters were evaluated at six sites for each tooth (mesial, centre and distal, both buccal sides and both the palatal/lingual surfaces).
Periodontitis was diagnosed when the patient presented interdental CAL at two or more non-adjacent teeth or buccal/oral CAL of ≥3 mm, with pocketing of >3 mm, at two or more teeth, and if the CAL observed was not attributable to non-periodontal causes,19 as described by Tonetti et al.23 Subsequently, periodontitis was also categorized into stages I, II, III and IV, as also highlighted by Tonetti et al.23
The presence or absence of biofilm on the buccal and/or lingual surfaces of all teeth was recorded and the results used to calculate the prevalence of dental surfaces with dental plaque. Bleeding on probing (BOP) was calculated based on the index proposed by Ainamo and Bay.24 This index refers to the presence or absence of BOP, even when visible clinical features with marginal changes are absent. BOP was considered as positive when bleeding occurred within10 seconds after probing.
Patients who were not diagnosed with periodontitis were evaluated according to the presence/absence of gingivitis. Patients with <10% of sites with BOP were classified as healthy, those with 10%–30% of sites with BOP were diagnosed with localized gingivitis and those who had >30% sites with BOP were diagnosed with generalized gingivitis.25
Statistical analysis
Data were analysed using IBM SPSS Statistics for Windows Version 25.0 (released 2017; IBM Corp., Armonk, NY, USA). For sample size calculation, the logistic regression analysis protocol proposed by Hosmer and Lemeshow26 was used, which requires at least 15 study subjects for each independent variable inserted in the initial logistic model. Thus, the primary outcome was dichotomised – periodontitis (0, without periodontitis; 1, with periodontitis) – and four independent variables were inserted in the logistic regression model, which required a total of 60 patients in the sample. As this study included 111 patients, the sample was considered to be representative.
Initially, the normality of the variables was tested using the Kolmogorov–Smirnov test. Once the testing hypotheses were met, the results for each study variable present in the study were analysed using the appropriate test. The t-test was adopted for numerical variables with a normal distribution (age, height, waist circumference, prevalence of dental plaque). For quantitative variables that did not follow a normal distribution and for ordinal qualitative variables (weight, BMI, hip circumference, WHR, risk of developing cardiovascular diseases, education level, prevalence of sites with BOP, PPD, prevalence of sites with PPD ≥ 4 mm, CAL, prevalence of sites with CAL ≥ 4 mm and severity of periodontitis) the Mann–Whitney U-test was applied. Nominal qualitative variables (sex, presence of periodontitis, presence of gingivitis) were assessed using the chi-square test.
After the bivariate analysis, independent variables with P < 0.20 were included in the logistic regression model to assess their association with the outcome. The Hosmer–Lemeshow test, collinearity and residual analysis were applied to clarify the results obtained from logistic regression. A significance level of 5% was used.
Results
The sample consisted of 54 morbidly obese individuals diagnosed with hypertension (G1) and 57 morbidly obese individuals without arterial hypertension (G2). The mean ± SD age of patients in G1 was 39.75 ± 7.41 years and that of patients in G2 was 32.96 ± 8.58 years. There were no differences between the groups with regard to sex (P = 0.532); however, the entire study sample of obese individuals had a higher proportion of women than men (Table 3). Patients in G1 had a lower level of education than those in G2 (P = 0.002).
Table 3.
Comparison of contextual variables in morbidly obese individuals with (G1) and without (G2) arterial hypertension
| Variable | G1 (n = 54) | G2 (n = 57) | P |
|---|---|---|---|
| Weight (kg) | 124.25 (112.50–136.70) | 126.00 (117.75–145.12) | 0.211† |
| Height (m) | 1.63 ± 0.05 | 1.65 ± 0.08 | 0.126* |
| BMI (kg/m2) | 46.58 (42.84–50.32) | 47.17 (42.81–52.53) | 0.551† |
| Circumf. waist (cm) | 120.20 ± 12.54 | 120.64 ± 13.25 | 0.859* |
| Circumf. hip (cm) | 138 (131–146) | 143 (136.00–151.25) | 0.029† |
| WHR | 0.85 (0.79–0.92) | 0.83 (0.77–0.86) | 0.067† |
| RDCV | 4 (3–4) | 3 (2–4) | |
| Low | 1 (1.8) | 4 (7.1) | 0.018† |
| Moderate | 11 (20.4) | 18 (31.6) | |
| High | 14 (25.9) | 17 (29.8) | |
| Very high | 28 (51.9) | 18 (31.5) | 0.002† |
| Education level Gender | 3 (2–3) | 3 (3–5) | |
| Male | 8 (14.8) | 11 (19.3) | 0.532‡ |
| Female | 46 (85.2) | 46 (80.7) |
Bold values mean that a statistical difference was found between the groups. Values are given as mean ± SD, median (1st-3rd quartiles) and n (%). BMI, body mass index; circumf., circumference; P, significance level; RDCV, risk of developing cardiovascular diseases; WHR, waist-to-hip ratio
t-test.
