Abstract
BACKGROUND
Patients with von Hippel–Lindau (VHL) disease have a high incidence of renal cell carcinoma owing to VHL gene inactivation and constitutive activation of the transcription factor hypoxia-inducible factor 2α (HIF-2α).
METHODS
In this phase 2, open-label, single-group trial, we investigated the efficacy and safety of the HIF-2α inhibitor belzutifan (MK-6482, previously called PT2977), administered orally at a dose of 120 mg daily, in patients with renal cell carcinoma associated with VHL disease. The primary end point was objective response (complete or partial response) as measured according to the Response Evaluation Criteria in Solid Tumors, version 1.1, by an independent central radiology review committee. We also assessed responses to belzutifan in patients with non–renal cell carcinoma neoplasms and the safety of belzutifan.
RESULTS
After a median follow-up of 21.8 months (range, 20.2 to 30.1), the percentage of patients with renal cell carcinoma who had an objective response was 49% (95% confidence interval, 36 to 62). Responses were also observed in patients with pancreatic lesions (47 of 61 patients [77%]) and central nervous system hemangioblastomas (15 of 50 patients [30%]). Among the 16 eyes that could be evaluated in 12 patients with retinal hemangioblastomas at baseline, all (100%) were graded as showing improvement. The most common adverse events were anemia (in 90% of the patients) and fatigue (in 66%). Seven patients discontinued treatment: four patients voluntarily discontinued, one discontinued owing to a treatment-related adverse event (grade 1 dizziness), one discontinued because of disease progression as assessed by the investigator, and one patient died (of acute toxic effects of fentanyl).
CONCLUSIONS
Belzutifan was associated with predominantly grade 1 and 2 adverse events and showed activity in patients with renal cell carcinomas and non–renal cell carcinoma neoplasms associated with VHL disease. (Funded by Merck Sharp and Dohme and others; MK-6482-004 ClinicalTrials.gov number, NCT03401788.)
A rare autosomal dominant hereditary disorder, von Hippel–Lindau (VHL) disease is caused by germline pathogenic variants in the VHL gene. VHL disease occurs in approximately 1 in every 27,300 to 39,000 live births.1,2 The condition is associated with benign and malignant neoplasms, including clear-cell renal cell carcinoma, pancreatic neuroendocrine tumors, and hemangioblastomas in the central nervous system and retina.1,2
Renal cell carcinomas develop in approximately 70% of patients with VHL disease during their lifetime.3 Although clinical judgment informs the risk–benefit assessment for surgery, surgical intervention, such as partial nephrectomy, is recommended to decrease the risk of metastatic disease in the case of renal tumors that grow beyond a 3-cm-diameter threshold and in the case of renal tumors that grow rapidly.1,4,5 A nephron-sparing approach is used in the resection of renal tumors when feasible, and early surgical intervention for renal cell carcinoma tumors less than 3 cm in diameter is generally not recommended because these tumors are associated with a low risk of metastasis.3 Patients typically undergo several surgical procedures during their lifetime for resection of renal tumors and other VHL-disease–associated neoplasms.6 Systemic therapy could benefit patients with renal cell carcinomas related to VHL disease by preventing tumor growth beyond 3 cm in diameter, thereby reducing the need for surgery and the risk of consequent renal insufficiency and metastases. Systemic therapy could provide similar benefits for patients with other neoplasms associated with VHL disease.
The VHL protein acts as an E3 ubiquitin ligase and leads to ubiquitination of the alpha subunit of hypoxia-inducible factor (HIF) in an oxygen-dependent fashion, which results in proteolysis of HIF.7 Pathogenic VHL variants reduce VHL protein activity, which results in stabilization of HIF subunits and the subsequent constitutive activation of HIF-mediated transcriptional pathways, independent of oxygen concentrations.7 In particular, HIF-mediated transcription facilitates gene expression of vascular endothelial growth factor (VEGF), cyclin D1, glucose transporter 1, and erythropoietin.7 These factors normally function to counteract the effects of hypoxia by promoting vascularization, enhancing glucose utilization, and increasing red-cell production through transcriptional signals.8
Constitutive activation of the HIF transcription factor is responsible for the hypervascularization that occurs in VHL-disease–associated renal cell carcinoma.1,7,8 Accordingly, VEGF-targeted therapy has been evaluated for use in VHL disease.5,9-14 Preclinical data indicate that HIF-2α subunit antagonists, which block HIF pathway activation at its most proximal source, inhibit tumor growth in clear-cell renal cell carcinoma.15,16 HIF-2α overexpression is ubiquitous in VHL-disease–associated renal cell carcinoma and is associated with sensitivity to HIF-2α inhibitor therapy in xenograft models.15 Preliminary data from phase 1 studies involving patients with advanced clear-cell renal cell carcinoma indicate the potential efficacy of HIF-2α blockade in patients with sporadic clear-cell renal cell carcinoma.17,18
Belzutifan (MK-6482, previously called PT2977) is a second-generation small-molecule HIF-2α inhibitor that offers better pharmacologic properties than the first-generation compound MK-3795 (previously called PT2385) and has shown efficacy and safety in a phase 1 trial involving patients with advanced clear-cell renal cell carcinoma.18,19 Given the role of VHL inactivation and the resulting HIF-2α activation in tumorigenesis associated with VHL disease, the objective of the present trial was to evaluate the efficacy and safety of belzutifan in patients with VHL-disease–associated renal cell carcinoma.
