Abstract
Introduction
This study was designed to assess whether a dental caries management protocol combining a single application of 38% silver diamine fluoride (SDF) with comprehensive oral health education will successfully divert high-risk children from dental treatment under dental general anaesthesia (DGA), arrest active caries in primary teeth, and improve parent-reported child oral health–related quality of life (OHRQoL).
Methods
Children aged 2 to 10 years, who attended two public dental agencies in Victoria, Australia, and were unable to tolerate restorative treatments in the clinic setting, elected to participate in either a 38% SDF intervention protocol or, alternatively, referral for DGA. Follow-up examinations were completed at 6 months to assess caries progression, decayed missing filled tooth index, PUFA index (pulpal involvement, ulceration, fistula, abscess), DGA referral rates, and OHRQoL (Early Childhood Oral Health Impact Scale [ECOHIS]).
Results
Of the total sample, 89.5% of children (n = 102) [mean (SD) age, 4.1 (1.0) years] with 401 active carious lesions elected to participate in the 38% SDF protocol; 10.5% (n = 12) of parents opted for referral for treatment under DGA. The proportion of active caries subsequently arrested at follow-up (number of arrested lesions/number of lesions treated) was 0.78 (95% CI, 0.69 to 0.87). There was an 88% reduction in referrals for DGA in eligible children over the 6-month period. The 38% SDF intervention group showed a significant improvement in ECOHIS scores at follow-up (P < .001).
Discussion
Adoption of the 38% SDF intervention protocol resulted in a significant reduction in the rate of preventable dental hospitalisations. Most parents opted against referral for DGA. Parent-reported OHRQoL for children improved significantly.
Key words: Silver diamine fluoride, Preventable dental hospitalisations, Dental general anaesthesia, Oral health–related quality of life, Early childhood caries
Introduction
Dental caries is the most prevalent chronic disease affecting children worldwide, including Australia.1 Dental caries experience in children negatively impacts their quality of life and leads to poor health outcomes by causing pain, infection, sleep disturbances, chewing difficulties, weight loss, and changes in behaviour.2,3
In Australia, the prevalence of dental caries among young children is high. Australia's most comprehensive National Child Oral Health Study showed that more than 40% of children aged 5 to 10 years experienced caries in their primary teeth.4 Oral diseases are a key marker of disadvantage. Substantial social patterning of dental disease, dental service use, and oral health behaviour is observed among children in Australia.5 Around 20% of 5- to 10-year-old and 17% of 11- to 14-year-old children in Australia experience more than 80% of the total population burden of dental caries in the primary and permanent dentition, respectively.4 Similarly, in Victoria, Australia, a study undertaken among preschool-aged children showed that more than 56% of children aged between 3 and 5 years presented with signs of dental caries, with significantly worse caries experience reported for children of Health Care Card holders, Aboriginal Torres Strait Islanders, and those from non-English-speaking backgrounds.6
Dental caries is currently the highest cause of potentially preventable hospital admissions in Victoria for children in the 0-to-19-year age bracket.7 Approximately 4,500 Victorian children aged 0 to 14 years are hospitalised every year due to preventable dental conditions.8 Many of these children require dental treatment under general anaesthetic due to high levels of dental disease exacerbated by a general lack of compliance among children.
Evidence from a range of studies emphasises the need to provide alternatives to dental general anaesthesia (DGA) for children and shift the focus away from surgical management of dental caries towards the provision of largely preventative services in high-risk regions and populations.9 Such preventive strategies include the establishment of key oral health behaviours such as toothbrushing with fluoride toothpaste, one-to-one dietary interventions in the dental setting,10 and controlling sugar snacking.11 In addition, there is a strong drive to use patient-reported outcomes within clinical practice and prevention protocols to enable clinicians to understand patient perspectives of care, involve patients in their health care, and evaluate outcomes of clinical and preventive care.12
Silver diamine fluoride (SDF), a topical cavity cleanser and desensitiser, has been considered as an effective and viable noninvasive alternative for managing dental caries in high-risk children.13 For several years, 38% SDF has been used in conjunction with a range of other preventative oral health interventions in Australia, Argentina, China, Japan, Brazil, and the United States in the management of dental caries.14 The hallmark of SDF is the visible dark black staining that indicates caries arrest on treated dentin lesions. This is thought to affect parental acceptance of SDF.15,16 However, the staining can be reduced by topical application of potassium iodide (KI), and where possible, and, for children who are compliant, KI can be used alongside SDF. Within the SDF protocol, patient-reported outcomes represent an important concept to explore as they provide valuable insight into how children and parents perceive the effects of a specific treatment and how it might affect children's oral health–related quality of life (OHRQoL). Such insight may aid in clinical decision-making regarding which patients may benefit from SDF treatment and which patients may be more appropriately managed with conventional surgical treatment.17
There is a dearth of studies worldwide that use 38% SDF in conjunction with other preventive interventions in the management of dental caries in high-risk children who have been referred for DGA care. This is the first study that aimed to assess whether the adoption of an alternative preventive protocol for caries management that incorporated the application of 38% SDF, comprehensive oral health education including twice-daily toothbrushing with fluoride toothpaste, and diet modification would divert high-risk children from undergoing DGA and subsequently reduce the rate of preventable dental hospitalisation in Victorian children aged between 2 and 10 years. The study also examined the clinical caries progression following the preventive intervention with 38% SDF and parent assessment of their child's quality of life before and after the management of dental caries with SDF protocol.
