Abstract
Aims
Late gadolinium enhanced cardiovascular magnetic resonance (LGE‐CMR) is a valuable test to detect myocardial damage in patients with sarcoidosis; however, the clinical significance of LGE in sarcoidosis patients with preserved left ventricular ejection fraction (LVEF) is not defined. We aim to characterize the prevalence of LGE, its associated cardiac findings, and its clinical implications in sarcoidosis patients with preserved LVEF.
Methods and results
One hundred and fifty‐two patients with biopsy proven extra‐cardiac sarcoidosis, no known cardiac sarcoidosis, and LVEF ≥50% referred for LGE‐CMR were included in this observational study. The presence of LGE in the left ventricular myocardium was considered diagnostic for cardiac sarcoidosis. The cohort was divided into two groups based on the presence or absence of LGE. Twenty‐nine patients (19%) had LGE involving 11 ± 9% of the left ventricle. The modified Japanese Ministry of Health and Welfare (JMHW) criteria for diagnosing cardiac sarcoidosis only had a sensitivity of 52% and specificity of 83% for identifying myocardial LGE in these patients. Compared with those patients without LGE, those with LGE had a higher heart rate (84 ± 19 vs. 76 ± 18 b.p.m., P= 0.002), greater prevalence of an abnormal electrocardiogram (76 vs. 31%, P< 0.001), diastolic dysfunction (67 vs. 33%, P= 0.05), reduced right ventricular ejection fraction (49 ± 8 vs. 55 ± 6%, P= 0.012), and evidence of non‐sustained ventricular tachycardia (33 vs. 6%).
Conclusions
In patients with sarcoidosis and preserved systolic function, myocardial damage is commonly present and may increase the risk of ventricular tachy‐arrhythmias. The JMHW Criteria were neither sensitive nor specific for predicting the presence of myocardial LGE.
Keywords: Cardiac sarcoidosis, Cardiomyopathy, Cardiac magnetic resonance, Late gadolinium enhancement, Myocarditis, Infiltrative cardiomyopathy
Introduction
Sarcoidosis is a multisystem disorder characterized by the infiltration of various organs with non‐caseating granulomas. Infiltration and resultant damage of the myocardium is an important cause of death. Autopsy series suggest that the heart is involved in 20–30% of patients;1,2 however, just a fifth of those patients have grossly visible cardiac involvement while the rest only have histological evidence of cardiac sarcoidosis. In one series, two‐thirds of patients with histological evidence of cardiac sarcoidosis at autopsy had sudden cardiac death.3 Currently, the diagnosis of cardiac sarcoidosis is made using clinical criteria outlined by the Japanese Ministry of Health and Welfare (JMHW)4 which requires a tissue diagnosis of sarcoidosis, the presence of an abnormal electrocardiogram, and evidence of structural deformation or functional abnormality as diagnosed by echocardiography, nuclear imaging, or cardiac catheterization. Using the JMHW diagnostic criteria, the 5‐year mortality from cardiac sarcoidosis, when structural deformation or functional abnormality is present, is ∼25% with the cause of death being progression of heart failure or cardiac arrest.5 Based on the poor prognosis and the relatively high prevalence of sudden cardiac death, recent guidelines have stated implantable cardioverter‐defibrillators ‘(ICD) implantation is reasonable for patients with cardiac sarcoidosis (Class IIa; level of evidence C)’.6 Additionally, some have suggested that ventricular arrhythmias may not be prevented with corticosteroid therapy.7 There is currently no consensus on the optimal approach to medical therapy for cardiac sarcoidosis patients.