METHODS
PATIENTS
Patients 18 years of age or older were eligible for enrollment if they had VHL disease that was diagnosed on the basis of a germline VHL alteration and at least one measurable renal cell carcinoma tumor (≥10 mm in the longest diameter as measured with computed tomography [CT] or magnetic resonance imaging [MRI]), defined according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; if they had no renal cell carcinoma tumors larger than 3 cm that necessitated immediate surgical intervention and no evidence of metastatic disease; and if they had an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 or 1 (on a 5-point scale, with 0 indicating no symptoms and higher scores indicating greater disability). Complete eligibility criteria are provided in Section 7.2 of the protocol, available with the full text of this article at NEJM.org.
TRIAL DESIGN AND TREATMENT
In this phase 2, open-label, single-group trial, patients were enrolled at 11 centers in the United States, Denmark, France, and the United Kingdom between May 31, 2018, and March 29, 2019. Patients received belzutifan administered orally at a dose of 120 mg once daily (in three 40-mg tablets) unless unacceptable adverse events or disease progression occurred. The primary end point was objective response to treatment with belzutifan (complete response or partial response), as defined according to RECIST, version 1.1, in patients with VHL-disease–associated renal cell carcinoma. Secondary end points were duration of response, time to response, and progression-free survival; other secondary end points were the efficacy of belzutifan in the treatment of non–renal cell carcinoma neoplasms associated with VHL disease (including retinal and central nervous system hemangioblastomas and pancreatic lesions [i.e., serous cystadenomas and pancreatic neuroendocrine tumors]) and the safety of belzutifan.
TRIAL OVERSIGHT
This ongoing trial is sponsored by Merck Sharp and Dohme and was designed by the sponsor in collaboration with academic advisors. The protocol and its amendments were approved by the appropriate institutional review board or independent ethics committee at each center, and the trial was conducted according to International Council for Harmonisation Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. All patients provided written informed consent. Data were collected by trial investigators and site personnel. The authors and sponsor representatives were responsible for the analysis and interpretation of the data. All authors had access to the trial data and reviewed and edited the manuscript before submission. The authors vouch for the completeness and accuracy of the reported data and the fidelity of the trial to the protocol. Medical-writing and editorial assistance with earlier drafts of the manuscript was provided by ApotheCom and was paid for by the sponsor.
ASSESSMENTS
Assessment of safety end points was conducted throughout the trial and included the recording of adverse events, laboratory results, vital signs, findings from physical examinations, and electrocardiography results. Adverse events were coded according to the terminology used in the Medical Dictionary for Regulatory Activities, version 23.0. Severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Imaging of renal tumors with CT or MRI was performed at baseline, within 7 days before the week 13 visit, and every 12 weeks thereafter. When available, assessments of target tumors in VHL-disease–associated renal cell carcinoma were obtained and evaluated by independent central radiology reviewers two or more times before screening imaging was performed, to estimate growth kinetics before treatment. Among patients with non–renal cell carcinoma neoplasms, radiologic imaging and ophthalmic evaluations (dilated fundus examination, color fundus photography, and best-corrected visual acuity measurement) were performed at baseline and were performed during the trial treatment period only in patients who had neoplasms that were documented at baseline.
Tumor assessments of solid lesions were performed by an independent review committee with the use of RECIST, version 1.1, for each organ system affected by VHL disease. A maximum of five target lesions and five nontarget lesions could be identified for assessment in each affected organ system. In assessments of central nervous system hemangioblastomas with solid and cystic components, both components were included in the measurements. In assessments of pancreatic lesions, pancreatic neuroendocrine tumors and serous cystadenomas were measured. Qualitative assessment of retinal hemangioblastomas included multiple factors, such as size, location, and number of lesions, as well as the degree of feeder or drainer vessel engorgement, exudate, and fibrosis.
The linear growth of target lesions before treatment was calculated in patients who underwent at least three pretreatment imaging assessments, including the screening scan. Linear growth of lesions during treatment was calculated in patients who had a screening and at least two imaging assessments while they were receiving treatment. Linear regression was applied to lesion sizes, with time as a continuous variable and individual tumor as a categorical variable. The linear growth rate was derived as the coefficient of time.
STATISTICAL ANALYSIS
The data cutoff date was December 1, 2020. The full statistical analysis plan is available in Section 13.0 of the protocol. We calculated that a planned sample size of approximately 50 patients would provide the trial with 80% power to detect a significant difference (at a one-sided alpha level of 0.05) between an objective response of 30% and the null hypothesis of an objective response of 15%. Confidence intervals for objective responses were calculated with the use of the two-sided Clopper–Pearson method.