Methods
Approval for this study was obtained from the Royal Children's Hospital of Melbourne Human Research Ethics Committee (HREC/17/RCHM/290).
Participants and data collection
Dental Health Services Victoria is the lead public dental agency in Victoria that provides specialist oral health services through the Royal Dental Hospital of Melbourne (RDHM) for people from disadvantaged backgrounds. Children who cannot tolerate comprehensive restorative treatment in the dental chair or comply with dental treatment in community dental agencies across the state of Victoria are referred to RDHM for DGA care. An analysis of the statewide DGA referral trend data between 2015 and 2016 indicated that the community dental agencies with the highest referral rates of children were located in areas where a high proportion of people from low socioeconomic backgrounds resided.
A further assessment of the RDHM day surgery data between 2015 and 2016 revealed that 2 metropolitan community dental agencies based in the North West region of Melbourne had the highest referral rate for children for DGA care compared to the state average. Convenience sampling was used to recruit children from these 2 community dental agencies. Parents of high-risk children in the 2-to-10-year age bracket who were unable to tolerate or comply with restorative treatment in the dental clinic setting upon initial assessment and met the criteria for care under DGA were invited to participate in the study. Following informed consent from parents, recruitment commenced in November 2017 over a 6-month period. Follow-up continued until December 2018.
Parents of eligible children who consented to participate were offered to enrol their children in either the SDF protocol intervention group or the DGA care group. The intervention group received topical 38% SDF application, instruction on twice-daily tooth brushing with fluoride toothpaste, and comprehensive diet counselling. The DGA group consisted of children whose parents opted for a referral for treatment under DGA at RDHM. The DGA group, similar to the SDF group, received instruction on twice-daily tooth brushing with fluoride toothpaste and diet counselling.
The risks and benefits of SDF application and the study process and materials were methodically explained to the parents by the investigators, and informed consent was obtained from parents prior to application of SDF.
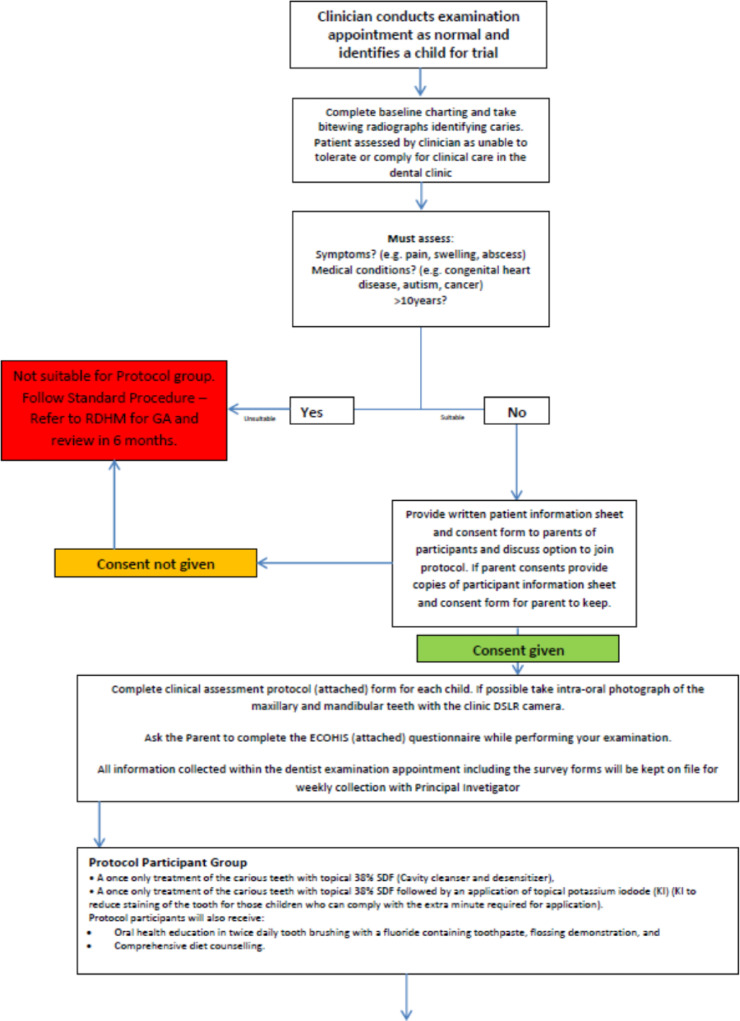
Investigators followed a strict clinical protocol and inclusion criteria to determine eligibility of children to be part of the SDF intervention group (Figure 1). This included having asymptomatic active caries; having no presenting symptoms including pain, swelling, and abscess; and being fit and healthy with no concomitant health conditions such as congenital heart disease, cancer, adrenal insufficiency, or autism.
