Abstract
Translational research requires good quality specimens to ensure the integrity of research results. Clinical research must rely not only on quality specimens, but as well on clinical annotation for consistent, accurate and verifiable scientific and clinical outcomes. In laboratory research performed on a specimen by a single investigator, quality control is easily maintained. In a multi-site clinical research network, the numerous steps for biospecimens from procurement through transport, processing, storage and ultimately testing requires strict standardization of operational workflows and procedures. The practices of a central biorepository can inform and contribute to best practices regarding clinical research specimen integrity for multi-site clinical research.
1. Introduction
Clinically annotated, well-characterized, high-quality biological specimens are a critical prerequisite in translational research. Such specimens are needed for a range of research applications including genomics and proteomics, discoveries of novel biomarkers associated with certain stages or subtypes of a cancer and as new diagnostic, prognostic and therapeutics targets [1,2]. Just as an accurate clinical diagnosis depends on a properly collected and appropriately representative specimen, the outcome of any clinical trial and its scientific and clinical impact rely on the quality of its study samples [3–5]. The path of study specimens from procurement through testing greatly impacts the study results [6].
The technological advances brought on by the internet and web-access have facilitated large consortium multi-site clinical trials and research programs. This has been accompanied by an exponential growth of local institutional biobanks in the last 30 years. Multisite clinical trials can allow each site to hold their own specimens in what is referred to as federated biobanking. In this case, each site processes, stores and distributes specimens according to the protocol. However, often when biospecimens are stored in separate point-of-collection repositories, they have been found to be unfit for analyses due to the influence of variable factors surrounding collection, processing, handling, storage and data entry [7,8]. This can be especially problematic when biospecimens from different institutions are pooled for comparative analyses.
To overcome these challenges for multi-site projects, a central biobank which follows current industry best practices affords a pathway to successful research outcomes both nationally and internationally. We describe the elements of a central biorepository, including its governance, standards, operational workflows, specimen handling procedures, and problem solving based on our combined experience in managing biorepositories of cancer clinical research specimens from multi-site networks.
2. Elements of a central biorepository
2.1. Standards and certifications
A central biorepository needs to follow best practices that are evidence/consensus-based for collection, long-term storage, retrieval, and distribution of specimens and data. Examples of guidance include best practice guidelines from the Biorepositories and Biospecimen Research Branch (BBRB) of the National Institute of Health (NIH)-National Cancer Institute (NCI) [9], the International Society for Biological and Environmental Repositories (ISBER) [10,11], the European Union [12] and the International Organization for Standardization [13]. Additionally, biobank certification and accreditation programs exist to demonstrate compliance with quality standards and management of both specimens and associated data [14–17]. Fig. 1 shows the areas most covered by biorepository best practices.