Unlike other cardiac imaging tests, late gadolinium enhanced cardiovascular magnetic resonance (LGE‐CMR) has been used not only to evaluate global and regional abnormalities in cardiac function but also to directly visualize the presence of even small amounts of myocardial damage.8 Recent data suggest that LGE‐CMR is more sensitive than the clinical JMHW criteria used for detecting cardiac sarcoidosis9,10 and may be present in as many as 20–35% of patients with extra‐cardiac sarcoidosis. Others have shown that LGE of the myocardium may be present in patients with sarcoidosis who have normal electrocardiograms, echocardiograms, nuclear tests, and 24 h ambulatory electrocardiograms.11 Late gadolinium enhanced cardiovascular magnetic resonance is emerging as the imaging test of choice for diagnosing cardiac sarcoidosis12 and has recently been shown to identify patients with sarcoidosis who are at increased risk of death.13 Studies performed prior to routine incorporation of LGE‐CMR into the clinical evaluation of sarcoidosis have shown that cardiac sarcoidosis patients with a left ventricular ejection fraction (LVEF) <50% may have a 5‐year survival rate of as low as 59%.5 However, LGE‐CMR readily identifies the presence of cardiac sarcoidosis even in those with preserved systolic function (LVEF≥50%).13 The clinical significance of myocardial LGE in patients with sarcoidosis and preserved systolic function has yet to be systematically determined. The goals of this study are (i) to better define the prevalence of cardiac sarcoidosis diagnosed by LGE‐CMR in patients with sarcoidosis with preserved systolic function and no known history of cardiac sarcoidosis; (ii) to identify the presence of associated structural abnormalities; and (iii) to determine whether a relationship to ventricular tachy‐arrhythmias exists.
Methods
Population
An observational cohort of 152 individuals with LVEF≥50% (as determined by CMR), biopsy proven extra‐cardiac sarcoidosis, and no known history of cardiac sarcoidosis were selected from 174 consecutive patients referred for LGE‐CMR at the University of Chicago Medical Center between January 2007 and December 2010 to evaluate for possible cardiac sarcoidosis [from the 174 patients, 16 were excluded for having LVEF<50%, 2 for having an alternative cardiac diagnosis (apical variant of hypertrophic cardiomyopathy and cardiac amyloidosis), and 4 for poor image quality] (Figure 1). The LGE‐CMR was ordered at the discretion of the referring physician. Patients with severe renal impairment (glomerular filtration rate <30 mL/min/1.73 m2) and those with typical contra‐indications for magnetic resonance imaging (ICD, pacemaker, cerebral aneurysm clips, severe claustrophobia, etc.) were excluded. The Institutional Review Board approved this study.
Figure 1. Selection of patient cohort.
Cardiovascular magnetic resonance protocol
All patients underwent LGE‐CMR imaging performed on a 1.5 T magnetic resonance imaging scanner (Achieva, Philips Healthcare, Best, Netherlands) using a five‐channel flexible surface coil. Retrospectively gated steady‐state free precession cines (repetition time [TR] 2.9 ms, echo time [TE] 1.5 ms, flip angle 60°, temporal resolution 25–40 ms) of the left ventricular (LV) two‐chamber, three‐chamber, four‐chamber, and contiguous short axis slices spanning from the LV base to the LV apex were acquired. Late gadolinium enhanced images of the same views were obtained 10–20 min after infusion of gadolinium‐diethylene triamine pentaacetic acid (0.15–0.2 mmol/kg) typically using a phased sensitive inversion recovery pulse sequence (TR 4.5 ms, TE 2.2 ms, inversion time 200–300 ms, flip angle 30°, phase sensitive inversion recovery flip angle 5°, voxel size 2 × 2 × 10 mm, sense factor 2). Philips Extended MR Workspace software was used to quantify CMR data. Left ventricular end‐diastolic volume (LVEDV), LV end‐systolic volume (LVESV), LVEF, right ventricular ejection fraction (RVEF) and LV mass were measured from the LV short axis cines using the method of discs. The diagnosis of cardiac sarcoidosis was made when any amount of LGE was present anywhere in the LV myocardium. The presence or absence of LGE was determined by consensus of two reviewers (with an arbitrator if needed) for each of the 17 segments of the American Heart Association LV model. Late gadolinium enhancement was considered to be present if seen in two different views and the mean signal intensity was >4 SD higher than the noise of remote normal myocardium. The extent of LV LGE was semi‐quantitatively determined as the number of myocardial segments that had any LGE expressed as a percentage.