Fig. 1.
38% silver diamine fluoride protocol intervention group.
The examinations were performed by dentists and oral health therapists. All 6 clinicians underwent standardised training in the protocol procedure and application of 38% SDF. This training programme involved a workshop discussing the trial protocol, patient selection, informed consent procedure, and video demonstration of correct technique of SDF and KI application. Clinicians were then provided with a laminated trial protocol, copies of the clinical forms to be completed, and the 38% SDF and KI. The presentation was identical for all clinicians and was delivered by a single experienced instructor. A standardised baseline examination was completed for each child, which included radiographs and clinical photographs taken where possible and where compliance allowed to assist clinicians in determining changes in lesions. Active caries was recorded by visual assessment of lesion size and colour and tactile assessment with a blunt probe that, when applied with light force, easily penetrated the dentine.
The SDF protocol included a 2-step application procedure. The first step included application of 38% SDF (Riva Star TM, SDI Ltd.) to identified carious lesions on primary teeth. Prior to application, teeth with carious lesions were dried and isolated with gauze and cotton rolls. The teeth were not subject to any caries removal or preparation. Food debris and plaque were removed if required utilising a microbrush, spoon excavator, or toothbrush. Following this, 38% SDF was applied directly to the lesion with a microbrush for 1 minute. In the second step, KI was applied to the lesion for a further 1 minute or until a white precipitate formed. Parents were instructed to prevent the child from eating or drinking for 1 hour after the treatment.
Parents received oral health education on twice-daily toothbrushing with a fluoride-containing toothpaste, a flossing demonstration, and comprehensive diet counselling. To supplement the clinical indicators, child OHRQoL measures were captured using the Early Childhood Oral Health Impact Scale (ECOHIS).18 ECOHIS has been developed from a defined conceptual model of health-related quality of life and has been subjected to psychometric evaluation. The construct validity, internal consistency, and test/retest reliability of the ECOHIS questionnaire has been confirmed by several studies.18, 19, 20 Parents of the children in both the SDF protocol intervention group and those who opted to be in the DGA group completed the ECOHIS survey at baseline and 6-month follow-up.
To examine the caries level and progression in the SDF intervention group, data were collected on the number and location of carious lesions, lesion colour, lesion hardness, decayed missing filled tooth (DMFT) index, and PUFA index (pulpal involvement, ulceration, fistula, abscess). At both the baseline and recall visit, 38% SDF–treated lesions were assessed and records taken for lesion colour (yellow, light brown, dark brown, black) and lesion texture (soft or hard, assessed using gentle pressure with a probe). The presence or absence of pain and infection was observed through the PUFA index at baseline and recall visit through examination and parent interview. The effectiveness of the treatment with 38% SDF was evaluated based on these clinical outcomes. That is, hard and black lesions with no pain or infection were considered positive outcomes.
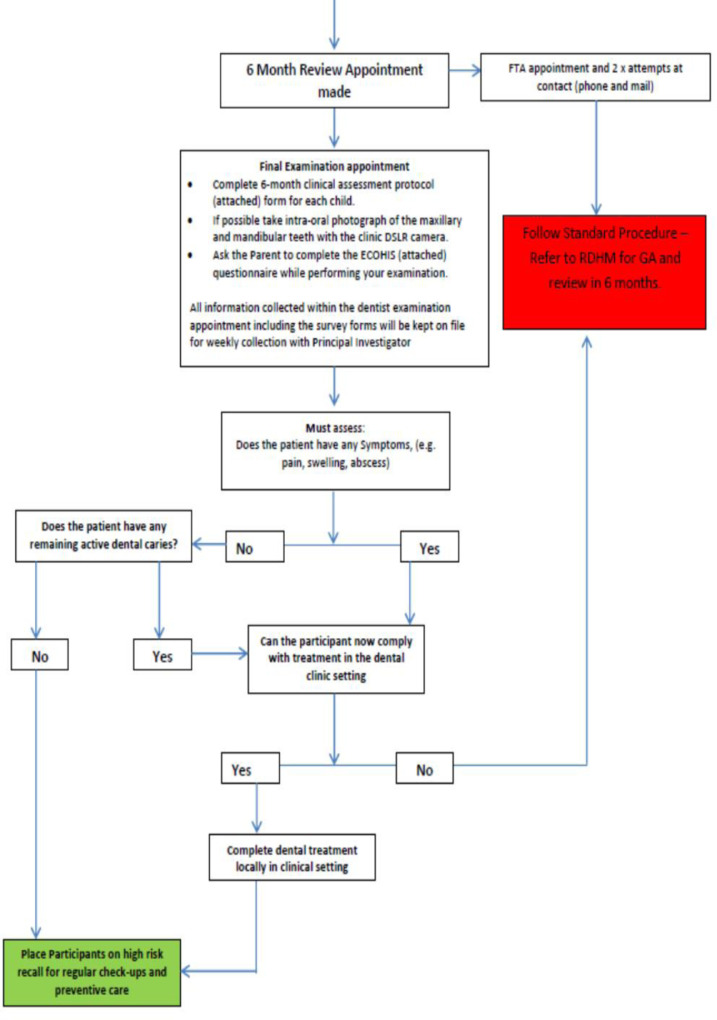
Children were reexamined and lesions were reevaluated approximately 6 months after the baseline examination by the same clinician who conducted the baseline evaluation. At this visit, if the carious lesion was not arrested, a second application of 38% SDF was delivered and recorded. Where children exhibited arrested caries, parents were placed on a 6-month recall to continually monitor the lesions. In some instances, if improved cooperation and behaviour were noted in the child, definitive restoration such as stainless-steel crown or restoration was placed. Children who presented with symptoms of pain and infection following the treatment with 38% SDF were directly referred to RDHM for DGA. These children were seen at the earliest possible appointment to ensure that the children's condition did not deteriorate.
