Abstract
Objectives
The aim of this work was to review the current uses of chlorhexidine (CHX) in dentistry based on its mechanism of action, whilst highlighting the most effective protocols that render the highest clinical efficacy whilst limiting adverse drug reactions.
Methods
A literature search was conducted using the key words chlorhexidine, mechanism of action, adverse effects, and dentistry using databases in the University of Toronto library system. The titles and abstracts were read, and relevant articles were selected.
Results
A total of 1100 publications were identified, 100 were investigated, and 67 of them were used. Out of the 67 selected articles, 12 were reviews on CHX; 5 articles focussed on CHX gels; 13 focussed on CHX mouthwashes; 8 focussed on CHX products; 13 discussed adverse effects associated with CHX; 13 focussed on periodontal pathology and treatment; 6 focussed on implant periodontal and dental surgeries; 7 evaluated effects on caries; 6 looked at the mechanisms of action; and 12 focussed on the antibacterial and antimicrobial impact on the oral biome. There were multiple areas of overlap amongst the articles, and results showed that CHX provides different uses, but mainly as an adjunct to various treatments. Mouthwash was the most superior medium when used in short time spans when mechanical prophylaxis was not possible for the prevention of gingivitis and maintenance of oral hygiene. CHX products are often used in periodontics, post–oral surgical procedures, and as a prophylaxis for multiple invasive procedures with minimal adverse effects. Tooth staining was the most negative adverse effect reported by patients.
Conclusions
CHX's antimicrobial properties make it an ideal prophylactic when mechanical debridement is not possible. CHX mouthwash appears to be more effective compared to gels. Concentrations of 0.12% to 0.2% are recommended; any mouthwash with concentrations above 0.2% will unnecessarily increase the unwanted side effects. CHX is useful amongst various areas of dentistry including oral surgery, periodontics, and even general dentistry. For long-term treatments, especially in periodontitis patients (stage I-III) undergoing nonsurgical treatments, CHX chips are recommended. CHX chips are also recommended as an adjunct to implant debridement in patients with peri-implant mucositis and peri-implantitis over CHX mouthwash and gels.
Key words: Chlorhexidine, Mechanism of action, Adverse effects, Dentistry
Introduction
Chlorhexidine (CHX) is a bisbiguanide that was developed in the 1940s in the UK and has been marketed as a general disinfectant.1 In the 1970s, its antiplaque activity was discovered, and by 1976 it was available as a mouthwash.1,2 Oral biofilm and its associated bacteria have been linked to the pathogenesis of various oral diseases including halitosis, caries, gingivitis, and periodontitis.3, 4, 5 Periodontal disease in the US has been diagnosed in approximately 47.2% of adults 30 years and older and 70.1% of adults 65 years of age and older.6,7 Therefore, biofilm control is critical to prevent the development of such conditions; specifically, manual or electronically powered toothbrushing is recommended as the main method for prevention as well as reduction of plaque and gingivitis.3, 4, 5,7,8
Effective mechanical biofilm control requires time, willingness, and dexterity-related skills.3,4 However, this may not be feasible in patients who are mentally or physically disabled, as well as in postsurgical situations where oral hygiene becomes almost impossible.2, 3, 4,9,10 Currently, CHX mouthwash is the most potent chemotherapeutic agent and gold standard in reducing S mutans and oral biofilm.11,12 In dentistry, CHX products are available by prescription and include formulations such as mouthwashes, gels, chips, and varnishes. 1,13,14
There are many scholarly articles and manufacturer reports describing the uses and benefits of CHX products; however, there are conflicting data amongst them. Moreover, there is limited clinical information regarding effective protocols. This review will examine the mechanism of action (MOA) of CHX and investigate the most effective protocols regarding CHX uses in dentistry whilst highlighting important adverse drug reactions (ADRs) relevant to a dental setting based on the most recent literature and clinical trials. Amongst protocols, general, periodontal, prophylactic, and postsurgical uses of CHX will be investigated and addressed and any associated ADRs examined.
Methods
A literature review was commenced to identify the MOA and to investigate the most effective adjunctive uses of CHX in dental procedures. Such procedures include the treatment of periodontal diseases as well as prophylactic uses. Databases in the University of Toronto library system were searched to locate relevant peer-reviewed articles written in English and published in the past 5 years. Search words entered included “chlorhexidine,” “mechanism of action,” “adverse effects,” and “dentistry.” Dentistry textbooks were also obtained from the National Dental Examination Board as well as the University of Toronto Library and reviewed for relevant information. A total of 1100 publications were identified, the abstracts were read, and out of the 1100 a total of 100 were investigated. Analysis of articles revealed that CHX mouthwash is the most effective out of the CHX products due to its ability to successfully inhibit significantly more plaque when compared with gels and varnishes.14, 15, 16, 17 Therefore, this review will focus on CHX mouthwashes.
Results and discussion
MOA
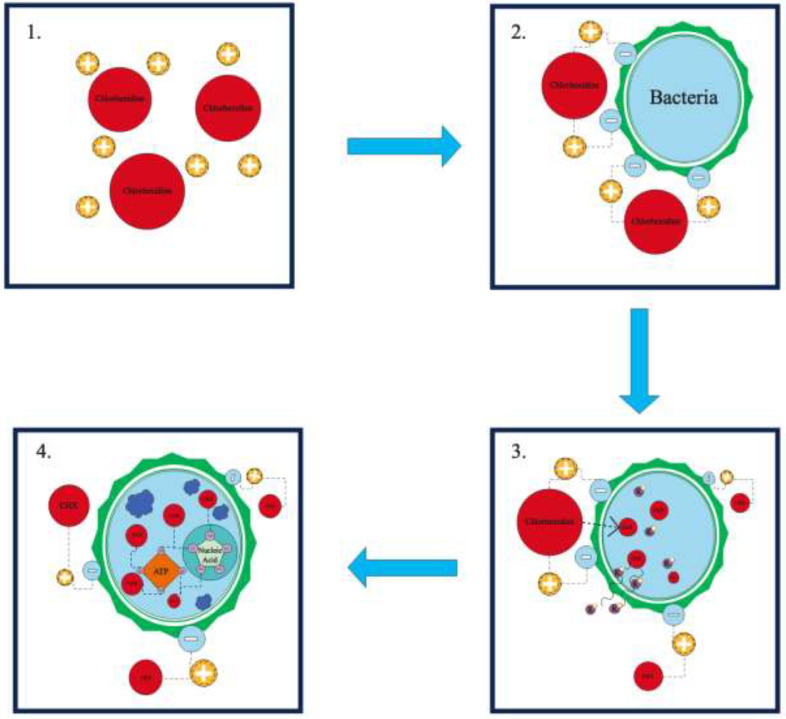
The MOA (refer to Figure) begins with rapid attraction of a cationic CHX molecule to the surface of a negatively charged bacterial cell containing phosphates and sulphate groups.18, 19, 20 The cationic properties of CHX result in a bond to negatively charged sites within the biofilm including the bacteria, extracellular polysaccharides, and glycoproteins.18,21 This causes specific and strong adsorption to phosphate-containing components forming the surface of the bacterial cell.19,22 Penetration through the bacterial cell wall occurs, as a result of passive diffusion, attracting towards the cytoplasmic membrane of the cell, damaging it, and compromising its integrity.19 This event allows CHX to infiltrate the inner cell membrane, resulting in greater permeability.18,19 The result is an outflow of low-molecular-weight molecules and cytoplasmic components escaping from the microorganism, such as potassium ions, leading to the inhibition of activity of some of the enzymes associated with the cytoplasmic membrane.18,19,21
Figure.