Echocardiogram
From the first 125 consecutive patients enrolled into the study, those with adequate Doppler and tissue Doppler datasets (n= 75) underwent assessment of diastolic function and LV filling pressures using comprehensive transthoracic echocardiography including two‐dimensional echocardiography, Doppler echocardiography, and tissue Doppler echocardiography. Studies were performed using the iE33 imaging system (Philips Healthcare). Pulsed wave mitral inflow was used to evaluate diastolic dysfunction according to traditional classification of E/A ratio and E wave deceleration time. The American Society of Echocardiography criteria for diastolic dysfunction were not used for the determination of diastolic dysfunction because a large number of patients in our cohort had conflicting data points leading to an ambiguous grading of diastolic dysfunction. Pulsed wave tissue Doppler was obtained of the septal and lateral mitral annulus according to usual practice. Left atrial volume was determined using the area‐length model and was indexed to body size by dividing by body surface area.14 The presence or absence of elevated LV filling pressures was determined using the septal mitral annular tissue Doppler e' and LA volume index using a previously published algorithm.15 Right ventricular systolic pressure was estimated from the tricuspid valve regurgitation jet velocity according to the modified Bernoulli equation plus estimated right atrial pressure based on inferior vena cava size and its variation with respiration.
Resting and ambulatory electrocardiogram
A 12‐lead surface electrocardiogram was recorded. The findings were interpreted by an experienced cardiologist and classified as abnormal when right or left bundle branch block, left axis deviation, atrio‐ventricular nodal conduction disease, ventricular tachycardia, premature ventricular contraction, pathological Q‐wave, or ST‐T abnormalities were noted. Ambulatory cardiac rhythm monitoring for 24–72 h was performed at the discretion of the patient's physician (n= 73) and were considered abnormal if there was >100 premature ventricular contractions per day,11 non‐sustained ventricular tachycardia (NSVT) (3–30 consecutive premature ventricular contractions), sustained ventricular tachycardia (>30 s), or atrioventricular block.
Implantable cardioverter‐defibrillator selection
Patients with LGE‐positive CMR and biopsy proven extra‐cardiac sarcoidosis were considered candidates for ICD implantation. Patient risk stratifying contributors without specific importance included: the presence of NSVT, abnormal electrocardiographic data, and/or abnormal echocardiographic findings as detailed by the methods above. The risks and benefits of ICD implantation were described to the patients in detail with the implantation of an ICD at the discretion of the patient and their cardiologist/electrophysiologist. Most of the mismatch between qualifying abnormalities and lack of an ICD implantation were due to patient refusal of the procedure.
Statistical analysis
Patients were divided into two groups (those with LGE and those without LGE). Analysis was performed using Prism (GraphPad Software, San Diego, CA). Continuous variables were expressed as mean ± standard deviation and were compared using unpaired t‐tests. Dichotomous variables were compared using the χ 2 test. A P‐value <0.05 was considered statistically significant. Statistical analysis was not performed on data regarding the prevalence of NSVT in LGE‐positive and LGE‐negative populations because of the non‐uniform monitoring that these populations may have received.
Results
As predetermined by the study design, all 152 patients had an LVEF ≥ 50%. Twenty‐nine (19%) patients had evidence of LGE. The typical patient had a small burden of LGE (11 ± 7% of the myocardial segments). Figure 2 shows the distribution of LGE lesions among the myocardial segments. Figure 3 is an example of a patient with preserved LV systolic function and a region of LGE. Patients were followed for 651 ± 340 days. Fifteen (52%) of these 29 patients underwent ICD implantation at the discretion of the patient's treating physician. Two of these patients received successful anti‐tachycardic pacing for ventricular tachycardia. One patient was inappropriately shocked for atrial fibrillation and another was inappropriately shocked for T‐wave over‐sensing.
Figure 2. Percentage of total late gadolinium enhanced lesions detected in each of the American Heart Association myocardial segments.
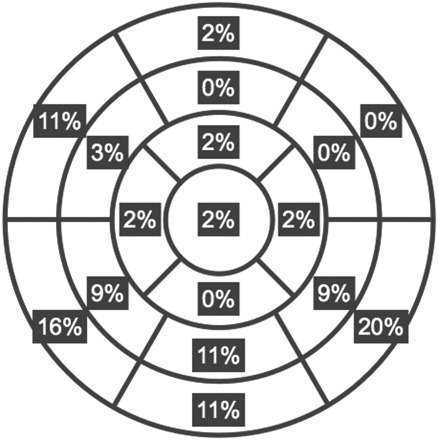
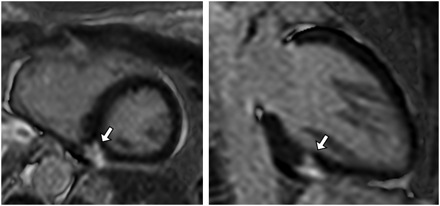
Figure 3. Late gadolinium enhanced cardiovascular magnetic resonance images are shown of a left ventricular short axis slice (left) and a left ventricular two‐chamber view (right). The arrows point to two different views of a small region of late gadolinium enhancement.