Mann-Whitney U-test.
Chi-square test.
No differences in anthropometric parameters were found between the groups; however, patients in G2 had a smaller hip circumference value than those in G1 (P = 0.029). Additionally, although there was no difference between the groups for WHR, when considering this parameter along with sex and age, 78% of the patients in G1 had a high/very high risk of developing cardiovascular diseases (Table 3).
All patients in both groups reported daily toothbrushing using a toothbrush and fluoride-containing toothpaste. There was no difference between groups in the frequency of daily toothbrushing, with an average of three times per day recorded for all patients. Nevertheless, patients in G1 had worse periodontal parameters related to the prevalence of bacterial plaque (P = 0.005), BOP (P = 0.002) and CAL (P = 0.008), but no differences were found between groups for PPD (P = 0.226). Regarding the classification of periodontitis, 72.2% (n = 39) of the individuals in G1 had periodontitis: 33.3% (n = 18) with stage I, 27.8% (n = 15) with stage II, 7.4% (n = 4) with stage III and 3.7% (n = 2) with stage IV. By contrast, only 38.6% (n = 22) of individuals in G2 had periodontitis: 29.8% (n = 17) with stage I, 1.7% (n = 1) with stage II and 7.1% (n = 4) with stage III. In G2, 27 (47.3%) of the patients had gingivitis, which was localized in one (1.7%) and generalised in 26 (45.6%) (Table 4).
Table 4.
Comparison of periodontal parameters in morbidly obese individuals with (G1) and without (G2) arterial hypertension
| Parameter | G1 (n = 54) | G2 (n = 57) | P |
|---|---|---|---|
| Prev. of bacterial plaque (%) | 40.59 ± 22.90 | 29.03 ± 20.00 | 0.005* |
| Prev. of BOP (%) | 63.96 (40.90–1.96) | 51.85 (26.78–67.85) | 0.002† |
| PPD (mm) | 1.71 (1.51–2.02) | 1.66 (1.50–1.86) | 0.226† |
| Sites with PPD ≥ 4 mm | 3 (0–6) | 1 (0–2) | <0.001† |
| CAL (mm) | 1.88 (1.64–2.19) | 1.70 (1.60–1.90) | 0.008† |
| Sites with CAL ≥ 4 mm | 5 (3–11) | 1 (0–4.25) | <0.001† |
| Periodontitis | |||
| No | 15 (27.8) | 35 (61.4) | 0.0004‡ |
| Yes | 39 (72.2) | 22 (38.6) | |
| Periodontitis stage | |||
| I | 18 (33.3) | 17 (29.8) | 0.0001† |
| II | 15 (27.8) | 1 (1.7) | |
| III | 4 (7.4) | 4 (7.1) | |
| IV | 2 (3.7) | 0 | |
| Gingivitis | |||
| No | 0 | 8 (14.1) | 0.045‡ |
| Yes | 15 (27.8) | 27 (47.3) | |
| Localized | 3 (5.6) | 1 (1.7) | |
| Generalized | 12 (22.2) | 26 (45.6) |
Bold values mean that a statistical difference was found between the groups. Values are given as mean ± SD, median (1st-3rd quartiles) and n (%). BOP, bleeding on probing; CAL, clinical attachment loss; P, significance level; PPD, probing pocket depth; Prev., prevalence.
t-test.
Mann–Whitney U-test.
Chi-square test.