Efficacy was assessed in the intention-to-treat population. Safety was assessed in all the patients who received at least one dose of belzutifan. Efficacy and safety outcomes were summarized descriptively.
RESULTS
PATIENT CHARACTERISTICS
A total of 61 patients were enrolled in this trial. The median age was 41 years (range, 19 to 66); of the 61 patients, 32 (52%) were men, and 50 (82%) had an ECOG performance-status score of 0 (Table 1). A total of 59 patients (97%) had undergone at least one previous tumor reduction procedure (e.g., partial nephrectomy, craniotomy, or cryoablation); 46 patients (75%) with renal tumors had undergone tumor reduction procedures, including 40 patients (66%) who had undergone a partial or radical nephrectomy. At baseline, patients had a median of 2.0 (range, 1.0 to 5.0) renal cell carcinoma target tumors, 1.0 (range, 1.0 to 3.0) pancreatic target lesion, and 1.5 (range, 1.0 to 5.0) central nervous system target hemangioblastomas. All the patients had localized renal cell carcinoma and pancreatic lesions (22 patients [36%] had pancreatic neuroendocrine tumors), 50 patients (82%) had central nervous system hemangioblastomas, and 12 patients (20%) had retinal hemangioblastomas at baseline that could be evaluated by the independent central review committee.
Table 1.
Baseline Demographics and Disease Characteristics in the Safety Population.*
| Characteristic | All Patients (N=61) |
|---|---|
| Age — yr | |
| Median (range) | 41 (19–66) |
| At time of VHL disease diagnosis — median (range) | 32 (4–66) |
| At time of diagnosis of VHL-disease–associated renal cell carcinoma — median (range) | 30 (15–62) |
| Sex — no. (%) | |
| Male | 32 (52) |
| Female | 29 (48) |
| ECOG performance-status score — no. (%)† | |
| 0 | 50 (82) |
| 1 | 10 (16) |
| 2 | 1 (2) |
| VHL disease subtype — no. (%)‡ | |
| 1 | 51 (84) |
| 2A | 2 (3) |
| 2B | 6 (10) |
| 2C | 0 |
| Missing | 2 (3) |
| Previous surgery or ablative procedure — no. (%) | |
| Total | 59 (97) |
| Surgery for renal cell carcinoma | 46 (75) |
| Partial or radical nephrectomy§ | 40 (66) |
| Ablative procedures¶ | 20 (33) |
| Central nervous system surgery | 47 (77) |
| Pancreas-related surgery | 9 (15) |
| Previous surgical or ablative procedures per patient — median (range) | 4 (0–15) |
| Procedures — no. of patients (%) | |
| 0 | 2 (3) |
| 1 | 5 (8) |
| 2 | 6 (10) |
| 3 | 8 (13) |
| ≥4 | 40 (66) |
| Non–renal cell carcinoma neoplasms — no. (%)∥ | |
| Pancreatic lesions∥ | 61 (100) |
| Pancreatic neuroendocrine tumors | 22 (36) |
| Central nervous system hemangioblastomas | 50 (82) |
| Retinal hemangioblastomas | 12 (20) |
| Median size of target lesions (range) — mm | |
| Renal cell carcinomas | 22 (10–61) |
| Pancreatic lesions** | 20 (10–89) |
| Pancreatic neuroendocrine tumors | 21 (10–52) |
| Central nervous system hemangioblastoma | 16 (10–87) |
VHL denotes von Hippel–Lindau.
Eastern Cooperative Oncology Group (ECOG) performance-status scores are assessed on a 5-point scale, with higher scores indicating greater disability. A waiver was requested by the investigator and approved by the institutional review board before enrollment of the patient with an ECOG performance-status score of 2.
Type 1 disease manifests as retinal and central nervous system hemangioblastomas, renal cell carcinoma, pancreatic cysts, neuroendocrine tumors, and a decreased risk of pheochromocytomas; type 2A disease as pheochromocytomas, retinal and central nervous system hemangioblastomas, and a decreased risk of renal cell carcinoma; type 2B disease as renal cell carcinoma, pheochromocytomas, and retinal and central nervous system hemangioblastomas; and type 2C disease as pheochromocytomas only.3
Partial or radical nephrectomy also includes renal surgery and renal tumor excision.
Ablative procedures include cryotherapy and kidney ablation.
Included are neoplasms that could be evaluated by the independent central review committee.
Pancreatic lesions include pancreatic neuroendocrine tumors and serous cystadenomas.
EFFICACY IN RENAL CELL CARCINOMA
As of December 1, 2020, the median follow-up time, defined as the time from the first dose to the date of data cutoff, was 21.8 months (range, 20.2 to 30.1). Median exposure was 21.7 months (range, 1.9 to 30.1). A total of 54 patients (89%) continued to receive treatment with belzutifan as of the data cutoff date.