Patient characteristics
The average age of the study population was 49 ± 11 years. The majority of patients were women (74%). African‐American race was most common (49%). There was no difference in gender between those with or without LGE (Table 1); however, those with LGE were more likely to be African American (69 vs. 45%, P= 0.019) and older (54 vs. 48 years, P= 0.021). In both groups the majority had lung involvement and were equally likely to have been treated with corticosteroids or non‐steroidal immunosuppressive therapy. More patients with LGE were on angiotensin‐converting enzyme inhibitors (ACE‐I) or angiotensin receptor blockers (ARB) therapy (44 vs. 23%, P= 0.021). Patients with LGE also had a longer duration of sarcoidosis than those without LGE (12 ± 12 vs. 8 ± 9 years, P= 0.021). More patients with LGE satisfied the JMHW criteria for the diagnosis of cardiac sarcoidosis (CS) than patients without LGE (48 vs. 17%, P= 0.0001). Using the presence of LGE as the gold standard for the diagnosis of CS, the JMHW criteria had a sensitivity of 52% and a specificity of 83%. The positive predictive value of JMHW criteria was 40% and the negative predictive value was 89% in our cohort.
Table 1.
Patient characteristics and their relationship to cardiac sarcoidosis
| LGE+ (n= 29) | LGE− (n= 123) | P‐value | |
|---|---|---|---|
| Age (years) | 54 ± 11 | 48 ± 11 | 0.021 |
| Gender (female) | 21/29 (72%) | 92/123 (75%) | 0.792 |
| Race (African American) | 20/29 (69%) | 55/123 (45%) | 0.019 |
| Years since diagnosis | 12 ± 12 | 8 ± 9 | 0.021 |
| Steroid use | 12/27 (44%) | 50/116 (43%) | 0.900 |
| Non‐steroidal immunosuppressive therapy | 6/25 (24%) | 36/116 (31%) | 0.485 |
| ACE‐I or ARB use | 12/27 (44%) | 27/119 (23%) | 0.021 |
| Beta‐blocker use | 5/27 (19%) | 17/119 (14%) | 0.589 |
| Statin use | 8/27 (30%) | 21/119 (18%) | 0.159 |
| Lung involvement | 23/27 (83%) | 97/122 (80%) | 0.500 |
| Satisfy JMHW criteria | 14/27 (48%) | 21/123 (17%) | <0.001 |
The changing denominators reflect data points that were not available for analysis.
Cardiac morphology and function
Those with LGE and those without LGE had no significant differences in LVEDV, LVESV, LV mass, left atrial volume, and right ventricular end‐diastolic volume (Table 2). Although likely influenced by the systematic exclusion of patients with depressed LV function, both groups had similar LVEF (LGE 60 ± 5% vs. no LGE 61 ± 6%, P= 0.149). Patients with LGE had greater RVESV (86 ± 28 vs. 72 ± 24 mL, P= 0.008) and lower RVEF (49 ± 8 vs. 55 ± 6%, P< 0.001) when compared with those without LGE suggestive of right ventricle (RV) involvement and/or pulmonary hypertension in these patients. Patients with LGE were more likely to have a wall motion abnormality (41.4 vs. 13.8%, P= 0.0007) and regional wall thickening (10.3 vs. 1.6%, P= 0.017) when compared with those without LGE. A subgroup of 75 patients with adequate echocardiographic images underwent assessment of diastolic dysfunction and estimation of LV filling pressures using mitral inflow and tissue Doppler indices. Left ventricular diastolic dysfunction was present in 67% of patients with LGE and in 33% of subjects without LGE (P= 0.05). Elevated LV filling pressures were present uncommonly in both groups, and the frequency of elevated LV filling pressures were not different between the two groups of subjects (23% of patients with LGE vs. 25% of patients without LGE, P= 0.86). Estimated right ventricular systolic pressure was higher (but did not reach statistical significance) in patients with LGE than in those without (46.0 vs. 36.1 mmHg, P= 0.068). Tricuspid regurgitation jet velocity was higher in patients with LGE than those without (290 vs. 250 cm/s, P= 0.05); however, only 12 patients with LGE had an adequate jet of tricuspid regurgitation from which to estimate right ventricular systolic pressure and tricuspid regurgitation jet velocity.