Statistical analysis
IBM SPSS Statistics 19 was used for analyses. Descriptive statistics [frequency and percentage or mean and standard deviation (SD)] were used to summarise the characteristics of the children and their parents or guardians. The proportion of arrested lesions before and after 38% SDF application was calculated to assess the effectiveness of caries arrests.
For the ECOHIS surveys, repeated analysis of variance (ANOVA) was performed to detect overall differences between related mean scores, with adjustments for multiple comparisons using the Bonferroni tests with the confidence interval significance level set at 5%. Questionnaires with more than 30% missing responses were excluded from the analysis. The effect size was calculated by dividing the mean of change score by the standard deviation of the baseline score. An effect size of <0.2 indicated a small but clinically meaningful magnitude of change; 0.2 to 0.7, a moderate change; and >0.7, a large change.
Results
One hundred two children [mean (SD) age, 4.1 (1.0) years] with 401 active carious lesions were enrolled in the SDF protocol intervention group (Table 1). Parents of 12 children opted for referral for DGA at RDHM. Overall, 89.5% of participants chose to participate in the SDF protocol intervention group. Follow-up was completed for 83% (n = 85) of the children [mean (SD) age, 4.1 (1.0) years] and for 296 baseline active carious lesions. There was an even distribution of males and females (43:42).
Table 1.
Demographic characteristics of children enrolled in the silver diamine fluoride (SDF) protocol at baseline and at 6-month follow-up.
| Characteristics | Baseline enrolled n = 102 | Completed 6-month follow-up n = 85 |
|---|---|---|
| Mean age (years) of sample (SD) | 4.1 (1.0) | 4.08 (1.0) |
| Sex [N (%)] | ||
| Female | 62 (51) | 42(50) |
| Male | 50 (49) | 43 (50) |
| Number of SDF-treated teeth | 401 | 296 |
| Race/ethnicity [N (%)] | ||
| Caucasian | 50 (49) | 44 (52) |
| Aboriginal/Torres Strait Islander | 10 (10) | 8 (9) |
| Asian | 26 (25) | 19 (22) |
| Other | 16 (16) | 14 (17) |
| Marital status [N (%)] | ||
| Partnered | 69 (68) | 59 (69) |
| Single | 33 (32) | 26 (31) |
A total of 44 children did not meet the inclusion criteria and were excluded from the study entirely and referred for DGA care. Reasons for excluding these children included the following: pain, 21 (47%); abscess, 9 (20%); surgical extraction, 7 (16%); medical condition excluding from trial, 3 (6%); supernumerary, 2 (5%); trauma, 1 (2%); lip tie, 1 (2%); and submerged tooth, 1 (2%).
Compliance for 38% SDF application was 100% both at initial presentation and 6-month follow-up. The average number of 38% SDF applications per child at baseline was 5 teeth. The average number of appointments required for application at baseline was 1.3. For children who completed the follow-up (n = 85), the mean (SD) DMFT was 4.4 (2.7) at baseline (Table 2). At baseline, 72% (213/296) of treated lesions were in posterior teeth and 28% (83/296) in anterior teeth.
Table 2.
Features of 38% silver diamine fluoride (SDF)–treated carious lesions at baseline and follow-up.
| Baseline | 6-month review | |
|---|---|---|
| Distribution of teeth | ||
| Molars | 72% | 83% |
| Incisors | 28% | 17% |
| DMFT index, mean (SD) | 4.37 (2.7) | 4.51 (3.3) |
| No. of active carious lesions per patient, mean (SD) | 3.48 (2.6) | 0.77 (0.8) |
DMFT, decayed missing filled tooth.