Logistic regression analysis was performed to identify the factors associated with primary outcome (0, without periodontitis; 1, with periodontitis) (Table 5). The variables presence of hypertension, age, education level and WHR were inserted in the initial model. On multicollinearity analysis, all independent variables showed tolerance values of >0.20 and variance inflation factor values of <2. The final model – X2(2) = 20.50; P < 0.001; Nagelkerke R2 = 0.225 – revealed that age [adjusted odds ratio (OR) = 1.07; 95% CI: 1.01–1.13; P = 0.008) and the presence of arterial hypertension (OR = 2.77; 95% CI: 1.17–6.56; P = 0.019) were associated with the presence of periodontitis. The global precision of the model was 70.27%. Results of Hosmer–Lemeshow analysis showed a chi-square value of 6.56 with 8 degrees of freedom for the final model (P = 0.584).
Table 5.
Final model of logistic regression with the independent variables related to the outcome (periodontitis)
| Variables | β | P | Adjusted OR | 95% CI | |
|---|---|---|---|---|---|
| Final model | Age | 0.07 | 0.008 | 1.07 | 1.01–1.13 |
| Hypertension | 1.02 | 0.019 | 2.77 | 1.17–6.56 | |
| Constant | –2.89 | 0.003 |
β, constant; OR, odds ratio; P, significance level.
Discussion
This study highlights that morbidly obese patients with arterial hypertension, who are candidates for bariatric surgery, are at a high risk of developing cardiovascular diseases. These patients also had worse periodontal parameters related to the presence of dental plaque, prevalence of BOP and loss of clinical attachment level, which were reflected in the higher prevalence and severity of periodontitis.
Worldwide, the prevalence of obesity has increased from 30.5% in 1999–2000 to 42.4% in 2017–2018. Meanwhile, in Brazil, 55.4% of the population is overweight and 20.3% is obese. Obesity is related to several comorbidities, such as type 2 diabetes mellitus, arterial hypertension, atherosclerosis, cancer and metabolic syndrome.1,3,6,20,27,28 A previous study showed that abdominal obesity, measured using WHR, can be an important predictor of the development of cardiovascular diseases.20 In the present study, morbidly obese patients diagnosed with arterial hypertension had a higher risk of developing cardiovascular diseases than those without arterial hypertension, when assessed according to abdominal fat accumulation, sex and age. Approximately 78% of these patients had a risk of developing cardiovascular diseases classified as ‘high’ and ‘very high’ (P = 0.018) (Table 3).
Strong scientific evidence shows a positive association between periodontitis and obesity13, 14, 15 as obese patients are in a generalized state of inflammation caused by the actions of inflammatory cytokines and adipokines, such as tumour necrosis factor alpha (TNF-α),6,29 interleukin-6 (IL-6),5 leptin,7 adiponectin,8 adipocytokines9 and cytoplasmic enzymes.30 Thus, even with a small amount of bacterial plaque on teeth, patients with obesity may have an exacerbated local inflammatory response.13, 14, 15
In respect of hypertension, almost 45% of the population worldwide is affected by this condition and the estimate increases steeply with age.17 In Brazil, approximately 24.5% of the overall population is diagnosed with hypertension. Scientific findings have pointed out that hypertension is directly associated with stroke, myocardial infarction, sudden death, heart failure and peripheral artery disease, as well as end-stage renal disease.17
Previous studies sought to assess the association between arterial hypertension and periodontitis.16,17,31, 32, 33, 34, 35, 36, 37, 38, 39 Although Czesnikiewicz-Guzik et al.39 demonstrated a causal relationship between periodontitis and hypertension and also were the first to provide genetic and experimental evidence that periodontitis is linked to hypertension, systematic reviews with meta-analyses showed heterogeneous findings, which hampered the understanding of pathophysiological mechanisms and the temporal association of these outcomes.16,17
Current evidence shows that gingival bleeding, an easily accessible marker of periodontal health status, contributes to shaping the relationship between periodontal diseases and blood pressure.40 According to Pietropaoli et al., the unique contribution of specific and diverse inflammatory pathways in acute and chronic periodontal disease might be a possible explanation for the differences observed between gingivitis and stable/unstable periodontitis in the reported association. The potential immunological and metabolic effects of fluctuations in local microflora might also exert a pathogenic role in the same relation.40 As aforementioned, gingival bleeding contributes to shaping the relationship between periodontal diseases and blood pressure, but the burden represented by periodontitis is remarkable.40
For a better understanding of the association between periodontitis and arterial hypertension, the presence of some confounding factors, such as obesity – a potential risk factor for hypertension – must be considered.35 In this study, all patients included in the sample cohort were morbidly obese; therefore, the results obtained for the periodontal condition were not influenced by the presence of obesity, reinforcing the association between periodontitis and arterial hypertension.