Table 2.
Cardiac morphology and function and their relationship to cardiac sarcoidosis
| LGE+ (n= 29) | LGE− (n= 123) | P‐value | |
|---|---|---|---|
| LVEDV (mL) | 142 ± 38 | 148 ± 38 | 0.415 |
| LVESV (mL) | 56 ± 18 | 58 ± 19 | 0.643 |
| LVEF (%) | 60 ± 5 | 61 ± 6 | 0.149 |
| LV mass (g) | 99 ± 40 | 89 ± 27 | 0.115 |
| LAV (mL) | 56 ± 21 | 53 ± 21 | 0.571 |
| RVEDV (mL) | 165 ± 36 | 158 ± 42 | 0.424 |
| RVESV (mL) | 86 ± 28 | 72 ± 24 | 0.008 |
| RVEF (%) | 49 ± 8 | 55 ± 6 | < 0.001 |
Electrocardiography
Seventy‐six per cent of those with LGE had an abnormal electrocardiogram compared with only 31% of those without LGE (P< 0.001). As demonstrated in Table 3, patients with LGE had higher heart rates (84 ± 19 vs. 76 ± 18 b.p.m., P= 0.035), longer PR intervals (171 ± 49 vs. 156 ± 28 ms, P= 0.036), longer QRS duration (98 ± 20 vs. 90 ± 14 ms, P= 0.024), and longer QTc intervals (450 ± 38 vs. 432 ± 28 ms, P= 0.005) than those without LGE. In the patients who had an ambulatory electrocardiogram performed, it was abnormal in 40% of those with LGE and in only 19% of those without LGE (P= 0.087).
Table 3.
Electrocardiography features and their relationship to cardiac sarcoidosis
| LGE+ (n= 29) | LGE− (n= 123) | P‐value | |
|---|---|---|---|
| Abnormal EKG | 22/29 (76%) | 34/111 (31%) | <0.001 |
| Heart rate (b.p.m.) | 84 ± 19 | 76 ± 18 | 0.035 |
| PR interval (ms) | 171 ± 49 | 156 ± 28 | 0.036 |
| QRS duration (ms) | 98 ± 20 | 90 ± 14 | 0.024 |
| QT interval (ms) | 390 ± 40 | 391 ± 36 | 0.913 |
| QTc interval (ms) | 450 ± 38 | 432 ± 28 | 0.005 |
| Any NSVT | 5/15 (33%) | 4/67 (6%) | Not calculated |
| Abnormal Holter | 6/15 (40%) | 13/67 (19%) | 0.087 |
Non‐sustained ventricular tachycardia
In our cohort a higher percentage of patients with LGE had documented NSVT (on either 12‐lead electrocardiogram, 24 h ambulatory electrocardiogram, cardiac event monitor, or device interrogation) than those without LGE (33 vs. 6%). The subgroup of patients with LGE plus NSVT (when compared with patients with LGE minus NSVT) had a statistically non‐significant higher percentage of myocardial segments involved with LGE (15 ± 12 vs. 10 ± 6%, P= 0.12).
Discussion
We used LGE‐CMR to better define the prevalence and clinical significance of myocardial damage in sarcoidosis patients with preserved LV systolic function (LVEF >50%) and no known history of cardiac sarcoidosis. In our cohort, 19% of patients had evidence of LGE despite having preserved LV performance. We additionally demonstrated that, despite preserved LV systolic function, those with LGE were more likely to have diastolic dysfunction, a modest reduction in RV systolic function, higher heart rates, and longer PR, QRS, and QTc intervals compared with those without LGE.