Fig. 1.
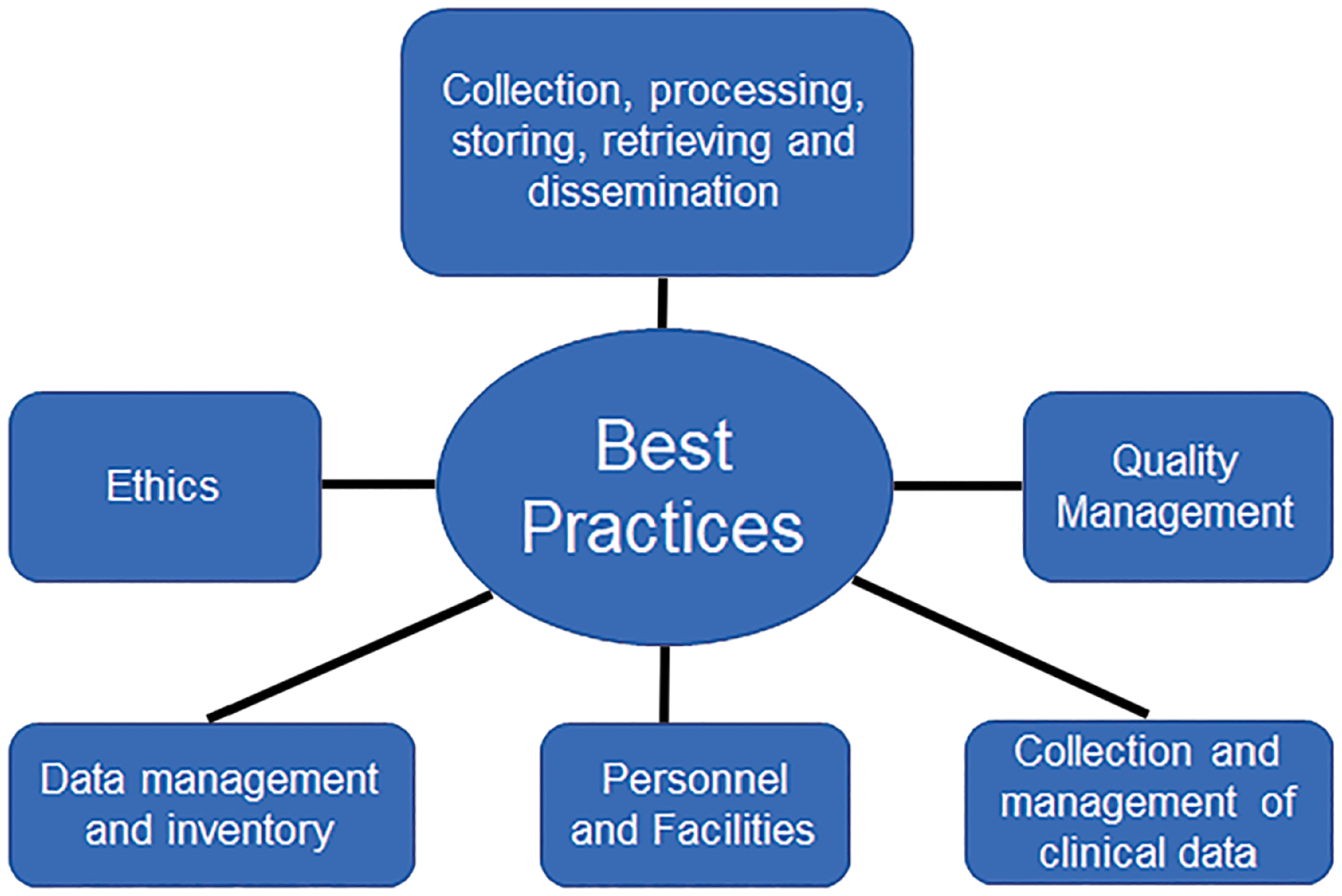
Overview of areas covered by Biorepository Best Practices with standards to ensure specimen and data integrity.
2.2. Governance
A biorepository could be located in an academic medical center or a hospital and should have a governance structure to ensure accountability and compliance with good clinical practices and ethical and legal operations. The functions of the biorepository should be overseen by an Oversight or Advisory Committee that ensures the operations are in the hands of a qualified director. The biorepository director guarantees a qualified staff, establishes technical and administrative policies and standard operating procedures (SOPs) and ensures positive interactions with the specimen collecting sites and the multiple sites that use the specimens for testing and/or research. All biorepository staff maintain human subjects training requirements including good clinical and laboratory practice training.
Usually, a clinical research organization (CRO) oversees the operations, data management and statistical support in large multisite clinical trials. The CRO generates protocol-specific Manuals of Operations (MOOs) and a related biospecimen Manual of Procedures (bMOP) per protocol as well as Standard Operation Procedures (SOPs) in close collaboration with the central biorepository. SOPs describe each procedure and each step and are an integral part of good quality management. The bMOP sets the functions of a central biorepository within a particular protocol. Using a CRO is not mandatory; alternatively, these processes can be handled within the network by committees or a program manager.
The research protocol needs to obtain institutional review board (IRB) approval, and in the case of international studies, human research ethics approval. IRB approval can either be received by local IRBs affiliated with individual research sites or by the use of a centralized review process. A central IRB is defined as a single IRB of record for all participating sites. The advantage of a single or central IRB is greater efficiency by eliminating duplicative or redundant IRB review in multisite studies, by avoiding delays of trial conduct, and by providing a review process to sites that may not have an easy access to a local IRB [18]. In addition, a central IRB may reduce the diffusion of responsibility and thereby the potential exposure of participants to undue risks. It also may reduce the risk of compromising the ethical integrity of the study by required changes in the informed consent form used by a local IRB that are not communicated to other IRBs [19]. Although the Food and Drug Administration (FDA) and the Office of Human Research Protections (OHRP) support the use of central IRBs for multi-site trials, there are concerns about deferring to a central IRB review surrounding lack of attention to local concerns, and regulatory and legal liability of the participating site [20].