Mechanism of action (MOA) for chlorhexidine. Stage 1: Positively charged chlorhexidine is attracted to the negative charge on the bacterial cell wall.18, 19, 20 Stage 2: Chlorhexidine forms specific and strong adsorption to phosphate-containing molecules that are on the surface of the bacterial cell.19,22 Stage 3 (bacteriostatic): Penetration through the bacterial cell wall occurs, damaging it and compromising its integrity. The result is an outflow of low-molecular-weight cytoplasmic components, such as potassium ions, and inhibition in the activity of some of the enzymes associated with the cytoplasmic membrane.19 Stage 4 (bactericidal): Cytoplasmic coagulation and precipitation occur by forming complexes with phosphorylated compounds, such as adenosine triphosphate (ATP) and nucleic acids.18,19,22
At this point, CHX's antimicrobial action remains in the bacteriostatic stage, but it can be reversed if CHX is removed.18,19,22 However, if the concentration of CHX remains stable over time, or increases, this will lead to irreversible cell damage and a bacteriocidal stage.18,22 In the bacteriocidal stage, cytoplasmic coagulation and precipitation occurs by forming complexes with phosphorylated compounds, such as adenosine triphosphate and nucleic acids.18,19,22 Due to the negative charge of most oral surfaces (including mucous membranes, teeth, and salivary glycoproteins), the cationic nature of CHX molecules exhibits good adherence to these surfaces and therefore interferes with bacterial adhesion, allowing substantivity for up to 12 hours.18,21
Uses in dentistry
In dentistry, CHX products are used therapeutically as well as prophylactically.7,11,12,23 For these purposes, the ideal dosage of CHX is 18 to 20 mg per application, which maximises efficacy whilst minimising side effects4,7,18,24,25 (refer to Table 1 for details on mouthwash uses).
Table 1.
| Chlorhexidine usage | Evidence-based recommendations |
|---|---|
| Presurgical | Utilise 0.12% or 0.2% CHX preoperative rinse for 1 minute to reduce oral microbial load prior to implant placement. |
| Postoperative (short-term maintenance) | If toothbrushing is not possible due to postoperative pain, it is recommended that a 0.12% to 0.2% 1-minute rinse is performed 3 times a day for 7 days or until sutures are removed and oral hygiene in the form of toothbrushing can resume. |
| Postoperative (long-term maintenance) | Locally administered sustained-release CHX, in the form of CHX chips, may be considered for use as an adjunct to nonsurgical treatment of peri-implant mucositis and peri-implantitis. Specifically, the use of PeriochipTM over a maximum of 6 months has been suggested in the reduction of implant pocket depth, with a minimum of 2-week recalls. |
CHX demonstrates swift antimicrobial and antifungal activity, and it maintains efficacy even at low concentrations.7,20,26 CHX can affect both aerobic and anaerobic bacteria.7,27 It can even destroy DNA and RNA viruses and inactivate lipophilic-enveloped viruses, including human immunodeficiency viruses, influenza A, parainfluenza, hepatitis B, herpes simplex virus, and cytomegalovirus.7,27, 28, 29, 30 The next sections expand on the clinical relevance and uses of CHX, focusing mainly on effective protocols.
Dental caries and oral hygiene
CHX mouthwash with a 0.1% to 0.2% concentration demonstrates significant antiplaque effects when used daily over 2 weeks in the absence of mechanical cleaning and as a long-term adjunct to oral hygiene at 4- to 6-week and 6-month intervals.4,18,22,31 However, mouthwashes with concentrations below 0.1% are not reliably used because efficacy regarding the inhibition of plaque formation is not clear.4 There is contrasting evidence, some studies indicating no differences and others indicating a significantly reduced antiplaque action with concentrations below 0.12%.4 It should be noted that some components of toothpaste such as calcium and anionic surfactants like sodium lauryl sulfate (SLS), sodium dodecyl sulfate (SDS), and cocamidopropyl betaine (CAPB) reduce the substantivity and overall effectiveness of CHX.22,32,33 In order to avoid these interactions, the use of CHX mouthwash should be delayed for a minimum of 30 minutes after toothbrushing if it is to be used as an adjunct in oral hygiene.22,31, 32, 33 Furthermore, if a fluoridated toothpaste is used, cocamidopropyl betaine toothpaste is recommended because it is the surfactant that allows for the greatest enamel remineralisation when combined with a 30-minute delayed CHX oral rinse.33 When comparing CHX dentifrices or gels of 1% and 0.12% to CHX mouthwashes of 0.12% and 0.2%, there is a superiority in the performance delivered by mouthwash in respect to plaque inhibition.15,17 Although CHX gels do inhibit some plaque growth, when CHX gels and dentifrices are incorporated into a non-brushing model, where mechanical oral hygiene is not feasible, CHX mouthwash should be the first product of choice.17
In regard to S mutans, CHX is known to have a minimum inhibitory concentration (MIC) that does not exceed 1 μg/mL.19,34 CHX mouthwashes have demonstrated significant reductions in S mutans, but this is not the case for CHX gels.25 CHX gels at concentrations of 0.12% and 0.2% as well CHX varnishes of 10% and 40% concentrations failed to reduce S mutans when applied to teeth surfaces.14,25 Although CHX mouthwashes have demonstrated significant reductions in S mutans, this does not translate into direct clinical benefits, such as caries reduction.25 In fact, no CHX product appears to prevent caries when used alone, and there is little evidence supporting such claims.14
In the past, fluoride and CHX were studied together for their compatibility in the hope of creating a favourable product that incorporated the antimicrobial and remineralising benefit of each.35 Previous studies looked at monofluorophosphate and CHX at clinically relevant concentrations and found that a large portion of free CHX was eliminated.35 This led to the belief that fluoride and CHX are incompatible25,35; however, this does not appear to be the case for sodium fluoride (NaF).35 NaF appears to be compatible in rinse and toothpaste formulations.35 When CHX and fluoride are combined, the result is CHX difluoride, which ionises to the same extent as NaF in aqueous solutions.35 Currently, it appears that CHX mouthwash can be combined with NaF without losing CHX's effect on plaque, gingivitis, and even S mutans35,36; however, it is unclear whether CHX affects the properties of fluoride.35 Therefore, further clinical research is required before a CHX-sodium fluoride mouthwash can be recommended for use.35
Periodontal disease
Periodontal disease is a complex multifactorial inflammatory oral disease categorised by destruction of periodontal tissues and loss of the connective tissue attachment.37, 38, 39 Once the diagnosis is made, it must be categorised by the process of staging and grading; this is a significant change from the previous classification system.40 Gingivitis and periodontitis are a continuum of the same inflammatory disease, and although not all patients with gingivitis will progress to periodontitis, the management of gingivitis is the primary prevention strategy for periodontitis.41 It is also a secondary prevention strategy for recurrent periodontitis.41,42