The mean (SD) number of SDF-treated teeth was 3.5 (2.6) at baseline. The mean (SD) number of remaining active carious lesions treated at 6-month follow-up was 0.8 (0.8). The average proportion of treated teeth with arrested lesions at follow-up (number of arrested lesions /number of treated) in the SDF group was 0.78 (95% CI; 0.69 to 0.87). More than 60% of children in the SDF protocol intervention group had 100% of lesions arrested (52/85).
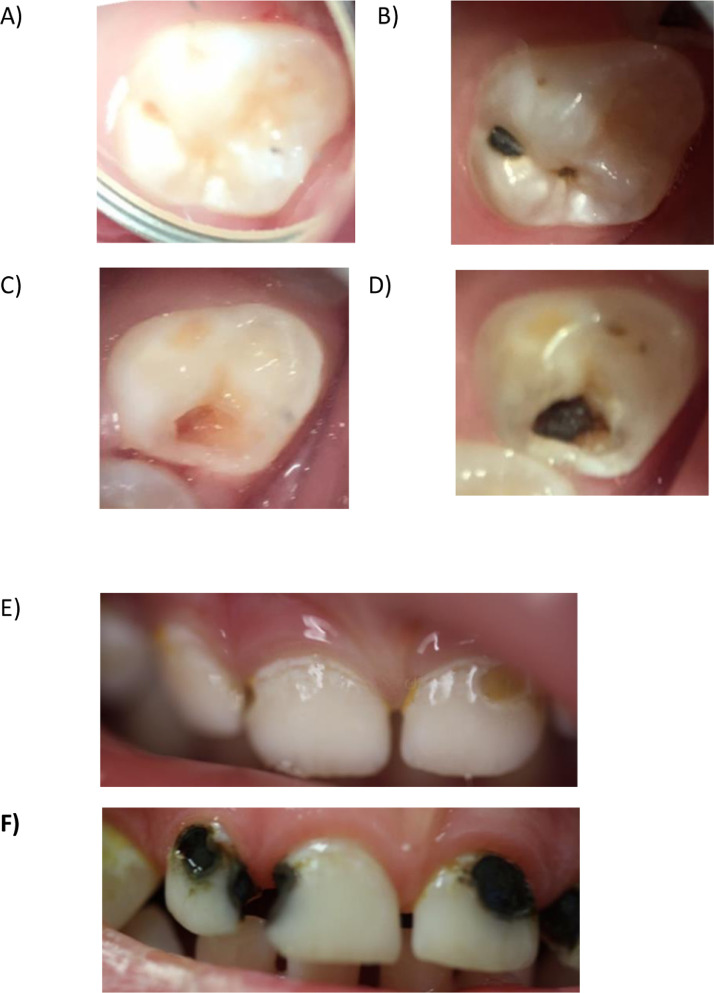
No adverse events were reported by parents of children who were treated using 38% SDF (gingival or soft tissue stomatitis or ulcerative lesions); however, one clinician noted gingival irritation in 2 children, which resolved spontaneously. The arrested carious lesions were identified as “black” and “hard” in all cases (Figure 2).
Fig. 2.
Clinical photographs of silver diamine fluoride protocol–managed teeth at baseline and at 6-month follow-up in a 4-year-old boy (A–D) and a 2.5-year -old boy (E and F).
At follow-up, the remaining active carious lesions were managed by repeating the SDF protocol and placing the patient on 6-month recall. During the 6-month follow-up period, 2 patients in the SDF protocol group developed a PUFA index of 1 and were referred for DGA at RDHM.
A total of 64 participants completed ECOHIS surveys at 6-month review. The overall ECOHIS, child impact section, and family impact section scores decreased significantly (P < .001), demonstrating moderate effect sizes (Table 3). The greatest decrease in the ECOHIS scores were for the domains of child psychology (30.8%) and parental distress (32.3%). The lowest ECOHIS scores were found for the domain of image (6.1%) and function (15.8%) in the child impact scale and family function (13.9%) in the family impact section. The P value corresponding to the repeated ANOVA was slightly higher than 0.05 for these impacts, suggesting that the changes in parent reported child self-image, child function, and family function were not significantly different for that level of significance. The overall OHRQoL of the children significantly improved following the 38% SDF protocol.
Table 3.
Overall Early Childhood Oral Health Impact Scale (ECOHIS), child impact section (CIS), and family impact section (FIS) scores (pretreatment and 6 months posttreatment) for children who received the silver diamine fluoride protocol (N = 64).