2.3. Biospecimen collection
The key to a high-quality collection of specimens is a very detailed and distinct protocol bMOP. The bMOP provides detailed instructions on the following: number and types of specimens to be collected and the type of collection medium depending on the intent of future research (specimen purpose), i.e., DNA, RNA or protein analysis; the specimen collection schedule; specimen shipment schedule; shipping instructions; required supplies; supply orders; equipment needed; institutional specimen collection, handling and processing procedures. Biobanking staff expertise is essential during the development of the bMOP and the particular SOPs that detail the implementation of the bMOP at each site, since specimen collection sites have diverse environments and settings. The central biorepository assists in the writing of the SOPs and bMOPs by providing guidance, review and feedback, and also serves as a quality control check point.
SOPs help promote proper handling of specimens through detailed step-by-step work instructions. The biorepository staff can offer individual assistance to collection sites for unique situations; for example, how to ensure viability when the sample is collected quite distant from the lab where the sample is to be processed, stored short-term and then prepared for shipping. Some sites must transport the sample from one building to another, across a campus or even a town, county or international border. Infrastructure and regulation limitations that affect courier reliability, access and costs in developing countries with limited resources can potentially negatively impact biospecimen shipping and handling and endanger specimen integrity. A recent study showed that shipping duration between different regions of Africa averaged 5 days and that the DNA quality before and after shipment did not significantly differ, although shipments sent at uncontrolled ambient temperature fluctuated between 5.6 °C and 32.7 °C [21]. Likewise in our experience, the shipping duration from African states to the US also averages 5 days. The use of stabilizing agents such as RNAlater may help to mitigate the risk of RNA degradation and allow for longer exposures to ambient temperatures during transport of tissues or cells. If overnight shipments cannot be guaranteed in these circumstances, a strict weekly shipping schedule of Mondays or Tuesdays can help avoid delivery delays [21]. A central biorepository serving clinical trials within resource limited countries should reach out prior to trial commencement to ensure compliance with country regulatory issues (e.g., need for export or import permits) and ensure smooth specimen movement through “mock runs.”
2.4. Specimen storage
The biospecimen storage temperatures and appropriate size for aliquots and samples should be determined in advance as part of the bMOP to ensure continued stability of the analytes of interest. The determination of storage temperature depends on the biospecimen types and stabilization media, the anticipated length of storage, the biomolecules of interest (e.g., RNA, DNA, proteins or lipids), and the intended downstream analytic procedures. For example, for long-term storage, and when future analytical research methods are yet uncertain, liquid biospecimens such as blood and its derivatives should be kept in the vapor phase of liquid nitrogen freezers or at −80 °C. The temperature of freezer interiors should be regularly mapped to ensure uniformity and identify hot spots. This can be achieved by placing two sensors in different spots into the cold units.
The purpose of aliquoting specimens is to avoid thawing and refreezing and to maximize the availability of the specimens for multiple analyses. One needs to determine the adequate volume needed for downstream testing and realize that small volumes will result in a higher number of aliquots which might not always be practical. For example, in order to extract DNA from whole blood 200 μl are sufficient. However, depending on the trial size this approach is not always feasible. For example, we currently participate in one trial with 22 sites and an anticipated 1.5 million biospecimens to be collected by trial completion. In order to limit lab space requirements, number of cold units and costs, we increased the aliquot size to 1 ml and 2 ml per sample. We also received 5 ml aliquots destined for certain tests. In order to maximize storage space, we assigned cold units to either 5 ml or 2 ml cryovials. Specimens should be stored in a secure location with limited access only by authorized personnel.
2.5. Data management system
Multi-site clinical trials ideally should implement centralized information systems that ensure the tracking of specimens, annotation of data collected with patient consent and standardized nomenclature. The data management system is essential for the optimization in the value of the biospecimens.
The quality of a specimen is essential and influenced by every parameter associated with it. Consequently, in addition to epidemiologic, clinical and medical patient data, many data points for each sample should be recorded. A well-designed inventory management database executes and keeps track of all aspects of managing biorepository activities [22,23]. The database serves as a collection center of recorded information encompassing specimen classification, processing, storage conditions, and chain-of-custody (all stages of acquisition, processing, handling, banking, quality control, retrieval and dissemination, and ultimate disposition). Audit trails and configurable reports are routinely generated to provide the most complete information on the specimen. For all studies, the biorepository receives only de-identified specimens. Clinical data and regulated participant health information including the link is kept at the CRO, thereby maintaining patient confidentiality.
To ensure complete and accurate data, specimens added to the inventory are checked by another staff member who reviews the inventory entries against specimen data forms. Additionally, an inventory quality check is performed each quarter. The Biobank director generates a list representing 10% of new specimens randomly selected to have checks on correctness of data entry and freezer location; two biobank associates work together in accomplishing these tasks.