Oral biofilm in the gingival margin is a primary etiologic factor for the development of periodontitis.4 It appears that certain bacteria cause gingivitis, as their removal results in the reversal of gingival inflammatory response.43 Therefore, a reduction of biofilm is critical in the restoration of gingival tissues to a healthy biological state.18,44,45 CHX mouthwash with concentrations between 0.1% and 0.2% exhibits significant anti-inflammatory and antiplaque effects on the gingiva and teeth.4,7,26,45,46 Rinsing daily with a 0.2% CHX mouthwash for 4 to 6 weeks also resulted in reduced clinical signs of gingivitis in several studies.25 However, CHX mouthwash at 0.12% is most effective in preventing the development of gingivitis on a plaque-free dental surface; therefore, it is most effective if the patients’ teeth were professionally cleaned prior to its application.7,21 Moreover, it failed to elicit any bacterial change after 48 hours from application if these pathogens were already established in the oral biome.21 This is why CHX should not be considered a universal product for all plaque-induced gingival or periodontal disease.
The development of gingivitis occurs after 2 to 3 weeks of undisturbed plaque buildup alongside a shift in the composition of subgingival bacteria from gram-positives to gram-negatives.43 However, the biocidal activity of CHX is more effective against gram-positive bacteria because their cell has a larger negative charge.19,27,47 Therefore, if CHX mouthwash is used, it must be used for the control of gingival inflammation as an adjunct to toothbrushing and interdental cleaning in periodontitis patients (stage I-III; refer to Table 2 for details).25,48 CHX mouthwash may also be considered for a limited period of time in periodontitis therapy as an adjunct to mechanical debridement in some cases (refer to Table 3 for additional considerations).48
Table 2.
Adjunctive uses of chlorhexidine products in the treatment of stage I-III periodontitis.55
| Purpose of intervention | Evidence-based recommendations |
|---|---|
| Improve clinical outcome of subgingival instrumentation | Chlorhexidine mouthwash may be used for a limited period of time, in periodontitis therapy, as an adjunct to mechanical debridement in specific cases. |
| Locally administered sustained-release chlorhexidine may be considered for use as an adjunct to subgingival instrumentation in patients with periodontitis. | |
| Control of gingival inflammation in patients with periodontitis receiving supportive periodontal care | If antiseptic dentifrice formulation is to be used adjunctively, products containing chlorhexidine, triclosan-copolymer, and stannous fluoride-sodium hexametaphosphate are suggested |
| If antiseptic mouthwash is to be used adjunctively, mouthwash containing chlorhexidine, essential oils, or cetylpyridinium chloride is suggested |
Table 3.
Factors to consider in periodontitis therapy, if chlorhexidine is to be used adjunctively to mechanical debridement.55,62
| • | It is not clear whether this should be a general recommendation for initial therapy. |
| • | Optimised mechanical plaque control may be necessary before considering the adjunctive use of chlorhexidine to mechanical debridement. |
| • | Specific recommendations can be made by the clinician when used in conjunction with full mouth disinfection approaches and/or systemic antimicrobials. |
| • | The medical status of the patient should be considered, especially if the patient has hypertension or cardiovascular disease. Chlorhexidine appears to increase systolic blood pressure and could have detrimental effects on the healthy microbiome and therefore cardiovascular health. |
| • | Adverse effects, especially staining. |
| • | Economic status of the patient as well as additional costs of treatment. |
Even with the addition of CHX mouthwash to mechanical debridement, there is potential for a subgingival biofilm and calculus to remain in deep pockets.48,49 This is possible when anatomical variability is considered; some individuals have poor access to the base of deep pockets due to complexities of furcation involvement and root concavities that may prevent the end goal of periodontal therapy from being achieved.48,50
As a result, the concept of local delivery of antibacterial agents into periodontal pockets was developed.51, 52, 53 The outcome was a lower-dose administration at the site of action (the periodontal pocket) maintained over a longer duration of time.52 This is seen in the local administration of 2.5 mg of CHX gluconate via biodegradable CHX “chips” Periochip™.25,50,54
Currently, the European Federation of Periodontology believes locally administered sustained-release CHX used as an adjunct to subgingival instrumentation in patients with periodontitis stage 1-3 may be considered.48 When Periochip™ was utilised as an adjunct to root surface debridement, there were small improvements in periodontal pocketing and clinical attachment loss (less than 1 mm).25 When adjunctive subgingival application of CHX gel, ranging between 0.5% and 2.0%, was utilised as a nonsurgical periodontal treatment for periodontitis, there was a slight benefit in probing pocket depths that were previously 4 mm or deeper.49 Nevertheless, the management of gingivitis is the primary prevention strategy for periodontitis.41,48
Prophylactic use and post–oral surgery procedures
CHX mouthwash is frequently used after surgical intervention in dentistry, usually prescribed after periodontal and implant surgeries.4,55 CHX mouthwash is considered the easiest to apply, allowing good patient compliance.4 Upon completion of periodontal surgeries, CHX mouthwashes are often prescribed for a duration of 2 weeks and at concentrations between 0.1% and 0.2%.4,18,55 After surgical procedures in dentistry, mechanical maintenance of oral hygiene may become difficult or even unmanageable due to trauma and discomfort.3,4,7 Thus, the use of CHX is critical as a prophylactic in the prevention of oral biofilm accumulation and to allow for adequate wound healing in surgical areas where mechanical brushing is not possible, therefore decreasing the risk of postsurgical complications.4,18,25,46,55 Oral biofilms are also the most common etiologic factors responsible in the development of peri-implantitis following implant surgery and negatively affect the process of wound healing.4,7,18,55 Hence, biofilm reduction is critical in the restoration of gingival tissues to a healthy biological state.4,7,18,55