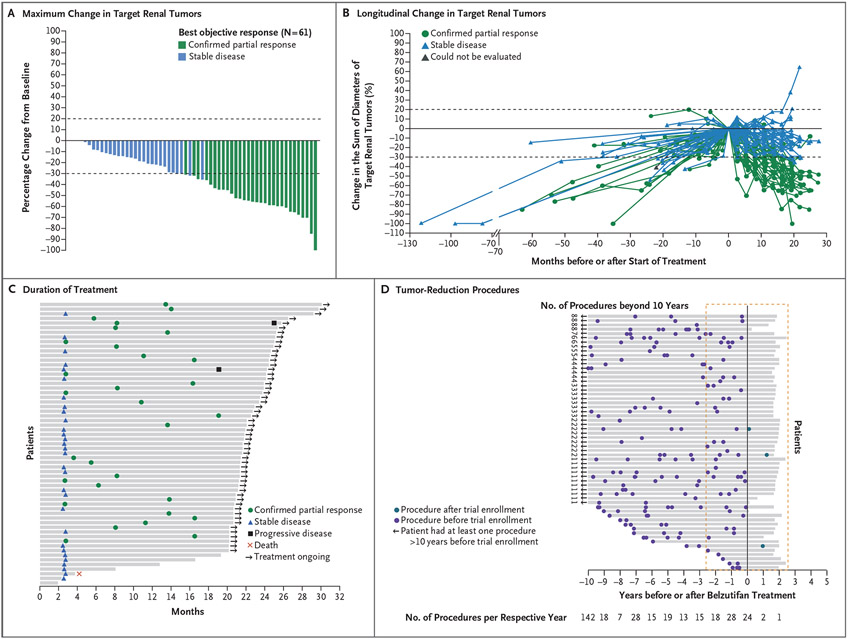
A total of 30 patients had confirmed partial responses, for an objective response in renal cell carcinoma of 49% (95% confidence interval [CI], 36 to 62) (Table 2). An additional 30 patients (49%) had a best response of stable disease. Two patients (3%) had disease progression, as assessed by the independent central radiology review committee. In addition, one patient was assessed by the central radiology review committee as having had a best objective response of stable disease but was categorized by the treating physician as having progressive disease. A reduction in the sum of all target lesion diameters was observed in 56 patients (92%) (Fig. 1A, and Table S1 in the Supplementary Appendix). Most patients had growing tumors before treatment, followed by an observed reduction in the sum of the largest tumor diameters after treatment began (Fig. 1B and Fig. S1). At 24 months, the percentage of patients with progression-free survival was 96% (95% CI, 87 to 99) (Fig. S2).
Table 2.
Best Objective Response in Renal Cell Carcinoma Associated with VHL Disease.*
| Variable | Efficacy Population (N=61) |
|---|---|
| Objective response — no. (% [95% CI]) | 30 (49 [36 to 62]) |
| Best response — no. (%) | |
| Complete response | 0 |
| Partial response | 30 (49) |
| Stable disease | 30 (49) |
| Disease progression | 0 |
| Unable to be evaluated† | 1 (2) |
| Median time to response (range) — mo | 8.2 (2.7 to 19.1) |
| Median duration of response (range) — mo‡ | NR (2.8+ to 22.3+) |
The best objective response was assessed according to Response Evaluation Criteria in Solid Tumors, version 1.1. NR denotes not reached.
One patient discontinued the trial before the first post-baseline tumor assessment.
Plus sign indicates ongoing response.
Figure 1 (facing page). Change in Lesions from Baseline, Duration of Treatment, and Tumor-Reduction Procedures.
Panel A shows the maximum change from baseline in target renal tumors. Panel B shows the longitudinal change from baseline in target renal tumors. In Panels A and B, the dashed lines represent a 20% increase in target tumors from baseline and a 30% reduction in target tumors from baseline, which correspond to the definitions of progressive disease and partial response, respectively, according to Response Evaluation Criteria in Solid Tumors, version 1.1. Panel C shows duration of treatment and time to response in patients with renal cell carcinoma. Panel D shows the distribution of tumor-reduction procedures: adrenalectomy, craniotomy, cryoablation, cryotherapy, eye removal, intradural resection, laser ablation, laser surgery, laminectomy, laser photocoagulation, pancreatectomy, partial nephrectomy, radiation therapy, radiofrequency ablation, retinal surgery, total nephrectomy, tumor enucleation, and ventriculoperitoneal shunt placement. In Panel D, the solid line indicates the start of belzutifan treatment, and the red dashed line shows the maximum duration of treatment and the equivalent period before treatment started. Two patients had no tumor-reduction procedures before treatment or while receiving belzutifan.