2.6. Flow charts
Protocols are written depending on the specific research aims of each study protocol and delineate the specific steps to be taken for each component. Biorepository staff are familiar with each protocol and produce flow charts, of which both electronic and hardcopies are available in both the biorepository director’s office and working areas within the biorepository, including the specimen processing laboratories where they are easily accessible to staff. Each protocol is color-coded with the particular color carried onto protocol labels and documentation. Any approved amendment to a protocol requires an update to the flow chart and processing protocols.
The flow charts represent specimen flow from receipt through processing, storage and disbursement. The flow charts take into account such things as the specimen type, visit number, specimen purpose, specimen processing procedures, number and volume of aliquots to be created and the type of storage, i.e., 4 °C, −20 °C, −80 °C or in liquid nitrogen, depending upon the analytes intended to be examined and when and where aliquots are to be distributed.
2.7. Quality checkpoints
To prepare sending specimens to the central biorepository, the clinical sites should create electronic manifests containing individual specimen information such as participant-code, visit number, specimen type and specimen purpose. These manifests are entered into a central tracking software hosted and usually maintained by the CRO. These digital shipping manifests are emailed to the biorepository beforehand, alerting it to the specimens that are to arrive the next day. This allows the biorepository to be prepared for the next day’s specimen workload such as staff scheduling, task assignments, and preparing the workspace with materials needed.
Upon specimen arrival, the data in the manifest are compared with the content of the shipment. At this first quality checkpoint the biorepository staff, using the study protocol design, determines when a particular type of specimen is anticipated for each participant and visit number. Thus, the biorepository staff is able to determine that a specimen is correct or appears erroneously related to a particular participant and/or time-point or stage in the study. If a discrepancy occurs, a query is made to the study site on the appropriateness and correctness of the shipment or the specimen in question. For example, in one of our studies we had a site that failed to procure blood collected into lavender top (EDTA) tubes from its participants at an early study visit, as required by the study protocol; a deviation from protocol. Our central biorepository noticed the discrepancy, reported it to the study Principal Investigator (PI) and CRO and corrected the error with the site early on in the study. In a federated model this major error may not have been identified at all or perhaps not until much later in the study.
For international sites, mock runs are executed prior to commencement of the shipping of the study specimens to the biorepository to pre-empt and resolve any possible logistical and/or regulatory issues that might occur. Such issues may include permit requirements, chain of custody, inventory management system checks, receiving workflows, shipping duration and cold chain management. Mock runs assist in indicating adherence to SOPs or identify necessary adjustments needed to be made to the SOPs. This general preparedness for international shipping assists not only the sites involved, but also the biorepository.
2.8. Specimen history and reports
The biorepository maintains an inventory and tracking record of specimen movements. To create a specimen’s history, the data points shown in Table 1 are recorded.
Table 1.
Data points that are recorded in a specialized database to create a specimen’s history.
| Biospecimen Data Fields |
|---|
| Participant ID (de-identified) |
| Visit number |
| Specimen ID code |
| Specimen barcode |
| Collection date and time |
| Collection clinical site ID |
| Specimen arrival at biorepository (date and time) |
| Temperature at arrival (inner specimen holding shipping box) |
| Processing time after arrival at biorepository |
| Processing technician name |
| Volumes received |
| Conditions of shipments and specimens (compliance with IATA regulations) |
| Specimen type (i.e. blood, swabs, biopsies) |
For specimen storage history, all freezer units are equipped with temperature sensors that send hourly temperature readings to a central server and staff have the ability to be remotely monitoring temperature twenty-four hours a day, every day. In the event that a freezer’s temperature begins to go out of the set range, alarm notifications are immediately sent to designated staff members’ cell phone and email address. Monthly reports for each cold storage unit are created and kept on record (Fig. 2).
Fig. 2.