Often the bacterial contamination of implant placement may result in biofilm formation and early failure; therefore, a 1-minute pre-operative rinse of 0.2% CHX is recommended to reduce the bacterial load.56 Furthermore, if toothbrushing is not possible due to postoperative pain from implant placement, it is recommended that a 1-minute rinse of 0.2% CHX is used 3 times a day for 7 days or until sutures are removed.56 In the past, it was believed that the use of CHX mouthwash and gels an as adjunct to mechanical prophylaxis were also effective for the treatment of peri-implant mucositis over a period of 1 to 3 months57; however, more recent data reveal that periodontal basic therapy consisting of oral hygiene instruction, motivation, and full-mouth scaling and root planing yielded similar results with no significant difference when compared to the additional use of 0.12% CHX mouthwash over a period of 1, 3, and 6 months.58 The CHX products that are effective as an adjunct for treatment of peri-implant mucositis and peri-implantitis are CHX chips, such as Periochip™.59, 60, 61 Data reveal for patients with peri-implant mucositis that bleeding on probing and peri-implant pocket depth were significantly improved when CHX chips were used as an adjunct to mechanical prophylaxis instead of 1% CHX gel.59 Similar protocols involving CHX chips as an adjunct to nonsurgical therapy (implant debridement) also appear to be effective in the treatment of peri-implantitis.60,61 Patients with peri-implantitis with implant pocket depths of 5 to 8 mm were treated with subgingival implant surface debridement followed by repeated biweekly supragingival plaque removal and CHX chip application (2.5-mg chip, Periochip™) with a maximum application of 2 chips per pocket for 12 weeks.61 At weeks 8, 12, and 16, implant pocket depth (IPD), recession, and bleeding on probing were recorded, and final measurements were recorded at 6 months. The results revealed significant IPD reduction and a greater percentile of sites with IPD reduction ≥2 mm compared to the control group, which received the same treatment without the application of the CHX chips.61 There were no significant differences in bleeding on probing or recession.61 Ultimately, CHX products may be used as an adjunct for the nonsurgical treatment of peri-implant mucositis and peri-implantitis, but they should be used correctly. Refer to Table 4 for recommendations specific to CHX, dental implant surgery, and peri-implantitis.
Table 4.
| Clinical condition | Recommended treatment | Active ingredient(s) | Instructions for use |
|---|---|---|---|
| Gingivitis | Antimicrobial, as an adjunct to mechanical plaque control | 0.12% CHX | 15 mL oral rinse, swish and spit for 30 seconds, 2 times a day (mornings and evenings) following toothbrushing and/or scaling and root planing (SRP) 2-4 weeks, with a maximum of 30 days before reevaluation, if deemed necessary by the practitioner. |
| Prophylactic in periodontal, implant, and extraction surgeries (presurgical and postsurgical) | Antimicrobial, as an adjunct to proper surgical technique | 0.12% or 0.2% CHX | 0.2% CHX 10 mL rinse for 30 seconds immediately before surgery, and 0.12% CHX 15 mL rinse for 60 seconds twice a day following surgery for a maximum of 2 weeks depending on procedure performed. |
| Oral candidiasis | Antimicrobial, soak dentures in use mouthwash | 0.2% CHX | Soak dentures in mouthwash for 15 minutes once or twice a day. |
| Halitosis | Antimicrobial, as an adjunct to oral hygiene and antibiotics | 0.12% or 0.2% CHX | 0.2% CHX 10 mL rinse for 30 seconds. |
| Prophylaxis in the transmission of COVID-19 | Antimicrobial, as preprocedural rinse in the prevention of SARS-CoV-2 transmission | 0.12% or 0.2% CHX | 0.2% or 0.12% CHX 10 mL rinse for 60 seconds, prior to procedure. |
Note: Chlorhexidine can shift the oral microbiome to biofilms where Fusobacterium can predominate. Therefore, this should be closely monitored by a dental practitioner if incorporated as an adjunct.
A systemic review examined plaque reduction after rinsing with 0.12% to 0.2% CHX mouthwash; significantly less plaque buildup was reported by all studies in the CHX rinse groups compared to the control groups.18 When sulcus bleeding index was evaluated, there was also a significant improvement when CHX was used following surgery, compared to the placebo rinse.18 However, there was no significant difference after 1 and 3 months when routine oral hygiene was resumed.18 This further strengthens the ideology that inflammation and mechanical plaque control that is challenging due to postoperative discomfort can be controlled with CHX mouth rinses on a short-term basis. This may explain why the American Dental Association recommends the use of CHX before any surgical procedure in the field of oral and maxillofacial surgery.7,20,27,62
ADRs and effects
An ADR can be defined as any undesirable effect of a drug.63 Any drug has the potential for adverse drug reactions; thus, a risk-benefit analysis is always required when a drug is prescribed.64 CHX mouthwash may result in some ADRs, even at low concentrations between 0.06% and 0.2% within the therapeutic range.4,7,26,27,31,45
Throughout 21 days of CHX usage, some self-reported ADRs included taste alteration, numbness in mouth and tongue, pain in mouth and tongue, xerostomia, and subjective discolouration.7,45 Although the “loss of taste” and “numb feeling” were significantly more frequent with the 0.12% and 0.2% concentrations compared to 0.06%, severe ADRs including erosion and ulceration of oral mucosa were not reported.7,45
Some of the most frequent ADRs of CHX mouthwash and gel also included xerostomia, hypogeusia, and a discolouration of tongue; as well as calculus and extrinsic tooth staining in long-term use.25,31 Whereas less common ADRs include, swelling of the parotid gland, oral paraesthesia glossodynia, and desquamation of the oral mucosa.25 However tooth staining is the number one ADR that discourages patients from using chlorhexidine.25
Fortunately, there are ways to overcome staining with the incorporation of 1.5% hydrogen peroxide (H2O2).66,66 For example, the use of 1.5% H2O2 mouthwash as an adjunct following the application of 0.2% CHX mouthwash resulted in significantly less staining of teeth without decreasing the efficacy of CHX.7,65,66 Even more promising is the recent development of CHX mouthwash with antidiscolouration system (CHX-MW-ADS).67 It appears that CHX-MW-ADS significantly reduced stain scores compared to traditional CHX mouthwash; moreover, there were no differences in bleeding, gingivitis, and plaque scores between CHX-MW-ADS and traditional CHX mouthwash.67 In fact, some studies have achieved better results when utilising 0.2% CHX-MW-ADS when incorporated into oral hygiene of adolescents with fixed orthodontic appliances over a duration of 18 weeks.68 Using 0.2% CHX-MW-ADS in patients with ceramic brackets resulted in a better gingival status compared to patients who used 0.2% CHX mouthwash.68
Another evolving issue with the use of CHX is the development of antimicrobial resistance (AMR), which is a serious adverse effect.25,69 Low-level exposure to CHX may cause cross-resistances to antibiotics.69 Moreover, some mechanisms that allow for resistance of CHX in bacterial organisms include efflux pumps and mutations in the cell membrane structure.69 Multidrug resistance and virulence are growing in ESKAPE pathogens which include Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.70,71 The Infectious Diseases Society of America has labeled them as ESKAPE pathogens because they are capable of escaping the MOA of antibiotics and eventually developing resistance.70 These pathogens are the number one reason for the development of hospital-acquired infection, or nosocomial infection.70,71 In fact, E faecalis plays a critical role in the development of endodontic infections.72 When this species is found in the form of biofilm, it is considered one of the most resistant species in oral cavity, due to its resistance to CHX and sodium hypochlorite.72 However, little is known about the risk of resistance to CHX in oral bacteria and cross-resistances to antibiotics; therefore, future research should focus on related effects of CHX on bacteria in oral biofilm.