Morbidly obese patients with hypertension had a higher prevalence of dental surfaces with plaque (P = 0.005) and sites with BOP (P = 0.002) (Table 4) than their study counterparts without hypertension. Furthermore, approximately 72% had periodontitis: 33.3% with stage I, 27.8% with stage II, 7.4% with stage III and 3.7% with stage IV periodontitis (P = 0.0001). By contrast, only 38.6% of the morbidly obese patients without arterial hypertension had periodontitis, and the periodontitis was of a relatively low degree of severity (stage I periodontitis) in just over three-quarters.
Clinical and experimental evidence suggests that the association between periodontitis and arterial hypertension could be mediated by hypertension itself, which causes microcirculatory alterations in the periodontium, resulting in ischemia, exacerbated inflammation and/or altered composition of microbes (e.g., numbers and species) in dental plaque.17,32,41,42 The composition of dental biofilm was not biologically evaluated in this study. However, morbidly obese patients with hypertension had more dental surfaces with plaque than their study counterparts without hypertension (Table 4), even though the daily toothbrushing frequency was not different between the two patient groups.
In this context, it must be emphasized that social inequality plays a fundamental role in the health condition of individuals.43 Thus, although there were no differences between the two groups regarding oral hygiene behaviours, less knowledge and access to information about health, characterized by a lower level of education (P = 0.029), might explain the worse periodontal parameters observed in morbidly obese patients with high blood pressure (G1). This finding reinforces that, although the Brazilian public health system has universality and comprehensiveness as its fundamental pillars, greater investments in prevention and health education of morbidly obese patients are necessary such that a larger portion of the population is assisted, which would eventually result in health equity.
It is also hypothesized that periodontitis is a risk factor for hypertension, and this can be elucidated by some mechanisms, as follows:
-
•
Periodontitis is associated with systemic inflammation, the mediators of which (C-reactive protein, IL-6 and TNF-α) – can impair endothelial function and consequently cause hypertension17
-
•
Some findings suggest possible direct effects of bacteraemia, caused by to the oral microbiota, in mediating vascular dysfunction. Experimental evidence in animals indicates that an immune response to a common periodontal pathogen results in elevated blood pressure, vascular inflammation and endothelial dysfunction17,44
-
•
Another hypothesis is that T cells, B cells and monocytes/macrophages from the inflamed periodontium are more prone to chemotactic recruitment to the perivascular adipose tissue (adventitious fat layers), a phenomenon that has been shown to precede the development of vascular dysfunction, hypertension and atherosclerosis.17,45,46
It is important to highlight that periodontal treatment plays a fundamental role in improving systemic conditions. In addition, professionals must consider that conditions such obesity and hypertension are related to the effect and success of periodontal therapy on systemic disease.47,48 This evidence draws the attention of doctors and dentists to the importance of prevention and multidisciplinary and interdisciplinary care for patients with systemic impairments.
As previously mentioned, the patient groups in this study differed with regard to the level of education, which should be considered an important social determinant of health that is related to the periodontal parameters found in this study. However, it is important to note that when binary logistic regression was performed to understand which independent variables would affect the outcome of periodontitis, education level did not remain in the final logistic model (Table 5). The results of logistic regression analysis reinforced known facts that the age of patients and the presence of arterial hypertension are variables associated with periodontitis.
Our findings are in line with those reported by previous studies. Zhao et al.38 also found an influence of age on the association between periodontal disease and hypertension (OR = 1.27 for age between 40 and 60 years; OR = 1.01 for age ≥60 years). However, as in the present study, the influence of age on the association of the investigated outcomes was weak. They also found an association between periodontal disease and hypertension from multivariate adjustments and propensity score adjustments. Likewise, Martin-Cabezas et al.16 suggested that periodontal disease significantly increased, by 1.5-fold, the risk of hypertension in the Asian population (OR = 1.50; 95% CI: 1.27–1.78), while Lamster and Pagan found that the development of hypertension was associated with a community periodontal index code of ≥3.27 Kawabata et al.34 reported a significant risk of hypertension associated with periodontitis in their Japanese cohort (OR = 2.07; 95% CI: 1.29–6.29).