The use of LGE‐CMR to identify sarcoidosis patients who have myocardial involvement is appealing. Early validation studies have clearly demonstrated LGE‐CMR's ability to accurately identify even small myocardial scars.8,16 The strength of the technique lies in its ability to directly visualize abnormalities in the myocardium rather than relying on secondary sequelae such as reduced systolic function, wall motion abnormalities, wall thinning, wall thickening, or abnormal haemodynamics to make the diagnosis of cardiac sarcoidosis.4 Previous investigators have shown that LGE‐CMR is more sensitive than electrocardiography, echocardiography, or the JMHW criteria for the diagnosis of cardiac sarcoidosis.10,11 Our data suggest that the fulfilment of JMHW criteria may also not be specific for the presence of myocardial involvement (as defined by the presence of LGE) in patients with sarcoidosis. Limited data are available regarding the role of biomarkers such as N‐terminal pro b‐type natriuretic peptide (NT‐proBNP) and troponin for the detection of cardiac sarcoidosis. However Handa et al. 17 have suggested that NT‐proBNP may be a useful biomarker to identify cardiac involvement. In another study, Yasutake et al.18 showed that BNP levels (but not troponin levels) were higher in patients with cardiac sarcoidosis. The relationship between biomarkers and LGE is yet undefined.
Based on recent LGE‐CMR studies the frequency of cardiac sarcoidosis is believed to be as high as 35%.10,12,13 Previously, in a study of 31 patients, Cheong et al.19 reported that 26% of patients with no known history of cardiac involvement with sarcoid had evidence of myocardial late gadolinium enhancement. In another study,13 which included 81 patients with extra‐cardiac sarcoidosis, 6 patients had LGE despite having a normal LVEF; however, the overall prevalence of LGE in patients with preserved function could not be determined based on the data reported. In our significantly larger cohort (which by design only included patients with preserved LVEF), nearly one out of every five patients (19%) had evidence of myocardial damage. We also found that patients with LGE had a reduction in RV systolic function likely suggestive of biventricular involvement from cardiac sarcoidosis and/or the concomitant presence of pulmonary hypertension.
It is commonly believed that cardiac sarcoidosis is associated with the presence of diastolic dysfunction, however, to the best of our knowledge, this has not been systematically investigated in the past. In our study, we found that two‐thirds of patients with LGE had evidence of diastolic dysfunction compared with only one‐third of those without LGE, supporting the notion that cardiac sarcoidosis is associated with diastolic dysfunction. However, our results also highlight the fact that a third of patients with LGE did not have evidence of diastolic dysfunction, suggesting that diastolic dysfunction would be neither sensitive nor specific for diagnosing cardiac sarcoidosis.
Identification of patients with cardiac sarcoidosis is clinically important because this disease may result in sudden cardiac death or heart failure.2,3,5,13 The presence of increased mortality has also been confirmed in patients with cardiac sarcoidosis when cardiac involvement is identified by LGE‐CMR.13 Current guidelines suggest that it may be reasonable to implant an ICD for primary prevention of sudden cardiac death in this patient cohort;6 however, these guidelines are predominantly based on data collected prior to the routine incorporation of LGE‐CMR into the clinical management of patients with sarcoidosis. In fact, the guidelines were likely to have been influenced by the work of investigators such as Yazaki et al.5 who demonstrated that patients with cardiac sarcoidosis and LVEF < 50% had a 5‐year survival of only 59%. Three of the patients who were excluded from our cohort because of reduced systolic function (LVEF < 50%) received appropriate shocks for treatment of ventricular tachycardia from their ICD. However, as seen in our cohort, LGE‐CMR can readily identify cardiac sarcoidosis even in patients with LVEF > 50%, and it remains to be determined whether evidence of myocardial damage in the setting of preserved systolic function has an equally poor prognosis as in those with an LVEF < 50%. Interestingly, two of the patients in our cohort with preserved systolic function received appropriate anti‐tachycardic pacing for ventricular tachycardia suggesting that these patients may still be at risk for sudden cardiac death. However, two patients received inappropriate shocks as discussed above. More data are needed to determine whether there is a benefit from ICD implantation in sarcoidosis patients with LGE and a preserved systolic function. Some investigators have advocated the use of programmed ventricular stimulation for further risk stratification of these patients.20