The most serious ADRs associated with the oral use of CHX are type I and type IV hypersensitivity reactions followed by severe anaphylaxis.25,73 However, these are only reported at an incidence of 0.78 per 100,000 exposures.25 Therefore, clinicians should not be discouraged from utilising CHX when indicated, as long as the allergy status of the patient is negative.74
Conclusions
CHX has proven to be an extremely useful antimicrobial in the field of health. In dentistry, its versatility is unmatched as a chemotherapeutic agent when mechanical prophylaxis is not possible. A recommended 18 to 20 mg per application can be achieved with CHX mouthwash at concentrations between 0.12% and 0.2%. CHX mouthwash is recommended over gels and dentifrices due to significantly superior plaque inhibition with no serious side effects. CHX has provided benefits as an adjunct to oral health, in periodontics, and as a prophylaxis. It has also provided significant aid in various oral surgical procedures, including involvement in implantology and periodontology. Locally administered and sustained CHX in the form of CHX chips is the most advantageous for long-term use as an adjunct to oral hygiene in patients with periodontitis (stage I-III) as well as patients experiencing peri-implant mucositis and peri-implantitis.
Author contributions
Concept: A.O.; design: A.O., F.P.D.; supervision: A.O.; resources: A.O., F.P.D.; materials: F.P.D.; processing: F.P.D.; analysis and/or interpretation: A.O., F.P.D.; literature search: F.P.D.; writing manuscript: F.P.D., A.O.; critical review: A.O.
Funding
This study did not receive any financial support.
Ethics
This research was conducted in full accordance with the World Medical Association Declaration of Helsinki.
Conflict of interest
None disclosed.
REFERENCES
- 1.Raszewski Z, Nowakowska-Toporowska A, Wezgowiec J, Nowakowska D. Design and characteristics of new experimental chlorhexidine dental gels with anti-staining properties. Adv Clin Exp Med. 2019;28(7):885–890. doi: 10.17219/acem/94152. https://pubmed.ncbi.nlm.nih.gov/30888120/ Available from. [DOI] [PubMed] [Google Scholar]
- 2.Balagopal S, Arjunkumar R. Chlorhexidine: the gold standard antiplaque agent. J Pharm Sci Res. 2013;5(12):270–274. [Google Scholar]
- 3.Farah CS, McIntosh L, McCullough MJ. Vol. 32. Australian Prescriber. Australian Government Publishing Service; Available from; 2009. pp. 162–164.https://www.oralhealthgroup.com/features/mouthwashes-and-their-use-in-dentistry-a-review/ (Mouthwashes). [Google Scholar]
- 4.Chye RML, Perrotti V, Piattelli A, Iaculli F, Quaranta A. Effectiveness of different commercial chlorhexidine-based mouthwashes after periodontal and implant surgery. Implant Dent. 2019;28(1):74–85. doi: 10.1097/ID.0000000000000854. http://journals.lww.com/00008505-201902000-00012 Available from. [DOI] [PubMed] [Google Scholar]
- 5.Joshipura KJ, Munoz-Torres FJ, Morou-Bermudez E, Patel RP. Over-the-counter mouthwash use and risk of pre-diabetes/diabetes. Nitric Oxide. 2017;71:14–20. doi: 10.1016/j.niox.2017.09.004. Available from: https://doi.org/10.1016/j.niox.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Periodontal Disease | Oral Health Conditions | Division of Oral Health | CDC [Internet]. Center for Disease Control and Prevention. Available from: https://www.cdc.gov/oralhealth/conditions/periodontal-disease.html. Accessed 27 November 2020.
- 7.Poppolo Deus F, Ouanounou A. Mouthwashes and their use in dentistry: a review. Oral Health. 2021:22–34. https://www.oralhealthgroup.com/features/mouthwashes-and-their-use-in-dentistry-a-review/ Available from. [Google Scholar]
- 8.Chapple ILC, Van Der Weijden F, Doerfer C, et al. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42:S71–S76. doi: 10.1111/jcpe.12366. https://pubmed.ncbi.nlm.nih.gov/25639826/ Available from: [DOI] [PubMed] [Google Scholar]
- 9.Van Der Weijden FA, Van Der Sluijs E, Ciancio SG, et al. Can Chemical Mouthwash Agents Achieve Plaque/Gingivitis Control? Dent Clin NA. 2015;59:799–829. doi: 10.1016/j.cden.2015.06.002. Available from: [DOI] [PubMed] [Google Scholar]
- 10.American Dental Association. Mouthwash (mouthrinse). 2019. Available from: https://www.ada.org/en/member-center/oral-health-topics/mouthrinse. Accessed 17 November 2020.