For the evaluation of linear growth of renal tumors, 57 patients met the pretreatment criteria, 58 met the on-treatment criteria, and 54 met pretreatment and on-treatment criteria. At the patient level, the median linear growth rate of tumors before treatment was 3.6 mm per year (range, −3.1 to 18.6) as compared with −3.7 mm per year (range, −9.5 to 10.1) during the on-treatment period. Among patients with a partial response who could be evaluated (28 before treatment and 30 during the on-treatment period), the median linear growth rate was 4.1 mm per year (range, −3.1 to 18.6) before treatment, as compared with −5.6 mm per year (range, −9.5 to −1.3) during the on-treatment period. Among patients with stable disease who could be evaluated (28 patients), the median linear growth rate was 3.4 mm per year (range, −0.5 to 8.9) before treatment and −1.6 mm per year (range, −7.2 to 10.1) during the on-treatment period. At the tumor level, the median linear growth rate was 3.3 mm per year (range, −3.1 to 18.6) in 110 tumors before treatment and −3.6 mm per year (range, −16.6 to 10.1) in 105 tumors during treatment.
The median time to response was 8.2 months (range, 2.7 to 19.1) (Fig. 1C), and treatment was ongoing in 54 patients at the time of data cutoff. The median duration of response was not reached (range, 2.8+ to 22.3+ months [with the plus sign indicating ongoing response at the time of data cutoff]) (Fig. S3). A total of 30 patients (49%) had a partial response; all these patients continued to receive treatment as of the time of data cutoff. One patient had disease progression after an initial partial response. A total of 24 patients (39%) with a best response of stable disease were continuing treatment at the time of data cutoff. As of the data cutoff date, 3 patients had had a tumor reduction procedure (partial nephrectomy in 1 patient, cerebellar radiation in 1 patient, and resection of a nontarget central nervous system hemangioblastoma in 1 patient) after treatment was started; all 3 patients were receiving ongoing treatment as of the data cutoff. No patient underwent pancreatic surgery; 1 patient underwent vitrectomy for retinal detachment, which was not considered by the trial investigators to be related to active disease. In contrast, 327 procedures had been performed before treatment began, 64 of which had been performed in the 2.5 years before treatment was started (Fig. 1D).
EFFICACY IN NON–RENAL CELL CARCINOMA NEOPLASMS
All 61 patients in the trial had pancreatic lesions; a confirmed response was observed in 47 patients (77%), including 6 patients (10%) who had a complete response (Table S2). Among 22 patients with pancreatic neuroendocrine tumors, 20 patients (91%) had a confirmed response (including 3 patients [14%] who had a complete response). Among patients with central nervous system hemangioblastomas, 15 of 50 patients (30%) had a confirmed response (including 3 who had a complete response [6%]). A total of 60 target central nervous system hemangioblastomas were identified; 27 originated in the cerebellum, 23 in the spine, and 10 in another location (e.g., the brain stem or frontal lobe). The median target-lesion change in cerebellar hemangioblastomas was −30% (range, −71 to 0); in spinal hemangioblastomas, −51% (range, −100 to −5); and in other hemangioblastomas, −35% (range, −50 to 0). The median time to response was 8.4 months (range, 2.5 to 19.1) for all pancreatic lesions, 5.5 months (range, 2.5 to 16.4) for pancreatic neuroendocrine tumors, and 3.2 months (range, 2.3 to 16.6) for central nervous system hemangioblastomas. At the time of data cutoff, all responses were ongoing for 2.6+ to 22.3+ months in patients with pancreatic lesions, 2.9+ to 22.3+ months in patients with pancreatic neuroendocrine tumors, and 2.8+ to 22.3+ months in patients with central nervous system hemangioblastomas. Among the 16 eyes in 12 patients identified at baseline as having retinal hemangioblastomas that could be evaluated, all (100%) were graded by the independent review committee as showing improvement at the time of data cutoff (Table S3).
SAFETY
All patients reported at least one treatment-related adverse event. The most common adverse events of any cause were anemia, fatigue, headache, and dizziness (Table 3 and Table 4, and Table S4). Treatment was interrupted in 26 patients (43%) and the treatment dose was reduced in 9 patients (15%) because of adverse events. Seven patients discontinued treatment: 4 patients voluntarily discontinued treatment, 1 discontinued owing to a treatment-related adverse event (grade 1 dizziness), 1 discontinued because of disease progression as assessed by the investigator, and 1 patient died (as the result of acute toxic effects of fentanyl). The median duration of treatment for the 7 patients who discontinued was 8.1 months (range, 1.9 to 19.3).
Table 3.
Adverse Events of Any Cause in the Full Safety Population.
| Adverse Event | Safety Population (N = 61) |
|---|---|
| no. (%) | |
| Any grade | |
| Total | 61 (100) |
| Treatment-related | 61 (100) |
| Grade 3 to 5 | 20 (33) |
| Grade 3 treatment-related | 9 (15) |
| Grade 4 or 5 treatment-related | 0 |
| Treatment discontinuation | |
| Total* | 2 (3) |
| Due to a treatment-related adverse event | 1 (2) |
| Death | |
| Total† | 1 (2) |
| Due to a treatment-related adverse event | 0 |
One patient death unrelated to treatment was recorded as an adverse event.
Death was caused by acute toxic effects of fentanyl.
Table 4.