Detection of cardiac sarcoidosis in patients with preserved systolic function may also have important treatment implications; in fact, some have suggested that treatment with long‐term corticosteroids may prevent adverse LV remodelling and deterioration of LVEF.21 The role of steroid sparing agents in the treatment of cardiac sarcoidosis is currently unknown.22–24 In our cohort of sarcoidosis patients with LGE and preserved systolic function, we found that their clinical course commonly included the presence of NSVT suggesting a pro‐arrhythmic milieu. In fact, patients with LGE also had longer PR, QRS, and QTc intervals than those without LGE suggesting an increased prevalence of conduction system disease. Although it is unclear whether the pro‐arrhythmic state in cardiac sarcoidosis is mediated by re‐entry circuits created around the regions of myocardial damage7,25 or whether it is a result of the prolongation of the QTc interval, the high prevalence of NSVT (33%) is noteworthy. Recently, in a cohort of cardiac sarcoidosis patients with less severe LV systolic dysfunction, Jefec et al.26 reported that they were able to induce a single ventricular tachycardia (VT) morphology in one‐third of patients, which potentially arose from lesions such as those detected in our study. These small regions of myocardial damage might provide a sufficient substrate to create the re‐entry circuit needed to initiate VT in patients with cardiac sarcoidosis. Further studies are required to determine the natural history and the best therapeutic strategy for this cohort of patients. The role of immunosuppressive agents to reduce the burden of arrhythmias in these patients is not well defined.7,27,28
There are several limitations of our study. First, it is a retrospective study. The specific testing, including CMR, that any individual patient underwent was performed at the discretion of the patient's physician. As such, any bias created by test choice cannot be accurately quantified. Also, since our study was performed at a tertiary care centre, a referral bias likely influenced our results. Additionally, long‐term follow‐up of our cohort (patients with LGE and normal LV systolic function) is not yet available. Most previous studies that have shown that patients with cardiac sarcoidosis have a poor prognosis typically used the JMHW criteria to make the diagnosis and likely represented patients with a higher burden of myocardial involvement. It is unclear whether these previous outcomes data could be applied to a cohort like ours.
Conclusions
In this study, we have demonstrated that myocardial damage is commonly present in patients with sarcoidosis who have preserved systolic function. Importantly, patients with evidence of late gadolinium enhancement and preserved systolic function were more likely to have diastolic dysfunction, reduced RV systolic function, abnormalities in regional wall motion and thickening, and evidence of conduction system disease. The optimal treatment strategy for patients with cardiac sarcoidosis and preserved systolic function remains to be determined. Multicentre trials will be required to guide therapy in this patient population.
Acknowledgements
The authors would like to acknowledge E. Bruce Jamison RT(MR) for his help with image acquisition and data analysis.
Conflict of interest: none declared.
References
- 1. Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital Medicine 1952. 31 1–132 [PubMed] [Google Scholar]
- 2. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis Circulation 1978. 58 1204–1211 [DOI] [PubMed] [Google Scholar]
- 3. Roberts WC, McAllister HA Jr., Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11) Am J Med 1977. 63 86–108 [DOI] [PubMed] [Google Scholar]
- 4. Hiraga H, Yuwai K, Hiroe M. Guidelines for the diagnosis of cardiac sarcoidosis: study report of diffuse pulmonary diseases Jpn. Ministry Health Welfare 1993. 23–24 [Google Scholar]
- 5. Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, Izumo T, Sekiguchi M. Prognostic determinants of long‐term survival in Japanese patients with cardiac sarcoidosis treated with prednisone Am J Cardiol 2001. 88 1006–1010 [DOI] [PubMed] [Google Scholar]
- 6. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC Jr., Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 Guidelines for Device‐Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons J Am Coll Cardiol 2008. 51 e1–e62 [DOI] [PubMed] [Google Scholar]