- 11.Shrimathi S, Kemparaj U, Umesh S, Karuppaiah M, Pandian P, Krishnaveni A. Comparative evaluation of cocoa bean husk, ginger and chlorhexidine mouth washes in the reduction of Steptococcus mutans and Lactobacillus count in saliva: a randomized controlled trial. Cureus. 2019;11(6) doi: 10.7759/cureus.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugar SS, Patil S, Metgud R, Nanjwade B, Hugar SM. Influence of application of chlorhexidine gel and curcumin gel as an adjunct to scaling and root planing: an interventional study. J Nat Sci Biol Med. 2016;7(2):149–154. doi: 10.4103/0976-9668.184701. https://pubmed.ncbi.nlm.nih.gov/27433065/ Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asadoorian J. Therapeutic oral rinsing with commercially available products: position paper and statement from the Canadian Dental Hygienists Association. Can J Dent Hyg. 2016;50(3):126–139. [Google Scholar]
- 14.Walsh T, Oliveira-Neto JM, Moore D. Chlorhexidine treatment for the prevention of dental caries in children and adolescents. Cochrane Database Syst Rev. 2015:CD008457. doi: 10.1002/14651858.CD008457.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards D. Chlorhexidine mouthwash more effective than dentifrice or gel. Evid Based Dent. 2015;16:59. doi: 10.1038/sj.ebd.6401102. www.nature.com/ebd Available from. [DOI] [PubMed] [Google Scholar]
- 16.Richards D. Caries prevention - little evidence for use of chlorhexidine varnishes and gels. Evid Based Dent. 2015;16:43–44. doi: 10.1038/sj.ebd.6401091. www.nature.com/ebd Available from: [DOI] [PubMed] [Google Scholar]
- 17.Supranoto SC, Slot DE, Van Der Weijden GA, et al. The effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration: a systematic review. Int J Dent Hyg. 2015;13:83–92. doi: 10.1111/idh.12078. [DOI] [PubMed] [Google Scholar]
- 18.Solderer A, Kaufmann M, Hofer D, Wiedemeier D, Attin T, Schmidlin PR. Efficacy of chlorhexidine rinses after periodontal or implant surgery: a systematic review. Clin Oral Investig. 2019;23:21–32. doi: 10.1007/s00784-018-2761-y. Available from: [DOI] [PubMed] [Google Scholar]
- 19.Łukomska-Szymańska M, Sokołowski J, Łapińska B. Chlorhexidine – mechanism of action and its application to dentistry. J Stomatol. 2017;70:405–417. [Google Scholar]
- 20.Lexicomp-Chlorhexidine Gluconate (Oral). 2020. Available from: http://online.lexi.com.myaccess.library.utoronto.ca/lco/action/doc/retrieve/docid/patch_f/6532095?cesid=9rLlQSM949M&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dchlorhexidine%26t%3Dname%26va%3Dchlorhexidine#pha. Accessed 5 August 2020.
- 21.Zanatta FB, Antoniazzi RP, Rösing CK. The effect of 0.12% chlorhexidine gluconate rinsing on previously plaque-free and plaque-covered surfaces: a randomized, controlled clinical trial. J Periodontol. 2007;78(11):2127–2134. doi: 10.1902/jop.2007.070090. http://doi.wiley.com/10.1902/jop.2007.070090 Available from. [DOI] [PubMed] [Google Scholar]
- 22.Thangavelu A, Kaspar S, Kathirvelu R, Srinivasan B, Srinivasan S, Sundram R. Chlorhexidine: an elixir for periodontics. J Pharm Bioallied Sci. 2020;12(5):57. doi: 10.4103/jpbs.JPBS_162_20. http://www.jpbsonline.org/text.asp?2020/12/5/57/292842 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrimathi S, Kemparaj U, Umesh S, Karuppaiah M, Pandian P, Krishnaveni A. Comparative evaluation of cocoa bean husk, ginger and chlorhexidine mouth washes in the reduction of Steptococcus mutans and Lactobacillus count in saliva: a randomized controlled trial. Cureus. 2019;11(6) doi: 10.7759/cureus.4968. Available from: /pmc/articles/PMC6701906/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang N, Lindhe J, editors. Clinical periodontology and implant dentistry. 6th ed. Wiley-Blackwell; Chichester, UK: 2015. [Google Scholar]
- 25.Brookes ZLS, Bescos R, Belfield LA, Ali K, Roberts A. Current uses of chlorhexidine for management of oral disease: a narrative review. J Dent. 2020;103 doi: 10.1016/j.jdent.2020.103497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoun G, Saadeh M, Berberi A. Effectiveness of hexetidine 0.1% compared to chlorhexidine digluconate 0.12% in eliminating Candida albicans colonizing dentures: a randomized clinical in vivo study. J Int Oral Heal JIOH. 2015;7(8):5–8. http://www.ncbi.nlm.nih.gov/pubmed/26464531 Available from: [PMC free article] [PubMed] [Google Scholar]
- 27.DynaMed-Chlorhexidine gluconate. 2020. Available from: https://www.dynamed.com/drug-monograph/chlorhexidine-gluconate#GUID-B9F49950-C025-4766-AA85-7B72A89A4088. Accessed 5 August 2020.
- 28.Gupta J, Thomas M, Radhakrishna M, Srikant N, Ginjupalli K. Effect of silver diamine fluoride-potassium iodide and 2% chlorhexidine gluconate cavity cleansers on the bond strength and microleakage of resin-modified glass ionomer cement. J Conserv Dent. 2019;22(2):201–206. doi: 10.4103/JCD.JCD_485_18. Available from: /pmc/articles/PMC6519189/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J, Wei P, Zhao C, He C, Yan Z, Hua H. In vitro antifungal effect and inhibitory activity on biofilm formation of seven commercial mouthwashes. Oral Dis. 2014;20:815–820. doi: 10.1111/odi.12242. [DOI] [PubMed] [Google Scholar]
- 30.Vergara-Buenaventura A, Castro-Ruiz C. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg. 2020;58:924–927. doi: 10.1016/j.bjoms.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James P, Worthington HV, Parnell C, et al. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev. 2017;(3) doi: 10.1002/14651858.CD008676.pub2. http://doi.wiley.com/10.1002/14651858.CD008676.pub2 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeSpain EB. Prevention in Clinical Oral Health Care. Mosby Inc.; St. Louis, MO: 2008. Prevention strategies for periodontal diseases; pp. 213–229. [Google Scholar]
- 33.Almohefer SA, Levon JA, Gregory RL, Eckert GJ, Lippert F. Caries lesion remineralization with fluoride toothpastes and chlorhexidine - effects of application timing and toothpaste surfactant. J Appl Oral Sci. 2018;26 doi: 10.1590/1678-7757-2017-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn KB, Baik JE, Park OJ, Yun CH, Han SH. Lactobacillus plantarum lipoteichoic acid inhibits biofilm formation of Streptococcus mutans. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0192694. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0192694 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elkerbout T, Slot D, Van Loveren C, Van der Weijden G. Will a chlorhexidine-fluoride mouthwash reduce plaque and gingivitis? Int J Dent Hyg. 2019;17(1):3–15. doi: 10.1111/idh.12329. http://doi.wiley.com/10.1111/idh.12329 Available from. [DOI] [PubMed] [Google Scholar]
- 36.Sadat Sajadi F, Moradi M, Pardakhty A, Yazdizadeh R, Madani F. Effect of fluoride, chlorhexidine and fluoride-chlorhexidine mouthwashes on salivary Streptococcus mutans count and the prevalence of oral side effects. J Dent Res Dent Clin Dent Prospects. 2015;9(1):49–52. doi: 10.15171/joddd.2015.010. https://pubmed.ncbi.nlm.nih.gov/25973155/ Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramesh Reddy B, Chava V, Kumar AJ. Effect of chlorhexidine chip in the treatment of chronic periodontitis. J Nat Sci Biol Med. 2014;5(2):268. doi: 10.4103/0976-9668.136159. http://www.jnsbm.org/text.asp?2014/5/2/268/136159 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ubertalli J. Overview of periodontal disease - dental disorders - Merck Manuals Professional Edition. 2020. Available from: https://www.merckmanuals.com/en-ca/professional/dental-disorders/periodontal-disorders/overview-of-periodontal-disease?query=periodontitis. Accessed 6 August 2020.