Adverse Events in at Least 10% of the Safety Population (61 Patients).
| Event | Any Grade | Grade 1 | Grade 2 | Grade 3* |
|---|---|---|---|---|
| number (percent) | ||||
| Anemia | 55 (90) | 24 (39) | 26 (43) | 5 (8) |
| Fatigue | 40 (66) | 29 (48) | 8 (13) | 3 (5) |
| Headache | 25 (41) | 20 (33) | 5 (8) | 0 |
| Dizziness | 24 (39) | 20 (33) | 4 (7) | 0 |
| Nausea | 21 (34) | 15 (25) | 6 (10) | 0 |
| Dyspnea | 14 (23) | 13 (21) | 0 | 1 (2) |
| Arthralgia | 12 (20) | 10 (16) | 2 (3) | 0 |
| Constipation | 12 (20) | 10 (16) | 2 (3) | 0 |
| Myalgia | 12 (20) | 9 (15) | 2 (3) | 1 (2) |
| Upper respiratory tract infection | 11 (18) | 4 (7) | 7 (11) | 0 |
| Alanine aminotransferase level increase | 10 (16) | 10 (16) | 0 | 0 |
| Hypertension | 10 (16) | 3 (5) | 2 (3) | 5 (8) |
| Vision blurred | 10 (16) | 6 (10) | 4 (7) | 0 |
| Abdominal pain | 9 (15) | 5 (8) | 4 (7) | 0 |
| Diarrhea | 8 (13) | 7 (11) | 0 | 1 (2) |
| Weight increase | 8 (13) | 5 (8) | 2 (3) | 1 (2) |
| Peripheral edema | 7 (11) | 6 (10) | 1 (2) | 0 |
| Aspartate aminotransferase level increase | 7 (11) | 7 (11) | 0 | 0 |
| Urinary tract infection | 7 (11) | 1 (2) | 5 (8) | 1 (2) |
| Muscle spasms | 7 (11) | 5 (8) | 2 (3) | 0 |
One patient reported asymptomatic grade 3 hypoxia that did not result in treatment.
Adverse events were generally grade 1 or 2. Grade 3 to 5 adverse events of any cause were reported in 20 patients (33%). Grade 3 events were considered to be treatment-related in 9 patients (15%). Only 1 patient (2%) had a grade 4 adverse event (a retinal detachment), and it was considered to be unrelated to treatment. There were no deaths from a treatment-related adverse event. The patient death attributed to acute toxic effects of fentanyl was considered to be unrelated to the trial drug.
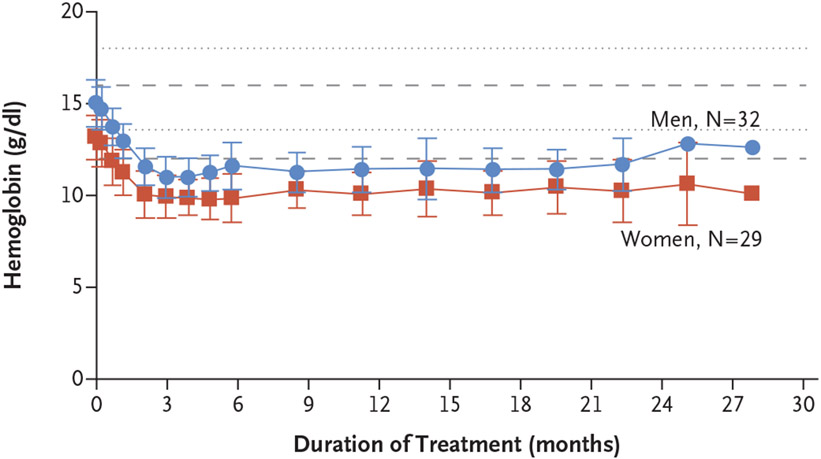
All patients had a decrease in hemoglobin levels from baseline of at least 1.9 g per deciliter during the first 13 weeks of treatment, as was expected from on-target inhibition of the EPO gene, which reduces erythropoietin production (Fig. 2 and Fig. S4); thereafter, the hemoglobin levels stabilized. Four patients (7%) received blood transfusions owing to anemia; one of the patients received three blood transfusions. A total of 12 patients (20%) received erythropoietin-stimulating agents, with a median of 2.5 administrations (range, 1 to 17); 3 of the 12 patients received both an erythropoietin-stimulating agent and a blood transfusion. One patient (2%) had grade 3 transient hypoxia, which resolved with dose interruption for 1 week followed by dose reduction to 80 mg; the patient did not receive supplemental oxygen or other treatment.
Figure 2. Hemoglobin Levels in All Patients over Time.
Dashed lines represent upper and lower boundaries of normal values for women; dotted lines represent upper and lower boundaries of normal values for men. I bars represent standard deviations.
DISCUSSION
Neoplasms associated with VHL disease are currently managed with surgical resection or ablation with the goal of reducing the risk of metastatic disease and controlling local or systemic sequelae. Since patients with VHL disease have a lifelong risk of tumors in affected organs, most patients undergo several surgical procedures during their lives, with considerable attendant complications. An effective systemic alternative might reduce the surgical burden in patients with VHL disease and represents a new approach to the management of VHL-disease–associated neoplasms that are confined to organs.