- 7. Banba K, Kusano KF, Nakamura K, Morita H, Ogawa A, Ohtsuka F, Ogo KO, Nishii N, Watanabe A, Nagase S, Sakuragi S, Ohe T. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis Heart Rhythm 2007. 4 1292–1299 [DOI] [PubMed] [Google Scholar]
- 8. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function Circulation 1999. 100 1992–2002 [DOI] [PubMed] [Google Scholar]
- 9. Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands Chest 2005. 128 30–35 [DOI] [PubMed] [Google Scholar]
- 10. Mehta D, Lubitz SA, Frankel Z, Wisnivesky JP, Einstein AJ, Goldman M, Machac J, Teirstein A. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing Chest 2008. 133 1426–1435 [DOI] [PubMed] [Google Scholar]
- 11. Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Cheriex EC, Gorgels AP, Crijns HJ. The additional value of gadolinium‐enhanced MRI to standard assessment for cardiac involvement in patients with pulmonary sarcoidosis Chest 2005. 128 1629–1637 [DOI] [PubMed] [Google Scholar]
- 12. Smedema JP, Snoep G, van Kroonenburgh MPG, van Geuns RJ, Dassen WRM, Gorgels AP, Crijns HJ. Evaluation of the accuracy of gadolinium‐enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis J Am Coll Cardiol 2005. 45 1683–1690 [DOI] [PubMed] [Google Scholar]
- 13. Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ. Detection of myocardial damage in patients with sarcoidosis Circulation 2009. 120 1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology J Am Soc Echocardiogr 2005. 18 1440–1463 [DOI] [PubMed] [Google Scholar]
- 15. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography J Am Soc Echocardiogr 2009. 22 107–133 [DOI] [PubMed] [Google Scholar]
- 16. Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast‐enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study Lancet 2003. 361 374–379 [DOI] [PubMed] [Google Scholar]
- 17. Handa T, Nagai S, Ueda S, Chin K, Ito Y, Watanabe K, Tanizawa K, Tamaya M, Mishima M, Izumi T. Significance of plasma NT‐proBNP levels as a biomarker in the assessment of cardiac involvement and pulmonary hypertension in patients with sarcoidosis Sarcoidosis Vasc Diffuse Lung Dis 2010. 27 27–35 [PubMed] [Google Scholar]
- 18. Yasutake H, Seino Y, Kashiwagi M, Honma H, Matsuzaki T, Takano T. Detection of cardiac sarcoidosis using cardiac markers and myocardial integrated backscatter Int J Cardiol 2005. 102 259–268 [DOI] [PubMed] [Google Scholar]
- 19. Cheong BY, Muthupillai R, Nemeth M, Lambert B, Dees D, Huber S, Castriotta R, Flamm SD. The utility of delayed‐enhancement magnetic resonance imaging for identifying nonischemic myocardial fibrosis in asymptomatic patients with biopsy‐proven systemic sarcoidosis Sarcoidosis Vasc Diffuse Lung Dis 2009. 26 39–46 [PubMed] [Google Scholar]
- 20. Mehta D, Mori N, Goldbarg SH, Lubitz S, Wisnivesky JP, Teirstein A. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: role of programmed ventricular stimulation Circ Arrhythm Electrophysiol 2011. 4 43–48 [DOI] [PubMed] [Google Scholar]
- 21. Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K. Prevention of left ventricular remodeling by long‐term corticosteroid therapy in patients with cardiac sarcoidosis Am J Cardiol 2005. 95 143–146 [DOI] [PubMed] [Google Scholar]
- 22. Sweiss NJ, Welsch MJ, Curran JJ, Ellman MH. Tumor necrosis factor inhibition as a novel treatment for refractory sarcoidosis Arthritis Rheum 2005. 53 788–791 [DOI] [PubMed] [Google Scholar]
- 23. Sweiss NJ, Baughman RP. Tumor necrosis factor inhibition in the treatment of refractory sarcoidosis: slaying the dragon? J Rheumatol 2007. 34 2129–2131 [PubMed] [Google Scholar]
- 24. Sweiss NJ, Curran J, Baughman RP. Sarcoidosis, role of tumor necrosis factor inhibitors and other biologic agents, past, present, and future concepts Clin Dermatol 2007. 25 341–346 [DOI] [PubMed] [Google Scholar]
- 25. Redheuil AB, Paziaud O, Mousseaux E. Ventricular tachycardia and cardiac sarcoidosis: correspondence between MRI and electrophysiology Eur Heart J 2006. 27 1430 [DOI] [PubMed] [Google Scholar]
- 26. Jefic D, Joel B, Good E, Morady F, Rosman H, Knight B, Bogun F. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry Heart Rhythm 2009. 6 189–195 [DOI] [PubMed] [Google Scholar]
- 27. Shimada T, Shimada K, Sakane T, Ochiai K, Tsukihashi H, Fukui M, Inoue S, Katoh H, Murakami Y, Ishibashi Y, Maruyama R. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium‐DTPA‐enhanced magnetic resonance imaging Am J Med 2001. 110 520–527 [DOI] [PubMed] [Google Scholar]
- 28. Hiramastu S, Tada H, Naito S, Oshima S, Taniguchi K. Steroid treatment deteriorated ventricular tachycardia in a patient with right ventricle‐dominant cardiac sarcoidosis Int J Cardiol 2009. 132 e85–e87 [DOI] [PubMed] [Google Scholar]