- 39.Newman M, Takei H, Klokkevold P, Carranza F. 13th ed. Saunders; Los Angeles: Available from; 2018. Newman and Carranza's clinical periodontology; pp. 50–125.https://www.elsevier.com/books/newman-and-carranzas-clinical-periodontology/newman/978-0-323-52300-4 [Google Scholar]
- 40.Tonetti MS, Sanz M. Implementation of the new classification of periodontal diseases: decision-making algorithms for clinical practice and education. J Clin Periodontol. 2019;46(4):398–405. doi: 10.1111/jcpe.13104. https://onlinelibrary.wiley.com/doi/abs/10.1111/jcpe.13104 Available from. [DOI] [PubMed] [Google Scholar]
- 41.Chapple ILC, Van Der Weijden F, Doerfer C, et al. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42:71–76. doi: 10.1111/jcpe.12366. [DOI] [PubMed] [Google Scholar]
- 42.Arweiler NB, Auschill TM, Sculean A. Patient self-care of periodontal pocket infections. Periodontology. 2000;76:164–179. doi: 10.1111/prd.12152. https://pubmed.ncbi.nlm.nih.gov/29197129/ Available from. [DOI] [PubMed] [Google Scholar]
- 43.Curtis MA, Diaz PI, Van Dyke TE. The role of the microbiota in periodontal disease. Periodontol 2000. 2020;83(1):14–25. doi: 10.1111/prd.12296. https://onlinelibrary.wiley.com/doi/abs/10.1111/prd.12296 Mariano RJ, ed. Available from. [DOI] [PubMed] [Google Scholar]
- 44.Parashar A. Mouthwashes and their use in different oral conditions. Sch J Dent Sci J Dent Sci. 2015;2:186–191. [Google Scholar]
- 45.Haydari M, Bardakci AG, Koldsland OC, Aass AM, Sandvik L, Preus HR. Comparing the effect of 0.06%, 0.12% and 0.2% chlorhexidine on plaque, bleeding and side effects in an experimental gingivitis model: a parallel group, double masked randomized clinical trial. BMC Oral Health. 2017;17(1):1–8. doi: 10.1186/s12903-017-0400-7. https://link.springer.com/articles/10.1186/s12903-017-0400-7 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filetici P, Troiano G, Laino L, Longo F, D'Onofrio I, Canullo L. Chlorhexidine in extractive, periodontal and implant surgery–a systematic review and meta-analysis. Clin Oral Implants Res. 2019;30(S19):397. doi: 10.11607/jomi.8216. https://onlinelibrary.wiley.com/doi/abs/10.1111/clr.340_13509 Available from: [DOI] [PubMed] [Google Scholar]
- 47.Hagi A, Iwata K, Nii T, Nakata H, Tsubotani Y, Inoue Y. Bactericidal effects and mechanism of action of olanexidine gluconate, a new antiseptic. Antimicrob Agents Chemother. 2015;59(8):4551–4559. doi: 10.1128/AAC.05048-14. Available from: https://dx.doi.org/10.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanz M, Herrera D, Kebschull M, et al. Treatment of stage I–III periodontitis—the EFP S3 level clinical practice guideline. J Clin Periodontol. 2020;47(S22):4–60. doi: 10.1111/jcpe.13290. https://pubmed.ncbi.nlm.nih.gov/32383274/ Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H, Hu J, Zhao L. Adjunctive subgingival application of chlorhexidine gel in nonsurgical periodontal treatment for chronic periodontitis: a systematic review and meta-analysis. BMC Oral Health. 2020;20 doi: 10.1186/s12903-020-1021-0. https://pubmed.ncbi.nlm.nih.gov/32005169/ Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain R, Jhinger N, Kapoor D. Comparison of Periochip (chlorhexidine gluconate 2.5 mg) and Arestin (Minocycline hydrochloride 1 mg) in the management of chronic periodontitis. Indian J Dent. 2015;6(1):20. doi: 10.4103/0975-962X.151697. Available from: /pmc/articles/PMC4357074/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreno Villagrana AP, Gómez Clavel JF. Antimicrobial or subantimicrobial antibiotic therapy as an adjunct to the nonsurgical periodontal treatment: a meta-analysis. ISRN Dent. 2012 doi: 10.5402/2012/581207. In: Behr M, Widmalm SE, Magnusson I, eds. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pattnaik S, Anand N, Chandrasekaran SC, Chandrashekar L, Mahalakshmi K, Satpathy A. Clinical and antimicrobial efficacy of a controlled-release device containing chlorhexidine in the treatment of chronic periodontitis. Eur J Clin Microbiol Infect Dis. 2015;34(10):2103–2110. doi: 10.1007/s10096-015-2459-x. https://pubmed.ncbi.nlm.nih.gov/26210387/ Available from. [DOI] [PubMed] [Google Scholar]
- 53.Kaur A, Bhavikatti SK, Das SS, Khanna S, Jain M, Kaur A. Efficacy of ozonised water and 0.2% chlorhexidine gluconate in the management of chronic periodontitis when used as an irrigant in conjugation with phase i therapy. J Contemp Dent Pract. 2019;20(3):318–323. [PubMed] [Google Scholar]
- 54.Nair SC, Anoop KR. Intraperiodontal pocket: an ideal route for local antimicrobial drug delivery. J Adv Pharm Technol Res. 2012;3(1):9–15. doi: 10.4103/2231-4040.93558. www.japtr.org Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gartenmann SJ, Dörig I, Sahrmann P, Held U, Walter C, Schmidlin PR. Influence of different post-interventional maintenance concepts on periodontal outcomes: an evaluation of three systematic reviews. BMC Oral Health. 2016;17(1):19. doi: 10.1186/s12903-016-0244-6. http://bmcoralhealth.biomedcentral.com/articles/10.1186/s12903-016-0244-6 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bryce G, Bomfim DI, Bassi GS. Pre- and post-operative management of dental implant placement. Part 2: management of early-presenting complications. Br Dent J. 2014;217(4):171–176. doi: 10.1038/sj.bdj.2014.702. https://pubmed.ncbi.nlm.nih.gov/25146803/ Available from. [DOI] [PubMed] [Google Scholar]