This study has some limitations, including the fact that it has a cross-sectional design, which makes it impossible to understand the temporal and the cause-and-effect relationship of the investigated outcomes. In addition, the difference found between the study groups with regard to education level may have some influence on the patients’ oral condition because worse oral conditions are associated with low socioeconomic status. Moreover, in this study, patient data on triglyceride and cholesterol levels were not accessed, therefore making it impossible to understand the prevalence of metabolic syndrome in the study sample and how it could influence the outcomes. Finally, biological analyses of the composition of dental biofilm should be performed longitudinally for patients diagnosed with arterial hypertension who are undergoing non-surgical periodontal treatment; this would contribute to the understanding of the pathophysiological mechanism of the outcomes studied. In view of these limitations, prospective cohorts with a larger sample size are required in future studies, aiming to exclude the bias of socio-economic/cultural factors; and access to patients’ triglyceride and cholesterol levels and analyses of the biological composition of dental biofilm are also necessary.
Despite the limitations of the present study, our findings have some strengths because they draw the attention of researchers, clinicians and health managers to the associations of oral impairments with chronic diseases. Our findings highlight the need for reinforcement and investment in preventive and health education and for multidisciplinary, comprehensive and equitable treatment of morbidly obese patients, especially those in patients who are vulnerable. In view of this, it is important for clinicians to understand that morbidly obese patients with hypertension may be at higher risk of cardiovascular disease and worse systemic parameters, requiring regular follow-up and appropriate periodontal intervention.
In conclusion, patients with morbid obesity and arterial hypertension, scheduled for bariatric surgery, are associated with a higher prevalence of cardiovascular diseases. Moreover, morbidly obese patients with hypertension have a higher prevalence of periodontitis and a greater periodontal disease severity than morbidly obese people without arterial hypertension.
Authors contributions
GAFJ and SHCP worked on the conception and design of the study, analysis and interpretation of data; drafting the article; and approval of the version to be published. ESO worked on the analysis and interpretation of data; drafting the article; and approval of the version to be published. LSM and CCM worked on data collection and analysis and interpretation of results; drafting the article; and approval of the version to be published.
Acknowledgments
Acknowledgements
The authors thank the Coordination of Superior Level Staff Improvement - Brazil (CAPES - Finance Code 001) and São Paulo Research Foundation (FAPESP - 2013/19691-3) for supporting this study. It is important to highlight, however, that CAPES and FAPESP had no role in study design, data analysis or manuscript drafting/approval.
Ethical approval
Respecting the Declaration of Helsinki of 1964 and its subsequent amendments, the present study was approved by the Research Ethics Committee of the Bauru School of Dentistry, University of São Paulo (CAAE 50987315.5.00005434). All patients provided written consent before participating in the research.
Conflict of interest
The authors declare that there are no conflicts of interest related to this study.
REFERENCES
- 1.Saito T, Shimazaki Y. Metabolic disorders related to obesity and periodontal disease. Periodontol. 2000;2007:254–266. doi: 10.1111/j.1600-0757.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 2.Foratori GA, Jr, Andrade FJ, Mosquim V, et al. Presence of serum ferritin before and after bariatric surgery: analysis in dentate and edentulous patients. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosquim V, Foratori-Junior GA, Hissano WS, et al. Obesity, bariatric surgery and the impact on oral health: a literature review. Salusvita. 2019;38:117–132. [Google Scholar]
- 4.Foratori-Junior GA, Máscoli LS, Jesuino BG, et al. Evaluation of systemic conditions, tooth loss, body image, and quality of life of women with obesity and women who underwent gastric bypass surgery. Spec Care Dentist. 2020;40:151–159. doi: 10.1111/scd.12453. [DOI] [PubMed] [Google Scholar]