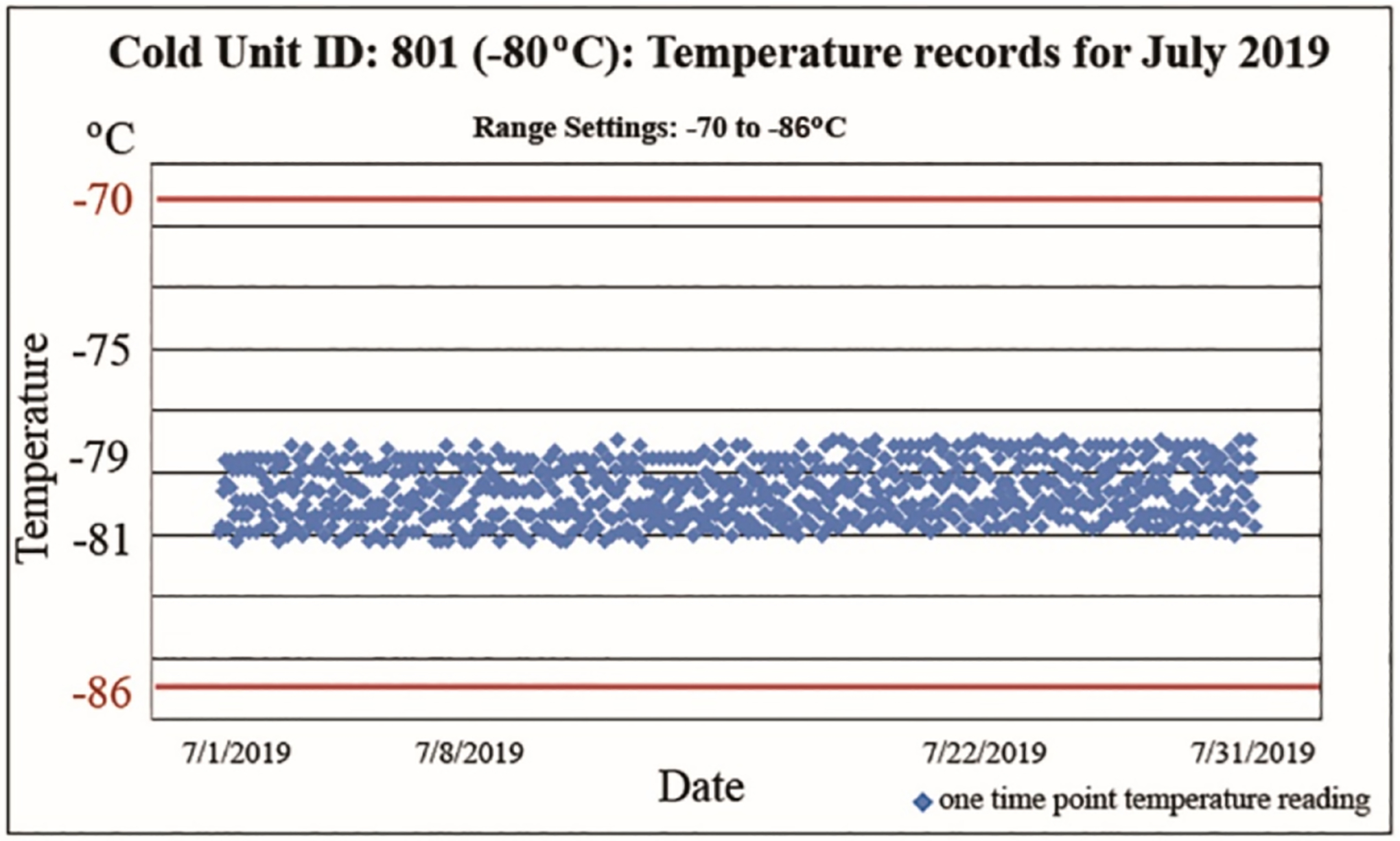
Monthly report of temperature recordings of a −80 °C cold unit as part of a specimen’s history. Every hour, temperature readings are automatically sent to a server and recorded in the log. The temperature range is set from −86 °C to −70 °C indicated by the red lines. The 24 daily data points were entered for entire months, here for the month of July 2019, showing the temperature history of the specimens stored in this specific unit.
In case of a freezer failure, reports of specimen rescue are created including the time of arrival of the rescue team when an incident occurs outside of regular operation hours, the highest temperature of the failing freezer at the time when specimens were removed, and the information of the backup freezer to where the specimens have been moved. Designated freezers are kept empty and cold at corresponding temperatures as backup locations for freezers holding specimens. At all times a specimen rescue schedule is in place with two staff members assigned to the freezer failure response team. The temperature sensors are routinely calibrated according to the American National Standards by an ANAB (ANSI National Accreditation Board owned by the American National Standard Institute) certified laboratory. Furthermore, regular routine maintenance schedules of equipment such as defrosting or cleaning, battery health reports and change, and calibration of pipettes are recorded.
2.9. Shipments to central biorepository
The timing of shipping specimens is very important. For international shipments best practice is to ascertain the duration of the shipment beforehand and then decide on the particular day of shipment. For example, for a 5-day shipment, mostly Fridays are best for shipping, because potential issues with customs can be dealt with during week days thus avoiding delays.