| ECOHIS domain (No. of items) | Pretreatment, mean (SD) | 6 months posttreatment, mean (SD) | P values | Percentage change from baseline to 6-month follow-up | Effect size (baseline to 6-month follow-up) |
|---|---|---|---|---|---|
| ECOHIS (13) | 20.9 (10.5) | 16.7 (9.35) | < .001 | 19.8 | 0.4 |
| CIS (9) | 14.3 (7.9) | 11.6 (6.39) | < .001 | 18.4 | 0.4 |
| Oral symptoms | 2.0 (1.5) | 1.5 (0.67) | < .05 | 24.2 | 0.4 |
| Function | 6.3 (4.6) | 5.3 (2.40) | > .05 | 15.8 | 0.3 |
| Psychology | 3.2 (1.7) | 2.2 (0.56) | < .001 | 30.8 | 0.8 |
| Image | 2.8 (1.6) | 2.6 (1.67) | > .05 | 6.1 | 0.1 |
| FIS (4) | 6.6 (2.5) | 5.1 (2.94) | < .001 | 23.0 | 0.5 |
| Parental distress | 3.3 (1.9) | 2.2 (1.16) | < .001 | 32.3 | 0.7 |
| Family function | 3.3 (2.1) | 2.9 (1.68) | > .05 | 13.9 | 0.2 |
Of the 12 parents who opted to be in the DGA group, only 3 returned the ECOHIS surveys. No analysis was undertaken for changes in the ECOHIS scores for those who underwent DGA, as the small size of this sample was unlikely to provide viable results.
The rate of acceptance to participate in the SDF protocol was 89% (102/114). Of the 102 children who participated in the SDF protocol group, only 2 children subsequently required referral for DGA prior to completion of the study. When added to the 12 children in the DGA care group, the reduction in referrals of eligible children for DGA at RDHM over the 6 month period was 88%, showing that a significant proportion of children were diverted from potentially having treatment under DGA.
Discussion
The number of Victorian children who have had dental care under DGA has significantly increased over the last 15 years.21 This study aimed at diverting dental care under DGA through a protocol of prevention and management of caries utilising 38% SDF in young healthy children who were asymptomatic and not compliant for routine dental treatment. The findings of this study demonstrate that a single application of 38% SDF in combination with oral hygiene and dietary instruction is effective in arresting dental caries. The trial was successful in diverting 88% of at-risk children from DGA over the 6-month trial period, with the vast majority of parents opting to participate in the SDF protocol intervention group.
In this study, single application of 38% SDF was effective in arresting 78% of dental caries at the 6-month review. This finding is similar to those found in international studies22,23 in which randomised clinical trials showed caries arrest levels above 75% with 6 monthly 38% SDF applications. These findings are also supported by a systematic review that showed that the caries arrest rate for SDF treatment was almost 86% at 6 months.14 Results from other randomised clinical trials conducted internationally using different SDF concentrations and application frequencies indicate that caries arrest using SDF is both effective and safe for preschool-aged children.24,25 However, the findings represent specific demographics of children with diet patterns, oral hygiene regimen, and water fluoridation levels different from Australia. Although there are trials under way in Australia, there are no comparable published studies.
The ECOHIS questionnaire uses parental reports on the child OHRQoL. The work of Parsons et al.26 suggests that parents or caregivers are frequently the primary decision-makers in regard to a child's health. As such, their perceptions can have a great influence on treatment choices. Moreover, children do not acquire the capacity for abstract thinking until approximately 6 years of age.27 Therefore, parents can be reliably used as a surrogate measure to assess child's OHRQoL in the absence of child's self-reports.28,29
The findings of this study illustrated that children who participated in the study were in great need of dental treatment and their OHRQoL was seriously impaired prior to treatment. There was a significant improvement in these children's OHRQoL following treatment with 38% SDF and a positive impact on the family's quality of life. Specifically, marked improvements were observed in the child psychology and parental distress (P < .001) impacts after enrolling in the 38% SDF protocol intervention group. The most commonly reported impacts in this study are similar to those of previous studies conducted in Australia, which have also demonstrated more pronounced improvements in the child psychology, parental distress, and family impact sections of the ECOHIS questionnaire.30,31 In this study, the percentile reduction in the overall ECOHIS score following 38% SDF protocol at 6-month review was 19.8%.
The results from the ECOHIS questionnaire indicate that all domains demonstrated a significant improvement except the parent-reported child self-image, parent-reported function, and family function domains, which showed only small effect sizes after SDF application. A post hoc power analysis was conducted using the software package G*Power. The sample size of 85 was used for the statistical power analyses and a 2-predictor variable equation was used as a baseline. The alpha level used for this analysis was P < .05. The post hoc analyses revealed the statistical power for this study was .95 for detecting a small effect, whereas the power exceeded .99 for the detection of a moderate to large effect size. Thus, there was more than adequate power at all effect size levels.
There are several studies that have explored parental perception and acceptance of SDF staining. These studies have found that staining on anterior teeth was perceived as undesirable; however, most parents preferred this option as it might avoid the need for advanced behavioral guidance techniques such as sedation or DGA to deliver traditional restorative care.15,16 In this study, despite the potential for visible permanent tooth staining caused by SDF application, there was a high participation rate of children in the SDF group compared to the group who opted for care under DGA. In addition, there was a slight improvement in parent-reported child self-image. Although we did not explore these domains further, one of the possible reasons could be the level of importance placed on dental aesthetics among the high-risk group who participated in the SDF protocol.
