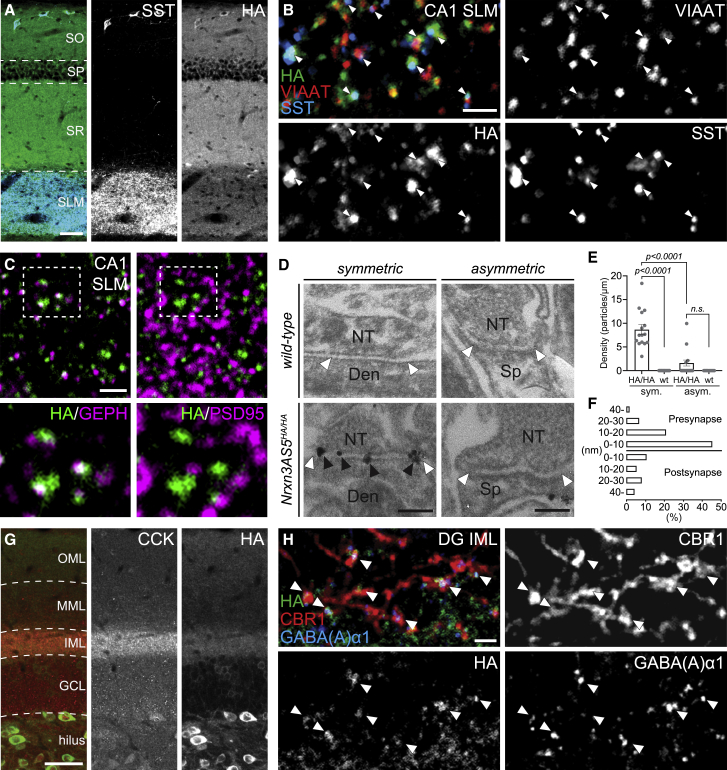
Figure 3.
Presynaptic localization of NRXN3 AS5+ isoforms
(A) Overview of NRXN3-AS5HA protein (HA, green) and somatostatin (SST, blue) labeling in hippocampal CA1, SO, stratum oriens, SP, stratum pyramidale, SR, stratum radiatum, and SLM, stratum lacunosum moleculare.
(B) High-magnification view of NRXN3-AS5HA protein (HA, green) colocalization with VIAAT (red) and somatostatin (SST, blue) in CA1 SLM.
(C) High-magnification view of NRXN3-AS5HA protein (HA, green) colocalization with gephyrin (GEPH, magenta) and PSD-95 (magenta) in CA1 SLM, lower panels magnified view of indicated area.
(D) Pre-embedding immunoelectron microscopy on glyoxal-fixed tissue for NRXN3-AS5HA protein localization at symmetric and asymmetric synapses in SLM of wild-type control and Nrxn3 AS5HA/HA mice. The edge of the postsynaptic specializations at asymmetrical and symmetrical synapses are each indicated by a pair of white arrowheads. Each immunogold particle is indicated by a black arrowhead, NT, nerve terminal, Den, dendrite, Sp, spine. For overview images, see Figure S3.
(E) The density of metal particles detected per 1 μm of synaptic cleft calculated from 15 symmetric and 20 asymmetric synapses from n = 4 sections and N = 2 Nrxn3ASHA/HA mice, and 17 symmetric and 18 asymmetric synapses from n = 4 sections and N = 2 wild-type mice. Note that there is no statistically significant difference in labeling density of asymmetric synapses as compared with wild-type mice.
(F) Vertical distribution of 78 particles from the midline of synaptic cleft across 15 symmetric synapses from n = 4 sections and N = 2 Nrxn3ASHA/HA mice.
(G) Overview of NRXN3-AS5HA protein (HA, green) and cholecystokinin (CCK, red) labeling in the hilus of the dentate gyrus, OML, outer molecular layer, MML, middle molecular layer, IML, inner molecular layer, and GCL, granule cell layer.
(H) High-magnification view of NRXN3-AS5HA protein (HA, green) colocalization with cannabinoid receptor 1 (CBR1, red) and GABAA-receptor subunit alpha 1 (GABAAα1, blue) in dentate gyrus IML.
Scale bars, 50 μm in (A and G); 2 μm in (B, C, and H); and 200 nm in (D). Note that all experiments (except G) were performed on glyoxal-fixed tissue.
Mean and SEM, one-way ANOVA followed by Tukey’s multiple comparison.