For domestic shipments, it is best to ship only from Monday through Thursday by using overnight express to avoid an unsuccessful delivery attempt at a closed receiving dock of the biorepository on a weekend in case of a courier delay. In addition, the biorepository communicates with the sites well ahead of time around any holiday closures and advises on the last date to ship prior to closures.
Couriers mostly use central hubs. The biorepository advises the sites to check on the weather conditions at the location of these central hubs during hurricane and winter storm seasons, and if necessary, request a hold on shipments. To mitigate the risk to specimen integrity by courier delays, approximately ten pounds of dry ice are required for shipments of frozen specimens allowing for an additional day of delay. For shipment of refrigerated specimens, altering the recommended number of cold packs from winter to summer seasons may prevent shipping temperatures to go out of recommended range (2–8 °C). For example, in summer heat we recommend to the sites to add an additional cold pack. In winter when we observe shipments arriving with temperatures below 2 °C, the recommendation would be to reduce the number of cold packs [24]. In rare cases, when shipping temperatures are way out of the allowed range in the absence of delivery delays and we cannot identify the cause, we have the sites place temperature data loggers in the shipments.
2.10. Disbursement of specimens
When clinical sites participating in multi-site clinical trials collect study specimens, hold them on-site until distribution to the appropriate outside laboratories contracted for specific explorative studies, they may not aliquot to the minimum needed volumes. Therefore, core and other laboratories may receive more of the specimens than needed, resulting in specimen waste and minimizing specimen availability for possible additional studies. Specimens held at separate international sites might be delayed in arriving at their destinations causing disruption of testing. Furthermore, at both national and international sites, these sites may batch and hold specimens until the achievement of a cost-effective sample number to establish a “run.” Excess specimens may be refrozen to have “on hand” in case technical repeats are needed, or to return after the completion of the protocol studies, possibly compromising the integrity of biospecimens by freeze-thaw cycling. In our experience, study sites often do not have the resources and the time to optimize aliquoting. A central biorepository operating under Best Practices would most likely have processed and stored specimens to the amounts necessary for further downstream testing. Therefore, upon request or at specified times per study protocol, specimens to be disbursed will ideally already be aliquoted to meet the needs for designated study research locations.
Working in pairs, the central biorepository staff removes only the number of aliquoted samples requested for particular tests from freezers, produces labels for distribution, creates the shipping manifest and packages the specimens for shipping. Two staff handle these procedures with each person checking each procedure along each step. During these processes, temperatures of specimens, as determined per protocol, are maintained all the time by use of benchtop freezers, mobile ice containers or cryo-carts until packaging for shipping is completed. Depending on the protocol, shipping will occur at specified temperatures using ambient temperatures, ice packs, dry ice or shippers that can accommodate liquid nitrogen. If international shipping is specified, companies that will follow the specimens through customs and ensure a proper temperature history are used.
2.11. Problem solving
One of the main advantages to having a central biorepository is that it serves as a critical checkpoint in maintaining the standardization and integrity of specimen collection through the implementation of a robust quality management system or plan. For example, if deviations from the bMOP are noted, the biorepository can immediately report the issue to the clinical site and provide feedback on the issue requesting corrections if possible. This centralized checkpoint ensures that downstream procedures will occur on the proper specimens.
To minimize or correct problems, one of the main advantages of a central biorepository is having one entity communicate with the sites. The biorepository by immediately communicating any problems uncovered to the clinical site, tries to get an understanding of what exactly the obstacle is that the site faces and helps develop a site-specific solution. For example, we had one site sending sample vials on which the barcode label was sticking out over the vial edge. This caused problems placing the vials in the slots of the freezer storage boxes. Upon inquiry it turned out that the site was operated by a physician without an assistant. The physician had to label the vials personally but had difficulty placing the barcode labels correctly due to arthritic hands. From then on, the biorepository sent the physician barcode-labeled empty vials.
Further actions to mitigate problems can include clarifying and updating the bMOP on a regular basis and providing training and instruction materials such as pictures, PowerPoint slides and/or demonstration videos. If recurring issues cannot be resolved by regular communication, re-training can occur. The implementation of individual site SOPs may be helpful to avoid most issues. In general, attention to detail and careful reading of the bMOP and SOP, implementing color coding for different specimen types and internal checklists may help with a smoother workflow and avoid issues.