A recommendation for future studies on the use of SDF to reduce DGAs would be the use of a control group. This study had a 6-month follow-up and observation period. Importantly, previous studies have demonstrated that the effectiveness of caries lesion arrest by 38% SDF decreases as the period of assessment increases.14 Long-term studies are needed to determine whether the caries arrest and prevention can be sustained and whether more frequent applications of SDF would enhance efficacy.
SDF has been recommended for children with high caries risk, including those with behavioral complications, those who require multiple treatment visits, or those without access to dental care.32 The findings of this study indicate that DGA can be avoided for high-risk children using the SDF protocol in conjunction with other prevention strategies such as twice-daily tooth brushing with fluoride toothpaste and comprehensive diet counselling. The main disadvantage of SDF is the non-aesthetic black discolouration of carious lesions after its application, which can be reduced using KI. SDF as a topical cleanser and desensitiser is effective in managing dentine caries, and the ease of application can result in the ability to expand delivery of care to a larger population of children with untreated caries. It is minimally invasive, low-cost and simple to use and has demonstrated its use as an alternative treatment option for active caries in young children at high risk for caries and emergency presentation.33 The use of SDF has important implications in paediatric dentistry and the provision of minimally invasive preventive care in the community dental agencies, outreach services, and school dental programmes.
Conflict of interest
None disclosed.
REFERENCES
- 1.Phantumvanit P, Makino Y, Ogawa H, et al. WHO global consultation on public health intervention against early childhood caries. Community Dent Oral Epidemiol. 2018;46:280–287. doi: 10.1111/cdoe.12362. [DOI] [PubMed] [Google Scholar]
- 2.Abanto J, Carvalho T, Mendes F, Wanderley M, Bönecker M, Raggio D. Impact of oral diseases and disorders on oral health-related quality of life of preschool children. Community Dent Oral Epidemiol. 2011;39(2):105–114. doi: 10.1111/j.1600-0528.2010.00580.x. [DOI] [PubMed] [Google Scholar]
- 3.Krisdapong S, Somkotra T, Kueakulpipat W. Disparities in early childhood caries and its impact on oral health-related quality of life of preschool children. Asia Pac J Public Health. 2014;26(3):285–294. doi: 10.1177/1010539512438608. [DOI] [PubMed] [Google Scholar]
- 4.Spencer A, Do L. University of Adelaide;; South Australia: 2016. Oral Health of Australian Children: The National Child Oral Health Study 2012–14. editors. [Google Scholar]
- 5.Peres M, Ju X, Mittinty M, Spencer A, Do L. Modifiable factors explain socioeconomic inequalities in children's dental caries. J Dent Res. 2019;98(11):1211–1218. doi: 10.1177/0022034519866628. [DOI] [PubMed] [Google Scholar]
- 6.Graesser H, Sore R, Rogers J, Cole D, Hegde S. Early Childhood Caries in Victorian pre-schoolers: a cross-sectional study. Int Dent J (Manuscript accepted to be published) 2021 doi: 10.1016/j.identj.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Australian Institute of Health and Welfare. Oral health and dental care in Australia. Available fromhttps://www.aihw.gov.au/reports/dental-oral-health/oral-health-and-dental-care-in-australia. Accessed 25 May 2019.
- 8.Rogers J, Adams G, Wright F, Roberts-Thomson K, Morgan M. Reducing potentially preventable dental hospitalizations of young children: a community-level analysis. JDR Clin Trans Res. 2018;3(3):272–278. doi: 10.1177/2380084418764312. [DOI] [PubMed] [Google Scholar]
- 9.Marinho V, Worthington H, Walsh T, Clarkson J. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2013;(7) doi: 10.1002/14651858.CD002279.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans D. Some evidence that one-to-one dietary interventions in the dental setting can change behaviour. Evid Based Dent. 2012;13(2):42. doi: 10.1038/sj.ebd.6400855. [DOI] [PubMed] [Google Scholar]
- 11.Adair P, Burnside G, Pine C. Analysis of health behaviour change interventions for preventing dental caries delivered in primary schools. Caries Res. 2013;47(Suppl 1):2–12. doi: 10.1159/000351829. [DOI] [PubMed] [Google Scholar]
- 12.Gilchrist F, Rodd H, Deery C, Marshman Z. Assessment of the quality of measures of child oral health-related quality of life. BMC Oral Health. 2014;14:40. doi: 10.1186/1472-6831-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crystal YO, Marghalani AA, Ureles SD, et al. Use of silver diamine fluoride for dental caries management in children and adolescents, including those with special health care needs. Pediatr Dent. 2017;39(5) 135E–45E. [PubMed] [Google Scholar]