- 57.De Siena F, Francetti L, Corbella S, Taschieri S, Del Fabbro M. Topical application of 1% chlorhexidine gel versus 0.2% mouthwash in the treatment of peri-implant mucositis. An observational study. Int J Dent Hyg. 2013;11(1):41–47. doi: 10.1111/idh.12002. https://pubmed.ncbi.nlm.nih.gov/22998456/ Available from. [DOI] [PubMed] [Google Scholar]
- 58.Menezes KM, Fernandes-Costa AN, Silva-Neto RD, Calderon PS, Gurgel BCV. Efficacy of 0.12% chlorhexidine gluconate for non-surgical treatment of peri-implant mucositis. J Periodontol. 2016;87(11):1305–1313. doi: 10.1902/jop.2016.160144. https://pubmed.ncbi.nlm.nih.gov/27389963/ Available from. [DOI] [PubMed] [Google Scholar]
- 59.Sahrmann P, Bettschart C, Wiedemeier DB, Al-Majid A, Attin T, Schmidlin PR. Treatment of peri-implant mucositis with repeated application of chlorhexidine chips or gel during supportive therapy - a randomized clinical trial. Dent J. 2019;7(4) doi: 10.3390/dj7040115. https://pubmed.ncbi.nlm.nih.gov/31835899/ Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Machtei E, Frankenthal S, Levi G, et al. Treatment of peri-implantitis using multiple applications of chlorhexidine chips: a double-blind, randomized multi-centre clinical trial. J Clin Periodontol. 2012;39(12):1198–1205. doi: 10.1111/jcpe.12006. https://pubmed.ncbi.nlm.nih.gov/23020659/ Available from. [DOI] [PubMed] [Google Scholar]
- 61.Machtei EE, Romanos G, Kang P., et al. Repeated delivery of chlorhexidine chips for the treatment of peri-implantitis: a multicenter, randomized, comparative clinical trial. J Periodontol. 2021;92(1):11–20. doi: 10.1002/JPER.20-0353. https://pubmed.ncbi.nlm.nih.gov/33111988/ Available from. [DOI] [PubMed] [Google Scholar]
- 62.American Dental Association; 2019. Osteoporosis Medications and Medication-Related Osteonecrosis of the Jaw.https://www.ada.org/en/member-center/oral-health-topics/osteoporosis-medications Available from. Accessed 7 August 2020. [Google Scholar]
- 63.Ouanounou A, Ng K, Chaban P. Adverse drug reactions in dentistry. Int Dent J. 2020;70(2):79–84. doi: 10.1111/idj.12540. https://onlinelibrary.wiley.com/doi/abs/10.1111/idj.12540 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marsh D. Adverse drug reactions - clinical pharmacology - Merck Manuals Professional Edition. 2018. Available from: https://www.merckmanuals.com/en-ca/professional/clinical-pharmacology/adverse-drug-reactions/adverse-drug-reactions?query=adversedrugreactions. Accessed 28 August 2020.
- 65.Greenwall L. 2nd ed. CRC Press; London, England: 2017. Tooth whitening techniques; pp. 1–352. [Google Scholar]
- 66.Jhingta P, Bhardwaj A, Sharma D, Kumar N, Bhardwaj VK, Vaid S. Effect of hydrogen peroxide mouthwash as an adjunct to chlorhexidine on stains and plaque. J Indian Soc Periodontol. 2013;17(4):449–453. doi: 10.4103/0972-124X.118315. Available from: /pmc/articles/PMC3800406/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Swaaij BWM, van der Weijden GA, Bakker EWP, Graziani F, Slot DE. Does chlorhexidine mouthwash, with an anti-discoloration system, reduce tooth surface discoloration without losing its efficacy? A systematic review and meta-analysis. Int J Dent Hyg. 2020;18(1):27–43. doi: 10.1111/idh.12402. https://pubmed.ncbi.nlm.nih.gov/31054209/ Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jurišić S, Verzak Jurišić G, Jurić H. Assessment of efficacy of two chlorhexidine mouthrinses on oral hygiene and gingival health in adolescents wearing two types of orthodontic brackets. Int J Dent Hyg. 2018;16(2):e52–e57. doi: 10.1111/idh.12299. [DOI] [PubMed] [Google Scholar]
- 69.Cieplik F, Jakubovics NS, Buchalla W, Maisch T, Hellwig E, Al-Ahmad A. Resistance toward chlorhexidine in oral bacteria-is there cause for concern? Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00587. Available from: /pmc/articles/PMC6439480/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghosh A, Saran N, Saha S. Survey of drug resistance associated gene mutations in Mycobacterium tuberculosis, ESKAPE and other bacterial species. Sci Reports. 2020;10(1):1–11. doi: 10.1038/s41598-020-65766-8. https://www.nature.com/articles/s41598-020-65766-8 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol. 2019;10(APR):539. doi: 10.3389/fmicb.2019.00539. Available from: /pmc/articles/PMC6452778/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakonieczna J, Wozniak A, Pieranski M, Rapacka-Zdonczyk A, Ogonowska P, Grinholc M. Photoinactivation of ESKAPE pathogens: overview of novel therapeutic strategy. Future Med Chem. 2019;11(5):443–461. doi: 10.4155/fmc-2018-0329. https://pubmed.ncbi.nlm.nih.gov/30901231/ Available from. [DOI] [PubMed] [Google Scholar]
- 73.Pemberton MN, Gibson J. Chlorhexidine and hypersensitivity reactions in dentistry. Br Dent J. 2012;213(11):547–550. doi: 10.1038/sj.bdj.2012.1086. http://www.resus.org.uk/pages/MEdental.pdf Available from. [DOI] [PubMed] [Google Scholar]
- 74.Veale B. Alveolar osteitis: a critical review of the aetiology and management. Oral Surg. 2015;8(2):68–77. http://doi.wiley.com/10.1111/ors.12130 Available from. [Google Scholar]