3. Discussion
Centralized biobanking offers benefits like standardization and harmonization of procedures, quality management, tracking, distribution and implementation of all regulatory requirements by designated trained personnel. Without a central biorepository, the collection and storage of specimens for multisite clinical trials occurs at multiple individual sites, which are often academic medical centers and private physician offices. One argument for the federated model may be the perception that the site PI has control over the specimens [25]. However, research plans for use of clinical research specimens are often approved as part of the study protocol. Any use of specimens that were collected by NIH-funded trials must be reviewed and approved by oversight committees such as the Clinical and Translational Epidemiology Branch (CTEB). Moreover, since site PIs often are not involved in bench research, they may not have a vested interest in controlling the specimens they collect. In addition, these individual facilities must have certain capabilities as a prerequisite to participate in the trials, including appropriate infrastructure related to specimen accrual, processing, short term storage and distribution.
Although a central biorepository cannot control specimen collections and preanalytical variables from a multisite clinical trial, it can harmonize the processes that occur until the specimens reach the testing sites specified in the protocol. The establishment of one well-defined quality standard that provides requirements, specifications and guidelines is necessary to ensure that materials and processes are fit for their purpose, so important in clinical research. A uniform approach at every level of operation including standards for handling, storage, data collection and data entry is required. Possible errors at every stage in protocols can be avoided through correction of data or resending of correct specimens. Through these means, a central biorepository can ensure the proper fit for purpose of protocol specimen testing and support of publication of results.
In summary, a central biorepository for a multi-site clinical trial network adds value to a multisite clinical trial by ensuring accountability, traceability and quality control. A central biorepository that follows best practices and follows a quality management system will provide high quality, standardized specimens. Also important is the development of the specimen collection bMOP and the training and education of the staff. The quality management of specimen integrity brought through the use of a central biorepository can be of benefit for the planning and execution of multi-site investigations. In our experience, the use of a centralized biorepository model has greatly increased the availability and use of high-quality specimens for meaningful and relevant research outcomes. Various authors have demonstrated that intra-laboratory variability in the handling and processing of clinical research specimens occurs [26–28]. Some differences are due to use of different instrumentation but differences in the handling and processing of specimens is also cited. As the biorepository that handles the specimens for the clinical trials, we do not hold outcome experience, but our contribution is to assure a centralized practice of quality management once the participant specimens reach us and continues through processing, storage and distribution to the laboratories for testing.
The specifics discussed here can be a framework for implementation for a multi-site clinical trial network or other multi-site research network.
Abbreviations:
- ANSI
American National Standard Institute
- ANAB
ANSI National Accreditation Board
- BBRB
Biorepositories and Biospecimen Research Branch
- bMOP
Biospecimen Manual of Procedures
- CRO
Clinical Research Organization
- CTEB
Clinical and Translational Epidemiology Branch
- FDA
Food and drug Administration
- ISBER
International Society for Biological and Environmental Repositories
- IRB
Institutional Review Board
- MOOs
Manuals of Operations
- NIH
National Institute of Health
- NCI
National Cancer Institute
- OHRB
Office of Human Research Protections
- PI
Principal Investigator
- SOPs
Standard Operation Procedures
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Al Diffalha S, Sexton KC, Watson PH, et al. , The importance of human tissue bioresources in advancing biomedical research, Biopreserv. Biobank. 17 (2019) 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ginsburg GS, Burke RW, Febbo P, Centralized biorepositories for genetic and genomic research, JAMA 299 (2008) 1359–1361. [DOI] [PubMed] [Google Scholar]
- [3].De Souza YG, Greenspan JS, Biobanking past, present and future: responsibilities and benefits, AIDS 27 (2013) 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Simeon-Dubach D, Burt AD, Quality really matters: the need to improve specimen quality in biomedical research, J. Pathol 228 (2012) 431–433. [DOI] [PubMed] [Google Scholar]
- [5].Engel KB, Vaught J, Moore HM, National Cancer Institute biospecimen evidence-based practices: a novel approach to pre-analytical standardization, Biopreserv. Biobank. 12 (2014) 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hartman V, Matzke L, Watson PH, Biospecimen complexity and the evolution of biobanks, Biopreserv. Biobank. 17 (2019) 264–270. [DOI] [PubMed] [Google Scholar]
- [7].Rudloff U, Bhanot U, Gerald W, et al. , Biobanking of human pancreas cancer tissue: impact of ex-vivo procurement times on RNA quality, Ann. Surg. Oncol 17 (2010) 2229–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jewell SD, Srinivasan M, McCart LM, et al. , Analysis of the molecular quality of human tissues: an experience from the cooperative human tissue network, Am. J. Clin. Pathol 118 (2002) 733–741. [DOI] [PubMed] [Google Scholar]
- [9].National Cancer Institute, NCI Best Practices for Biospecimen Resources. https://biospecimens.cancer.gov/bestpractices/2016-NCIBestPractices.pdf, 2016.