Belzutifan is a novel pharmacologic agent that targets HIF-2α. In this trial, 49% of patients with renal cell carcinomas associated with VHL disease who received belzutifan had a confirmed objective response; most patients had a reduction in renal tumor size. These data indicate that belzutifan has activity against renal cell carcinoma associated with VHL disease. Two patients (3%) had progressive disease, as assessed by independent central review, but a mechanism of possible resistance to belzutifan is unknown. Acquired resistance from prolonged treatment with HIF-2α inhibitors was observed in a previous preclinical study with PT2399 and a phase 1 study of PT2385 (now called MK-3475) and may have been the result of a HIF-2α G323E gatekeeper mutation that prevented HIF-2 dissociation.15,19
Extrarenal manifestations are also associated with substantial morbidity and mortality among patients with VHL disease,6 and effective systemic therapy could decrease the frequency of surgical interventions for these lesions. In this trial, 30% of patients with central nervous system hemangioblastomas had a response after treatment with belzutifan, as did 91% of patients with pancreatic neuroendocrine tumors, which indicates clear signs of activity in these VHL-disease–associated neoplasms. Because tumor size is a primary determinant of the need for surgical intervention, reduction in tumor size is likely to result in fewer indications for surgery. As observed in the current trial, patients often underwent multiple surgical or ablative procedures before treatment with belzutifan. After treatment initiation, only three patients underwent an intervention directed at a neoplasm associated with VHL disease. The appropriate use of belzutifan in patients with VHL remains to be determined. However, data from the current trial suggest that belzutifan might serve as an alternative treatment or a complement to surgical treatment in these patients. Therefore, belzutifan might play an integral role in the treatment of patients with VHL disease by delaying or obviating the need for serial surgeries that are associated with substantial complications.
Side effects of belzutifan were mainly low-grade: only one patient (2%) discontinued treatment because of a treatment-related adverse event (dizziness). Adverse events were consistent with expectations for a HIF-2α inhibitor given the integral role of HIF-2α in erythropoietin production and erythropoiesis.20-22 Anemia, considered an on-target effect of HIF-2α inhibition, was the most common adverse event, but the number of patients who received transfusion or growth factor support was low. Hemoglobin levels also stabilized, typically without intervention, after an initial decrease in levels. Hypoxia was previously reported in patients with advanced renal cell carcinoma who received belzutifan18; however, in the current trial, only one transient hypoxemic event was reported, which might reflect differences in the overall health of the participants in the two studies. As compared with the population of persons with advanced renal cell carcinoma, patients in this trial were younger (median age, 41 years vs. 62 years), and a higher percentage had an ECOG performance-status score of 0 (82% vs. 36%).18
HIF-2α inhibition may offer a more favorable safety profile than antiangiogenic agents, which are associated with cardiovascular adverse events, hematologic disturbances, hepatotoxicity, diarrhea, and metabolic disturbances.10,11,13 Studies that evaluated the tyrosine kinase inhibitors dovitinib (involving 6 patients with VHL disease)12 and sunitinib (one study involving 15 patients and one involving 5 patients with VHL disease)12,14 showed modest efficacy and were limited by undesirable side effects related to treatment and by the low number of patients enrolled. Results of a phase 2 study of pazopanib involving patients with VHL disease showed that 42% of patients (13 of 31) had an objective response (all partial responses) across kidney, pancreas, and central nervous system neoplasms, but 23% of patients (7 of 31) discontinued treatment because of adverse events, including 4 patients who had grade 3 or 4 increases in liver aminotransferase levels.11 Furthermore, only 10 of 31 patients (32%) who received pazopanib were able to receive a full dose of 800 mg per day without unacceptable adverse events, and 16 patients (52%) continued to receive treatment after 24 weeks. In this trial, 54 patients (89%) continued to receive treatment as of the time of data cutoff, and most patients (52 [85%]) did not have a dose reduction because of adverse events.
The interpretation of outcomes in this trial is limited by the lack of a comparator group and by the modest sample size. Given that no other nonsurgical treatment for VHL disease has been approved to date, design of a randomized, controlled trial is an ethical challenge.
We found that belzutifan has mainly low-grade side effects that affect most patients who take it and has shown activity in patients with renal cell carcinoma associated with VHL disease, pancreatic neuroendocrine tumors, and hemangioblastomas by targeting the underlying pathophysiology of the disease.
Supplementary Material
Acknowledgments
Supported by Merck Sharp and Dohme, a subsidiary of Merck; the Intramural Research Program of the National Institutes of Health, National Cancer Institute (NCI) Center for Cancer Research; and a grant (UO1 CA236489) from the NCI.