- 5.Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 6.Genco RJ, Grossi SG, Ho A, et al. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–2084. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 7.Martin A, David V, Malaval L, et al. Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-I pathway. Endocrinology. 2007;148:3419–3425. doi: 10.1210/en.2006-1541. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi N, Kukita T, Li YJ, et al. Adiponectin inhibits osteoclast formation stimulated by lipopolysaccharide from Actinobacillus actinomycetemcomitans. FEMS Immunol Med Microbiol. 2007;49:28–34. doi: 10.1111/j.1574-695X.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 9.Shimada Y, Komatsu Y, Ikezawa-Suzuki I, et al. The effect of periodontal treatment on serum leptin, interleukin-6, and C-reactive protein. J Periodontol. 2010;81:1118–1123. doi: 10.1902/jop.2010.090741. [DOI] [PubMed] [Google Scholar]
- 10.Leong XF, Ng CY, Badiah B, et al. Association between hypertension and periodontitis: possible mechanisms. Scientific World Journal. 2014 2014 doi: 10.1155/2014/768237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macedo Paizan ML, Vilela-Martin JF. Is there an association between periodontitis and hypertension? Curr Cardiol Rev. 2014;10:355–361. doi: 10.2174/1573403X10666140416094901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathus-Vliegen EM, Nikkel D, Brand HS. Oral aspects of obesity. Int Dent J. 2007;57:249–256. doi: 10.1111/j.1875-595x.2007.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 13.Moura-Grec PG, Marsicano JA, Carvalho CA, et al. Obesity and periodontitis: systematic review and meta-analysis. Cien Saude Colet. 2014;19:1763–1772. doi: 10.1590/1413-81232014196.13482013. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Herrera M, Silvestre-Rangil J, Silvestre FJ. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med Oral Patol Oral Cir Bucal. 2017;22:e708–e715. doi: 10.4317/medoral.21786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan S, Barrington G, Bettiol S, et al. Is overweight/obesity a risk factor for periodontitis in young adults and adolescents? A systematic review. Obes Rev. 2018;19:852–883. doi: 10.1111/obr.12668. [DOI] [PubMed] [Google Scholar]
- 16.Martin-Cabezas R, Seelam N, Petit C, et al. Association between periodontitis and arterial hypertension: a systematic review and meta-analysis. Am Heart J. 2016;180:98–112. doi: 10.1016/j.ahj.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz Aguilera E, Suvan J, Buti J, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. 2020;116:28–39. doi: 10.1093/cvr/cvz201. [DOI] [PubMed] [Google Scholar]
- 18.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Caracho RA, Foratori-Junior GA, Fusco NDS, et al. Systemic conditions and oral health-related quality of life of pregnant women of normal weight and who are overweight. Int Dent J. 2020;70:287–295. doi: 10.1111/idj.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 21.Heyward VH, Stolarczyk LM. Avaliação da composição corporal aplicada. Manole; São Paulo: 2000. organizators. [Google Scholar]
- 22.Foratori-Junior GA, Jesuino BG, Caracho RA, et al. Association between excessive maternal weight, periodontitis during the third trimester of pregnancy, and infants’ health at birth. J Appl Oral Sci. 2020;28 doi: 10.1590/1678-7757-2019-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45:S149–S161. doi: 10.1111/jcpe.12945. [DOI] [PubMed] [Google Scholar]
- 24.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–235. [PubMed] [Google Scholar]
- 25.Trombelli L, Farina R, Silva CO, et al. Plaque-induced gingivitis: case definition and diagnostic considerations. J Clin Periodontol. 2018;45:S44–S67. doi: 10.1111/jcpe.12939. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer D, Lemeshow S. Applied logistic regression. Wiley; New York, NY: 2000. organizators. [Google Scholar]
- 27.Lamster IB, Pagan M. Periodontal disease and the metabolic syndrome. Int Dent J. 2017;67:67–77. doi: 10.1111/idj.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foratori-Junior GA, Andrade FJ, Mosquim V, et al. Association of metabolic syndrome with oral and systemic conditions in morbidly obese patients. Braz J Oral Sci. 2019;18 [Google Scholar]
- 29.Lundin M, Yucel-Lindberg T, Dahllöf G, et al. Correlation between TNFalpha in gingival crevicular fluid and body mass index in obese subjects. Acta Odontol Scand. 2004;62:273–277. doi: 10.1080/00016350410000172. [DOI] [PubMed] [Google Scholar]