- [10].Campbell LD, Astrin JJ, DeSouza Y, et al. , The 2018 revision of the ISBER best practices: summary of changes and the editorial team’s development process, Biopreserv. Biobank. 16 (2018) 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].ISBER (International Society for Biological and Environmental Repositories), 2012 Best Practices for repositories: collection, storage, retrieval, and distribution of biological materials for research, Biopreserv. Biobank 10 (2012) 79–161. [DOI] [PubMed] [Google Scholar]
- [12].Organisation for Economic Co-operation and Development (OECD), Chemical Safety and Biosafety, Available at, https://www.oecd.org/chemicalsafety, 2021.
- [13].International Organization for Standardization (ISO), Standards, Available at, https://www.iso.org/standards.html, 2021.
- [14].Hallmans G, Vaught JB, Best practices for establishing a biobank, Methods Mol. Biol 675 (2011) 241–260. [DOI] [PubMed] [Google Scholar]
- [15].Borisova AL, Pokrovskaya MS, Meshkov AN, et al. , ISO 20387 Biobanking Standard. Analysis of requirements and experience of implementation, Klin. Lab. Diagn 65 (2020) 587–592, 10.18821/0869-2084-2020-65-9-587-592. [DOI] [PubMed] [Google Scholar]
- [16].Canadian Tissue Repository Network (CTRNet), Certification Program. https://www.ctrnet.ca/en/home/, 2021.
- [17].College of American Pathologists (CAP), Biorepository Accreditation Program. https://www.cap.org/laboratory-improvement/accreditation/biorepository-accreditation-program, 2021.
- [18].Gordon VM, Culp MA, Wolinetz CD, Final NIH policy on the use of a single institutional review board for multisite research, Clin. Transl. Sci 10 (2017) 130–132, 10.1111/cts.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Menikoff J, The paradoxical problem with multiple-IRB review, N. Engl. J. Med 363 (2010) 1591–1593. [DOI] [PubMed] [Google Scholar]
- [20].Jansen LA, Local IRBs, multicenter trials, and the ethics of internal amendments, IRB. 27 (2005) 7–11. [PubMed] [Google Scholar]
- [21].Croxton T, Swanepoel C, Musinguzi H, et al. , Lessons learned from biospecimen shipping among the human heredity and health in Africa biorepositories, Biopreserv. Biobank. 15 (2017) 103–110, 10.1089/bio.2017.0009. [DOI] [Google Scholar]
- [22].Moore HM, Kelly AB, Jewell SD, et al. , Biospecimen reporting for improved study quality (BRISQ), Cancer Cytopathol. 119 (2011) 92–101. [DOI] [PubMed] [Google Scholar]
- [23].Vaught JB, Henderson MK, Biological sample collection, processing, storage and information management, IARC Sci. Publ 163 (2011) 23–42. [PubMed] [Google Scholar]
- [24].Gordy D, Tashjian RS, Lee H, et al. , Domestic and international shipping of biospecimens, Methods Mol. Biol 1897 (2019) 433–443, 10.1007/978-1-4939-8935-5_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hewitt R, Hainaut P, Biobanking in a fast moving world: an international perspective, J. Natl. Cancer Inst. Monogr 2011 (2011) 50–51, 10.1093/jncimonographs/lgr005. [DOI] [PubMed] [Google Scholar]
- [26].Bainbridge J, Wilkening CL, Rountree W, et al. , The immunology quality assessment proficiency testing program for CD3+4+ and CD3+8+ lymphocyte subsets: a ten year review via longitudinal mixed effects modeling, J. Immunol. Methods 409 (2014) 82–90, 10.1016/j.jim.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schöffl C, Haas A, Herrmann M, et al. , The crux of C1-INH testing in everyday lab work, J. Immunol. Methods 497 (2021) 113109, 10.1016/j.jim.2021.113109. [DOI] [PubMed] [Google Scholar]
- [28].Fei DT, Paxton H, Chen AB, Difficulties in precise quantitation of CD4+ T lymphocytes for clinical trials: a review, Biologicals. 21 (1993) 221–231, 10.1006/biol.1993.1079. [DOI] [PubMed] [Google Scholar]