- 14.Gao S, Zhao I, Hiraishi N, et al. Clinical trials of silver diamine fluoride in arresting caries among children: a systematic review. JDR Clin Trans Res. 2016;1(3):201–210. doi: 10.1177/2380084416661474. [DOI] [PubMed] [Google Scholar]
- 15.Crystal YO, Janal MN, Hamilton DS, Niederman R. Parental perceptions and acceptance of silver diamine fluoride staining. J Am Dent Assoc. 2017;148(7):510–518. doi: 10.1016/j.adaj.2017.03.013. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crystal Y, Kreider B, Raveis V. Parental expressed concerns about silver diamine fluoride (SDF) treatment. J Clin Pediatr Dent. 2019;43(3):155–160. doi: 10.17796/1053-4625-43.3.2. [DOI] [PubMed] [Google Scholar]
- 17.Sihra R, Schroth RJ, Bertone MF, et al. The effectiveness of silver diamine fluoride and fluoride varnish in arresting caries in young children and associated oral health-related quality of life. Ethnicity. 2019;26 65.0. [PubMed] [Google Scholar]
- 18.Pahel BT, Rozier RG, Slade GD. Parental perceptions of children’s oral health: the early childhood oral health impact scale (ECOHIS) Health Qual Life Outcomes. 2007;5(1):6. doi: 10.1186/1477-7525-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arrow P, Klobas E. Evaluation of the early childhood oral health impact scale in an Australian preschool child population. Aust Dent J. 2015;60(3):375–381. doi: 10.1111/adj.12236. [DOI] [PubMed] [Google Scholar]
- 20.Jokovic A, Locker D, Stephens M, Kenny D, Tompson B, Guyatt G. Measuring parental perceptions of child oral health-related quality of life. J Public Health Dent. 2003;63(2):67–72. doi: 10.1111/j.1752-7325.2003.tb03477.x. [DOI] [PubMed] [Google Scholar]
- 21.Rogers JG. Dental hospitalisation of Victorian children and young adults-prevalence, determinants, impacts and policy implications 2016.
- 22.Fung M, Duangthip D, Wong M, Lo E, Chu C. Randomized clinical trial of 12% and 38% silver diamine fluoride treatment. J Dent Res. 2018;97(2):171–178. doi: 10.1177/0022034517728496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhi QH, Lo ECM, Lin HC. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent. 2012;40(11):962–967. doi: 10.1016/j.jdent.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Milgrom P, Horst JA, Ludwig S, et al. Topical silver diamine fluoride for dental caries arrest in preschool children: a randomized controlled trial and microbiological analysis of caries associated microbes and resistance gene expression. J Dent. 2018;68:72–78. doi: 10.1016/j.jdent.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yee R, Holmgren C, Mulder J, Lama D, Walker D, van Palenstein Helderman W. Efficacy of silver diamine fluoride for arresting caries treatment. J Dent Res. 2009;88(7):644–647. doi: 10.1177/0022034509338671. [DOI] [PubMed] [Google Scholar]
- 26.Parsons SK, Barlow SE, Levy SL, Supran SE, Kaplan SH. Health-related quality of life in pediatric bone marrow transplant survivors: according to whom? Int J Cancer. 1999;83(S12):46–51. doi: 10.1002/(sici)1097-0215(1999)83:12+<46::aid-ijc9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 27.Locker D, Jokovic A, Clarke M. Assessing the responsiveness of measures of oral health-related quality of life. Community Dent Oral Epidemiol. 2004;32(1):10–18. doi: 10.1111/j.1600-0528.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- 28.Khin Hla T, Hegarty M, Russell P, Drake-Brockman TF, Ramgolam A, von Ungern-Sternberg BS. Perception of pediatric pain: a comparison of postoperative pain assessments between child, parent, nurse, and independent observer. Pediatr Anesth. 2014;24(11):1127–1131. doi: 10.1111/pan.12484. [DOI] [PubMed] [Google Scholar]
- 29.Rajasagaram U, Taylor DM, Braitberg G, Pearsell JP, Capp BA. Paediatric pain assessment: differences between triage nurse, child and parent. J Paediatr Child Health. 2009;45(4):199–203. doi: 10.1111/j.1440-1754.2008.01454.x. [DOI] [PubMed] [Google Scholar]
- 30.Arrow P. Responsiveness and sensitivity of the early childhood oral health impact scale to primary dental care for early childhood caries. Community Dent Oral Epidemiol. 2016;44(1):1–10. doi: 10.1111/cdoe.12183. [DOI] [PubMed] [Google Scholar]
- 31.Yawary R, Anthonappa RP, Ekambaram M, McGrath C, King NM. Changes in the oral health-related quality of life in children following comprehensive oral rehabilitation under general anaesthesia. Int J Paediatr Dent. 2016;26(5):322–329. doi: 10.1111/ipd.12200. [DOI] [PubMed] [Google Scholar]
- 32.Horst JA, Ellenikiotis H, Milgrom PM, Committee USCA. UCSF protocol for caries arrest using silver diamine fluoride: rationale, indications, and consent. J Calif Dent Assoc. 2016;44(1):16. [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas ML, Magher K, Mugayar L, Dávila M, Tomar SL. Silver diamine fluoride helps prevent emergency visits in children with early childhood caries. Pediatr Dent. 2020;42(3):217–220. [PubMed] [Google Scholar]