Footnotes
Presented in part at the American Society of Clinical Oncology 2020 (ASCO 2020) Virtual Scientific Program, May 29–31, 2020; the European Society of Medical Oncology 2020 Virtual Congress, October 16–18, 2020; and the ASCO 2021 Virtual Scientific Program, June 4–8, 2021.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank Robert Steger, Ph.D., and Matthew Grzywacz, Ph.D., (ApotheCom) for medical writing and editorial assistance with an earlier version of the manuscript, funded by Merck Sharp and Dohme.
The MK-6482-004 investigators are listed in the Supplementary Appendix, available at NEJM.org.
Contributor Information
Eric Jonasch, University of Texas M.D. Anderson Cancer Center, Houston
Frede Donskov, Aarhus University Hospital, Aarhus, Denmark
Othon Iliopoulos, Massachusetts General Hospital Cancer Center and Harvard Medical School, Boston
W. Kimryn Rathmell, Vanderbilt University Medical Center, Nashville
Vivek K. Narayan, University of Pennsylvania, Philadelphia
Benjamin L. Maughan, University of Utah, Salt Lake City
Stephane Oudard, Hôpital Européen Georges-Pompidou, University of Paris, Paris
Tobias Else, University of Michigan, Ann Arbor
Jodi K. Maranchie, University of Pittsburgh, Pittsburgh
Sarah J. Welsh, Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom
Sanjay Thamake, Merck, Kenilworth, NJ
Eric K. Park, Merck, Kenilworth, NJ
Rodolfo F. Perini, Merck, Kenilworth, NJ
W. Marston Linehan, Center for Cancer Research, National Cancer Institute, Bethesda, MD
Ramaprasad Srinivasan, Center for Cancer Research, National Cancer Institute, Bethesda, MD
REFERENCES
- 1.Couch V, Lindor NM, Karnes PS, Michels VV. von Hippel-Lindau disease. Mayo Clin Proc 2000;75:265–72. [DOI] [PubMed] [Google Scholar]
- 2.Binderup MLM, Galanakis M, Budtz-Jørgensen E, Kosteljanetz M, Luise Bisgaard M. Prevalence, birth incidence, and penetrance of von Hippel-Lindau disease (vHL) in Denmark. Eur J Hum Genet 2017; 25:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet 2011; 19:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmid S, Gillessen S, Binet I, et al. Management of von Hippel-Lindau disease: an interdisciplinary review. Oncol Res Treat 2014;37:761–71. [DOI] [PubMed] [Google Scholar]
- 5.Kim E, Zschiedrich S. Renal cell carcinoma in von Hippel-Lindau disease — from tumor genetics to novel therapeutic strategies. Front Pediatr 2018;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binderup MLM, Jensen AM, Budtz-Jørgensen E, Bisgaard ML. Survival and causes of death in patients with von Hippel-Lindau disease. J Med Genet 2017;54: 11–8. [DOI] [PubMed] [Google Scholar]
- 7.Choueiri TK, Kaelin WG Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat Med 2020;26:1519–30. [DOI] [PubMed] [Google Scholar]
- 8.Haase VH. The VHL tumor suppressor: master regulator of HIF. Curr Pharm Des 2009;15:3895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meléndez-Rodríguez F, Roche O, Sanchez-Prieto R, Aragones J. Hypoxia-inducible factor 2-dependent pathways driving von Hippel-Lindau-deficient renal cancer. Front Oncol 2018;8:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Kidney cancer, version 4. Clinical practice guidelines in oncology. 2021. (https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf).
- 11.Jonasch E, McCutcheon IE, Gombos DS, et al. Pazopanib in patients with von Hippel-Lindau disease: a single-arm, single-centre, phase 2 trial. Lancet Oncol 2018; 19:1351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilié P, Hasanov E, Matin SF, et al. Pilot study of dovitinib in patients with von Hippel-Lindau disease. Oncotarget 2018; 9:23390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonasch E, McCutcheon IE, Waguespack SG, et al. Pilot trial of sunitinib therapy in patients with von Hippel-Lindau disease. Ann Oncol 2011;22:2661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oudard S, Elaidi R, Brizard M, et al. Sunitinib for the treatment of benign and malignant neoplasms from von Hippel-Lindau disease: a single-arm, prospective phase II clinical study from the PREDIR group. Oncotarget 2016;7:85306–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Hill H, Christie A, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 2016;539:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho H, Du X, Rizzi JP, et al. On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature 2016; 539:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtney KD, Infante JR, Lam ET, et al. Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2alpha antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J Clin Oncol 2018;36:867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choueiri TK, Bauer TM, Papadopoulos KP, et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med 2021;27:802–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courtney KD, Ma Y, Diaz de Leon A, et al. HIF-2 complex dissociation, target inhibition, and acquired resistance with PT2385, a first-in-class HIF-2 inhibitor, in patients with clear cell renal cell carcinoma. Clin Cancer Res 2020;26:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rankin EB, Biju MP, Liu Q, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 2007;117:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scortegagna M, Ding K, Zhang Q, et al. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood 2005;105:3133–40. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL. Pharmacologic targeting of hypoxia-inducible factors. Annu Rev Pharmacol Toxicol 2019;59:379–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.