- 30.Gursoy UK, Marakoglu I, Ersan S. Periodontal status and cytoplasmic enzyme activities in gingival crevicular fluid of type 2 diabetic and/or obese patients with chronic periodontitis. J Int Acad Periodontol. 2006;8:2–5. [PubMed] [Google Scholar]
- 31.Tsakos G, Sabbah W, Hingorani AD, et al. Is periodontal inflammation associated with raised blood pressure? Evidence from a National US survey. J Hypertens. 2010;28:2386–2393. doi: 10.1097/HJH.0b013e32833e0fe1. [DOI] [PubMed] [Google Scholar]
- 32.Tsioufis C, Kasiakogias A, Thomopoulos C, et al. Periodontitis and blood pressure: the concept of dental hypertension. Atherosclerosis. 2011;219:1–9. doi: 10.1016/j.atherosclerosis.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 33.Zeigler CC, Wondimu B, Marcus C, et al. Pathological periodontal pockets are associated with raised diastolic blood pressure in obese adolescents. BMC Oral Health. 2015;15:41. doi: 10.1186/s12903-015-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawabata Y, Ekuni D, Miyai H, et al. Relationship between prehypertension/hypertension and periodontal disease: a prospective cohort study. Am J Hypertens. 2016;29:388–396. doi: 10.1093/ajh/hpv117. [DOI] [PubMed] [Google Scholar]
- 35.Goulart AC, Armani F, Arap AM, et al. Relationship between periodontal disease and cardiovascular risk factors among young and middle-aged Brazilians. Cross-sectional study. Sao Paulo Med J. 2017;135:226–233. doi: 10.1590/1516-3180.2016.0357300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Oh JY, Youk TM, et al. Association between periodontal disease and non-communicable diseases: a 12-year longitudinal health-examinee cohort study in South Korea. Medicine. 2017;96:e7398. doi: 10.1097/MD.0000000000007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita T, Yamazaki Y, Fujiharu C, et al. Association between the duration of periodontitis and increased cardiometabolic risk factors: a 9-year cohort study. Metab Syndr Relat Disord. 2016;14:475–482. doi: 10.1089/met.2016.0018. [DOI] [PubMed] [Google Scholar]
- 38.Zhao MJ, Qiao YX, Wu L, et al. Periodontal disease is associated with increased risk of hypertension: a cross-sectional study. Front Physiol. 2019;10:440. doi: 10.3389/fphys.2019.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. 2019;40:3459–3470. doi: 10.1093/eurheartj/ehz646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietropaoli D, Monaco A, D'Aiuto F, et al. Active gingival inflammation is linked to hypertension. J Hypertens. 2020;38:2018–2027. doi: 10.1097/HJH.0000000000002514. [DOI] [PubMed] [Google Scholar]
- 41.Demmer RT, Papapanou PN, Jacobs DR, Jr, et al. Bleeding on probing differentially relates to bacterial profiles: the Oral Infections and Vascular Disease Epidemiology Study. J Clin Periodontol. 2008;35:479–486. doi: 10.1111/j.1600-051X.2008.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonato CF, do-Amaral CCF, Belini L, et al. Hypertension favors the inflammatory process in rats with experimentally induced periodontitis. J Periodontal Res. 2012;47:783–792. doi: 10.1111/j.1600-0765.2012.01496.x. [DOI] [PubMed] [Google Scholar]
- 43.Rebelo MA, de Castro PH, Rebelo Vieira JM, et al. Low social position, periodontal disease, and poor oral health-related quality of life in adults with systemic arterial hypertension. J Periodontol. 2016;87:1379–1387. doi: 10.1902/jop.2016.160204. [DOI] [PubMed] [Google Scholar]
- 44.Czesnikiewicz-Guzik M, Nosalski R, Mikolajczyk TP, et al. Th1-type immune responses to Porphyromonas gingivalis antigens exacerbate angiotensin II-dependent hypertension and vascular dysfunction. Br J Pharmacol. 2019;176:1922–1931. doi: 10.1111/bph.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikolajczyk TP, Nosalski R, Szczepaniak P, et al. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. FASEB J. 2016;30:1987–1999. doi: 10.1096/fj.201500088R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzik TJ, Skiba DS, Touyz RM, et al. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113:1009–1023. doi: 10.1093/cvr/cvx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slade GD, Ghezzi EM, Heiss G, et al. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med. 2003;163:1172–1179. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- 48.Offenbacher S, Beck JD, Moss K, et al. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. 2009;80:190–201. doi: 10.1902/jop.2009.080007. [DOI] [PMC free article] [PubMed] [Google Scholar]